Modelling and Kinetic Study of Novel and Sustainable Microwave-Assisted Dehydration of Sugarcane Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Sugarcane Juice Extraction
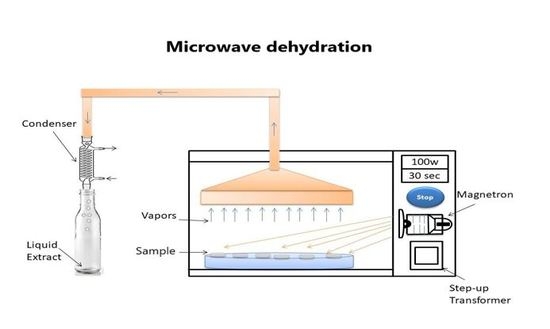
2.2. Concentration of Sugarcane Juice through Microwave
2.3. Evaporation Rate
2.4. Moisture Ratio (MR)
2.5. Free radical Scavenging Activity (DPPH)
2.6. Energy Consumption
2.7. Solubility Index
2.8. Color
2.9. Statistical Analysis
3. Results and Discussions
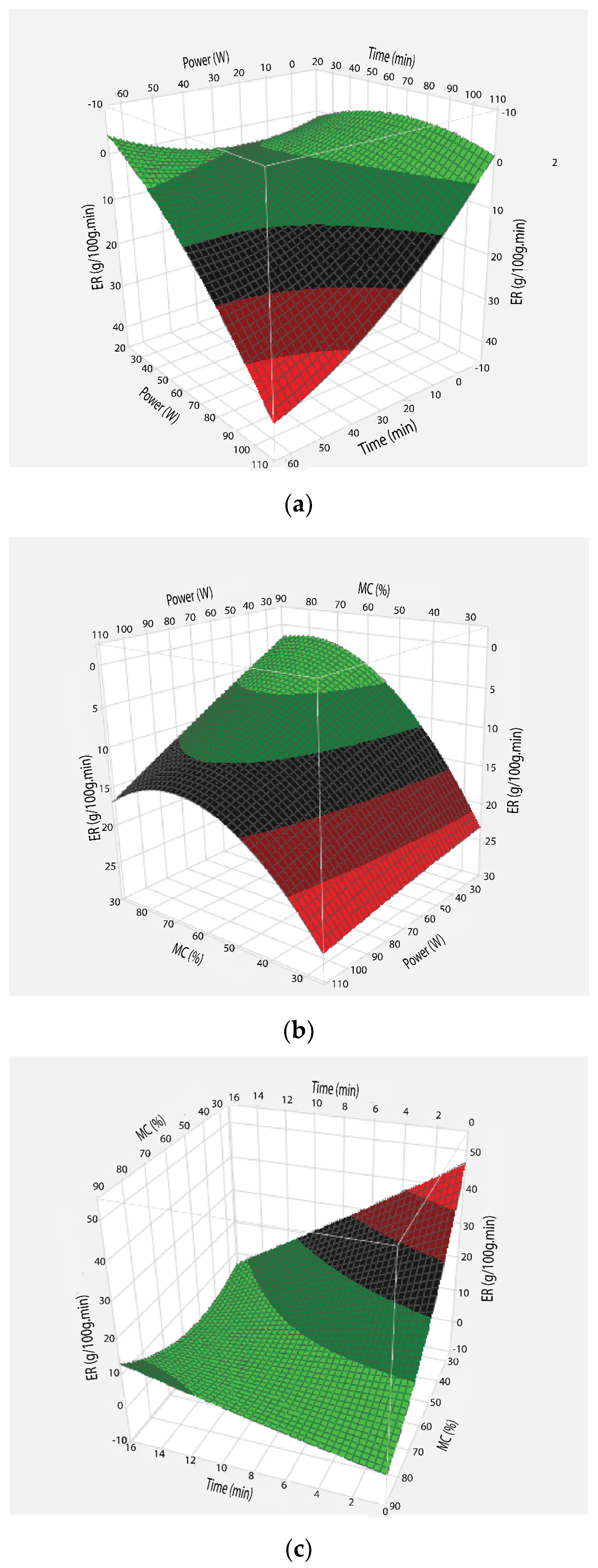
3.1. Evaporation Rate
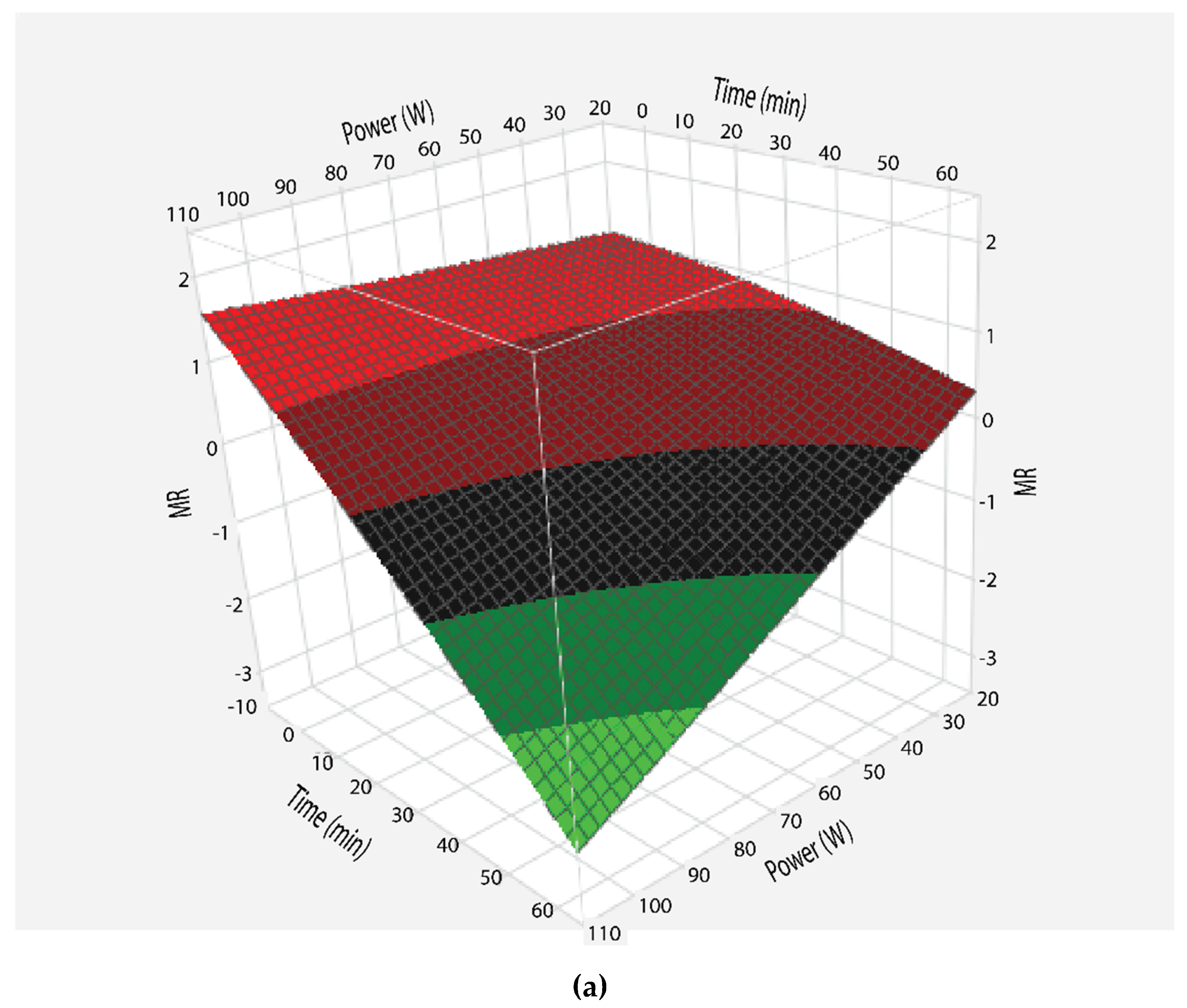
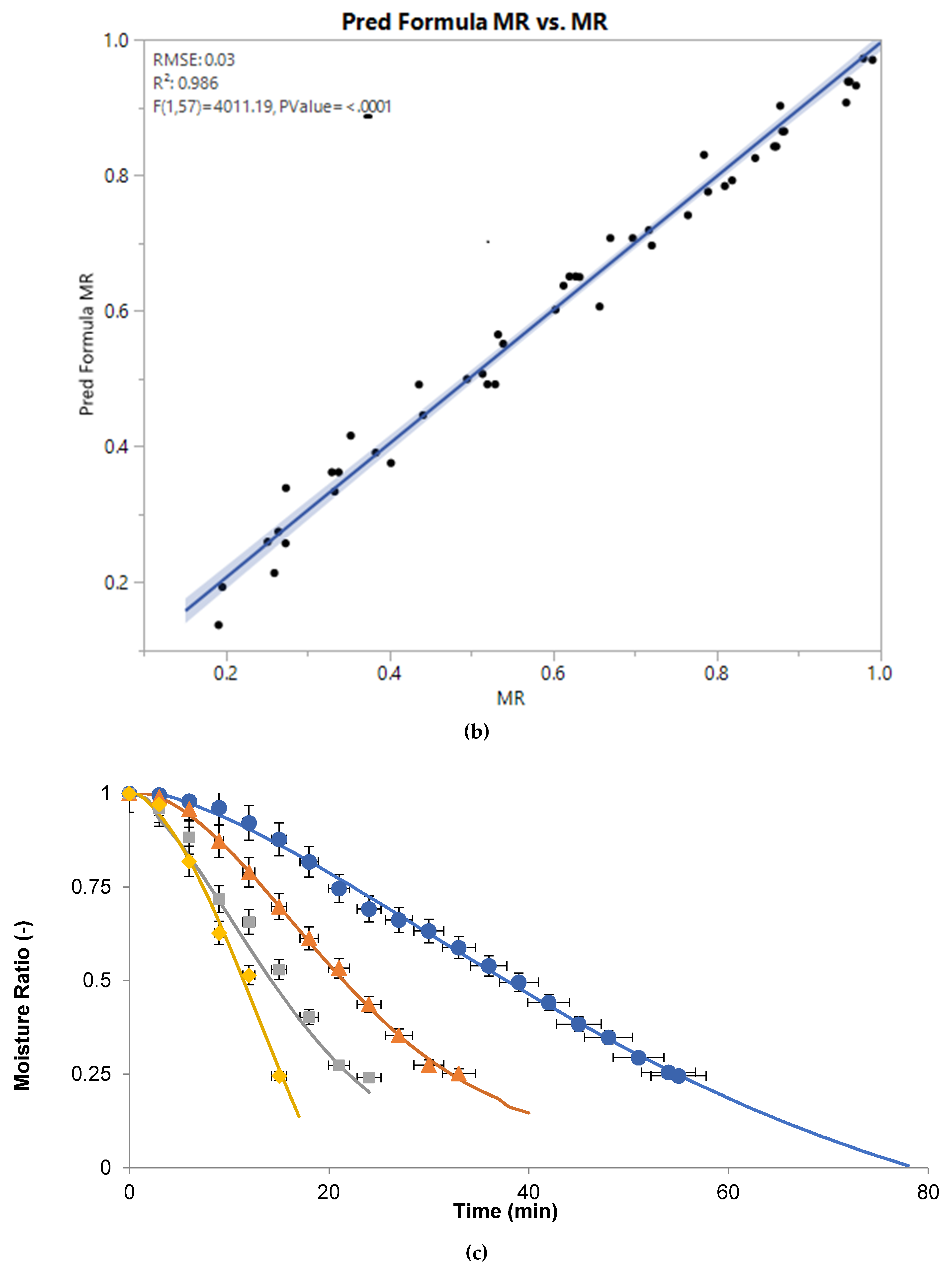
3.2. Moisture Ratio
3.3. Effective moisture Diffusivity as Function of Microwave Power
3.4. Antioxidant Activity and Energy Consumption
3.5. Solubility Index & Color Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, M.K.I.; Ansar, M.; Nazir, A.; Maan, A.A. Sustainable dehydration of onion slices through novel microwave hydro-diffusion gravity technique. Innov. Food Sci. Emerg. Technol. 2016, 33, 327–332. [Google Scholar] [CrossRef]
- Sharma, E.R.; Yadav, K.C. Study on Effect of Microwave Drying on Drying and Quality Characteristics of Guava (Psidium guajava). Int. J. Sci. Eng. Technol. 2017, 6, 150–154. [Google Scholar] [CrossRef]
- Chahbani, A.; Fakhfakh, N.; Balti, M.A.; Mabrouk, M.; El-Hatmi, H.; Zouari, N.; Kechaou, N. Microwave drying effects on drying kinetics, bioactive compounds and antioxidant activity of green peas (Pisum sativum L.). Food Biosci. 2018, 25, 32–38. [Google Scholar] [CrossRef]
