A New Concept of Stirred Multiphase Reactor Using a Stationary Catalytic Foam
Abstract
1. Introduction
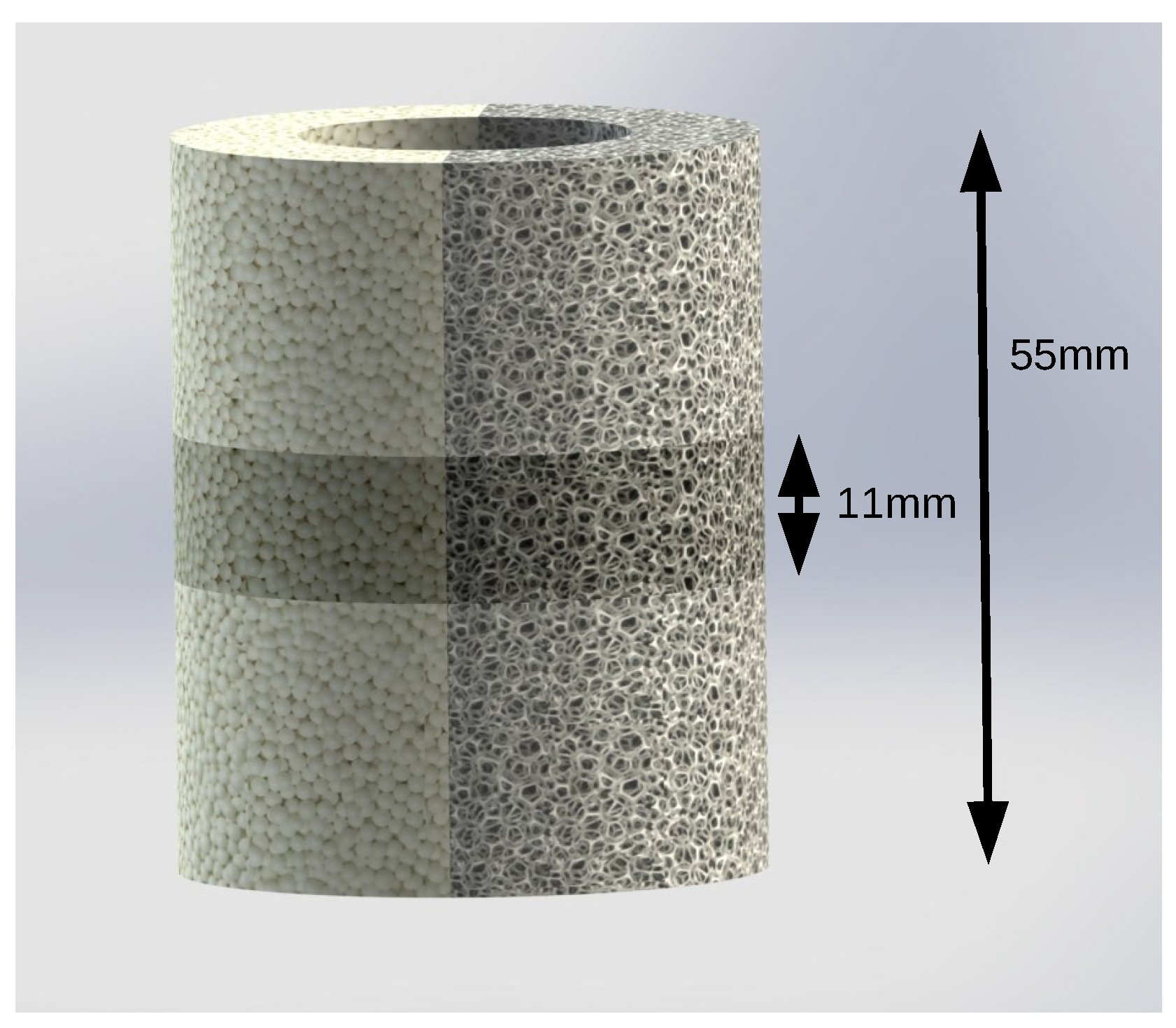
2. Materials and Methods
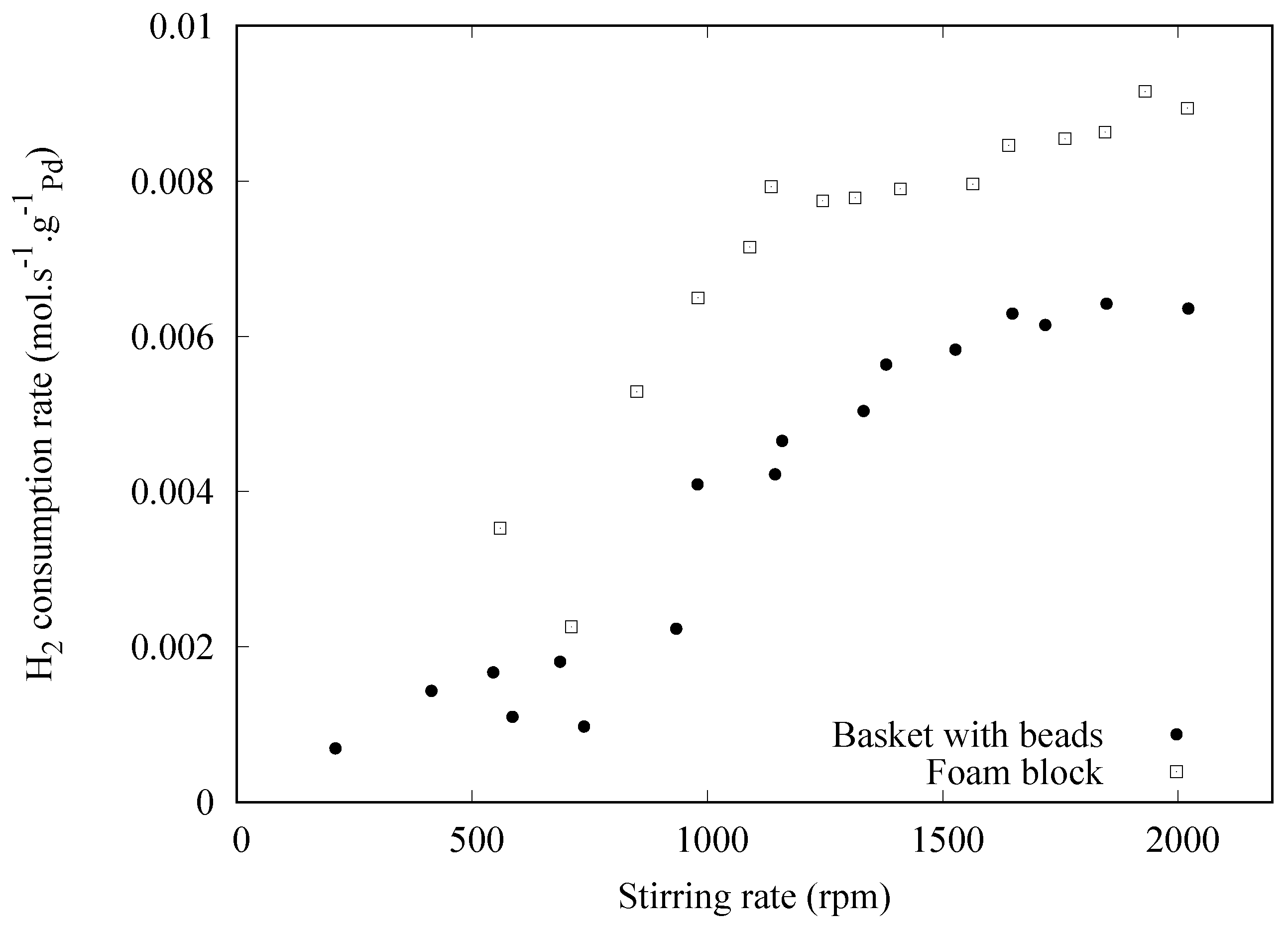
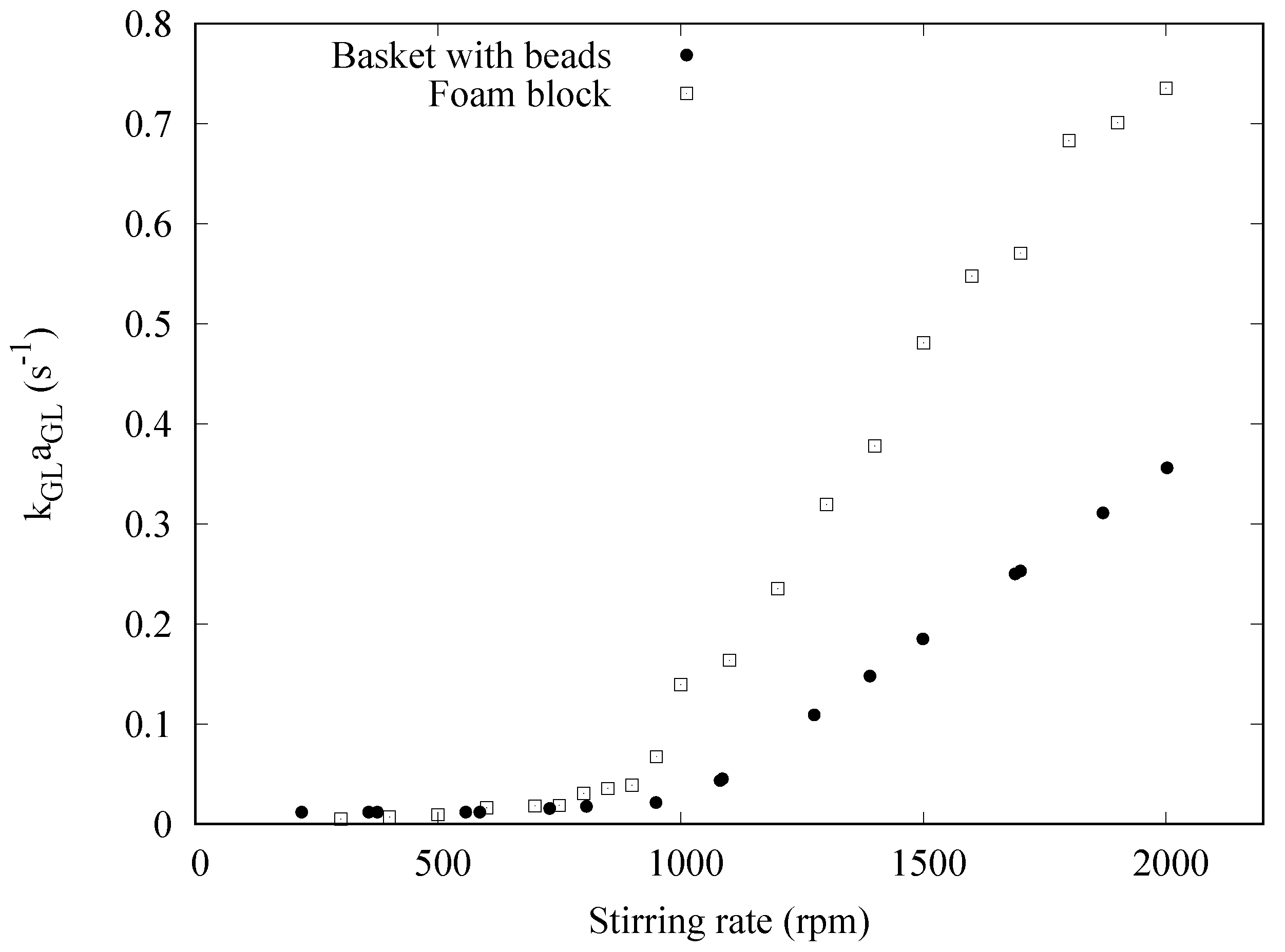
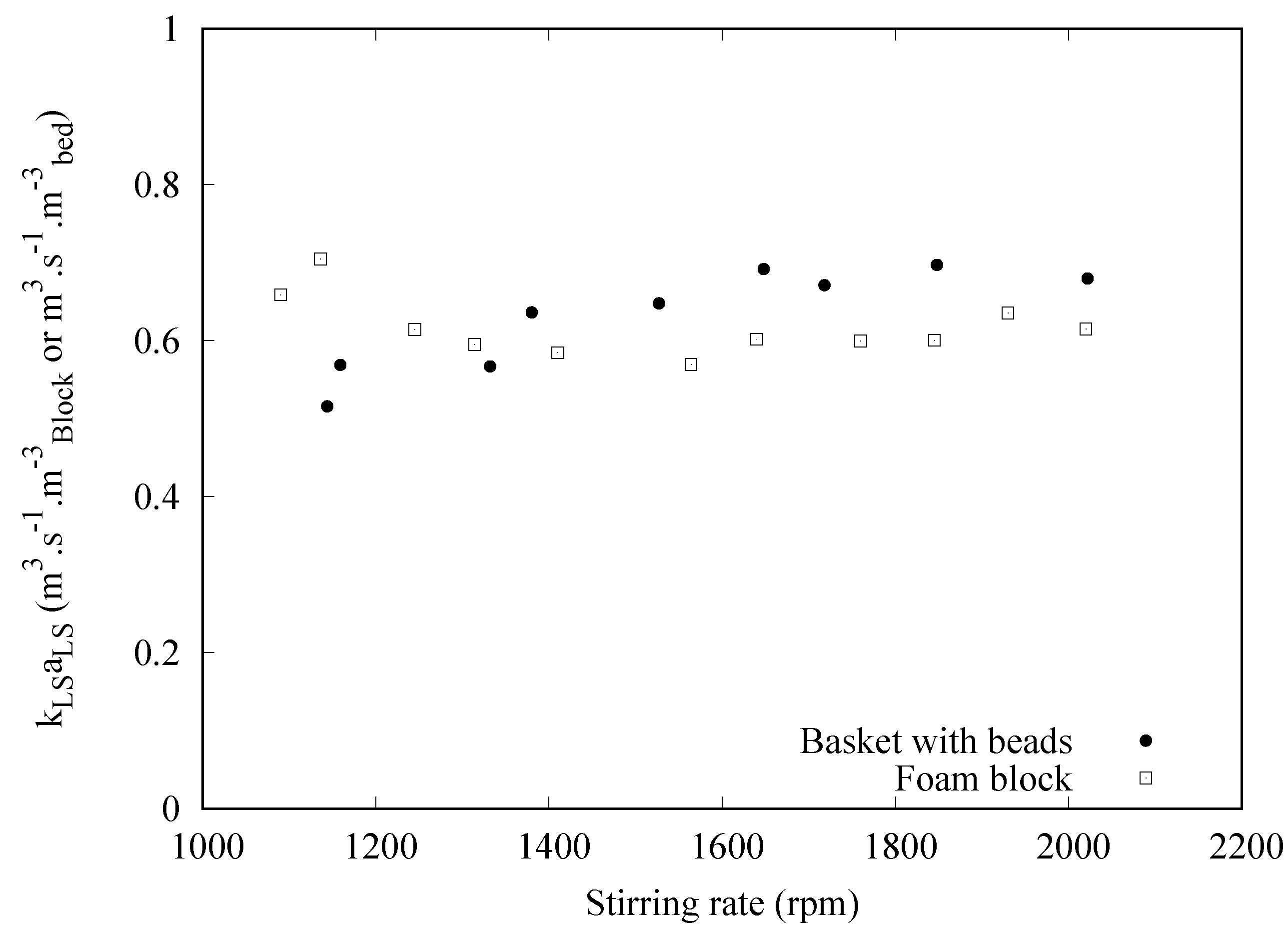
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Specific area of gas–liquid interface (m) | |
| Specific area of liquid–solid interface (mm) or (mm) | |
| Concentration of hydrogen solved in liquid phase (molm) | |
| Concentration of hydrogen solved in liquid phase at saturation (equilibrium) (molm) | |
| Concentration of hydrogen solved in liquid phase at solid interface (molm) | |
| Particle diameter (m) | |
| Diffusivity of -methylstyrene in methylcyclohexane (ms) | |
| Activation energy of reaction (Jmol) | |
| Kinetic parameter of reaction (mskg) | |
| Gas–liquid mass transfer coefficient (ms) | |
| Adsorption equilibrium constant of hydrogen (mmol) | |
| Liquid–solid mass transfer coefficient (ms) | |
| Pre-exponential kinetic parameter of reaction (molskg) | |
| Number of moles of hydrogen in the reservoir (mol) | |
| Number of moles of hydrogen in the liquid phase (mol) | |
| N | Stirring speed (rpm) |
| Critical Stirring Speed (rpm) | |
