1. Introduction
Zinc phosphate conversion coatings are integral to industrial pretreatment lines for automotive and metal-forming applications due to their established ability to enhance corrosion resistance, improve coating adhesion, and stabilize interfacial properties prior to subsequent finishing processes [
1,
2,
3]. The phosphating reaction forms an insoluble crystalline layer through coupled electrochemical dissolution and precipitation at the metal–solution interface, resulting in a durable conversion coating that supports subsequent organic finishes [
4]. Because these reactions are governed by local chemical and electrochemical conditions, coating morphology and surface topography are inherently sensitive to pretreatment chemistry, rendering surface roughness a quality-critical parameter in industrial zinc phosphating [
2].
In high-throughput manufacturing environments, surface roughness functions not merely as a descriptive surface metric but as a specification-bound functional indicator. Variations in roughness directly influence sealing performance, coating thickness uniformity, tribological behavior, and dimensional tolerances, particularly for components incorporating sealing interfaces or tight assembly requirements [
5,
6]. When roughness exceeds defined acceptance limits, corrective actions such as in-facility rework or the segregation and external return of nonconforming components are frequently required [
7]. These responses extend the implications of pretreatment variability beyond surface quality, affecting operational efficiency, logistics flows, and environmental performance [
8,
9].
The mechanisms governing zinc phosphate coating formation have been extensively investigated in laboratory and pilot-scale studies. Coating initiation is known to occur at discrete electrochemical microcells on the steel surface, where local pH, ion availability, and mass-transport conditions determine nucleation density and crystal growth behavior [
4,
10]. Consequently, bath chemistry parameters—including pickling acid composition, dissolved metal ion concentration, accelerator chemistry, temperature, and immersion time—play a decisive role in defining coating morphology and surface roughness [
1]. While this body of work has provided valuable mechanistic insight, its direct translation to industrial process control is often limited by the highly controlled conditions under which such studies are conducted [
11,
12].
Collectively, existing experimental studies on zinc phosphating have provided important insight into the influence of individual bath parameters on coating morphology and surface roughness. However, the majority of these investigations remain limited to laboratory or pilot-scale conditions, where process variables are tightly controlled and statistical designs are optimized for mechanistic isolation rather than industrial decision-making. As a result, reported findings often emphasize qualitative trends or single-factor effects, while providing limited guidance on the relative dominance, practical effect magnitude, or prioritization of control levers under real production constraints. In addition, roughness outcomes are rarely interpreted in terms of acceptance thresholds or downstream operational consequences. These limitations motivate the need for production-scale, statistically grounded studies that translate experimental findings into actionable process control strategies.
Among controllable pretreatment variables, pickling acid chemistry plays a foundational role by defining the surface condition prior to phosphating. Sulfuric acid and phosphoric acid differ substantially in dissolution kinetics, buffering capacity, and interaction with steel substrates. Sulfuric acid is commonly associated with more aggressive dissolution behavior, which can enhance surface activity but also increase susceptibility to localized over-etching [
10]. In contrast, phosphoric acid provides a more buffered chemical environment that promotes uniform surface conditioning. These differences directly influence phosphate nucleation density and subsequent crystal growth, with clear implications for surface roughness evolution under industrial operating conditions [
4].
Dissolved ferrous iron (Fe
2+) is another well-recognized factor in zinc phosphating systems and has been investigated extensively in prior mechanistic studies. In industrial lines, Fe
2+ accumulates naturally in both pickling and phosphating baths as a consequence of continuous steel dissolution. While controlled Fe
2+ levels may contribute to surface activation, excessive accumulation has been associated with heterogeneous nucleation, coarser crystal growth, and increased roughness variability [
4,
12]. Despite the breadth of mechanistic knowledge available, the relative importance of Fe
2+ compared with other bath chemistry parameters under real production conditions remains insufficiently quantified, and Fe
2+ management is therefore often based on empirical practice rather than statistically prioritized control.
Accelerator chemistry constitutes an additional and often decisive control lever in industrial zinc phosphating. Nitrate-based accelerators are widely employed to enhance reaction kinetics, refine phosphate crystal morphology, and promote uniform surface coverage by shifting the balance between anodic metal dissolution and cathodic reduction reactions [
13]. These effects are directly relevant to roughness stability and reproducibility, particularly under operating conditions where bath chemistry evolves continuously with throughput and aging. Further refinement of coating microstructure has been reported through the use of additives such as α-zirconium phosphate, which can enhance coating uniformity and stability when appropriately integrated into the phosphating system [
14].