- Mahjoorian, A.; Mokhtarian, M.; Fayyaz, N.; Rahmati, F.; Sayyadi, S.; Ariaii, P. Modeling of drying kiwi slices and its sensory evaluation. Food Sci. Nutr. 2017, 5, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Gautam, S.; Sharma, A. Shelf Life Extension of Sugarcane Juice Using Preservatives and Gamma Radiation Processing. J. Food Sci. 2011, 76, M573–M578. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Huang, Y.; Wang, Y.; Shi, T.; Zhang, L.; Chen, Y.; Xie, M. Comparison of (poly)phenolic compounds and antioxidant properties of pomace extracts from kiwi and grape juice. Food Chem. 2019, 271, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, H.; Azadbakht, M.; Rezaeiasl, A.; Farhang, A. Drying characteristics of sardine fish dried with microwave heating. J. Saudi Soc. Agric. Sci. 2013, 12, 121–127. [Google Scholar] [CrossRef]
- Jafari, S.M.; Ghalenoei, M.G.; Dehnad, D. Influence of spray drying on water solubility index, apparent density, and anthocyanin content of pomegranate juice powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Kortei, N.K.; Tawia Odamtten, G.; Obodai, M.; Appiah, V.; Toah Akonor, P. Determination of color parameters of gamma irradiated fresh and dried mushrooms during storage. Hrvat. Časopis Za Prehrambenu Tehnol. Biotehnol. I Nutr. 2015, 10, 66–71. [Google Scholar]
- Khan, M.K.I.; Cakmak, H.; Tavman, Ş.; Schutyser, M.; Schroën, K. Anti-browning and barrier properties of edible coatings prepared with electrospraying. Innov. Food Sci. Emerg. Technol. 2014, 25, 9–13. [Google Scholar] [CrossRef]
- Mota, C.; Luciano, C.; Dias, A.; Barroca, M.J.; Guiné, R. Convective drying of onion: Kinetics and nutritional evaluation. Food Bioprod. Process. 2010, 88, 115–123. [Google Scholar] [CrossRef]