| P | Instantaneous pressure of hydrogen (bar) |
| Stabilized pressure of hydrogen (bar) | |
| Hydrogen pressure in the reservoir (bar) | |
| Maximal pressure of hydrogen (bar) | |
| Initial pressure of hydrogen (bar) | |
| Pores per inch | |
| Reaction rate of hydrogenation of -methylstyrene (molsm) | |
| R | Perfect Gas constant (JmolK) |
| Sherwood number (-), | |
| t | Time (s) |
| T | Temperature (K) |
| Temperature in the reservoir (K) | |
| Volume of liquid (m) | |
| Volume of reservoir (m) | |
| W | Mass of catalyst (kg) |
| Mass fraction of palladium atoms in the alumina (-) | |
| Volume fraction of foam block or of catalytic bed in the liquid bulk (mm) or (mm) | |
| Efficiency factor (-) |
References
- Chaudhari, R.V.; Mills, P.L. Multiphase catalysis and reaction engineering for emerging pharmaceutical processes. Chem. Eng. Sci. 2004, 59, 5337–5344. [Google Scholar] [CrossRef]
- Porta, R.; Benaglia, M.; Puglisi, A. Flow Chemistry: Recent Developments in the Synthesis of Pharmaceutical Products. Org. Process. Res. Dev. 2016, 20, 2–25. [Google Scholar] [CrossRef]
- Le Doan, T.V.; Stavarek, P.; de Bellefon, C. A Method to Identify Best Available Technologies (BAT) for Hydrogenation Reactors in the Pharmaceutical Industry. J. Flow Chem. 2012, 2, 77–82. [Google Scholar] [CrossRef]