Despite decades of mechanistic investigation, a critical gap persists between laboratory-scale understanding and robust industrial implementation. Most published studies isolate individual parameters under tightly controlled conditions, whereas industrial phosphating lines operate under dynamic and constrained environments characterized by continuously evolving bath chemistry, variability in incoming substrate surface history, and limited flexibility for extended experimentation [
15,
16,
17]. As a result, production-scale, statistically defensible evidence identifying which bath chemistry parameters genuinely dominate surface roughness variability—and which exert secondary or interaction-dependent effects—remains scarce.
In addition, although surface roughness is widely recognized as a key quality indicator in zinc phosphating, its implications are typically discussed only in terms of coating performance and downstream paint quality. The environmental consequences of roughness instability—specifically its role in triggering rework, segregation, and external return logistics—have received comparatively little explicit attention. Product carbon footprint methodologies, as formalized in ISO 14067 [
18] and the GHG Protocol [
19], provide structured frameworks for quantifying greenhouse gas emissions associated with manufacturing processes. However, few studies have linked surface-quality deviations in pretreatment operations to product-level carbon footprint outcomes under real production conditions, particularly with explicit treatment of uncertainty [
20,
21].
In the context of surface pretreatment operations, carbon footprint assessment is particularly sensitive to quality-driven process deviations, as rework and external return logistics introduce additional energy use and transport-related emissions that are not captured under nominal production conditions. Accordingly, carbon footprint analysis in this study is framed as a decision-conditioned evaluation, focusing on how roughness-induced quality outcomes alter process-level emission profiles rather than on absolute emission minimization.
While prior studies have established the mechanistic roles of bath chemistry in zinc phosphating, industrial implementation typically operates under dynamic constraints that limit experimentation and hinder statistically defensible prioritization of control levers. The novelty of this work lies in providing production-scale, uncertainty-aware evidence from a fully operational line that ranks the relative influence of pickling acid chemistry, Fe2+ levels, and nitrate acceleration on surface roughness stability using effect sizes and bootstrap confidence intervals, and independently validates the identified trends with paired measurements from routine production parts. In addition, the study explicitly translates roughness-driven quality decisions (first-pass production, rework, and external return logistics) into scenario-based product carbon footprint outcomes within an ISO 14067-aligned gate-to-gate framework with Monte Carlo uncertainty propagation.
Accordingly, the primary motivation of this study is process-level quality stabilization, with carbon footprint assessment employed as a consequence-based evaluation of how quality-driven production decisions propagate into environmental performance, rather than as an independent or parallel research objective. In industrial pretreatment lines, surface roughness therefore acts not only as a quality descriptor but as a decision-triggering parameter that determines subsequent production pathways. When roughness exceeds defined acceptance limits, components are routed into first-pass acceptance, in-facility rework, or external return logistics, each associated with distinct material, energy, and transport requirements. In this study, carbon footprint assessment is explicitly conditioned on these roughness-driven production scenarios, treating surface roughness as an upstream decision-linked driver rather than evaluating environmental performance as an independent or parallel process metric.
2. Materials and Methods
2.1. Industrial Zinc Phosphating Line and Process Boundary
The experiments were conducted on a fully operational industrial zinc phosphating line used for high-throughput steel components. For clarity, the main process steps illustrated in
Figure 1 are also described textually in this section, and the figure is provided solely as a visual aid to support process flow understanding. The line, consists of eight primary pretreatment stages, with an additional final rinsing step operated depending on customer specifications. The sequential stages include degreasing, rinsing, pickling, surface activation, zinc phosphating, and final rinsing operations, all arranged in an immersion-based configuration under continuous production conditions.
To isolate the influence of chemical pretreatment on surface roughness evolution, the experimental boundary was restricted to the process stages up to and including zinc phosphating. Any subsequent sealing, lubrication, or oiling steps were deliberately excluded, as such treatments can modify apparent surface roughness and confound interpretation of phosphate coating effects. All non-investigated stages were operated at standard production setpoints for temperature, residence time, and line speed throughout the experimental campaign.
The line was maintained under quasi-steady-state conditions during data acquisition. Chemical adjustments were avoided during experimental runs except where explicitly required by the experimental design, ensuring that observed variations in surface roughness reflect controlled changes in bath chemistry rather than transient operational disturbances.
Machined steel components representative of routine automotive production were used as substrates. The steel grade conforms to supplier specifications for automotive pretreatment applications. To minimize confounding effects arising from excessive machining damage or surface defects, all components were pre-screened prior to pretreatment. Initial surface roughness was measured before exposure to the pretreatment line, and only components exhibiting an initial roughness of Rz < 4 μm were included in the study. This threshold was selected to ensure that subsequent roughness evolution is primarily governed by chemical pretreatment rather than pre-existing surface irregularities. Components were randomly allocated to experimental conditions to avoid systematic bias related to machining history.
