Influence of Particle Contact Number on Triboelectric Separation Selectivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
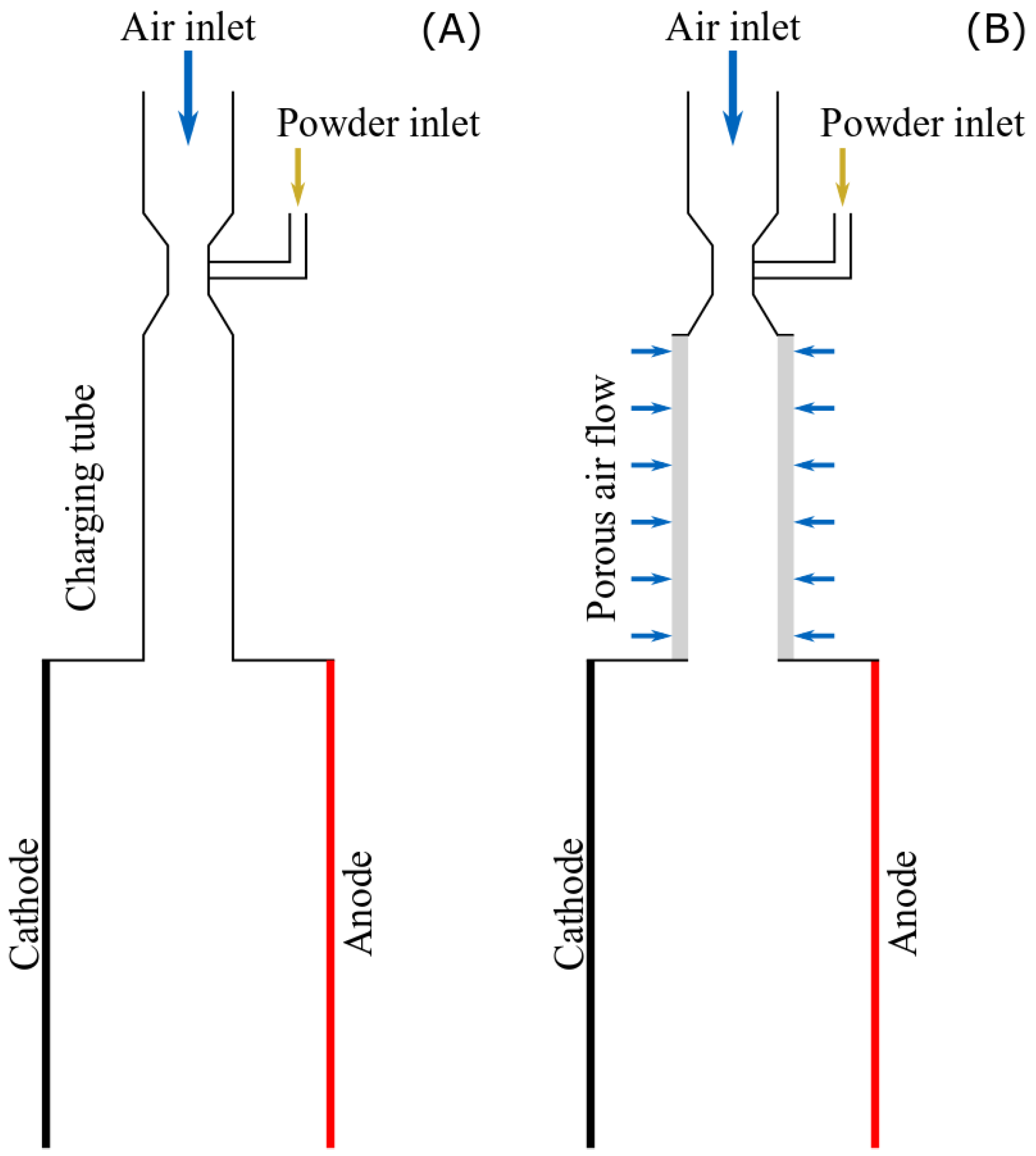
2.2.1. Experimental Setup
2.2.2. Particle Analysis
2.2.3. Particle Interaction Parameter
2.2.4. Statistics
3. Results
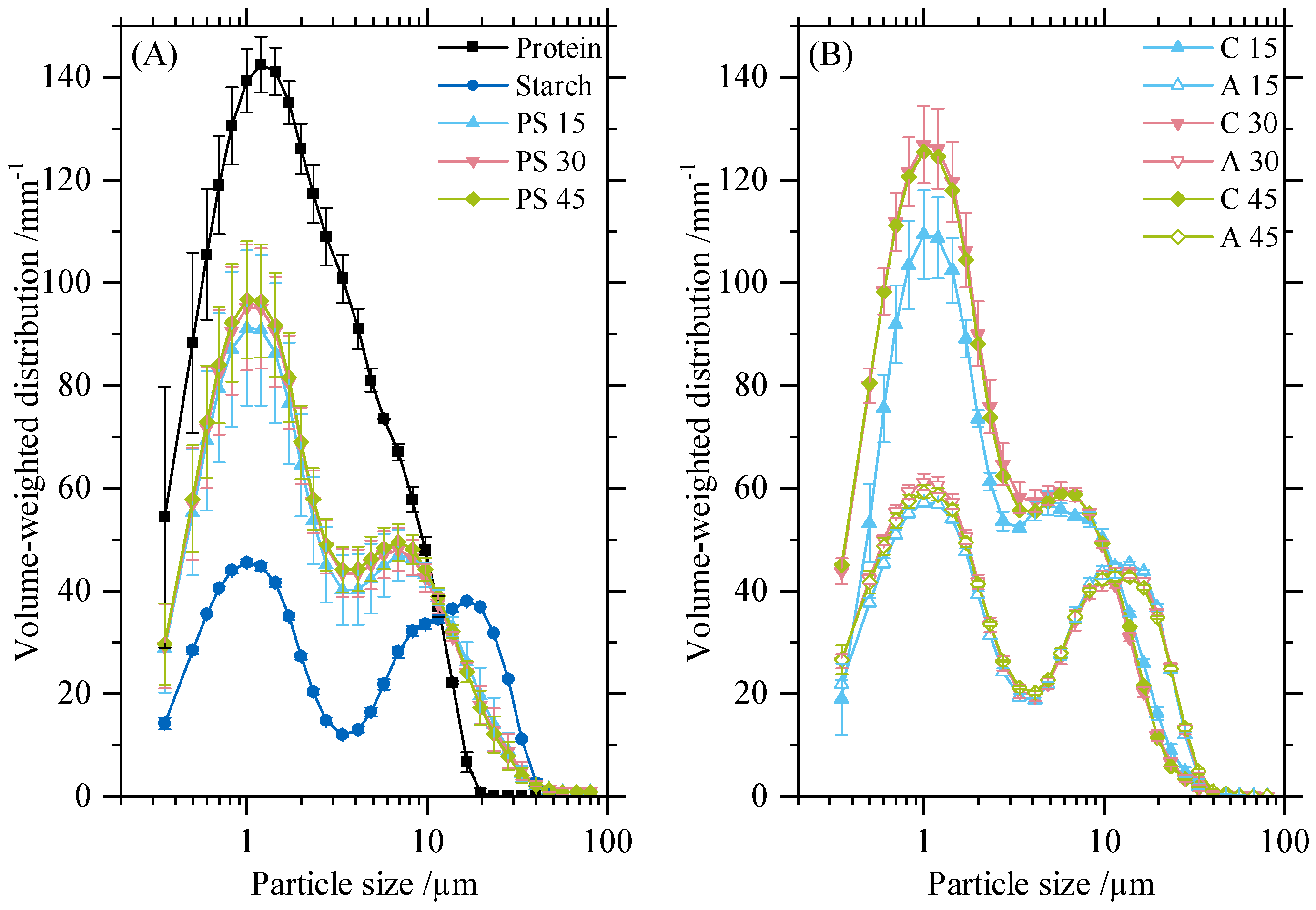
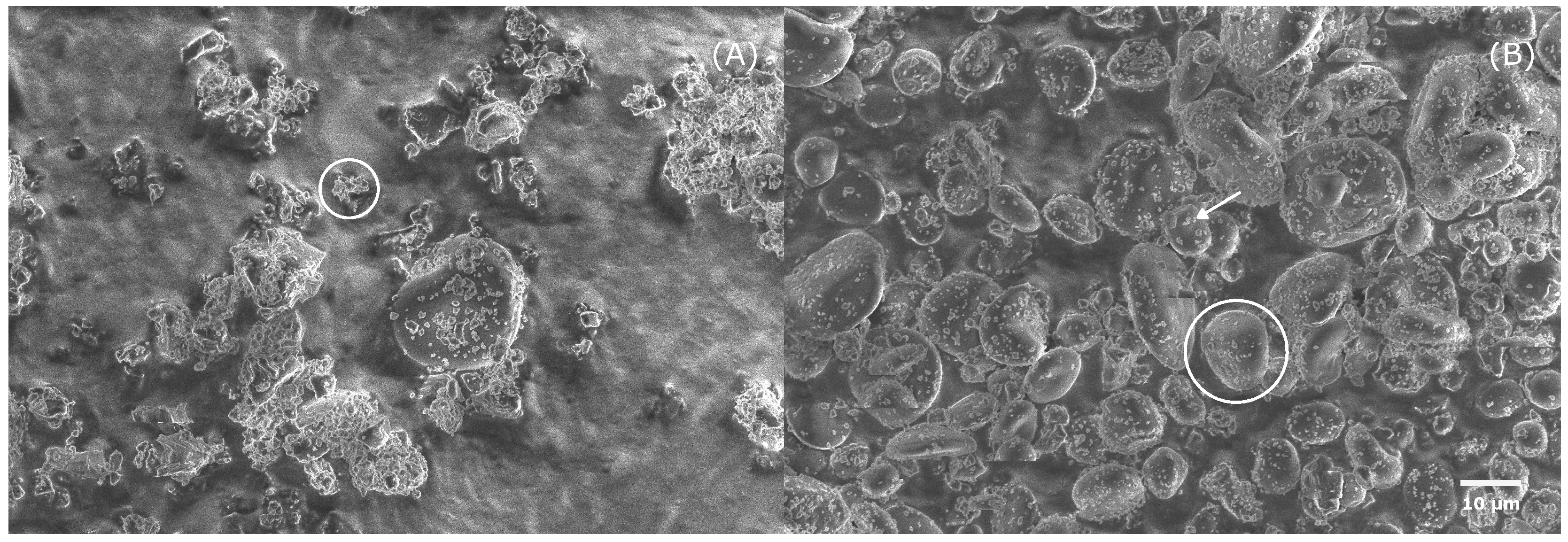
3.1. Particle Size Distribution and SEM of Separated Powders
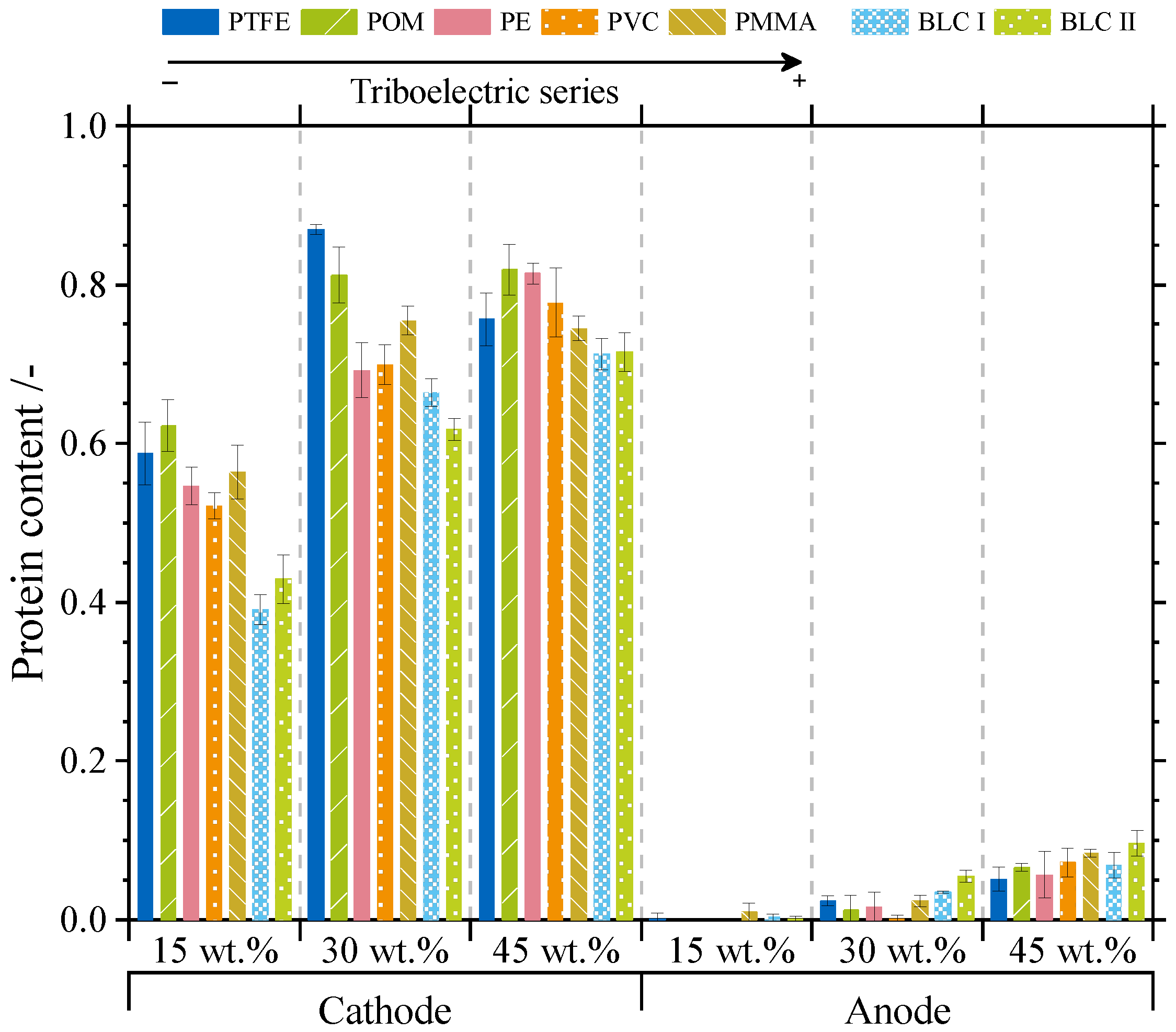
3.2. Influence on Separation Selectivity
3.3. Particle Interaction
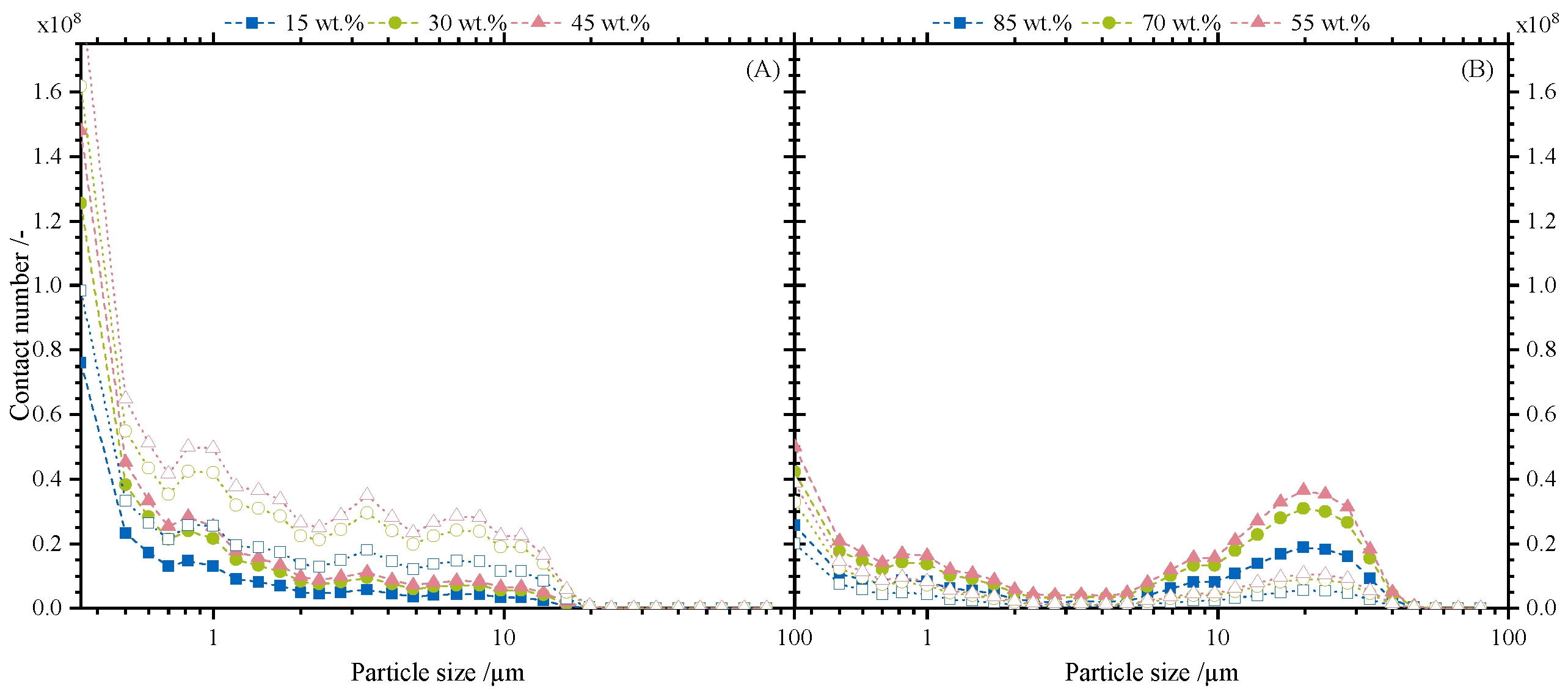
3.3.1. Contact Number Distribution Depending on Particle Size
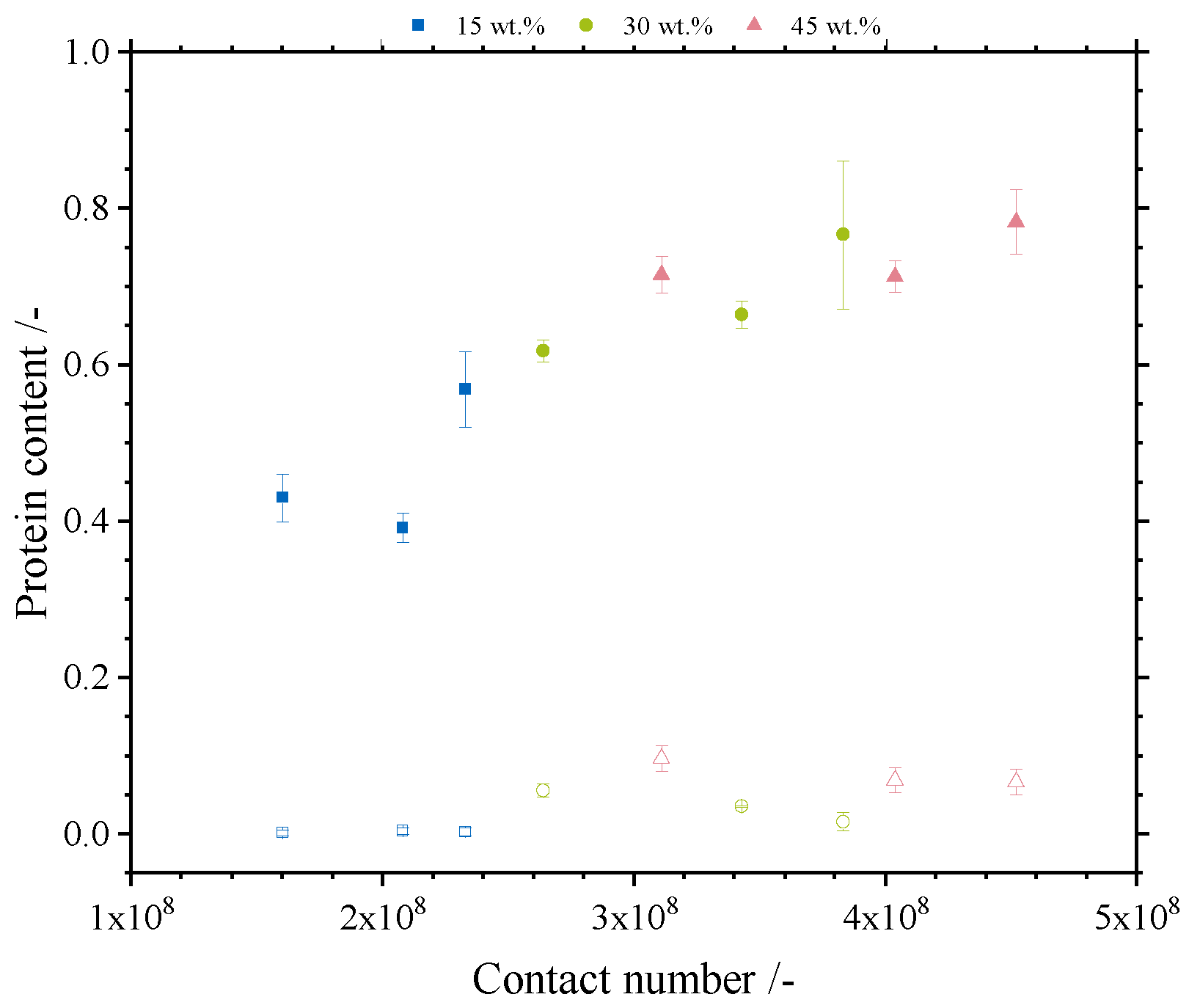
3.3.2. Separation Selectivity and Contact Numbers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BLC | Bondary-layer control |
References
- Lacks, D.J.; Mohan Sankaran, R. Contact electrification of insulating materials. J. Phys. D Appl. Phys. 2011, 44, 453001. [Google Scholar] [CrossRef]
- Matsusaka, S.; Masuda, H. Electrostatics of particles. Adv. Powder Technol. 2003, 14, 143–166. [Google Scholar] [CrossRef]
- Matsusaka, S.; Maruyama, H.; Matsuyama, T.; Ghadiri, M. Triboelectric charging of powders: A review. Chem. Eng. Sci. 2010, 65, 5781–5807. [Google Scholar] [CrossRef]
- Baytekin, H.T.; Patashinski, A.Z.; Branicki, M.; Baytekin, B.; Soh, S.; Grzybowski, B.A. The mosaic of surface charge in contact electrification. Science 2011, 333, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Lacks, D.J.; Shinbrot, T. Long-standing and unresolved issues in triboelectric charging. Nat. Rev. Chem. 2019, 3, 465–476. [Google Scholar] [CrossRef]
