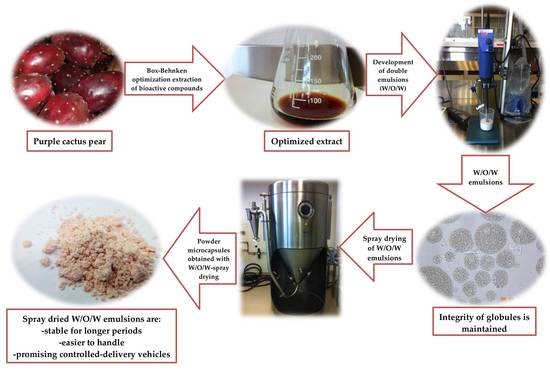
Microencapsulation of Purple Cactus Pear Fruit (Opuntia ficus indica) Extract by the Combined Method W/O/W Double Emulsion-Spray Drying and Conventional Spray Drying: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Purple Cactus Pear Fruit for Extraction of Bioactive Compounds
2.2. Extraction of Bioactive Compounds for the Characterization of Purple Cactus Pear Fruit
2.3. Optimization of Extraction Parameters of Bioactive Compounds from Purple Cactus Pear Fruit Peel and Pulp
2.4. W/O/W Emulsion Preparation
2.5. Spray Drying
2.6. Preparation of Microcapsules for Analysis
2.6.1. W/O/W Emulsion before and after SP
2.6.2. Conventional SP
2.7. Total Phenolic Compounds Determination
2.8. Antioxidant Capacity Determination
2.9. Betalain Determination
2.10. Ascorbic Acid Determination
2.11. Determination of Moisture Content and Total Solids
2.12. Retention and Encapsulation Efficiency Determination
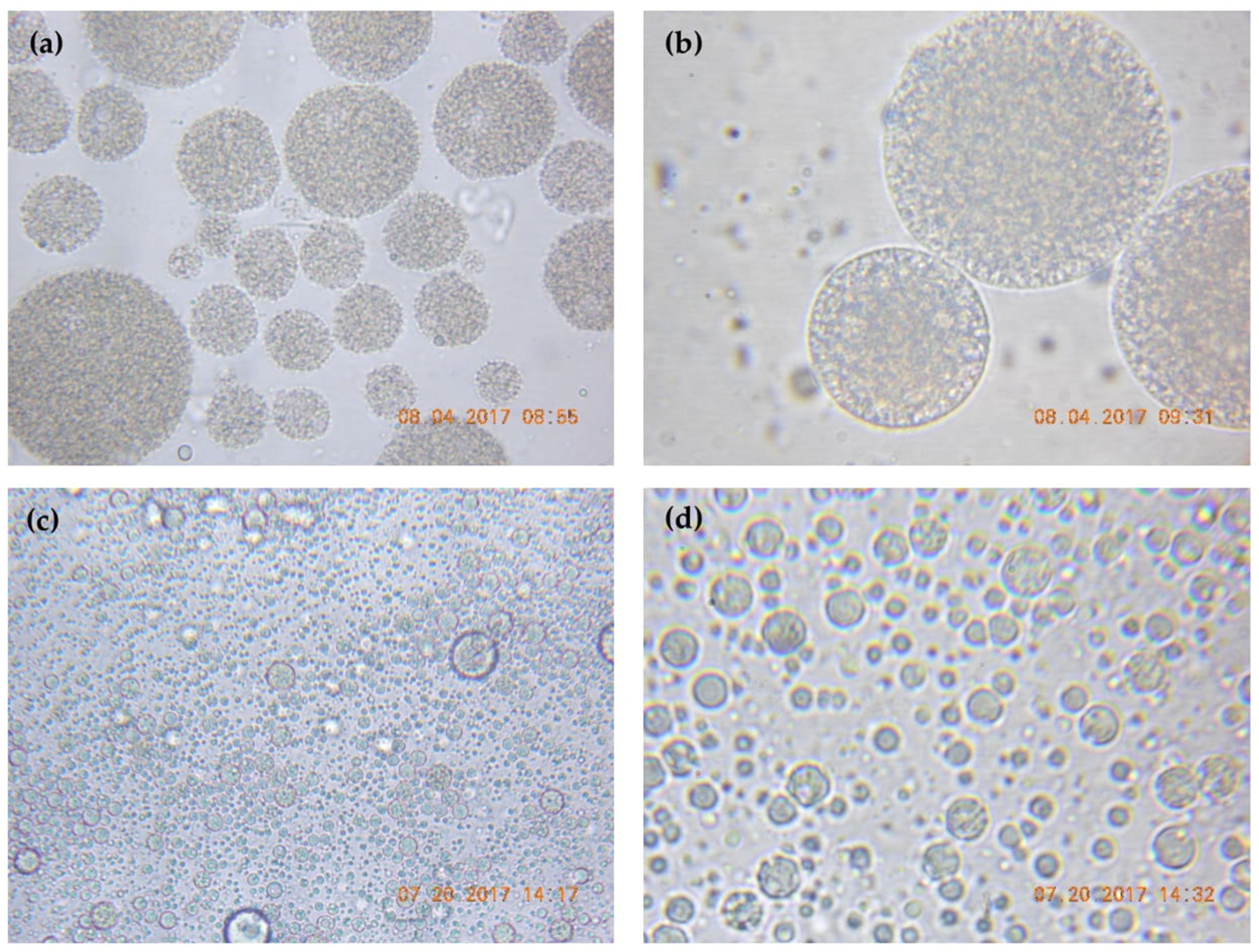
2.13. Integrity of W/O/W before and after Spray Drying