Two mineral acids commonly employed in industrial pretreatment were evaluated as a categorical factor: sulfuric acid (H2SO4) and phosphoric acid (H3PO4). Both pickling baths were operated at production-relevant setpoints for acid concentration, temperature, and immersion time. These parameters were held constant throughout the experimental campaign to isolate the effect of acid chemistry. Sulfuric acid (H2SO4) and phosphoric acid (H3PO4) were supplied by Merck, Darmstadt, Germany. Dissolved ferrous iron (Fe2+) accumulation in the pickling bath was identified as a critical process variable due to its continuous generation during steel dissolution. Fe2+ concentration was monitored using standard titrimetric procedures routinely applied in industrial pretreatment facilities. Two levels, designated low and high, were defined based on nominal industrial operating ranges. Fe2+ control was implemented using bath management practices consistent with normal production, thereby preserving industrial realism.
Zinc phosphating was carried out using a commercial zinc phosphate formulation representative of automotive pretreatment practice. Bath temperature, pH, total acidity, and free acidity were maintained at standard production setpoints throughout the study. A nitrate-based accelerator was selected due to its widespread industrial use. The nitrate-based accelerator was a commercial formulation supplied by a commercial supplier in Türkiye. The manufacturer is located in Türkiye, and the formulation represents a standard industrial product used in routine automotive pretreatment operations. Accelerator dosage was investigated at two levels: 0 g L−1 (absence of acceleration) and 0.5 g L−1, corresponding to typical industrial application levels. These levels were chosen to represent the presence and absence of acceleration effects without departing from routine operating practice. Dissolved Fe2+ concentration in the phosphating bath was monitored independently of the pickling bath and controlled at predefined low and high levels consistent with industrial norms. This approach enabled evaluation of both direct and interaction-dependent effects of Fe2+ across consecutive process stages.
2.2. Experimental Design and Process Variables
A Taguchi L16 (2
4) orthogonal array was employed to evaluate the combined influence of four factors at two levels (
Table 1):
Pickling acid chemistry (H2SO4 vs. H3PO4),
Fe2+ concentration in the pickling bath (low vs. high),
Fe2+ concentration in the phosphating bath (low vs. high),
Nitrate accelerator dosage (0 vs. 0.5 g L−1).
The Taguchi approach was selected due to its suitability for screening dominant process variables under industrial constraints, where full factorial experimentation is impractical. Taguchi orthogonal arrays provide a statistically efficient framework for identifying and prioritizing the main effects of multiple control factors with a substantially reduced number of experimental runs, making them particularly suitable for high-throughput industrial environments where time, cost, and operational flexibility are limited [
17,
22,
23]. The effectiveness of the Taguchi method for process optimization and surface quality control has been extensively demonstrated across a wide range of industrial manufacturing operations, including machining, injection molding, welding, and composite processing [
24,
25,
26,
27,
28,
29,
30].
Surface roughness (Rz) was defined as the response variable, with minimization specified as the objective (“smaller-the-better”). Experimental runs were executed in a sequence compatible with production scheduling to avoid disruption of routine operations.
The Taguchi L16 (24) orthogonal array was applied with a screening-oriented objective, aiming to identify and prioritize dominant process control parameters under realistic industrial constraints. Consistent with standard Taguchi methodology, the statistical analysis was therefore restricted to main effects. Interaction terms were not explicitly modeled, as the primary goal was to establish robust main effect dominance within a production-scale operating window rather than to resolve higher-order interactions. Formal interaction quantification would require a full factorial or response surface design, which was not feasible within the operational, scheduling, and cost constraints of an active industrial zinc phosphating line.
For clarity, the “low” and “high” Fe2+ levels investigated in this study correspond to concentration ranges routinely encountered in industrial zinc phosphating practice. Low Fe2+ conditions represent freshly controlled or well-managed bath states, whereas high Fe2+ conditions reflect accumulated iron levels approaching the upper limits of routine industrial operation prior to corrective bath maintenance. Specific Fe2+ concentrations were maintained consistently within these predefined industrially relevant ranges throughout each experimental run. This approach ensures industrial realism while avoiding artificial laboratory conditions that are not representative of production-scale pretreatment lines.
Similarly, Fe2+ concentration levels in the phosphating bath were defined as low and high based on routine industrial operating ranges recommended for stable zinc phosphating performance. These levels were selected to represent realistic bath aging conditions rather than extreme or non-operational concentrations.
