Energy and Exergy Analysis of the S-CO2 Brayton Cycle Coupled with Bottoming Cycles
Abstract
:1. Introduction
2. System Configuration, Modeling, and Simulation Environment
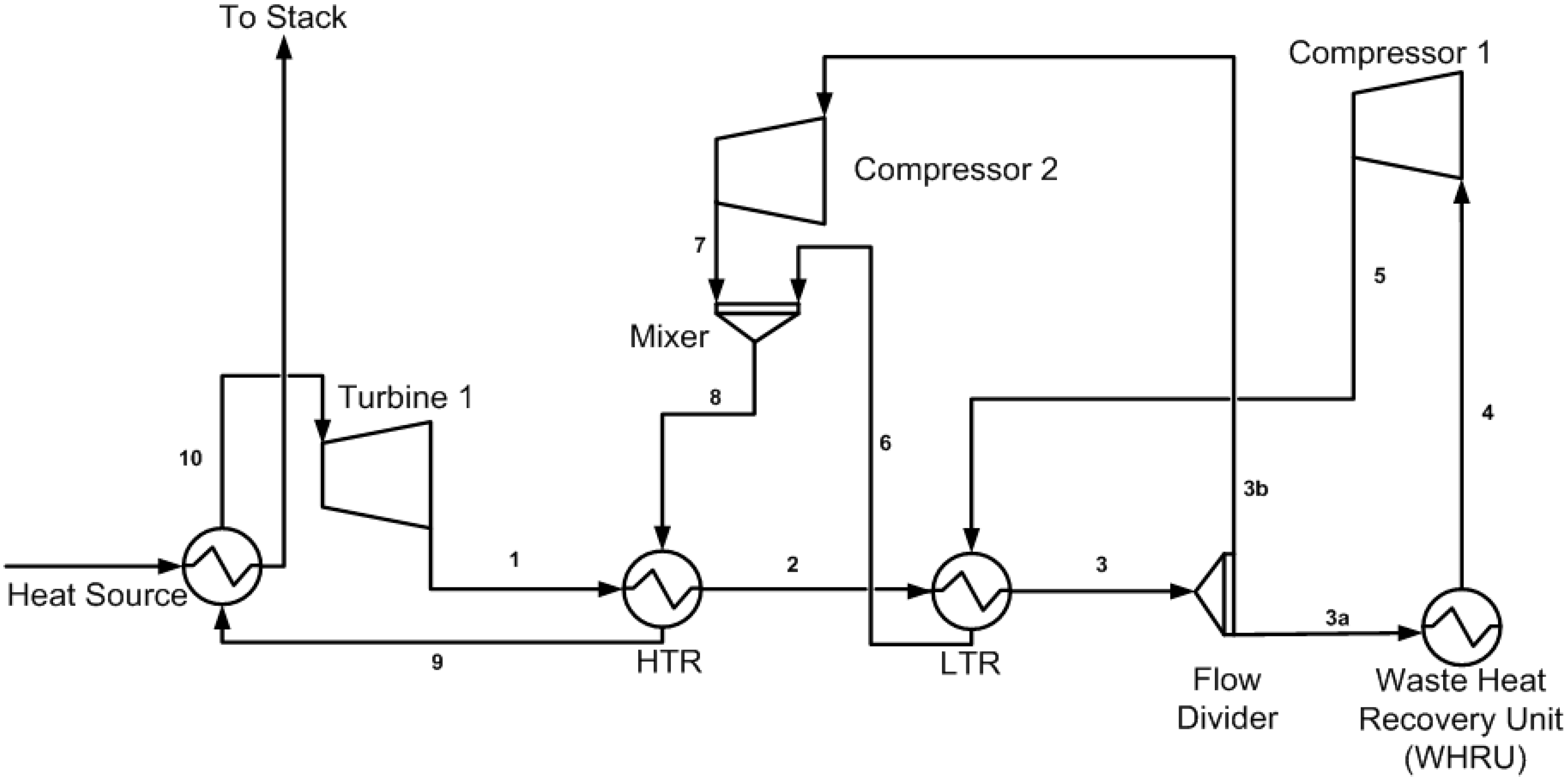
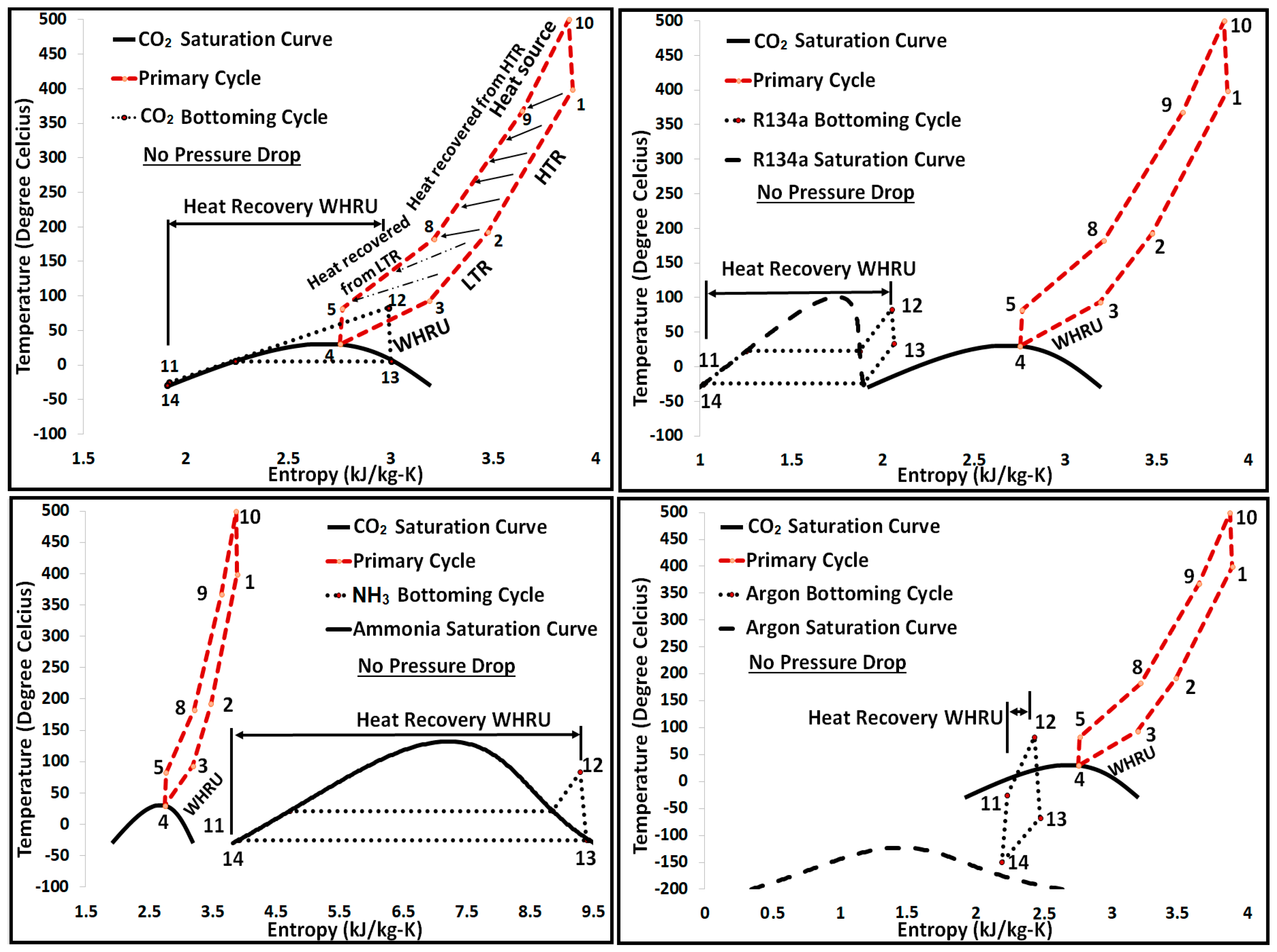
2.1. Primary Cycle (Base Model) Layout for the S-CO2 Cycle
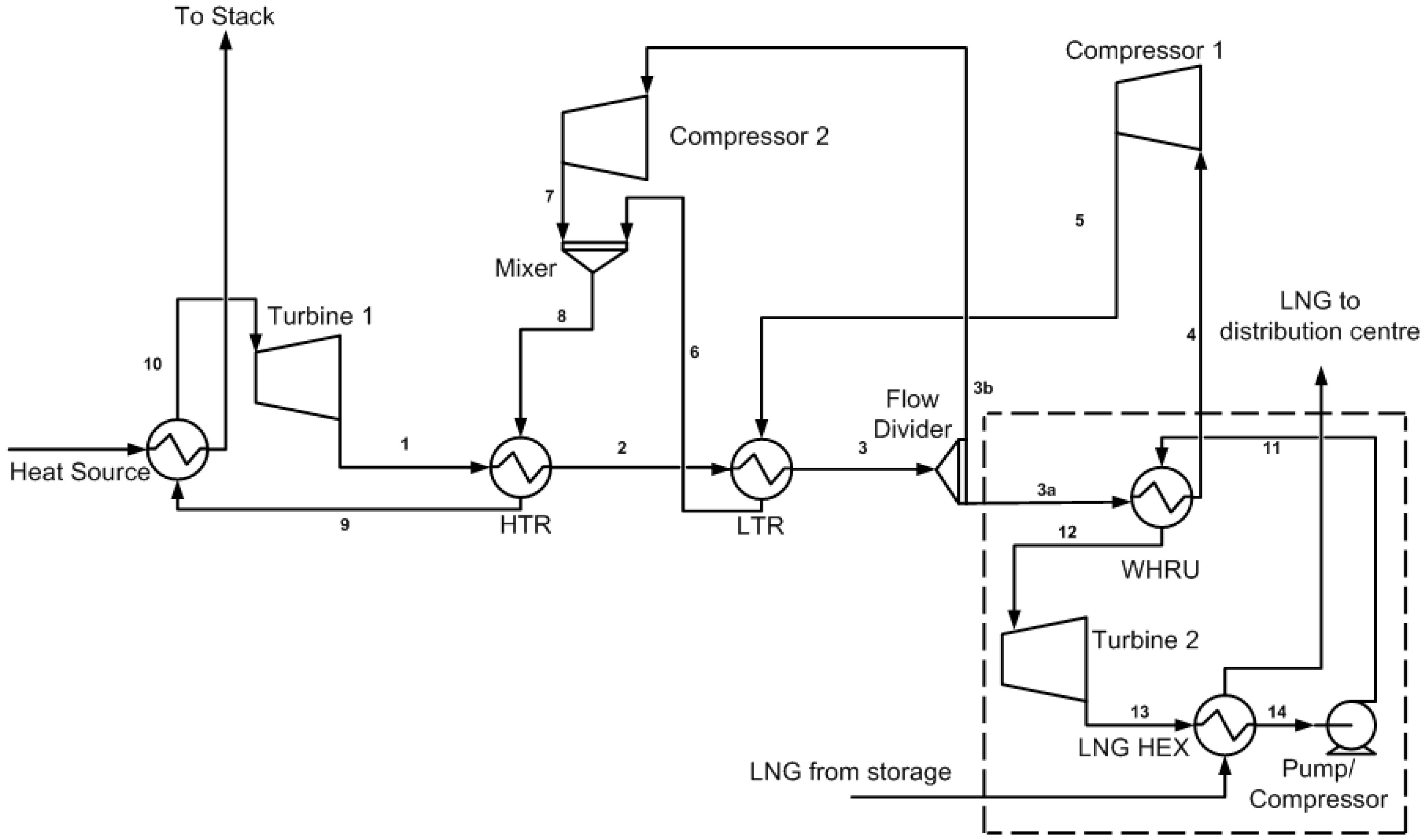
2.2. Cycle Cascade of the S-CO2 Combined Cycle
3. Mathematical Model
3.1. Energy Analysis—Governing Equations
3.2. Exergy Analysis—Governing Equations
4. Simulation Environment and Procedure
- The cycle operates under steady-state conditions.
- Surrounding temperature is 25 °C.
- Primary cycle mass flow rate is 100 kg/s.
- LNG in the storage tank is maintained at −162 °C.
- Energy losses in the pipelines are neglected.
- Compression and expansion processes are adiabatic.
- Compressor and turbine adiabatic efficiencies are 85% and 90%, respectively.
- Adiabatic efficiency of a centrifugal pump in the secondary cycle is 80%.
- Minimum temperature approach is set to 10 degrees in HTR, LTR, WHRU, and LNG HEX.
- The state of CO2 is kept close to the critical point at the inlet of Compressor 1, (P = 7.2 MPa and T = 30 °C).
