Preparation and Potential Applications of Super Paramagnetic Nano-Fe3O4
Abstract
:1. Introduction
2. Methods for Preparation of Nano-Fe3O4
2.1. Hydrothermal Method
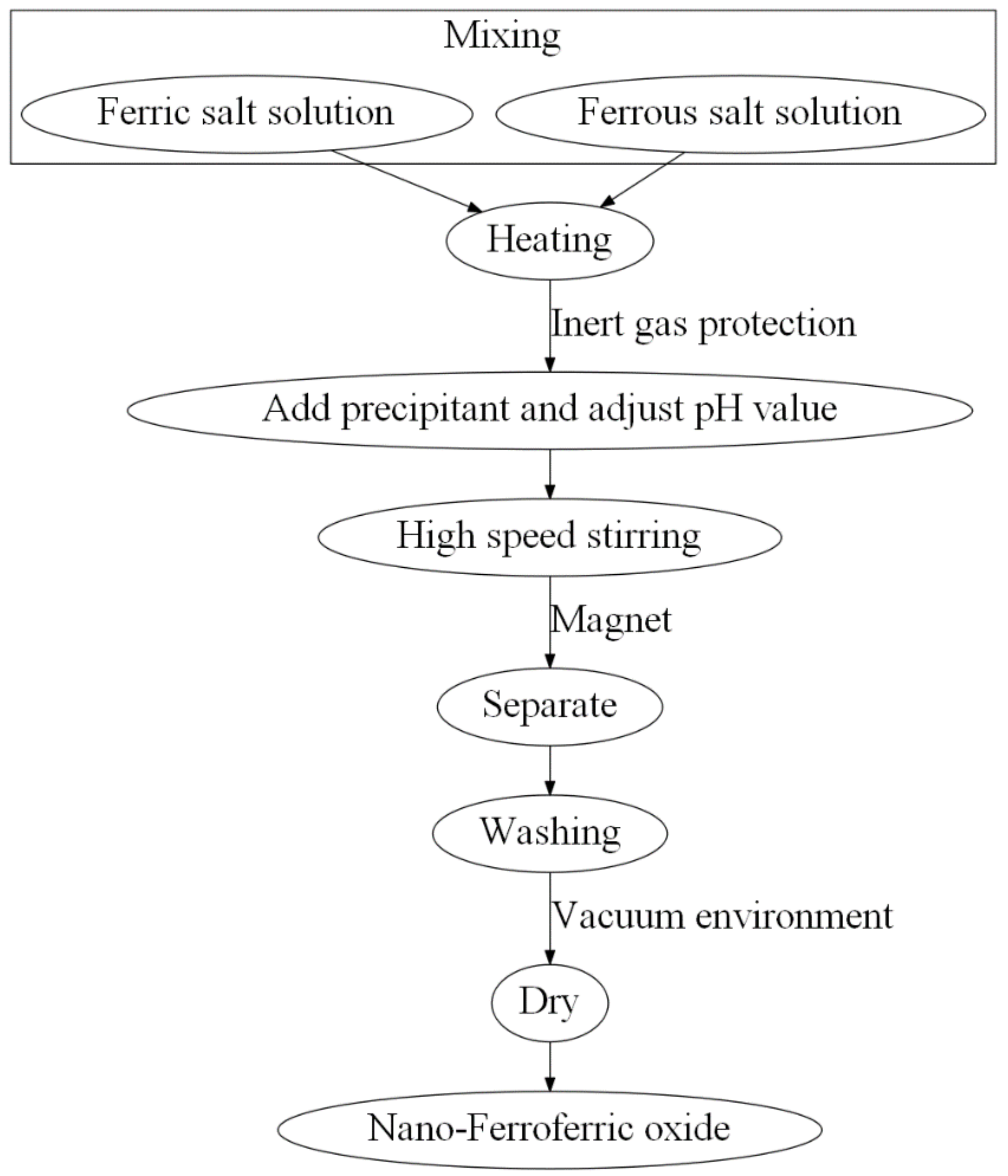
2.2. Coprecipitation Method
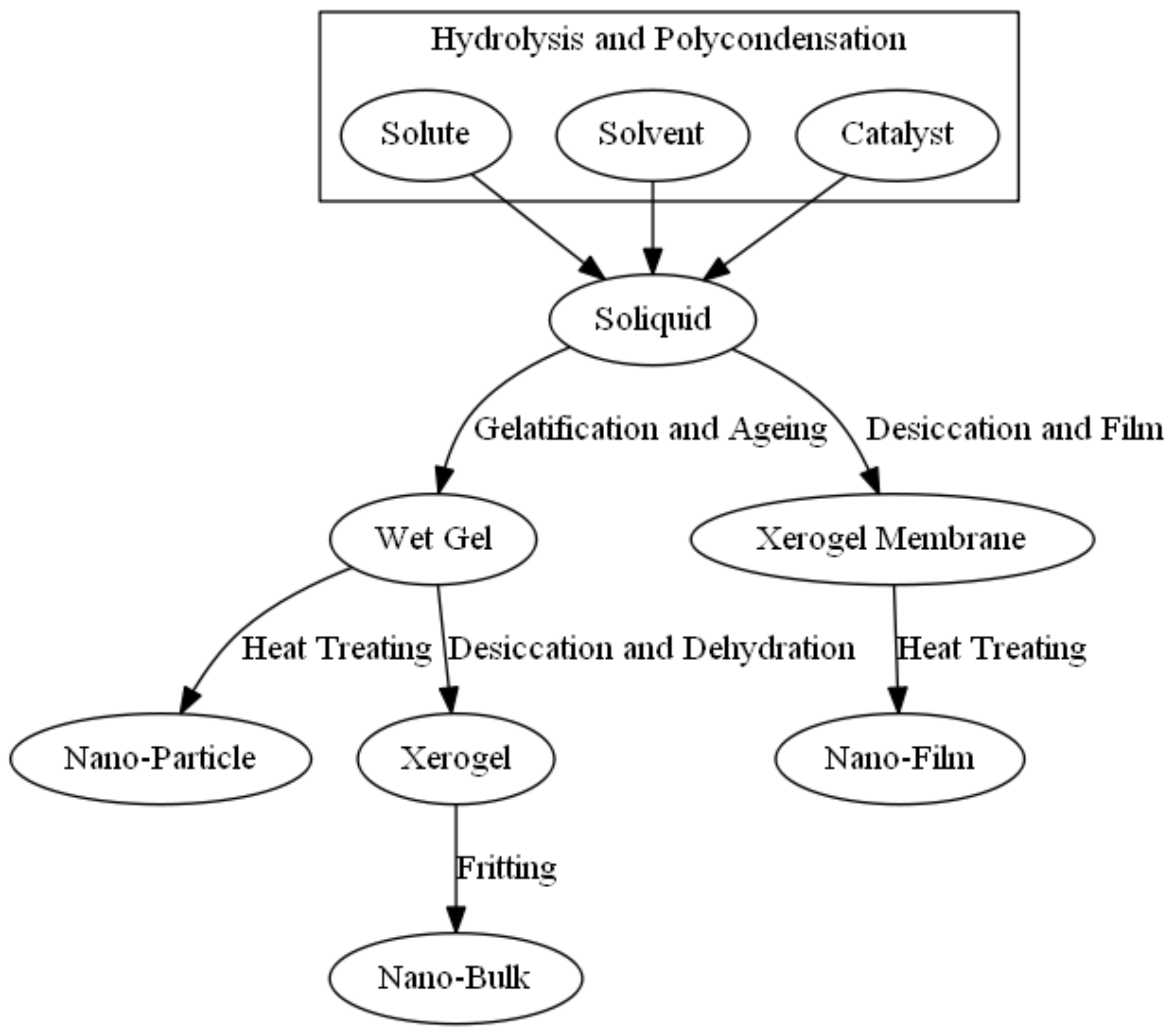
2.3. Sol-Gel Method
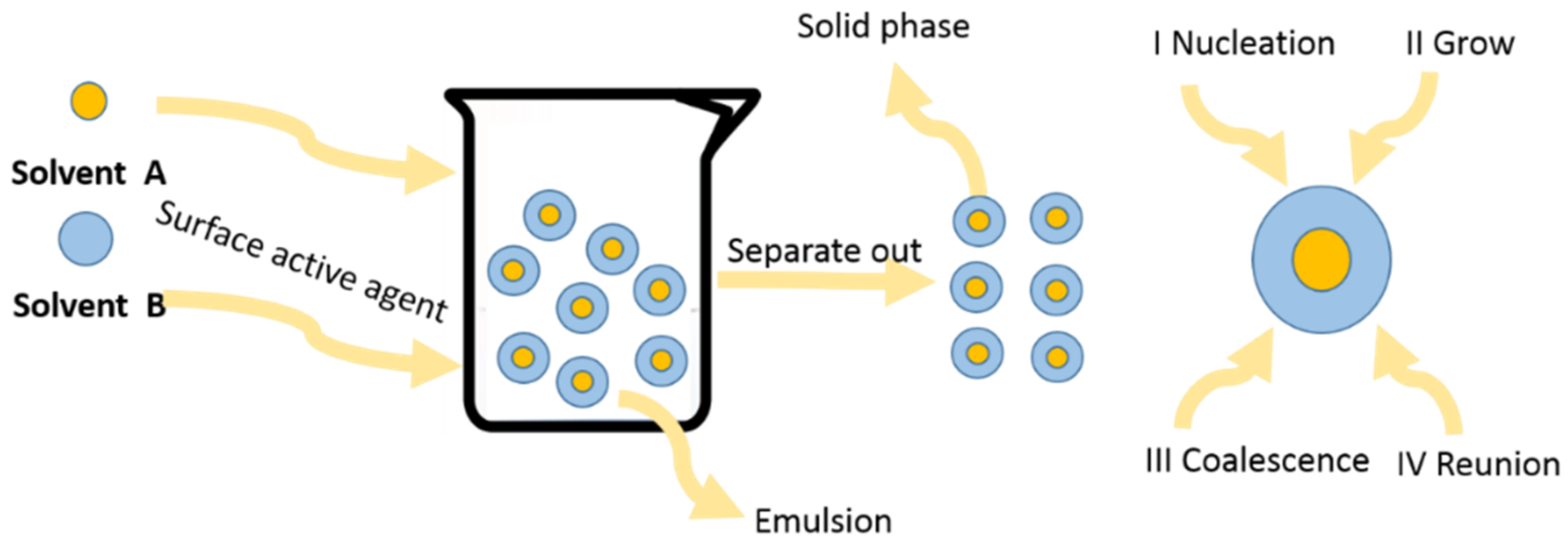
2.4. Micro-Emulsion Method
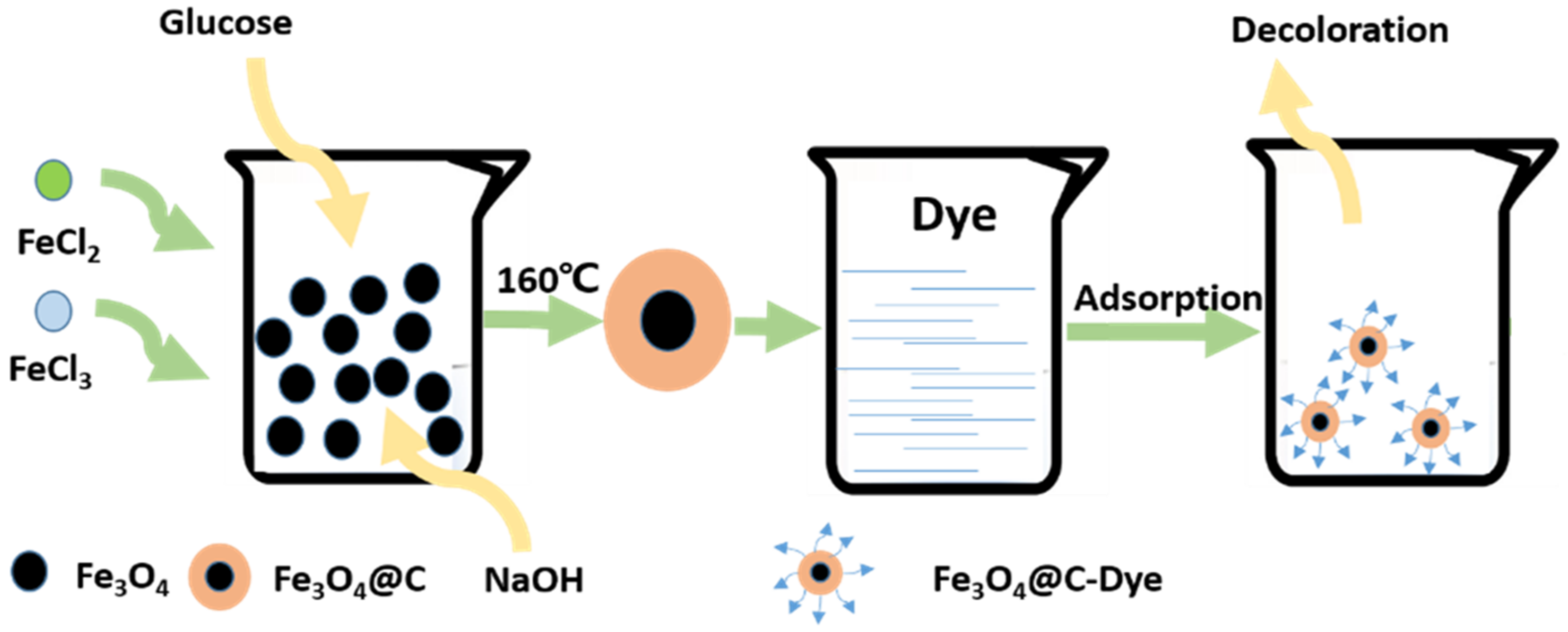
2.5. Solvent Heat Method
3. The Modification of Nano-Fe3O4
3.1. Modification of Small Inorganic Molecules
3.2. Modification of Small Organic Molecules
3.3. Modification of Organic Polymers
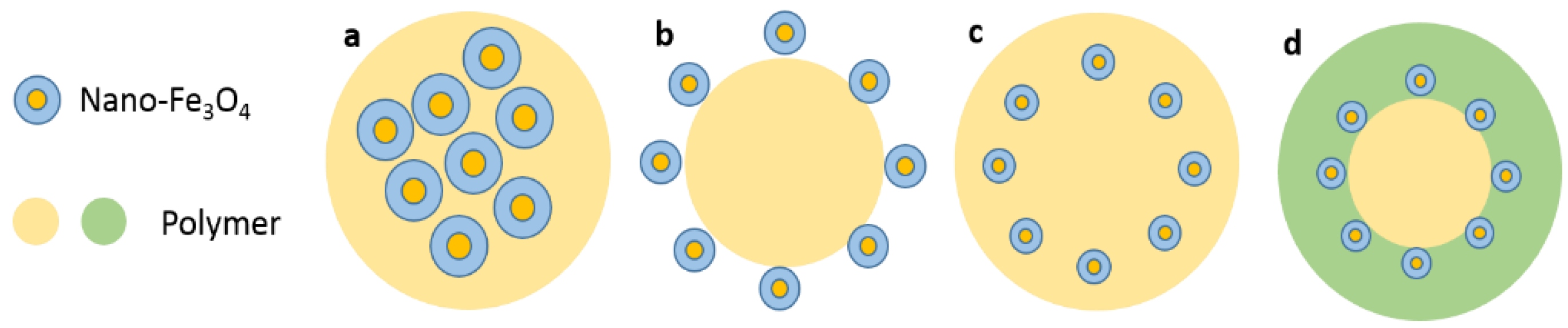
3.4. Structure of Nano-Fe3O4 Composite Materials
4. Applications of Nano-Fe3O4
4.1. Biomedical Sciences
4.2. Removal of Heavy Metals from Aqueous Systems
4.3. Electrochemical Sensor and Energy Storage
4.4. Chemical Catalysis
4.5. Others
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Salem, I.A.; Salem, M.A.; El-Ghobashy, M.A. The dual role of ZnO nanoparticles for efficient capture of heavy metals and Acid blue 92 from water. J. Mol. Liquids 2017, 248, 527–538. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Ziccarelli, I.; Espro, C.; Galvaqno, S.; Giofré, S.V.; Romeo, R.; Cicero, N.; Bua, G.D.; Lanza, G.; et al. Removal of heavy metal ions from wastewaters using dendrimer-functionalized multi-walled carbon nanotubes. Environ. Sci. Pollut. Res. 2017, 24, 14735–14747. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Sharma, J.; Kaith, B.S.; Yadav, S.; Sharma, A.K.; Aayushi, G. Gum xanthan-psyllium-clpoly(acrylic acid-co-itaconic acid) based adsorbent for effective removal of cationic and anionic dyes: Adsorption isotherms, kinetics and thermodynamic studies. Ecotoxicol. Environ. Saf. 2018, 149, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Hernandez, C.; Ahsan, M.A.; Pardo, A.; Wang, H.; Noveron, J.C. Sulfonated resorcinol-formaldehyde microspheres as high-capacity regenerable adsorbent for the removal of organic dyes from water. J. Environ. Chem. Eng. 2017, 5, 5270–5279. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, Y.H. Application of ferro-cobalt magnetic fluid for oil sealing. J. Magn. Magn. Mater. 2003, 267, 105–110. [Google Scholar] [CrossRef]
- Beydoun, D.; Amal, R.; Low, G.K.C.; McEvor, S. Novel Photocatalyst: Titania-Coated Magnetite. Activity and Photodissolution. J. Phys. Chem. B 2000, 104, 4387–4396. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanomaterials for removal of toxic elements from water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- Chen, M.; Hou, C.; Huo, D.; Fa, H.; Zhao, Y.; Shen, C. A sensitive electrochemical DNA biosensor based on three-dimensional nitrogen-doped graphene and Fe3O4 nanoparticles. Sens. Actuators B Chem. 2017, 239, 421–429. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Yu, J.; Xia, J.; Zhang, C.; Yin, D.; Häfeli, U.O. Preparation and radiolabeling of surface-modified magnetic nanoparticles with rhenium-188 for magnetic targeted radiotherapy. J. Magn. Magn. Mater. 2004, 277, 165–174. [Google Scholar] [CrossRef]
- Huang, Y.S.; Lu, Y.J.; Chen, J.P. Magnetic graphene oxide as a carrier for targeted delivery of chemotherapy drugs in cancer therapy. J. Magn. Magn. Mater. 2017, 427, 34–40. [Google Scholar] [CrossRef]
- Pistone, A.; Iannazzo, D.; Panseri, S.; Montesi, M.; Tampieri, A.; Galvaqno, S. Hydroxyapatite-magnetite-MWCNT nanocomposite as a biocompatible multifunctional drug delivery system for bone tissue engineering. Nanotechnology 2014, 25, 425701. [Google Scholar] [CrossRef] [PubMed]
- Prabha, G.; Raj, V. Preparation and characterization of polymer nanocomposites coated magnetic nanoparticles for drug delivery applications. J. Magn. Magn. Mater. 2016, 408, 26–34. [Google Scholar] [CrossRef]
- Ji, F.; Ceng, K.; Zhang, K.; Li, J.; Zhang, J. Synthesis and Properties of PEG Modifiers of Fe3O4 Magnetic Nanoparticles. Acta Polym. Sin. 2016, 1704–1709. [Google Scholar] [CrossRef]
- Gupta, J.; Prakash, A.; Jaiswal, M.K.; Agarrwal, A.; Bahadur, D. Superparamagnetic iron oxide-reduced graphene oxide nanohybrid-a vehicle for targeted drug delivery and hyperthermia treatment of cancer. J. Magn. Magn. Mater. 2018, 448, 332–338. [Google Scholar] [CrossRef]
- Butter, K.; Kassapidou, K.; Vroege, G.J.; Philipse, A.P. Preparation and properties of colloidal iron dispersions. J. Colloid Interface Sci. 2005, 287, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Samanta, S.; Maji, S.; Ganguli, S.; Bhaumik, A. Magnetic properties of α-Fe2O3 nanoparticle synthesized by a new hydrothermal method. J. Magn. Magn. Mater. 2005, 285, 296–302. [Google Scholar] [CrossRef]
- Eken, A.E.; Ozenbas, M. Characterization of nanostructured magnetite thin films produced by sol-gel processing. J. Sol-Gel Sci. Technol. 2009, 50, 321–327. [Google Scholar] [CrossRef]
- Liang, X.; Jia, X.; Cao, L.; Sun, J.; Yang, Y. Microemulsion Synthesis and Characterization of Nano-Fe3O4 Particles and Fe3O4 Nanocrystalline. J. Dispers. Sci. Technol. 2010, 31, 1043–1049. [Google Scholar] [CrossRef]
- Zhu, H.; Han, T.; Zhang, J.; Zhou, Z.; Yang, S. The Synthesis and Application of Water Soluble Fe3O4 Nanoparticles. Guangzhou Chem. Ind. 2014, 42, 88–89. [Google Scholar]
