A Process Study of Lactic Acid Production from Phragmites australis Straw by a Thermophilic Bacillus coagulans Strain under Non-Sterilized Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain, Culture Medium, and Fermentation
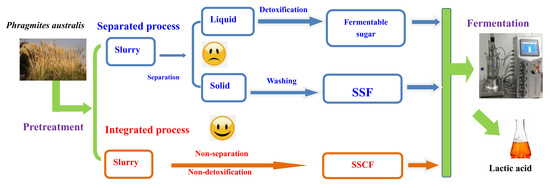
2.2. Pretreatment of Raw Materials and Dilute Acid Hydrolysates Fermentation
2.3. SSF Process
2.4. SSCF Process
2.5. Analytical Methods
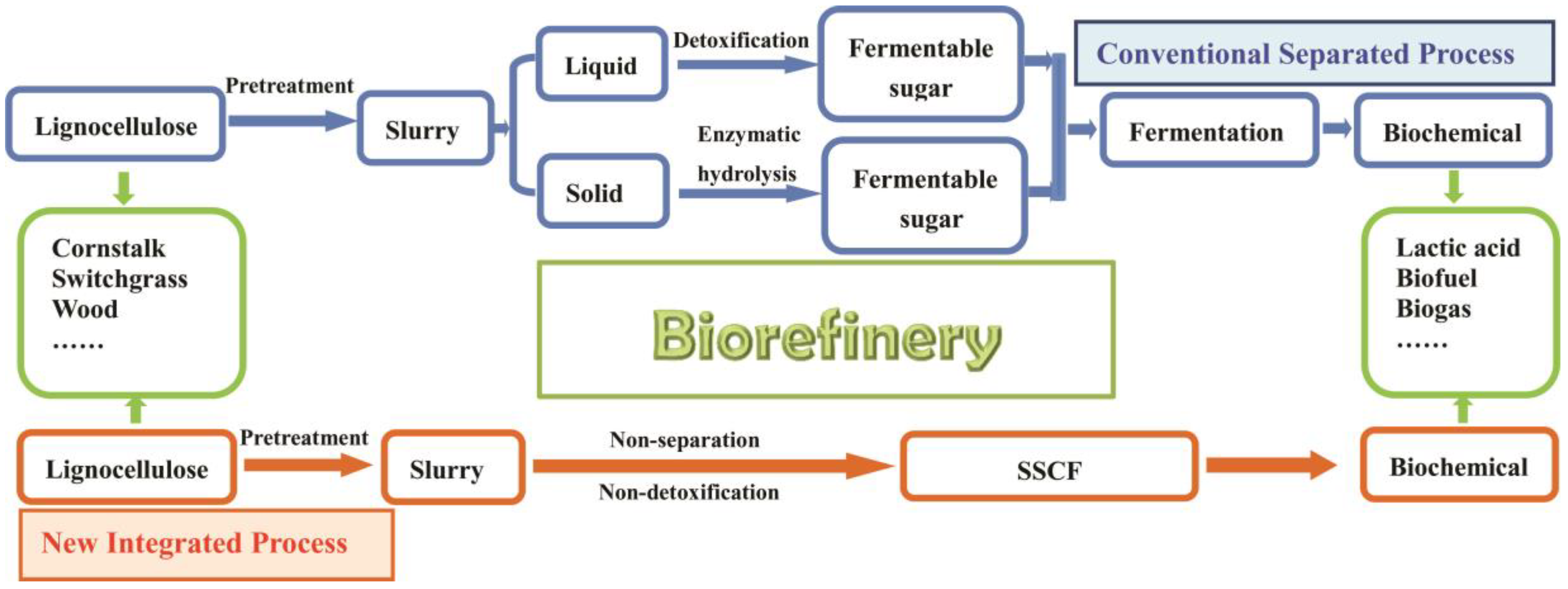
3. Results
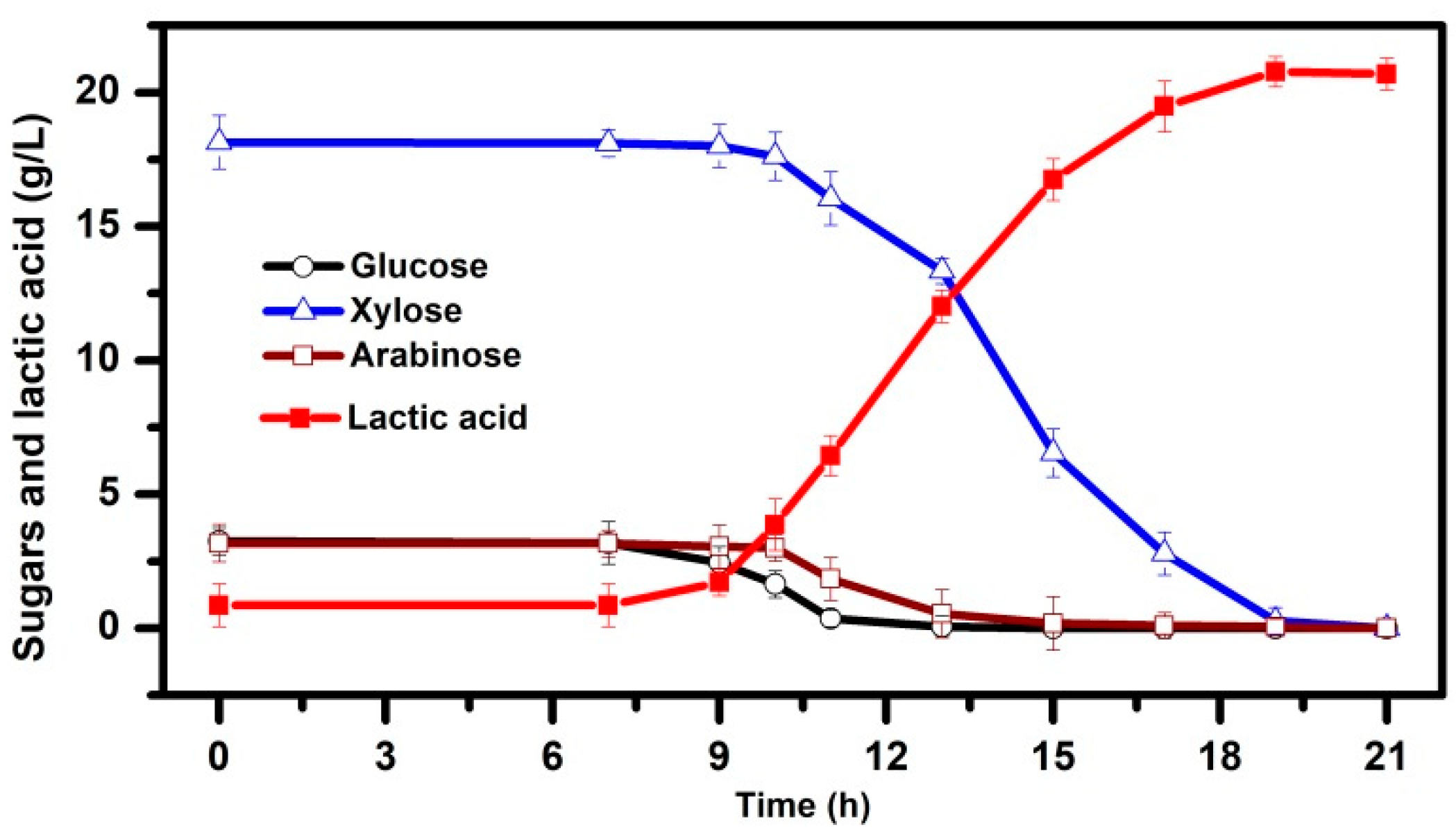
3.1. Pretreatment of PAS and Bioconversion of Sugars in Dilute acid Hydrolysates to LA
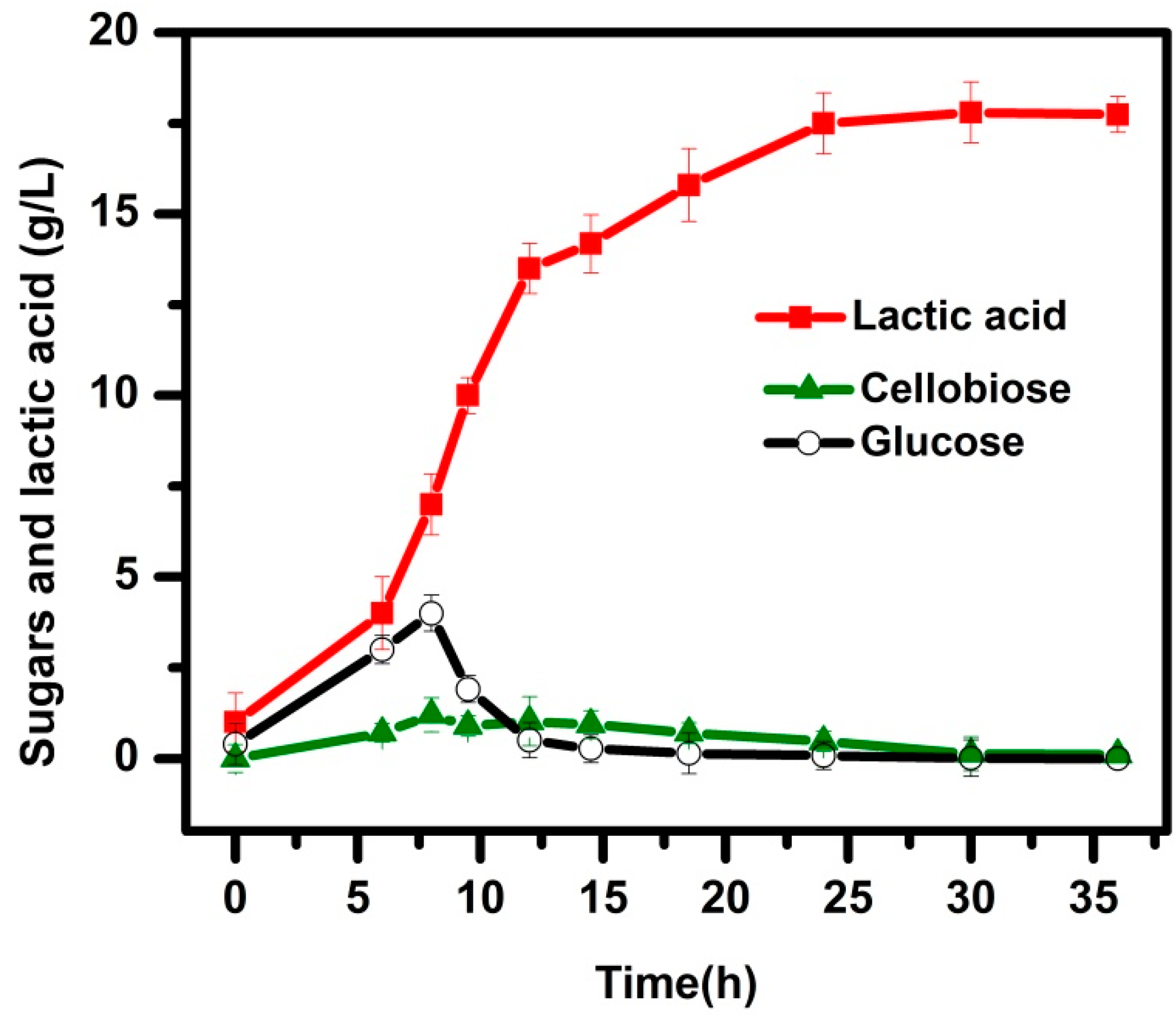
3.2. SSF Process
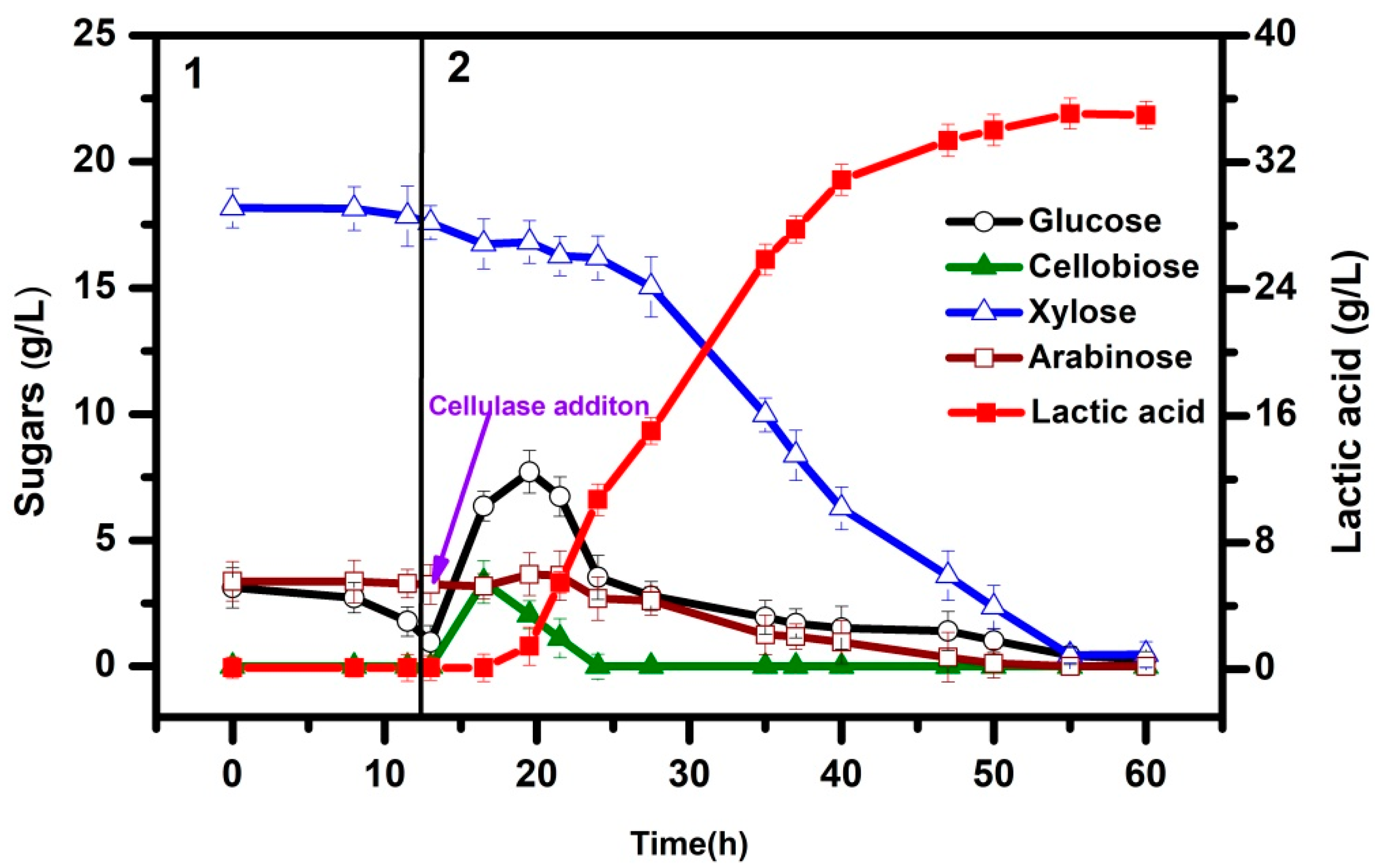
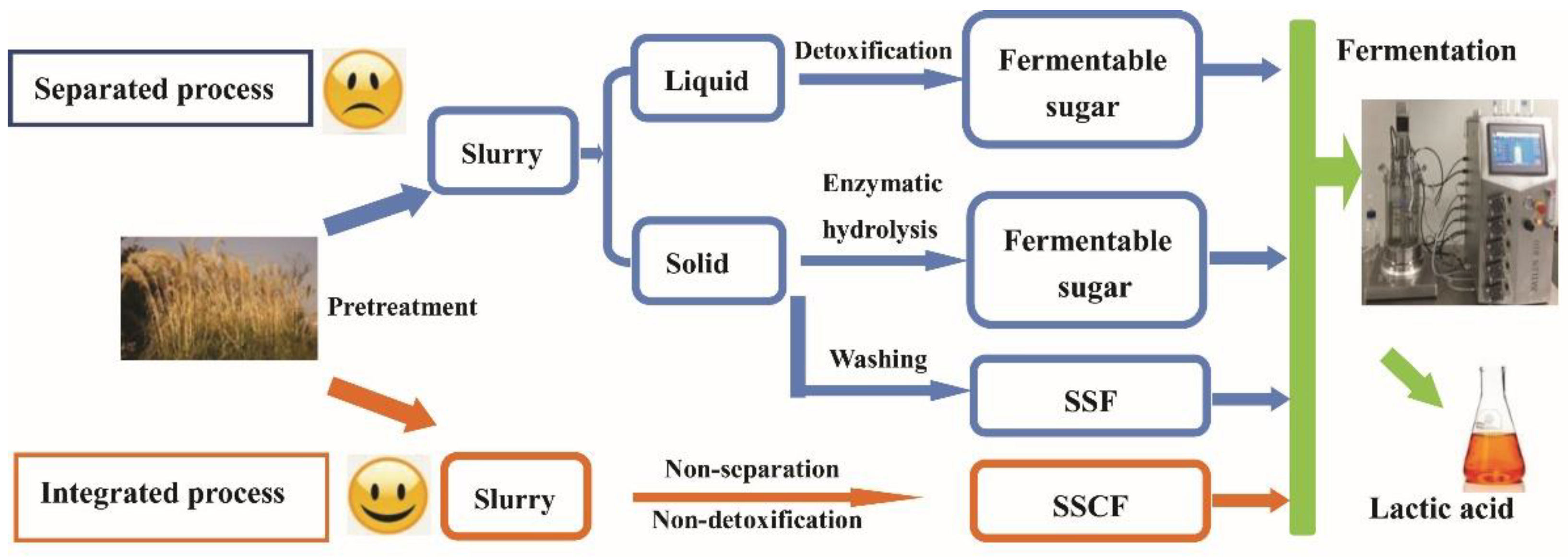
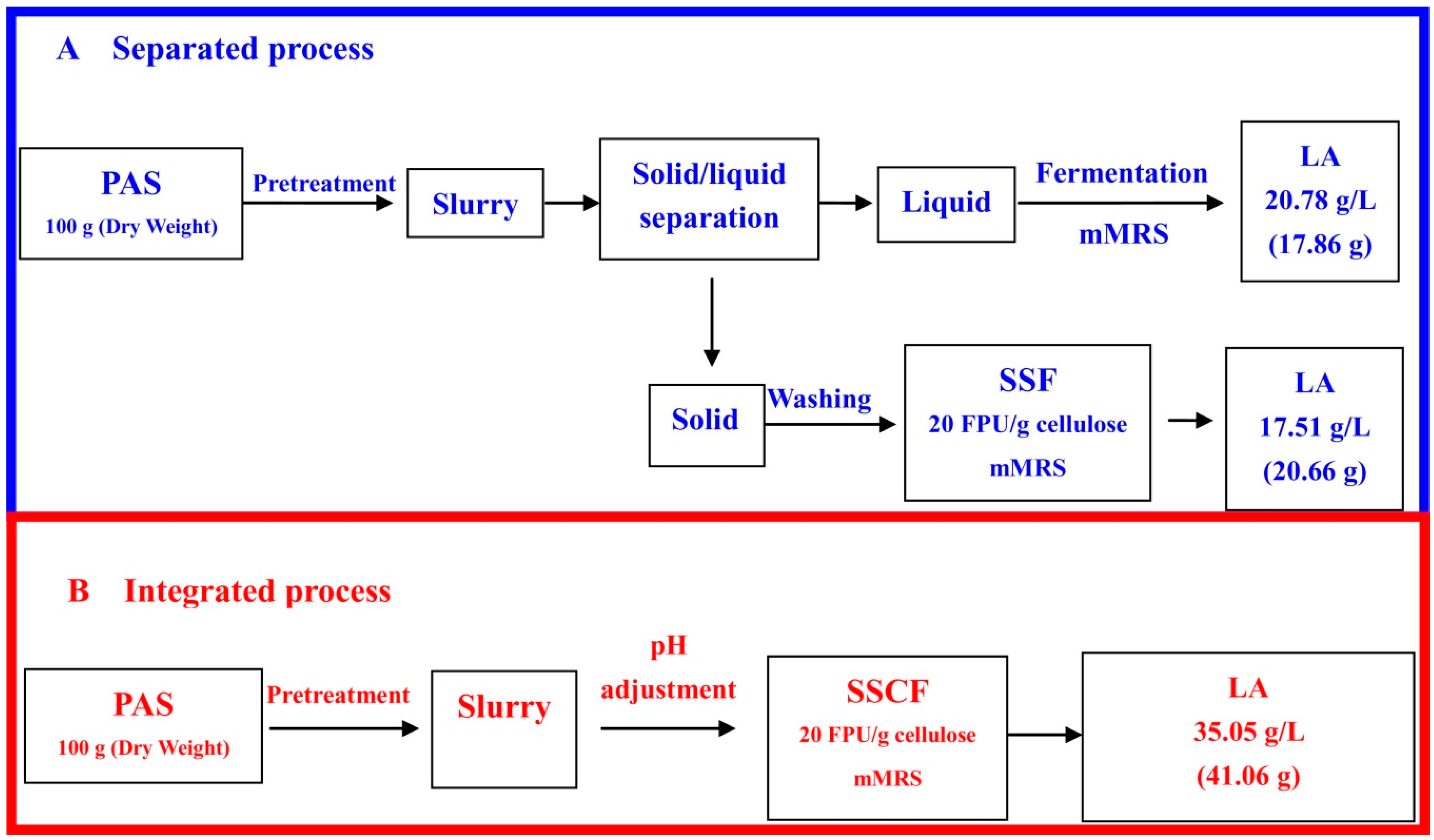
3.3. Integrated Process for LA Production via SSCF
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.X.; Yoshida, M.; Vadlani, P.V. Biosynthesis of d-lactic acid from lignocellulosic biomass. Biotechnol. Lett. 2018, 40, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Q.H.; Xu, Z.; Zhang, W.Y.; Xiang, J. Effect of Fermentation Conditions on L-Lactic Acid Production from Soybean Straw Hydrolysate. J. Microbiol. Biotechnol. 