Experimental Investigation of Sludge Treatment Using a Rotor-Stator Type Hydrodynamic Cavitation Reactor and an Ultrasonic Bath
Abstract
1. Introduction
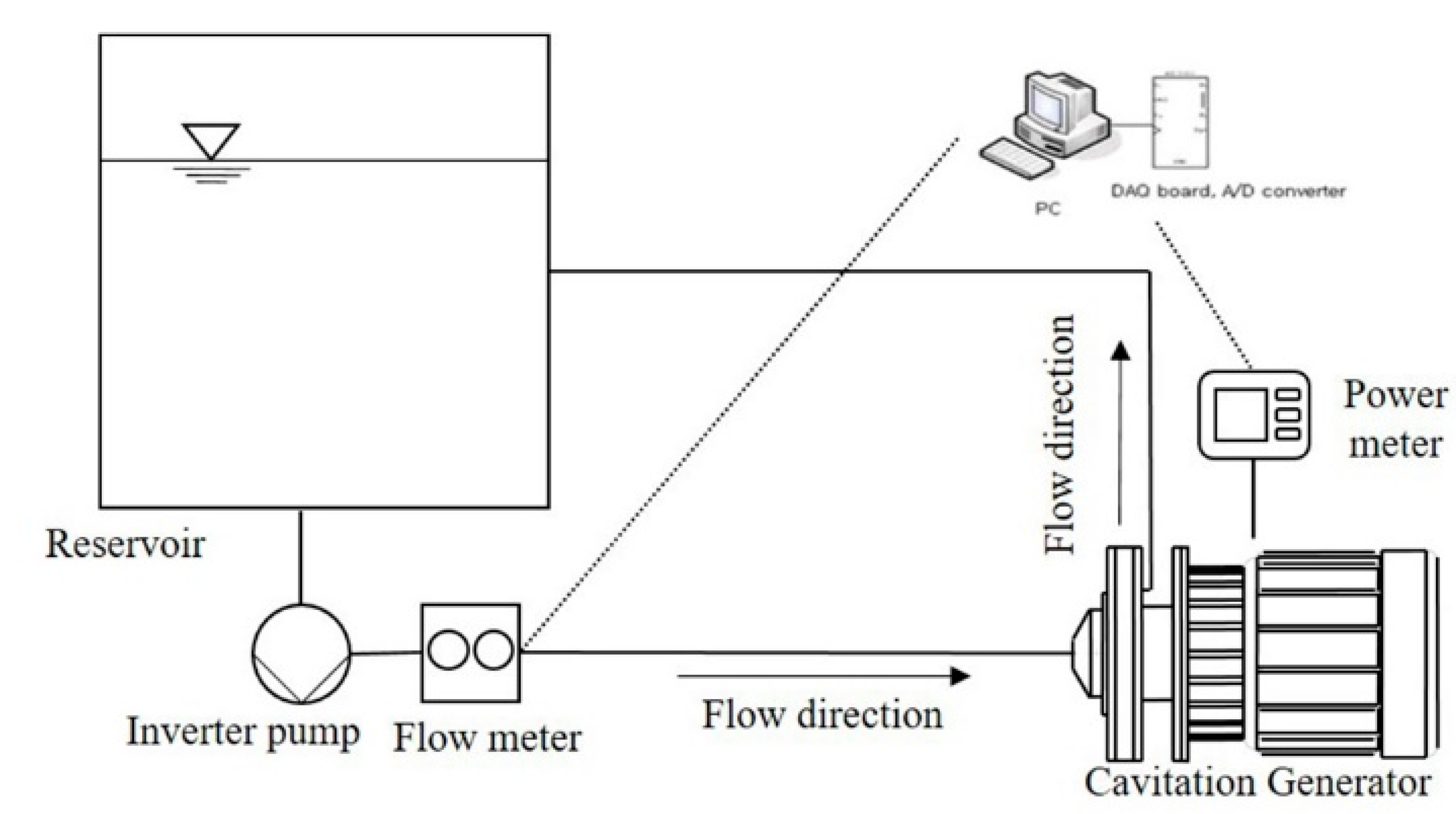
2. Experimental Methods
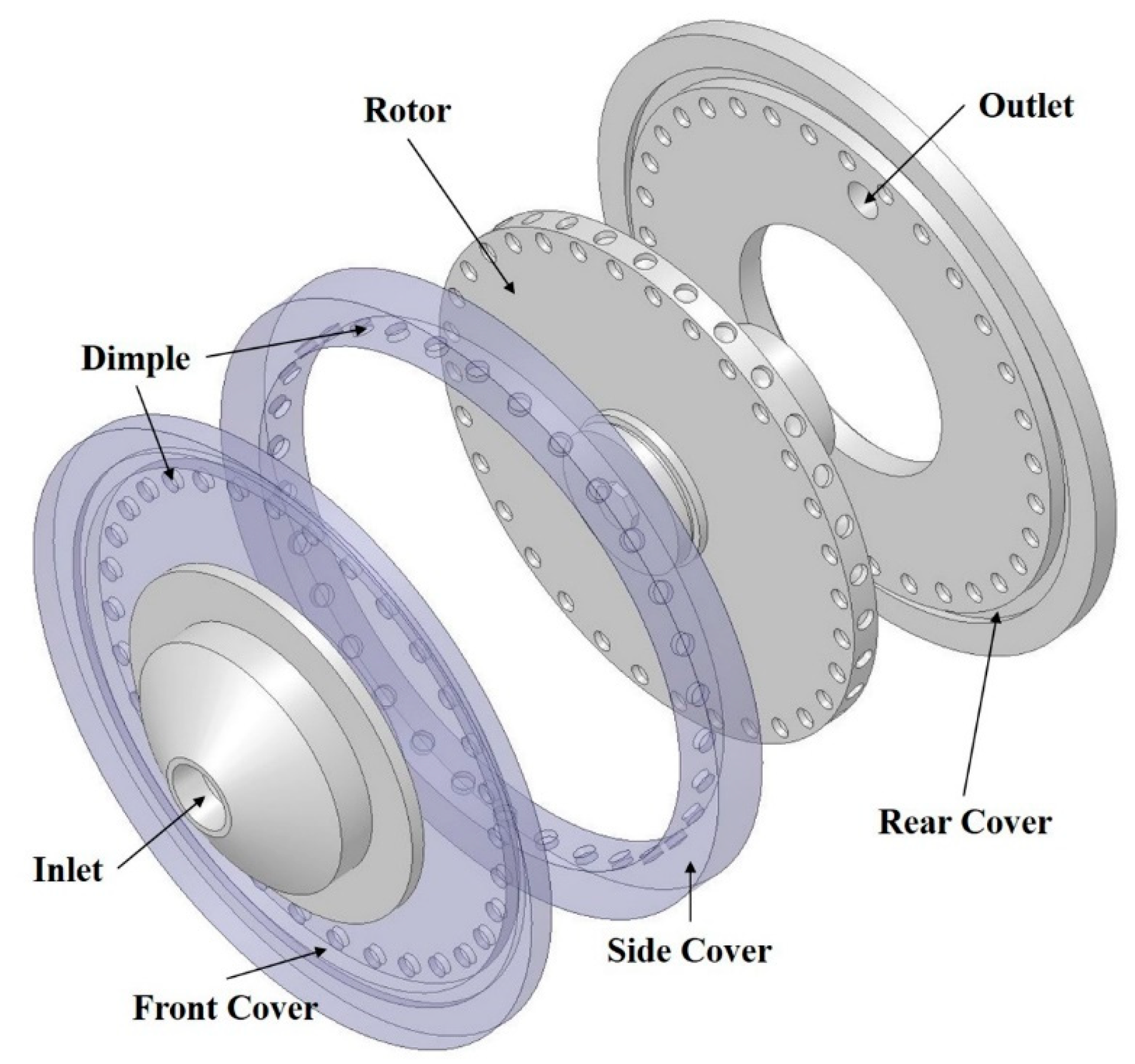
2.1. Rotor-Stator Type Hydrodynamic Cavitation Reactor
2.2. Ultrasonic Bath
2.3. Properties of Sludge