- Grosshans, H.; Papalexandris, M.V. Large Eddy simulation of triboelectric charging in pneumatic powder transport. Powder Technol. 2016, 301, 1008–1015. [Google Scholar] [CrossRef]
- Bunchatheeravate, P.; Curtis, J.; Fujii, Y.; Matsusaka, S. Prediction of particle charging in a dilute pneumatic conveying system. AChE J. 2013, 59, 2308–2316. [Google Scholar] [CrossRef]
- Fotovat, F.; Bi, X.T.; Grace, J.R. Electrostatics in gas-solid fluidized beds: A review. Chem. Eng. Sci. 2017, 173, 303–334. [Google Scholar] [CrossRef]
- Naik, S.; Mukherjee, R.; Chaudhuri, B. Triboelectrification: A review of experimental and mechanistic modeling approaches with a special focus on pharmaceutical powders. Int. J. Pharm. 2016, 510, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Mareev, E.A.; Dementyeva, S.O. The role of turbulence in thunderstorm, snowstorm, and dust storm electrification. J. Geophys. Res. Atmos. 2017, 122, 6976–6988. [Google Scholar] [CrossRef]
- Forward, K.M.; Lacks, D.J.; Sankaran, R.M. Particle-size dependent bipolar charging of Martian regolith simulant. Geophys. Res. Lett. 2009, 36, 139. [Google Scholar] [CrossRef]
- Lee, V.; Waitukaitis, S.R.; Miskin, M.Z.; Jaeger, H.M. Direct observation of particle interactions and clustering in charged granular streams. Nat. Phys. 2015, 11, 733–737. [Google Scholar] [CrossRef]
- Dwari, R.K.; Mohanta, S.K.; Rout, B.; Soni, R.K.; Reddy, P.; Mishra, B.K. Studies on the effect of electrode plate position and feed temperature on the tribo-electrostatic separation of high ash Indian coking coal. Adv. Powder Technol. 2015, 26, 31–41. [Google Scholar] [CrossRef]
