Mechanism of Acetyl Salicylic Acid (Aspirin) Degradation under Solar Light in Presence of a TiO2-Polymeric Film Photocatalyst
Abstract
:1. Introduction
2. Experimental Setup
2.1. Materials and Methods
2.2. High Performance Liquid Chromatography
2.3. Photodegradation and LC/MS Analysis
2.4. Fourier Transform Infrared (FTIR)
3. Results and Discussion
3.1. Results
3.2. Mechanism of Photodegradation of ASA
3.3. Reaction Pathways of Acetyl Salicylic Acid
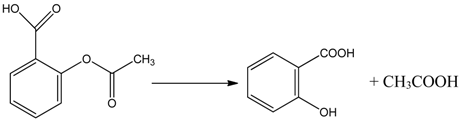
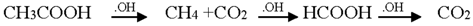
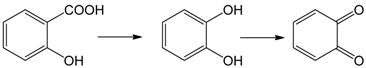
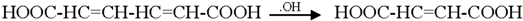
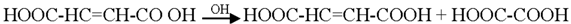
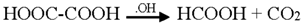
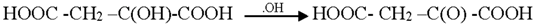
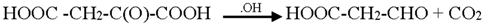
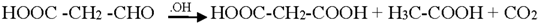

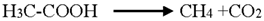
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TiO2 | Titatium Dioxide |
| FTIR | Fourier Transform Infra Red |
| LC/MS | Liquid Chromatography Mass Spectroscopy |
| TOC | Total Organic Carbon content |
| Ppm | Parts per million |
References
- Terry, L.A. Water Pollution. Environ. Law Pract. 1996, 4, 19–29. [Google Scholar]
- Calza, P.; Sakkas, V.A.; Medana, C.; Baiocchi, C.; Dimou, A.; Pelizzetti, E.; Albanis, T. Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl. Catal. B Environ. 2006, 67, 197–205. [Google Scholar] [CrossRef]