- Robinson, K.K.; Mahoney, J.A. Gradientless Reactors for Process Kinetic Studies and Catalyst Testing. Abst. P. Am. Chem. Soc. 1977, 174, 15–16. [Google Scholar]
- Pitault, I.; Fongarland, P.; Koepke, D.; Mitrovic, M.; Ronze, D.; Forissier, M. Gas–liquid and liquid–solid mass transfers in two types of stationary catalytic basket laboratory reactor. Chem. Eng. Sci. 2005, 60, 6240–6253. [Google Scholar] [CrossRef]
- Mitrovic, M.; Pitault, I.; Forissier, M.; Simoens, S.; Ronze, D. Liquid–Solid Mass Transfer in a Three-Phase Stationary Catalytic Basket Reactor. AIChE J. 2005, 51, 1747–1757. [Google Scholar] [CrossRef]
- Visscher, F.; van der Schaaf, J.; Nijhuis, T.; Schouten, J. Rotating reactors—A review. Chem. Eng. Res. Des. 2013, 91, 1923–1940. [Google Scholar] [CrossRef]
- Leon, M.A.; Tschentscher, R.; Nijhuis, T.A.; van der Schaaf, J.; Schouten, J.C. Rotating Foam Stirrer Reactor: Effect of Catalyst Coating Characteristics on Reactor Performance. Ind. Eng. Chem. Res. 2011, 50, 3184–3193. [Google Scholar] [CrossRef]
- Leon, M.; Geers, P.; Nijhuis, T.; van der Schaaf, J.; Schouten, J. Effect of foam stirrer design on the catalytic performance of rotating foam stirrer reactors. Chem. Eng. J. 2012, 207–208, 209–217. [Google Scholar] [CrossRef]