- Mohanta, S.K.; Rout, B.; Dwari, R.K.; Reddy, P.; Mishra, B.K. Tribo-electrostatic separation of high ash coking coal washery rejects: Effect of moisture on separation efficiency. Powder Technol. 2016, 294, 292–300. [Google Scholar] [CrossRef]
- Bouhamri, N.; Zelmat, M.E.; Tilmatine, A. Micronized plastic waste recycling using two-disc tribo-electrostatic separation process. Adv. Powder Technol. 2019, 30, 625–631. [Google Scholar] [CrossRef]
- Messafeur, R.; Mahi, I.; Ouiddir, R.; Medles, K.; Dascalescu, L.; Tilmatine, A. Tribo-electrostatic separation of a quaternary granular mixture of plastics. Part. Sci. Technol. 2019, 37, 760–765. [Google Scholar] [CrossRef]
- Xing, Q.; de Wit, M.; Kyriakopoulou, K.; Boom, R.M.; Schutyser, M.A. Protein enrichment of defatted soybean flour by fine milling and electrostatic separation. Innov. Food Sci. Emerg. Technol. 2018, 50, 42–49. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, F.; Wang, L.; Li, Y.; Wang, R.; Chen, Z. Tribocharging properties of wheat bran fragments in air–solid pipe flow. Food Res. Int. 2014, 62, 262–271. [Google Scholar] [CrossRef]
- Albrecht, V.; Janke, A.; Drechsler, A.; Schubert, G.; Németh, E.; Simon, F. Visualization of Charge Domains on Polymer Surfaces. In Characterization of Polymer Surfaces and Thin Films; Progress in Colloid and Polymer Science; Grundke, K., Stamm, M., Adler, H.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 132, pp. 48–53. [Google Scholar]
- Harper, W.R. The Volta Effect as a Cause of Static Electrification. Proc. R. Soc. A Math. Phys. Eng. Sci. 1951, 205, 83–103. [Google Scholar] [CrossRef]
- Kamiyama, M.; Maeda, M.; Okutani, H.; Koyama, K.; Matsuda, H.; Sano, Y. Effect of functional groups on the triboelectric charging property of polymer particles. J. Appl. Polym. Sci. 1994, 51, 1667–1671. [Google Scholar] [CrossRef]
