Abstract
Recovering valuable compounds from waste streams of bio-based production processes is in line with the circular economy paradigm, and is achievable by implementing “simple-to-use” and well-established process separation technologies. Such solutions are acceptable from industrial, economic and environmental points of view, implying relatively easy future implementation on pilot- and full-scale levels in the bio-based industry. Reviewing such technologies is therefore the focus here. Considerations about technology readiness level (TRL) and Net Present Value (NPV) are included in the review, since TRL and NPV contribute significantly to the techno-economic evaluation of future and promising process solutions. Based on the present review, a qualitative guideline for resource recovery from bio-based production processes is proposed. Finally, future approaches and perspectives toward identification and implementation of suitable resource recovery units for bio-based production processes are discussed.
1. Introduction
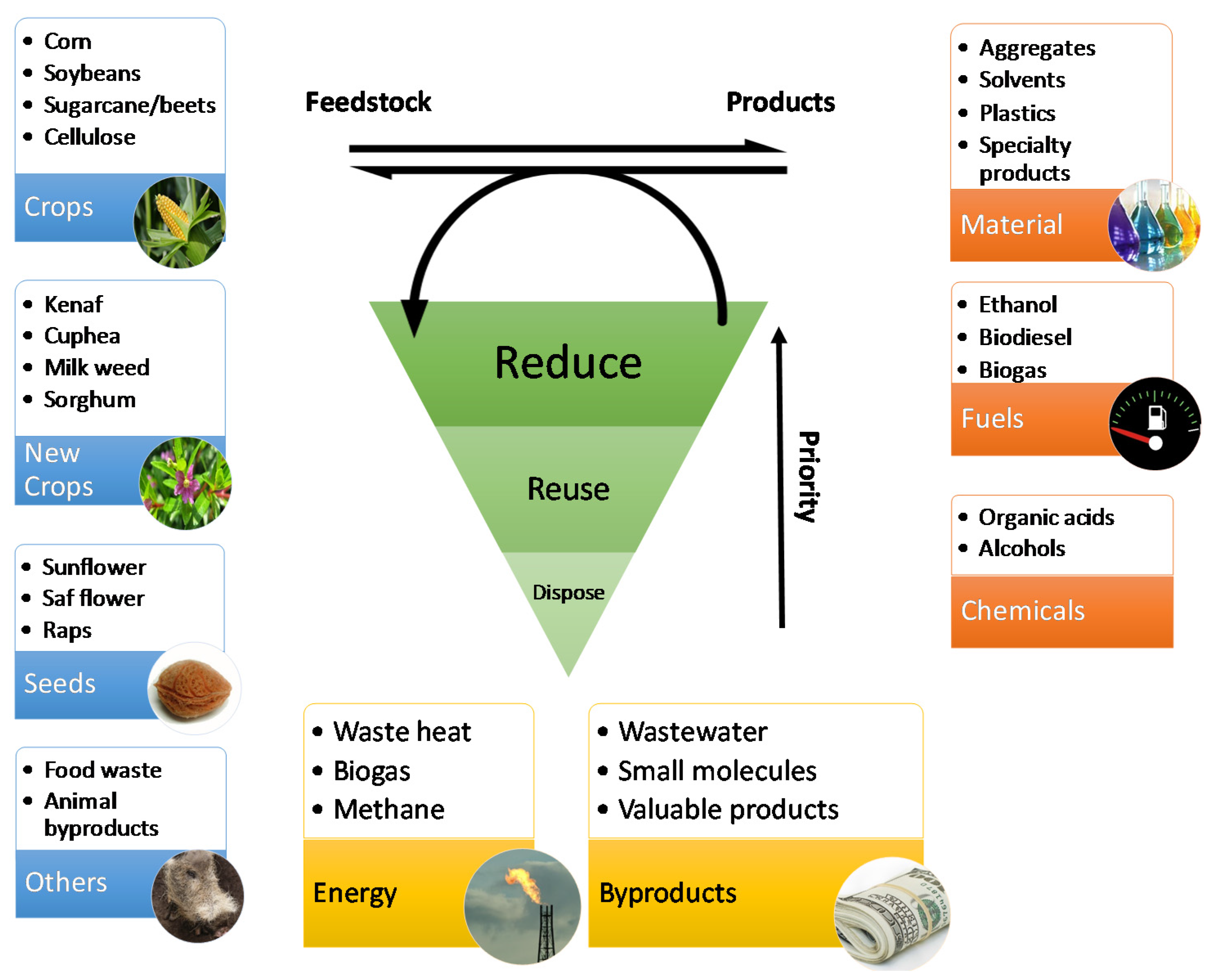
The efficient identification, design and development of appropriate processes are important priorities for the industry to remain competitive in a global and rapidly evolving market place [1,2,3]. Potential reductions in greenhouse gas emissions and decreased dependence on fossil resources are fueling interest in the development of bio-based products and consequently their production processes [4]. We define bio-based production processes as “processes that convert a set of bio-based renewable raw materials through chemical reactions and/or microbial fermentation into products” and have also elaborated on its necessity elsewhere [5,6]. As depicted in Figure 1, a large array of products can be obtained through bio-based production processes. These products range from commodity chemicals to specialty products [7].
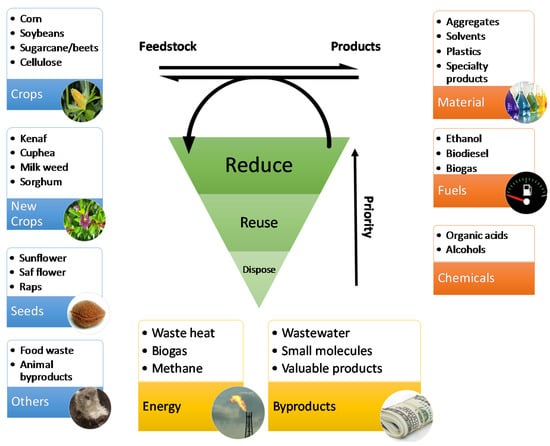
Figure 1.
An overview of feedstock, typical products and waste generated from bio-based production processes. Waste reduction has priority over reuse/resource recovery, and disposal is the least preferable option.
However, a considerable amount of waste is also generated from bio-based production processes. Therefore, the search for productivity improvements in bioreactors is a major driver in the modern biotech-based industry [8]. Furthermore, striving towards realization of a zero waste policy in downstream processing plants is attractive [9], and the circular economy concept has therefore gained increased popularity in recent years, with the main goal to capture remaining valuable compounds from waste streams as well as process streams that no longer contain the main product of interest that is produced in a production process [10]. Hydraulic and pollutant loads to industrial wastewater treatment plants are to be reduced in this way, leading to more sustainable and economic processes.
It must be noted that when many of the existing bio-based processes were implemented, energy costs were low, water scarcity was less of a concern, climate change was not a consideration and there was no demand for reuse of potentially valuable compounds found in wastewater. Therefore, the goal, traditionally, has been to prevent environmental harm and protect public health. To this end, wastewater is transported to wastewater treatment facilities where potentially harmful and polluting agents are being removed. The remaining effluent and residual materials are discharged into local water bodies. However, nowadays it is of utmost importance to recover economically lucrative resources from wastewater streams to obtain (1) clean water and (2) value-added resources from wastewater to face resource scarcity, since there is indeed much value to be recovered. This need is further strengthened by introduction of tighter environmental regulations which require certain harmful agents to be reduced to acceptable levels. While wastewater does contain salts and pathogens that must be removed, it consists of 99.9 percent water, and furthermore contains valuable organics recoverable as energy, for example via anaerobic digestion processes, and nutrients, such as nitrogen and phosphorus recoverable as fertilizer. The main challenge for resource recovery is to develop cost-effective means for recovery and purification of these valuable commodities as well as to achieve a sufficiently high technology readiness for candidate techniques associated with recovering the valuable compounds from a waste stream. From an industrial perspective, a successful application of resource recovery from waste streams improves the economic performance of a process at little to no added operating costs. There are various types of wastewater, largely originating from fermentation processes and downstream operations, with different availability of resources. Furthermore, depending on the availability and condition of the wastewater, various separation and recovery approaches are employed to recover a set of target resources.
The remainder of this manuscript is organized as follows. In Section 2 the lucrative economics behind recovering resources available in bio-based production processes are highlighted, while in Section 3 key platform technologies are identified that can be applied to recover these resources. To this end, this work will focus on identifying promising separation technologies with the purpose of achieving resource recovery from bio-based production processes, as many economically lucrative raw materials are already present in these waste streams and can be recovered without the need for other conversion processes. In situations where raw materials need to be converted into a more economically lucrative form, other processes such as bio-conversion [11,12] or hydrothermal processing [13,14,15] can be applied. After establishing the economics of resource recovery and identifying the platform technologies, the techno-economic considerations that need to be considered when developing a resource recovery platform technology from a concept to an implementation are discussed in Section 4. In Section 5, the role of process systems engineering in facilitating this transition from concept to implementation is discussed. In the subsequent section the strengths, weaknesses as well as opportunities and threats concerning the implementation of resource recovery projects are highlighted and discussed. Finally, future perspectives to direct efforts towards implementation of resource recovery units are given followed by conclusions.
2. Role of Economics in Resource Recovery
The initial decision to explore the possibility of transforming a wastewater treatment process of an industrial bio-based production site into a resource recovery unit will be based on an economic incentive. This incentive might be due to market demand for a valuable resource found in the waste stream or due to economic incentives of recovery and recycling resources. Reuse of process water is a good example, and can be attractive due to tough environmental regulations in place or due to water scarcity. Either way, identifying the demand and the subsequent economic incentive of recovering valuable resources from bio-based waste streams will be a vital initial step. Table 1 indicates a variety of valuable resources, not all, to be potentially recovered from industrial bio-based waste streams, and that could subsequently be sold again as raw materials assuming that they can be isolated from the waste streams in a sufficiently high purity.

Table 1.
Potentially valuable products present in wastewater streams and waste from fermentation processes, with their present status and potential future application, with estimated product prices, market size and market value (The values are based on a white paper published by Deloitte [16]).
Table 1 indeed shows a large variety of resources that can be potentially available in a waste stream of a bio-based production process. These resources also have varying market demand as well as financial value. In general, the size of the market for many resources found in bio-based production waste streams is large, such as the organic acid market worth in excess of 3 billion USD. As such, from a scalability point of view, if resource recovery from bio-based production processes becomes an industry standard in the future there is also a need for sufficient market size to absorb the products of these resource recovery units. A closer examination of these resources and their commercial values also shows us that in comparison to other industries and their waste streams, the relative commercial value of resources in bio-based waste streams such as vitamins, organic acids, antibiotics and industrial acids is potentially much higher. Thus, from a purely economic point of view the concept of resource recovery for bio-based production waste streams would be a more attractive idea. If there are available technologies to recover these resources at reasonable costs, resource recovery units have a good opportunity to be commercialized in the area of bio-based production processes. In the dairy industry this is already the case for the whey protein where membrane separation is performed as an initial step to produce highly valuable whey protein powder [17].
