Adsorptive Properties of Poly(1-methylpyrrol-2-ylsquaraine) Particles for the Removal of Endocrine-Disrupting Chemicals from Aqueous Solutions: Batch and Fixed-Bed Column Studies
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
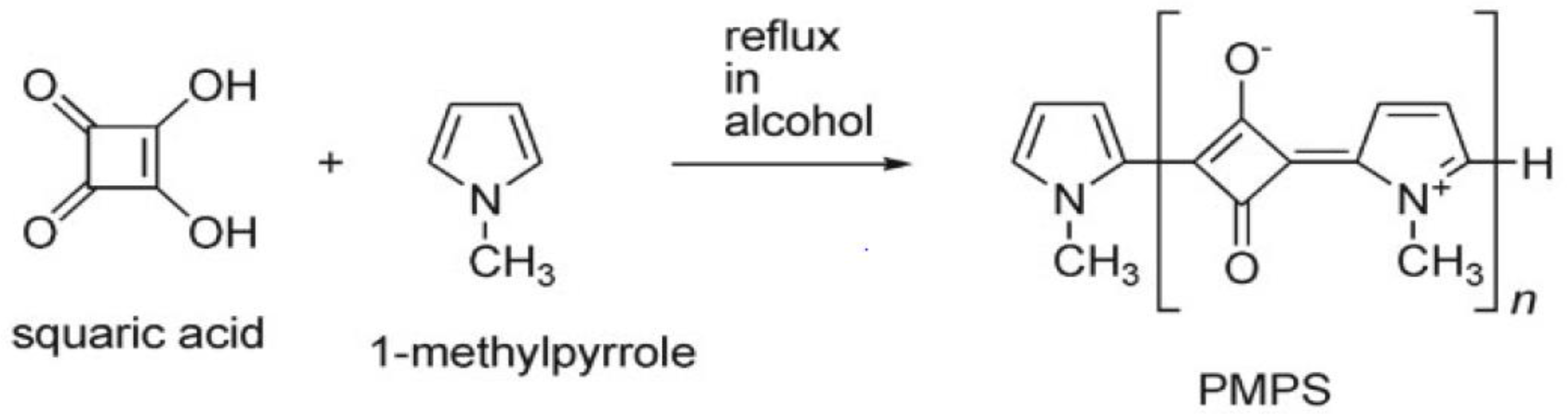
2.2. PMPS Particles
2.3. Batch Adsorption Experiments
2.4. Column Adsorption Experiment
2.5. Analytical Procedures
3. Results and Discussion
3.1. Batch Adsorption Experiments
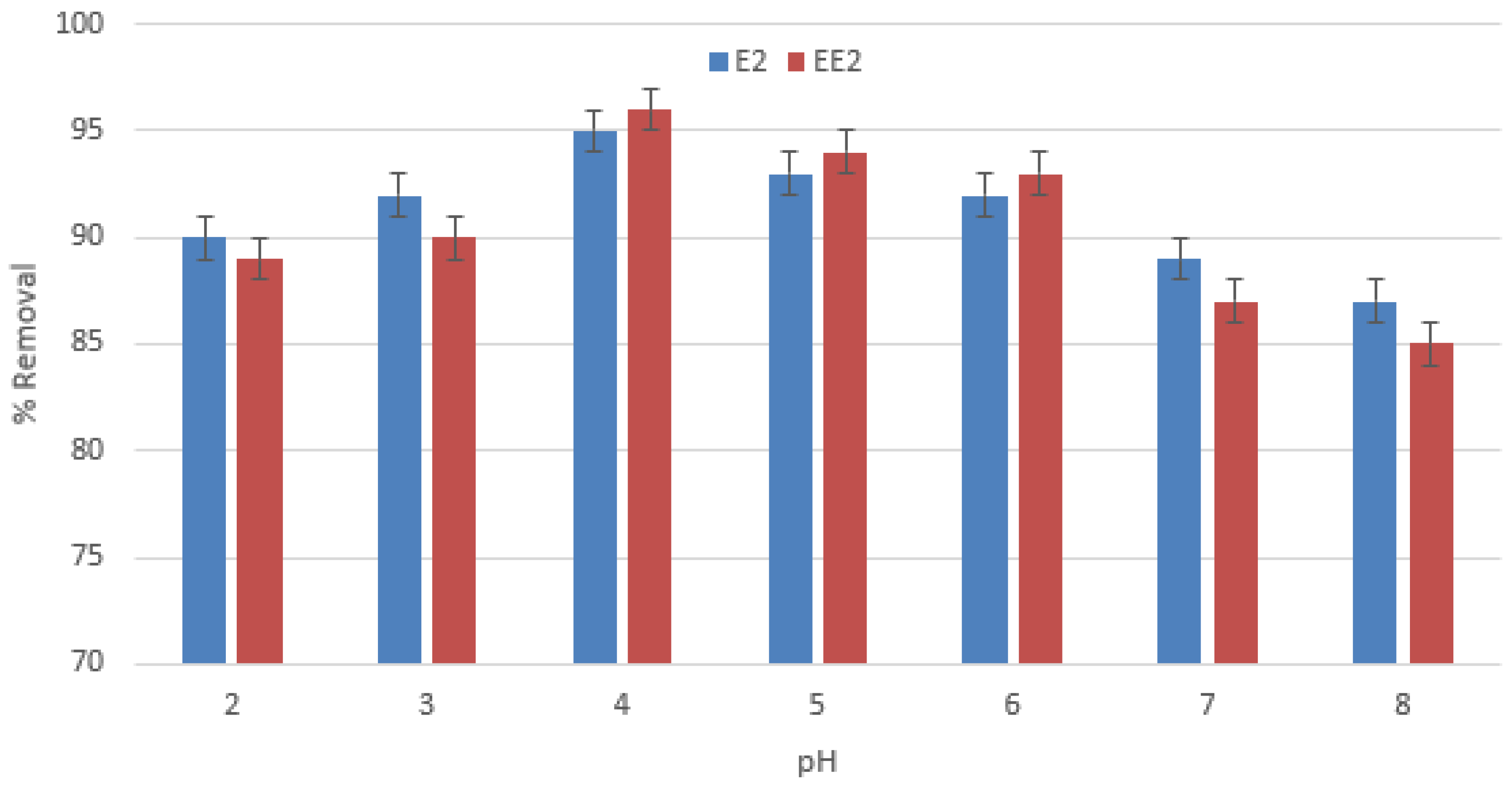
3.1.1. Influence of pH on Adsorptive Capacity of PMPS Particles
3.1.2. Effects of Contact Time and Adsorption Kinetics
3.1.3. Adsorption Isotherm
Langmuir Adsorption Isotherm
- qe (mg/g) is the amount of PMPS adsorbed per unit mass,
- Qm (mg/g) is the maximum amount of PMPS per unit mass to form a monolayer on the surface,
- KL (L/Mg) is the isotherm constant related to the affinity of the binding site.
Freundlich Adsorption Isotherm
- n is a measure of the adsorption intensity,
- Ce (mg/L) is the adsorbate concentration at equilibrium,
- Qe (mg/g) is the amount of PMPS adsorbed per unit mass,
Temkin Adsorption Isotherm
3.1.4. Adsorption Thermodynamics
3.2. Column Studies
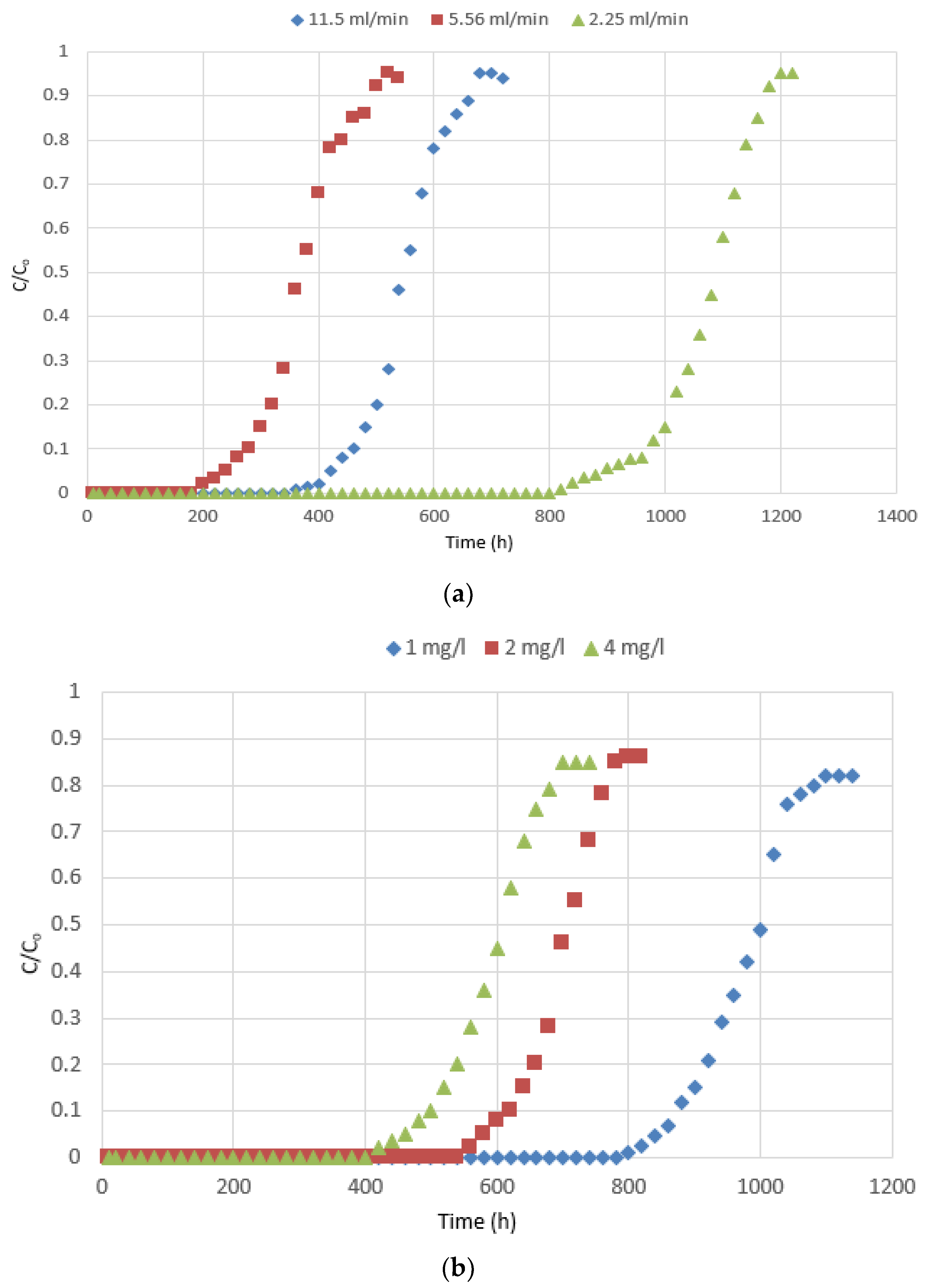
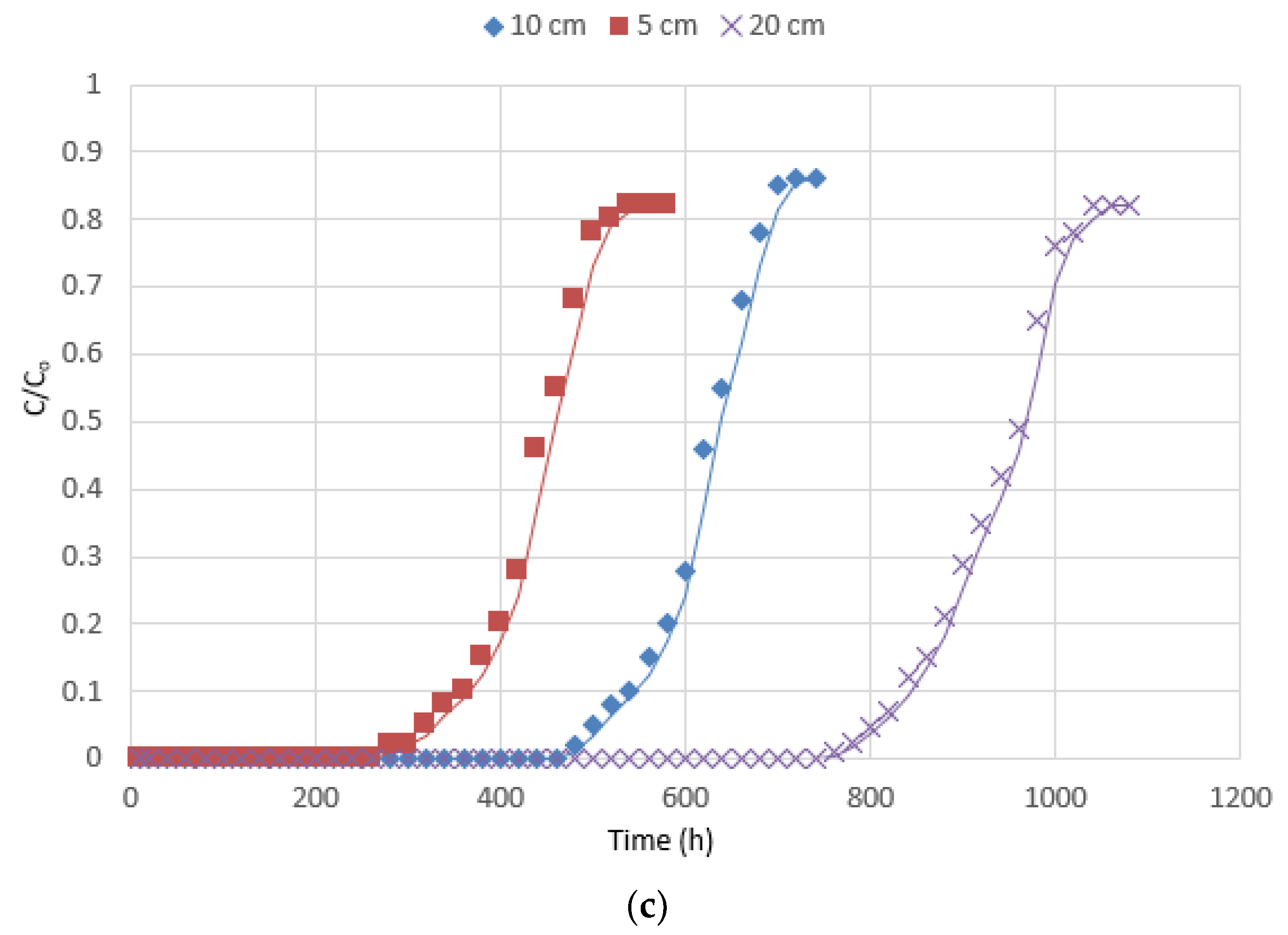
3.2.1. Effects of the Operating Parameters on the Breakthrough Curve
3.2.2. Breakthrough Modelling
Thomas Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Surface area (m2) |
| AT | Tempkin isotherm equilibrium binding constant (L/g) |
| EBCT | Empty bed contact time (h) |
| EDCs | Endocrine-disruptive compounds |
| E2 | estradiol |
| EE2 | 17α-ethynylestradiol |
| Ce | E2 and EE2 concentration at equilibrium (mg/L) |
| Co | E2 and EE2 concentration at time ‘0’ (mg/L) |
| k1 | Pseudo first-order rate constants (min−1) |
| k2 | Pseudo-second-order rate constants (g/mg min) |
| kL | Langmuir’s sorption isotherm constant (L/mg) |