- Forward, K.M.; Lacks, D.J.; Sankaran, R.M. Triboelectric Charging of Granular Insulator Mixtures Due Solely to Particle—Particle Interactions. Ind. Eng. Chem. Res. 2009, 48, 2309–2314. [Google Scholar] [CrossRef]
- Trigwell, S.; Grable, N.; Yurteri, C.U.; Sharma, R.; Mazumder, M.K. Effects of surface properties on the tribocharging characteristics of polymer powder as applied to industrial processes. IEEE Trans. Ind. Appl. 2003, 39, 79–86. [Google Scholar] [CrossRef]
- Schönert, K.; Eichas, K.; Niermöller, F. Charge distribution and state of agglomeration after tribocharging fine particulate materials. Powder Technol. 1996, 86, 41–47. [Google Scholar] [CrossRef]
- Atroune, S.; Tilmatine, A.; Alkama, R.; Samuila, A.; Dascalescu, L. Comparative Experimental Study of Triboelectric Charging of Two Size Classes of Granular Plastics. Part. Sci. Technol. 2015, 33, 652–658. [Google Scholar] [CrossRef]
- Lacks, D.J.; Levandovsky, A. Effect of particle size distribution on the polarity of triboelectric charging in granular insulator systems. J. Electrost. 2007, 65, 107–112. [Google Scholar] [CrossRef]
- Mukherjee, R.; Gupta, V.; Naik, S.; Sarkar, S.; Sharma, V.; Peri, P.; Chaudhuri, B. Effects of particle size on the triboelectrification phenomenon in pharmaceutical excipients: Experiments and multi-scale modeling. Asian J. Pharm. Sci. 2016, 11, 603–617. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Shimada, S.; Kashiwabara, H. Mechanoions produced by mechanical fracture of solid polymer. 6. A generation mechanism of triboelectricity due to the reaction of mechanoradicals with mechanoanions on the friction surface. Macromolecules 1990, 23, 5038–5040. [Google Scholar] [CrossRef]