- Niu, W.; Shen, Y.; Xu, J.; Ma, L.; Zhao, Y.; Shen, M. Solvothermal Synthesis of Fe3O4 Nanospheres and Study on the Catalytic Degradation of Xylenol Orange. Chin. J. Inorg. Chem. 2013, 29, 2110–2118. [Google Scholar]
- Fuskele, V.; Sarviya, R.M. Recent developments in Nanoparticles Synthesis, Preparation and Stability of Nanofluids. Mater. Today Proc. 2017, 4, 4049–4060. [Google Scholar] [CrossRef]
- Dutta, B.; Shetake, N.G.; Gawali, S.L.; Barick, B.K.; Barick, K.C.; Babu, P.D.; Pandey, B.N.; Priyadarsini, K.I.; Hassan, P.A. PEG mediated shape-selective synthesis of cubic Fe3O4 nanoparticles for cancer therapeutics. J. Alloys Compd. 2018, 737, 347–355. [Google Scholar] [CrossRef]
- Nadimpalli, N.K.V.; Bandyopadhyaya, R.; Runkana, V. Thermodynamic analysis of hydrothermal synthesis of nanoparticles. Fluid Phase Equilib. 2018, 456, 33–45. [Google Scholar] [CrossRef]
- Ge, S.; Shi, X.; Sun, K.; Li, C.; Baker, J.R., Jr.; Banaszak Holl, M.M.; Orr, B.G. A Facile Hydrothermal Synthesis of Iron Oxide Nanoparticles with Tunable Magnetic Properties. J. Phys. Chem. C Nanomater. Interfaces 2009, 113, 13593–13599. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, W.; Liu, L.; Chen, B.; Wu, S.; Sun, D.; Li, F. One-step hydrothermal synthesis of highly water-soluble secondary structural Fe3O4 nanoparticles. J. Magn. Magn. Mater. 2012, 324, 2249–2257. [Google Scholar] [CrossRef]
- Wu, R.; Liu, J.H.; Zhao, L.; Zhang, X.; Xie, J.; Yu, B.; Ma, X.; Yang, S.T.; Wang, H.; Liu, Y. Hydrothermal preparation of magnetic Fe3O4@C nanoparticles for dye adsorption. J. Environ. Chem. Eng. 2014, 2, 907–913. [Google Scholar] [CrossRef]
- Xia, T.; Xu, X.; Wang, J.; Xu, C.; Meng, F.; Shi, Z.; Lian, J.; Bassat, J. Facile complex-coprecipitation synthesis of mesoporous Fe3O4 nanocages and their high lithium storage capacity as anode material for lithium-ion batteries. Electrochim. Acta 2015, 160, 114–122. [Google Scholar] [CrossRef]
- Wu, J.H.; Ko, S.P.; Liu, H.L.; Kim, S.; Ju, J.S.; Kim, Y.K. Sub 5 nm magnetite nanoparticles: Synthesis, microstructure, and magnetic properties. Mater. Lett. 2007, 61, 3124–3129. [Google Scholar] [CrossRef]
- Qin, R.; Jiang, W.; Liu, H.; Li, F. Preparation and Characterization of Nanometer Magnetite. Mater. Rev. 2003, 17, 66–68. [Google Scholar]
- Meng, H.; Zhang, Z.; Zhao, F.; Qiu, T.; Yang, J. Orthogonal optimization design for preparation of Fe3O4 nanoparticles via chemical coprecipitation. Appl. Surf. Sci. 2013, 280, 679–685. [Google Scholar] [CrossRef]
- Lemine, O.M.; Omri, K.; Zhang, B.; Mir, L.; Sajieddine, M.; Alyamani, A.; Bououdina, M. Sol-gel synthesis of 8 nm magnetite (Fe3O4) nanoparticles and their magnetic properties. Superlattices Microstruct. 2012, 52, 793–799. [Google Scholar] [CrossRef]
- Chen, G.; Yang, S.; Wang, D.; Zhao, L.; Zhou, T.; Jiang, J. Review of the Preparation of Fe3O4 Based on Sol-gel Method. Guangdong Chem. Ind. 2017, 44, 41–42. [Google Scholar]
- Cai, W.; Wan, J. Facile synthesis of superparamagnetic magnetite nanoparticles in liquid polyols. J. Colloid Interface Sci. 2007, 305, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chai, C.P.; Luo, Y.J.; Wang, L.; Li, G.P. Synthesis, structure and electromagnetic properties of mesoporous Fe3O4 aerogels by sol–gel method. Mater. Sci. Eng. B 2014, 188, 13–19. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, Z. Preparation of magnetic Fe3O4/SiO2 Nano-composite Particles by the Methods of Sol-Gel. J. Jilin Inst. Archit. Civ. Eng. 2011, 28, 78–80. [Google Scholar]
- Lu, T.; Wang, J.; Yin, J.; Wang, A.; Wang, X.; Zhang, T. Surfactant effects on the microstructures of Fe3O4 nanoparticles synthesized by microemulsion method. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 675–683. [Google Scholar] [CrossRef]
- Hao, J.J.; Chen, H.L.; Ren, C.L.; Yan, N.; Geng, H.J.; Chen, X.G. Synthesis of superparamagnetic Fe3O4 nanocrystals in reverse microemulsion at room temperature. Mater. Res. Innov. 2010, 14, 324–326. [Google Scholar] [CrossRef]
- Sun, L.; Zhan, L.; Shi, Y.; Chu, L.; Ge, G.; He, Z. Microemulsion synthesis and electromagnetic wave absorption properties of monodispersed Fe3O4/polyaniline core–shell nanocomposites. Synth. Met. 2014, 187, 102–107. [Google Scholar] [CrossRef]
- Lv, H.; Jiang, R.; Li, Y.; Zhang, X.; Wang, J. Microemulsion-mediated hydrothermal growth of pagoda-like Fe3O4 microstructures and their application in a lithium–air battery. Ceram. Int. 2015, 41, 8843–8848. [Google Scholar] [CrossRef]
- Abdullaeva, Z.; Kelgenbaeva, Z.; Nagaoka, S.; Matsuda, M.; Masayuki, T.; Koinuma, M.; Nishiyama, T. Solvothermal Synthesis of Surface-Modified Graphene/C and Au-Fe3O4 Nanomaterials for Antibacterial Applications. Mater. Today Proc. 2017, 4, 7044–7052. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, F.; Cai, F.; Zhang, X. Trisodium citrate-assisted synthesis of highly water-dispersible and superparamagnetic mesoporous Fe3O4 hollow microspheres via solvothermal process. J. Alloys Compd. 2015, 636, 34–39. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, B.; Li, X.; Li, R.; Yu, R. Solvothermal synthesis of hollow Fe3O4 sub-micron spheres and their enhanced electrochemical properties for supercapacitors. Mater. Des. 2016, 101, 35–43. [Google Scholar] [CrossRef]
