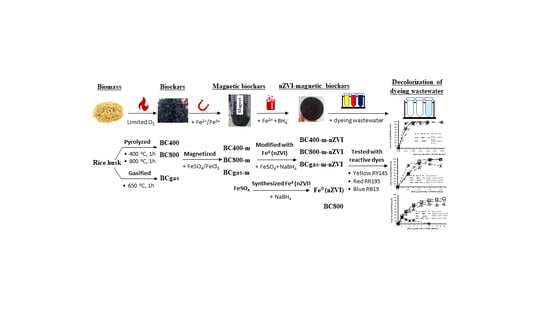
Rice Husk Biochars Modified with Magnetized Iron Oxides and Nano Zero Valent Iron for Decolorization of Dyeing Wastewater
Abstract
1. Introduction
2. Materials and Methods
- Full-scale gasification rice husk biochar: A full-scale gasifier (BiGchar 2200 fast rotary hearth, ϕ = 2.2 m, h = 2 m, nominal capacity of 300 kg biochar/h, designed and fabricated by Pyrocal Pty Ltd, Wellcamp, Queensland, Australia) was used to produce gasification biochar (Figure 1). The gasifier has four chambers equipped temperature and air controllers. Briefly, rice husk was continuously fed on top and moved down by a rotary hand in every chamber. The top three chambers were heated in a range of temperature of 300–650 °C by the energy of the gasification process under air controlled conditions. The bottom chamber was used for cooling. Retention time of rice husk in the gasifier was approximately 1 h. Gasification biochar after cooling was collected by a screw conveyor system and labeled as BCgas.
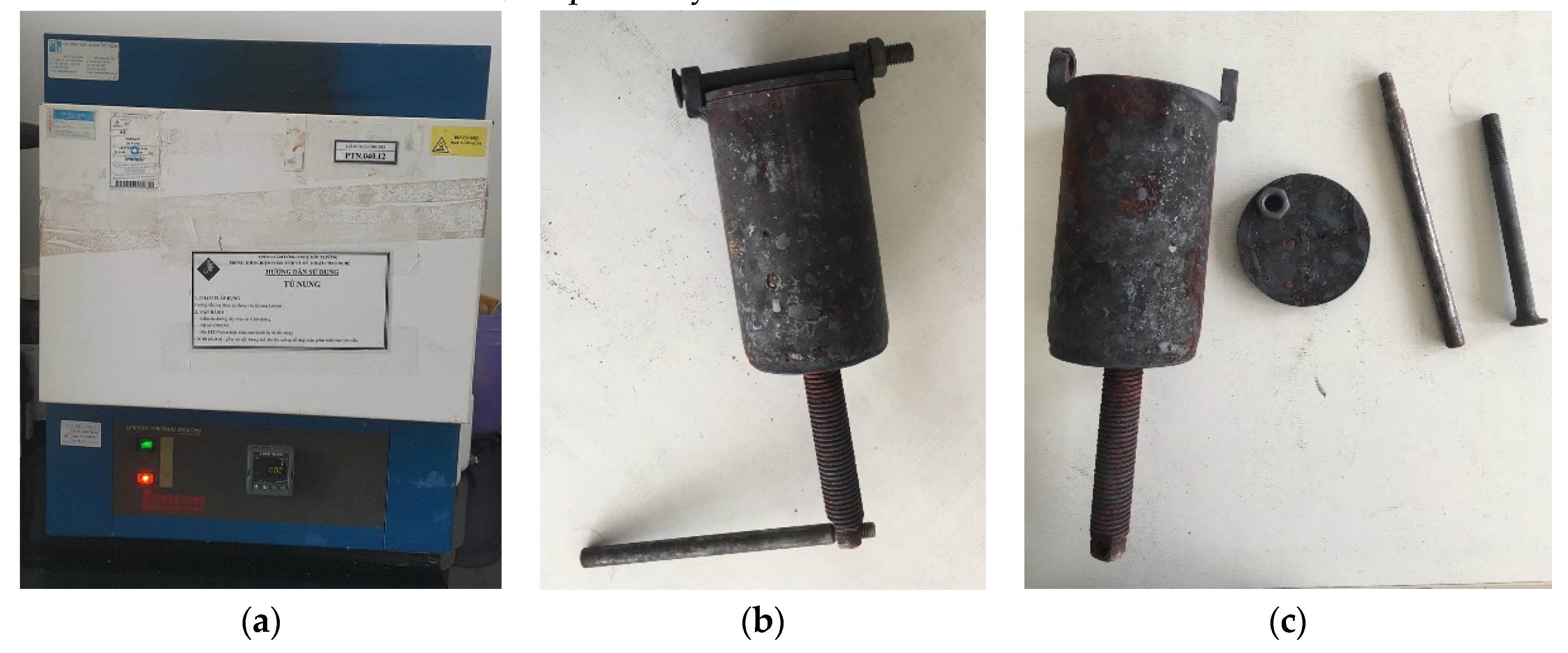
- Lab-scale pyrolysis rice husk biochar: A lab-scale closed furnace (EF 11/8B, Lenton, Hope Valley, UK) and other associated equipments including the closed-steel cylinder and its components (Hoang Ha Co. Ltd, Binh Duong, Vietnam) were used for producing pyrolysis biochar (Figure 2). Briefly, rice husk (40 g) was transferred to the steel-cylinder (ϕ = 2.5 cm, L = 10 cm, Figure 2b,c), tightly closed at one end and screwed tight by hand at the other end with a piston-screw mechanic system. This step aimed to minimize available air in the cylinder. It was then placed in the furnace and heated (heating rate of approx. 40 °C/min) to a pre-set temperature and retain for 1 h. Two thresholds of temperature were set to be 400 °C and 800 °C. The furnace was finally turned off for cooling down for approximately 4 h. Biochars at 400 °C and 800 °C were collected and labeled as BC400 and BC800, respectively.