2.4. Experimental Cases
2.5. Analytical Methods
3. Results and Discussion
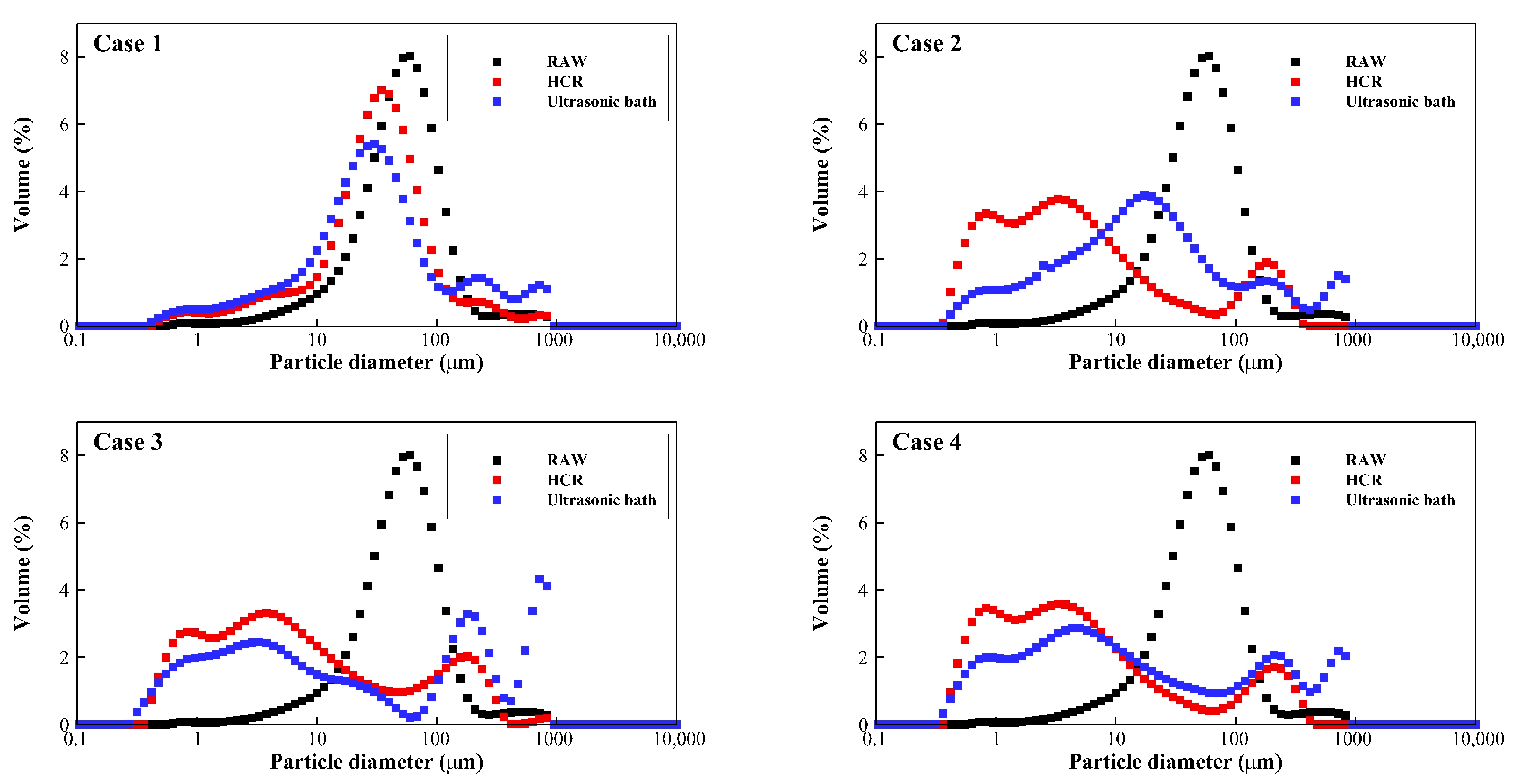
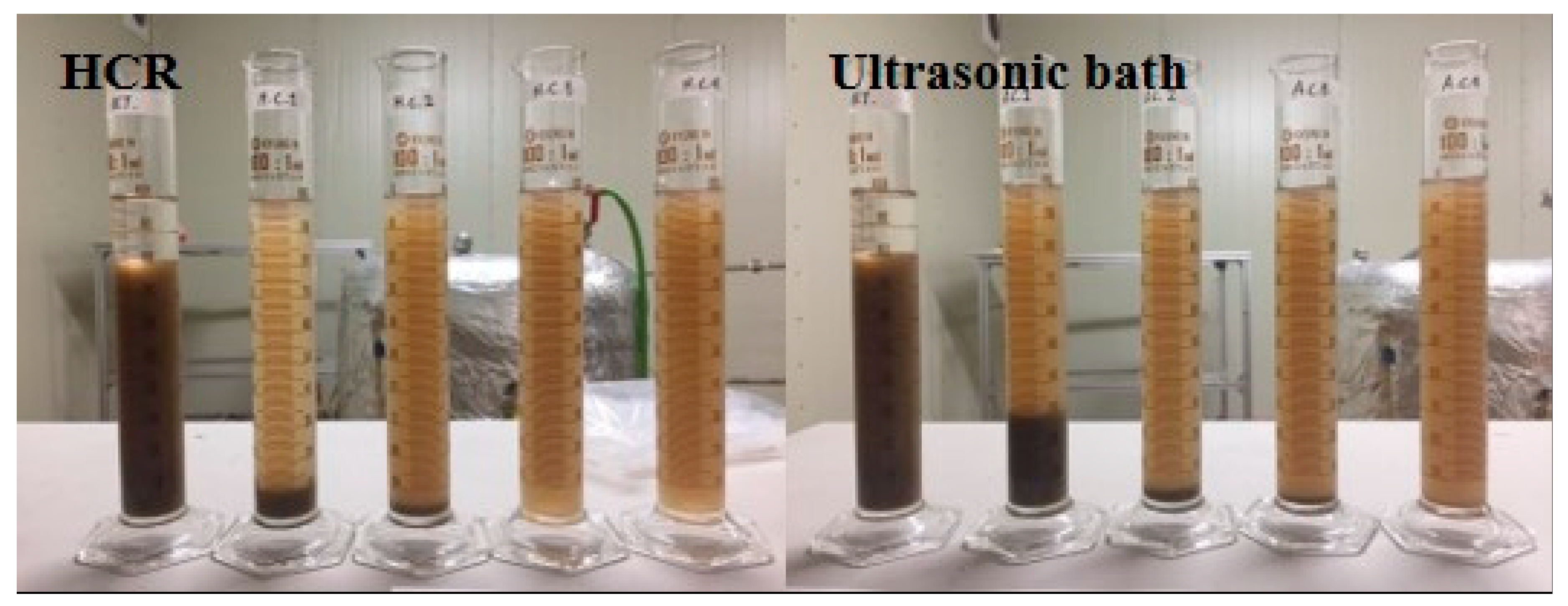
3.1. Decomposition Performance
3.2. Oxidation Performance
3.3. Solubilization Performance
4. Conclusions
- Particle decomposition of the rotor-stator type HCR is superior to that of the ultrasonic bath. After 10 treatments, the median particle size decreased by 92.7% in the rotor-stator type HCR, compared to a median particle size reduction of 67.6% at the same specific energy input in an ultrasonic bath. Also, through the results of SVI, it was discovered that the rotor-stator type HCR decomposed the sludge much more uniformly compared to the ultrasonic bath.