- Horn, R.G.; Smith, D.T.; Grabbe, A. Contact electrification induced by monolayer modification of a surface and relation to acid–base interactions. Nature 1993, 366, 442–443. [Google Scholar] [CrossRef]
- Kolehmainen, J.; Sippola, P.; Raitanen, O.; Ozel, A.; Boyce, C.M.; Saarenrinne, P.; Sundaresan, S. Effect of humidity on triboelectric charging in a vertically vibrated granular bed: Experiments and modeling. Chem. Eng. Sci. 2017, 173, 363–373. [Google Scholar] [CrossRef]
- Wistuba, H. The effect of an external electric field on the operation of an aluminium oxide–cast iron sliding contact joint. Wear 1997, 208, 113–117. [Google Scholar] [CrossRef]
- Watanabe, H.; Ghadiri, M.; Matsuyama, T.; Maruyama, H.; Matsusaka, S.; Ghadiri, M.; Matsuyama, T.; Ding, Y.L.; Pitt, K.G.; Maruyama, H.; et al. Triboelectrification of pharmaceutical powders by particle impact. Int. J. Pharm. 2007, 334, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ireland, P.M. Impact tribocharging of soft elastic spheres. Powder Technol. 2019, 348, 70–79. [Google Scholar] [CrossRef]
- Ghori, M.U.; Supuk, E.; Conway, B.R. Tribo-electric charging and adhesion of cellulose ethers and their mixtures with flurbiprofen. Eur. J. Pharm. Sci. 2014, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Murtomaa, M.; Laine, E. Electrostatic measurements on lactose–glucose mixtures. J. Electrost. 2000, 48, 155–162. [Google Scholar] [CrossRef]