| kf | Sorption capacity derived from Freundlich’s isotherm model (L/mg) |
| kTH | Thomas kinetic constant (mL/h/mg) |
| Q | Flow rate (mL/min) |
| Qm | Sorption capacity of PMPS from the Langmuir’s isotherm (mg/g) |
| qe | Solid phase concentration of EE2 at contact time (t, min)(mg/g) |
| MTZ | Mass transfer zone to length of the column ratio (m) |
| t | Contact time (min) |
| tb | Time to breakthrough (h) |
| te | Time to exhaustion (h) |
| Z | Total bed depth (cm) |
| 1/n | Sorption intensity derived from Freundlich's isotherm model |
| ΔGo | Change in free energy (kJ/mol) |
| ΔHo | Change in enthalpy (kJ/mol) |
| ΔSo | Change in entropy (J/kmol) |
References
- Nakada, N.; Tanishima, T.; Shinohara, H.; Kiri, K.; Takada, H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res. 2006, 40, 3297–3303. [Google Scholar] [CrossRef] [PubMed]
- Ifelebuegu, A.O.; Ukpebor, J.E.; Obidiegwu, C.C.; Kwofi, B.C. Comparative potential of black tea leaves waste to granular activated carbon in adsorption of endocrine disrupting compounds from aqueous solution. Glob. J. Environ. Sci. Manag. 2015, 1, 205–214. [Google Scholar]
- Ifelebuegu, A.O.; Ukpebor, J.; Nzeribe-Nwedo, B. Mechanistic evaluation and reaction pathway of UV photo-assisted Fenton-like degradation of progesterone in water and wastewater. Int. J. Environ. Sci. Technol. 2016, 13, 2757–2766. [Google Scholar] [CrossRef]
- Dalrymple, O.K.; Yeh, D.H.; Trotz, M.A. Removing pharmaceuticals and endocrine-disrupting compounds from wastewater by photocatalysis. J. Chem. Technol. Biotechnol. 2007, 82, 121–134. [Google Scholar] [CrossRef]
- Ifelebuegu, A.O. The fate and behaviour of selected endocrine disrupting chemicals in full scale wastewater and sludge treatment unit processes. Int. J. Environ. Sci. Technol. 2011, 8, 245–254. [Google Scholar] [CrossRef]