2.14. Particle Size Distribution Determination
2.15. Surface Morphology Determinaton
2.16. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Fresh Whole Purple Cactus Pear Fruit
3.2. Characterization of Dry Whole Purple Cactus Pear Fruit and Each of Its Separate Parts (Peel, Pulp, and Seeds)
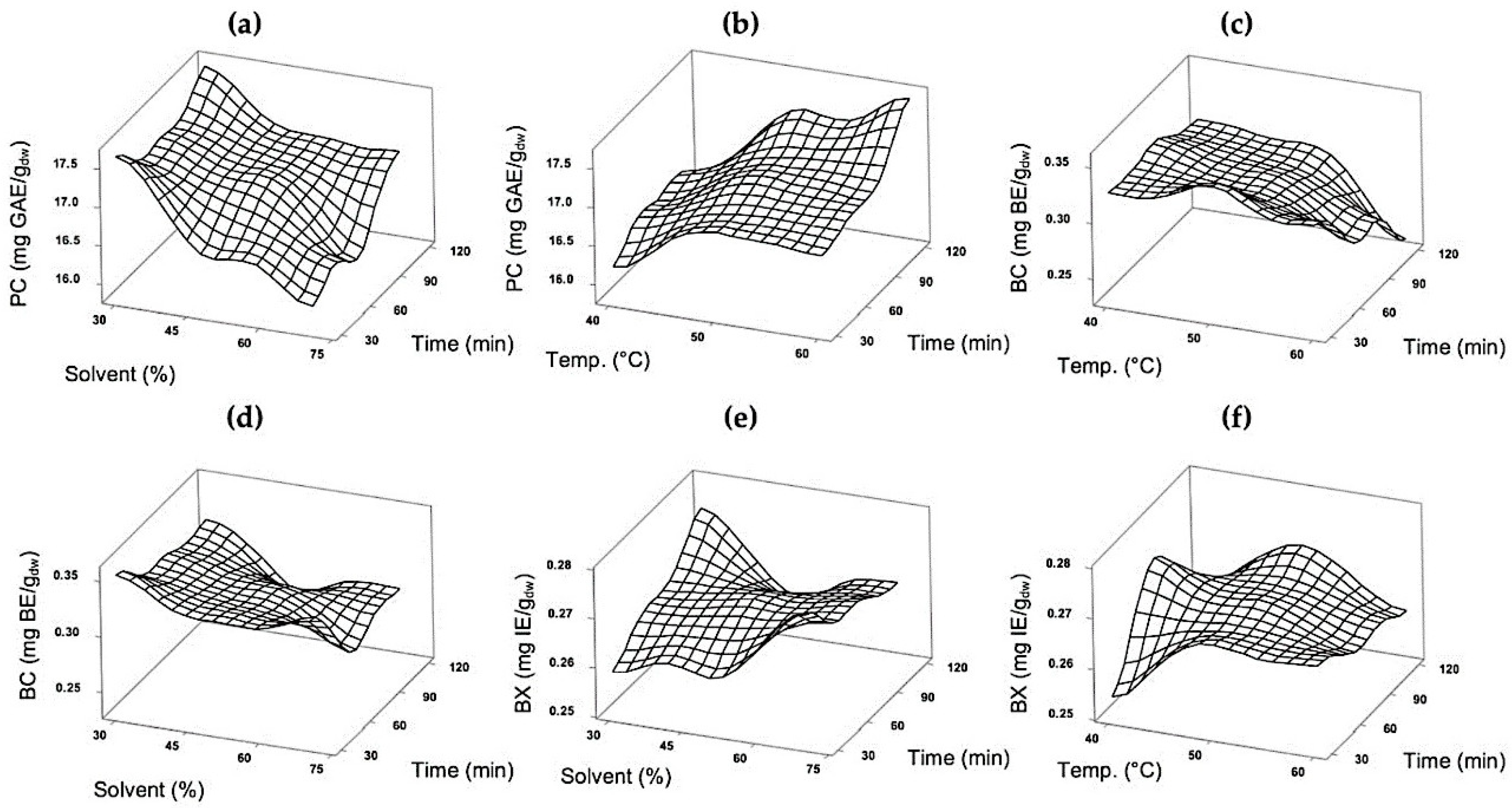
3.3. Optimization of Extraction Parameters of Bioactive Compounds from Purple Cactus Pear Fruit Peel and Pulp
3.3.1. Effect of Process Variables in Phenolic Compounds and Betalain Extraction