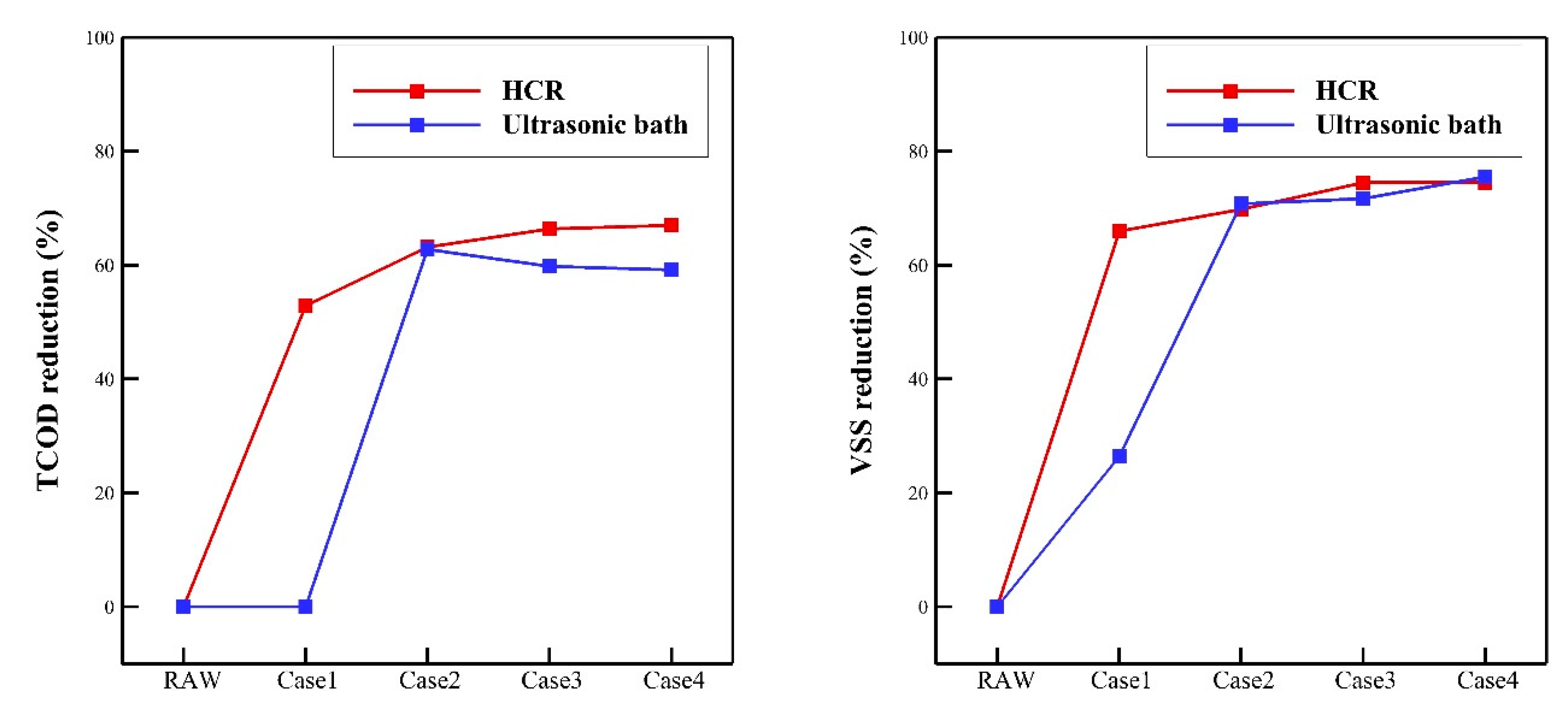
- A comparison of the TCOD and VSS reduction rates revealed that the rotor-stator type HCR had superior oxidation performance over the ultrasonic bath. In case 1, which had the lowest specific energy input, the rotor-stator type HCR reduced the TCOD and VSS by 53% and 66%, respectively. On the other hand, the ultrasonic bath reduced the TCOD and VSS by 0% and 26%, respectively. In case 4, which had the highest specific energy input, the rotor-stator type HCR showed oxidation performance; the reduction rate of TCOD and VSS were calculated as 67% and 74%, respectively.