In capturing the total benefits of resource recovery in bio-based wastewater streams it is also important to look at the water as a potential resource. Water makes up the majority of many waste streams and in many industrial wastewater treatment facilities will be treated adequately to be discharged into nature. However, if a resource recovery unit instead upgrades part of this wastewater to potable water quality, this can create a significant revenue stream. More specifically, in regions where the value of potable water is high, e.g., due to water scarcity, it can potentially lead to reduced water consumption, and as additional benefit also reduced investment in “end of pipe” wastewater treatment due to reduced flow rates of water to be discharged. As such, identifying the revenue that production of potable quality water can bring to a resource recovery project should also be considered when designing these resource recovery units.
3. Resource Recovery Technologies
3.1. Membrane-Based Processes
Membrane-based processes are receiving significant attention in the modern biotech industry. Their high suitability for the recovery of solvents, energy and materials from waste streams leads to economic benefits and additionally reduced environmental impacts. Such an approach is becoming a major driver in modern downstream processing plants [18]. Separation processes based on membrane technology can be divided in five different groups according to the pore size. Table 2 illustrates different types of membranes and additionally implies on the selection of molecules that could be separated by applying specific membrane types [19,20].

Table 2.
Types of membranes applicable for resource recovery in the biotechnological industry (Adapted from [21] with permission from The Royal Society of Chemistry).
Applications of membrane-based operations are already widely spread in the bio-based production processes with focus on purifying a desired group of products. Modifying such an approach into the recovery of potentially valuable products from waste streams, and then to bring these products to the global market is slowly becoming a driving force in the modern manufacturing of food, alcoholic drinks, sodas, dairy products, enzymes, microorganisms, proteins, sugars, and so on.
Recovery of water from the wastewater streams is the most suitable illustration of the resource recovery concept. Applications of desalination processes are already well-known for producing bottled water for example [22], as well as for producing process water for breweries [23]. A steady water quality can be achieved, implying lower fluctuations of the quality of the products resulting from the fermentation processes. The dairy industry is an additional example where a resource recovery strategy can achieve plenty of benefits by using membrane-based separation processes. Removal of whey in producing cheese is a very well-known case where a valuable by-product could be successfully separated and concentrated afterwards by using membranes. Such a by-product is a great source for whey proteins for example [24,25]. Production of beverages on the other hand includes plenty of membrane-based processes for clarification of beers, wine and juices in order to remove suspended solids, yeast and thermally resistant biological species. Furthermore, up-concentration of juices can also be done by applying membranes [26].
The biochemical industry has additionally a significant demand for the application of membrane based processes [27]. Applications of microfiltration in enzymatic processes are receiving more attention. More precisely, membranes can reject enzymes allowing only reaction products to pass through [28]. In such cases, productivities of bioreactors are significantly improved, yielding a better exploitation of available resources. Membrane based technology has therefore a strong potential to maximize efficiency of the resource recovery concept in bio-based production processes. Such unit operations could easily replace some of the commonly used operations such as distillation and thus avoid decomposition of temperature sensitive desired side products to be recovered. A good example is the avoidance of evaporation and distillation in order to preserve stability of complex sugars and flavor components in the production of beverages [29]. New and hybrid combinations are additionally becoming interesting, such as a combo of micro-scale technologies and membrane processes for separating immiscible liquids with similar boiling points and densities [30]. In such a case, centrifugation could be avoided and a maximized exploitation of the transport phenomena could be achieved enabling increased efficiency of the new hybrid operations.
3.2. Precipitation
Minerals precipitation heavily interacts with other physico-chemical reactions [31] and influences water composition dynamically by sequestering solubles [32]. In addition, minerals precipitation causes inorganic scale formation which is a well-known maintenance problem [33] and can negatively impact biological processes occurring in water treatment streams, such as in anaerobic sludge blanket reactors treating pulp and paper wastewater [34,35]. On the other hand, precipitation can also have positive impacts in wastewater treatment, for example sulfate and sulfide precipitation which limits the occurrence of the toxic dissolved sulfide in anaerobic digesters [36,37], or with nutrient recovery as mineral fertilizers [38].
The latter has received substantial interest during the past years since it is a good alternative for: (1) traditional phosphorus removal techniques; and (2) potential recovery of P from aqueous waste streams. One of the most widely studied phosphorus recovery technologies is struvite crystallization. Crystallization of struvite (MgNH4PO4·6H2O) is a simple method for simultaneous precipitation of ammonium and orthophosphate from water streams. The other alternative is calcium phosphate (CaPs), for example in the form of dicalcium phosphate dihydrate (CaHPO4·2H2O) and octacalcium phosphate (Ca8H2(PO4)6·5H2O) or amorphous calcium phosphate (Ca3(PO4)2·nH2O). These compounds are precursors of the most stable compound, hydroxylapatite (Ca5(PO4)3OH). In water streams with enough P, the formation of these compounds can occur upon the addition of (slightly) soluble salts of Ca and Mg. In both cases, reagent solubility, pH, retention time, temperature, crystal size and impurities might play an important role [39,40]. The economic feasibility of these compounds is strongly dependent of the Ca and Mg input cost. Therefore, there is substantial ongoing research dealing with efficient ways of cation usage covering electro-chemical systems [41], improved reactor design and modelling strategies [42,43].
3.3. Extraction
Extraction is one way to separate a desired substance when it is mixed with and/or dissolved into others. The mixture is brought into contact with a solvent in which the substance of interest is soluble, but the other substances present are insoluble. Therefore, extraction is often a term used when two immiscible phases are used to separate a solute into one phase. Many of the resources that are found in the waste streams of bio-based production processes are often found in aqueous form. That is, they are mixed or dissolved in water. The extraction processes that can be applied to separate resources from waste streams can be classified into: ion exchange, solvent extraction and adsorption techniques.
3.3.1. Ion Exchange
Ion exchange is a technique that mainly involves a chemical reaction that takes place between an electrolyte in the solution and an insoluble electrolyte that is in contact with this solution. This technique is mainly used as an alternative to physical separation techniques such as precipitation. There is a wide range of reported applications for recovering metal compounds from various solutions, including the removal of mercury, cadmium, calcium, copper, nickel and zinc among others [44]. However, ion exchange usually requires a high capital cost for equipment as well as high operational cost due to the use of chemicals for resin regeneration. Thus, it is not a very popular method for remediation of metal compounds in very low quantities from wastewaters [45]. Therefore, identification and development of resins towards reduction of operating costs of these processes is necessary to make ion exchange an attractive unit operation to be used in the frame of resource recovery projects. For example, there have been some recent developments on nitrogen recovery from wastewater stream using a new method of ion-exchange resin ammonium recovery combined with membrane [46].
3.3.2. Adsorption
Adsorption is a separation process where material (adsorbate) is concentrated from a bulk vapor or liquid phase on to the surface of a porous solid (adsorbent). Usually the amount adsorbed is only a fraction of a monolayer. Thus, to adsorb a substantial amount of material, the adsorbent must have a large specific surface area. There are several advantages associated with this separation technique such as usually low cost of adsorbent, easy availability and industrial implementation, low operating cost and easy operation [21,47]. These advantages make it a popular separation technique. The activated carbon is an adsorbent which is widely used for separation of metals and inorganic compounds (copper, zinc, chromium, cyanides, mercury, etc.) from wastewater streams [48,49,50], but also for isolation of organic compounds [51]. This wide interest in application of activated carbon is mainly due to its large specific surface area, high adsorption capacity and special surface chemical properties [52]. Apart from activated carbon as absorbent, there are other adsorbents such as synthetic and/or naturally occurring metal oxides (e.g., iron oxides/hydroxides/oxyhydroxides [53], aluminum oxides/hydroxides [54]) and biosorbents (e.g., micro-organism based biosorbents [55], agricultural based biosorbents [56], and agro-industrial waste materials [57]). Specifically, for resource recovery, there is a need to develop low-cost adsorbents specifically aimed at recovering resources available in waste streams in low quantities.
3.3.3. Solvent Extraction
Solvent extraction is a well-established and powerful technique to recover and separate both metal ions and organic compounds from aqueous solutions [58]. The governing principle in this technique is that when a solution containing a solute of interest is in contact with a solvent, the solute is distributed between two phases. Liquid-liquid extraction from aqueous solutions is a mature area of research for recovering both metal ions [59] and organic compounds [60]. However, the disadvantage remaining with this technique is that usually large amounts of solvent are required, and the extracted phase (solvent and solute) must usually be refreshed in a stripping step. The stripping costs are usually very high, which is a clear disadvantage. It is worth noting that this disadvantage is not universal, but depends to a large extent on the choice of the solvent. Therefore, the choice of solvent is a first and most important consideration for an extraction process. Two desirable characteristics for a solvent are a high capacity for the solute and a high selectivity for the solute over water [61]. These characteristics, combined with the amount and value of the resource to be recovered, determine the economic viability of a solvent extraction process.
3.4. Distillation
Distillation as a concept has been in use for almost 5000 years [62]. Today, traditional (thermal) distillation is used to carry out 90–95% of all industrial separation processes [63,64] while using almost 40% of the energy in chemical and refining processes [65]. Classical thermal distillation works by boiling a mixture consisting of multiple components into a two phase region (both vapor and liquid), where components with higher volatility (low boiling point) will preferentially concentrate in the vapor phase [63]. The relationship between vapor and liquid concentration is described by vapor-liquid equilibrium (VLE) data. In many industrial scale distillation units (for example methanol recovery [66]), this separation process happens in trays or packing material where vapor flowing upwards in a column comes into contact with liquid flowing down, where mass transfer between the streams leads to a VLE. This is then repeated throughout the column which results in higher volatility components concentrating at the top of the column, while lower volatility components concentrate at the bottom [63,67,68]. In terms of operations, distillation units can be operated as continuous, batch or semi batch processes while different types of trays and packing are also available to promote better mass transfer.
While all distillation columns use this basic principle of thermal separation, there are situations where special precautions or changes are required to achieve the desired separation. In situations where the components of a mixture are sensitive to elevated pressures or have ultra-high boiling points, vacuum distillation can be used to lower the boiling point of mixtures [69,70]. Similarly, in situations where a mixture forms an azeotrope that makes it impossible to distill conventionally, it is necessary to take remedial action [70]. In these situations, two columns that are set up at low pressure and high pressure respectively can be used to shift the azeotrope around to achieve separation beyond azeotrope concentrations. Alternatively, an entrainer can be introduced to influence the volatility of one component in the mixture so an azeotrope no longer forms [71].
Distillation has been commonly used in bio-based production processes, including the refining of biofuels and their derivatives [72,73,74,75,76,77]. In the area of bio-oil production, a distillation has been used in many studies. In particular, vacuum distillation seems to be a popular choice in upgrading the bio-oil/bio-oil residue to transport fuel [73,78,79], while other work has looked at making a range of products from bio-oils [80]. In these examples vacuum conditions are used to lower the boiling point of bio-oil products, while in Silvestre et al. [75] vacuum distillation was used to carry out a separation on a heat sensitive compound. Reactive distillation is another form of distillation that is employed in the separation of bio-based production processes [73,74,81], where reaction and distillation steps are combined in one column.
An analysis of the available literature on distillation applications in the bio-based production industry shows that it is applied in selected areas. However, in comparison to the widespread use of classical distillation in the chemicals and energy sector, bio-based production processes in fact lack the widespread use of classical distillation. This is due to the following factors:
- (1)
- Some of the resources that need to be recovered from bio-based waste streams contain compounds that cannot be boiled at reasonable temperature and pressure (e.g., phosphate). Hence, from a practical point of view, classical distillation is not an applicable separation technology for this class of components.