3.3.2. Determination of Optimum Extraction Conditions
3.4. Microencapsulation with W/O/W Emulsion
3.5. Spray Drying
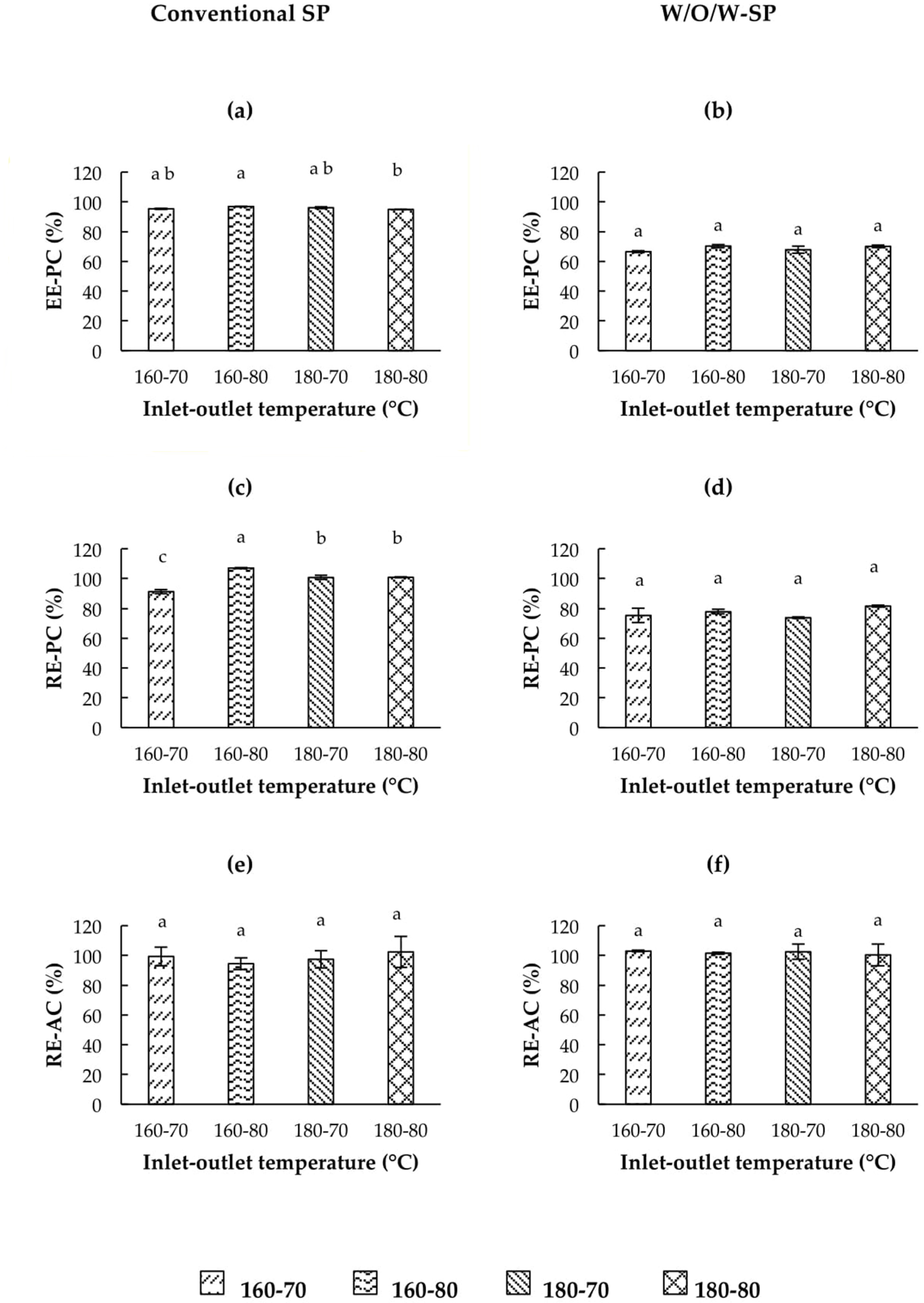
3.5.1. Encapsulation Efficiency of Phenolic Compounds (EE-PC)
3.5.2. Phenolic Compounds Retention
3.5.3. Antioxidant Capacity Retention
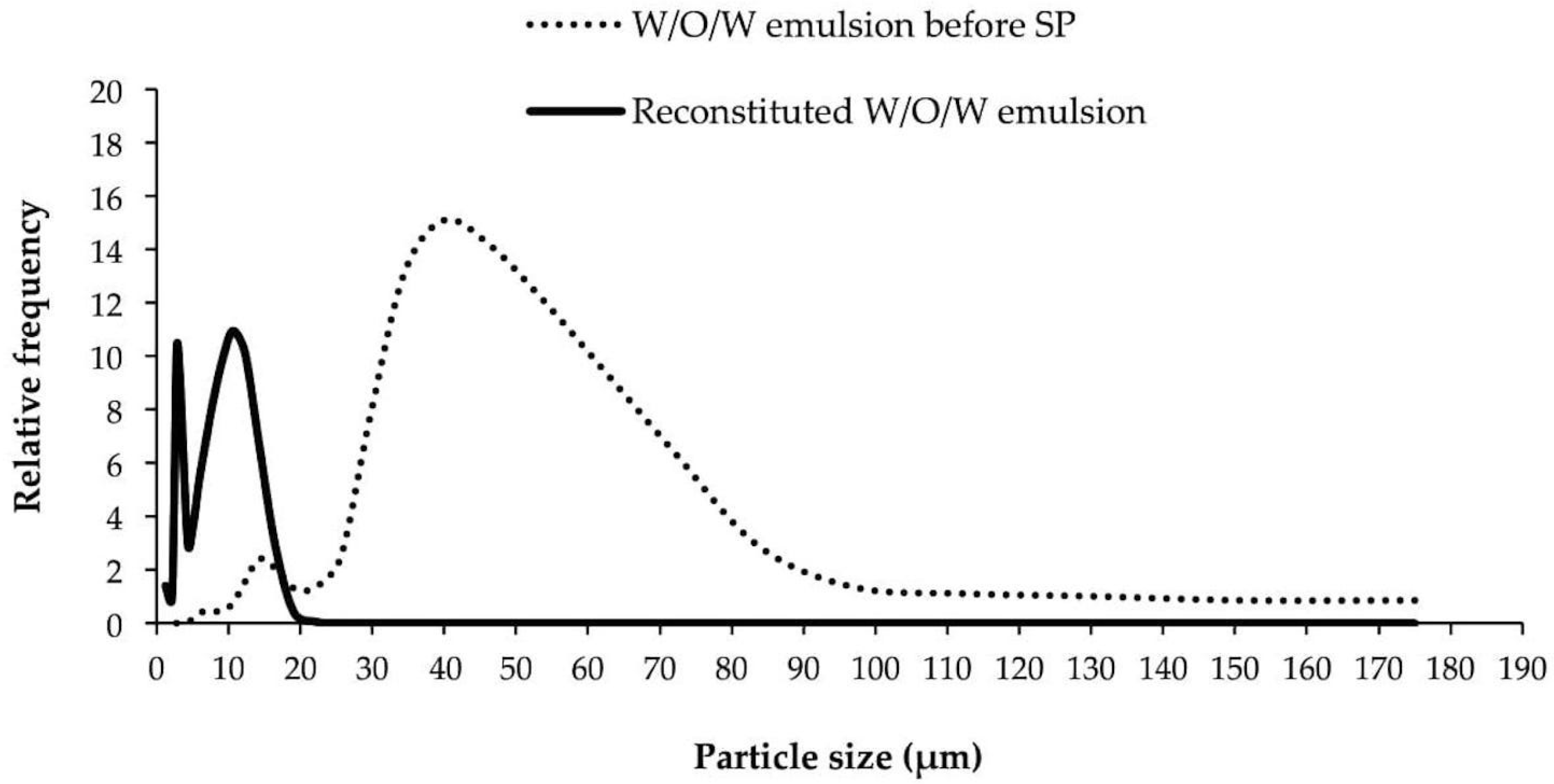
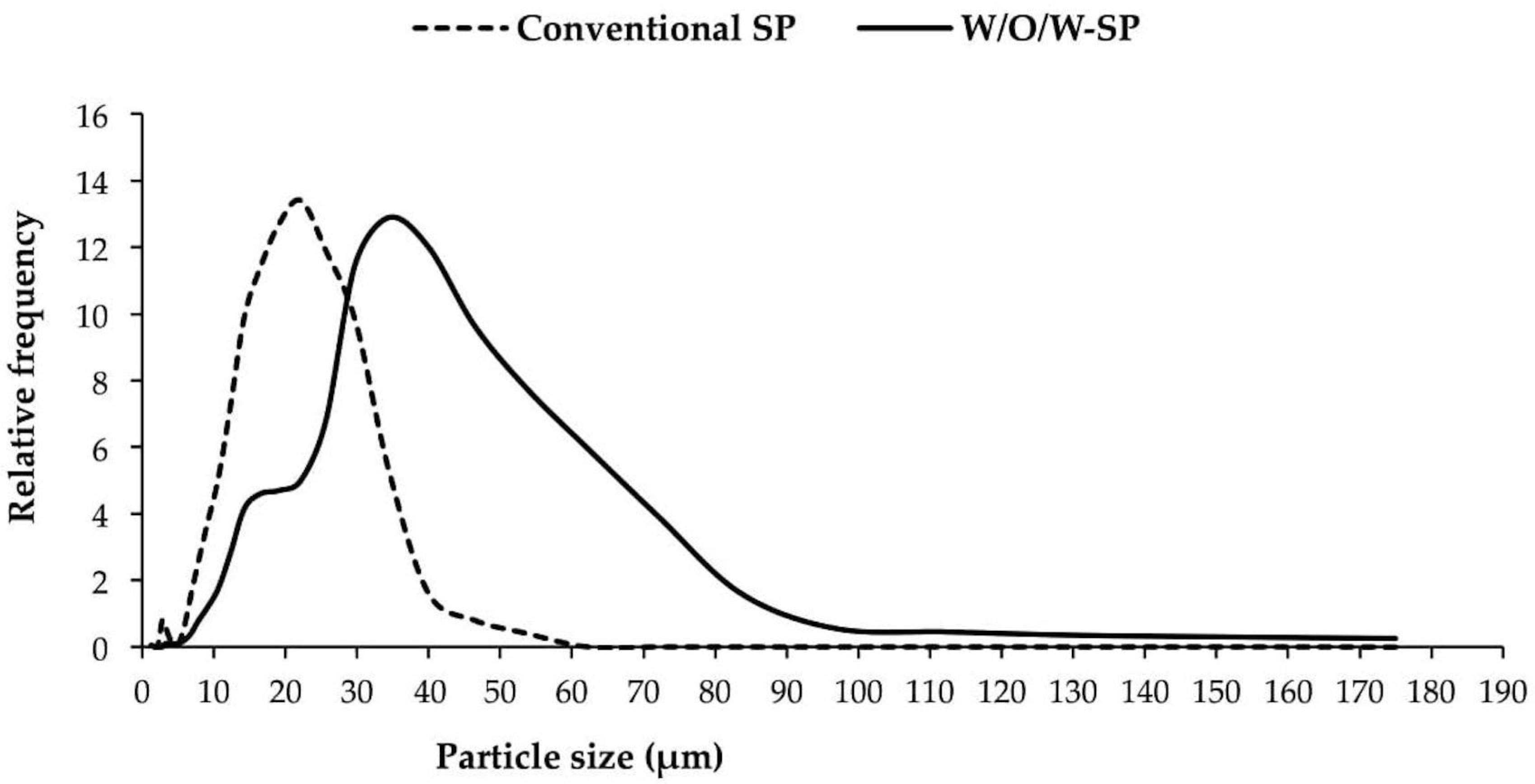
3.6. Particle Size and Particle Size Distribution
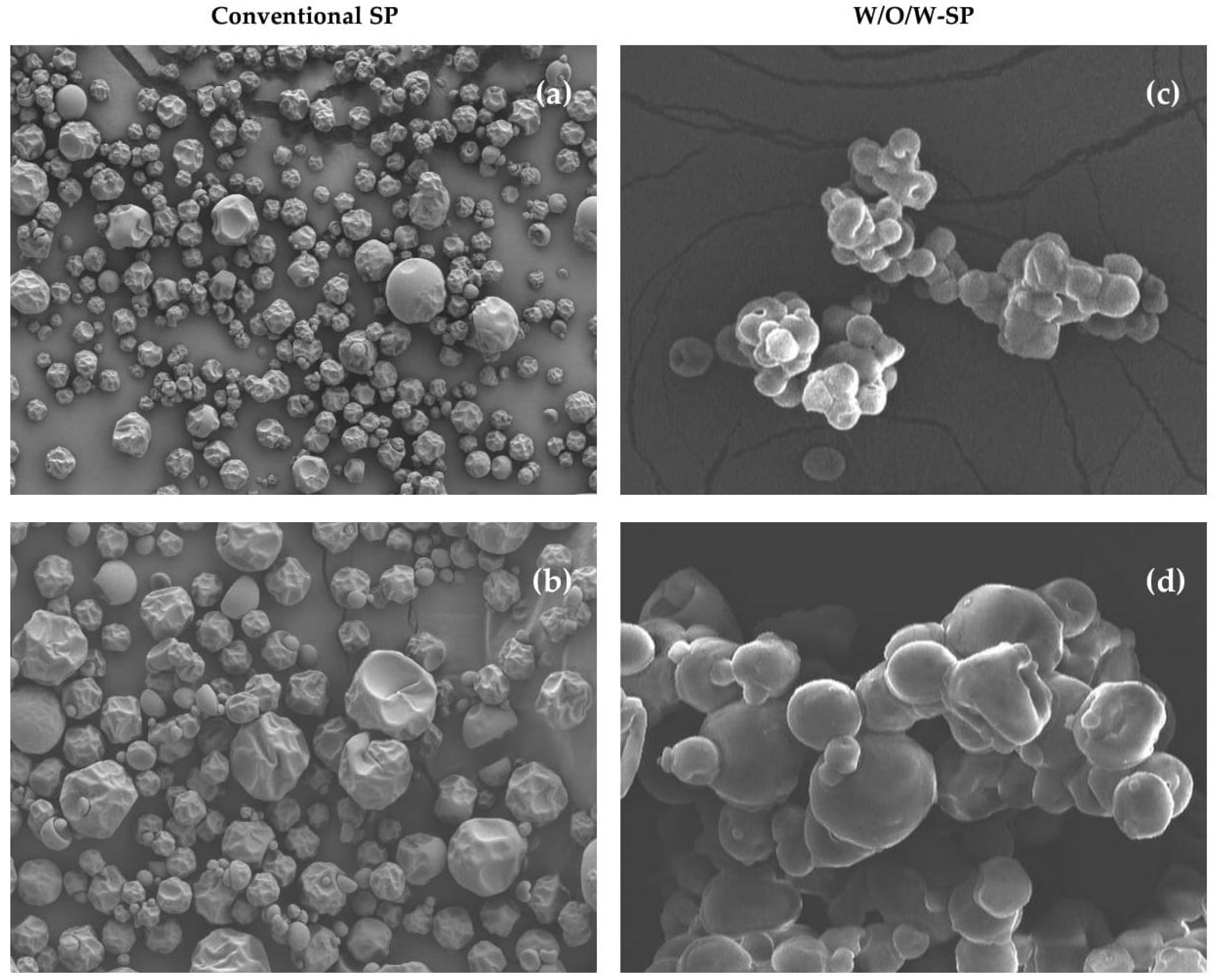
3.7. Surface Morphology of Microcapsules
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carbonell-Capela, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccesibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Sumaya-Martínez, M.T.; Cruz-Jaime, S.; Madrigal-Santillán, E.; García-Paredes, J.D.; Cariño-Cortés, R.; Cruz-Cansino, N.; Valadez-Vega, C.; Martinez-Cardenas, L.; Alanís-García, E. Betalain, Acid Acorbic, Phenolic Contents and Antioxidant Properties of Purple, Red, Yellow and White Cactus Pears. Int. J. Mol. Sci. 2011, 12, 6452–6468. [Google Scholar] [CrossRef] [PubMed]
- En 2017, La Producción Nacional de Tuna y Xoconostle Fue Superior a 470 mil Toneladas. Financiero Rural, 2018. Available online: https://www.gob.mx/siap/articulos/en-2017-la-produccion-nacional-de-tuna-y-xoconostle-fue-superior-a-470-mil-toneladas (accessed on 18 June 2018).
- LA TUNA, Una Fruta Muy Mexicana. Financiero Rural, 6 September 2017. Available online: https://www.gob.mx/firco/articulos/la-tuna-una-fruta-muy-mexicana?idiom=es (accessed on 18 June 2018).