5. Primary Cycle Parametric Adjustments
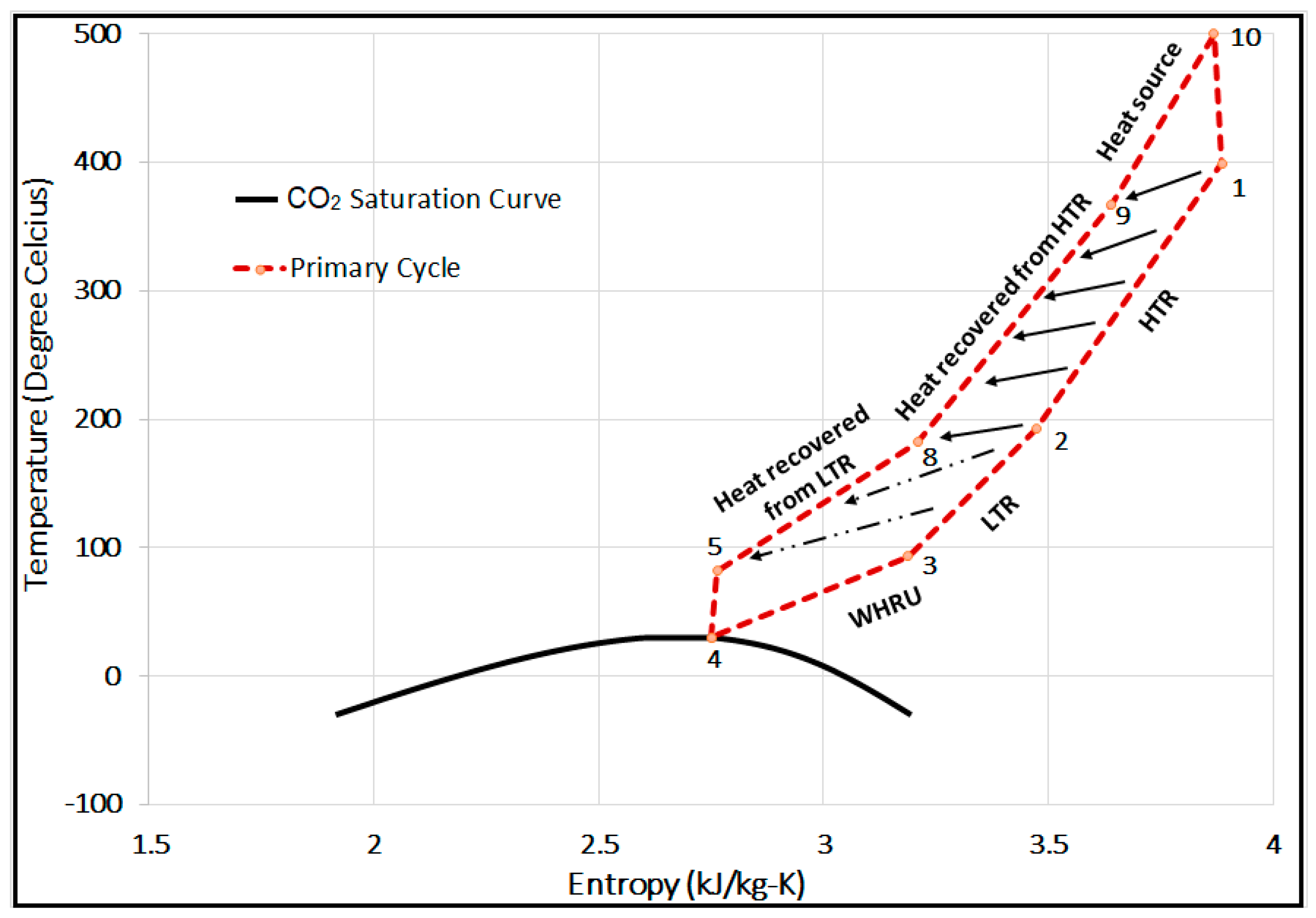
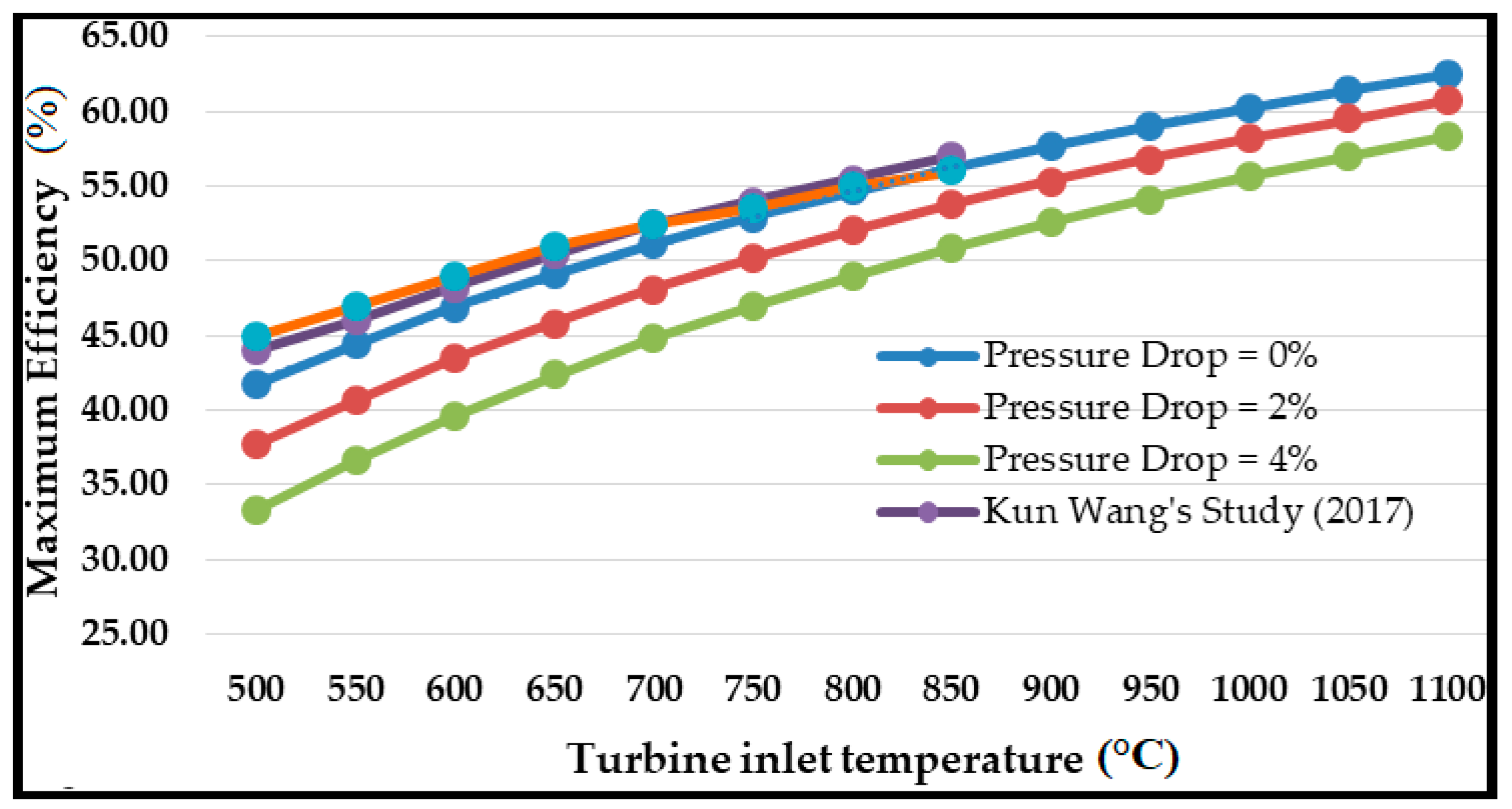
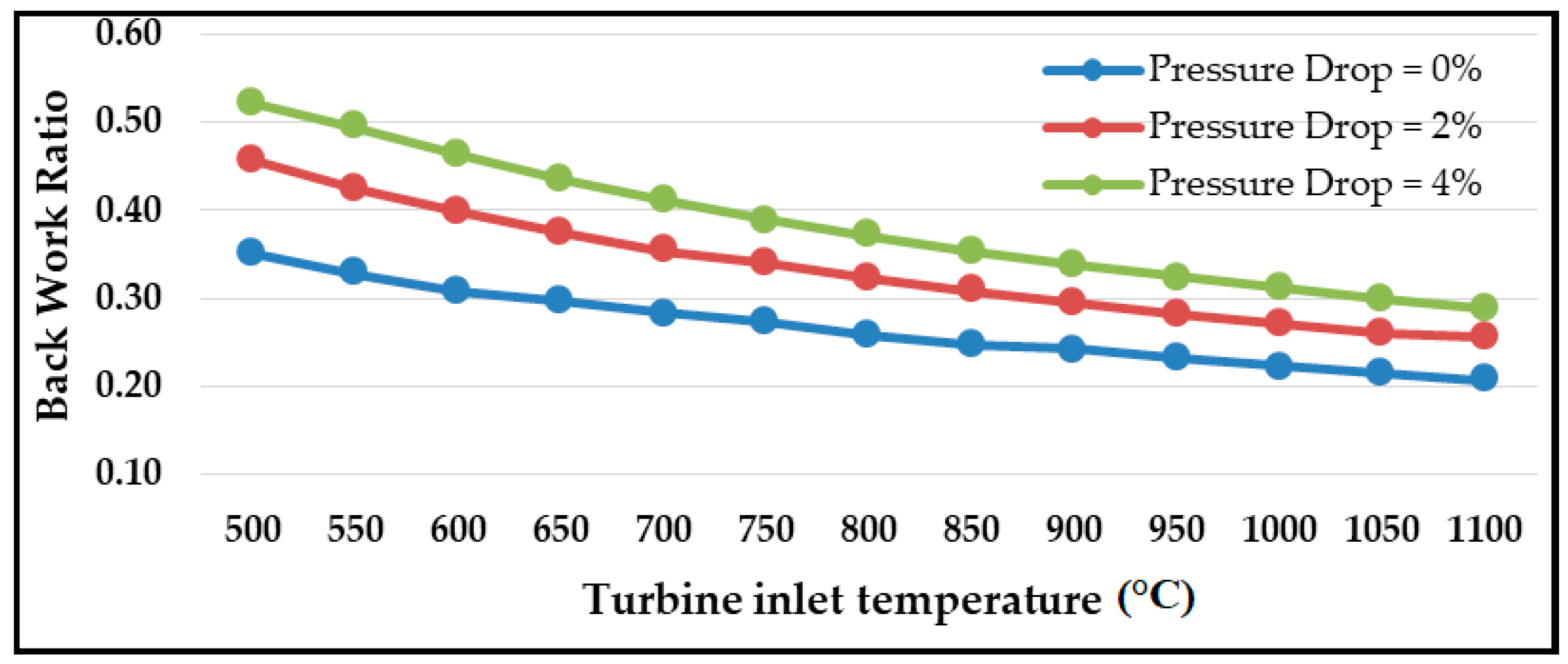
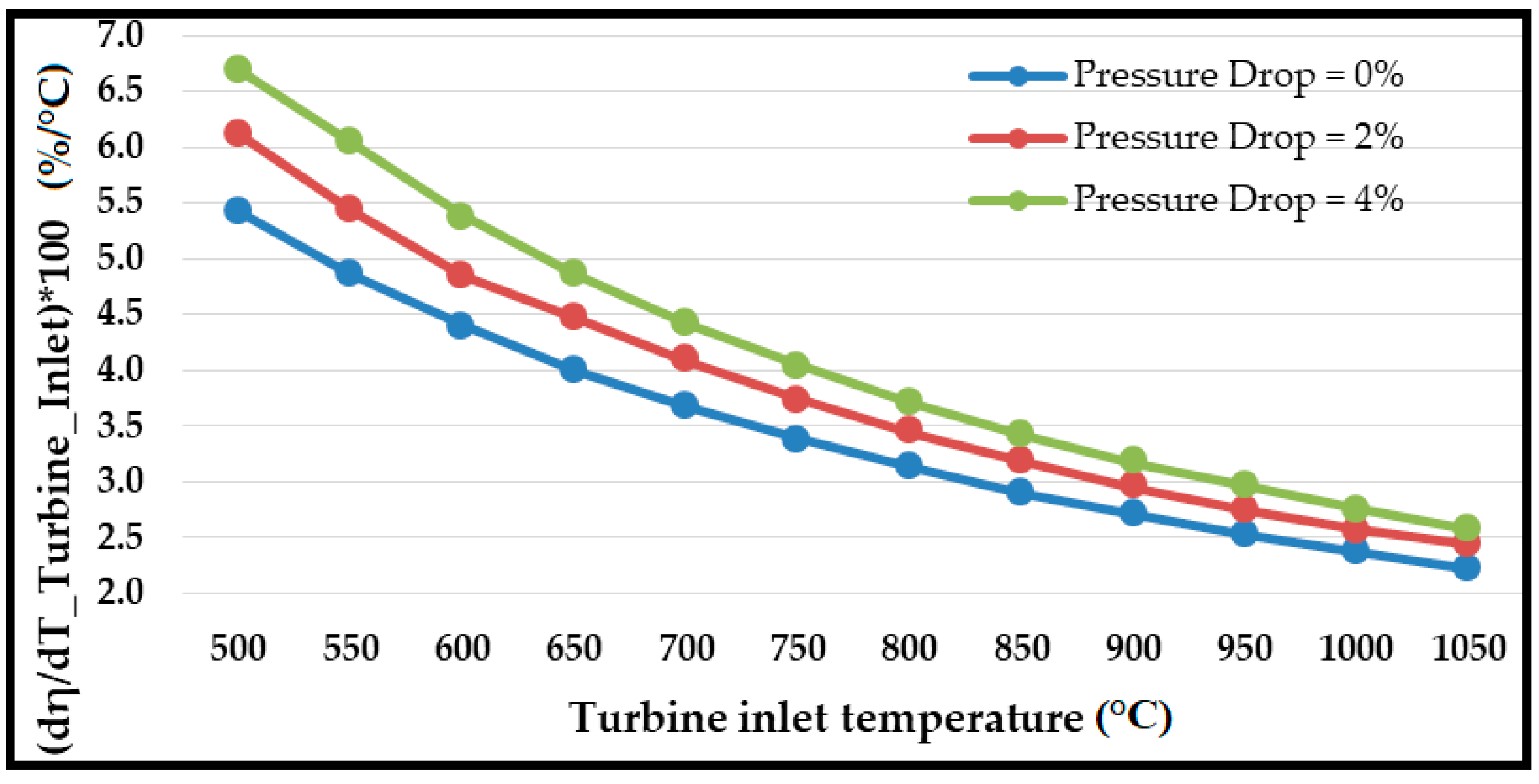
6. Primary Cycle (Base Model) Validation and Performance
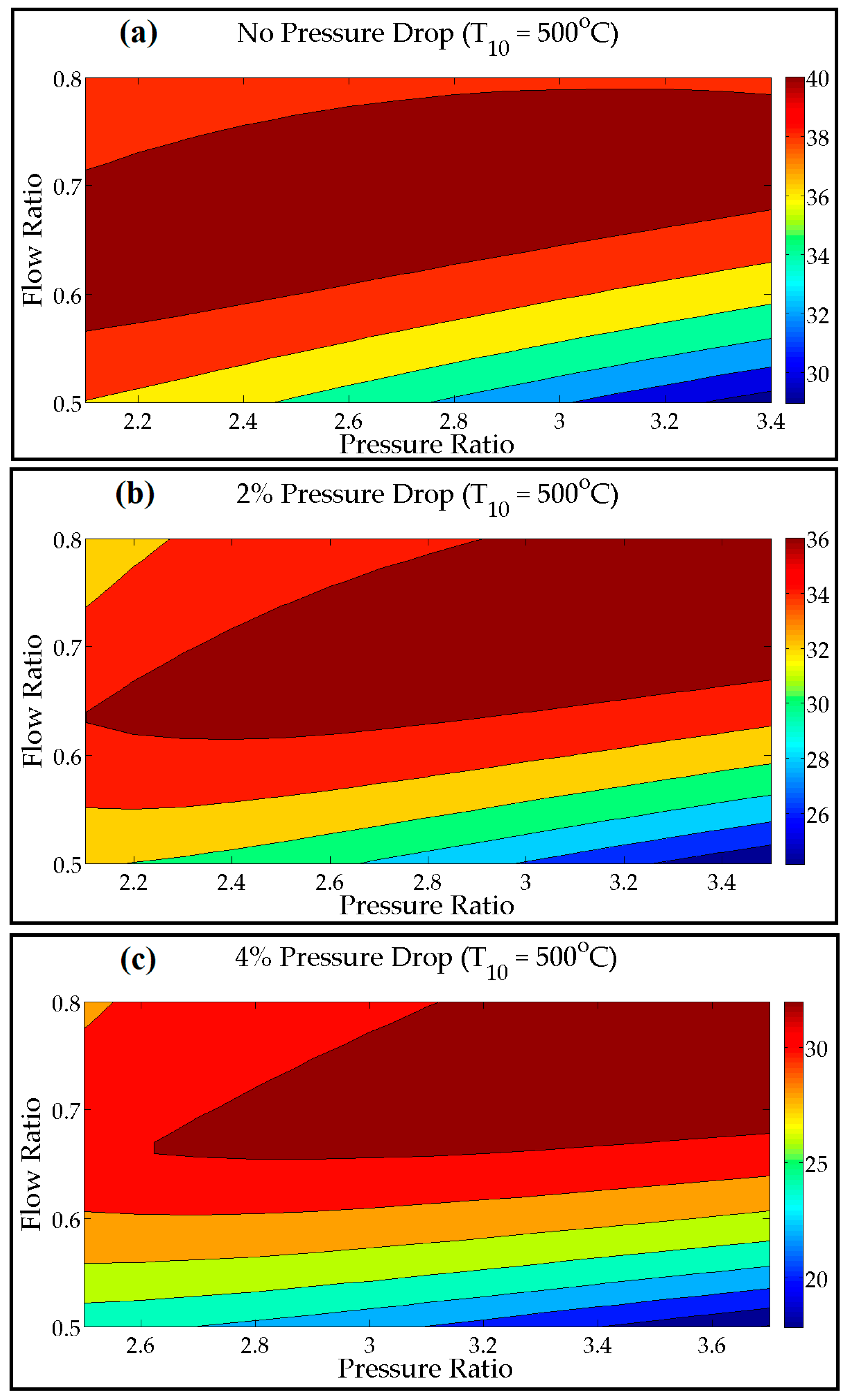
7. Combined Cycle Parametric Adjustments
8. Combined Cycle Performance and Overall Improvement
8.1. Combined Cycle Energy Analysis
8.2. Combined Cycle Exergy Analysis
9. Conclusions
- The supercritical CO2 recompression Brayton cycle is efficient, with a potential to provide high-efficiency values for medium-range source temperatures.
- As expected, the S-CO2 RBC thermal efficiency declined with the pressure drop. The pressure drop in the heat exchangers resulted in increased compressor work required to maintain the optimum cycle pressure. The optimum cycle pressure was 16.60 MPa, which raised to 24.45 MPa for a 4% pressure drop.
- Implementing the bottoming cycle is an attractive option with a sink temperature as low as −50 °C.
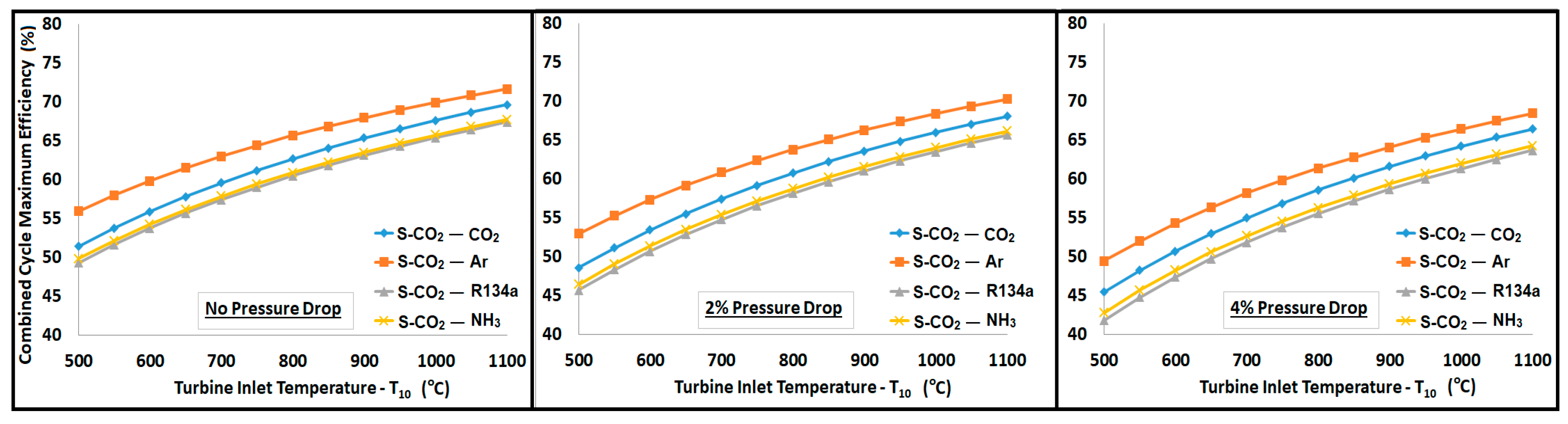
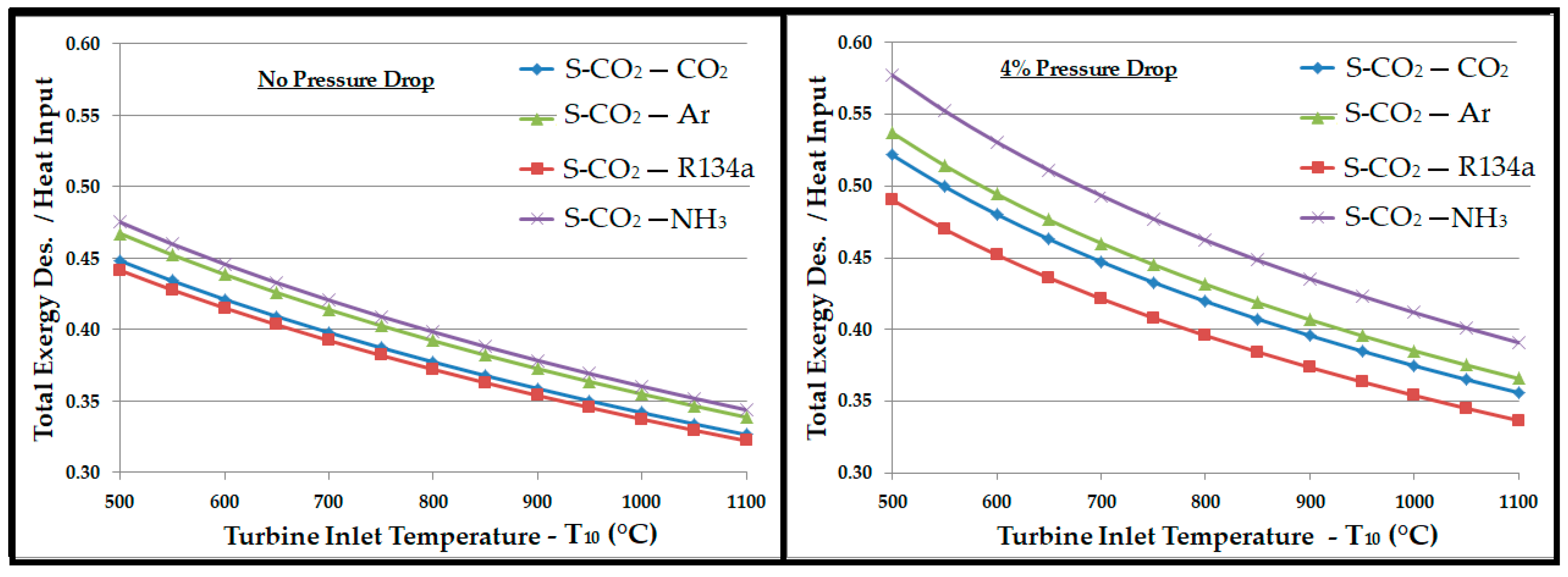
- The pressure drop in the primary cycle reduced the efficiency but, in turn, offered higher temperature and exergy available to the WHRU, which appeared as an increased contribution from the secondary cycle.
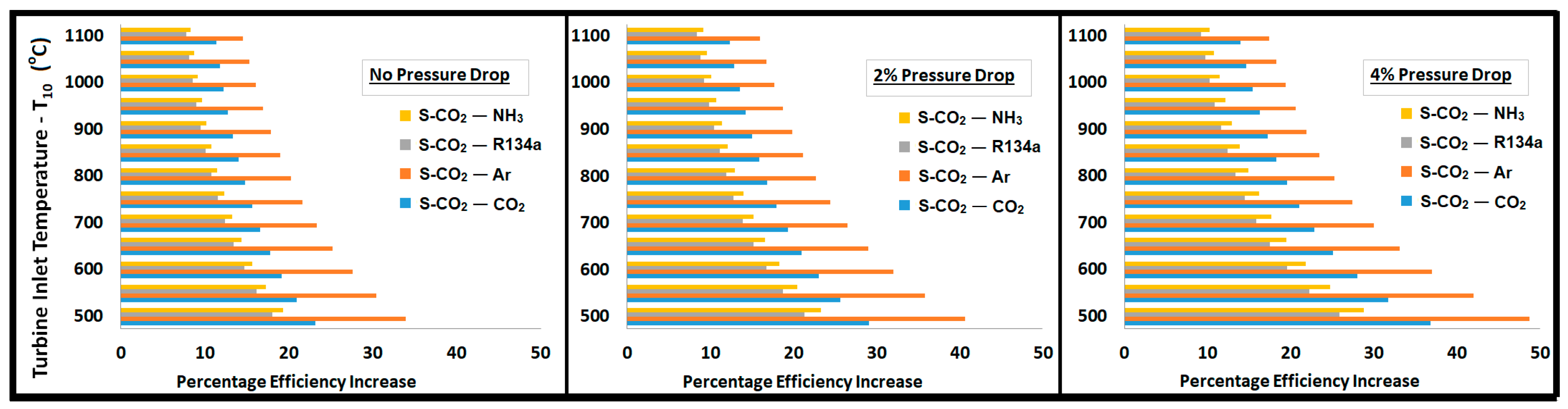
- The combined cycle efficiency increased monotonically with the turbine inlet temperature (T10); the rise was more evident for temperatures up to 850 °C, beyond which the curve appeared to saturate.
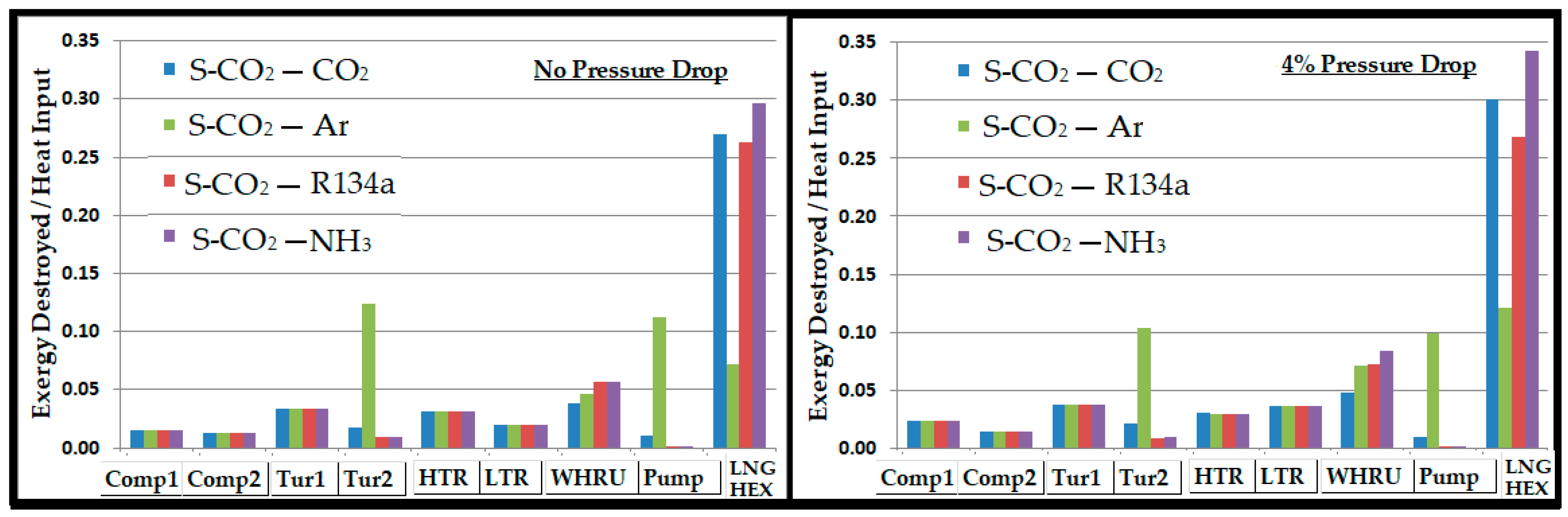
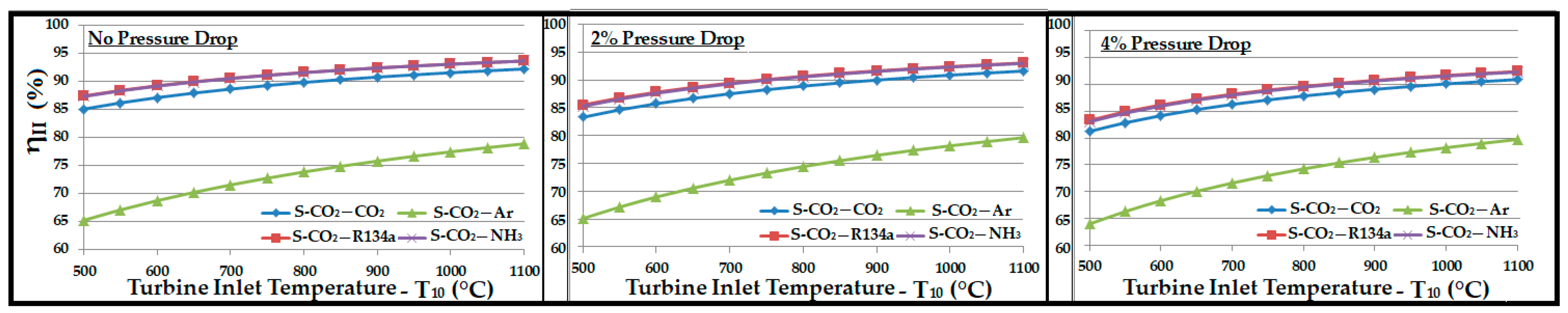
- Regardless of the pressure drop in the system, the bottoming cycle with argon as a working fluid gave the highest thermal efficiency. On the other side, it required a mass flow rate approximately 5 times higher than R134a and CO2. A high mass flow rate would require a large equipment (turbine, compressor, heat exchangers, etc.), which ultimately increases the capital and operational costs. Significantly high exergy losses in compressor, turbine, and WHRU were also associated with argon, which was manifested by the smaller second law efficiency.