Experimental Methods for Investigation of Color Removal Efficiency (ɳ,%) of the Modified Biochars
3. Results and Discussion
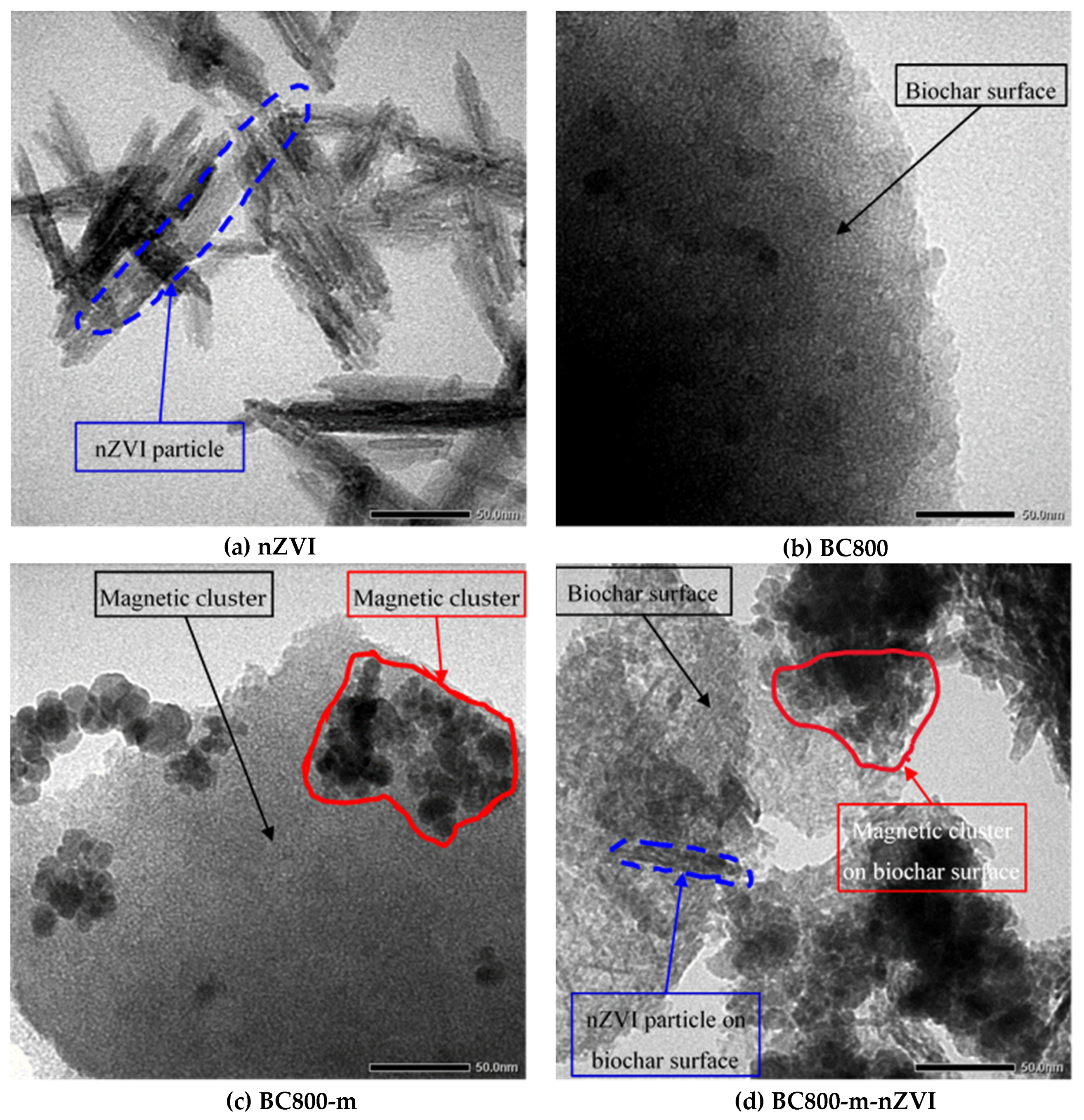
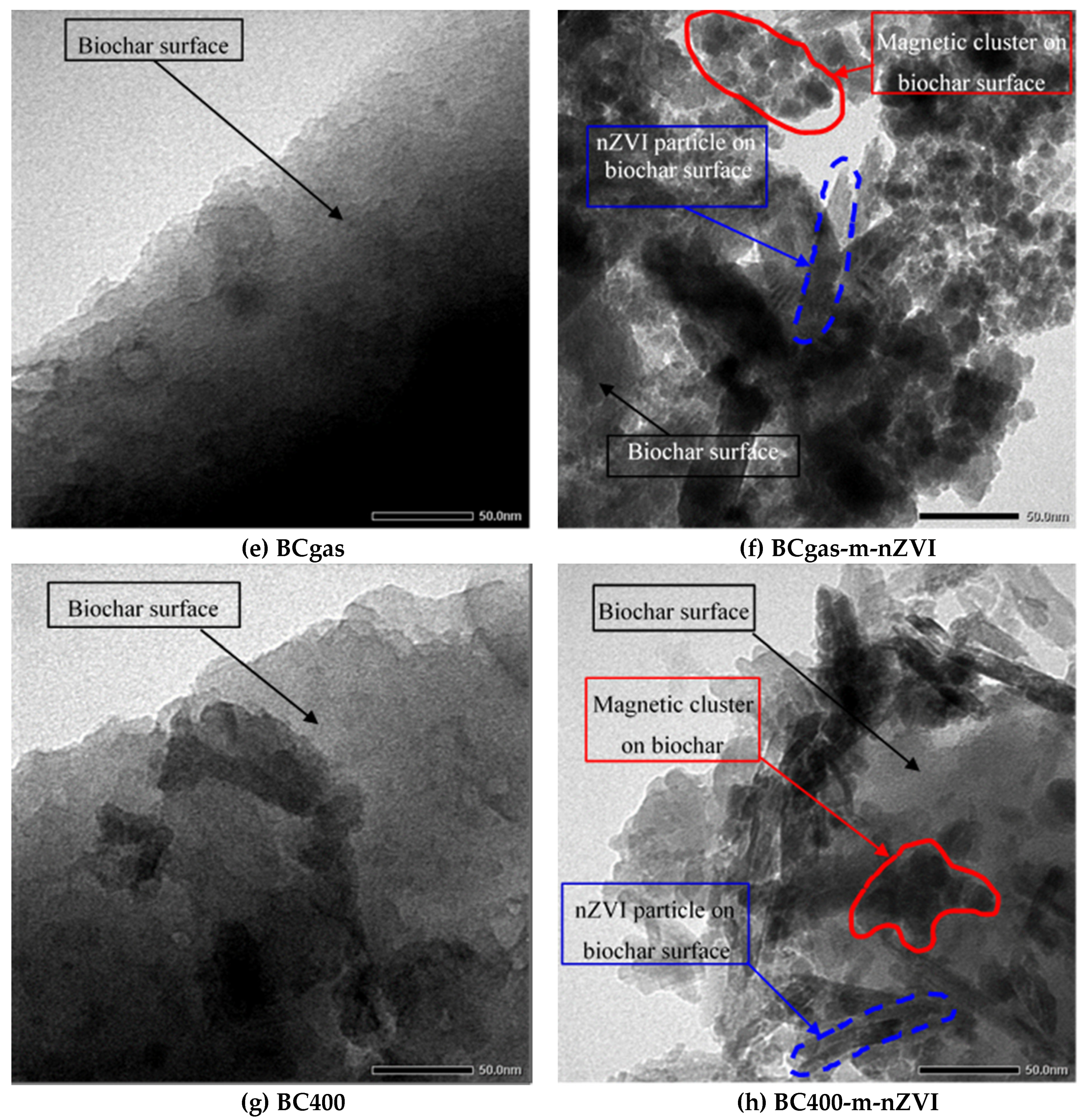
3.1. Physico-Chemical Properties of the Materials
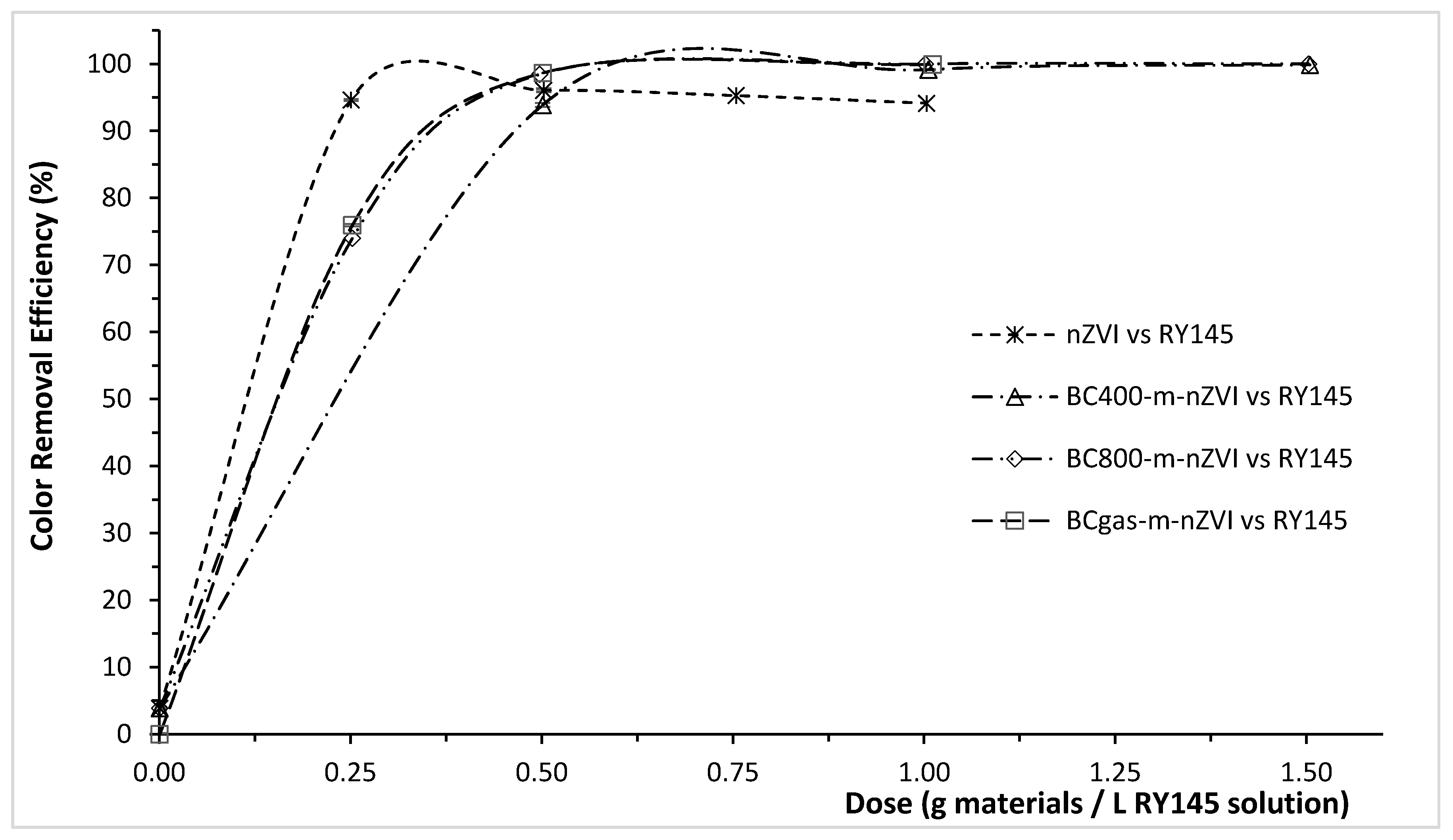
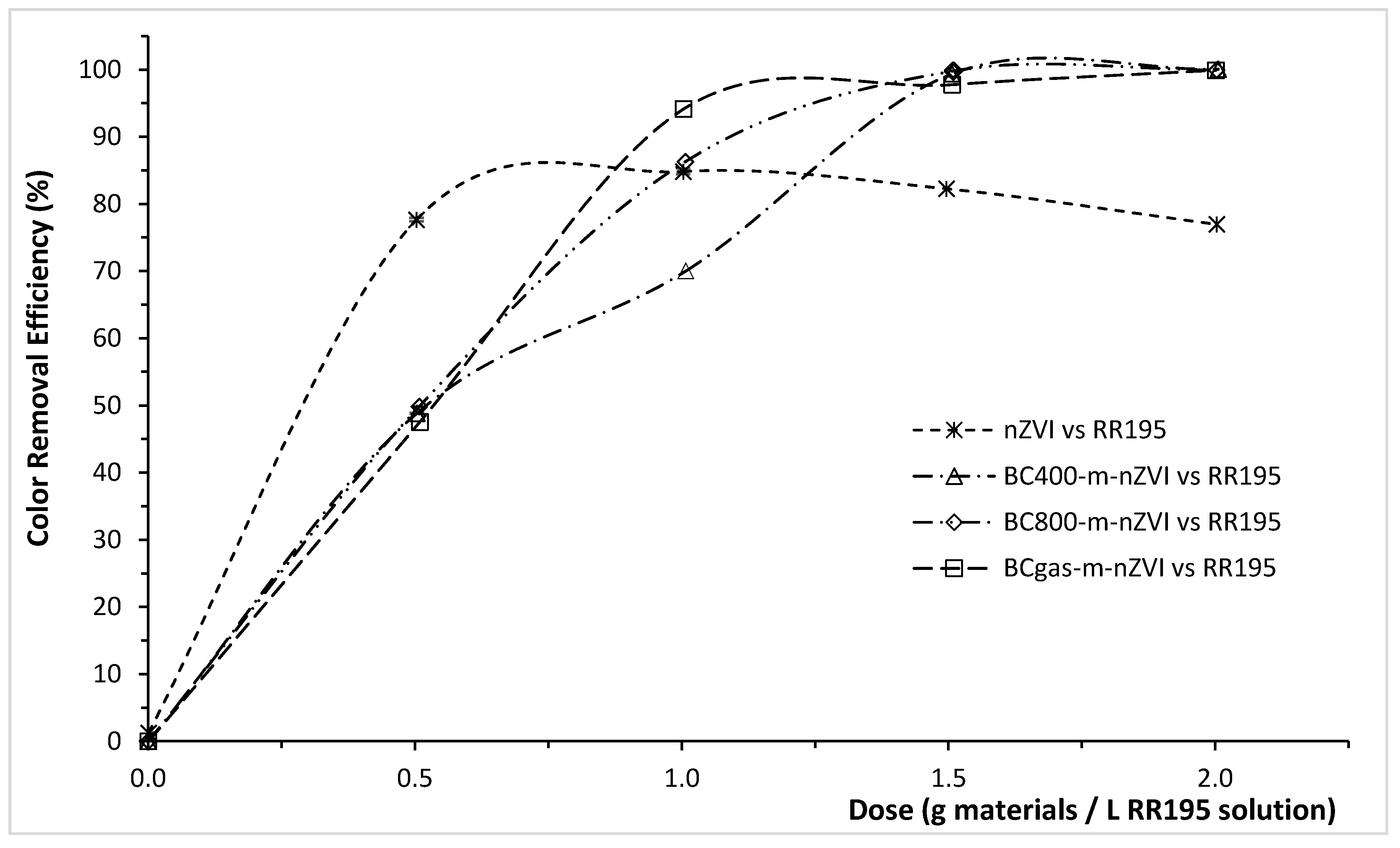
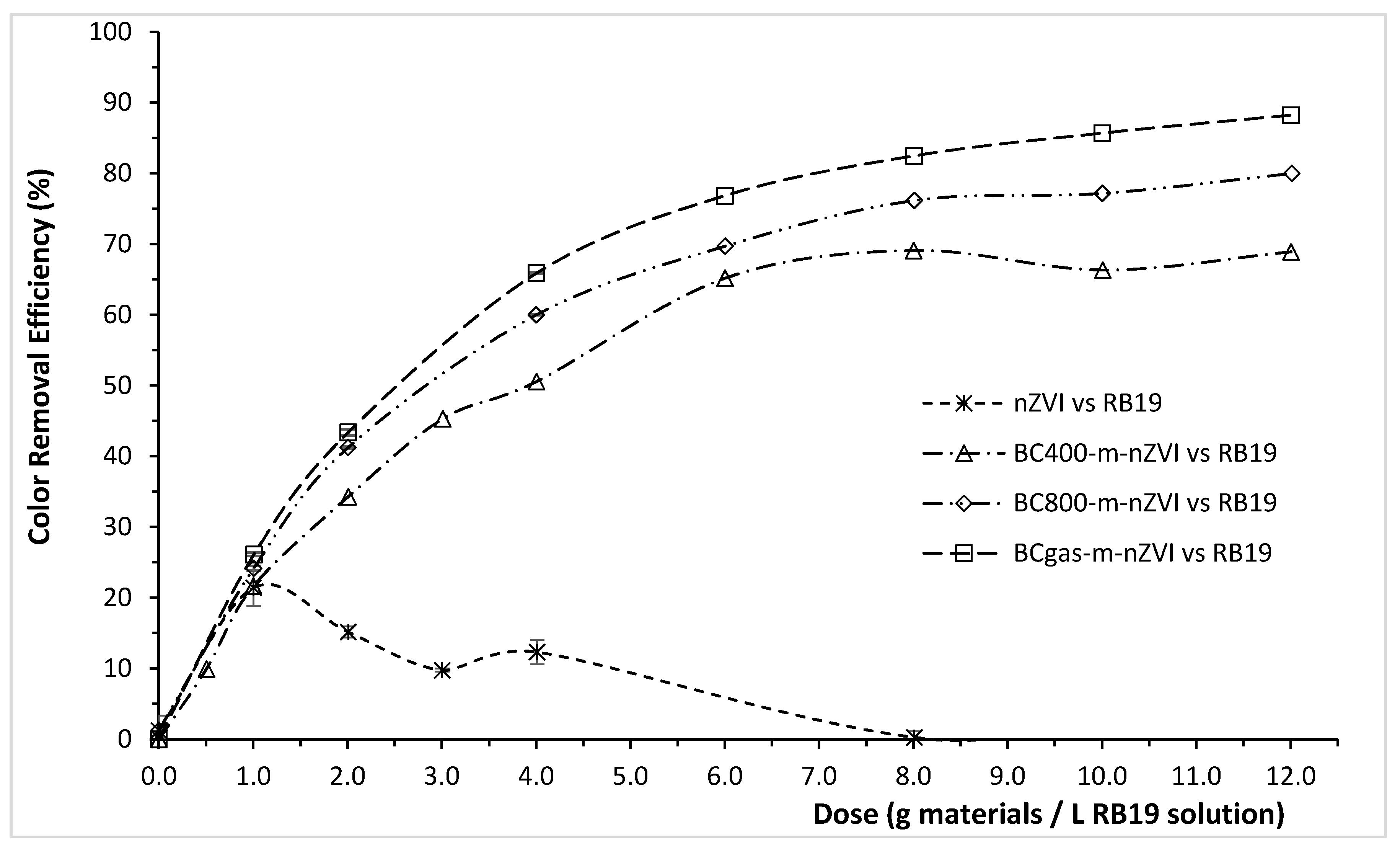
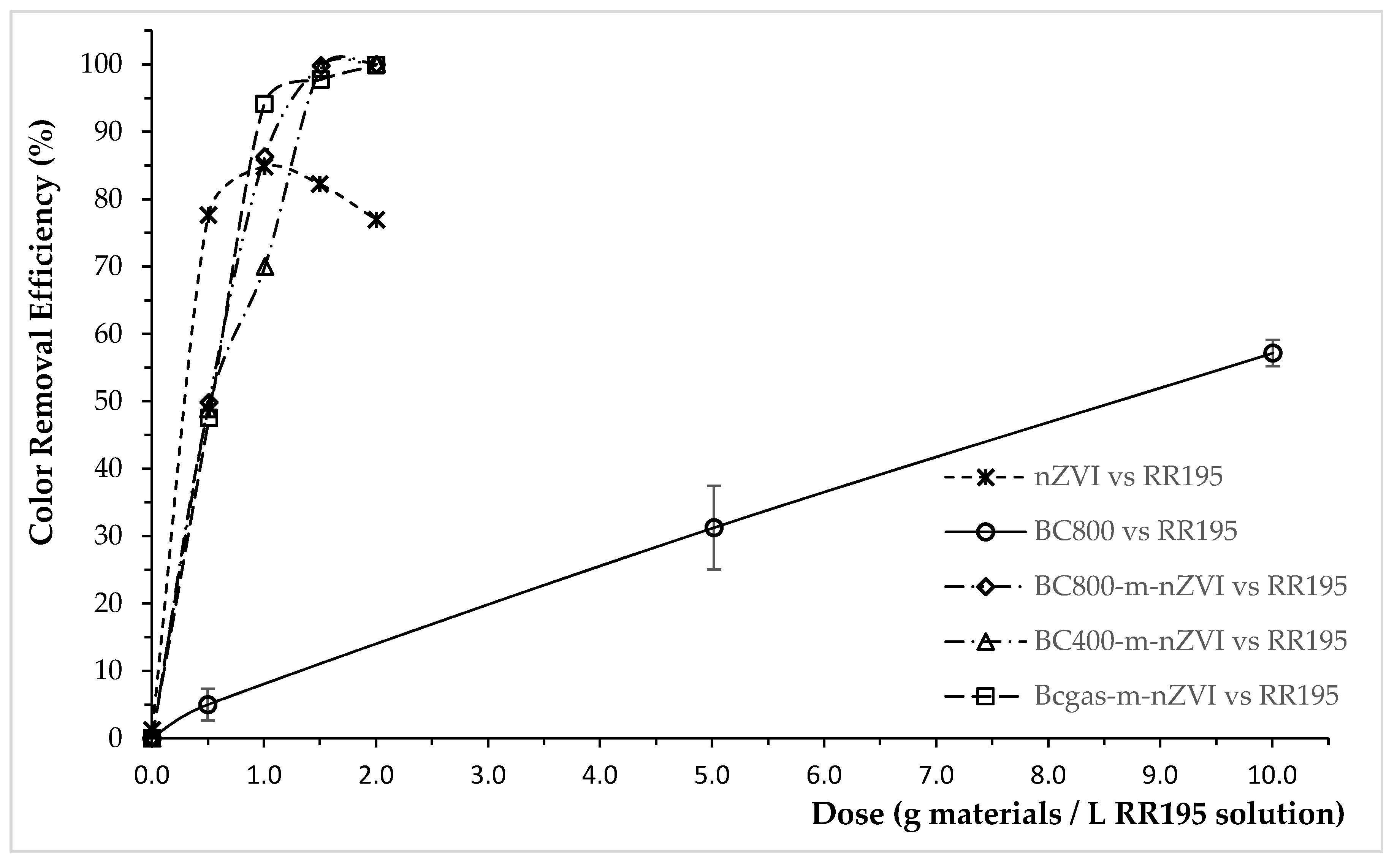
3.2. Color Removal Efficiency of the Modified Biochars for RY145, RR195, and RB19
3.3. Color Removal Mechanisms of the Modified Biochars
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boateng, A.A.; Garcia-Perez, M.; Masek, O.; Brown, R.; Campo del, B. Biochar production technology. In Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Earthscan from Routledge: London, UK, 2015. [Google Scholar]