- Chemseddine, A.; Boehm, H.P. A study of the primary step in the photochemical degradation of acetic acid and chloroacetic acids on a TiO2 photocatalyst. J. Mol. Catal. 1990, 60, 295–311. [Google Scholar] [CrossRef]
- Muggli, S.D.; Falconer, J.L. Parallel pathways for photocatalytic decomposition of acetic acid on TiO2. J. Catal. 1999, 187, 230–237. [Google Scholar] [CrossRef]
- Dai, Q.; Xia, Y.; Chen, J. Mechanism of enhanced electrochemical degradation of highly concentrated aspirin wastewater using a rare earth La-Y co-doped PbO2 electrode. Electrochim. Acta 2016, 188, 871–881. [Google Scholar] [CrossRef]
- Dai, Q.; Xia, Y.; Jiang, L.; Li, W.; Wang, J.; Chen, J. Enhanced degradation of aspirin by electrochemical oxidation with modified PbO2 electrode and hydrogen peroxide. Int. J. Electrochem. Sci. 2012, 7, 12895–12906. [Google Scholar]
- He, Y.; Huang, W.; Chen, R.; Zhang, W.; Lin, H.; Li, H. Anodic oxidation of aspirin on PbO2, BDD and porous Ti/BDD electrodes: Mechanism, kinetics and utilization rate. Sep. Purif. Technol. 2015, 156, 124–131. [Google Scholar] [CrossRef]
- Xia, Y.; Dai, Q.; Chen, J. Electrochemical degradation of aspirin using a Ni doped PbO2 electrode. J. Electroanal. Chem. 2015, 744, 117–125. [Google Scholar] [CrossRef]
- Carlsson, D.O.; Hua, K.; Forsgren, J.; Mihranyan, A. Aspirin degradation in surface-charged TEMPO-oxidized mesoporous crystalline nanocellulose. Int. J. Pharm. 2014, 461, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Z.; Wei, L.F.; Zhang, Z.H.; Jiang, Q.J.; Wei, Y.J.; Xie, B.; Wei, M.B. Research on photocatalytic H2 production from acetic acid solution by Pt/TiO2 nanoparticles under UV irradiation. Int. J. Hydr. Energy 2009, 34, 9033–9041. [Google Scholar] [CrossRef]