- Roberts, J.S.; Kidd, D.R.; Padilla-Zakour, O. Drying kinetics of grape seeds. J. Food Eng. 2008, 89, 460–465. [Google Scholar] [CrossRef]
- Zarein, M.; Samadi, S.H.; Ghobadian, B. Investigation of microwave dryer effect on energy efficiency during drying of apple slices. J. Saudi Soc. Agric. Sci. 2015, 14, 41–47. [Google Scholar] [CrossRef]
- Arimandi, M.; Aguayo Giménez, E.P.; Otón Alcaraz, M.; Artés Calero, F. Continuous and Conventional Microwave Heating Effects on the Antioxidant Capacity of Tomato Juice; III Workshop en Investigación Agroalimentaria: Cartagena, Spain, 2014. [Google Scholar]

| Model Name | Power | Model Constants | R2 | Chi (χ2) | SSE | RMSE | RPD (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | k | a | b | c | ko | k1 | g | |||||||
| Midilli | 30 | 1.613 | 0.002 | 1.016 | −0.002 | - | - | - | - | 0.997 | 2.164 × 10−4 | 2.291 × 10−4 | 0.014 | 0.107 |
| 50 | 1.866 | 0.003 | 1.004 | 0.002 | - | - | - | - | 0.998 | 1.053 × 10−4 | 1.17 × 10−4 | 0.010 | 0.072 | |
| 80 | 1.413 | 0.003 | 1.014 | −0.021 | - | - | - | - | 0.986 | 0.001 | 0.001 | 0.031 | 0.458 | |
| 100 | 2.194 | 0.002 | 1.013 | −0.017 | - | - | - | - | 0.989 | 0.001 | 0.002 | 0.030 | 0.712 | |
| Page | 30 | 0.001 | 1.742 | - | - | - | - | - | - | 0.996 | 0.125 | 2.735 × 10−4 | 0.344 | 69.566 |
| 50 | 1.830 | 0.002 | - | - | - | - | - | - | 0.998 | 1.492 × 10−4 | 1.658 × 10−4 | 0.012 | 0.345 | |
| 80 | 1.711 | 0.006 | - | - | - | - | - | - | 0.994 | 5.383 × 10−4 | 6.28 × 10−4 | 0.022 | 0.220 | |
| 100 | 2.122 | 0.004 | - | - | - | - | - | - | 0.986 | 0.152 | 0.002 | 0.349 | 74.049 | |
