An Intensified Reactive Separation Process for Bio-Jet Diesel Production
Abstract
1. Introduction
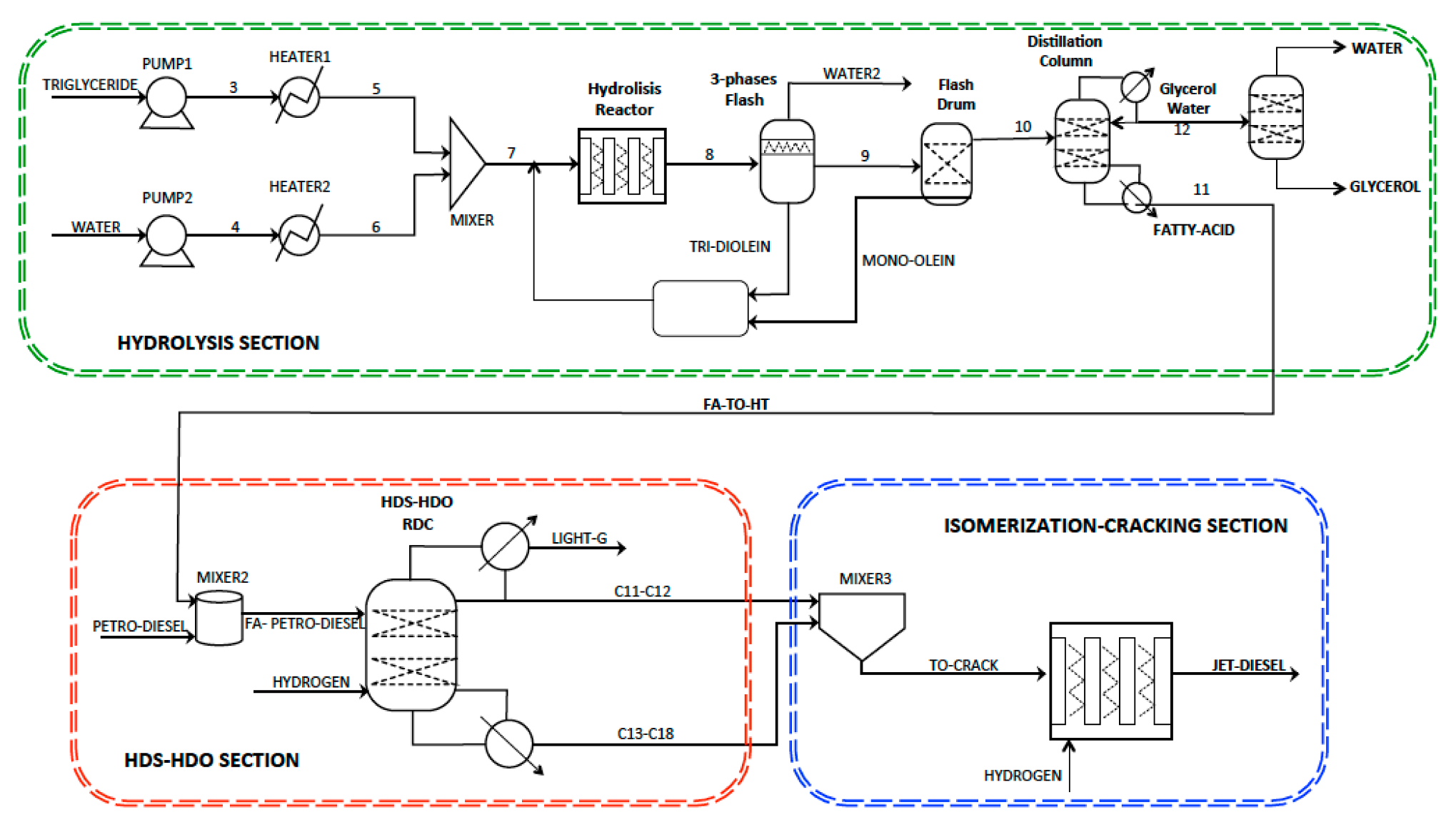
2. The Intensified Reactive Separation Process
2.1. The Triglycerides Hydrolysis Section
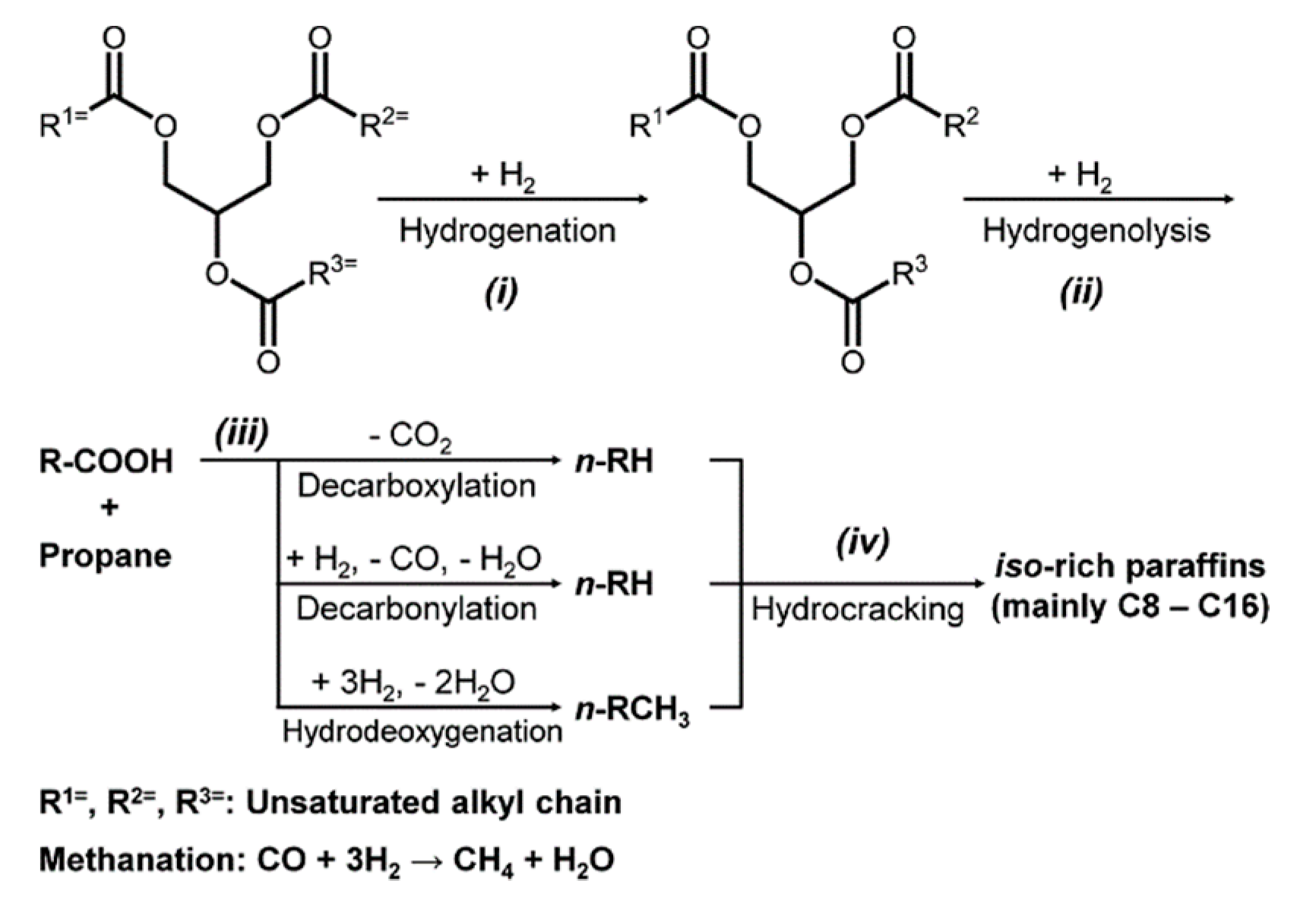
2.2. The HDS-HDO Reactive Distillation Section
2.3. The Isomerization-Hydrocracking Section

3. Results and Discussion
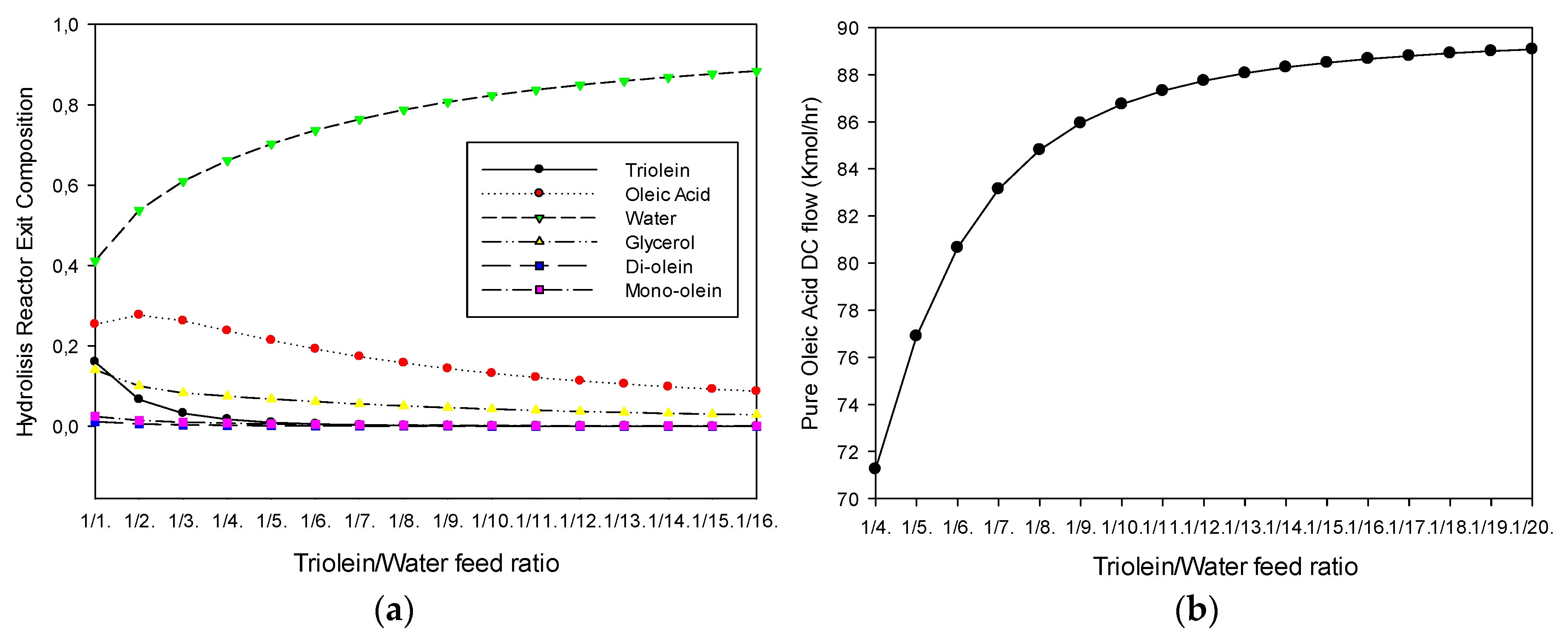
3.1. Hydrolysis Section
Effect of the Triolein-Water Feed Ratio
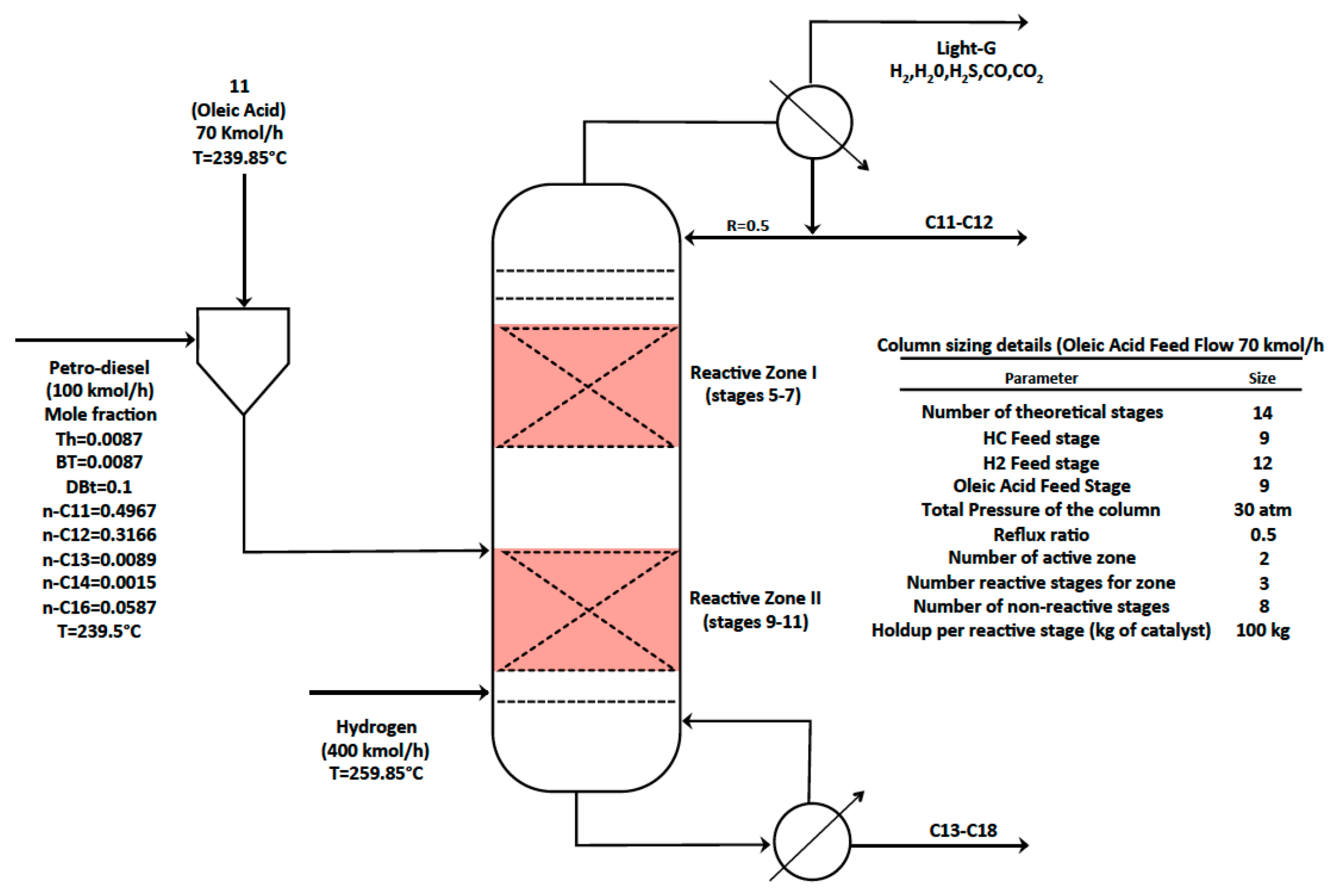
3.2. HDS-HDO Section
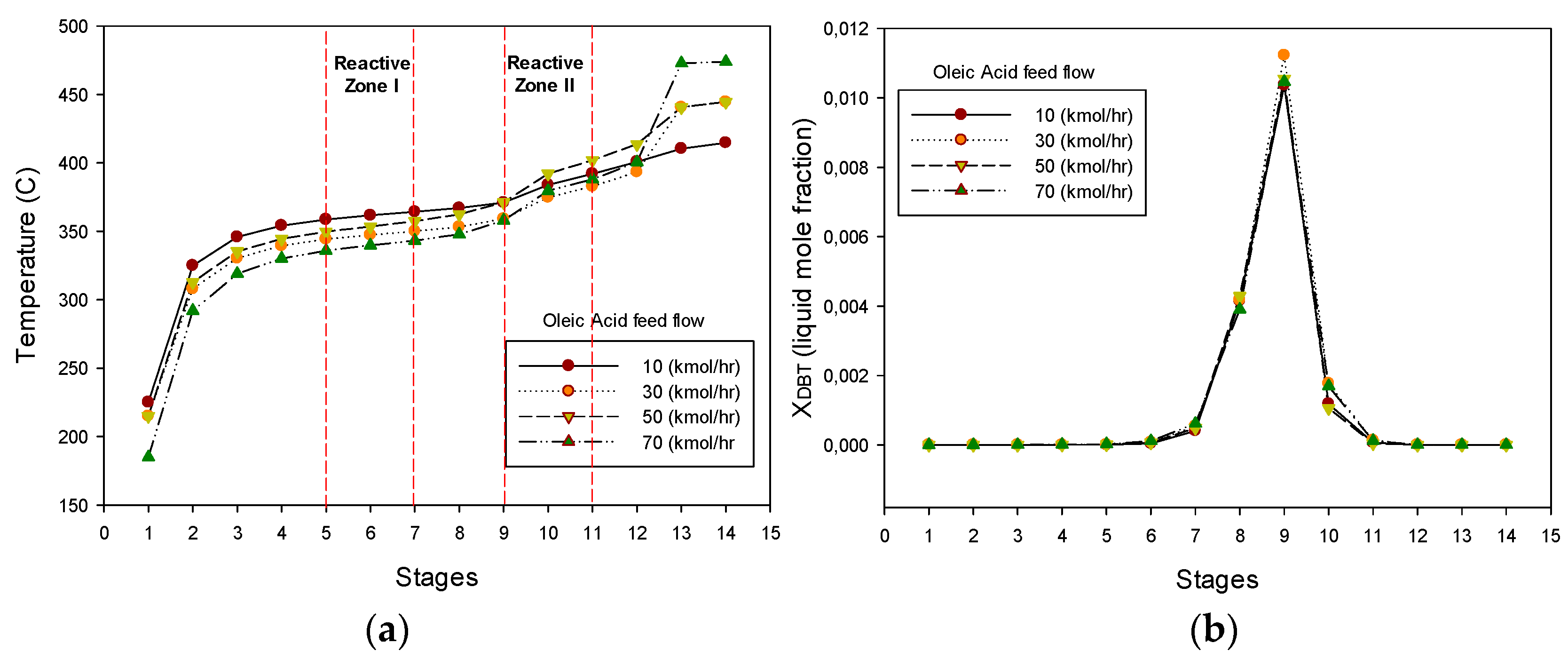
3.2.1. Effect of the Oleic Acid-Petro-Diesel Feed Ratio
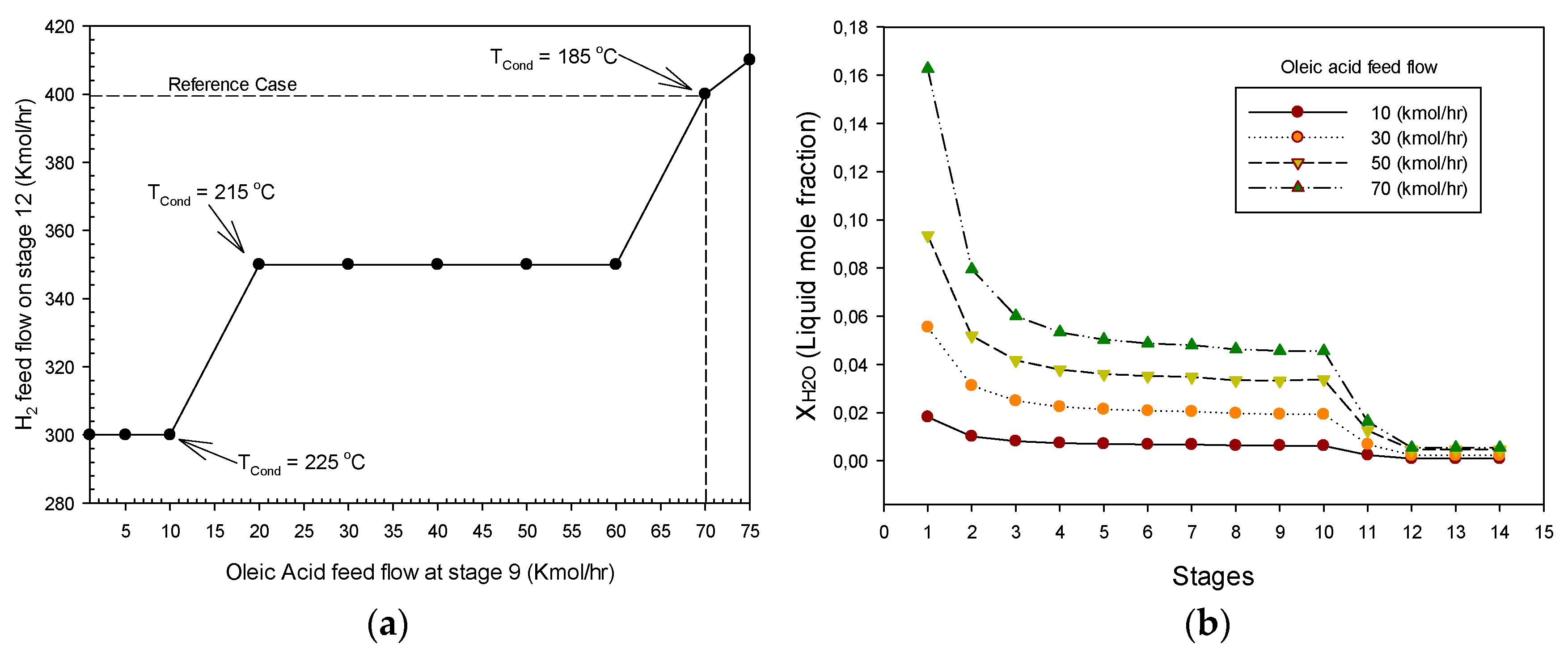
3.2.2. Hydrogen Consumption and Liquid Water Production
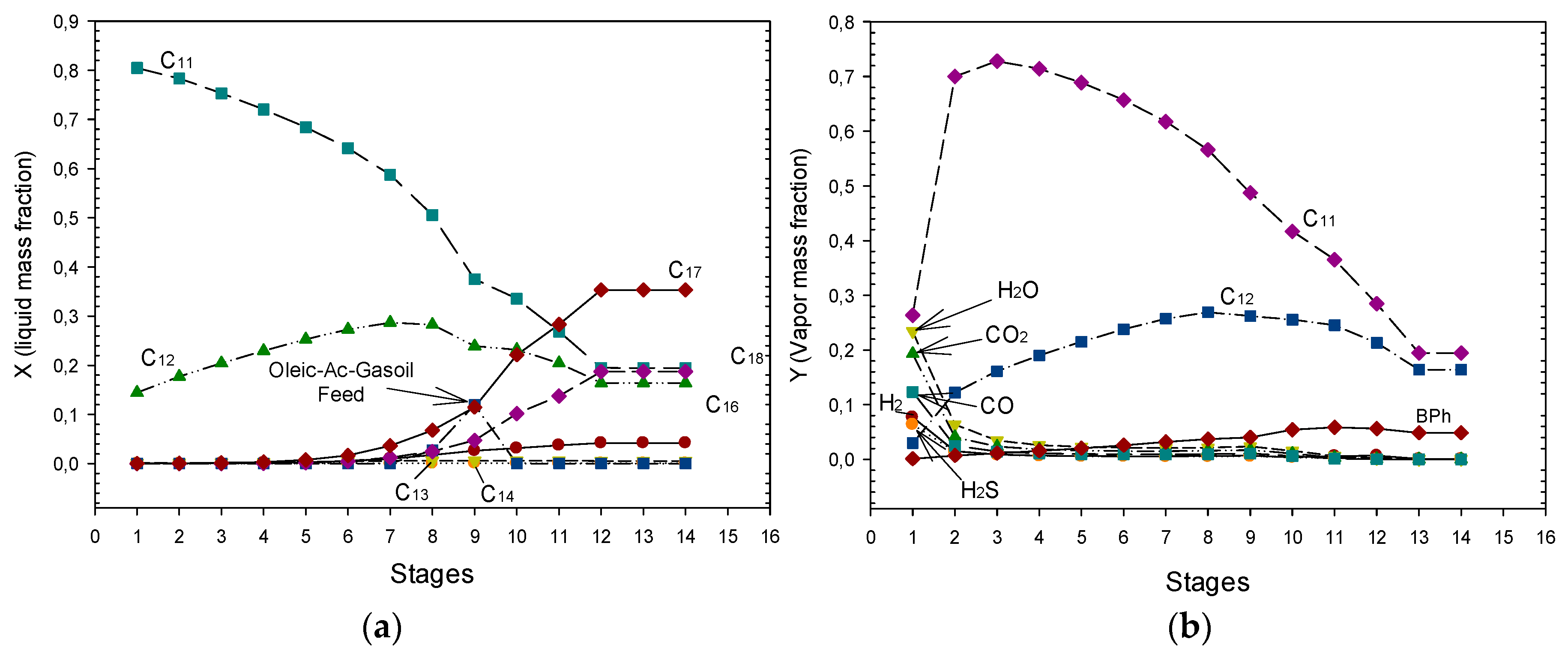
3.2.3. Hydrocarbon Distribution and Release of Generated Gases
3.2.4. Operability and Controllability of the HDO-HDS Reactive Distillation Section