The full Taguchi L16 experimental matrix and corresponding roughness statistics are provided in
Appendix A Table A1.
2.3. Surface Roughness Measurement
Surface roughness was measured using a contact stylus profilometer in accordance with ISO 4287 terminology and definitions. Surface roughness measurements were performed using a MarSurf GD 120 contact stylus profilometer (Mahr GmbH, Göttingen, Germany), operated under standard industrial metrology procedures and in compliance with ISO 4287. The instrument employs a diamond stylus to record surface height variations along a defined traverse length with minimal contact force, ensuring accurate profiling without surface alteration. Extended traverse capability (up to 120 mm) enabled reliable measurement of representative surface areas [
31].
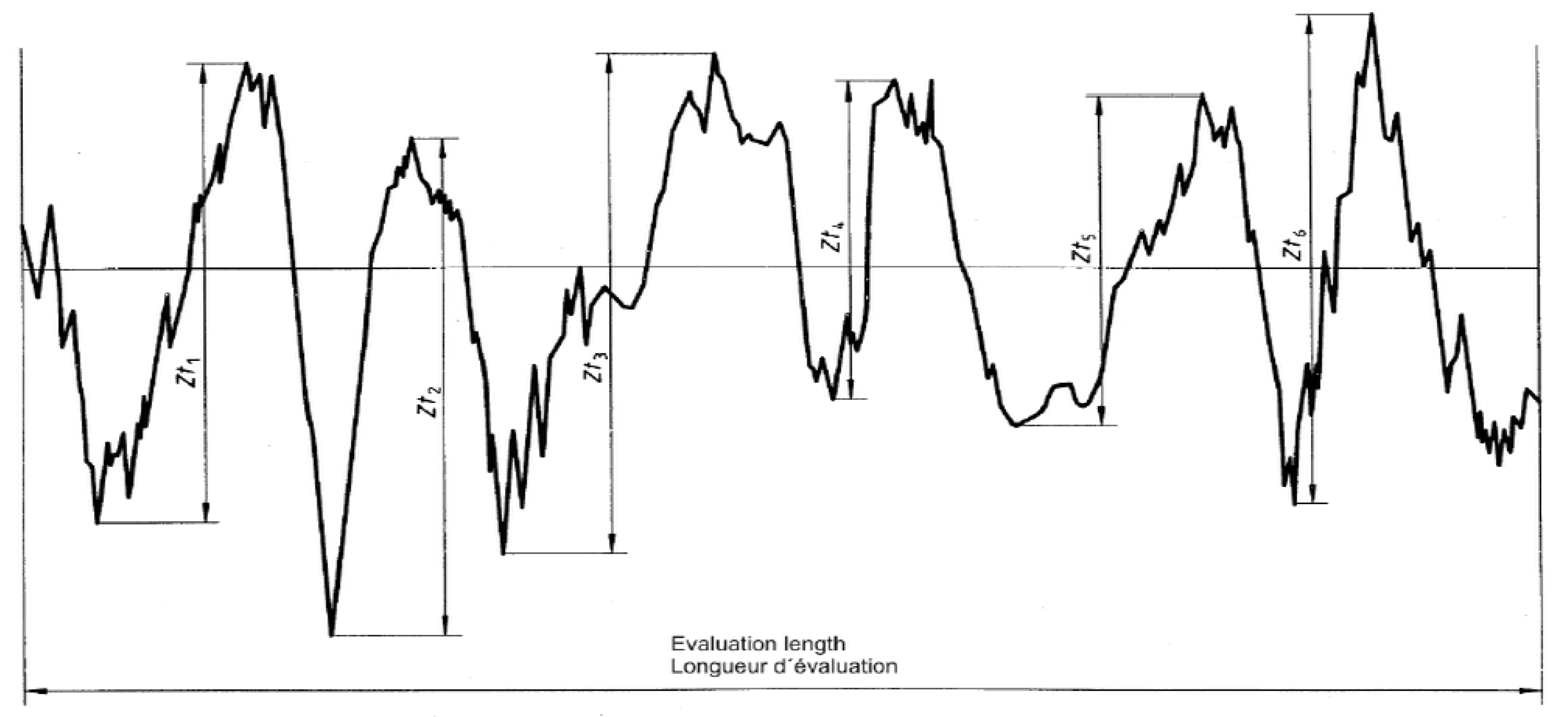
Figure 2 demonstrates the Rz surface roughness parameter and the associated sampling length definition. The Rz parameter was selected as the primary roughness metric because it is particularly sensitive to peak-to-valley surface features that govern sealing performance, coating thickness uniformity, and pass/fail acceptance decisions in industrial zinc phosphating lines.