- Landauer, J.; Aigner, F.; Kuhn, M.; Foerst, P. Effect of particle-wall interaction on triboelectric separation of fine particles in a turbulent flow. Adv. Powder Technol. 2019, 30, 1099–1107. [Google Scholar] [CrossRef]
- Horn, R.G.; Smith, D.T. Contact electrification and adhesion between dissimilar materials. Science 1992, 256, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Haeberle, J.; Schella, A.; Sperl, M.; Schröter, M.; Born, P. Double origin of stochastic granular tribocharging. Soft Matter 2018, 14, 4987–4995. [Google Scholar] [CrossRef] [PubMed]
- Landauer, J.; Foerst, P. Triboelectric separation of a starch-protein mixture – Impact of electric field strength and flow rate. Adv. Powder Technol. 2018, 29, 117–123. [Google Scholar] [CrossRef]
- Schlichting, H.; Gersten, K. Boundary-Layer Theory; Springer: Berlin/Heidelberg, Germay, 2017. [Google Scholar] [CrossRef]
- Landauer, J.; Tauwald, S.M.; Foerst, P. A Simple\textmu-PTV Setup to Estimate Single-Particle Charge of Triboelectrically Charged Particles. Front. Chem. 2019, 7, 1. [Google Scholar] [CrossRef]
- Meyer, C.J.; Deglon, D.A. Particle collision modeling—A review. Miner. Eng. 2011, 24, 719–730. [Google Scholar] [CrossRef]
- Schmideder, S.; Kirse, C.; Hofinger, J.; Rollié, S.; Briesen, H. Modeling the Separation of Microorganisms in Bioprocesses by Flotation. Processes 2018, 6, 184. [Google Scholar] [CrossRef]
- Saffman, P.G.; Turner, J.S. On the collision of drops in turbulent clouds. J. Fluid Mech. 1956, 1, 16. [Google Scholar] [CrossRef]
- Smoluchowski, M. Versuch einer mathematischen Theorie der Koagulationskinetik kolloider Lösungen. Z. Phys. Chem. (Leipzig) 1917, 92, 129–168. [Google Scholar] [CrossRef]