3.3. The Isomerization-Cracking Section
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sargent, R.W.H. Integrated design and optimization of processes. Chem. Eng. Prog. 1967, 63, 71–78. [Google Scholar]
- Sargent, R.W.H. Forecasts and trends in systems engineering. Chem. Eng. 1972, 262, 226. [Google Scholar]
- Grossmann, I.E.; Westerberg, A.W. Research challenges in process systems engineering. AIChE J. 2000, 46, 1700–1703. [Google Scholar] [CrossRef]
- Pantelides, C.C. New challenges and opportunities for process modelling. In Computer Aided Chemical Engineering; Gani, R., Jorgensen, S.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Moulijn, J.A.; Stankiewicz, A.; Grievink, J.; Gorak, A. Process intensification and process systems engineering: A friendly symbiosis. Comput. Chem. Eng. 2008, 32, 3–11. [Google Scholar] [CrossRef]
- Zhang, L.; Babi, D.K.; Gani, R. New vistas in chemical product-Process design . Ann. Rev. Chem. Biomol. Eng. 2016, 7, 557–582. [Google Scholar] [CrossRef]
- Gani, R. Chemical product design: Challenges and opportunities. Comput. Chem. Eng. 2004, 28, 2441–2457. [Google Scholar] [CrossRef]
- Rosillo-Calle, F.; Teelucksingh, S.; Thrän, D.; Seiffert, M. IEA Bioenergy. 2012. Available online: http://task40.ieabioenergy.com/iea-publications/task-40-library/ (accessed on 22 July 2019).
- McCall, M.J.; Kocal, J.A.; Bhattacharyya, A.; Kalnes, T.N.; Brandvold, T.A. Production of Aviation Fuel from Renewable Feedstocks. U.S. Patent 8,039,682B2, 18 October 2011. [Google Scholar]
- Lamprecht, D. Fischer−Tropsch Fuel for Use by the U.S. Military as Battlefield-Use Fuel of the Future. Energy Fuels 2007, 21, 1448–1453. [Google Scholar] [CrossRef]