- Leon, M.; Maas, R.; Bieberle, A.; Schubert, M.; Nijhuis, T.; van der Schaaf, J.; Hampel, U.; Schouten, J. Hydrodynamics and gas–liquid mass transfer in a horizontal rotating foam stirrer reactor. Chem. Eng. J. 2013, 217, 10–21. [Google Scholar] [CrossRef]
- Teshima, H.; Ohashi, Y. Particle to Liquid Mass Transfer in a Rotating Catalyst Basket Reactor. Chem. Eng. J. Jpn. 1977, 10, 70–72. [Google Scholar] [CrossRef]
- Goto, S.; Saito, T. Liquid–solid mass transfer in basket type three-phase reactors. Chem. Eng. J. Jpn. 1984, 17, 324–327. [Google Scholar] [CrossRef]
- Pitault, I.; Fongarland, P.; Mitrovic, M.; Ronze, D.; Forissier, M. Choice of laboratory scale reactors for HDT kinetic studies or catalyst tests. Catal. Today 2004, 98, 31–42. [Google Scholar] [CrossRef]
- Braga, M. Study of External Transport Phenomena and Hydrodynamics in a Stirred Catalytic Basket Reactor. Ph.D. Thesis, University of Lyon, Lyon, France, 2014. [Google Scholar]
- Tourvieille, J.N.; Philippe, R.; de Bellefon, C. Milli-channel with metal foams under an applied gas–liquid periodic flow: External mass transfer performance and pressure drop. Chem. Eng. J. 2015, 267, 332–346. [Google Scholar] [CrossRef]