| Henderson and Pabis | 30 | - | 0.022 | 1.123 | - | - | - | - | - | 0.949 | 0.004 | 0.004 | 0.059 | 4.048 |
| 50 | - | 0.037 | 1.121 | - | - | - | - | - | 0.939 | 0.005 | 0.006 | 0.069 | 4.906 | |
| 80 | - | 0.047 | 1.081 | - | - | - | - | - | 0.951 | 0.005 | 0.005 | 0.063 | 5.167 | |
| 100 | - | 0.067 | 1.094 | - | - | - | - | - | 0.887 | 0.012 | 0.016 | 0.098 | 8.620 | |
| Logarithmic | 30 | - | 3.041 × 10−6 | 4.881 × 103 | - | 4.88 × 103 | - | - | - | 0.992 | 5.526 × 10−4 | 5.851 × 10−4 | 0.023 | 0.365 |
| 50 | - | 6.202 × 10−6 | 4.125 × 103 | - | 4.125 × 103 | - | - | - | 0.987 | 0.001 | 0.001 | 0.032 | 0.417 | |
| 80 | - | - | - | - | - | 0.987 | 0.001 | 0.001 | 0.032 | 0.922 | ||||
| 100 | - | 1.042 × 10−5 | 4.878 × 103 | - | −4.877 × 103 | - | - | - | 0.959 | 0.004 | 0.006 | 0.059 | 3.090 | |
| Two term | 30 | - | - | 356.226 | −355.234 | - | 0.049 | 0.049 | - | 0.995 | 3.612 × 10−4 | 3.825 × 10−4 | 0.018 | 0.848 |
| 50 | - | - | 0.707 | 0.414 | - | 0.037 | 0.037 | - | 0.938 | 0.005 | 0.006 | 0.069 | 4.906 | |
| 80 | - | - | 0.684 | 0.398 | - | 0.047 | 0.047 | - | 0.951 | 0.005 | 0.005 | 0.063 | 5.167 | |
| 100 | - | - | 0.689 | 0.406 | - | 0.067 | 0.067 | - | 0.887 | 0.012 | 0.016 | 0.098 | 8.620 | |
| Two term exponentials | 30 | - | 0.035 | 2.097 | - | - | - | - | - | 0.993 | 4.403 × 10−4 | 4.662 × 10−4 | 0.020 | 1.205 |
| 50 | - | 8.74 × 106 | 3.592 × 10−9 | - | - | - | - | - | 0.898 | 0.009 | 0.010 | 0.089 | 3.970 | |
| 80 | - | 0.074 | 1.998 | - | - | - | - | - | 0.982 | 0.002 | 0.002 | 0.038 | 2.056 | |
| 100 | - | 0.124 | 2.207 | - | - | - | - | - | 0.975 | 0.003 | 0.003 | 0.045 | 3.723 | |