2015, 25, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Oonkhanond, B.; Jonglertjunya, W.; Srimarut, N.; Bunpachart, P.; Tantinukul, S.; Nasongkla, N.; Sakdaronnarong, C. Lactic acid production from sugarcane bagasse by an integrated system of lignocellulose fractionation, saccharification, fermentation, and ex-situ nanofiltration. J. Environ. Chem. Eng. 2017, 5, 2533–2541. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Maciel, R. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Boiano, S.; Marzocchella, A.; Rehmann, L. Cellulosic butanol production from alkali-pretreated switchgrass (Panicum virgatum) and phragmites (Phragmites australis). Bioresour. Technol. 2014, 174, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; Xin, F.X.; Chang, Y.K.; Zhao, Y.; Wong, W.C. Feasibility of reed for biobutanol production hydrolyzed by crude cellulase. Biomass Bioenerg. 2015, 76, 24–30. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Direct lactic acid fermentation: Focus on simultaneous saccharification and lactic acid production. Biotechnol. Adv. 2009, 27, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Moldes, A.B.; Torrado, A.; Converti, A.; Dominguez, J.M. Complete bioconversion of hemicellulosic sugars from agricultural residues into lactic acid by Lactobacillus pentosus. Appl. Biochem. Biotechnol. 2006, 135, 219–227. [Google Scholar] [CrossRef]