- Ghasemi, E.; Heydari, A.; Sillanpää, M. Superparamagnetic Fe3O4@EDTA nanoparticles as an efficient adsorbent for simultaneous removal of Ag(I), Hg(II), Mn(II), Zn(II), Pb(II) and Cd(II) from water and soil environmental samples. Microchem. J. 2017, 131, 51–56. [Google Scholar] [CrossRef]
- Ding, C.; Pan, G.; Zhang, M. Study on preparation of starch-coated Fe3O4 and its phosphate removal properties. Chin. J. Environ. Eng. 2011, 5, 2167–2172. [Google Scholar]
- Zhou, Q.; Wang, X.; Liu, J.; Zhang, L. Phosphorus removal from wastewater using nano-particulates of hydrated ferric oxide doped activated carbon fiber prepared by Sol–Gel method. Chem. Eng. J. 2012, 200–202, 619–626. [Google Scholar] [CrossRef]
- Wang, T.; Gao, Y.; Jin, X.; Chen, Z. Simultaneous removal of Pb (II) and Cr(III) from wastewater by magnetite nanoparticles. Chin. J. Environ. Eng. 2013, 7, 3476–3482. [Google Scholar]
- Yan, L.; Li, S.; Yu, H.; Shan, R.; Du, B.; Liu, T. Facile solvothermal synthesis of Fe3O4/bentonite for efficient removal of heavy metals from aqueous solution. Powder Technol. 2016, 301, 632–640. [Google Scholar] [CrossRef]
- Zarei, S.; Niad, M.; Raanaei, H. The removal of mercury ion pollution by using Fe3O4-nanocellulose: Synthesis, characterizations and DFT studies. J. Hazard. Mater. 2018, 344, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Moloney, M.G.; Zhang, F.; Ji, W. Surface Hydrophobic Modification of Polymers with Fluorodiazomethanes. Mater. Lett. 2018, 210, 295–297. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, H.; Tang, J.; Deng, C.; Zhang, X. Facile Synthesis of Mercaptophenylboronic Acid-Functionalized Core−Shell Structure Fe3O4@C@Au Magnetic Microspheres for Selective Enrichment of Glycopeptides and Glycoproteins. J. Phys. Chem. C 2010, 114, 9221–9226. [Google Scholar] [CrossRef]
- Yu, X.; Tian, X.; Wang, S. Adsorption of Ni, Pd, Pt, Cu, Ag and Au on the Fe3O4 (111) surface. Surf. Sci. 2014, 628, 141–147. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, LV.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Wang, S.; Wu, Q.; Liu, C. Study on nanomagnets supported TiO2 photocatalysts prepared by a sol-gel process in reverse microemulsion combining with solvent-thermal technique. J. Hazard. Mater. 2009, 169, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Hu, S.H.; Liu, K.H.; Liu, D.M.; Chen, S.Y. Study on controlled drug permeation of magnetic-sensitive ferrogels: Effect of Fe3O4 and PVA. J. Control. Release 2008, 126, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Abu-Much, R.; Meridor, U.; Frydman, A.; Gedanken, A. Formation of a three-dimensional microstructure of Fe3O4-poly(vinyl alcohol) composite by evaporating the hydrosol under a magnetic field. J. Phys. Chem. B 2006, 110, 8194–8203. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Liu, Y.; Rao, H.; Wang, Y.; Wang, X. Fluorescence and magnetic nanocomposite Fe3O4@SiO2@Au MNPs as peroxidase mimetics for glucose detection. Anal. Biochem. 2017, 538, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ga, Y.; Zhu, G.; Ma, T. Progress in Fe3O4 magnetic nanoparticles and its application in biomedical fields. Chem. Ind. Eng. Prog. 2017, 36, 973–980. [Google Scholar]
- Jiao, L.; He, X.; Wang, L.; Zhang, L.; Ma, Y. Preparation of Fe3O4 and Their Modification. Guangdong Chem. Ind. 2016, 43, 127–128. [Google Scholar]
- Han, C.J.; Sheng, N.; Zhu, C.; Akiyama, T. Cotton-assisted combustion synthesis of Fe3O4/C composites as excellent anode materials for lithium-ion batteries. Mater. Today Proc. 2017, 5, 187–195. [Google Scholar]
- Wang, P.; Wang, X.; Yu, S.; Zou, Y.; Wang, J.; Chen, Z.; Alharbi, N.S.; Ahmed, A.; Hayat, T.; Chen, Y.; et al. Silica coated Fe3O4 magnetic nanospheres for high removal of organic pollutants from wastewater. Chem. Eng. J. 2016, 306, 280–288. [Google Scholar] [CrossRef]
- Mostafaei, M.; Hosseini, S.N.; Khatami, M.; Javidanbardan, A.; Sepahy, A.A.; Asadi, E. Isolation of recombinant Hepatitis B surface antigen with antibody-conjugated superparamagnetic Fe3O4/SiO2 core-shell nanoparticles. Protein Expr. Purif. 2018, 145, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Yang, H.; Jiang, P. The Research Progress of Magnetic Fe3O4/Mesoporous Silica Composite Microspheres. Chin. J. Biomed. Eng. 2017, 36, 348–353. [Google Scholar]
- Gao, F.; Botella, P.; Corma, A.; Blesa, J.; Dong, L. Monodispersed Mesoporous Silica Nanoparticles with Very Large Pores for Enhanced Adsorption and Release of DNA. J. Phys. Chem. B 2009, 113, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Zhang, L.; Peng, L.; Wang, L.; Zhang, J. Multifunctional magnetic mesoporous silica nanocomposites with improved sensing performance and effective removal ability toward Hg(II). Langmuir 2012, 28, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, X.; Yao, N.; Deng, C.; Yang, P.; Zhang, X. Facile synthesis of C8-functionalized magnetic silica microspheres for enrichment of low-concentration peptides for direct MALDI-TOF MS analysis. Proteomics 2008, 8, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xu, S.; Zhu, Q.; Qiang, L.; Ma, J. Preparation and Adsorption Properties of Amino Modified Magnetic Mesoporous Microsphere Fe3O4@SiO2@mSiO2. Chin. J. Inorg. Chem. 2016, 32, 1503–1511. [Google Scholar]