- Ammonia required a mass flow rate approximately 4 to 5 times lower than those of R134a and CO2. Exergy analysis revealed a higher second law efficiency associated with ammonia. However, a substantial amount of exergy was lost in the WHRU as a result of the high latent heat of vaporization for ammonia. Thus, a significantly smaller exergy was available for the secondary cycle, which resulted in a smaller contribution in the overall thermal efficiency in comparison to other working fluids.
- R134a can be a good candidate for the bottoming cycle. It offered an overall thermal efficiency improvement between 20% and 25%, as shown in Figure 10. It showed minimum exergy loss and high second law efficiency.
- CO2 provided a significantly higher contribution than ammonia and R134a in the overall cycle efficiency improvement, with a sink temperature of about −25 °C. It provided a thermal efficiency improvement of 30% to 35%.
Author Contributions
Funding
Conflicts of Interest
References
- Chacartegui, R.; Sánchez, D.; Muñoz, J.M.; Sánchez, T. Alternative ORC bottoming cycles for combined cycle power plants. Appl. Energy 2009, 86, 2162–2170. [Google Scholar] [CrossRef]
- Cao, Y.; Dai, Y. Comparative analysis on off-design performance of a gas turbine and ORC combined cycle under different operation approaches. Energy Convers. Manag. 2017, 135, 84–100. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y. Exergoeconomic analysis of utilizing the transcritical CO2 cycle and the ORC for a recompression supercritical CO2 cycle waste heat recovery: A comparative study. Appl. Energy 2016, 170, 193–207. [Google Scholar] [CrossRef]
- Santini, L.; Accornero, C.; Cioncolini, A. On the adoption of carbon dioxide thermodynamic cycles for nuclear power conversion: A case study applied to Mochovce 3 Nuclear Power Plant. Appl. Energy 2016, 181, 446–463. [Google Scholar] [CrossRef]