While average-based roughness parameters such as Ra and Rq are widely used for general surface characterization, they tend to smooth localized surface extremes that are operationally decisive under specification-bound industrial quality criteria. In contrast, Rz captures the mean maximum height difference within the evaluation length and is therefore more representative of worst-case surface conditions that trigger rework or rejection in high-throughput pretreatment operations. Accordingly, the use of Rz in this study reflects industrial quality control practice rather than purely descriptive surface analysis, consistent with ISO 4287 definitions [
31].
For each experimental condition, three independent profilometric scans were performed at representative locations on the treated surface. Measurement locations were selected to avoid edges, corners, and visually apparent defects. Mean Rz values, standard deviations, and coefficients of variation were calculated to distinguish process-induced roughness variability from measurement-related uncertainty, thereby ensuring robustness of the statistical evaluation (
Table 2). This repetition strategy enables separation of process-driven roughness effects from instrument-related measurement scatter under industrial production conditions.
Alternative three-dimensional optical or laser-based surface roughness measurement techniques were not employed in this study. The deliberate use of contact stylus profilometry reflects its widespread acceptance in industrial pretreatment quality control and its direct compatibility with specification-based acceptance criteria applied in automotive zinc phosphating lines.
2.4. Scanning Electron Microscopy (SEM)
Scanning electron microscopy was employed to provide qualitative microstructural context for the surface roughness trends identified through profilometric measurements and statistical analysis. SEM was not intended for quantitative surface evaluation or to replace numerical roughness metrics, but to support mechanistic interpretation by visualizing zinc phosphate coating morphology under representative roughness regimes.
Specimens corresponding to statistically distinct low- and high-roughness conditions were selected based on the outcomes of the design of experiments and general linear model analysis, ensuring direct linkage between SEM observations and experimentally validated roughness differences.
Surface morphology was examined using a ZEISS Gemini 300 field-emission scanning electron microscope (ZEISS, Oberkochen, Germany). Prior to imaging, samples were coated with a thin gold layer to enhance electrical conductivity. Images were acquired using a secondary electron detector at an accelerating voltage of 10 kV, with magnifications of 150× and 400×.
SEM observations focused on qualitative features such as crystal size, distribution, compactness, and surface coverage. These observations are interpreted strictly as supportive, mechanistic evidence complementing the statistically derived roughness trends and are not used for independent quantitative comparison or inferential analysis.
2.5. Statistical Analysis
Initial factor screening was conducted using Taguchi signal-to-noise (S/N) ratios calculated under the “smaller-the-better” criterion (
Table A2). Statistical analyses were performed using IBM SPSS Statistics, version 26.0 (IBM Corp., Armonk, NY, USA). To enable inferential interpretation, mean Rz values were analyzed using a general linear model (GLM) including main effects (
Table 3 and
Table A3). Model assumptions were evaluated using Shapiro–Wilk tests for residual normality and Levene’s tests for homogeneity of variances. Statistical significance was assessed at a 95% confidence level (
Table 3).
Practical relevance was quantified using partial eta-squared (ηp
2) effect sizes (
Table 4). In addition, production relevance was independently validated using a paired dataset of 25 components measured before and after phosphating. Paired
t-tests were employed, with non-parametric alternatives applied where normality assumptions were not fully satisfied.
To quantify uncertainty and reduce sensitivity to distributional assumptions, bootstrap resampling (10,000 iterations) was applied to key contrasts, yielding 95% confidence intervals (
Table 5 and
Table A4). Monte Carlo uncertainty analysis was conducted using MATLAB, version R2023a (The MathWorks, Inc., Natick, MA, USA).
2.6. Carbon Footprint Assessment and Uncertainty Analysis
Carbon footprint calculations were not conducted as an independent environmental assessment. Instead, they were explicitly conditioned on surface-roughness-driven quality decisions identified in the experimental program. Surface roughness evaluation determined whether a production batch followed a first-pass acceptance, in-facility rework, or external return pathway, and carbon footprint results were calculated separately for each decision-based scenario. Thus, carbon footprint analysis in this study serves to evaluate the environmental consequences of quality-driven process outcomes, not to optimize emissions independently of surface roughness control.
The carbon footprint assessment was based entirely on real operational data obtained from the same fully operational industrial zinc phosphating line on which the experimental investigations were conducted. The carbon footprint assessment was conducted using site-specific operational data obtained from the industrial pretreatment line, including electricity and natural gas consumption, chemical usage, and transport distances associated with quality-driven routing decisions. No simulated, generic, or hypothetical plant data were used at any stage of the carbon footprint calculation. The system boundary was limited to a gate-to-gate perspective in order to isolate the effects of pretreatment-related quality outcomes and to avoid confounding influences from upstream material production or downstream use phases. Product carbon footprint evaluation was performed using a gate-to-gate system boundary aligned with ISO 14067 principles [
18]. The functional unit was defined as one production batch of
N = 5520 components.