- Kortenkamp, A. Endocrine disruptors: The burden of endocrine-disrupting chemicals in the USA. Nat. Rev. Endocrinol. 2017, 13, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Kim, S.G.; Kim, C.W.; Kim, S.H. Effects of activated carbon types and service life on removal of endocrine disrupting chemicals: Amitrol, nonylphenol, and bisphenol-A. Chemosphere 2005, 58, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Ifelebuegu, A.O.; Lester, J.N.; Churchley, J.; Cartmell, E. Removal of an endocrine disrupting chemical (17α-ethinyloestradiol) from wastewater effluent by activated carbon adsorption: Effects of activated carbon type and competitive adsorption. Environ. Technol. 2006, 27, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Mohan, S.V. Endocrine disruptive synthetic estrogen (17α-ethynylestradiol) removal from aqueous phase through batch and column sorption studies: Mechanistic and kinetic analysis. Desalination 2011, 276, 66–74. [Google Scholar] [CrossRef]
- Ifelebuegu, A.O. Removal of steriod hormones by activated carbon adsorption—Kinetic and thermodynamic studies. J. Environ. Prot. 2012, 3, 469. [Google Scholar] [CrossRef]
- Joseph, L.; Zaib, Q.; Khan, I.A.; Berge, N.D.; Park, Y.G.; Saleh, N.B.; Yoon, Y. Removal of bisphenol A and 17α-ethinyl estradiol from landfill leachate using single-walled carbon nanotubes. Water Res. 2011, 45, 4056–4068. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Flora, J.R.; Her, N.; Park, Y.G.; Cho, J.; Son, A.; Yoon, Y. Removal of bisphenol A and 17β-estradiol in single walled carbon nanotubes–ultrafiltration (SWNTs–UF) membrane systems. Sep. Purif. Technol. 2012, 90, 39–52. [Google Scholar] [CrossRef]
- Zaib, Q.; Khan, I.A.; Saleh, N.B.; Flora, J.R.; Park, Y.G.; Yoon, Y. Removal of bisphenol A and 17β-estradiol by single-walled carbon nanotubes in aqueous solution: Adsorption and molecular modeling. Water Air Soil Pollut. 2012, 223, 3281–3293. [Google Scholar] [CrossRef]