- Dietrich, E.; Mathieu, C.; Delmas, H.; Jenck, J. Raney-nickel catalyzed hydrogenations: gas–liquid mass transfer in gas-induced stirred slurry reactors. Chem. Eng. Sci. 1992, 47, 3597–3604. [Google Scholar] [CrossRef]
- Johnson, D.L.; Saito, H.; Polejes, J.D.; Hougen, O.A. Effects of bubbling and stirring on mass transfer coefficients in liquids. AIChE J. 1957, 3, 411–417. [Google Scholar] [CrossRef]
- Meille, V.; de Bellefon, C. Effect of water on alpha-methylstyrene hydrogenation on Pd/Al2O3. Can. J. Chem. Eng. 2004, 82, 190–193. [Google Scholar] [CrossRef]
- Meille, V.; de Bellefon, C.; Schweich, D. Kinetics of α-Methylstyrene Hydrogenation on Pd/Al2O3. Ind. Eng. Chem. Res. 2002, 41, 1711–1715. [Google Scholar] [CrossRef]
- Martin, H. The generalized Lévêque equation and its practical use for the prediction of heat and mass transfer rates from pressure drop. Chem. Eng. Sci. 2002, 57, 3217–3223. [Google Scholar] [CrossRef]
- Simescu-Lazar, F.; Meille, V.; Bornette, F.; Campoli, F.; de Bellefon, C. In situ electrochemical regeneration of deactivated coated catalyst on basket foam in Robinson-Mahoney reactor: Example of Pd/C for nitrobenzene hydrogenation. Catal. Today 2015, 249, 52–58. [Google Scholar] [CrossRef]