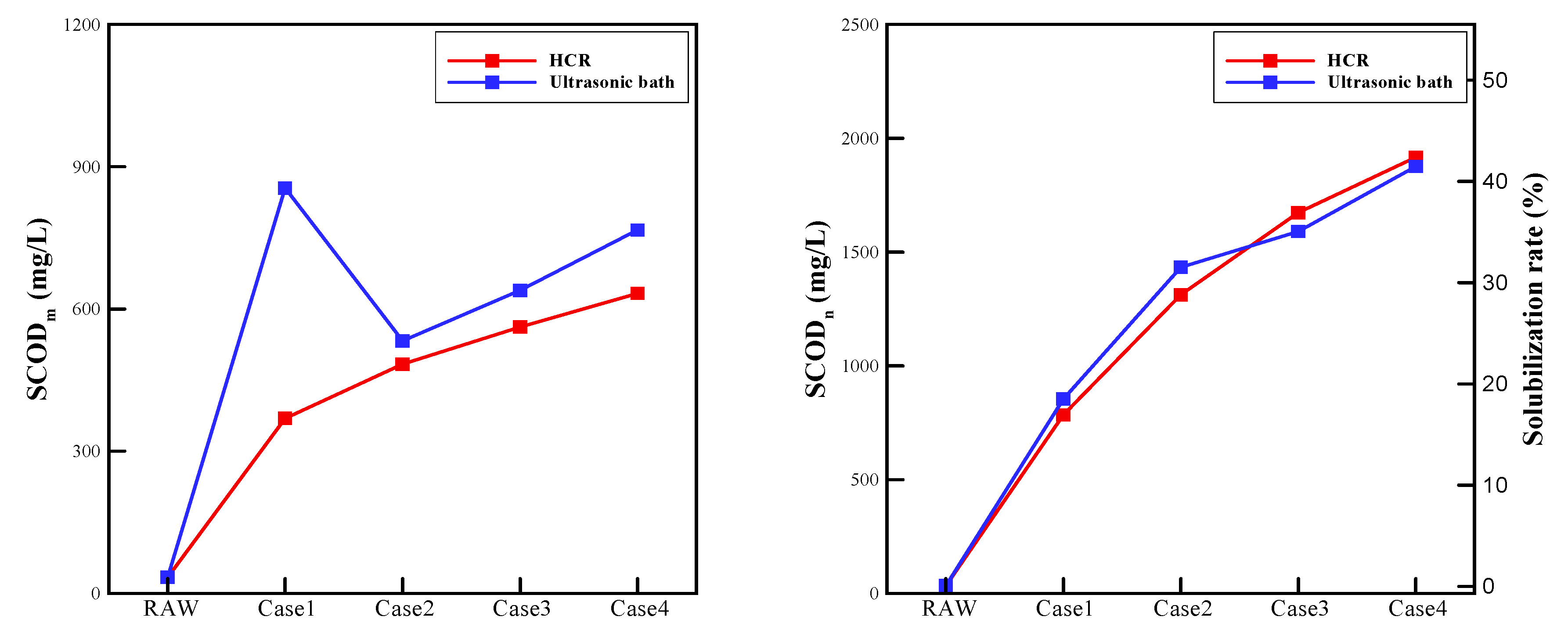
- As shown through and the solubilization rate, the two devices showed similar solubilization performance. Since particle disintegration and oxidation counteract each other, in case 1 and case 2, the ultrasonic bath exhibited only a slightly higher solubilization rate. In contrast, the rotor-stator type HCR in case 3 and case 4 showed a slightly higher solubilization rate. In case 1, which shows significant different oxidation performances, the values were considerably different.
Author Contributions
Funding
Conflicts of Interest
References
- Bagal, M.V.; Gogate, P.R. Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: A review. Ularason. Sonochem. 2014, 21, 1–14. [Google Scholar] [CrossRef]
- Cvetković, M.; Kompare, B.; Klemenčič, A.K. Application of hydrodynamic cavitation in ballast water treatment. Environ. Sci. Pollut. R 2015, 22, 7422–7438. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Ai, S.; Liu, H.; Wu, M.; Zeng, G.; Yang, C. Roles of acid-producing bacteria in anaerobic digestion of waste activated sludge. Front. Env. Sci. Eng. 2018, 12, 3. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Su, L.; Kobayashi, T.; Kumar, G.; Zhou, T.; Xu, K.; Li, Y.-Y.; Zhu, X.; Zhao, Y. Unraveling the catalyzing behaviors of different iron species (Fe2+ vs. Fe0) in activating persulfate-based oxidation process with implications to waste activated sludge dewaterability. Water Res. 2018, 134, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R. Treatment of wastewater streams containing phenolic compounds using hybrid techniques based on cavitation: A review of the current status and the way forward. Ularason. Sonochem. 2008, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.N.; Gogate, P.R.; Csoka, L.; Dregelyi-Kiss, A.; Horvath, M. Intensification of biogas production using pretreatment based on hydrodynamic cavitation. Ularason. Sonochem. 2016, 30, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Rayleigh, L., VIII. On the pressure developed in a liquid during the collapse of a spherical cavity. Philos. Mag. 1917, 34, 94–98. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Dębowski, M.; Kisielewska, M.; Nowicka, A.; Rokicka, M.; Szwarc, K. Comparison of Ultrasonic and Hydrothermal Cavitation Pretreatments of Cattle Manure Mixed with Straw Wheat on Fermentative Biogas Production. Waste Biomass Valori. 2017, 10, 747–754. [Google Scholar] [CrossRef]
- Ruiz-Hernando, M.; Martinez-Elorza, G.; Labanda, J.; Llorens, J. Dewaterability of sewage sludge by ultrasonic, thermal and chemical treatments. Chem. Eng. J. 2013, 230, 102–110. [Google Scholar] [CrossRef]
- Pawar, S.K.; Mahulkar, A.V.; Pandit, A.B.; Roy, K.; Moholkar, V.S. Sonochemical effect induced by hydrodynamic cavitation: Comparison of venturi/orifice flow geometries. AIChE J. 2017, 63, 4705–4716. [Google Scholar] [CrossRef]
- Tao, Y.; Cai, J.; Huai, X.; Liu, B. A novel antibiotic wastewater degradation technique combining cavitating jets impingement with multiple synergetic methods. Ularason. Sonochem. 2018, 44, 36–44. [Google Scholar] [CrossRef]
- Pilli, S.; Bhunia, P.; Yan, S.; LeBlanc, R.J.; Tyagi, R.D.; Surampalli, R.Y. Ultrasonic pretreatment of sludge: A review. Ularason. Sonochem. 2011, 18, 1–18. [Google Scholar] [CrossRef]
- Jorand, F.; Zartarian, F.; Thomas, F.; Block, J.C.; Bottero, J.Y.; Villemin, G.; Urbain, V.; Manem, J. Chemical and structural (2D) linkage between bacteria within activated sludge flocs. Water Res. 1995, 29, 1639–1647. [Google Scholar] [CrossRef]
- Sivakumar, M.; Pandit, A.B. Wastewater treatment: A novel energy efficient hydrodynamic cavitational technique. Ularason. Sonochem. 2002, 9, 123–131. [Google Scholar] [CrossRef]
- Badve, M.; Gogate, P.; Pandit, A.; Csoka, L. Hydrodynamic cavitation as a novel approach for wastewater treatment in wood finishing industry. Sep. Purif. Technol. 2013, 106, 15–21. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Highly effective degradation of selected groups of organic compounds by cavitation based AOPs under basic pH conditions. Ularason. Sonochem. 2018, 45, 257–266. [Google Scholar] [CrossRef]