- (2)
- Some of the biological resources to be recovered from bio-based production streams can be heat sensitive and would degrade at higher temperature levels. In principle vacuum distillation can be used to recover these compounds; however, from an economic and practical point of view the level of vacuum (reduced pressure) might be high, leading to a high operating cost, and as such an alternative separation might be preferred.
- (3)
- In many bio-based separation units the resources to be recovered are dissolved in aqueous streams and are typically present at relatively low concentrations. As such, even if the resource can be separated using distillation, it might not be economically viable. This is because the aqueous stream (mainly water) needs to be brought into a two-phase region (boil) to recover a relatively small quantity of resources. Since water has a large heat capacity and heat of vaporization, this will result in significant energy expenditure. Hence rather than employing classical distillation, it might be more cost effective to use other methods such as membrane separation or precipitation to recover these resources from an aqueous waste stream.
Despite these difficulties, it is important to note that distillation is a well-established reliable technology with a large amount of research work that has been done in the past, and a wide variety of applications, while operators and engineers are very familiar with the principles and modes of distillation operations. Therefore, from a technical point of view, in any instance where distillation does a satisfactory job at separating a resource from a waste stream it will be preferred over other separation technologies, unless there is an overwhelming economic reason to switch to a different technology.
3.5. Hybrid and Intensified Processes
Development in the chemical process industry is directed toward the low energy/high throughput separation technologies. Therefore, one way to tackle this objective is by using process intensification and integration (PII) options where the overall cost is reduced and the sustainability of industrial processes is improved. As defined by Stankiewicz [82], an intensified process is “any chemical engineering development that leads to a substantially smaller, cleaner, and more energy efficient technology”. A more elaborate definition of PI is given by Lutze et al. [83] as “a process development/design option which focuses on improvements of a whole process by adding/enhancing of phenomena through integration of unit operations, integration of functions, and integration of phenomena and/or targeted enhancement of a phenomenon within an operation”. There is an increasing interest in application of intensified and multi-functional processes in the chemical industry [84]. Several applications of process intensification principles are realized so far on an industrial scale including reactive distillation [85] (with already over 150 industrial applications, it is one of the most successful intensified processes on an industrial scale [71]), micro-reactors [86], rotating packed bed reactors [87], etc.
PII can be beneficial to enhance the performance of currently available separation techniques/processes, which is essential toward developing sustainable bio-based production processes in industry. However, there are many ways and options to achieve PII such that a feasible and optimal solution could be found. A disadvantage is that intensified processes possess specific and/or unique properties that may result in a more difficult or complex operation in the presence of disturbances. This is mainly due to the loss in degrees of freedom because of integration of unit operations, functions or phenomena [88,89,90,91].
4. Technology Selection, Readiness and Economics
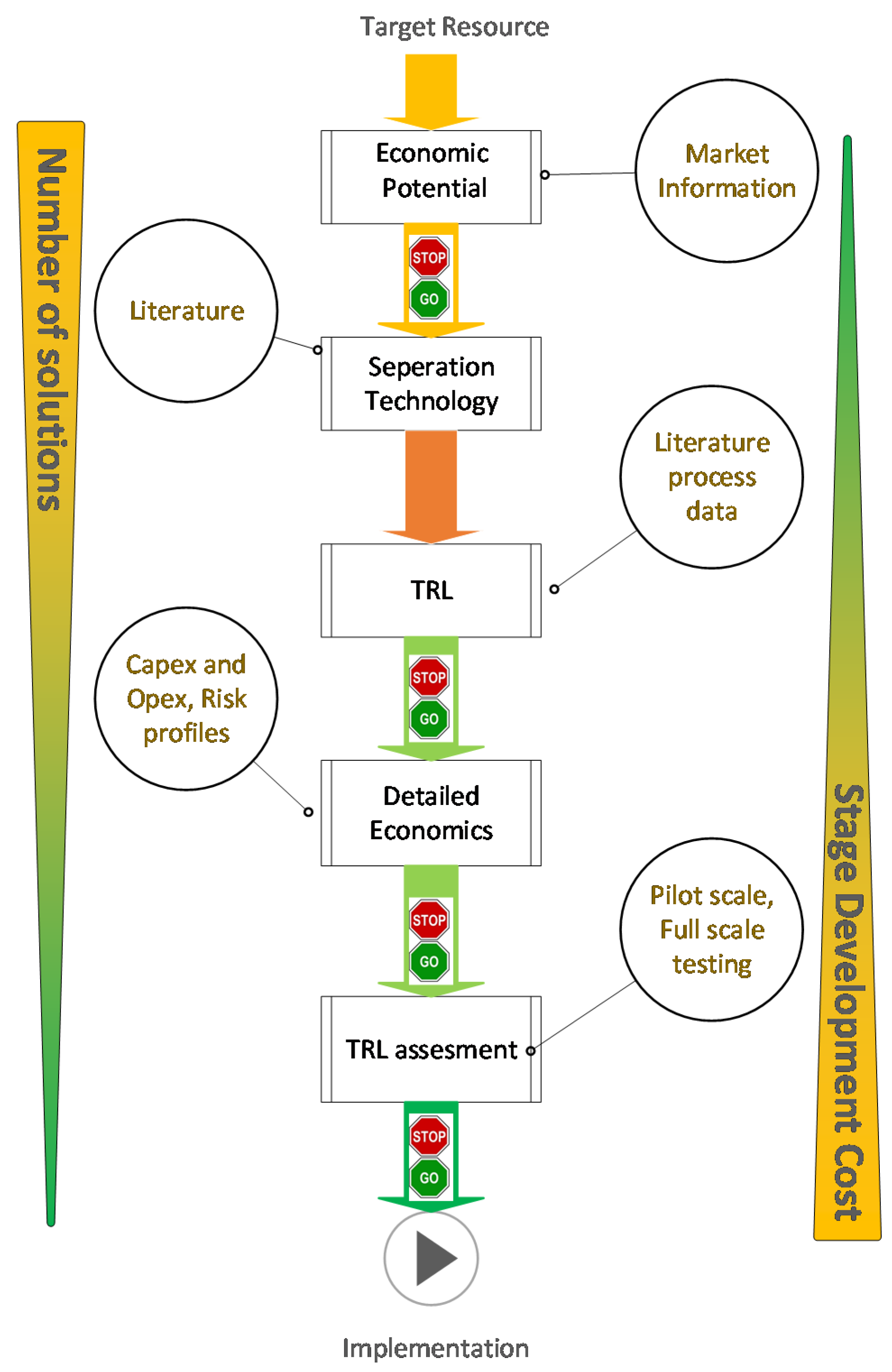
A concise review of the available literature into the recovery of resources from bio-based waste streams illustrates that there are multiple types of separation technologies that have been proposed. However, the scale, maturity and applicability of these technologies differ [17,41,92]. The development of a separation technology to be implemented for recovering a target resource from a bio-based production waste stream is based on both the technical as well as the economic aspect of the proposal. If the economic incentive to pursue recovering a resource is not present in current or future economic conditions, the research would only add to the scientific knowledge. Similarly, having the economic incentive to pursue the recovery of a target resource from a waste stream will only have economic value on paper if a suitable separation technology cannot be developed. There are established and industrially accepted concepts such as technology readiness level (TRL) to assess the technical aspects of a project [93] while concepts like net present value (NPV) analysis can be used to assess the economic aspects of a project [94]. However, from a practical point of view, the concurrent assessment of both technical and economic aspects would allow the efficient screening of multiple solutions, which will result in the best technologies being further developed and industrially implemented. The idea of techno-economic evaluation is not a new concept in bio-based manufacturing. For example, in [94] a techno-economic approach has been adapted to carry out an analysis in bio-diesel production taking into account the market fluctuations. Others have also used techno-economics for evaluation purposes [95,96]. However, most of this techno-economic analysis has been focused on assessing the economic variability and uncertainty of prices while not considering the economic implications to production due to failure of novel technology that might be developed. This is an important aspect for any resource recovery project in the area of bio-based production as this concept is relatively new and potential resource recovery projects will require some initial development work to be carried out. As such, it is important to concurrently asses both the techno-economics of a project taking into account the role of TRL. In Figure 2 a general guideline is suggested identifying key technical and economic evaluations that should be carried out during the development of a bio-based resource recovery project. The objective of this guideline is to systematically narrow down the number of candidate technologies that potentially can become the best separation technology for a given resource recovery project in a process. As such, the aspects are presented loosely in the order in which they should be assessed, while the development of a successful project will usually result from an iterative approach.
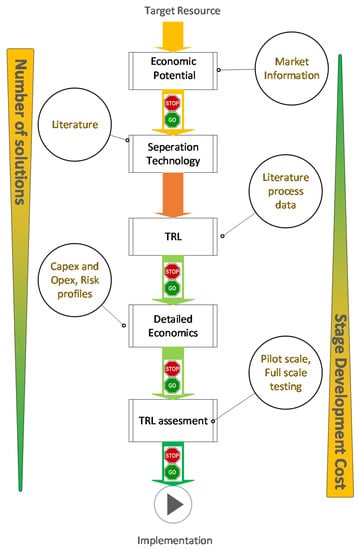
Figure 2.
“Plug ‘n’ Play” guideline for identification and development of resource recovery projects from bio-based production processes. Note: the number of solutions and the development costs per each stage decreases/increase dramatically when moving from target resource identification to implementation.
- Initial economic potential: is one of the key aspects that need to be assessed at an early stage of a resource recovery project. Assessment of this aspect would require the identification of a target resource as well as raw materials that might be required to carry out the resource recovery project. The economic potential aspect should also include any reduction in costs that are achieved when transforming a potential pollutant into a resource (e.g., reduction in waste treatment cost). It is also important to investigate if the resources available in waste streams are being recovered in a valuable form (i.e., burning an ethanol rich waste stream to produce bio energy instead of recovering the ethanol). The general approach that can be used in this step could be simple cash flow analysis or a discounted cash flow analysis as this provides sufficient information to evaluate the stop go criteria.
- Separation technology search: is another key aspect that needs to be carried out in the early stage of a resource recovery project. This process begins with a literature review to identify potential separation technologies that can be employed to recover the target resource. It is likely that there exists more than one established recovery pathway/technology, and all of these technologies can be further evaluated. In situations where the target resource has not been recovered previously, a more fundamental approach needs to be taken. If a similar resource has been recovered as part of previous research, the applicability of the technology should be investigated. This assessment would allow an individual to get a thorough understanding of all the technologies that are available for a given resource recovery project, which allows for an informed decision to be made.