- Conboy, T.; Pasch, J.; Fleming, D. Control of a supercritical CO2 recompression Brayton cycle demonstration loop. J. Eng. Gas Turbines Power 2013, 135, 111701. [Google Scholar] [CrossRef]
- Conboy, T.; Wright, S.; Pasch, J.; Fleming, D.; Rochau, G.; Fuller, R. Performance characteristics of an operating supercritical CO2 Brayton cycle. J. Eng. Gas Turbines Power 2012, 134, 111703. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Zang, J.; Liu, G. Research Activities on Supercritical Carbon Dioxide Power Conversion Technology in China. In Proceedings of the ASEM Turbo Expo 2014: Turbine Technical Conference and Exposition, Dusseldorf, Germany, 16−20 June 2014. [Google Scholar]
- Vesely, L.; Dostal, V.; Hajek, P. Design of Experimental Loop With Supercritical Carbon Dioxide. In Proceedings of the 2014 22nd International Conference on Nuclear Engineering, Prague, Czech Republic, 7−11 July 2014. [Google Scholar]
- Angelino, G.; Colonna Di Paliano, P. Multicomponent working fluids for organic Rankine cycles (ORCs). Energy 1998, 23, 449–463. [Google Scholar] [CrossRef]
- Astolfi, M.; Romano, M.C.; Bombarda, P.; Macchi, E. Binary ORC (Organic Rankine Cycles) power plants for the exploitation of medium-low temperature geothermal sources—Part B: Techno-economic optimization. Energy 2014, 66, 435–446. [Google Scholar] [CrossRef]
- Chen, Y.; Lundqvist, P.; Johansson, A.; Platell, P. A comparative study of the carbon dioxide transcritical power cycle compared with an organic Rankine cycle with R123 as working fluid in waste heat recovery. Appl. Therm. Eng. 2006, 26, 2142–2147. [Google Scholar] [CrossRef]
- Chen, H.; Goswami, D.Y.; Stefanakos, E.K. A review of thermodynamic cycles and working fluids for the conversion of low-grade heat. Renew. Sustain. Energy Rev. 2010, 14, 3059–3067. [Google Scholar] [CrossRef]
- Al-Sulaiman, F.A.; Atif, M. Performance comparison of different supercritical carbon dioxide Brayton cycles integrated with a solar power tower. Energy 2015, 82, 61–71. [Google Scholar] [CrossRef]