| Wang and Sing | 30 | - | - | −0.009 | −8.782 × 10−5 | - | - | - | - | 0.990 | 6.814 × 10−4 | 7.215 × 10−4 | 0.025 | 0.760 |
| 50 | - | - | −0.016 | −2.641 × 10−4 | - | - | - | - | 0.984 | 0.001 | 0.001 | 0.035 | 0.932 | |
| 80 | - | - | −0.023 | −2.714 × 10−4 | - | - | - | - | 0.989 | 0.073 | 0.001 | 0.030 | 0.116 | |
| 100 | - | - | −0.016 | −0.002 | - | - | - | - | 0.989 | 0.001 | 0.002 | 0.030 | 0.104 | |
| Diffusion approximation | 30 | - | 0.018 | 0.655 | 1 | - | - | - | - | 0.907 | 0.007 | 0.007 | 0.080 | 3.340 |
| 50 | - | 0.086 | −334.513 | 0.996 | - | - | - | - | 0.997 | 2.082 × 10−4 | 2.313 × 10−4 | 0.014 | 0.784 | |
| 80 | - | 0.099 | −201.879 | 0.995 | - | - | - | - | 0.983 | 0.002 | 0.002 | 0.037 | 1.889 | |
| 100 | - | 0.058 | 0.639 | 1 | - | - | - | - | 0.855 | 0.015 | 0.020 | 0.111 | 6.965 | |
| Verma et al | 30 | - | 0.018 | 0.781 | - | - | - | - | 0.018 | 0.907 | 0.007 | 0.007 | 0.080 | 3.340 |
| 50 | - | 0.031 | 0.399 | - | - | - | - | 0.031 | 0.898 | 0.009 | 0.010 | 0.089 | 3.970 | |
| 80 | - | 0.042 | 0.500 | - | - | - | - | 0.042 | 0.931 | 0.006 | 0.008 | 0.075 | 4.455 | |
| 100 | - | 0.058 | 0.500 | - | - | - | - | 0.058 | 0.855 | 0.015 | 0.020 | 0.111 | 6.965 | |
| Power W | Effective Diffusivity m2/s (× 10−15) | Specific Energy Consumption kJ/kg | Antioxidant Activity % |
|---|---|---|---|
| 30 | 0.68 d | 452 ± 0.001 b | 72.98 ± 0.03 a |
| 50 | 1.45 c | 444 ± 0.01 c | 69.24 ± 0.05 c |
| 80 | 3.36 b | 555 ± 0.005 a | 70.54 ± 0.03 b |
| 100 | 6.61 a | 411 ± 0.002 d | 64.93 ± 0.03 d |
| p-value | <0.001 | 0.2049 | 0.0007 |