- Le Noir, M.; Plieva, F.; Hey, T.; Guieysse, B.; Mattiasson, B. Macroporous molecularly imprinted polymer/cryogel composite systems for the removal of endocrine disrupting trace contaminants. J. Chromatogr. A 2007, 1154, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wu, D.; Chen, X.; Lin, Y. Adsorption of bisphenol A from water by surfactant-modified zeolite. J. Colloid Interface Sci. 2010, 348, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.E. Pyrrolyl Squaraines—Fifty Golden Years. Metals 2015, 5, 1349–1370. [Google Scholar] [CrossRef]
- Triebs, A.; Jacob, K. Cyclotrimethine dyes derived from squaric acid. Angew. Chem. Int. Ed. 1965, 4, 694. [Google Scholar] [CrossRef]
- Lynch, D.E.; Nawaz, Y.; Bostrom, T. Preparation of sub-micrometer silica shells using poly (1-methylpyrrol-2-ylsquaraine). Langmuir 2005, 21, 6572–6575. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.E.; Bennett, J.B.; Bateman, M.J.; Reeves, C.R. The uptake of metal elements into poly(1-methylpyrrol-2-ylsquaraine) particles and a study of their porosity. Adsorpt. Sci. Technol. 2016, 34, 176–192. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Q.J.; He, J.H.; Xu, Q.F.; Li, H.; Li, N.J.; Chen, D.Y.; Lu, J.M. Polysquaraines: Novel humidity sensor materials with ultra-high sensitivity and good reversibility. Sens. Actuators B Chem. 2018, 255, 1147–1152. [Google Scholar] [CrossRef]
- Begum, S.; Jones, I.P.; Jiao, C.; Lynch, D.E.; Preece, J.A. Characterisation of hollow Russian doll microspheres. J. Mater. Sci. 2010, 45, 3697–3706. [Google Scholar] [CrossRef]
- Ifelebuegu, A.O.; Theophilus, S.C.; Bateman, M.J. Mechanistic evaluation of the sorption properties of endocrine disrupting chemicals in sewage sludge biomass. Int. J. Environ. Sci. Technol. 2010, 7, 617–622. [Google Scholar] [CrossRef]