- Kleber, M.; Hockaday, W.; Nico, N.P. Characteristics of biochar: Macro-molecular properties. In Biochar for Environmental Management: Science, Technology and Implementation; Johannes, L., Stephan, J., Eds.; Earthscan from Routledge: London, UK; New York, NY, USA, 2015; pp. 111–137. [Google Scholar]
- Trinh, B.-S.; Werner, D.; Reid, B.J. Application of a full-scale wood gasification biochar as a soil improver to reduce organic pollutant leaching risks. J. Chem. Technol. Biotechnol. 2017, 92, 1928–1937. [Google Scholar] [CrossRef]
- Ghosh, U.; Luthy, R.G.; Cornelissen, G.; Werner, D.; Menzie, C.A. In-situ Sorbent Amendments: A New Direction in Contaminated Sediment Management. Environ. Sci. Technol. 2011, 45, 1163–1168. [Google Scholar] [CrossRef]
- Werner, D.; Hale, S.E.; Ghosh, U.; Luthy, R.G. Polychlorinated Biphenyl Sorption and Availability in Field-Contaminated Sediments. Environ. Sci. Technol. 2010, 44, 2809–2815. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Rios, R.V.R.A.; Fabris, J.D.; Garg, V.; Sapag, K.; Lago, R.M. Activated carbon/iron oxide magnetic composites for the adsorption of contaminants in water. Carbon 2002, 40, 2177–2183. [Google Scholar] [CrossRef]
- Tan, X.-F.; Liu, Y.-G.; Gu, Y.-L.; Xu, Y.; Zeng, G.-M.; Hu, X.-J.; Liu, S.-B.; Wang, X.; Liu, S.-M.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef]
- Tseng, H.-H.; Su, J.-G.; Liang, C. Synthesis of granular activated carbon/zero valent iron composites for simultaneous adsorption/dechlorination of trichloroethylene. J. Hazard. Mater. 2011, 192, 500–506. [Google Scholar] [CrossRef]