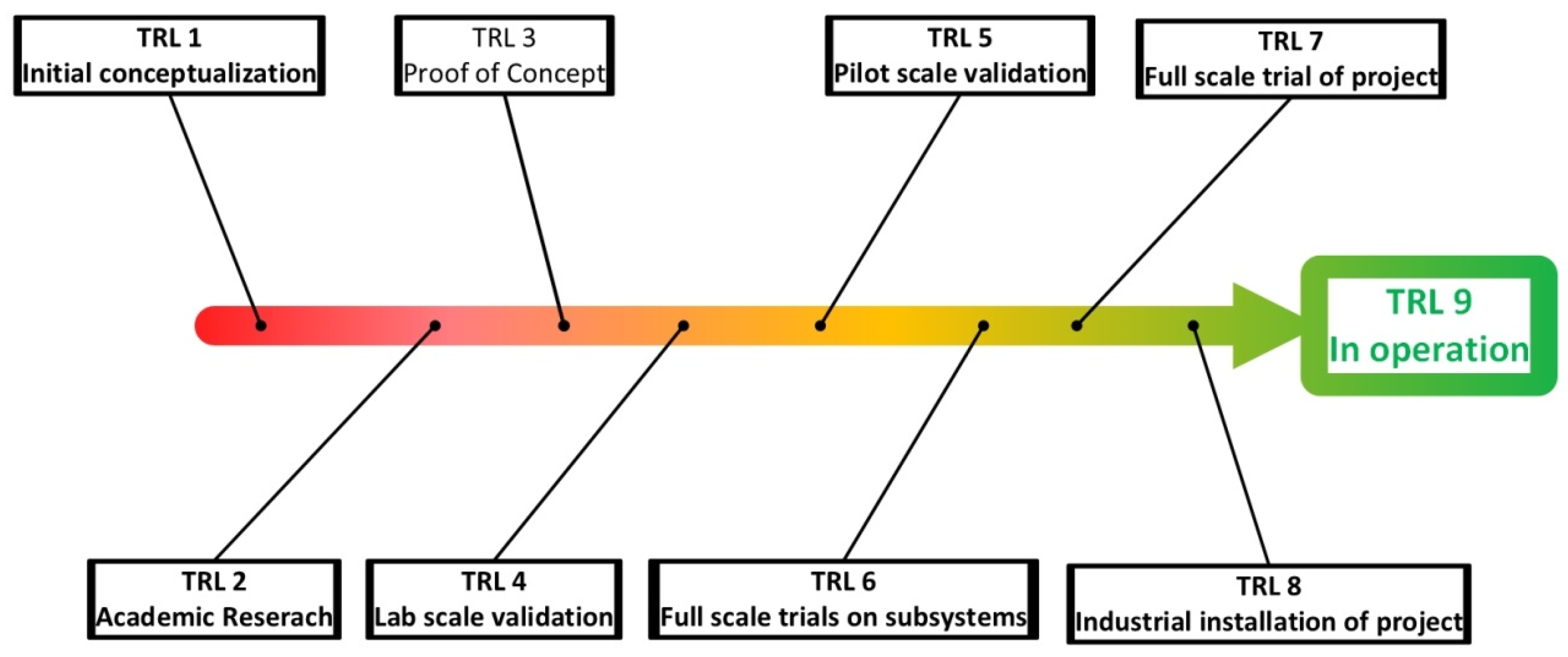
- TRL assessment (Technology readiness level): is a matrix developed by NASA to assess the maturity of a technology, ranging from TRL1, where one has to do with a basic concept, to TRL5 where the concept has been proven in pilot scale, and finally to TRL9 where the technology has been implemented “flight proven” [97,98]. This concept has been adopted by many other fields including bio-manufacturing [99]. In most instances academic bioprocess development tends to focus on TRL1 and TRL2 where conceptually an idea is tested and proven in lab scale [100]. Figure 3 shows different TRL levels that are available, and how they would apply to a resource recovery project. However, determining the TRL of a resource recovery project can be subjective as many separation technologies are adapted from other areas of commercial application and are not purely developed for resource recovery, which requires engineers to make an informed decision of the impact on the TRL of a technology with respect to a specific project. The objective of this assessment is to narrow down the number of resource recovery solutions by understanding and estimating TRL. In general, technologies with low levels of TRL require a large economic and time commitment to be developed to TRL9. As such the TRL assessment will allow the screening of technologies that are not sufficiently developed and can form a stop/go decision point for a specific technology. The “acceptable” level of TRL for a project is subjective to any specific project, although many investors would prefer high TRL projects.
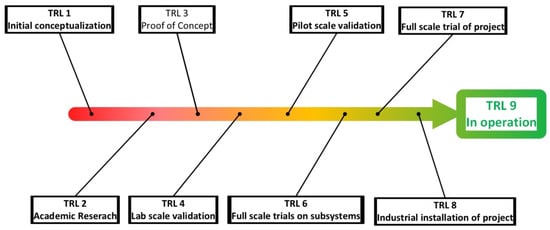 Figure 3. An overview of different TRL levels as interpreted for resource recovery projects.
Figure 3. An overview of different TRL levels as interpreted for resource recovery projects. - Detailed economics: The use of TRL assessment in the previous section allows to identify a number of possible process technology solutions that are at a sufficiently high TRL to be applied successfully in industry. However, prior to proceeding to testing and implementation of a resource recovery project it is important to further narrow down to a single technology. This can be done by considering the overall economics of the project where higher reward and the lowest risk projects will be preferred. It should be emphasized that the exact trade-off between risk and reward will be subjective to the project and investors. The use of detailed economic analysis of projects to identify the best process technology/operation is an established practice in bio-based production processes [94,101,102]. In general, these methodologies use the concept of net present value (NPV) analysis to develop economic models and combine these models with uncertainty analysis methods to quantify the economic risks and rewards. However, these methods typically do not address the inherent economic risk of implementing “new” process technology into an industrial environment, which is a key economic risk that needs to be considered in a detailed economic analysis. To this end a modified LOPA (layer of protection analysis) based analysis can be incorporated to these economic analysis methods to quantify the risk of technology failure. LOPA in its original form is a simplified method of safety risk assessment that uses information gathered during a process, such as Process Hazard Analysis (PHA). As with other hazard analysis methods, LOPA has been developed to identify if sufficient layers of protection are present to safeguard against accidents [103]. The idea of LOPA, however, has been extended beyond safety risk assessment into economic risk assessment. For example in [104] LOPA was used to compare the cost vs benefit of installing an extra layer of safety. In this guideline, a modified LOPA will be used to quantify economic risk brought on/mitigated by a firm who will develop and implement a separation technology to an existing production facility. As such, this modified LOPA should capture the risk of technology failure in development as well as in implementation. The combined LOPA and economic analysis (NPV with uncertainty analysis) provides sufficient information for an individual to screen out the best possible solution based on the levels of economic risk and reward, which are deemed acceptable.
- Testing: Once a suitable process technology is selected it is necessary to build prototypes and to carry out tests at pilot and full scales to confirm the feasibility of the technology and to carry out modifications to the process design to optimize the performance of the separation technology. This assessment is significantly costlier than the previous assessment as it requires physical assets to be successfully completed. As this assessment is conducted, the TRL of a separation technology would normally increase. In addition, information generated in this assessment can be fed back to the detailed economic assessment, as the TRL improvements of the process with better technology/process information would reduce the inherent risk of a project. The output of these assessments can be used to identify weaknesses in the proposed project/technology, and can furthermore be used to set goals for the next steps and to redesign/“tweak” the process design for better performances.
The five step guideline introduced in this section is specifically designed to efficiently identify resource recovery opportunities and then to systematically transform these resource recovery opportunities into implemented projects. To this end, each step of the guideline acts to identify and then narrow down suitable platform technologies for a given resource and waste stream combination. In each step of this guideline, the number of solutions is expected to reduce dramatically while the costs to complete each stage are also expected to increase significantly when moving through the different steps of this guideline.
Examples of Resource Recovery Projects
Example 1: Whey Protein
The development process of whey protein production from dairy industry effluent (specifically effluent from cheese production) is a good example where the key aspects illustrated in the guideline (see Figure 4) have played an influential role in identifying and developing a separation solution into industrial implementation. In the past, process water from cheese production was treated as a waste and was used as a raw material in cattle feed [17]. From an economic point of view this stream contains proteins (~700 USD/T) which could potentially be a significant source of extra income for the dairy industry. As such, there was a significant drive to identify and develop technologies that can separate and recover the whey protein in a valuable form [17]. However, originally there were no economically viable and sufficiently developed (sufficiently high TRL) separation technologies capable of recovering whey protein from dairy process water [17]. Hence, no detailed development work was carried out on these technologies. However, with the emergence of membrane technology [105,106], these assumptions were reassessed, where for the first time there was a technology with sufficiently high TRL available. This led to development/adaptation of membrane technologies to recover whey protein from dairy process water. The use of membrane technology in dairy production to recovery whey protein has led to significant financial benefits and is now an industry standard [17,107]. This process represents an example of a resource recovery process that has gone through all stop/go decisions and is now industrially implemented.
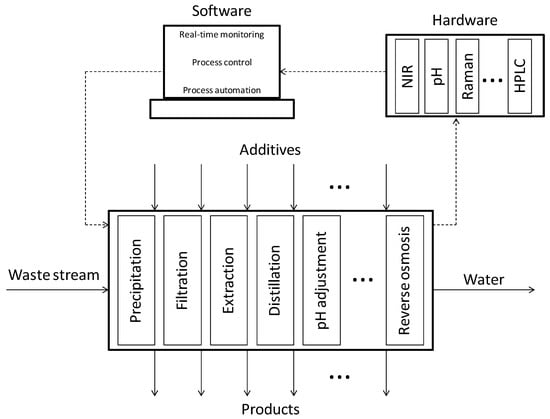
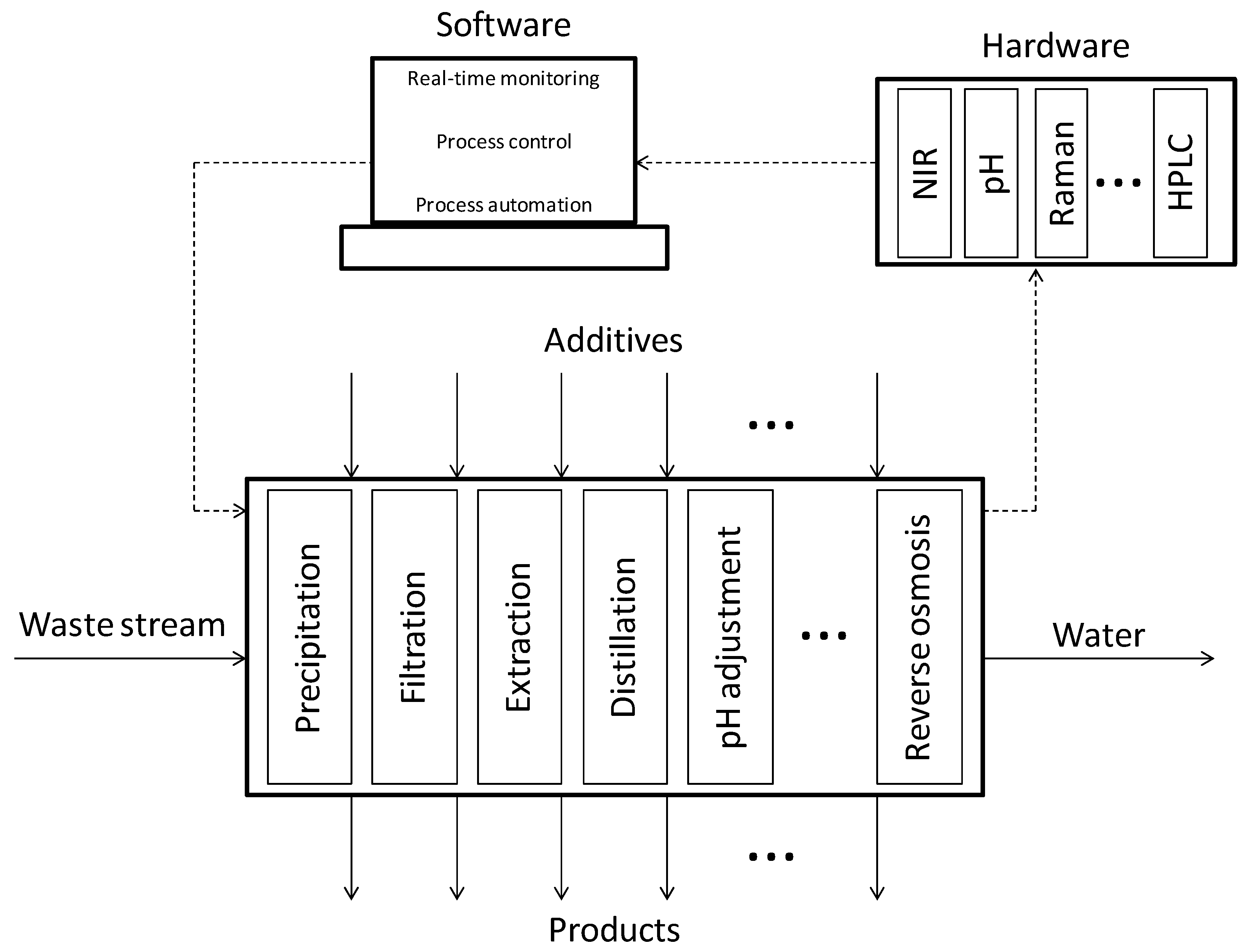
Figure 4.