- Ifelebuegu, A.O.; Ezenwa, C.P. Removal of endocrine disrupting chemicals in wastewater treatment by Fenton-like oxidation. Water Air Soil Pollut. 2011, 217, 213–220. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Sun, M.; Qiu, H.; Li, X. Synthesis of magnetic β-cyclodextrin–chitosan/graphene oxide as nanoadsorbent and its application in dye adsorption and removal. Colloids Surf. B Biointerfaces 2013, 103, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Shailaja, S.; Krishna, M.R.; Sarma, P.N. Adsorptive removal of phthalate ester (Di-ethyl phthalate) from aqueous phase by activated carbon: A kinetic study. J. Hazard. Mater. 2007, 146, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Mohan, S.V.; Sarma, P.N. Sorptive removal of endocrine-disruptive compound (estriol, E3) from aqueous phase by batch and column studies: Kinetic and mechanistic evaluation. J. Hazard. Mater. 2009, 164, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Kalavathy, M.H.; Karthikeyan, T.; Rajgopal, S.; Miranda, L.R. Kinetic and isotherm studies of Cu (II) adsorption onto H3PO4-activated rubber wood sawdust. J. Colloid Interface Sci. 2005, 292, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Langergren, S. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handlingnaar. 1898, 24, 1–39. [Google Scholar]
- Parab, H.; Joshi, S.; Shenoy, N.; Lali, A.; Sarma, U.S.; Sudersanan, M. Determination of kinetic and equilibrium parameters of the batch adsorption of Co (II), Cr (III) and Ni (II) onto coir pith. Process Biochem. 2006, 41, 609–615. [Google Scholar] [CrossRef]
- Pérez-Marín, A.; Zapata, V.M.; Ortuno, J.; Aguilar, M.; Sáez, J.; Lloréns, M. Removal of cadmium from aqueous solutions by adsorption onto orange waste. J. Hazard. Mater. 2007, 139, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Yuan, X.; Xia, W.; An, J.; Yin, J.; Zhou, X.; Yang, W. Kinetic and thermodynamic studies on the phosphate adsorption removal by dolomite mineral. J. Chem. 2015, 2015, 853105. [Google Scholar] [CrossRef]