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Joya, J.; De la Garza-Toledo, H.; Zugasti-Cruz, A.; Belmares-Cerda, R.; Aguilar-Cristóbal, N. The optimization of phenolic compounds extraction from cactus pear (Opuntia ficus-indica) skin in a reflux system using response surface methodology. Asia Pac. J. Biomed. 2013, 3, 436–442. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chen, Q.; Shi, J. Microencapsulation of Food Ingredients for Functional Foods. In Functional Food Ingredients and Nutraceuticals: Processing Technologies, 2nd ed.; Shi, J., Ed.; CRC Press: Boca Raton, FL, USA, 2015; p. 268. ISBN 9781482240641. [Google Scholar]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Nesterenko, A.; Alric, I.; Silvestre, F.; Durrieu, V. Vegetable proteins in microencapsulation: A review of recent interventions and their effectiveness. Ind. Crops Prod. 2013, 42, 469–479. [Google Scholar] [CrossRef]
- Bicudo, M.O.P.; Jó, J.; Oliveira, G.A.; Chaimsohn, F.P.; Sierakowski, M.R.; Freitas, R.A. Microencapsulation of juçara (Euterpe edulis M.) pulp by spray drying using different carriers and drying temperatures. Dry. Technol. 2015, 33, 153–161. [Google Scholar] [CrossRef]
- Tan, S.P.; Kha, T.C.; Parks, S.; Stathopoulos, C.; Roach, P.D. Optimising the Encapsulation of an Aqueous Bitter Melon Extract by Spray-Drying. Foods 2015, 4, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.I.; Stringheta, P.C.; Teófilo, R.F.; Oliveira, I.R.N. Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. J. Food Eng. 2013, 117, 538–544. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Lamba, H.; Sathish, K.; Sabikhi, L. Double Emulsions: Emerging Delivery System for Plant Bioactives. Food Bioprocess Technol. 2015, 8, 709–728. [Google Scholar] [CrossRef]
- Berendsen, R.; Güell, C.; Ferrando, M. Spray dried double emulsions containing procyanidin-rich extracts produced by premix membrane emulsification: Effect of interfacial composition. Food Chem. 2015, 178, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Muschiolik, G.; Dickinson, E. Double Emulsions Relevant to Food Systems: Preparation, Stability, and Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef]
- Giroux, H.; Constantineau, S.; Fustier, P.; Champagne, C.; St-Gelais, D.; Lacroix, M.; Britten, M. Cheese fortification using water-in-oil-in-water double emulsions as carrier for water soluble nutrients. Int. Dairy J. 2013, 29, 107–114. [Google Scholar] [CrossRef]
- Andrade, J.; Corredig, M. Vitamin D3 and phytosterols affect the properties of polyglicerol polyricinoleate (PGPR) and protein interfaces. Food Hydrocoll. 2015, 54, 278–283. [Google Scholar] [CrossRef]
- Dickinson, E. Double Emulsions Stabilized by Food Polymers. Food Biophys. 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Kaimainen, M.; Marze, S.; Järvenpää, E.; Anton, M.; Huopalahti, R. Encapsulation of betalain into w/o/w double emulsion and release during in vitro intestinal lipid digestion. LWT-Food Sci. Technol. 2015, 60, 899–904. [Google Scholar] [CrossRef]
- Benichou, A.; Aserin, A.; Garti, N. Polyols, High Pressure, and Refractive Indices Equalization for Improved Stability of W/O Emulsions for Food Applications. J. Dispers. Sci. Technol. 2001, 22, 269–280. [Google Scholar] [CrossRef]
- Cárdenas-Bailón, F.; Osorio-Revilla, G.; Gallardo-Velázquez, T. Microencapulation of insulin using a W/O/W double emulsion followed by complex coacervation to provide protection against gastrointestinal tract. J. Microencapsul. 2015, 32, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Hemar, Y.; Cheng, L.; Oliver, C.; Sanguansri, L.; Augustin, M. Encapsulation of Resveratrol using Water-in-oil-in-water Double Emulsion. Food Biophys. 2010, 5, 120–127. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepúlveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spay drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Rufino, M.S.; Alves, R.; Brito, E.; Morais, S.; Sampaio, C.; Pérez, J.; Saura, F. Metodología Científica: Determinação da Atividade Antioxidante Total em Frutas pela Captura do Radical Livre ABTS. Comunicado Técnico 2007, 128, 1–4. [Google Scholar]