- Kolehmainen, J.; Ozel, A.; Boyce, C.M.; Sundaresan, S. Triboelectric charging of monodisperse particles in fluidized beds. AIChE J. 2017, 63, 1872–1891. [Google Scholar] [CrossRef]
- Kolehmainen, J.; Ozel, A.; Gu, Y.; Shinbrot, T.; Sundaresan, S. Effects of Polarization on Particle-Laden Flows. Phys. Rev. Lett. 2018, 121, 124503. [Google Scholar] [CrossRef] [PubMed]
- Schella, A.; Herminghaus, S.; Schröter, M. Influence of humidity on tribo-electric charging and segregation in shaken granular media. Soft Matter 2017, 13, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Schella, A.; Weis, S.; Schröter, M. Charging changes contact composition in binary sphere packings. Phys. Rev. E 2017, 95, 062903. [Google Scholar] [CrossRef] [PubMed]
- Landauer, J.; Foerst, P. Influence of Particle Charge and Size Distribution on Triboelectric Separation—New Evidence Revealed by In Situ Particle Size Measurements. Processes 2019, 7, 381. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Jafari, M.; Rajabzadeh, A.R.; Legge, R.L. Solvent-free production of protein-enriched fractions from navy bean flour using a triboelectrification-based approach. J. Food Eng. 2016, 174, 21–28. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Jafari, M.; Rajabzadeh, A.R.; Legge, R.L. Development and optimization of a triboelectrification bioseparation process for dry fractionation of legume flours. Sep. Purif. Technol. 2016, 163, 48–58. [Google Scholar] [CrossRef]
- Wang, J.; de Wit, M.; Boom, R.M.; Schutyser, M.A. Charging and separation behavior of gluten–starch mixtures assessed with a custom-built electrostatic separator. Sep. Purif. Technol. 2015, 152, 164–171. [Google Scholar] [CrossRef]