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Durantini, E.N. Photodynamic properties and photoinactivation of microorganisms mediated by 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin covalently linked to silica-coated magnetite nanoparticles. J. Photochem. Photobiol. A Chem. 2017, 346, 452–461. [Google Scholar] [CrossRef]
- Sun, Q.; Huang, Y.; Wang, J.; Guan, Z.; Li, M.; Zhou, J.; Wang, Y. Experimental study on mercury removal efficiencies of magnetic Fe3O4-Ag composite nanoparticles. Chem. Ind. Eng. Prog. 2017, 36, 1101–1106. [Google Scholar]
- Wang, H.; Liu, Y.G.; Zeng, G.M.; Hu, X.; Hu, X.; Li, T.; Li, H.; Wang, Y.; Jiang, L. Grafting of β-cyclodextrin to magnetic graphene oxide via ethylenediamine and application for Cr(VI) removal. Carbohydr. Polym. 2014, 113, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Mallick, N.; Asfer, M.; Anwar, M.; Kumar, A.; Samim, M.; Talegaonkar, S.; Ahmad, F.J. Rhodamine-loaded, cross-linked, carboxymethyl cellulose sodium-coated super-paramagnetic iron oxide nanoparticles: Development and in vitro localization study for magnetic drug-targeting applications. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 51–62. [Google Scholar] [CrossRef]
- Li, L.; Mak, K.Y.; Leung, C.W.; Chan, W.K.; Zhong, W.; Pong, P.W.T. Effect of synthesis conditions on the properties of citric-acid coated iron oxide nanoparticles. Microelectron. Eng. 2013, 110, 329–334. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Ghasemi, Y.; Rasoul-Amini, S.; Barar, J.; Davaran, S. Preparation of novel magnetic fluorescent nanoparticles using amino acids. Colloids Surf. B Biointerfaces 2013, 102, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhuang, H.; Liu, Y. Surface Modification of Fe3O4 Nanoparticles. Chem. Res. 2003, 14, 11–13. [Google Scholar]
- Zhu, C.; Song, J.; Qiu, L.; Zhang, Q. Preparation of magnetic Fe3O4 nano-particles modified with oleic acid at low-temperature and washed with distilled water. Chem. Ind. Eng. Prog. 2011, 30, 1552–1555. [Google Scholar]
- Shi, W.; Yang, J.; Wang, T.; Jin, Y. Magnetic Fe3O4 particles surface organic modification. Acta Phys.-Chim. Sin. 2001, 17, 507–510. [Google Scholar]
- Gui, S.; Shen, X.; Lin, B. Surface organic modification of Fe3O4 nanoparticles by silane-coupling agents. Rare Metals 2006, 25, 426–430. [Google Scholar] [CrossRef]
- Lou, M.; Wang, D.; Huang, W.; Zhao, H. The preparation and characterization of the MNPs mondified by silane-coupling agents. Shanghai J. Biomed. Eng. 2004, 25, 14–19. [Google Scholar]
- Portet, D.; Denizot, B.; Rump, E.; Lejeune, J.J.; Jallet, P. Nonpolymeric Coatings of Iron Oxide Colloids for Biological Use as Magnetic Resonance Imaging Contrast Agents. J. Colloid Interface Sci. 2001, 238, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.C.; Wells, S.; Charles, S.; Curtis, A.S.G. Dextran and albumin derivatised iron oxide nanoparticles: Influence on fibroblasts in vitro. Biomaterials 2003, 24, 4551–4557. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.H.; Im, K.H.; Kim, K.M.; Shim, I.B.; Lee, M.H.; Lee, Y.K. Surface-modified magnetite nanoparticles for hyperthermia: Preparation, characterization, and cytotoxicity studies. Curr. Appl. Phys. 2006, 6, e242–e246. [Google Scholar] [CrossRef]
- Lewin, M.; Carlesso, N.; Tung, C.H.; Tang, X.W.; Cory, D.; Scadden, D.T.; Weisslder, R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotechnol. 2000, 18, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.S.; Nicolas, V.; Wilhelm, C.; Ménager, C.; Barratt, G.; Lesieur, S. The in vitro kinetics of the interactions between PEG-ylated magnetic-fluid-loaded liposomes and macrophages. Biomaterials 2007, 28, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Uner, B.; Ramasubramanian, M.K.; Zauscher, S.; Kadla, J.F. Adhesion interactions between poly (vinyl alcohol) and iron-oxide surfaces: The effect of acetylation. J. Appl. Polym. Sci. 2006, 99, 3528–3534. [Google Scholar] [CrossRef]
- Godovsky, D.Y.; Varfolomeev, A.V.; Efremova, G.D.; Moskvina, M.A. Magnetic properties of polyvinyl alcohol-based composites containing iron oxide nanoparticles. Adv. Funct. Mater. 1999, 9, 87–93. [Google Scholar] [CrossRef]
- Reddy, N.N.; Varaprasad, K.; Ravindra, S.; Reddy, G.V.S.; Reddy, K.M.S.; Reddy, K.M.M.; Raju, K.M. Evaluation of blood compatibility and drug release studies of gelatin based magnetic hydrogel nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2011, 385, 20–27. [Google Scholar] [CrossRef]
- Cheng, Z.; Dai, Y.; Kang, X.; Li, C.; Huang, S.; Lian, H.; Hou, Z.; Ma, P.; Lin, J. Gelatin-encapsulated iron oxide nanoparticles for platinum (IV) prodrug delivery, enzyme-stimulated release and MRI. Biomaterials 2014, 35, 6359–6368. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tang, W.; He, S.; Yang, L.; Zhang, W.; Xiu, R. Preparation of Fe3O4/polyethyleneimine and its application for phosphate adsorption. Acta Sci. Circumst. 2017, 37, 4129–4138. [Google Scholar]
- Zhang, W.; Shen, H.; Xia, J.; Zhang, X.; He, X. Absorption Properties of Aqueous Ferrofluid Modified by Polyelthylenemine. Nanotechnol. Precis. Eng. 2007, 5, 125–128. [Google Scholar]
- He, S.; Zhang, F.; Cheng, S.; Wang, W. Preparation and Pb2+/Cd2+ adsorption of encapsulated Fe3O4/sodium polyacrylate magnetic crosslinking polymer. CIESC J. 2016, 67, 4290–4299. [Google Scholar]