- Milly, P.J.; Toledo, R.T.; Chen, J.; Kazem, B. Hydrodynamic cavitation to improve bulk fluid to surface mass transfer in a nonimmersed ultraviolet system for minimal processing of opaque and transparent fluid foods. J. Food Sci. 2007, 72, M407–M413. [Google Scholar] [CrossRef] [PubMed]
- Milly, P.J.; Toledo, R.T.; Harrison, M.A.; Armstead, D. Inactivation of food spoilage microorganisms by hydrodynamic cavitation to achieve pasteurization and sterilization of fluid foods. J. Food Sci. 2007, 72, M414–M422. [Google Scholar] [CrossRef] [PubMed]
- Milly, P.J.; Toledo, R.T.; Kerr, W.L.; Armstead, D. Hydrodynamic cavitation: Characterization of a novel design with energy considerations for the inactivation of Saccharomyces cerevisiae in apple juice. J. Food Sci. 2008, 73, M298–M303. [Google Scholar] [CrossRef] [PubMed]
- Arrojo, S.; Benito, Y.; Tarifa, A.M. A parametrical study of disinfection with hydrodynamic cavitation. Ularason. Sonochem. 2008, 15, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Patil, L.; Gogate, P.R. Large scale emulsification of turmeric oil in skimmed milk using different cavitational reactors: A comparative analysis. Chem. Eng. Process. Process. Intensif. 2018, 126, 90–99. [Google Scholar] [CrossRef]
- Yi, C.; Lu, Q.; Wang, Y.; Wang, Y.; Yang, B. Degradation of organic wastewater by hydrodynamic cavitation combined with acoustic cavitation. Ularason. Sonochem. 2018, 43, 156–165. [Google Scholar] [CrossRef]
- Petkovšek, M.; Zupanc, M.; Dular, M.; Kosjek, T.; Heath, E.; Kompare, B.; Širok, B. Rotation generator of hydrodynamic cavitation for water treatment. Sep. Purif. Technol. 2013, 118, 415–423. [Google Scholar] [CrossRef]
- Badve, M.P.; Gogate, P.R.; Pandit, A.B.; Csoka, L. Hydrodynamic cavitation as a novel approach for delignification of wheat straw for paper manufacturing. Ularason. Sonochem. 2014, 21, 162–168. [Google Scholar] [CrossRef]
- Zupanc, M.; Kosjek, T.; Petkovšek, M.; Dular, M.; Kompare, B.; Širok, B.; Stražar, M.; Heath, E. Shear-induced hydrodynamic cavitation as a tool for pharmaceutical micropollutants removal from urban wastewater. Ularason. Sonochem. 2014, 21, 1213–1221. [Google Scholar] [CrossRef]
- Šarc, A.; Kosel, J.; Stopar, D.; Oder, M.; Dular, M. Removal of bacteria Legionella pneumophila, Escherichia coli, and Bacillus subtilis by (super) cavitation. Ularason. Sonochem. 2018, 42, 228–236. [Google Scholar] [CrossRef]
- Sun, X.; Kang, C.H.; Park, J.J.; Kim, H.S.; Om, A.S.; Yoon, J.Y. An experimental study on the thermal performance of a novel hydrodynamic cavitation reactor. Exp. Therm. Fluid Sci. 2018, 99, 200–210. [Google Scholar] [CrossRef]
- Sun, X.; Park, J.J.; Kim, H.S.; Lee, S.H.; Seong, S.J.; Om, A.S.; Yoon, J.Y. Experimental investigation of the thermal and disinfection performances of a novel hydrodynamic cavitation reactor. Ularason. Sonochem. 2018, 49, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Cerecedo, L.M.; Dopazo, C.; Gomez-Lus, R. Water disinfection by hydrodynamic cavitation in a rotor-stator device. Ularason. Sonochem. 2018, 48, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Langone, M.; Andreottola, G. A swirling jet-induced cavitation to increase activated sludge solubilisation and aerobic sludge biodegradability. Ularason. Sonochem. 2017, 35, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, T.; Nishimura, M.; Yoshino, H.; Kato, H.; Nonokuchi, M.; Fujii, T.; Satoh, H.; Terada, A.; Hosomi, M. High-pressure jet device for activated sludge reduction: Feasibility of sludge solubilization. Biochem. Eng. J. 2015, 100, 1–8. [Google Scholar] [CrossRef]
- Nabi, M.; Zhang, G.; Zhang, P.; Tao, X.; Wang, S.; Ye, J.; Zhang, Q.; Zubair, M.; Bao, S.; Wu, Y. Contribution of solid and liquid fractions of sewage sludge pretreated by high pressure homogenization to biogas production. Bioresour. Technol. 2019, 286, 121378. [Google Scholar] [CrossRef]
- Cai, M.; Hu, J.; Lian, G.; Xiao, R.; Song, Z.; Jin, M.; Dong, C.; Wang, Q.; Luo, D.; Wei, Z. Synergetic pretreatment of waste activated sludge by hydrodynamic cavitation combined with Fenton reaction for enhanced dewatering. Ularason. Sonochem. 2018, 42, 609–618. [Google Scholar] [CrossRef]