- Luning Prak, D.J.; Jones, M.H.; Trulove, P.; McDaniel, A.M.; Dickerson, T.; Cowart, J.S. Physical and Chemical Analysis of Alcohol-to-Jet (ATJ) Fuel and Development of Surrogate Fuel Mixtures. Energy Fuels 2015, 29, 3760–3769. [Google Scholar] [CrossRef]
- Olcay, H.; Subrahmanyam, A.V.; Xing, R.; Lajoie, J.; Dumesic, J.A.; Huber, G.W. Production of renewable petroleum refinery diesel and jet fuel feedstocks from hemicellulose sugar streams. Energy Environ. Sci. 2013, 6, 205–216. [Google Scholar] [CrossRef]
- Davis, R.; Biddy, M.J.; Tan, E.; Tao, L.; Jones, S.B. Biological Conversion of Sugars to Hydrocarbons Technology Pathway; Pacific Northwest National Lab.(PNNL): Richland, WA, USA, 2013. [Google Scholar]
- Wang, W.C.; Tao, L.; Markham, J.; Zhang, Y.; Tan, E.; Batan, L.; Warner, E.; Biddy, M. Bio-Jet Fuel Conversion Technologies. Review of Bio-Jet Fuel Conversion Technologies at 2016. NREL/TP-5100-66291. Available online: http://www.nrel.gov/publications (accessed on 13 January 2018).
- Douvartzides, S.L.; Charisiou, N.D.; Papageridis, K.N.; Goula, M.A. Green Diesel: Biomass Feedstocks, Production Technologies, Catalytic Research, Fuel Properties and Performance in Compression Ignition Internal Combustion Engines. Energies 2019, 12, 809. [Google Scholar] [CrossRef]
- Wang, W.C.; Tao, L. Bio-Jet fuel conversion technologies. Renew. Sustain. Energy Rev. 2016, 53, 801–822. [Google Scholar] [CrossRef]
- Martinez-Hernandez, E.; Ramírez-Verduzco, L.F.; Amezcua-Allieri, M.A.; Aburto, J. Process simulation and techno-Economic analysis of bio-Jet fuel and green diesel production—Minimum selling prices. Chem. Eng. Res. Des. 2019, 146, 60–70. [Google Scholar] [CrossRef]
- American Society for Testing and Materials; Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbons; ASTM International: West Conshohocken, PA, USA, 2014.