- Yan, M.; Zhang, Q.; Xie, H.; Kong, J.; Qu, H. Load of PANI on nano-Fe3O4 and synergy catalytic degradation of dyes. China Environ. Sci. 2017, 37, 1394–1400. [Google Scholar]
- Cendrowski, K.; Sikora, P.; Zielinska, B.; Horszczaruk, E.; Mijowska, E. Chemical and thermal stability of core-shelled magnetite nanoparticles and solid silica. Appl. Surf. Sci. 2017, 407, 391–397. [Google Scholar] [CrossRef]
- Li, C.L.; Chang, C.J.; Chen, J.K. Fabrication of sandwich structured devices encapsulating core/shell SiO2/Fe3O4 nanoparticle microspheres as media for magneto-responsive transmittance. Sens. Actuators B Chem. 2015, 210, 46–55. [Google Scholar] [CrossRef]
- Feng, W.; Zhou, X.; Nie, W.; Chen, L.; Qiu, K.; Zhang, Y.; He, C. Au/polypyrrole@ Fe3O4 nanocomposites for MR/CT dual-modal imaging guided-photothermal therapy: An in vitro study. ACS Appl. Mater. Interfaces 2015, 7, 4354–4367. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Sun, Y.; Zheng, J.; Yang, W. Boronic acid-functionalized core-shell-shell magnetic composite microspheres for the selective enrichment of glycoprotein. ACS Appl. Mater. Interfaces 2013, 5, 8351–8358. [Google Scholar] [CrossRef] [PubMed]
- Snoussi, Y.; Bastide, S.; Abderrabba, M.; Chehimi, M. Sonochemical synthesis of Fe3O4@NH2-mesoporous silica@Polypyrrole/Pd: A core/double shell nanocomposite for catalytic applications. Ultrason. Sonochem. 2018, 41, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Sun, K.; Tang, K. Extended Stöber method to synthesize core-shell magnetic composite catalyst Fe3O4@C-Pd for Suzuki coupling reactions. Mater. Chem. Phys. 2018, 207, 181–185. [Google Scholar] [CrossRef]
- Baskakov, A.O.; Solov Eva, A.Y.; Ioni, Y.V.; Starchikov, S.S.; Lyubutin, I.S.; Khodos, I.I.; Avilov, A.S.; Gubin, S.P. Magnetic and interface properties of the core-shell Fe3O4/Au nanocomposites. Appl. Surf. Sci. 2017, 422, 638–644. [Google Scholar] [CrossRef]
- Peng, Z.; Fang, X.; Yan, G.; Gao, M.; Zhang, X. Highly efficient enrichment of low-abundance intact proteins by core-shell structured Fe3O4-chitosan@graphene composites. Talanta 2017, 174, 845–852. [Google Scholar]
- Kandibanda, S.R.; Gundeboina, N.; Das, S.; Sunkara, M. Synthesis, characterisation, cellular uptake and cytotoxicity of functionalised magnetic ruthenium(II) polypyridine complex core-shell nanocomposite. J. Photochem. Photobiol. B Biol. 2018, 178, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Zhikun, W.; Qiang, L.; Chunling, L.; Shuangqing, S.; Song, H. Preparation of amino-functionalized Fe3O4@mSiO2 core-shell magnetic nanoparticles and their application for aqueous Fe3+ removal. J. Hazard. Mater. 2018, 341, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Khashan, S.; Dagher, S.; Tit, N.; Alazzam, A.; Obaidat, I. Novel method for synthesis of Fe3O4@TiO2 core/shell nanoparticles. Surf. Coat. Technol. 2017, 322, 92–98. [Google Scholar] [CrossRef]
- Murata, J.; Ueno, Y.; Yodogawa, K.; Sugiura, T. Polymer/CeO2–Fe3O4 multicomponent core–shell particles for high-efficiency magnetic-field-assisted polishing processes. Int. J. Mach. Tools Manuf. 2016, 101, 28–34. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Wykowska, U.; Satuła, D. Magnetic nanoparticles of core-shell structure. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 527–536. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Yang, X.; Lu, L.; Wang, X. The Development and Prospect of Nanometer Materials. Missiles Space Veh. 2000, 3, 11–16. [Google Scholar]
- Ramasamy, T.; Ruttala, H.B.; Gupta, B.; Poudel, B.K.; Choi, H.G.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J. Control. Release 2017, 258, 226–253. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Tu, Z.; Duan, H.; Zhou, S. Study on the Histocompatibility and Tissue Distribution of Fe3O4 Nanoparticles. Chin. J. Tissue Eng. Res. 2016, 20, 7872–7877. [Google Scholar]
- Isiamian, J.P.; Hatamian, M.; Aval, N.A.; Rashidi, M.R.; Mesbahi, A.; Mohammadzadeh, M.; Jafarabadi, M.A. Targeted superparamagnetic nanoparticles coated with 2-deoxy-d-gloucose and doxorubicin more sensitize breast cancer cells to ionizing radiation. Breast 2017, 33, 97–103. [Google Scholar]
- Fakhri, A.; Tahami, S.; Nejad, P.A. Preparation and characterization of Fe3O4-Ag2O quantum dots decorated cellulose nanofibers as a carrier of anticancer drugs for skin cancer. J. Photochem. Photobiol. B Biol. 2017, 175, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, G.; Fan, X.; Wang, H.; Wang, J.; Gao, Y.; Xie, B.; Chen, Y.; Song, Z. Superparamagnetic Fe3O4 Nanoparticles in the Application of Water Model of Magnetic Resonance Imaging and CT Imaging in Rat Brain. Guangdong Trace Elem. Sci. 2017, 24, 10–16. [Google Scholar]
- Cui, L.; Hu, L.; Guo, X.; Zhang, Y.; Wang, Y.; Wei, Q.; Du, B. Kinetic, isotherm and thermodynamic investigations of Cu2+ adsorption onto magnesium hydroxyapatite/ferroferric oxide nano-composites with easy magnetic separation assistance. J. Mol. Liquids 2014, 198, 157–163. [Google Scholar] [CrossRef]
- Tian, X.; Wang, W.; Tian, N.; Zhou, C.; Yang, C.; Komarneni, S. Cr(VI) reduction and immobilization by novel carbonaceous modified magnetic Fe3O4/halloysite nanohybrid. J. Hazard. Mater. 2016, 309, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhong, Z.; Xu, H.; Yao, Z.; Chen, R. Fabrication of Poly(γ-glutamic acid)-coated Fe3O4 Magnetic Nanoparticles and Their Application in Heavy Metal Removal. Chin. J. Chem. Eng. 2013, 21, 1244–1250. [Google Scholar] [CrossRef]