Schematic representation of the concept for improving reactor productivity and for the “plug-and-play” approach for integrated process design aiming at achieving a zero-waste policy.
Example 2: Phosphorus Recovery
Another interesting example to present is phosphorus recovery from wastewater. Numerous studies have demonstrated that phosphorus recovery might reduce scaling problems (pipes, pumps) [33], lower pollution levels linked to excess discharge of nutrients (N and P) in wastewater effluents [43,108], and even produce financial gains for wastewater companies when it is sold as fertilizer [32]. There are an increasing number of technologies capable to capture P from the aqueous phase of anaerobic digester supernatants, sewage sludge and sewage sludge ash [109,110]. The selection of a certain technology is not an easy task and it must be evaluated on the basis of the achieved P recovery rates, removal and destruction of potentially hazardous materials (heavy metals, organic micropollutants and pathogens), the quality of the obtained product (environmental risk, fertilizing effects) and process economics [111]. The latter strongly depends on (i) chemicals used for precipitation and sludge disposal; and, (ii) downtime for cleaning unwanted struvite formed during chemical and biological removal processes [112].
Other Examples
As another example, in second generation bio-ethanol production (from wheat straw as raw material [113]) the platform technology used is relatively well developed with industrial scale demonstration pilots [114]. However, considering today’s market condition, this type of projects will fail when investigating detailed economic aspects of such technology development, which has led to industrial scale implementation of this type of processes being halted. As such, this type of process can potentially be implemented in future markets when fuels represent a higher value. In contrast, converting glycerol to hydrogen, with glycerol being a surplus side product/waste from bio-diesel production [115], has a positive economic potential as well as a platform technology that is capable of performing the transformation. However, the level of technology readiness of this platform is not sufficient, i.e., further development work needs to be carried out. Even if the current low TRL can be resolved and a detailed economic analysis is carried out, the large uncertainties associated with a potential unproven technology and geographical constraints will still result in a negative economic evaluation. Lastly, the recovery of a compound such as hesperidin with a value of up to 250,000 USD/T will stop at the technology search aspect, as currently there are no platform technologies that can recover these compounds from waste streams from bio-based production processes. The analysis of these examples illustrates the critical importance that each of the aspects summarized in Figure 3 plays in the progression of a concept into an industrially implemented reality.
5. The Role of Process Systems Engineering (PSE) in Resource Recovery
The chemical and biochemical companies are continuously in need of effective and efficient process design and operation of their processes to remain competitive in today’s world. Therefore, the methods and tools which can facilitate industry to achieve this form a compelling aspect of Process Systems Engineering (PSE) [116]. However, the full impact of the PSE tools and research, especially with respect to bio-based production processes, is often not realized. A successful implementation of the symbiosis between Quality by Design (QbD), Process Systems Engineering (PSE) and Process Analytical Technology (PAT) is a major driver towards establishment of effective solutions for a successful recovery of resources from wastewater streams (see Figure 4).
The application of model-based methodologies and computer-aided tools in process systems engineering (PSE) have great potential in predicting product/process performance without the requirement to carry out costly experiments. Computer-aided methods and tools are therefore valuable to rapidly evaluate different physical/chemical properties of substrates, products, impurities and solvents, then to generate possible synthetic routes towards desired products and undesired impurities, as well as to evaluate process configurations/alternatives. On the other hand, the lean production system (LPS) approach and its possibility to minimize non-value added activities (NVAs) plays a significant role in decreasing total costs, as well as in improving adaptability of processing plants to quick changes of the global market. The LPS approach therefore can be a suitable approach towards developing a sustainable economy and more environmentally friendly processes. With this general conceptual idea in mind, Table 3 is proposed where several systematic methods are proposed to facilitate the assessment of key aspects discussed in the previous section.

Table 3.
Classification of methods for identification of suitable separation techniques for resource recovery.
Efforts directed towards development and application of these methodologies in the area of resource recovery from bio-based production waste streams can potentially facilitate the development of a comprehensive assessment framework. The benefit of such framework is to be ultimately applied by a user as an initial screening tool and as a road map in systematically assessing and then developing a resource recovery project in the area of bio-based production processes from conception to its implementation. However, the development of such a framework would require the commitment of high levels of resources from multiple academic and industrial partners.
In the PSE field, several methods exist for this and it is a very prominent area, in that product and process design were conventionally separate problems. The four-stage process in product engineering proposed by Cussler and Wei [123] is often solved sequentially. In product and process engineering there is an interest in integrating the product design (selection) and process design (manufacturing). This is termed integrated product-process design. As with the earlier Computer-aided molecular design (CAMD) methods, one of the first integrated product-process design methods involved the design and use of solvents. In [124], a method for integration of solvent and separation process design/synthesis is presented. The method, composed of seven steps, is a hybrid of mathematical modeling and heuristics. The heuristics are applied to simplify the mathematical problem by means of thermodynamic knowledge.
There are also academic studies where one is unsure which medium should be used to best recover a certain resource [125,126]. In particular, the issue can be very sensitive to the choice of solvent used for obtaining the optimal recovery. In addition, this sensitivity can extend to the process conditions itself and even the process design decisions. Thus, it can be necessary to integrate the product-process design problem to solve it simultaneously. As an example, the acetic acid separation from water is a frequently studied problem, which can be efficiently solved if the liquid-liquid extraction model and the solvent design model are solved in an integrated manner [127].
The integrated product-process synthesis and design is also of both academic and industrial interest. Linke and Kokossis [128], proposed a framework in which, the optimal process—including the reaction and separation process steps—is synthesized and optimal solvents are designed simultaneously. Similar methods are discussed under product property clustering by Solvason et al. [129], where product design is utilized in developing integrated product-process synthesis systems. Several methods have been presented [130,131,132] that use the property clustering techniques by targeting product property values that yield optimal process performance. Once the target properties are deduced, products are generated that satisfy these. The method was extended in the works of Kazantazi et al. [133,134] and Chemmangattuvalappil et al. [133,134]. The virtual product-process design laboratory (VPPD-lab) is a software developed by Conte et al. [135,136]. The purpose of the software is to systematically integrate product-process design problems. The software is template based and depending on the type of product (or here the resource to be recovered) to be investigated, an appropriate template can be selected. The VPPD-lab has been extended to include analysis and verification as well [137]. Further work has been made into this software that also includes several other types of design templates [138,139]. Recent work focuses on true integration of the two design problems, where the solution can be obtained through direct mathematical optimization. These methods can avoid problems with combinatorial explosions, and can be applied in situations where it is difficult to screen multiple solutions/products, due to sensitivity between the product and process properties [14,33]. These methods also require an increased accuracy, in particular in the area of property prediction of specialty molecules [117,140].
A holistic view of the current state of PSE illustrates there are multiple methods, concepts and approaches that can play a pivotal role in efficiently assessing and developing resource recovery solutions. However, to our opinion these PSE based ideas and tools would require a significant amount of work to make these concepts usable in the assessment and development of industrial resource recovery projects. In the next section, a SWOT analysis is performed into the general area of resource recovery in bio-based production processes.
6. SWOT Analysis
In this section, the Strengths Weaknesses Opportunities and Threats (SWOT) faced by resource recovery projects within bio-based production processes are identified. The objective of this analysis is to provide a balanced perspective on resource recovery in bio-based production processes.
Strengths
- As detailed in previous sections the strengths of resource recovery in the area of bio-based production processes lies in the promise of creating economically lucrative products out of raw materials that otherwise would have no economic value. Compared to the current state of operation, where the bio-based industrial waste streams are treated to meet environmental standards, resource recovery offers an economically, environmentally and social responsible alternative.
- The platform technologies that are required to recover resources from bio-based production streams through separation are relatively well-defined and developed for many resources (as outlined in Section 4). As such, in general, a potential implementation of resource recovery projects requires less effort to be spent on developing base technology.
- The composition and availability of resources in the waste streams of bio-based production processes are relatively constant for a given production process (due to stable composition of the raw material and reactions). As such the developing of a resource recovery system for bio-based production processes is relatively easier than developing a similar resource recovery system for municipal wastewater, where a large variation in resources can occur.
- To our opinion, the overall TRL of the technologies surveyed in Section 4 is relatively high in comparison to other competing technologies such as bioconversion [11,12] and hydrothermal processing [13,15]. As such, from an industrial point of view, the resource recovery through extraction/separation can be a more established technology choice.
Weaknesses
- Despite the availability of platform technologies, the actual number of resource recovery projects implemented in practice is low. Therefore, initial pioneering resource recovery projects in the area of bio-based production will require significant process development time
- In situations where the resources available are of low value and must be converted (reacted) into a valuable product, the path of bio-conversation or hydrothermal processing can potentially be a better solution [11,141], rather than a straight resource recovery implementation through platform technologies discussed in Section 3.
- Difficulties in identifying applicable separation technologies for a given resource stream due to lack of successful examples, and the lack of a supporting techno-economic framework makes it difficult to select a suitable separation technique for a given resource stream.
- Despite the availability of multiple, established platform technologies, some high value products such as hesperidin cannot be recovered using these platform technologies. As such, all resources that are available in the bio-based waste stream cannot always be recovered currently.
Opportunities
- The development of a comprehensive framework that considers the maturity of the technology and other underlying techno-economic aspects would allow for a structured approach for assessing the potential impact and future value of resource recovery projects
- The development of an efficient model-based screening tool to identify resource/technology pairs would allow for an efficient identification.
- The tightening environmental regulations and increasing value of resources will result in the concept of resource recovery being economically lucrative for a larger number of applications
- Development of new or adaptation of platform technologies to recover resources of high economic value that are hard to extract from the waste streams has a significant potential.
Threats
- Competing process technologies such as biochemical conversion [11,12] or hydrothermal processing [15,141] can become more economically lucrative than the separation technologies discussed in this work. There is also likelihood chance that a new technology makes a leapfrogging discovery that would provide more lucrative economic incentives.