- Safarik, I.; Horska, K.; Pospiskova, K.; Safarikova, M. Magnetically Responsive Activated Carbons for Bio- and Environmental Applications. Int. Rev. Chem. Eng. 2012, 4, 346–352. [Google Scholar]
- Thines, K.R.; Abdullah, E.C.; Mubarak, N.M.; Ruthiraan, M. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renew. Sustain. Energy Rev. 2017, 67, 257–276. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Rychoudhury, T.; Scheytt, T. Potential of zerovalent iron nanoparticles for remediation of environmental organic contaminants in water: A review. Water Sci. Technol. 2013, 68, 1425–1439. [Google Scholar] [CrossRef]
- Raman, C.D.; Kanmani, S. Textile dye degradation using nano zero valent iron: A review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Z.-H.; Han, L.; Qin, C.-H. Synthesis of nanoscale zero-valent iron supported on exfoliated graphite for removal of nitrate. Trans. Nonferrous Met. Soc. China 2006, 16, s345–s349. [Google Scholar] [CrossRef]
- Claoston, N.; Samsuri, A.W.; Ahmad Husni, M.H.; Mohd Amran, M.S. Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. Iswa 2014, 32, 331–339. [Google Scholar] [CrossRef]
- Phonphuak, N.; Chindaprasirt, P. 6—Types of waste, properties, and durability of pore-forming waste-based fired masonry bricks. In Eco-Efficient Masonry Bricks and Blocks; Pacheco-Torgal, F., Lourenço, P.B., Labrincha, J.A., Kumar, S., Chindaprasirt, P., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 103–127. [Google Scholar] [CrossRef]
- FAO. Countries by Commodity (Rice Paddy). Food and Agriculture Organization of the United Nations. 2017. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 18 March 2019).