- Jin, S.; Park, B.C.; Ham, W.S.; Pan, L.; Kim, K.Y. Effect of the magnetic core size of amino-functionalized Fe3O4-mesoporous SiO2 core-shell nanoparticles on the removal of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 133–140. [Google Scholar] [CrossRef]
- Shen, S.; Wen, G.; Zheng, Y. Preparation of Rhizopus oryzae-Fe3O4 Composites and Their Cu2+ Adsorption Experiments. Anhui Agric. Sci. Bull. 2016, 22, 15–16. [Google Scholar]
- Zhao, J.; Liu, J.; Li, N.; Wang, W.; Nan, J.; Zhao, Z.; Cui, F. Highly efficient removal of bivalent heavy metals from aqueous systems by magnetic porous Fe3O4-MnO2: Adsorption behavior and process study. Chem. Eng. J. 2016, 304, 737–746. [Google Scholar] [CrossRef]
- Kilianová, M.; Prucek, R.; Filip, J.; Kolařík, J.; Kvítek, L.; Panáček, A.; Tuček, J.; Zbořil, R. Remarkable efficiency of ultrafine superparamagnetic iron(III) oxide nanoparticles toward arsenate removal from aqueous environment. Chemosphere 2013, 93, 2690–2697. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y. Preparation of magnetic ferriferous oxide and its application in purification of heavy metal ions in waste water. Inorg. Chem. Ind. 2015, 47, 20–22. [Google Scholar]
- Luo, X.; Wang, C.; Luo, S.; Dong, R.; Tu, X.; Zeng, G. Adsorption of As (III) and As (V) from water using magnetite Fe3O4-reduced graphite oxide-MnO2 nanocomposites. Chem. Eng. J. 2012, 187, 45–52. [Google Scholar] [CrossRef]
- Zhou, L.; Deng, H.; Wan, J.; Shi, J.; Su, T. A solvothermal method to produce RGO-Fe3O4 hybrid composite for fast chromium removal from aqueous solution. Appl. Surf. Sci. 2013, 283, 1024–1031. [Google Scholar] [CrossRef]
- Gupta, P.L.; Jung, H.; Tiwari, D.; Kong, S.H.; Lee, S.M. Insight into the mechanism of Cd(II) and Pb(II) removal by sustainable magnetic biosorbent precursor to Chlorella vulgaris. J. Taiwan Inst. Chem. Eng. 2017, 71, 206–213. [Google Scholar]
- Yi, X.; He, J.; Guo, Y.; Han, Z.; Yang, M.; Jin, J.; Gu, J.; Ou, M.; Xu, X. Encapsulating Fe3O4 into calcium alginate coated chitosan hydrochloride hydrogel beads for removal of Cu (II) and U (VI) from aqueous solutions. Ecotoxicol. Environ. Saf. 2018, 147, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Ge, K.; Zhang, X.; Zhang, Y.; Liu, J.; Bi, H. Preparation and Capacitance Properties of Fe3O4-ODA/GO/PANI Nanocomposites. J. Mater. Sci. Eng. 2017, 35, 475–479. [Google Scholar]
- Guan, G.; Zou, M.; Feng, Q.; Lin, J.; Huang, Z.; Yan, G. Synthesis of Fe3O4/RGO composites and their electrochemical performance. J. Fuel Chem. Technol. 2017, 45, 362–369. [Google Scholar]
- Peng, H.; Liang, R.; Zhang, L.; Qiu, J.D. General preparation of novel core-shell heme protein-Au-polydopamine-Fe3O4 magnetic bionanoparticles for direct electrochemistry. J. Electroanal. Chem. 2013, 700, 70–76. [Google Scholar] [CrossRef]
- Zheng, N.; Zhou, X.; Yang, W.; Li, X.; Yuan, Z. Direct electrochemistry and electrocatalysis of hemoglobin immobilized in a magnetic nanoparticles-chitosan film. Talanta 2009, 79, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tan, S.; Tang, R. AFP immunosensor based on Fe3O4-ferrocene nanocomposites study. Chin. J. Anal. Lab. 2017, 36, 477–480. [Google Scholar]
- Cai, K.; Zhang, Y.; Xu, Y.; Ma, F.; Zhang, L.; Zhou, H. One-pot Synthesis of Silver/Fe3O4 Particles and Its Catalytic Performances for the Reduction of 4-Nitrophenol. Ind. Saf. Environ. Prot. 2017, 43, 21–23. [Google Scholar]
- Zou, T.; Yi, Q.; Zhang, Y.; Xiang, B.; Zhou, X. Preparation of PdSn/Fe3O4-C Catalysts and Their Electro-catalytic Activities for The Oxidation of Ethyl Alcohol. Chin. J. Synth. Chem. 2017, 25, 480–486. [Google Scholar]
- Gu, Y.; Liu, G.; Zou, C.; Zhang, Z.; Liu, J.; Sun, M.; Cheng, G.; Yu, L. Ultrasonic Synthesis and Application in Catalytic 4-Nitrophenols Reduction of Au/Fe3O4. Chin. J. Inorg. Chem. 2017, 33, 787–795. [Google Scholar]
- Gao, X. Effect of nano Fe3O4 on properties of PTFE-based high temperature resistant sealing material. China Synth. Resin Plast. 2016, 33, 38–40. [Google Scholar]
- Gao, Z.; Peng, X.; Zhang, H.; Luan, Z.; Fan, B. Montmorillonite-Cu(II)/Fe(III) oxides magnetic material for removal of cyanobacterial Microcystis aeruginosa and its regeneration. Desalination 2009, 247, 337–345. [Google Scholar] [CrossRef]
- Ito, D.; Nishimura, K.; Miura, O. Removal and recycle of phosphate from treated water of sewage plants with zirconium ferrite adsorbent by high gradient magnetic separation. J. Phys. Conf. Ser. 2009, 156, 012033. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, T.; Shang, Y.; Yang, K.; Wang, H. Biodegradation of phenol by entrapped cell of Debaryomyces sp. with nano-Fe3O4 under hypersaline conditions. Int. Biodeterior. Biodegrad. 2017, 123, 37–45. [Google Scholar] [CrossRef]
- Fang, Q.; Lin, J.; Zhan, Y.; Yang, M.; Zheng, W. Synthesis of Hydroxyapatite/Magnetite/Zeolite Composite for Congo Red Removal from Aqueous Solution. Environ. Sci. 2014, 35, 2992–3001. [Google Scholar]
- Yuan, J.; Mao, M.; Cao, Y.; Wang, J. Preparation and properties of Fe3O4 nanoparticles/natural rubber composites. New Chem. Mater. 2015, 43, 33–36. [Google Scholar]