- From a societal (as a result of a regulation) perspective, recycling/recovering raw materials from waste can be challenging. For example, would the society be comfortable with reusing food ingredients that has been recovered from waste streams? However, a counter argument can be made by referring to the whey protein example discussed previously. The concept of Societal readiness level (SRL) can be a good yardstick in understanding and evaluating these concerns [142].
- The economic motivation to extract valuable resources from waste streams is clear. However, from an environmental point of view, there can be situations where the establishment of operations to recover valuable resources can have a negative environmental impact compared to wastewater treatment. The concept of Life cycle analysis (LCA) can be one method to comprehensively evaluate this threat.
Taking into account the SWOT analysis provided in this section, a potential issue that can hinder development of resource recovery strategies is that industries working with specific bio-based production processes often do not want to disclose what kind of substrates they use. Under such circumstances, the analysis of the waste streams can reveal more about the substrates that have been used, and therefore it can be difficult to obtain a detailed characterization of specific waste streams, a prerequisite for the development of a resource recovery implementation. In addition, the efficient determination of SRL can be complicated and is influenced by changes in the prevailing public opinion. Similarly, the economics are also at the mercy of the prevailing market conditions that can change during the course of development. Both these dynamic changes possible in SRL and economics must be taken into account in the decision making process.
7. Future Perspectives
This work has surveyed the landscape of resource recovery from bio-based waste streams in terms of technology and economics, while exploring and identifying methodologies and obstacles faced when transforming a resource recovery project from a concept to an industrially implemented solution. Table 4 contains a summary of the key separation technologies that are available for resource recovery projects and what class of resources they can potentially recover from bio-based wastewater streams. This table also lists the pros and cons of each separation technology and provides an estimated TRL value for each separation technology.

Table 4.
Pros and cons of current separation technology applicable to resource recovery from bio-based waste streams.
With lucrative economics and availability of a vast array of separation technologies in the open literature, one would expect bio-based production industries to design and/or retrofit bio-based production plants with a resource recovery unit whenever possible. However, except for a handful of applications, resource recovery units are scarcely applied nowadays in bio-based production industries. For example, whey protein from dairy industry, phosphorus recovery and energy recovery in form of biogas production. The bio-based industry could take the dairy industry as an example, when it comes to extracting value out of waste streams originating from its processes. We are convinced that the bio-based industry can potentially yield several successful implementations of resource recovery examples (as identified in Table 1) that are as lucrative as the whey processing in the dairy industry [17]. As such, resource recovery from bio-based production processes should be a high priority in the forthcoming years. However, the realization of these potentials can be challenging and complicated.
8. Conclusions
It is clear that in the end, the establishment of a resource recovery unit on a production plant will depend on local factors specific to the particular plant. However, in order to promote increased future implementation of resource recovery operations, more focus should be on developing easy to use generic frameworks and model-based methodologies and computer-aided tools that can be used for rapid identification and evaluation of promising separation techniques that will work on recovering target resources from a particular waste stream. To achieve this objective in a relatively short timeframe, an international effort is required, where it will be necessary to conduct an exhaustive literature search combined with carrying out experiments to collect the following information:
- A mapping of resources that are present in typical waste streams from bio-based production processes;
- The media and the state in which the resource is contained in the bio-based waste stream;
- Type of separation techniques that have been used and/or may potentially be used in order to separate a particular resource from a particular stream (not limited to waste streams from bio-based production processes);
- Knowledge of how the presence of other components affects the performance of a particular separation technique when applying such a technique for resource recovery.
Once properly mapped, resources and solvents can then be categorized into areas of similar chemical and physical properties. The literature/experimental information on separation techniques will be used to identify key separation techniques and their characteristics that can be applied to separate a particular category of resources from a particular category of waste streams. This information can then help to narrow down potential separation techniques that might be applicable, based only on knowledge about the category of resource and the waste stream. This will then again allow for a more intensified and focused development, and will help streamline the development of a concept into an implemented resource recovery solution.
Acknowledgments
The authors received financial support from Innovation Fund Denmark through the BIOPRO2 Strategic Research center (Grant number 4105-00020B) and the REWARD project (Grant number 1308-0027B).
Author Contributions
I.A.U., S.S.M., A.M. and X.F.-A. collected information and wrote the manuscript. K.V.G. has conceived the research, and provided feedback to the content and participated in writing the manuscript.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Carvalho, A.; Gani, R.; Matos, H. Design of sustainable chemical processes: Systematic retrofit analysis generation and evaluation of alternatives. Process Saf. Environ. Prot. 2008, 86, 328–346. [Google Scholar] [CrossRef]
- Gollapalli, U.; Dantus, M.M.; High, K.A. Environment and control issues in design. Comput. Chem. Eng. 2000, 24, 1709–1712. [Google Scholar] [CrossRef]
- Mitic, A.; Gernaey, K.V. Process Intensification Tools in the Small-Scale Pharmaceutical Manufacturing of Small Molecules. Chem. Eng. Technol. 2015, 38, 1699–1712. [Google Scholar] [CrossRef]
- Miller, S.A.; Landis, A.E.; Theis, T.L. Feature: Environmental Trade-offs of Biobased Production. Environ. Sci. Technol. 2007, 41, 5176–5182. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.S.; Udugama, I.A.; Cignitti, S.; Mitic, A.; Flores-Alsina, X.; Gernaey, K.V. Resource recovery from bio-based production processes: A future necessity? Curr. Opin. Chem. Eng. 2017, 18, 1–9. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Cignitti, S.; Udugama, I.S.B.A.; Mitic, A.; Alsina, X.F.; Bryde-Jacobsen, J.; Gernaey, K. Ressourcegenvinding–Vejen til øget bæredygtighed i biobaserede produktionsprocesser. Dansk Kemi 2017, 98, 18–21. [Google Scholar]
- Chen, Y.; Nielsen, J. Biobased organic acids production by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 37, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Y.; Liu, Y.; Shi, H.; Su, Z. Novel in situ product removal technique for simultaneous production of propionic acid and vitamin B12 by expanded bed adsorption bioreactor. Bioresour. Technol. 2012, 104, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Budarin, V.L.; Shuttleworth, P.S.; Dodson, J.R.; Hunt, A.J.; Lanigan, B.; Marriott, R.; Milkowski, K.J.; Wilson, A.J.; Breeden, S.W.; Fan, J.; et al. Use of green chemical technologies in an integrated biorefinery. Energy Environ. Sci. 2011, 4, 471–479. [Google Scholar] [CrossRef]
- Pfaltzgraff, L.A.; De bruyn, M.; Cooper, E.C.; Budarin, V.; Clark, J.H. Food waste biomass: A resource for high-value chemicals. Green Chem. 2013, 15, 307. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar]
- Valentino, F.; Morgan-Sagastume, F.; Campanari, S.; Villano, M.; Werker, A.; Majone, M. Carbon recovery from wastewater through bioconversion into biodegradable polymers. New Biotechnol. 2017, 37, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Jatzwauck, M.; Schumpe, A. Kinetics of hydrothermal carbonization (HTC) of soft rush. Biomass Bioenergy 2015, 75, 94–100. [Google Scholar] [CrossRef]
- Waldner, M.H.; Vogel, F. Renewable production of methane from woody biomass by catalytic hydrothermal gasification. Ind. Eng. Chem. Res. 2005, 44, 4543–4551. [Google Scholar] [CrossRef]
- Deloitte. Opportunities for the Fermentation-Based Chemical Industry; Deloitte: New York, NY, USA, 2014; pp. 1–50. [Google Scholar]
- Smithers, G.W. Whey and whey proteins-From “gutter-to-gold”. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Guest, J.S.; Skerlos, S.J.; Barnard, J.L.; Beck, M.B.; Daigger, G.T.; Hilger, H.; Jackson, S.J.; Karvazy, K.; Kelly, L.; Macpherson, L.; et al. A new planning and design paradigm to achieve sustainable resource recovery from wastewater. Environ. Sci. Technol. 2009, 43, 6126–6130. [Google Scholar] [CrossRef] [PubMed]
- Nicolaisen, B. Developments in membrane technology for water treatment. Desalination 2003, 153, 355–360. [Google Scholar] [CrossRef]
- Moradi, A.; Farsi, A.; Mansouri, S.S.; Sarcheshmehpoor, M. A new approach for modeling of RO membranes using MD-SF-PF model and CFD technique. Res. Chem. Intermed. 2012, 38, 161–177. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Simate, G.S.; Cluett, J.; Iyuke, S.E.; Musapatika, E.T.; Ndlovu, S.; Walubita, L.F.; Alvarez, A.E. The treatment of brewery wastewater for reuse: State of the art. Desalination 2011, 273, 235–247. [Google Scholar] [CrossRef]
- Daufin, G.; Escudier, J.-P.; Carrère, H.; Bérot, S.; Fillaudeau, L.; Decloux, M. Recent and Emerging Applications of Membrane Processes in the Food and Dairy Industry. Food Bioprod. Process. 2001, 79, 89–102. [Google Scholar] [CrossRef]
- Zydney, A.L. Protein Separations Using Membrane Filtration: New Opportunities for Whey Fractionation. Int. Dairy J. 1998, 8, 243–250. [Google Scholar] [CrossRef]
- Girard, B.; Fukumoto, L. R.; Sefa Koseoglu, S. Membrane Processing of Fruit Juices and Beverages: A Review. Crit. Rev. Biotechnol. 2000, 20, 109–175. [Google Scholar] [CrossRef] [PubMed]
- Kalyanpur, M. Downstream processing in the biotechnology industry. Mol. Biotechnol. 2002, 22, 87–98. [Google Scholar] [CrossRef]
- Rios, G.M.; Belleville, M.P.; Paolucci, D.; Sanchez, J. Progress in enzymatic membrane reactors—A review. J. Membr. Sci. 2004, 242, 189–196. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Figueroa, R.R.; Muñoz, R.C. A two-step nanofiltration process for the production of phenolic-rich fractions from artichoke aqueous extracts. Int. J. Mol. Sci. 2015, 16, 8968–8987. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Padrell, A.E.; Morthensen, S.T.; Lewandowski, D.J.; Skovby, T.; Kiil, S.; Gernaey, K.V. Continuous hydrolysis and liquid-liquid phase separation of an active pharmaceutical ingredient intermediate using a miniscale hydrophobic membrane separator. Org. Process Res. Dev. 2012, 16, 888–900. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Wiley: Hoboken, NJ, USA, 1993. [Google Scholar]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus Recovery from Wastewater by Struvite Crystallization: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Van Langerak, E.P.A.; Ramaekers, H.; Wiechers, J.; Veeken, A.H.M.; Hamelers, H.V.M.; Lettinga, G. Impact of location of CaCO3 precipitation on the development of intact anaerobic sludge. Water Res. 2000, 34, 437–446. [Google Scholar] [CrossRef]