Three operational scenarios were evaluated:
- (i)
first-pass production,
- (ii)
in-facility rework, and
- (iii)
external return logistics of nonconforming components.
To avoid double counting, transport-related emissions were assigned exclusively to scenarios involving off-site logistics. Carbon footprint calculations incorporated site-specific energy consumption data and chemical usage associated with the pre-treatment line. Transport distances used in the analysis correspond to actual logistics routes employed by the industrial facility under quality-driven return conditions.
Figure 3 illustrates the decision-based framework linking surface roughness evaluation to quality outcomes and associated carbon footprint scenarios.
Uncertainty propagation was performed using Monte Carlo simulation (10,000 iterations) with triangular uncertainty distributions applied to energy and transport inputs. Sensitivity analysis was conducted to identify dominant contributors to scenario-specific carbon footprint outcomes. Uncertainty analysis was included to reflect operational variability inherent to industrial production and to ensure that scenario-level differences remain robust under realistic input fluctuations.
3. Results and Discussion
3.1. Descriptive Statistics and Production-Scale Roughness Response
Surface roughness (Rz) values obtained from the Taguchi L16 (2
4) experimental matrix ranged from 5.1 µm to 17.1 µm, corresponding to a more than threefold variation across operating conditions (
Table A1). Median values closely tracked mean responses, and interquartile ranges remained narrow relative to between-condition differences, indicating that roughness distributions were not strongly skewed and that central tendency measures are representative. Within-condition variability was consistently low. Across all experimental runs, the coefficient of variation (CV) of replicated profilometric measurements ranged from 10.8% to 15.0% (mean CV = 12.1%), which is considered acceptable for contact profilometry under industrial production conditions. Importantly, differences in mean Rz values between experimental conditions exceeded within-condition standard deviations by a factor of 3–6, confirming that the observed variability is predominantly process-induced rather than measurement-driven.
This observation is consistent with classical surface metrology studies demonstrating that, when standardized measurement protocols are applied, roughness variability on manufactured surfaces is primarily governed by process conditions rather than instrumental uncertainty [
5,
6].
Independent production validation using paired measurements from 25 components further substantiated these findings (
Table 6). Mean surface roughness increased from 2.99 ± 0.60 µm prior to pretreatment to 5.15 ± 0.89 µm after phosphating, corresponding to a mean change in ΔRz = +2.16 µm. This increase was statistically significant (paired
t-test:
t (24) = 13.33,
p = 1.38 × 10
−12) and associated with a very large standardized effect size (Cohen’s dz = 2.67). Robustness against non-normality was confirmed using a Wilcoxon signed-rank test (
p = 5.96 × 10
−8).
3.2. Main Effects Identified by GLM Analysis
Inferential analysis was performed using a general linear model including main effects only, consistent with the screening-oriented objective of the Taguchi L16 design and the constraints of production-scale experimentation. Interaction terms were not included in the formal statistical model in order to preserve model parsimony and avoid overfitting. The analysis revealed statistically significant main effects for pickling acid chemistry and nitrate accelerator dosage, whereas dissolved Fe2+ concentration in both the pickling and phosphating baths did not exhibit statistically significant independent effects within the investigated Fe2+ concentration ranges.
Pickling acid chemistry exerted a strong influence on surface roughness (F (1,11) = 20.92, p = 0.000798). Averaged across all other factors, sulfuric acid pickling yielded a mean roughness of 13.13 ± 2.63 µm, whereas phosphoric acid pickling resulted in a substantially lower mean roughness of 7.69 ± 2.39 µm, corresponding to a mean difference of +5.44 µm.
The dominant role of pickling acid chemistry observed here is consistent with mechanistic studies demonstrating that acid type governs dissolution kinetics, buffering behavior, and surface activation homogeneity, which in turn directly influence phosphate nucleation density and subsequent crystal growth [
4,
10].
Nitrate accelerator dosage exhibited the strongest effect (F (1,11) = 34.74, p = 0.000104). Conditions without nitrate acceleration produced a mean roughness of 13.80 ± 2.87 µm, compared with 6.79 ± 2.16 µm under accelerated conditions, yielding a mean difference of +7.01 µm.