- Liu, H.; Li, X.; Wei, B.; Xu, F. Synthesis of super-paramagnetic Fe3O4 nanoparticles and its Fenton-like properties. Chin. J. Environ. Eng. 2017, 11, 3525–3531. [Google Scholar]
- Guo, B.; Ouyang, J.; Yang, H. Adsorption Performance to Methylene Blue by Nano-Fe3O4Assembled in Lumen of Halloysite Nanotubes. J. Chin. Ceram. Soc. 2016, 44, 1655–1661. [Google Scholar]
- Zhou, L.; Jin, J.; Liu, Z.; Liang, X.; Shang, C. Adsorption of acid dyes from aqueous solutions by the ethylenediamine-modified magnetic chitosan nanoparticles. J. Hazard. Mater. 2011, 185, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhang, C.; Chen, Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J. Hazard. Mater. 2011, 192, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Badruddoza, A.Z.M.; Hidajat, K.; Uddin, M.S. Adsorptive removal of emerging contaminants from water using superparamagnetic Fe3O4 nanoparticles bearing aminated β-cyclodextrin. J. Environ. Chem. Eng. 2013, 1, 122–130. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Singh, V.K.; Alexandre-Franco, M.; Pittman, C.U., Jr. Development of magnetic activated carbon from almond shells for trinitrophenol removal from water. Chem. Eng. J. 2011, 172, 1111–1125. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, F.; Xu, X.Z.; Wang, D.S. Novel ferromagnetic nanoparticle composited PACls and their coagulation characteristics. Water Res. 2012, 46, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, X.; Zheng, H.; Wang, Y.; Sun, Y.; Zhao, C.; Zhang, S. Rapid and efficient removal of heavy metal and cationic dye by carboxylate-rich magnetic chitosan flocculants: Role of ionic groups. Carbohydr. Polym. 2018, 181, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zheng, H.; Wang, Y.; An, Y.; Luo, K.; Zhao, C.; Xiang, W. Poly (2-acrylamido-2-methylpropane sulfonic acid) grafted magnetic chitosan microspheres: Preparation, characterization and dye adsorption. Int. J. Biol. Macromol. 2018, 112, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Shan, A.; Zhao, Y. Synthesis of a novel magnetic polyacrylamide coagulant and its application in wastewater purification. Water Sci. Technol. 2017, 75, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, P.; Wei, G.; Dong, W.; Hui, F. Removal of algal blooms from freshwater by the coagulation–magnetic separation method. Environ. Sci. Pollut. Res. 2013, 20, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
| Preparation Methods | Particle Size | Additive | Optimum Ratio | Pollutants | Adsorption Manner | Optimization Effect | References |
|---|---|---|---|---|---|---|---|
| Coprecipitation method | 35 nm | Ethylenediaminetetraacetic acid | 15 mol FeCl3·6 H2O, 7.5 mol FeCl2·4 H2O,150 mL deionized water | Ag(I), Hg(II), Mn(II), Zn(II), Pb(II) and Cd(II) | Chemical adsorption and physical adsorption | The Fe3O4 modified by ethylenediaminetetraacetic acid had reactive functional groups such as carboxyl groups and amino groups on its surface and could undergo chemical ligand exchange with heavy metal ions in water. | [43] |
| Coprecipitation method | 60 nm | Soluble starch | Reaction time = 2 h, pH = 3, equilibrium concentration = 1.0 mg/L | P | Physical adsorption | The Fe3O4 modified by starch has good dispersion, shown a monolayer disperse. The starch coats on the surface of Fe3O4 nanoparticle through steric hindrance and charge repulsion overcome the van der Waals force and magnetic attraction force. | [44] |
| Sol-gel method | - | Activated carbon fiber | pH = 2.0–6.0 at room temperature | P | Chemical adsorption | After modification through activated carbon fiber, the Fe3O4 had a significant increase in surface area and total pore volume. The modified Fe3O4 was positively charged and negatively charged by the electrostatic adsorption of phosphate ions. The ion exchange reaction occurred between phosphate and hydroxide. | [45] |
| Coprecipitation method | Around 25 nm | - | pH = 6.0 at 25 °C, 4.0 g/L Fe3O4 | Pb and Cr ions | Single phase adsorption Pb ion, multiphase adsorption for Cr ion | The prepared Fe3O4 particle had a larger BET. Under the van der Waals force and magnetic attraction, it had the link structure of the polygon, which increased the three-dimensional space between the particles. It had a spinel structure and high crystallization degree. | [46] |
| Solvent heat method | - | Bentonite | Heating for 8 h at 200 °C | Pb2+, Cd2+ and Cu2+ ions | Chemical adsorption | The composites composed of Fe3O4 and bentonite had reactive functional groups such as hydroxyl and carboxyl groups on the surface, which exhibited better adsorption of heavy metal ions. The specific surface area and total pore volume of composites were larger than those of pure Fe3O4, with higher magnetization saturation and lower remanence. | [47] |
| Coprecipitation and Sol-gel method | 1–100 nm | Cellulose | 4.0 g FeCl2·4H2O, 8.0 g FeCl3·6H2O, 150 mL deionized water, Reaction temperature = 60 °C stirring time = 60 min | Mercury ion | Chemical adsorption | Cellulose slender nanostructures were more likely to adsorb mercury ions. Cellulose-modified Fe3O4 has a reactive functional group such as CH2 on its surface and interacts with mercury ions. | [48] |