- Nguyen, T.D.; Dinh, M.T. Thị trường lúa gạo Việt Nam: Cái cách để hội nhập—Cách tiếp cận cấu trúc thị trường, in English “Vietnam Paddy Rice Market: Reform to Develop—the Market Structure Approach; Hong Duc publisher: Hanoi, Vietnam, 2015; p. 55. [Google Scholar]
- Fernandes, I.J.; Calheiro, D.; Kieling, A.G.; Moraes, C.A.M.; Rocha, T.L.A.C.; Brehm, F.A.; Modolo, R.C.E. Characterization of rice husk ash produced using different biomass combustion techniques for energy. Fuel 2016, 165, 351–359. [Google Scholar] [CrossRef]
- Ghaly, A.; Ananthashankar, R.; Alhattab, M.; Ramakrishnan, V. Production, characterization and treatment of textile effluents: A critical review. J. Chem. Eng. Process. Technol. 2014, 5, 1–19. [Google Scholar]
- Mui, E.L.K.; Cheung, W.H.; Valix, M.; McKay, G. Dye adsorption onto char from bamboo. J. Hazard. Mater. 2010, 177, 1001–1005. [Google Scholar] [CrossRef]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar] [CrossRef]
- Shu, H.-Y.; Chang, M.-C.; Chen, C.-C.; Chen, P.-E. Using resin supported nano zero-valent iron particles for decoloration of Acid Blue 113 azo dye solution. J. Hazard. Mater. 2010, 184, 499–505. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Savova, D.; Apak, E.; Ekinci, E.; Yardim, F.; Petrov, N.; Budinova, T.; Razvigorova, M.; Minkova, V. Biomass conversion to carbon adsorbents and gas. Biomass Bioenergy 2001, 21, 133–142. [Google Scholar] [CrossRef]
- Shah, I.; Adnan, R.; Wan Ngah, W.S.; Mohamed, N. Iron Impregnated Activated Carbon as an Efficient Adsorbent for the Removal of Methylene Blue: Regeneration and Kinetics Studies. PLoS ONE 2015, 10, e0122603. [Google Scholar] [CrossRef]
- Castro, C.S.; Guerreiro, M.C.; Gonçalves, M.; Oliveira, L.C.A.; Anastácio, A.S. Activated carbon/iron oxide composites for the removal of atrazine from aqueous medium. J. Hazard. Mater. 2009, 164, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Sani, B.; Akkanen, J.; Abel, S.; Nybom, I.; Karapanagioti, H.K.; Werner, D. A critical evaluation of magnetic activated carbon’s potential for the remediation of sediment impacted by polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2015, 286, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jin, Z.-h.; Li, T.-l.; Zhang, H.; Gao, S. Preparation of spherical iron nanoclusters in ethanol–water solution for nitrate removal. Chemosphere 2006, 65, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-X.; Elliott, D.W. Applications of iron nanoparticles for groundwater remediation. Remediat. J. 2006, 16, 7–21. [Google Scholar] [CrossRef]
- Tran Phong, D.; Van Thong, N. Review of the textile coloration industry in Vietnam. Coloration Technol. 2008, 124, 331–340. [Google Scholar] [CrossRef]
- Kalkan, N.A.; Aksoy, S.; Aksoy, E.A.; Hasirci, N. Adsorption of reactive yellow 145 onto chitosan coated magnetite nanoparticles. J. Appl. Polym. Sci. 2012, 124, 576–584. [Google Scholar] [CrossRef]
- Nawahwi, M.Z.; Ibrahim, Z.; Yahya, A. Degradation of the Azo Dye Reactive Red 195 by Paenibacillus spp. R2. J. Bioremediation Biodegrad. 2012, 4. [Google Scholar] [CrossRef]
- Özcan, A.; Ömeroğlu, Ç.; Erdoğan, Y.; Özcan, A.S. Modification of bentonite with a cationic surfactant: An adsorption study of textile dye Reactive Blue 19. J. Hazard. Mater. 2007, 140, 173–179. [Google Scholar] [CrossRef]
- QCVN-40:2011/BNTMT; National Technical Regulation on Industrial Wastewater of Vietnam; Minister of Natural Resources and Environment: Hanoi, Vietnam, 2011.