- Dada, A.; Olalekan, A.; Olatunya, A.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin-radushkevich isotherms studies of equilibrium sorption of Zn2 Unto phosphoric acid modified rice husk. J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef]
- Horsfall, M.; Spiff, A.I.; Abia, A.A. Studies on the influence of mercaptoacetic acid (MAA) modification of cassava (Manihot sculenta cranz). waste biomass on the adsorption of Cu2+ and Cd2+ from aqueous solution. Bull. Korean Chem. Soc. 2004, 25, 969–976. [Google Scholar]
- Abdel Ghani, N.T.; Elchaghaby, G.A. Influence of operating conditions on the removal of Cu, Zn, Cd and Pb ion from wastewater by adsorption. Int. J. Environ. Sci. Technol. 2007, 4, 451–456. [Google Scholar] [CrossRef]
- Woumfo, E.D.; Siéwé, J.M.; Njopwouo, D. A fixed-bed column for phosphate removal from aqueous solutions using an andosol-bagasse mixture. J. Environ. Manag. 2015, 151, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Bhunia, P.; Dash, R.R. Modeling isotherms, kinetics and understanding the mechanism of phosphate adsorption onto a solid waste: ground burnt patties. J. Environ. Chem. Eng. 2014, 2, 1331–1342. [Google Scholar] [CrossRef]
- Nur, T.; Loganathan, P.; Nguyen, T.; Vigneswaran, S.; Singh, G.; Kandasamy, J. Batch and column adsorption and desorption of fluoride using hydrous ferric oxide: Solution chemistry and modeling. Chem. Eng. J. 2014, 247, 93–102. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, X.; Ma, F.; Li, J.; Shen, J.; Han, W.; Liu, X.; Wang, L. Removal of phosphate from etching wastewater by calcined alkaline residue: Batch and column studies. J. Taiwan Inst. Chem. Eng. 2014, 45, 1709–1716. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead (II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Zulfadhly, Z.; Mashitah, M.D.; Bhatia, S. Heavy metals removal in fixed-bed column by the macro fungus Pycnoporus sanguineus. Environ. Pollut. 2001, 112, 463–470. [Google Scholar] [CrossRef]