- Castellanos, E.; Yahia, E. Identification and Quantification of Betalains from the Fruits of 10 Mexican Prickly Pear Cultivars by High-Performance Liquid Chromatography and Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2008, 56, 5758–5764. [Google Scholar] [CrossRef] [PubMed]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products, 2nd ed.; Mc Graw Hill Publishing Co.: New Dehli, India, 1986; pp. 107–108. ISBN 978-0-07-451851-9. [Google Scholar]
- Albano, C.; Negro, C.; Tommasi, N.; Gerardi, C.; Mita, G.; Miceli, A.; De Bellis, L.; Blando, F. Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes. Antioxidants 2015, 4, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Almela, L.; Obón, J.; Castellar, R. Determination of antioxidant constituents in cactus pear fruit. Plants Foods Hum. Nutr. 2010, 65, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Kuti, J. Antioxidant compounds from four Opuntia cactus pear fruits varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- Prakash, J.; Manikandan, S.; Mekala, V. Modeling and optimization of betalain extraction from Opuntia ficus-indica using Box-Behnken design with desirability function. Ind. Crops Prod. 2013, 49, 304–311. [Google Scholar] [CrossRef]
- Laqui-Vilca, C.; Aguilar-Tuesta, S.; Mamani-Navarro, W.; Montaño-Bustamante, J.; Condezo-Hoyos, L. Ultrasound-assisted optimal extraction and thermal stability of betalains from colored quinoa (Chenopodium quinoa Willd) hulls. Ind. Crops Prod. 2018, 111, 606–614. [Google Scholar] [CrossRef]
- Muschiolik, G. Multiple emulsions for food use. Curr. Opin. Colloid Interface Sci. 2007, 12, 213–220. [Google Scholar] [CrossRef]
- McClements, D.J. Emulsion design to improve the delivery of functional lipophilic components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.S.; Mazz, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
| Result | |
|---|---|
| Moisture content (%) | 84.60 ± 1.09 |
| Total soluble solids (°Brix) | 13 ± 0.00 |
| PC (mg GAE/gdw) | 11.47 ± 0.14 |
| BC (mg BE/gdw) | 0.89 ± 0.00 |
| BX (mg IX/gdw) | 0.42 ± 0.00 |
| AA (mg AA/gdw) | 1.07 ± 0.06 |
| AC (µmol TE/gdw) | 73.95 ± 2.44 |
| Part of the Fruit | PC (mg GAE/gdw) | BC (mg BE/gdw) | BX (mg IE/gdw) | AC (µmol TE/gdw) |
|---|---|---|---|---|
| Whole fruit | 13.90 ± 0.17 | 0.41 | 0.32 | 96.11 ± 2.61 |
| Peel | 9.60 ± 0.14 | 0.17 | 0.15 | 48.25 ± 1.68 |
| Pulp | 2.99 ± 0.04 | 0.27 | 0.23 | 32.48 ± 0.23 |
| Seeds | 0.17 | -- | -- | 1.34 ± 0.06 |
| Analysis of Variance | |||
|---|---|---|---|
| PC | BC | BX | |
| Source | p-Value | p-Value | p-Value |
| Model | 0.037 | 0.015 | 0.259 |
| Linear | 0.007 | 0.003 | 0.773 |
| Square | 0.330 | 0.125 | 0.287 |
| 2-Way Interaction | 0.425 | 0.155 | 0.106 |
| Lack-of-Fit | 0.834 | 0.446 | 0.180 |
| Model summary | R2: 90.84% R2 adj: 74.34% | R2: 93.84% R2 adj: 82.74% | R2: 76.87% R2 adj: 35.23% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo-Madrid, K.; Gallardo-Velázquez, T.; Osorio-Revilla, G. Microencapsulation of Purple Cactus Pear Fruit (Opuntia ficus indica) Extract by the Combined Method W/O/W Double Emulsion-Spray Drying and Conventional Spray Drying: A Comparative Study. Processes 2018, 6, 189. https://doi.org/10.3390/pr6100189
Toledo-Madrid K, Gallardo-Velázquez T, Osorio-Revilla G. Microencapsulation of Purple Cactus Pear Fruit (Opuntia ficus indica) Extract by the Combined Method W/O/W Double Emulsion-Spray Drying and Conventional Spray Drying: A Comparative Study. Processes. 2018; 6(10):189. https://doi.org/10.3390/pr6100189
Chicago/Turabian StyleToledo-Madrid, Keren, Tzayhrí Gallardo-Velázquez, and Guillermo Osorio-Revilla. 2018. "Microencapsulation of Purple Cactus Pear Fruit (Opuntia ficus indica) Extract by the Combined Method W/O/W Double Emulsion-Spray Drying and Conventional Spray Drying: A Comparative Study" Processes 6, no. 10: 189. https://doi.org/10.3390/pr6100189
APA StyleToledo-Madrid, K., Gallardo-Velázquez, T., & Osorio-Revilla, G. (2018). Microencapsulation of Purple Cactus Pear Fruit (Opuntia ficus indica) Extract by the Combined Method W/O/W Double Emulsion-Spray Drying and Conventional Spray Drying: A Comparative Study. Processes, 6(10), 189. https://doi.org/10.3390/pr6100189