- Okano, K.; Tanaka, T.; Ogino, C.; Fukuda, H.; Kondo, A. Biotechnological production of enantiomeric pure lactic acid from renewable resources: Recent achievements, perspectives, and limits. Appl. Microbiol. Biotechnol. 2010, 85, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.; Khare, S.K. One-pot bioprocess for lactic acid production from lignocellulosic agrowastes by using ionic liquid stable Lactobacillus brevis. Bioresour. Technol. 2018, 251, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Block, D.E.; Mills, D.A. Simultaneous consumption of pentose and hexose sugars: An optimal microbial phenotype for efficient fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2010, 88, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Block, D.E.; Shoemaker, S.P.; Mills, D.A. Conversion of rice straw to bio-based chemicals: An integrated process using Lactobacillus brevis. Appl. Microbiol. Biotechnol. 2010, 86, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Cotana, F.; Cavalaglio, G.; Pisello, A.L.; Gelosia, M.; Ingles, D.; Pompili, E. Sustainable Ethanol Production from Common Reed (Phragmites australis) through Simultaneuos Saccharification and Fermentation. Sustainability 2015, 7, 12149–12163. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Chen, X.R.; Luo, J.Q.; Qi, B.K.; Wan, Y.H. An efficient process for lactic acid production from wheat straw by a newly isolated Bacillus coagulans strain IPE22. Bioresour. Technol. 2014, 158, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Xiao, Y.T.; Tashiro, Y.; Wang, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Fed-batch fermentation for enhanced lactic acid production from glucose/xylose mixture without carbon catabolite repression. J. Biosci. Bioeng. 2015, 119, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liu, D.; Ren, H.F.; Shi, X.C.; Zhao, N.; Chen, Y.; Ying, H.J. D-Lactic Acid Production by Sporolactobacillus inulinus Y2-8 Immobilized in Fibrous Bed Bioreactor Using Corn Flour Hydrolyzate. J. Microbiol. Biotechnol. 2014, 24, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Gao, M.; Wang, Q.; Wang, J.; Sun, X.; Chang, Q.; Tashiro, Y. Enhancement of l-lactic acid production via synergism in open co-fermentation of Sophora flavescens residues and food waste. Bioresour. Technol. 