- Ghasemi, M.; Keshtkar, A.R.; Dabbagh, R.; Safdari, S.J. Biosorption of uranium (VI) from aqueous solutions by Ca-pretreated Cystoseira indica alga.Breakthrough curves studies and modeling. J. Hazard. Mater. 2011, 189, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Nwabanne, J.; Igbokwe, P. Adsorption performance of packed bed column for the removal of lead (II) using oil palm fibre. Int. J. Appl. Sci. Technol. 2012, 2, 5. [Google Scholar]


| Physical State | solid |
| Color | black |
| Specific gravity | 1.3 to 1.5 g/cm3 |
| Average particle size | 1.92 ± 0.05 µm [21] |
| Mean nominal rupture stress | 493 ± 113 MPa |
| Mean deformation at rupture | +65% initial diameter |
| Surface area | ~450 m2/g |
| Adsorbate | Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | ||||
|---|---|---|---|---|---|---|
| Linear Equation | K | R2 | Linear Equation | K | R2 | |
| EE2 | 0.016 × −0.2366 | 0.017 | 0.941 | 0.0291 × −0.072 | 0.029 | 0.996 |
| E2 | 0.019 × −0.2207 | 0.019 | 0.959 | 0.038 × −0.2084 | 0.038 | 0.993 |
| Adsorbate | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qm | KL | R2 | Kf | n | R2 | AT | B | R2 | |
| E2 | 0.313 | 2.80 | 0.977 | 0.287 | 1.59 | 0.995 | 1.386 | 0.2518 | 0.975 |
| EE2 | 0.327 | 2.53 | 0.979 | 0.285 | 1.618 | 0.996 | 1.393 | 0.2517 | 0.981 |
| Thermodynamic Parameter | E2 | EE2 |
|---|---|---|
| Gibbs Free Energy (kJ/mol) | −8.32 | −6.6 |
| Enthalpy (kJ/mol) | 68 | 43 |
| Entropy (J/mol K) | 0.26 | 0.17 |
| Co (mg L−1) | Z (cm) | ν (mL min−1) | kAB (L mg−1 min−1) × 10−3 | No (mg L−1) | R2 |
|---|---|---|---|---|---|
| 1 | 10 | 5.56 | 6.70 | 239 | 0.955 |
| 2 | 10 | 5.56 | 7.55 | 275 | 0.940 |
| 4 | 10 | 5.56 | 6.75 | 377 | 0.899 |
| 2 | 5 | 5.56 | 7.75 | 182 | 0.946 |
| 2 | 20 | 5.56 | 7.55 | 364 | 0.927 |
| 2 | 10 | 2.25 | 7.00 | 170 | 0.988 |
| 2 | 10 | 11.50 | 8.0 | 761 | 0.974 |
| Co (mg/L) | Z (cm) | ν (mL/min) | kTH (mL/min·mg) × 10−3 | qo (mg/g) | R2 |
|---|---|---|---|---|---|
| 1 | 10 | 5.56 | 7.50 | 1.67 | 0.956 |