- Méndez-Arriaga, F.; Esplugas, S.; Giménez, J. Photocatalytic degradation of non-steroidal anti-inflammatory drugs with TiO2 and simulated solar irradiation. Water Res. 2008, 42, 585–594. [Google Scholar]
- Uges, D.R.A.; Bloemhof, H.; Juul Christensen, E.K. An HPLC method for the determination of salicylic acid, phenacetin and paracetamol in serum, with indications; two case-reports of intoxication. Pharm. World Sci. 1981, 3, 1309–1315. [Google Scholar] [CrossRef]
- Li, D.; Cheng, X.; Yu, X.; Xing, Z. Preparation and characterization of TiO2-based nanosheets for photocatalytic degradation of acetylsalicylic acid: Influence of calcination temperature. Chem. Eng. J. 2015, 279, 994–1003. [Google Scholar] [CrossRef]
- Christoph, K.; Scheck, F.F. Degradation of phenol and salicylic acid by ultraviolet radiation/hydrogen peroxide/oxygen. Water Res. 1995, 29, 2346–2352. [Google Scholar]
- Masende, Z.P.G.; Kuster, B.F.M.; Ptasinski, K.J.; Janssen, F.J.J.G.; Katima, J.H.Y.; Schouten, J.C. Kinetics of malonic acid degradation in aqueous phase over Pt/graphite catalyst. Appl. Catal. B Environ. 2004, 56, 189–199. [Google Scholar] [CrossRef]
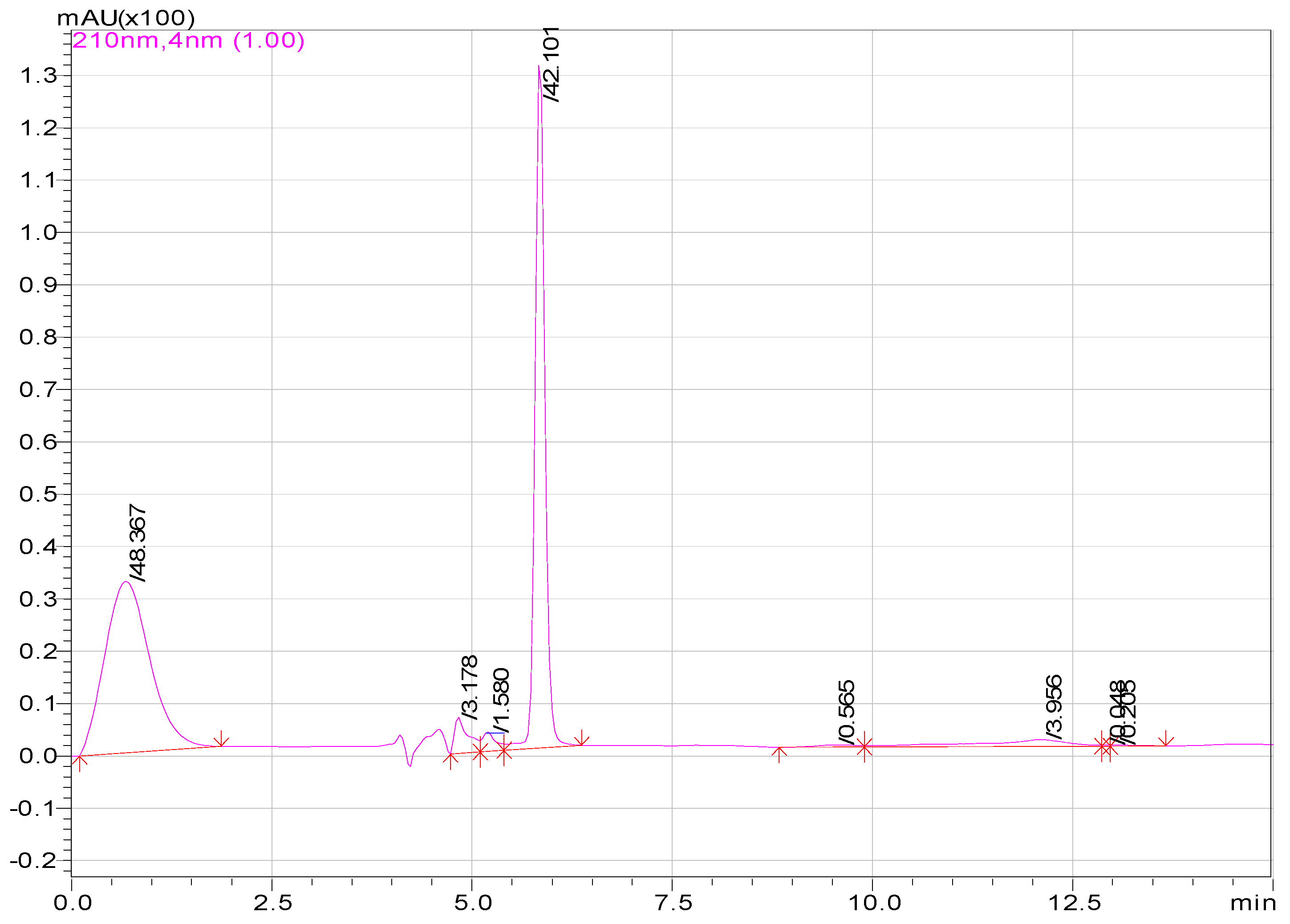
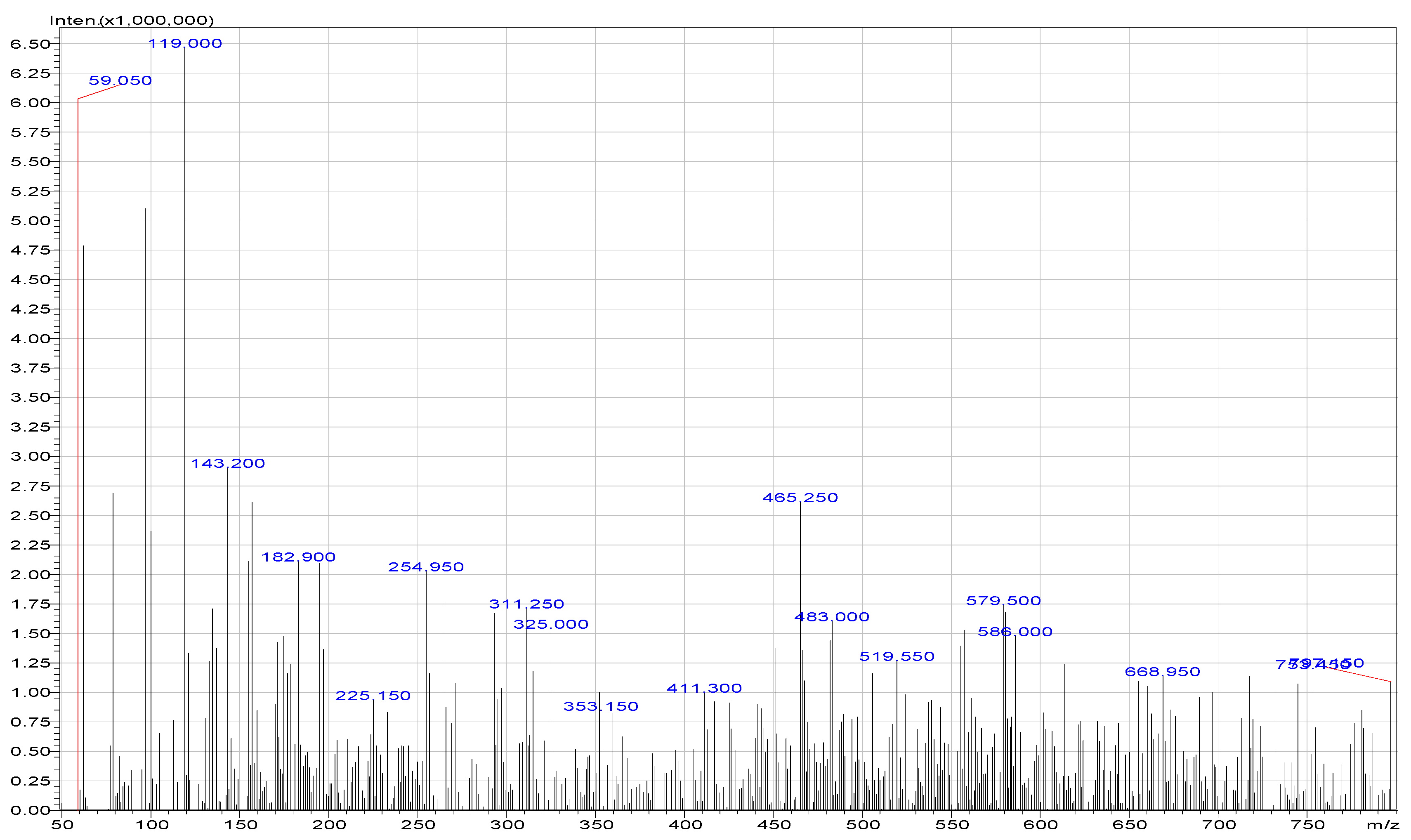
| Compounds | Area of Peaks | Reaction Time (min) | Residence Time (min) | Rates (ppm/min) |