- Han, Z.; Sani, B.; Mrozik, W.; Obst, M.; Beckingham, B.; Karapanagioti, H.K.; Werner, D. Magnetite impregnation effects on the sorbent properties of activated carbons and biochars. Water Res. 2015, 70, 394–403. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Liu, S.-C.; Chen, H.-R.; Chang, Y.-M.; Tsai, Y.-L. Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 2012, 89, 198–203. [Google Scholar] [CrossRef]
- Chompuchan, C.; Satapanajaru, T.; Suntornchot, P.; Pengthamkeerati, P. Decolorization of Reactive Black 5 and Reactive Red 198 using nanoscale zerovalent iron. World Acad. Sci. Eng. Technol. 2009, 49, 130–134. [Google Scholar]
- Fan, J.; Guo, Y.; Wang, J.; Fan, M. Rapid decolorization of azo dye methyl orange in aqueous solution by nanoscale zerovalent iron particles. J. Hazard. Mater. 2009, 166, 904–910. [Google Scholar] [CrossRef]
- Shu, H.-Y.; Chang, M.-C.; Yu, H.-H.; Chen, W.-H. Reduction of an azo dye Acid Black 24 solution using synthesized nanoscale zerovalent iron particles. J. Colloid Interface Sci. 2007, 314, 89–97. [Google Scholar] [CrossRef]
- Bokare, A.D.; Chikate, R.C.; Rode, C.V.; Paknikar, K.M. Iron-nickel bimetallic nanoparticles for reductive degradation of azo dye Orange G in aqueous solution. Appl. Catal. B: Environ. 2008, 79, 270–278. [Google Scholar] [CrossRef]
- Lapointe, M.; Barbeau, B. Understanding the roles and characterizing the intrinsic properties of synthetic vs. natural polymers to improve clarification through interparticle Bridging: A review. Sep. Purif. Technol. 2020, 231, 115893. [Google Scholar] [CrossRef]

| Chemicals | CAS | Empirical Formula | Weight (g/mol) | Molecular Formula |
|---|---|---|---|---|
| C.I. Reactive Red 195 (RR195) | 93050-79-4 | C31H19ClN7Na5O19S6 | 1136.3 | 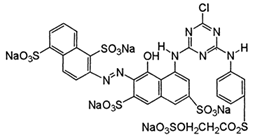 |
| C.I. Reactive Yellow 145 (RY145) | 93050-80-7 | C28H20ClN9Na4O16S5 | 1026.25 | 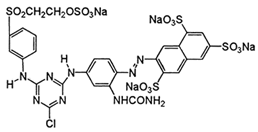 |
| C.I. Reactive Blue 19 (RB19) | 2580-78-1 | C22H16N2Na2O11S3 | 626.55 | 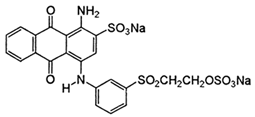 |
| Materials | Yield (%) | pH | pHZPC | Ash Content (%) | B.E.T. SSA (m2/g) |
|---|---|---|---|---|---|
| Bcgas | n.d. | 9.17 | 7.3 | 38.1 | 251.11 |
| BC400 | 42.1 | 9.37 | 6.95 | 30.3 | 141.23 |
| BC800 | 33.6 | 10.30 | 8.75 | 36.6 | 213.03 |
| nZVI | n.d. | n.d. | n.d. | n.d. | 158.27 |
| BCgas-m- nZVI | n.d. | n.d. | n.d. | n.d. | 192.07 |
| BC400-m- nZVI | n.d. | n.d. | n.d. | n.d. | 132.44 |
| BC800-m- nZVI | n.d. | n.d. | n.d. | n.d. | 181.92 |
| Materials | Color Removal Efficiency (ɳ, %) at a Specific Dose of the Materials (g/L) | ||
|---|---|---|---|
| RY145 | RR195 | RB19 | |
| nZVI | 94.62 ± 0.59 | 77.66 ± 0.41 | 21.40 ± 2.05 |
| (at 0.25 g/L) | (at 0.50 g/L) | (at 1.00 g/L) | |
| BCgas-m-nZVI | 98.66 ± 0.15 | 94.14 ± 0.96 | 76.84 ± 0.26 |
| (at 0.50 g/L) | (at 1.00 g/L) | (at 6.00 g/L) | |
| BC400-m-nZVI | 93.89 ± 0.26 | 70.03 ± 1.67 | 65.18 ± 0.27 |
| (at 0.50 g/L) | (at 1.00 g/L) | (at 6.00 g/L) | |
| BC800-m-nZVI | 98.44 ± 0.38 | 86.31 ± 2.22 | 69.72 ± 0.10 |
| (at 0.50 g/L) | (at 1.00 g/L) | (at 6.00 g/L) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, B.-S.; Le, P.T.K.; Werner, D.; Phuong, N.H.; Luu, T.L. Rice Husk Biochars Modified with Magnetized Iron Oxides and Nano Zero Valent Iron for Decolorization of Dyeing Wastewater. Processes 2019, 7, 660. https://doi.org/10.3390/pr7100660
Trinh B-S, Le PTK, Werner D, Phuong NH, Luu TL. Rice Husk Biochars Modified with Magnetized Iron Oxides and Nano Zero Valent Iron for Decolorization of Dyeing Wastewater. Processes. 2019; 7(10):660. https://doi.org/10.3390/pr7100660
Chicago/Turabian StyleTrinh, Bao-Son, Phung T. K. Le, David Werner, Nguyen H. Phuong, and Tran Le Luu. 2019. "Rice Husk Biochars Modified with Magnetized Iron Oxides and Nano Zero Valent Iron for Decolorization of Dyeing Wastewater" Processes 7, no. 10: 660. https://doi.org/10.3390/pr7100660
APA StyleTrinh, B.-S., Le, P. T. K., Werner, D., Phuong, N. H., & Luu, T. L. (2019). Rice Husk Biochars Modified with Magnetized Iron Oxides and Nano Zero Valent Iron for Decolorization of Dyeing Wastewater. Processes, 7(10), 660. https://doi.org/10.3390/pr7100660