- Batstone, D.J.; Landelli, J.; Saunders, A.; Webb, R.I.; Blackall, L.L.; Keller, J. The influence of calcium on granular sludge in a full-scale UASB treating paper mill wastewater. Water Sci. Technol. 2002, 45, 187–193. [Google Scholar] [PubMed]
- Tait, S.; Clarke, W.P.; Keller, J.; Batstone, D.J. Removal of sulfate from high-strength wastewater by crystallisation. Water Res. 2009, 43, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.M.; Batstone, D.J. Nucleation and growth kinetics of struvite crystallization. Water Res. 2013, 47, 2890–2900. [Google Scholar] [CrossRef] [PubMed]
- Ariyanto, E.; Sen, T.K.; Ang, H.M. The influence of various physico-chemical process parameters on kinetics and growth mechanism of struvite crystallisation. Adv. Powder Technol. 2014, 25, 682–694. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Carbonate and magnesium interactive effect on calcium phosphate precipitation. Environ. Sci. Technol. 2008, 42, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Hug, A.; Udert, K.M. Struvite precipitation from urine with electrochemical magnesium dosage. Water Res. 2013, 47, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, S.C.; Schneider, P.A.; Flood, A.E. Model-driven experimental evaluation of struvite nucleation, growth and aggregation kinetics. Water Res. 2014, 56, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Kazadi Mbamba, C.; Tait, S.; Flores-Alsina, X.; Batstone, D.J. A systematic study of multiple minerals precipitation modelling in wastewater treatment. Water Res. 2015, 85, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Mitchenko, T.; Stender, P.; Makarova, N. Optimization of sorption purification of industrial effluents, waste waters and technological solutions from polyvalent metal ions. Solvent Extr. Ion Exch. 1998, 16, 75–149. [Google Scholar] [CrossRef]
- Rao, K.; Mohapatra, M.; Anand, S.; Venkateswarlu, P. Review on cadmium removal from aqueous solutions. Int. J. Eng. Sci. Technol. 2011, 2, 81–103. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, H.; Zhang, Y.; Liang, P.; Wang, K. Nitrogen recovery from low-strength wastewater by combined membrane capacitive deionization (MCDI) and ion exchange (IE) process. Chem. Eng. J. 2017, 316, 1–6. [Google Scholar] [CrossRef]
- López, E.; Soto, B.; Arias, M.; Núñez, A.; Rubinos, D.; Barral, M.T. Adsorbent properties of red mud and its use for wastewater treatment. Water Res. 1998, 32, 1314–1322. [Google Scholar] [CrossRef]
- Monser, L.; Adhoum, N. Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Sep. Purif. Technol. 2002, 26, 137–146. [Google Scholar] [CrossRef]
- Mohan, D.; Gupta, V.; Srivastava, S.; Chander, S. Kinetics of mercury adsorption from wastewater using activated carbon derived from fertilizer waste. Colloids Surf. A Physicochem. Eng. Asp. 2001, 177, 169–181. [Google Scholar] [CrossRef]
- Cermakova, L.; Kopecka, I.; Pivokonsky, M.; Pivokonska, L.; Janda, V. Removal of cyanobacterial amino acids in water treatment by activated carbon adsorption. Sep. Purif. Technol. 2017, 173, 330–338. [Google Scholar] [CrossRef]
- García-Araya, J.F.; Beltrán, F.J.; Álvarez, P.; Masa, F.J. Activated Carbon Adsorption of Some Phenolic Compounds Present in Agroindustrial Wastewater. Adsorption 2003, 9, 107–115. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, K.-D. Adsorption Behaviors of CO2 and NH3 on Chemically Surface-Treated Activated Carbons. J. Colloid Interface Sci. 1999, 212, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Venema, P.; Hiemstra, T.; Weidler, P.G.; van Riemsdijk, W.H. Intrinsic Proton Affinity of Reactive Surface Groups of Metal (Hydr)oxides: Application to Iron (Hydr)oxides. J. Colloid Interface Sci. 1998, 198, 282–295. [Google Scholar] [CrossRef]
- Sen, T.K.; Sarzali, M.V. Removal of cadmium metal ion (Cd2+) from its aqueous solution by aluminium oxide (Al2O3): A kinetic and equilibrium study. Chem. Eng. J. 2008, 142, 256–262. [Google Scholar] [CrossRef]
- Han, Y.-L.; Lo, Y.-C.; Cheng, C.-L.; Yu, W.-J.; Nagarajan, D.; Liu, C.-H.; Li, Y.-H.; Chang, J.-S. Calcium ion adsorption with extracellular proteins of thermophilic bacteria isolated from geothermal sites—A feasibility study. Biochem. Eng. J. 2017, 117, 48–56. [Google Scholar] [CrossRef]
- Yap, M.W.; Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C. Microwave induced synthesis of magnetic biochar from agricultural biomass for removal of lead and cadmium from wastewater. J. Ind. Eng. Chem. 2017, 45, 287–295. [Google Scholar] [CrossRef]
- Cay, S.; Uyanik, A.; Ozasik, A. Single and binary component adsorption of copper(II) and cadmium(II) from aqueous solutions using tea-industry waste. Sep. Purif. Technol. 2004, 38, 273–280. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Zuo, X. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J. Power Sources 2005, 152, 278–284. [Google Scholar] [CrossRef]
- Yang, C.; Qian, Y.; Zhang, L.; Feng, J. Solvent extraction process development and on-site trial-plant for phenol removal from industrial coal-gasification wastewater. Chem. Eng. J. 2006, 117, 179–185. [Google Scholar] [CrossRef]
- Munson, C.L.; King, C.J. Factors influencing solvent selection for extraction of ethanol from aqueous solutions. Ind. Eng. Chem. Process Des. Dev. 1984, 23, 109–115. [Google Scholar] [CrossRef]
- Kiss, A.A. Distillation technology—Still young and full of breakthrough opportunities. J. Chem. Technol. Biotechnol. 2014, 89, 479–498. [Google Scholar] [CrossRef]
- Wankat, P.C. Separation Process Engineering: Includes Mass Transfer Analysisitle; Prentice Hall: Upper Saddle River, NJ, USA, 2016. [Google Scholar]
- Udugama, I.A.; Wolfenstetter, F.; Kirkpatrick, R.; Yu, W.; Young, B.R. A comparison of a novel robust decentralised control strategy and MPC for industrial high purity, high recovery, multicomponent distillation. ISA Trans. 2017, 69, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Department of Energy. U.S. Distillation Column Modelling Tools; U.S. Department of Energy: Washington, DC, USA, 2011.
- Udugama, I.A.; Mansouri, S.S.; Huusom, J.K.; Taube, M.A.; Maidl, A.; Young, B. Cost competitive “soft sensor” for determining product recovery in industrial methanol. In Proceedings of the 2017 Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 30–31 May 2017; IEEE: New York, NY, USA, 2017; pp. 23–28. [Google Scholar]
- Chang, A.-F.; Pashikanti, K.; Liu, Y.A. Refinery Engineering; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Udugama, I.A.; Munir, T.; Kirkpatrick, R.; Young, B.R.; Yu, W. High purity, high recovery, multi-component methanol distillation control. Comput. Aided Chem. Eng. 2015, 37, 1613–1618. [Google Scholar]
- Zhang, Y. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Ewell, R. H.; Harrison, J. M.; Berg, L. Azeotropic Distillation. Ind. Eng. Chem. 1944, 36, 871–875. [Google Scholar] [CrossRef]
- Harmsen, G.J. Reactive distillation: The front-runner of industrial process intensification. Chem. Eng. Process. Process Intensif. 2007, 46, 774–780. [Google Scholar] [CrossRef]
- Niesbach, A.; Adams, T.A.; Lutze, P. Semicontinuous distillation of impurities for the production of butyl acrylate from bio-butanol and bio-acrylic acid. Chem. Eng. Process. Process Intensif. 2013, 74, 165–177. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Y.; Chen, Q.; Lv, C.; Jia, S. Bio-oil upgrading by reactive distillation using p-toluene sulfonic acid catalyst loaded on biomass activated carbon. Biomass Bioenergy 2013, 56, 405–411. [Google Scholar] [CrossRef]
- Niesbach, A.; Lutze, P.; Górak, A. Reactive Distillation for Production of n-Butyl Acrylate from Bio-Based Raw Materials; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 32. [Google Scholar]
- Silvestre, W.P.; Agostini, F.; Muniz, L.A.R.; Pauletti, G.F. Fractionating of green mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. J. Food Eng. 2015, 178, 90–94. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Licht, S. Distillation for Recovery of Biofuels and Bio-Based Chemicals, 3rd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Xu, Y.; Zheng, X.; Peng, Y.; Li, B.; Hu, X.; Yin, Y. Upgrading the lubricity of bio-oil via homogeneous catalytic esterification under vacuum distillation conditions. Biomass Bioenergy 2015, 80, 1–9. [Google Scholar] [CrossRef]
- Capunitan, J.A.; Capareda, S.C. Characterization and separation of corn stover bio-oil by fractional distillation. Fuel 2013, 112, 60–73. [Google Scholar] [CrossRef]
- Huang, A.N.; Hsu, C.P.; Hou, B.R.; Kuo, H.P. Production and separation of rice husk pyrolysis bio-oils from a fractional distillation column connected fluidized bed reactor. Powder Technol. 2016. [Google Scholar] [CrossRef]
- Poddar, T.; Jagannath, A.; Almansoori, A. Biodiesel Production using Reactive Distillation: A Comparative Simulation Study. Energy Proced. 2015, 75, 17–22. [Google Scholar] [CrossRef]
- Stankiewicz, A.I.; Moulijn, J.A. Process Intensification: Transforming Chemical Engineering. Chem. Eng. Prog. 2000, 96, 22–34. [Google Scholar]
- Lutze, P.; Gani, R.; Woodley, J.M. Process intensification: A perspective on process synthesis. Chem. Eng. Process. Process Intensif. 2010, 49, 547–558. [Google Scholar] [CrossRef]
- Nikačević, N.M.; Huesman, A.E.M.; Van den Hof, P.M.J.; Stankiewicz, A.I. Opportunities and challenges for process control in process intensification. Chem. Eng. Process. Process Intensif. 2012, 52, 1–15. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Ismail, M.I.; Babi, D.K.; Simasatitkul, L.; Huusom, J.K.; Gani, R. Systematic Sustainable Process Design and Analysis of Biodiesel Processes. Processes 2013, 1, 167–202. [Google Scholar] [CrossRef]
- Fletcher, P.D.I.; Haswell, S.J.; Pombo-Villar, E.; Warrington, B.H.; Watts, P.; Wong, S.Y.F.; Zhang, X. Micro reactors: Principles and applications in organic synthesis. Tetrahedron 2002, 58, 4735–4757. [Google Scholar] [CrossRef]
- Chen, J.-F.; Shao, L.; Guo, F.; Wang, X.-M. Synthesis of nano-fibers of aluminum hydroxide in novel rotating packed bed reactor. Chem. Eng. Sci. 2003, 58, 569–575. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Huusom, J.K.; Gani, R.; Sales-Cruz, M. Systematic integrated process design and control of binary element reactive distillation processes. AIChE J. 2016, 62, 3137–3154. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Sales-Cruz, M.; Huusom, J.K.; Gani, R. Systematic integrated process design and control of reactive distillation processes involving multi-elements. Chem. Eng. Res. Des. 2016, 115, 348–364. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Sales-Cruz, M.; Huusom, J.K.; Woodley, J.M.; Gani, R. Integrated Process Design and Control of Reactive Distillation Processes. IFAC PapersOnLine 2015, 48, 1120–1125. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Sales-Cruz, M.; Huusom, J.K.; Gani, R. Integrated Process Design and Control of Multi-element Reactive Distillation Processes. IFAC PapersOnLine 2016, 49, 735–740. [Google Scholar] [CrossRef]
- Jeantet, R.; Maubois, J.; Boyaval, P. Semicontinuous production of lactic acid in a bioreactor coupled with nanofiltration membranes. Enzym. Microb. Technol. 1996, 19, 614–619. [Google Scholar] [CrossRef]
- Rybicka, J.; Tiwari, A.; Leeke, G.A. Technology readiness level assessment of composites recycling technologies. J. Clean. Prod. 2016, 112, 1001–1012. [Google Scholar] [CrossRef]
- Gargalo, C.L.; Carvalho, A.; Gernaey, K.V.; Sin, G. A framework for techno-economic & environmental sustainability analysis by risk assessment for conceptual process evaluation. Biochem. Eng. J. 2016, 116, 146–156. [Google Scholar]
- Gnansounou, E.; Dauriat, A. Bioresource Technology Techno-economic analysis of lignocellulosic ethanol: A review. Group 2010, 101, 4980–4991. [Google Scholar]
- Gargalo, C.L.; Cheali, P.; Posada, J.A.; Gernaey, K.V.; Sin, G. Economic Risk Assessment of Early Stage Designs for Glycerol Valorization in Biorefinery Concepts. Ind. Eng. Chem. Res. 2016, 55, 6801–6814. [Google Scholar] [CrossRef]
- Technology Readiness Level. Available online: https://www.nasa.gov/directorates/heo/scan/engineering/technology/txt_accordion1.html (accessed on 2 April 2017).