- Atif, M.; Al-Sulaiman, F.A. Energy and exergy analyses of solar tower power plant driven supercritical carbon dioxide recompression cycles for six different locations. Renew. Sustain. Energy Rev. 2017, 68, 153–167. [Google Scholar] [CrossRef]
- Wang, K.; He, Y.L.; Zhu, H.H. Integration between supercritical CO2 Brayton cycles and molten salt solar power towers: A review and a comprehensive comparison of different cycle layouts. Appl. Energy 2017, 195, 819–836. [Google Scholar] [CrossRef]
- Hou, S.; Wu, Y.; Zhou, Y. Performance analysis of the combined supercritical CO2 recompression and regenerative cycle used in waste heat recovery of marine gas turbine. Energy Convers. Manag. 2017, 151, 73–85. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Bai, Z.; Lei, J.; Jin, H. Thermodynamic analysis of the cascaded supercritical CO2 cycle integrated with solar and biomass energy. Energy Procedia 2017, 105, 445–452. [Google Scholar] [CrossRef]
- Cao, Y.; Ren, J.; Sang, Y.; Dai, Y. Thermodynamic analysis and optimization of a gas turbine and cascade CO2 combined cycle. Energy Convers. Manag. 2017, 144, 193–204. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Dai, Y.; Zhao, P. Thermodynamic analysis and optimization of a transcritical CO2 geothermal power generation system based on the cold energy utilization of LNG. Appl. Therm. Eng. 2014, 70, 531–540. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mehrpooya, M.; Pourfayaz, F. Thermodynamic and exergy analysis and optimization of a transcritical CO2 power cycle driven by geothermal energy with liquefied natural gas as its heat sink. Appl. Therm. Eng. 2016, 109, 640–652. [Google Scholar] [CrossRef]
- Amini, A.; Mirkhani, N.; Pakjesm Pourfard, P.; Ashjaee, M.; Khodkar, M.A. Thermo-economic optimization of low-grade waste heat recovery in Yazd combined-cycle power plant (Iran) by a CO2 transcritical Rankine cycle. Energy 2015, 86, 74–84. [Google Scholar] [CrossRef]