| Type | Materials | Advantages | References |
|---|---|---|---|
| Inorganic small molecules | (1) SiO2 and other oxides; (2) Au, Co, Ni and other inorganic metals | The modification of SiO2 and other oxides can shield the dipole interaction between the magnetic nanoparticles to prevent the particles from agglomerating and facilitate further functionalization of the particle surface. At the same time, it had a good biocompatibility, hydrophilicity and stability. Encapsulation of inorganic metals can synthesize composite particles of core-shell structure, giving the magnetic nanoparticles rich and excellent physical properties. | [57] |
| Organic small molecules | (1) Ethanol, organic carboxyl, sulfur and silane coupling agent oil-soluble substances; (2) Sodium oleate, sodium carboxymethylcellulose, β-cyclodextrin, citric acid, amino acids | Particle oil-soluble conversion to water-solubility was achieved by the interaction between the modifier and the stabilizer and the ligand exchange reaction resulting in water-soluble, oil-soluble and amphiphilic nanoparticles. Surfactant modification could control nanoparticle size and shape and changed surface properties of nanoparticle. Modification of silane coupling agents introduces reactive groups on the surface of nanoparticles to provide chemical selectivity for their further functionalization. | [58] |
| Organic polymers | (1) Glucose, starch, protein, peptides and other natural polymers; (2) Polyethylene glycol, polyvinyl alcohol and other synthetic polymers | Natural biomolecules had a good biodegradability and biocompatibility, greatly improving the biocompatibility of magnetic particles and giving them special biological activity. Synthetic polymer modification could give the material a variety of different properties to meet the actual requirement. Biomacromolecules had excellent bioactivity and were a synthetic polymer-rich chemical-selective organic combination. | [59] |
| Heavy Metal | Methods | Removal Mechanism | References |
|---|---|---|---|
| Pb | With different amounts of glycerol | Surface coordination, chemical adsorption. | [118] |
| As | With manganese dioxide and graphene oxide | Manganese dioxide can oxidize arsenic into pentavalent arsenic and graphene oxide can increase adsorption ability. | [119] |
| Cr | With Reduced graphene oxide | Electrostatic adsorption and acid groups adsorption. | [120] |
| Hg | With cellulose | The complex of mercury ion and cellulose on the surface of Fe3O4. | [48] |
| Pb and Cr | With micrococcus | Weak electrostatic forces between cadmium ions (II) and carboxyl groups or hydroxyl groups; chemical bonding of lead (II) ions and amino groups. | [121] |
| U and Cu | With calcium alginate containing-chitosan hydrogel beads | Chemical interaction of NH3-groups in Chitosan with -COOH groups in calcium alginate. Physical pore adsorption and electrostatic adsorption. | [122] |
| Pollutants | Methods for Modification of Nano-Fe3O4 | Removal Principles | References |
|---|---|---|---|
| Congo red | With hydroxyapatite and zeolite | The interaction of the dye and Nano-Fe3O4 through Surface coordination, hydrogen bonding, Lewis acid base reaction. | [135] |
| Natural rubber | With silane coupling agent | The Fe3O4 has high binding energy after modification and it was much easier to bond with rubber. | [136] |
| Rhodamine B | With Fenton reaction in the presence of H2O2 | The surface of Fe3O4 formed complexes and hydroxyl radicals, resulting of degradation of dye. | [137] |
| Methylene blue | With natural eloise under vacuum impregnation and high temperature pyrolysis | A large number of hydroxyl groups on the surface of rocky oxidized methylene blue. | [138] |
| Acid orange | With Chitosan | Interacting of ions of dye with the protonated amino ions of chitosan | [139] |
| Methylene blue | With Graphene | The positive charged oxygen-containing groups in Graphene attracted a negatively charged methylene blue. | [140] |
| Bisphenol A | With amine-containing β-cyclodextrin | Hybrid effects of electrostatic, hydrophobic and van der Waals. | [141] |
| Organics 2,4,6-trinitrophenol | With activated carbon | Porous physical adsorption on the surface of activated carbon; Electrostatic adsorption and hydrogen bonding; Surface reaction; In the presence of dissolved oxygen, the phenols on the surface of activated carbon are gathered. | [142] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, H.; Bian, Y.; Yuan, Q.; Ren, B.; Hursthouse, A.; Zhu, G. Preparation and Potential Applications of Super Paramagnetic Nano-Fe3O4. Processes 2018, 6, 33. https://doi.org/10.3390/pr6040033
Zhan H, Bian Y, Yuan Q, Ren B, Hursthouse A, Zhu G. Preparation and Potential Applications of Super Paramagnetic Nano-Fe3O4. Processes. 2018; 6(4):33. https://doi.org/10.3390/pr6040033
Chicago/Turabian StyleZhan, Hao, Yongning Bian, Qian Yuan, Bozhi Ren, Andrew Hursthouse, and Guocheng Zhu. 2018. "Preparation and Potential Applications of Super Paramagnetic Nano-Fe3O4" Processes 6, no. 4: 33. https://doi.org/10.3390/pr6040033
APA StyleZhan, H., Bian, Y., Yuan, Q., Ren, B., Hursthouse, A., & Zhu, G. (2018). Preparation and Potential Applications of Super Paramagnetic Nano-Fe3O4. Processes, 6(4), 33. https://doi.org/10.3390/pr6040033