2017, 225, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.L.; Zhang, H.Y.; Chai, Y.; Wang, L.J.; Mi, X.Y.; Zhang, L.Y.; Ware, M.A. Biogas properties and enzymatic analysis during anaerobic fermentation of Phragmites australis straw and cow dung: Influence of nickel chloride supplement. Biodegradation 2017, 28, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Wohler-Geske, A.; Moschner, C.R.; Gellerich, A.; Militz, H.; Greef, J.M.; Hartung, E. Yield, fermentation kinetics and the role of quality properties of thatching reed (Phragmites australis) during discontinuous anaerobic fermentation. Ind. Crop. Prod. 2016, 83, 701–709. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Van der Pol, E.C.; Eggink, G.; Weusthuis, R.A. Production of L(+)-lactic acid from acid pretreated sugarcane bagasse using Bacillus coagulans DSM2314 in a simultaneous saccharification and fermentation strategy. Biotechnol. Biofuels 2016, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Chen, X.R.; Qi, B.K.; Luo, J.Q.; Shen, F.; Su, Y.; Khan, R.; Wan, Y.H. Improving lactic acid productivity from wheat straw hydrolysates by membrane integrated repeated batch fermentation under non-sterilized conditions. Bioresour. Technol. 2014, 163, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Cubas-Cano, E.; Gonzalez-Fernandez, C.; Ballesteros, M.; Tomas-Pejo, E. Biotechnological advances in lactic acid production by lactic acid bacteria: Lignocellulose as novel substrate. Biofuels Bioprod. Biorefin. 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Lau, M.W.; Dale, B.E. Cellulosic ethanol production from AFEX-treated corn stover using Saccharomyces cerevisiae 424A(LNH-ST). Proc. Natl. Acad. Sci. USA 2009, 106, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.S.; Awasthi, D.; Nieves, I.; Wang, L.; Erickson, J.; Vermerris, W.; Ingram, L.O.; Shanmugam, K.T. Sweet Sorghum Juice and Bagasse as Feedstocks for the Production of Optically Pure Lactic Acid by Native and Engineered Bacillus coagulans Strains. Bioenerg. Res. 2016, 9, 123–131. [Google Scholar] [CrossRef]
- Hu, J.L.; Lin, Y.X.; Zhang, Z.T.; Xiang, T.; Mei, Y.X.; Zhao, S.M.; Liang, Y.X.; Peng, N. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour. Technol. 2016, 214, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.Y.; Gao, Q.Q.; Bao, J. Constructing xylose-assimilating pathways in Pediococcus acidilactici for high titer D-lactic acid fermentation from corn stover feedstock. Bioresour. Technol. 2017, 245, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, M.; Nie, T.; Ni, Z. A Process Study of Lactic Acid Production from Phragmites australis Straw by a Thermophilic Bacillus coagulans Strain under Non-Sterilized Conditions. Processes 2018, 6, 175. https://doi.org/10.3390/pr6100175
Zhang Y, Li M, Nie T, Ni Z. A Process Study of Lactic Acid Production from Phragmites australis Straw by a Thermophilic Bacillus coagulans Strain under Non-Sterilized Conditions. Processes. 2018; 6(10):175. https://doi.org/10.3390/pr6100175
Chicago/Turabian StyleZhang, Yuming, Mengran Li, Tian Nie, and Zhihua Ni. 2018. "A Process Study of Lactic Acid Production from Phragmites australis Straw by a Thermophilic Bacillus coagulans Strain under Non-Sterilized Conditions" Processes 6, no. 10: 175. https://doi.org/10.3390/pr6100175
APA StyleZhang, Y., Li, M., Nie, T., & Ni, Z. (2018). A Process Study of Lactic Acid Production from Phragmites australis Straw by a Thermophilic Bacillus coagulans Strain under Non-Sterilized Conditions. Processes, 6(10), 175. https://doi.org/10.3390/pr6100175