|---|---|---|---|---|
| Aspirin | 1520362 100 | 0–14.3 20 | 14.3 | - |
| Salicylic acid | 1398683 136.8 83.6 | 20 60 130 | 13.2 | 0.008 |
| Hydroquinone | 1091680 1091680 | 60 | 13 | 0.006 |
| Muconic acid | 289863 1052590 | 60 130 | 12.5 | 0.009 |
| Maleic acid | 15455 1000 | 130 220 | 10.5 | 0.005 |
| Malic acid | 505 14000 | 130 220 | 6.2 | 0.004 |
| Oxalic acid | 19005 | 130 | 1.5 | 0.008 |
| Time (min) | Carbon Content (mg/L) |
|---|---|
| 0 | 1.19 |
| 20 | 1.13 |
| 180 | 0.35 |
| 300 | 0.01 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, D.; Ray, A.K.; Barghi, S. Mechanism of Acetyl Salicylic Acid (Aspirin) Degradation under Solar Light in Presence of a TiO2-Polymeric Film Photocatalyst. Processes 2016, 4, 13. https://doi.org/10.3390/pr4020013
Mukherjee D, Ray AK, Barghi S. Mechanism of Acetyl Salicylic Acid (Aspirin) Degradation under Solar Light in Presence of a TiO2-Polymeric Film Photocatalyst. Processes. 2016; 4(2):13. https://doi.org/10.3390/pr4020013
Chicago/Turabian StyleMukherjee, Debjani, Ajay K. Ray, and Shahzad Barghi. 2016. "Mechanism of Acetyl Salicylic Acid (Aspirin) Degradation under Solar Light in Presence of a TiO2-Polymeric Film Photocatalyst" Processes 4, no. 2: 13. https://doi.org/10.3390/pr4020013
APA StyleMukherjee, D., Ray, A. K., & Barghi, S. (2016). Mechanism of Acetyl Salicylic Acid (Aspirin) Degradation under Solar Light in Presence of a TiO2-Polymeric Film Photocatalyst. Processes, 4(2), 13. https://doi.org/10.3390/pr4020013