- Walnum, H.T.; Nekså, P.; Nord, L.O.; Andresen, T. Modelling and simulation of CO2(carbon dioxide) bottoming cycles for offshore oil and gas installations at design and off-design conditions. Energy 2013, 59, 513–520. [Google Scholar] [CrossRef]
- Wu, C.; Yan, X.J.; Wang, S.S.; Bai, K.L.; Di, J.; Cheng, S.F.; Li, J. System optimisation and performance analysis of CO2 transcritical power cycle for waste heat recovery. Energy 2016, 100, 391–400. [Google Scholar] [CrossRef]
- Qiang, W.; Yanzhong, L.; Jiang, W. Analysis of power cycle based on cold energy of liquefied natural gas and low-grade heat source. Appl. Therm. Eng. 2004, 24, 539–548. [Google Scholar] [CrossRef]
- Kim, C.W.; Chang, S.D.; Ro, S.T. Analysis of the power cycle utilizing the cold energy of LNG. Int. J. Energy Res. 1995, 19, 741–749. [Google Scholar] [CrossRef]
- Zhang, N.; Lior, N. A novel near-zero CO2 emission thermal cycle with LNG cryogenic exergy utilization. Energy 2006, 31, 1666–1679. [Google Scholar] [CrossRef]
- Deng, S.; Jin, H.; Cai, R.; Lin, R. Novel cogeneration power system with liquefied natural gas (LNG) cryogenic exergy utilization. Energy 2004, 29, 497–512. [Google Scholar] [CrossRef]
- Feher, E.G. The Supercritical thermodynamic power cycle. Energy Convers. 1968, 8, 85–90. [Google Scholar] [CrossRef]
- Angelino, G. Carbon dioxide condensation cycles for power production. J. Eng. Gas Turbines Power 1968, 90, 287. [Google Scholar] [CrossRef]
- Dostal, V.; Driscoll, M.J.; Hejzlar, P. A Supercritical Carbon Dioxide Cycle for Next Generation Nuclear Reactors. Tech. Rep. MIT-ANP-TR-100 2004, 1–317. Available online: http://web.mit.edu/22.33/www/dostal.pdf (accessed on 28 August 2018).
- Ahn, Y.; Lee, J.; Kim, S.G.; Lee, J.I.; Cha, J.E.; Lee, S.W. Design consideration of supercritical CO2 power cycle integral experiment loop. Energy 2015, 86, 115–127. [Google Scholar] [CrossRef]