This behavior aligns with prior studies reporting that nitrate-based accelerators regulate cathodic reaction kinetics and refine phosphate crystal morphology by stabilizing electrochemical microcell activity during coating formation [
12]. The large effect size identified in the present study indicates that accelerator control functions as a primary roughness stabilization lever under industrial operating conditions rather than as a secondary optimization parameter.
By contrast, Fe
2+ concentration in the pickling bath (F (1,11) = 0.90,
p = 0.361) and phosphating bath (F (1,11) = 1.43,
p = 0.247) did not reach statistical significance as standalone factors within the investigated operating window defined by routine industrial practice. Model diagnostics confirmed validity of the inferential framework (Shapiro–Wilk
p = 0.226; Levene’s test
p = 0.673) (
Table 7).
3.3. Effect Size Analysis and Industrial Threshold Interpretation
Effect-size analysis demonstrated that nitrate accelerator dosage (ηp2 = 0.760) and pickling acid chemistry (ηp2 = 0.655) together account for the majority of explainable variance in surface roughness, representing large effects with direct industrial relevance. In contrast, Fe2+ concentration in the pickling (ηp2 = 0.076) and phosphating baths (ηp2 = 0.120) exhibited small effect sizes, indicating limited practical influence when considered independently within the Fe2+ concentration ranges investigated in this study.
The limited independent contribution of Fe
2+ concentration does not contradict the extensive mechanistic literature describing iron participation in zinc phosphating reactions [
2,
13] but rather indicates that under routinely managed industrial concentration windows, Fe
2+ acts as a secondary or context-dependent factor whose influence is overshadowed by stronger chemical controls.
From an industrial quality perspective, roughness increases on the order of 5–7 µm are highly consequential. In automotive pretreatment practice, such differences commonly shift components from within nominal acceptance limits into conditional acceptance or nonconformance categories, thereby triggering rework or segregation decisions. Accordingly, the effect sizes observed for acid chemistry and nitrate acceleration correspond not only to statistical significance but also to operational decision thresholds relevant to first-pass yield.
Bootstrap resampling (10,000 iterations) confirmed the robustness of these effects. Sulfuric acid pickling increased roughness relative to phosphoric acid pickling by +5.44 µm (95% CI: +1.51 to +9.57 µm), while omission of nitrate acceleration increased roughness by +7.01 µm (95% CI: +3.68 to +10.58 µm). The exclusion of zero from both confidence intervals confirms the stability of these findings.
3.4. Exploratory Interaction Patterns and Mechanistic Context
Given the screening-oriented nature of the experimental design, interaction effects are not treated as statistically inferred outcomes in this study. Instead, factor-level combinations are examined to identify qualitative response patterns that provide mechanistic context. These observations are therefore presented strictly as exploratory and descriptive, and not as validated interaction effects.
Although interaction terms were not explicitly included in the statistical model, the experimental results were examined across factor-level combinations to identify qualitative response patterns that may provide mechanistic context. These observations are presented strictly as exploratory and descriptive, rather than as statistically tested interaction effects.
Under phosphoric acid pickling combined with nitrate acceleration, surface roughness values remained consistently low (Rz ≈ 5–6 µm) across both investigated Fe2+ levels. In contrast, sulfuric acid pickling without nitrate acceleration—particularly at elevated Fe2+ levels—was associated with the highest roughness values (Rz > 16 µm) and increased variability. These patterns are not interpreted as statistically validated interaction effects, but as context-dependent response tendencies observed within the Fe2+ concentration ranges investigated in this study and within the screened experimental space.
These qualitative response patterns are mechanistically consistent with established zinc phosphating theory, which associates aggressive dissolution conditions with heterogeneous nucleation and coarser crystal growth, while buffered chemical environments favor uniform nucleation and stabilized coating morphology [
4].
From a mechanistic perspective, the observed trends are consistent with established zinc phosphating theory. Buffered surface conditioning under phosphoric acid pickling promotes more uniform surface activation, while nitrate-based acceleration enhances nucleation density and stabilizes crystal growth. Under these stabilized conditions, Fe2+ can participate in controlled microcell formation without inducing excessive heterogeneity. Conversely, the more aggressive dissolution associated with sulfuric acid pickling, when combined with elevated Fe2+ levels and the absence of acceleration, may favor heterogeneous nucleation and coarser crystal growth, resulting in increased surface roughness.
Given the screening-oriented nature of the Taguchi design and the absence of formal interaction modeling, these interpretations are intentionally limited to qualitative mechanistic context. Quantitative assessment of interaction effects, such as acid chemistry × Fe2+ concentration, would require a dedicated factorial or response surface methodology and therefore falls beyond the scope of the present production-scale screening study.