| 2 | 10 | 5.56 | 8.70 | 1.86 | 0.966 |
| 4 | 10 | 5.56 | 8.85 | 2.50 | 0.930 |
| 2 | 5 | 5.56 | 8.25 | 1.31 | 0.956 |
| 2 | 20 | 5.56 | 7.70 | 1.39 | 0.930 |
| 2 | 10 | 2.25 | 7.40 | 1.11 | 0.987 |
| 2 | 10 | 11.50 | 6.6 | 2.56 | 0.923 |
| C0 (mg/L) | Z (cm) | ν (mL/min) | kYN (mL/min) | τ (min) | R2 |
|---|---|---|---|---|---|
| 1 | 10 | 5.56 | 0.0197 | 999 | 0.956 |
| 2 | 10 | 5.56 | 0.0224 | 589 | 0.992 |
| 4 | 10 | 5.56 | 0.0259 | 409 | 0.998 |
| 2 | 5 | 5.56 | 0.0226 | 456 | 0.989 |
| 2 | 20 | 5.56 | 0.0206 | 954 | 0.983 |
| 2 | 10 | 2.25 | 0.0201 | 1100 | 0.989 |
| 2 | 10 | 11.50 | 0.0213 | 411 | 0.993 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ifelebuegu, A.O.; Salauh, H.T.; Zhang, Y.; Lynch, D.E. Adsorptive Properties of Poly(1-methylpyrrol-2-ylsquaraine) Particles for the Removal of Endocrine-Disrupting Chemicals from Aqueous Solutions: Batch and Fixed-Bed Column Studies. Processes 2018, 6, 155. https://doi.org/10.3390/pr6090155
Ifelebuegu AO, Salauh HT, Zhang Y, Lynch DE. Adsorptive Properties of Poly(1-methylpyrrol-2-ylsquaraine) Particles for the Removal of Endocrine-Disrupting Chemicals from Aqueous Solutions: Batch and Fixed-Bed Column Studies. Processes. 2018; 6(9):155. https://doi.org/10.3390/pr6090155
Chicago/Turabian StyleIfelebuegu, Augustine O., Habibath T. Salauh, Yihuai Zhang, and Daniel E. Lynch. 2018. "Adsorptive Properties of Poly(1-methylpyrrol-2-ylsquaraine) Particles for the Removal of Endocrine-Disrupting Chemicals from Aqueous Solutions: Batch and Fixed-Bed Column Studies" Processes 6, no. 9: 155. https://doi.org/10.3390/pr6090155
APA StyleIfelebuegu, A. O., Salauh, H. T., Zhang, Y., & Lynch, D. E. (2018). Adsorptive Properties of Poly(1-methylpyrrol-2-ylsquaraine) Particles for the Removal of Endocrine-Disrupting Chemicals from Aqueous Solutions: Batch and Fixed-Bed Column Studies. Processes, 6(9), 155. https://doi.org/10.3390/pr6090155