- Wang, K.; He, Y.L. Thermodynamic analysis and optimization of a molten salt solar power tower integrated with a recompression supercritical CO2 Brayton cycle based on integrated modeling. Energy Convers. Manag. 2017, 135, 336–350. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ma, Z.; Neises, T.W.; Wagner, M.J. Thermodynamic study of advanced supercritical carbon dioxide power cycles for concentrating solar power systems. J. Sol. Energy Eng. 2013, 135, 041007. [Google Scholar] [CrossRef]
- Kim, I.H.; No, H.C. Physical model development and optimal design of PCHE for intermediate heat exchangers in HTGRs. Nucl. Eng. Des. 2012, 243, 243–250. [Google Scholar] [CrossRef]
- Neises, T.; Turchi, C. A comparison of supercritical carbon dioxide power cycle configurations with an emphasis on CSP applications. Energy Procedia 2013, 49, 1187–1196. [Google Scholar] [CrossRef]


| Pressure Drop | Flow Ratio x | Compressor 1 Compression Ratio | Compressor 2 Compression Ratio | Turbine Inlet Pressure (P10) MPa |
|---|---|---|---|---|
| No Drop | 0.66 | 2.40 | 2.40 | 16.60 |
| 2% | 0.69 | 3.00 | 3.14 | 20.40 |
| 4% | 0.71 | 3.60 | 3.85 | 24.45 |
| Pressure Drop | S-CO2—CO2 | S-CO2—Ar | S-CO2—R134a | S-CO2—NH3 |
|---|---|---|---|---|
| No Drop | 33% | 156% | 36.5% | 6.5% |
| 2% | 34.4% | 164% | 37% | 7.7% |
| 4% | 36% | 168% | 38.6% | 8.7% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, M.E.; Taimoor, A.A.; Almitani, K.H. Energy and Exergy Analysis of the S-CO2 Brayton Cycle Coupled with Bottoming Cycles. Processes 2018, 6, 153. https://doi.org/10.3390/pr6090153
Siddiqui ME, Taimoor AA, Almitani KH. Energy and Exergy Analysis of the S-CO2 Brayton Cycle Coupled with Bottoming Cycles. Processes. 2018; 6(9):153. https://doi.org/10.3390/pr6090153
Chicago/Turabian StyleSiddiqui, Muhammad Ehtisham, Aqeel Ahmad Taimoor, and Khalid H. Almitani. 2018. "Energy and Exergy Analysis of the S-CO2 Brayton Cycle Coupled with Bottoming Cycles" Processes 6, no. 9: 153. https://doi.org/10.3390/pr6090153
APA StyleSiddiqui, M. E., Taimoor, A. A., & Almitani, K. H. (2018). Energy and Exergy Analysis of the S-CO2 Brayton Cycle Coupled with Bottoming Cycles. Processes, 6(9), 153. https://doi.org/10.3390/pr6090153