3.5. SEM-Supported Morphological Interpretation
Scanning electron microscopy was used to provide qualitative microstructural support for the statistically identified surface roughness regimes. The SEM micrographs presented in
Figure 4 correspond to representative experimental conditions associated with low- and high-roughness responses selected based on the outcomes of the design of experiments and GLM analysis, ensuring consistency between morphological observations and statistically validated roughness differences.
Under low-roughness conditions—most notably phosphoric acid pickling combined with nitrate acceleration—the coatings exhibit finer and more homogeneous crystal morphologies with relatively uniform surface coverage, indicative of stabilized nucleation and controlled crystal growth. In contrast, high-roughness conditions, particularly sulfuric acid pickling without nitrate acceleration, are characterized by coarser and more heterogeneous crystal structures, reflecting uneven nucleation behavior and locally intensified growth kinetics.
These observations are consistent with prior reports linking accelerated nucleation and controlled growth kinetics to refined phosphate crystal morphology, whereas intensified localized dissolution promotes heterogeneous crystal development and increased roughness [
4].
These SEM observations are interpreted strictly as qualitative, mechanistic support for the statistically derived roughness trends and are not used for quantitative comparison or independent inference. The observed morphological differences are consistent with the direction and magnitude of roughness variations measured profilometrically, reinforcing the plausibility of the identified dominant process parameters without extending the scope of SEM beyond its intended supportive role.
3.6. Statistical Interpretation of Carbon Footprint Outcomes
Scenario-based carbon footprint analysis demonstrated clear numerical separation between quality-driven operational outcomes (
Table 8).
It is emphasized that these differences arise from roughness-triggered production decisions rather than from marginal numerical variation in surface roughness itself.
The emission penalties associated with rework and external return logistics are consistent with broader manufacturing sustainability literature identifying quality deviations and reverse logistics as major contributors to avoidable energy use and transport-related emissions [
8,
20].
Median emissions for first-pass production were 9.85 tCO
2e (P5–P95: 9.19–10.51 tCO
2e), while in-facility rework increased emissions to 10.25 tCO
2e (P5–P95: 9.58–10.91 tCO
2e). Monte Carlo distributions for first-pass production and rework scenarios showed partial overlap; however, the median difference (+0.40 tCO
2e) consistently favored first-pass production, indicating a systematic emission penalty associated with rework. In contrast, return logistics scenarios exhibited minimal distribution overlap with first-pass production, with median emissions increasing to 18.60 tCO
2e (P5–P95: 16.85–20.35 tCO
2e) (
Table A5).
Transport-related processes accounted for approximately half of the total carbon footprint in the external return scenario and dominated the incremental emission increase relative to first-pass production. These results indicate that carbon footprint differences are not merely numerical artifacts but reflect decision-driven shifts between statistically distinguishable emission regimes, particularly when roughness deviations trigger external returns. (
Table 9).
4. Conclusions
This study provides production-scale evidence linking chemical bath control in industrial zinc phosphating to surface roughness stability and downstream carbon footprint performance. Within this framework, carbon footprint outcomes are interpreted as downstream consequences of surface roughness stabilization strategies, reinforcing process quality as the central motivation of the study. The results demonstrate that pickling acid chemistry and nitrate accelerator dosage are the dominant control levers governing roughness behavior under real manufacturing conditions. Phosphoric acid pickling combined with nitrate acceleration consistently yields lower and more stable roughness, directly improving first-pass yield and supporting robust industrial quality control.
Within the Fe2+ concentration ranges investigated—selected to reflect routine industrial operating windows—dissolved Fe2+ does not act as a primary independent driver of surface roughness but contributes in a secondary, context-dependent manner alongside the dominant chemical controls. Qualitative SEM observations support these findings by showing finer and more homogeneous phosphate crystal morphologies under stabilized chemical conditions, while roughness instability regimes correspond to coarser and more heterogeneous structures.
Beyond surface quality, the study demonstrates that roughness instability functions as an upstream carbon risk driver. Carbon footprint analysis reveals a clear hierarchy of impact, with first-pass production representing the baseline emission state, in-facility rework introducing a moderate penalty, and external return logistics generating disproportionately high emissions dominated by transport processes. Accordingly, stabilizing surface roughness at the pretreatment stage offers a high-leverage pathway for simultaneous quality improvement and carbon footprint mitigation.
Overall, the findings provide prioritized and decision-relevant guidance for industrial zinc phosphating, illustrating how statistically grounded process control can support both manufacturing efficiency and environmental performance within constrained production environments. Future work should extend this framework through formal interaction modeling, multi-site validation, and expansion toward full life cycle assessment.