- Grübel, K.; Suschka, J. Hybrid alkali-hydrodynamic disintegration of waste-activated sludge before two-stage anaerobic digestion process. Environ. Sci. Pollut. R 2015, 22, 7258–7270. [Google Scholar] [CrossRef]
- Jung, K.W.; Hwang, M.J.; Yun, Y.M.; Cha, M.J.; Ahn, K.H. Development of a novel electric field-assisted modified hydrodynamic cavitation system for disintegration of waste activated sludge. Ularason. Sonochem. 2014, 21, 1635–1640. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, P.; Zhang, G.; Jin, S.; Li, D.; Zhang, M.; Xu, X. Effect of alkaline addition on anaerobic sludge digestion with combined pretreatment of alkaline and high pressure homogenization. Bioresour. Technol. 2014, 168, 167–172. [Google Scholar] [CrossRef]
- Lee, I.; Han, J.-I. The effects of waste-activated sludge pretreatment using hydrodynamic cavitation for methane production. Ularason. Sonochem. 2013, 20, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, P.; Zhang, G.; Ma, W.; Wu, H.; Ma, B. Sewage sludge disintegration by combined treatment of alkaline+high pressure homogenization. Bioresour. Technol. 2012, 123, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, P.; Zhang, G.; Fan, J.; Zhang, Y. Enhancement of anaerobic sludge digestion by high-pressure homogenization. Bioresour. Technol. 2012, 118, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, K.; Asano, R.; Yokoyama, A.; Okazaki, M.; Sakamoto, A.; Nakai, Y. Reduction in excess sludge production in a dairy wastewater treatment plant via nozzle-cavitation treatment: Case study of an on-farm wastewater treatment plant. Bioresour. Technol. 2009, 100, 3161–3166. [Google Scholar] [CrossRef] [PubMed]
- Petkovšek, M.; Mlakar, M.; Levstek, M.; Stražar, M.; Širok, B.; Dular, M. A novel rotation generator of hydrodynamic cavitation for waste-activated sludge disintegration. Ularason. Sonochem. 2015, 26, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.W.; McCabe, B.K. Review of pre-treatments used in anaerobic digestion and their potential application in high-fat cattle slaughterhouse wastewater. Appl. Energy 2015, 155, 560–575. [Google Scholar] [CrossRef]
- Bandelin, J.; Lippert, T.; Drewes, J.E.; Koch, K. Cavitation field analysis for an increased efficiency of ultrasonic sludge pre-treatment using a novel hydrophone system. Ularason. Sonochem. 2018, 42, 672–678. [Google Scholar] [CrossRef]
- Kwon, W.C.; Yoon, J.Y. Experimental study of a cavitation heat generator. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2013, 227, 67–73. [Google Scholar] [CrossRef]
- Dewil, R.; Baeyens, J.; Goutvrind, R. Ultrasonic treatment of waste activated sludge. Environ. Prog. 2006, 25, 121–128. [Google Scholar] [CrossRef]
- Tiehm, A.; Nickel, K.; Zellhorn, M.; Neis, U. Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Water Res. 2001, 35, 2003–2009. [Google Scholar] [CrossRef]
- Feng, X.; Lei, H.; Deng, J.; Yu, Q.; Li, H. Physical and chemical characteristics of waste activated sludge treated ultrasonically. Chem. Eng. Process. Process. Intensif. 2009, 48, 187–194. [Google Scholar] [CrossRef]
- Mahmoud, N.; Zeeman, G.; Gijzen, H.; Lettinga, G. Solids removal in up flow anaerobic reactors, a review. Bioresour. Technol. 2003, 90, 1–9. [Google Scholar] [CrossRef]
- Huan, L.; Yiying, J.; Mahar, R.B.; Zhiyu, W.; Yongfeng, N. Effects of ultrasonic disintegration on sludge microbial activity and dewaterability. J. Hazard. Mater. 2009, 161, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.L.; Xu, Y.X.; Fang, Y.Y.; Chen, X.R. Sludge disintegration using a hydrocyclone to improve biological nutrient removal and reduce excess sludge. Sep. Purif. Technol. 2017, 177, 192–199. [Google Scholar] [CrossRef]
- Wu, Z.; Yuste-Córdoba, F.J.; Cintas, P.; Wu, Z.; Boffa, L.; Mantegna, S.; Cravotto, G. Effects of ultrasonic and hydrodynamic cavitation on the treatment of cork wastewater by flocculation and Fenton processes. Ularason. Sonochem. 2018, 40, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B.; Mdleleni, M.M.; Wong, M. Acoustic cavitation and its chemical consequences. Philos. TR Soc. A 1999, 357, 335. [Google Scholar] [CrossRef]