- Perez-Cisneros, E.S.; Sales-Cruz, M.; Lobo-Oehmichen, R.; Viveros-García, T. A reactive distillation process for co-hydrotreating of non-Edible vegetable oils and petro-Diesel blends to produce green diesel fuel. Comput. Chem. Eng. 2017, 105, 105–122. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Sales-Cruz, M.; Lopez-Arenas, T.; Viveros-García, T.; Ochoa-Tapia, A.; Lobo-Oehmichen, R.; Pérez-Cisneros, E.S. An Integrated Reactive Separation Process for Co-Hydrotreating of Vegetable Oils and Gasoil to Produce Jet Diesel. In Proceedings of the 28th European Symposium on Computer Aided Process Engineering, Graz, Austria, 10–13 June 2018; pp. 839–844. [Google Scholar] [CrossRef]
- Ngaosuwan, K.; Lotero, E.; Suwannakarn, K.; Goodwin, J.G.; Praserthdam, P. Hydrolysis of Triglycerides Using Solid Acid Catalysts. Ind. Eng. Chem. Res. 2009, 48, 4757–4767. [Google Scholar] [CrossRef]
- Calemma, V.; Peratello, S.; Perego, C. Hydroisomerization and hydrocracking of long chain n-Alkanes on Pt/amorphous SiO2–Al2O3 catalyst. Appl. Catal. A 2000, 190, 207–218. [Google Scholar] [CrossRef]
- Triantafyllidis, K.; Lappas, A.; Stöcker, M. The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Namdev, P.D.; Patil, T.A.; Raghunathan, T.S.; Shankar, H.S. Thermal Hydrolysis of Vegetable Oils and Fats. 3. An Analysis of Design Alternatives. Ind. Eng. Chem. Res. 1988, 27, 739–743. [Google Scholar] [CrossRef]
- Metzger, J.O.; Bornscheuer, U. Lipids as renewable resources: Current state of chemical and biotechnological conversion and diversification. Appl. Microbiol. Biotechnol. 2006, 71, 13–22. [Google Scholar] [CrossRef]
- Kubičková, I.; Snåre, M.; Eränen, K.; Mäki-Arvela, P.; Murzin, D.Y. Hydrocarbons for diesel fuel via decarboxylation of vegetable oils. Catal. Today 2005, 106, 197–200. [Google Scholar] [CrossRef]
- Moquin, P.H.L.; Temelli, F. Kinetic modeling of hydrolysis of canola oil in supercritical media. J. Supercrit. Fluids 2008, 45, 94–101. [Google Scholar] [CrossRef]
- Noureddini, H.; Harkey, D.W.; Gutsman, M.R. A continuous process for the glycerolysis of soybean oil. J. Am. Oil Chem. Soc. 2004, 81, 203–207. [Google Scholar] [CrossRef]
- Twitchell, E. Benzenestearosulphonic acid and other sulphonic acids containing the stearic radical. J. Am. Chem. Soc. 1900, 22, 22–26. [Google Scholar] [CrossRef][Green Version]
- Barnebey, H.L. Continuous Fat Splitting Plants Using the Colgate-Emery Process. J. Am. Chem. Soc. 1948, 25, 95–99. [Google Scholar] [CrossRef]
- Stumborg, M.; Wong, A.; Hogan, E. Hydroprocessed vegetable oils for diesel fuel improvement. Bioresour. Technol. 1996, 56, 13–18. [Google Scholar] [CrossRef]
- Egeberg, R.; Knudsen, K.; Nyström, S.; Lind, E.; Efraimsson, K. Industrial-Scale production of renewable diesel. Pet. Technol. Q. 2011, 16, 59–65. [Google Scholar]
- Scherzer, J.; Gruia, A.J. Hydrocracking Science and Technology; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Kim, M.Y.; Kim, J.K.; Lee, M.E.; Lee, S.; Choi, M. Maximizing Biojet Fuel Production from Triglyceride: Importance of the Hydrocracking Catalyst and Separate Deoxygenation/Hydrocracking Steps. ACS Catal. 2017, 7, 6256–6267. [Google Scholar] [CrossRef]
- Baur, R.; Taylor, R.; Krishna, R. Bifurcation analysis for TAME synthesis in a reactive distillation column: Comparison of pseudo-Homogeneous and heterogeneous reaction kinetics models. Chem. Eng. Process. 2003, 42, 211–221. [Google Scholar] [CrossRef]
- Wang, S.J.; Wong, D.S.H.; Lee, E.K. Effect of interaction multiplicity on control system design for a MTBE reactive distillation column. J. Process Control 2003, 13, 503–515. [Google Scholar] [CrossRef]