- Parasuraman, A. Technology Readiness Index (Tri): A Multiple-Item Scale to Measure Readiness to Embrace New Technologies. J. Serv. Res. 2000, 2, 307–320. [Google Scholar] [CrossRef]
- Tierney, R.; Hermina, W.; Walsh, S. The pharmaceutical technology landscape: A new form of technology roadmapping. Technol. Forecast. Soc. Chang. 2013, 80, 194–211. [Google Scholar] [CrossRef]
- Barta, D.J.; Castillo, J.M.; Fortson, R.E. The Biomass Production System for the Bioregenerative Planetary Life Support Systems Test Complex: Preliminary Designs and Considerations. In SAE Technical Paper; SAE International: Troy, MI, USA, 1999. [Google Scholar]
- Cheali, P.; Posada, J.A.; Gernaey, K.V.; Sin, G. Upgrading of lignocellulosic biorefinery to value-added chemicals: Sustainability and economics of bioethanol-derivatives. Biomass Bioenergy 2015, 75, 282–300. [Google Scholar] [CrossRef]
- Mellichamp, D.A. New discounted cash flow method: Estimating plant profitability at the conceptual design level while compensating for business risk/uncertainty. Comput. Chem. Eng. 2013, 48, 251–263. [Google Scholar] [CrossRef]
- Willey, R.J. Layer of Protection Analysis. Proced. Eng. 2014, 84, 12–22. [Google Scholar] [CrossRef]
- Johnson, R.W. Beyond-compliance uses of HAZOP/LOPA studies. J. Loss Prev. Process Ind. 2010, 23, 727–733. [Google Scholar] [CrossRef]
- Baldasso, C.; Barros, T.C.; Tessaro, I.C. Concentration and purification of whey proteins by ultrafiltration. Desalination 2011, 278, 381–386. [Google Scholar] [CrossRef]
- Cheang, B.; Zydney, A.L. A two-stage ultrafiltration process for fractionation of whey protein isolate. J. Membr. Sci. 2004, 231, 159–167. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, B.P.; Kumar, M.; Shahi, V.K. Membrane-based techniques for the separation and purification of proteins: An overview. Adv. Colloid Interface Sci. 2009, 145, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Solon, K.; Flores-Alsina, X.; Gernaey, K.V.; Jeppsson, U. Effects of influent fractionation, kinetics, stoichiometry and mass transfer on CH4, H2 and CO2 production for (plant-wide) modeling of anaerobic digesters. Water Sci. Technol. 2015, 71, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Egle, L.; Rechberger, H.; Zessner, M. Overview and description of technologies for recovering phosphorus from municipal wastewater. Resour. Conserv. Recycl. 2015, 105, 325–346. [Google Scholar] [CrossRef]
- Mehta, C.M.; Khunjar, W.O.; Nguyen, V.; Tait, S.; Batstone, D.J. Technologies to Recover Nutrients from Waste Streams: A Critical Review. Crit. Rev. Environ.Sci. Technol. 2015, 45, 385–427. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Schneider, P.; Jegatheesan, V.; Johnson, J. An economic evaluation of phosphorus recovery as struvite from digester supernatant. Bioresour. Technol. 2006, 97, 2211–2216. [Google Scholar] [CrossRef] [PubMed]
- Heerlen, N.L. DSM enzymes for cellulosic ethanol qualified by DONG Energy. Focus Catal. 2012, 2012, 3. [Google Scholar]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Martín, M.; Grossmann, I.E. On the systematic synthesis of sustainable biorefineries. Ind. Eng. Chem. Res. 2013, 52, 3044–3064. [Google Scholar]
- Grossmann, I.E.; Westerberg, A.W. Research challenges in process systems engineering. AIChE J. 2000, 46, 1700–1703. [Google Scholar] [CrossRef]
- Zhang, L.; Cignitti, S.; Gani, R. Generic mathematical programming formulation and solution for computer-aided molecular design. Comput. Chem. Eng. 2015, 78, 79–84. [Google Scholar] [CrossRef]
- Yeomans, H.; Grossmann, I.E. A systematic modeling framework of superstructure optimization in process synthesis. Comput. Chem. Eng. 1999, 23, 709–731. [Google Scholar] [CrossRef]
- Jaksland, C.A.; Gani, R.; Lien, K.M. Separation process design and synthesis based on thermodynamic insights. Chem. Eng. Sci. 1995, 50, 511–530. [Google Scholar] [CrossRef]
- Banga, J.; Moles, C.; Alonso, A. Global Optimization of Bioprocesses using Stochastic and Hybrid Methods. In Frontiers in Global Optimization SE-3; Nonconvex Optimization and Its Applications; Floudas, C.A., Pardalos, P., Eds.; Springer: New York, NY, USA, 2004; Volume 74, pp. 45–70. [Google Scholar]
- Buxton, A.; Livingston, A.G.; Pistikopoulos, E.N. Reaction path synthesis for environmental impact minimization. Comput. Chem. Eng. 1997, 21, S959–S964. [Google Scholar] [CrossRef]
- Shah, P.B.; Kokossis, A.C. Knowledge based models for the analysis of complex separation processes. Comput. Chem. Eng. 2001, 25, 867–878. [Google Scholar] [CrossRef]
- Cussler, E.L.; Wei, J. Chemical product engineering. AIChE J. 2003, 49, 1072–1075. [Google Scholar] [CrossRef]
- Hostrup, M.; Harper, P.M.; Gani, R. Design of environmentally benign processes: Integration of solvent design and separation process synthesis. Comput. Chem. Eng. 1999, 23, 1395–1414. [Google Scholar] [CrossRef]
- Gani, R. Chemical product design: Challenges and opportunities. Comput. Chem. Eng. 2004, 28, 2441–2457. [Google Scholar] [CrossRef]
- Gani, R.; Ng, K.M. Product design—Molecules, devices, functional products, and formulated products. Comput. Chem. Eng. 2015, 81, 70–79. [Google Scholar] [CrossRef]
- Linke, P.; Kokossis, A. Simultaneous Synthesis and Design of Novel Chemicals and Chemical Process Flowsheets; Elsevier Masson SAS: Amsterdam, The Netherlands, 2002; Volume 10. [Google Scholar]
- Solvason, C.C.; Chemmangattuvalappil, N.G.; Eden, M.R. A systematic method for integrating product attributes within molecular synthesis. Comput. Chem. Eng. 2009, 33, 977–991. [Google Scholar] [CrossRef]
- Eden, M.R.; Jørgensen, S.B.; Gani, R.; El-Halwagi, M. Property Integration—A New Approach for Simultaneous Solution of Process and Molecular Design Problems. Comput. Aided Chem. Eng. 2002, 12, 79–84. [Google Scholar]
- Eden, M.R.; Jorgensen, S.B.; Gani, R.; El-Halwagi, M.M. Property cluster based visual technique for synthesis and design of formulations. Comput. Aided Chem. Eng. 2003, 15, 1175–1180. [Google Scholar]
- Eden, M.R.; Jørgensen, S.B.; Gani, R.; El-Halwagi, M.M. A novel framework for simultaneous separation process and product design. Chem. Eng. Process. Process Intensif. 2004, 43, 595–608. [Google Scholar] [CrossRef]
- Kazantzi, V.; Qin, X.; El-Halwagi, M.; Eljack, F.; Eden, M. Simultaneous process and molecular design through property clustering techniques: A visualization tool. Ind. Eng. Chem. Res. 2007, 46, 3400–3409. [Google Scholar] [CrossRef]
- Chemmangattuvalappil, N.G.; Solvason, C.C.; Bommareddy, S.; Eden, M.R. Combined property clustering and GC+ techniques for process and product design. Comput. Chem. Eng. 2010, 34, 582–591. [Google Scholar] [CrossRef]
- Conte, E.; Gani, R.; Malik, T.I. The Virtual Product-Process Laboratory Applied to Personal Care Formulations; Elsevier B.V.: Amsterdam, The Netherlands, 2010; Volume 28. [Google Scholar]
- Conte, E.; Morales-Rodriguez, R.; Gani, R. The virtual product-process design laboratory for design and analysis of formulations. Comput. Aided Chem. Eng. 2009, 27, 825–830. [Google Scholar]
- Conte, E.; Gani, R.; Malik, T.I. The virtual Product-Process Design laboratory to manage the complexity in the verification of formulated products. Fluid Phase Equilib. 2011, 302, 294–304. [Google Scholar] [CrossRef]
- Kalakul, S.; Cignitti, S.; Zhang, L.; Gani, R. Integrated Computer-Aided Framework for Sustainable Chemical Product Design and Evaluation. Comput. Aided Chem. Eng. 2016, 38, 2343–2348. [Google Scholar]
- Gani, R.; Zhang, L.; Kalakul, S.; Cignitti, S. Computer-Aided Molecular Design and Property Prediction. In Tools for Chemical Product Design from Consumer Products to Biomedicine; Martín, M., Eden, M.R., Chemmangattuvalappil, N.G., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Chapter 6; Volume 39, pp. 153–196. [Google Scholar]
- Mondejar, M.E.; Cignitti, S.; Abildskov, J.; Woodley, J.M.; Haglind, F. Prediction of properties of new halogenated olefins using two group contribution approaches. Fluid Phase Equilib. 2017, 433, 79–96. [Google Scholar] [CrossRef]
- Jazrawi, C.; Biller, P.; Ross, A.B.; Montoya, A.; Maschmeyer, T.; Haynes, B.S. Pilot plant testing of continuous hydrothermal liquefaction of microalgae. Algal Res. 2013, 2, 268–277. [Google Scholar] [CrossRef]
- Yun, S.; Lee, J. Advancing societal readiness toward renewable energy system adoption with a socio-technical perspective. Technol. Forecast. Soc. Chang. 2015, 95, 170–181. [Google Scholar] [CrossRef]
- Nissen, O.; Technology, D. Whey processing with membrane technology. Eur. Dairy Mag. 2016, 5, 1–3. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).