- Wang, J.; de Wit, M.; Schutyser, M.A.; Boom, R.M. Analysis of electrostatic powder charging for fractionation of foods. Innov. Food Sci. Emerg. Technol. 2014, 26, 360–365. [Google Scholar] [CrossRef]
- Inculet, I.I.; Peter Castle, G.S.; Aartsen, G. Generation of bipolar electric fields during industrial handling of powders. Chem. Eng. Sci. 2006, 61, 2249–2253. [Google Scholar] [CrossRef]
- Zhao, H.; Castle, G.; Inculet, I.I.; Bailey, A.G. Bipolar charging of poly-disperse polymer powders in fluidized beds. IEEE Trans. Ind. Appl. 2003, 39, 612–618. [Google Scholar] [CrossRef]
- Sowinski, A.; Miller, L.; Mehrani, P. Investigation of electrostatic charge distribution in gas–solid fluidized beds. Chem. Eng. Sci. 2010, 65, 2771–2781. [Google Scholar] [CrossRef]
| Mean of Plain Wall | BLC I | BLC II | |
|---|---|---|---|
| / | 3.53 × 104 | 3.11 × 104 | 2.55 × 104 |
| / | 1.33 × 10−5 | ||
| / | 1330 | ||
| / | 1520 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landauer, J.; Foerst, P. Influence of Particle Contact Number on Triboelectric Separation Selectivity. Processes 2019, 7, 716. https://doi.org/10.3390/pr7100716
Landauer J, Foerst P. Influence of Particle Contact Number on Triboelectric Separation Selectivity. Processes. 2019; 7(10):716. https://doi.org/10.3390/pr7100716
Chicago/Turabian StyleLandauer, Johann, and Petra Foerst. 2019. "Influence of Particle Contact Number on Triboelectric Separation Selectivity" Processes 7, no. 10: 716. https://doi.org/10.3390/pr7100716
APA StyleLandauer, J., & Foerst, P. (2019). Influence of Particle Contact Number on Triboelectric Separation Selectivity. Processes, 7(10), 716. https://doi.org/10.3390/pr7100716