- Simescu-Lazar, F.; Chaieb, T.; Pallier, S.; Veyre, L.; Philippe, R.; Meille, V. Direct coating of carbon-supported atalysts on monoliths and foams—Singular behaviour of Pd/MWCNT. Appl. Catal. A Gen. 2015, 508, 45–51. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benamara, N.; Assoua, D.; Jaffeux, L.; Vanoye, L.; Simescu-Lazar, F.; Zanota, M.-L.; Bornette, F.; Meille, V.; Pitault, I. A New Concept of Stirred Multiphase Reactor Using a Stationary Catalytic Foam. Processes 2018, 6, 117. https://doi.org/10.3390/pr6080117
Benamara N, Assoua D, Jaffeux L, Vanoye L, Simescu-Lazar F, Zanota M-L, Bornette F, Meille V, Pitault I. A New Concept of Stirred Multiphase Reactor Using a Stationary Catalytic Foam. Processes. 2018; 6(8):117. https://doi.org/10.3390/pr6080117
Chicago/Turabian StyleBenamara, Nassima, Didier Assoua, Louis Jaffeux, Laurent Vanoye, Florica Simescu-Lazar, Marie-Line Zanota, Frédéric Bornette, Valérie Meille, and Isabelle Pitault. 2018. "A New Concept of Stirred Multiphase Reactor Using a Stationary Catalytic Foam" Processes 6, no. 8: 117. https://doi.org/10.3390/pr6080117
APA StyleBenamara, N., Assoua, D., Jaffeux, L., Vanoye, L., Simescu-Lazar, F., Zanota, M.-L., Bornette, F., Meille, V., & Pitault, I. (2018). A New Concept of Stirred Multiphase Reactor Using a Stationary Catalytic Foam. Processes, 6(8), 117. https://doi.org/10.3390/pr6080117