| Sample | ΔE | C* | WI | Solubility Index (%) |
|---|---|---|---|---|
| Fresh juice | 0 b | 21.13 ± 0.03 a | 28.90 ± 0.04 ab | N/A |
| Conventional concentrate | 18.01 ± 0.01 a | 3.85 ± 0.01 b | 27.06 ± 0.03 b | 45.28 ± 0.009 b |
| Microwave concentrate | 17.68 ± 0.04 a | 4.07 ± 0.08 b | 30.12 ± 0.005 a | 57.6 ± 0.01 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvi, T.; Khan, M.K.I.; Maan, A.A.; Nazir, A.; Ahmad, M.H.; Khan, M.I.; Sharif, M.; Khan, A.U.; Afzal, M.I.; Umer, M.; et al. Modelling and Kinetic Study of Novel and Sustainable Microwave-Assisted Dehydration of Sugarcane Juice. Processes 2019, 7, 712. https://doi.org/10.3390/pr7100712
Alvi T, Khan MKI, Maan AA, Nazir A, Ahmad MH, Khan MI, Sharif M, Khan AU, Afzal MI, Umer M, et al. Modelling and Kinetic Study of Novel and Sustainable Microwave-Assisted Dehydration of Sugarcane Juice. Processes. 2019; 7(10):712. https://doi.org/10.3390/pr7100712
Chicago/Turabian StyleAlvi, Tayyaba, Muhammad Kashif Iqbal Khan, Abid Aslam Maan, Akmal Nazir, Muhammad Haseeb Ahmad, Muhammad Issa Khan, Muhammad Sharif, Azmat Ullah Khan, Muhammad Inam Afzal, Muhammad Umer, and et al. 2019. "Modelling and Kinetic Study of Novel and Sustainable Microwave-Assisted Dehydration of Sugarcane Juice" Processes 7, no. 10: 712. https://doi.org/10.3390/pr7100712
APA StyleAlvi, T., Khan, M. K. I., Maan, A. A., Nazir, A., Ahmad, M. H., Khan, M. I., Sharif, M., Khan, A. U., Afzal, M. I., Umer, M., Abbas, S., & Qureshi, S. (2019). Modelling and Kinetic Study of Novel and Sustainable Microwave-Assisted Dehydration of Sugarcane Juice. Processes, 7(10), 712. https://doi.org/10.3390/pr7100712