- Yang, B.; Wu, J.; Zhao, G.; Wang, H.; Lu, S. Multiplicity analysis in reactive distillation column using ASPEN PLUS. Chin. J. Chem. Eng. 2006, 14, 301–308. [Google Scholar] [CrossRef]
- Cárdenas-Guerra, J.C.; López-Arenas, T.; Lobo-Oehmichen, R.; Pérez-Cisneros, E.S. A reactive distillation process for deep hydrodesulfurization of diesel: Multiplicity and operation aspects. Comput. Chem. Eng. 2010, 34, 196–209. [Google Scholar] [CrossRef]
| Category | Pathway | Key Conversion Steps | Catalyst | FeedStock | Companies |
|---|---|---|---|---|---|
| Alcohol to Jet (ATJ) | Ethanol to Jet | Ethanol dehydration | Al2O3, Transition metal oxides | Sugar cane Corn grain | Terrabon; Swedish biofuels; Coskata |
| Butanol to Jet | Butanol dehydration | Zirconia, Solid acid catalyst | Lignocellulose | Gevo; Byogy; Solazyme | |
| Oil to Jet (OTJ) | Hydroprocessed renewable Jet (HEFA) | Catalytic hydrogenation | Noble metals, Transition metals | Camelina oil Spybean oil Jatropha oil Waste oils Animal fat Microalgae | UOP; SG biofuels; Neste oil; PEMEX; Syntroleum-Tyson Food PetroChina |
| Cracking and isomerization | Pt, Ni, Precious metals | ||||
| Catalytic hydrothermolysis (CH) | Catalytic hydrothermolysis | Zinc Acetate | Camelina oils Lignocellulose | Aemetis/Chevron Lummus global | |
| Decarboxylation-Hydrotreating | Nickel | ||||
| Hydrotreated Depolymerized Cellulosic Jet (HDCJ) | Hydrodeoxygenation | MoCx/C, Pd-Mo | Lignocellulose | Kior/Hunt; Refining/Petrotech; Envergent; Dinamotive | |
| Gas to Jet (ATJ) | FT Synthesis | FT Process | Fe, Co, Ni and Ru | Lignocellulose | Syntrolleum; SynFuels; Shell |
| Sugar to Jet (ATJ) | Direct sugar biological to hydrocarbons | Acid Condensation Hydrodeoxygenation | Acid catalyst Ru/C | Sugar cane Lignocellulose | Amyris/Total; Solazyme, LS9 |
| Stream | Triolein | Water | 7 | 8 | 9 | Glycerol | 11 |
|---|---|---|---|---|---|---|---|
| Mole Flow kmol/h | |||||||
| Triolein | 30 | 0.0 | 30 | 5.21 | 5.21 | 0.0 | 0.0 |
| Oleic acid | 0.0 | 0.0 | 0.0 | 70.83 | 70.83 | 1.11 × 10−26 | 70.00 |
| Water | 0.0 | 265 | 265 | 194.16 | 47.00 | 9.27 × 10−3 | 1.44 × 10−33 |
| Glycerol | 0.0 | 0.0 | 0.0 | 21.89 | 21.55 | 21.54 | 1.67 × 10−16 |
| Diolein | 0.0 | 0.0 | 0.0 | 0.62 | 1.06 × 10−14 | 0.0 | 0.0 |
| Monolein | 0.0 | 0.0 | 0.0 | 2.25 | 2.25 | 0.0 | 0.0 |
| Mass fraction | |||||||
| Triolein | 1.0 | 0.0 | 0.8476 | 0.1474 | 0.1634 | 0.0 | 0.0 |
| Oleic acid | 0.0 | 0.0 | 0.0 | 0.6385 | 0.7077 | 1.58 × 10−27 | 1.00 |
| Water | 0.0 | 1.0 | 0.1524 | 0.1166 | 0.0300 | 8.41 × 10−5 | 1.29 × 10−36 |
| Glycerol | 0.0 | 0.0 | 0.0 | 0.0643 | 0.0702 | 0.9999 | 7.60 × 10−19 |
| Diolein | 0.0 | 0.0 | 0.0 | 0.0123 | 2.29 × 10−16 | 0.0. | 0.0 |
| Monolein | 0.0 | 0.0 | 0.0 | 0.0257 | 0.0284 | 0.0. | 0.0 |
| Total Flow (kg/h) | 26,563.47 | 4774.049 | 31,337.52 | 31,337.52 | 28,255.39 | 1985.16 | 19,772.67 |
| Temperature (K) | 298.15 | 298.15 | 494.26 | 553.15 | 410.28 | 557.18 | 631.04 |
| Pressure (atm) | 1.0 | 1.0 | 30.0 | 30.0 | 5.0 | 1.0 | 1.0 |
| Stream | Petro-Diesel | Hydrogen | Oleic Acid (11) | Light-G | C11–C12 | C13–C18 |
|---|---|---|---|---|---|---|
| Mole Flow kmol/h | ||||||
| H2 | 0.0 | 400 | 0.0 | 202.2064 | 0.0415 | 14.4201 |
| H2S | 0.0 | 0.0 | 0.0 | 9.8951 | 0.0157 | 0.0884 |
| Th | 0.8699 | 0.0 | 0.0 | 0.8363 | 0.0108 | 0.0228 |
| BT | 0.8699 | 0.0 | 0.0 | 0.0798 | 0.0122 | 0.7779 |
| DBT | 9.9999 | 0.0 | 0.0 | 6.73 × 10−7 | 9.72 × 10−7 | 7.61 × 10−4 |
| Biphenil | 0.0 | 0.0 | 0.0 | 0.0390 | 0.0126 | 9.9476 |
| n-C16 | 5.8899 | 0.0 | 0.0 | 7.79 × 10−5 | 9.84 × 10−5 | 5.8898 |
| n-C14 | 0.1500 | 0.0 | 0.0 | 5.84 × 10−5 | 3.57 × 10−5 | 0.14990 |
| n-C13 | 0.8899 | 0.0 | 0.0 | 3.46 × 10−3 | 1.24 × 10−3 | 0.8852 |
| n-C12 | 31.6599 | 0.0 | 0.0 | 0.9127 | 0.2000 | 30.5477 |