- Zorba Gozde, T.; Sanin, F.D. Disintegration of Sludge by Sonication and Improvement of Methane Production Rates in Batch Anaerobic Digesters. CLEAN-Soil Air Water 2012, 41, 396–402. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, S.R.; Nam, Y.K.; Yang, J.; Park, C.; Lee, M. Disintegration of excess activated sludge by hydrogen peroxide oxidation. Desalination 2009, 246, 275–284. [Google Scholar] [CrossRef]
- Weissler, A.; Cooper, H.W.; Snyder, S. Chemical Effect of Ultrasonic Waves: Oxidation of Potassium Iodide Solution by Carbon Tetrachloride. J. Am. Chem. Soc. 1950, 72, 1769–1775. [Google Scholar] [CrossRef]
- Bougrier, C.; Albasi, C.; Delgenès, J.P.; Carrère, H. Effect of ultrasonic, thermal and ozone pre-treatments on waste activated sludge solubilisation and anaerobic biodegradability. Chem. Eng. Process. Process. Intensif. 2006, 45, 711–718. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, P.; Zeng, G.; Liu, J.; Ye, J.; Zhang, H.; Fang, W.; Li, Y.; Fang, Y. Combined sludge conditioning of micro-disintegration, floc reconstruction and skeleton building (KMnO4/FeCl3/Biochar) for enhancement of waste activated sludge dewaterability. J. Taiwan Inst. Chem. E 2017, 74, 121–128. [Google Scholar] [CrossRef]
- Wang, R.; Liu, J.; Hu, Y.; Zhou, J.; Cen, K. Ultrasonic sludge disintegration for improving the co-slurrying properties of municipal waste sludge and coal. Fuel Process. Technol. 2014, 125, 94–105. [Google Scholar] [CrossRef]

| Characteristics | Unit | Value |
|---|---|---|
| pH | - | 6.48 |
| Total chemical oxygen demand | mg/L | 4480 |
| Soluble chemical oxygen demand | mg/L | 34 |
| Volatile suspended solids | mg/L | 2120 |
| Total solids | mg/L | 3726.5 |
| Specific Energy Input (kJ/kgTS) | Number of Passes (HCR) | Ultrasonication Time (Ultrasonic Bath) | |
|---|---|---|---|
| Case 1 | 84,530 | 5 | 26 min 15 s |
| Case 2 | 167,718 | 10 | 52 min 5 s |
| Case 3 | 248,491 | 15 | 1 h 17 min 10 s |
| Case 4 | 329,800 | 20 | 1 h 42 min 25 s |
| Item | Percentile Size (μm) | |||
|---|---|---|---|---|
| d (0.1) | d (0.5) | d (0.9) | ||
| Raw sludge | 17.286 | 54.921 | 127.023 | |
| Case 1 | HCR | 6.116 | 34.737 | 101.324 |
| Ultrasonic bath | 4.558 | 30.647 | 257.734 | |
| Case 2 | HCR | 0.778 | 4.029 | 142.733 |
| Ultrasonic bath | 1.820 | 17.804 | 227.587 | |
| Case 3 | HCR | 0.865 | 5.719 | 161.269 |
| Ultrasonic bath | 0.941 | 13.999 | 682.707 | |
| Case 4 | HCR | 0.778 | 4.053 | 153.402 |
| Ultrasonic bath | 0.992 | 9.685 | 389.672 | |
| Item | RAW | Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|---|---|
| TCOD (mg/L) | HCR | 4480 | 2110 | 1650 | 1505 | 1480 |
| Ultrasonic bath | 4482.5 | 1665 | 1800 | 1830 | ||
| VSS (mg/L) | HCR | 2120 | 720 | 640 | 540 | 540 |
| Ultrasonic bath | 1560 | 620 | 600 | 520 | ||
| Item | Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|---|
| (mg/L) | HCR | 369.5 | 483.5 | 562 | 633 |
| Ultrasonic bath | 855 | 532.5 | 639 | 766.5 | |
| (mg/L) | HCR | 784.5 | 1312.8 | 1672.9 | 1916.1 |
| Ultrasonic bath | 854.5 | 1432.8 | 1590.4 | 1876.5 | |
| Solubilization rate (%) | HCR | 16.9 | 28.8 | 36.9 | 42.3 |
| Ultrasonic bath | 18.5 | 31.5 | 35 | 41.4 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Sun, X.; Koo, B.; Yoon, J.Y. Experimental Investigation of Sludge Treatment Using a Rotor-Stator Type Hydrodynamic Cavitation Reactor and an Ultrasonic Bath. Processes 2019, 7, 790. https://doi.org/10.3390/pr7110790
Kim H, Sun X, Koo B, Yoon JY. Experimental Investigation of Sludge Treatment Using a Rotor-Stator Type Hydrodynamic Cavitation Reactor and an Ultrasonic Bath. Processes. 2019; 7(11):790. https://doi.org/10.3390/pr7110790
Chicago/Turabian StyleKim, Hyunsoo, Xun Sun, Bonchan Koo, and Joon Yong Yoon. 2019. "Experimental Investigation of Sludge Treatment Using a Rotor-Stator Type Hydrodynamic Cavitation Reactor and an Ultrasonic Bath" Processes 7, no. 11: 790. https://doi.org/10.3390/pr7110790
APA StyleKim, H., Sun, X., Koo, B., & Yoon, J. Y. (2019). Experimental Investigation of Sludge Treatment Using a Rotor-Stator Type Hydrodynamic Cavitation Reactor and an Ultrasonic Bath. Processes, 7(11), 790. https://doi.org/10.3390/pr7110790