| n-C11 | 49.6699 | 0.0 | 0.0 | 8.9238 | 1.2100 | 39.5333 |
| Oleic-Acid | 0.0 | 0.0 | 70 | 0.0 | 0.0 | 0.0 |
| n-C18 | 0.0 | 0.0 | 0.0 | 4.06 × 10−6 | 1.17 × 10−5 | 23.3332 |
| n-C17 | 0.0 | 0.0 | 0.0 | 5.05 × 10−5 | 1.02 × 10−4 | 46.6665 |
| Water | 0.0 | 0.0 | 0.0 | 68.7544 | 0.2980 | 0.9470 |
| CO2 | 0.0 | 0.0 | 0.0 | 23.2247 | 0.0224 | 0.08615 |
| CO | 0.0 | 0.0 | 0.0 | 23.2884 | 7.72 × 10−3 | 0.03714 |
| Mole fraction | ||||||
| H2 | 0.0 | 1.0 | 0.0 | 0.5979 | 0.0226 | 0.0831 |
| H2S | 0.0 | 0.0 | 0.0 | 0.0292 | 8.55 × 10−3 | 5.10 × 10−4 |
| Th | 0.0087 | 0.0 | 0 | 2.47 × 10−3 | 5.90 × 10−3 | 1.31 × 10−4 |
| BT | 0.0087 | 0.0 | 0.0 | 2.36 × 10−4 | 6.6.E-03 | 4.44 × 10−3 |
| DBT | 0.1 | 0.0 | 0.0 | 1.99 × 10−9 | 5.30 × 10−7 | 4.41 × 10−6 |
| Biphenyl | 0.0 | 0.0 | 0.0 | 1.15 × 10−4 | 6.84 × 10−3 | 0.0573 |
| n-C16 | 0.0589 | 0.0 | 0.0 | 2.31 × 10−7 | 5.36 × 10−5 | 0.0339 |
| n-C14 | 0.0015 | 0.0 | 0.0 | 1.73 × 10−7 | 1.94 × 10−5 | 8.64 × 10−4 |
| n-C13 | 0.0089 | 0.0 | 0.0 | 1.02 × 10−5 | 6.78 × 10−4 | 5.10 × 10−3 |
| n-C12 | 0.3166 | 0.0 | 0.0 | 2.69 × 10−3 | 0.1087 | 0.1762 |
| n-C11 | 0.4967 | 0.0 | 0.0 | 0.0263 | 0.6608 | 0.2280 |
| Oleic-Acid | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 |
| n-C18 | 0.0 | 0.0 | 0.0 | 1.20 × 10−8 | 6.37 × 10−6 | 0.1346 |
| n-C17 | 0.0 | 0.0 | 0.0 | 1.49 × 10−7 | 5.54 × 10−5 | 0.2692 |
| Water | 0.0 | 0.0 | 0.0 | 0.2033 | 0.1626 | 5.46 × 10−3 |
| CO2 | 0.0 | 0.0 | 0.0 | 0.0686 | 0.0122 | 4.97 × 10−4 |
| CO | 0.0 | 0.0 | 0.0 | 0.0688 | 4.20 × 10−3 | 2.15 × 10−4 |
| Total Flow (Kg/h) | 16,717.09 | 806.35 | 19,772.67 | 5296.10 | 235.52 | 31,764.49 |
| Temperature (K) | 513 | 533 | 513 | 458.15 | 458.15 | 746.89 |
| Pressure (atm) | 30 | 30 | 30 | 30 | 30 | 30 |
| Component | To Iso-Crack | Bio-Jet Diesel | Component | To Iso-Crack | Bio-Jet Diesel |
|---|---|---|---|---|---|
| Mole Flow (kmol/h) | Mole Fraction | ||||
| n-C16 | 5.8899 | 0.0 | n-C16 | 0.03968 | 0.0 |
| n-C14 | 0.1500 | 0.0 | n-C14 | 0.00101 | 0.0 |
| n-C13 | 0.8899 | 0.0 | n-C13 | 0.00600 | 0.0 |
| n-C12 | 30.7472 | 0.0 | n-C12 | 0.20716 | 0.0 |
| n-C11 | 40.7460 | 0.0 | n-C11 | 0.27453 | 0.0 |
| n-C18 | 23.3333 | 0.0 | n-C18 | 0.15721 | 0.0 |
| n-C17 | 46.6666 | 0.0 | n-C17 | 0.31441 | 0.0 |
| i-C16 | 0.0 | 5.8899 | i-C16 | 0.0 | 0.02697 |
| i-C14 | 0.0 | 0.15 | i-C14 | 0.0 | 0.00069 |
| i-C13 | 0.0 | 0.8899 | i-C13 | 0.0 | 0.00407 |
| i-C12 | 0.0 | 30.7472 | i-C12 | 0.0 | 0.14077 |
| i-C11 | 0.0 | 40.7460 | i-C11 | 0.0 | 0.18655 |
| i-C10 | 0.0 | 23.3333 | i-C10 | 0.0 | 0.10682 |
| i-C9 | 0.0 | 46.6666 | i-C9 | 0.0 | 0.21365 |
| i-C8 | 0.0 | 69.9999 | i-C8 | 0.0 | 0.32048 |
| Total Flow (kmol/h) | 148.423 | 218.4228 | |||
| Temperature (K) | 653 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Sánchez, M.; Sales-Cruz, M.; Lopez-Arenas, T.; Viveros-García, T.; Pérez-Cisneros, E.S. An Intensified Reactive Separation Process for Bio-Jet Diesel Production. Processes 2019, 7, 655. https://doi.org/10.3390/pr7100655
García-Sánchez M, Sales-Cruz M, Lopez-Arenas T, Viveros-García T, Pérez-Cisneros ES. An Intensified Reactive Separation Process for Bio-Jet Diesel Production. Processes. 2019; 7(10):655. https://doi.org/10.3390/pr7100655
Chicago/Turabian StyleGarcía-Sánchez, Miriam, Mauricio Sales-Cruz, Teresa Lopez-Arenas, Tomás Viveros-García, and Eduardo S. Pérez-Cisneros. 2019. "An Intensified Reactive Separation Process for Bio-Jet Diesel Production" Processes 7, no. 10: 655. https://doi.org/10.3390/pr7100655
APA StyleGarcía-Sánchez, M., Sales-Cruz, M., Lopez-Arenas, T., Viveros-García, T., & Pérez-Cisneros, E. S. (2019). An Intensified Reactive Separation Process for Bio-Jet Diesel Production. Processes, 7(10), 655. https://doi.org/10.3390/pr7100655





