Effect of Glycerol Concentration on the Properties of Semolina- and Farina-Based Biodegradable Films
Abstract
1. Introduction
1.1. Background on Biodegradable Materials
1.2. Overview of Semolina and Farina
1.3. Applications of Biodegradable Films
1.4. Objectives of the Study
2. Materials and Methods
2.1. Materials Propotions/Samples
- Glicerol
- By Biomus—Poland (Chemiczna 7, Lublin); class: pure; Nr CAS: 56-81-5; Nr EC 200-289-5
- Semolina
- By Radix-bis—Poland (Gerberowa 22, Rotmanka); From durum wheat from Austria
- Farina
- Made by Cenos Sp. z o. o.—Poland (Gen. Sikorskiego 22 Września); From common wheat from Poland
2.2. Film Preparation
2.3. Tensile Testing
2.4. Surface Properties
2.5. Colorimetric Properties
- L*: This represents the lightness of the color. Its value ranges from 0 (absolute black) to 100 (diffuse white).
- a*: This axis represents the green-red opponent colors. A negative a* value indicates a greener color, while a positive a* value indicates a redder color.
- b*: This axis represents the blue-yellow opponent colors. A negative b* value indicates a bluer color, while a positive b* value indicates a yellower color.
- D: Absorbance.
3. Results
3.1. Process for Obtaining Samples
3.2. Tensile Testing
3.3. Surface Free Energy
3.4. Surface Microscopic Analysis
3.4.1. Optical Images
3.4.2. SEM Images
3.4.3. Optical Roughness Measurement
3.5. Color
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1
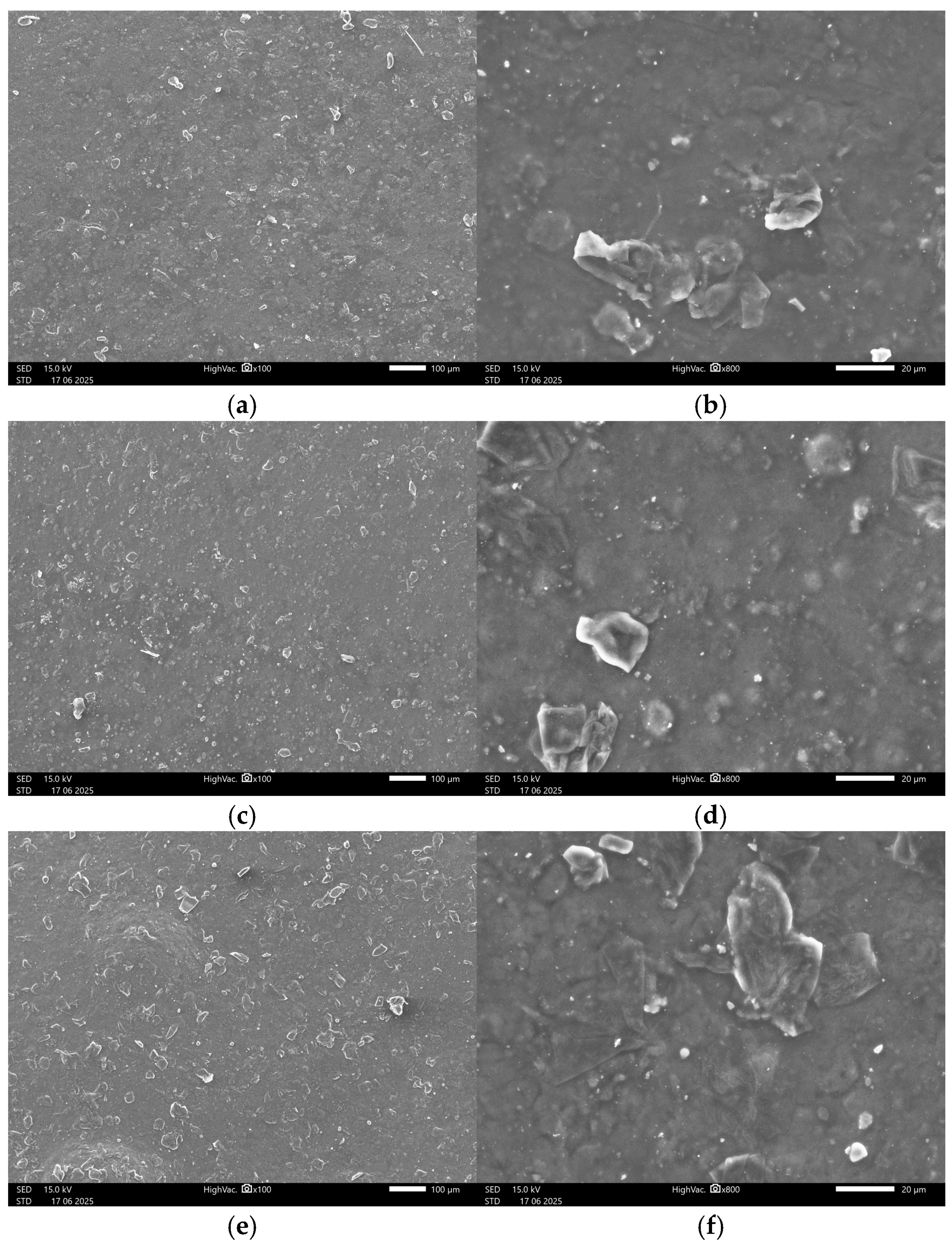
Appendix A.2
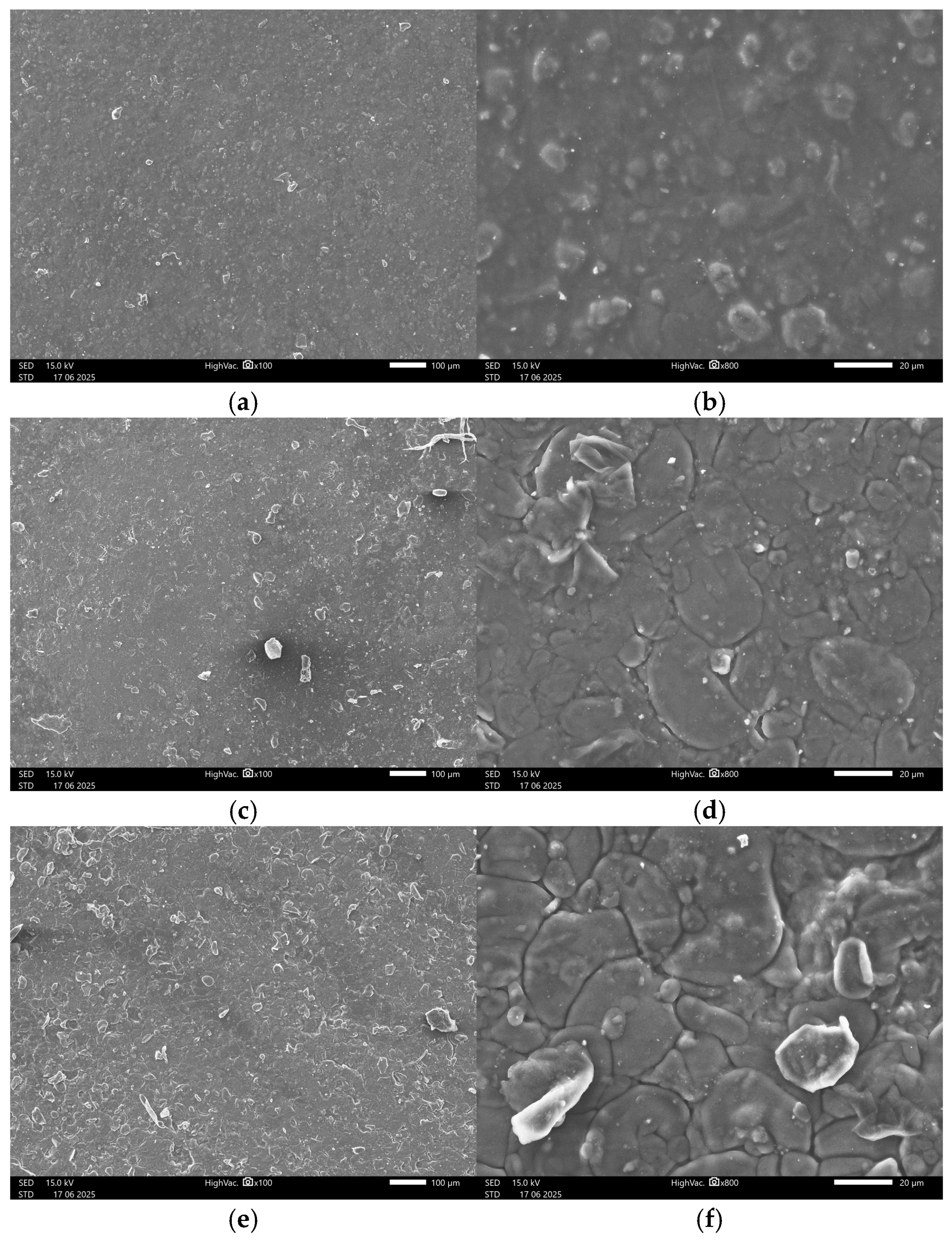
Appendix B
Appendix B.1
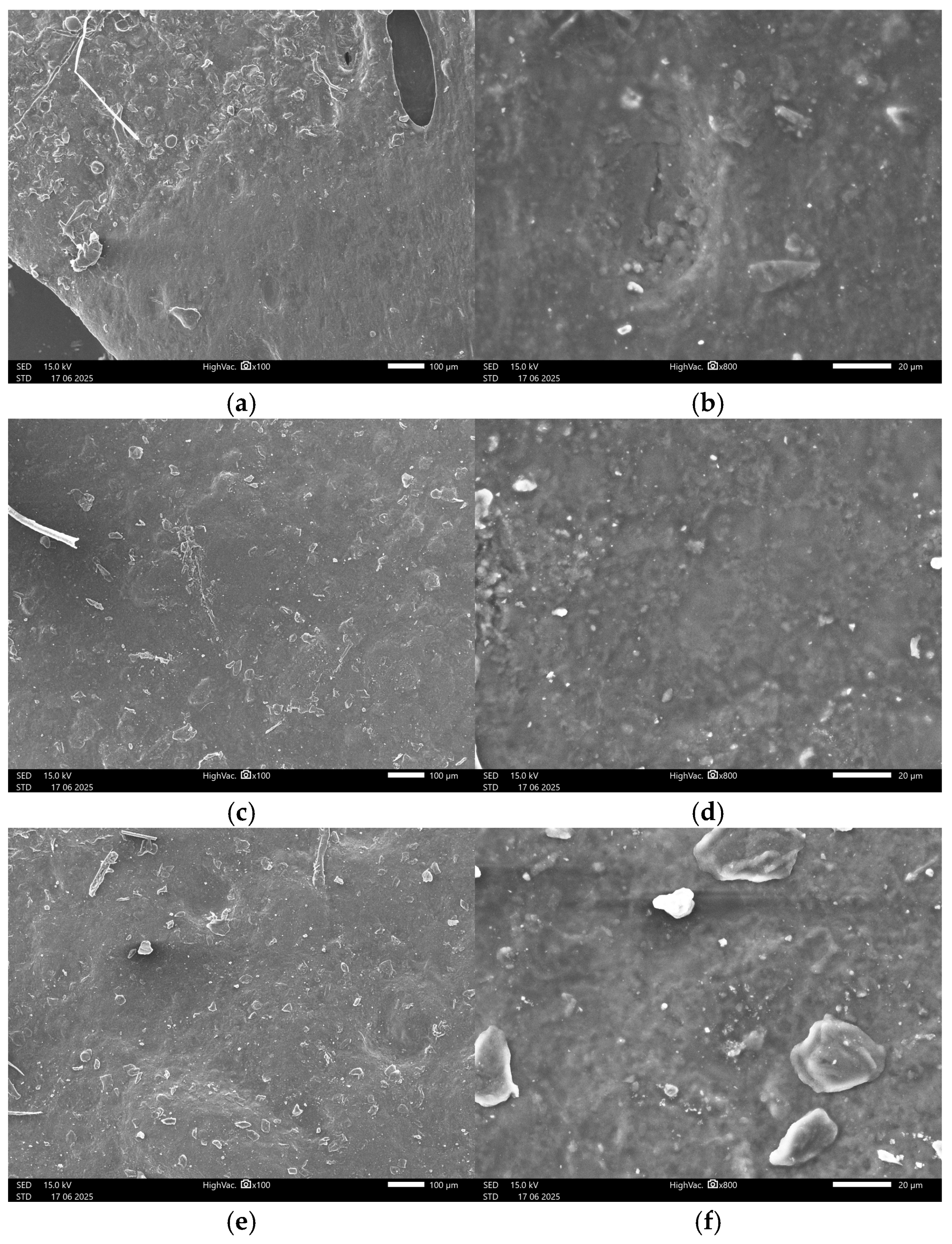
Appendix B.2
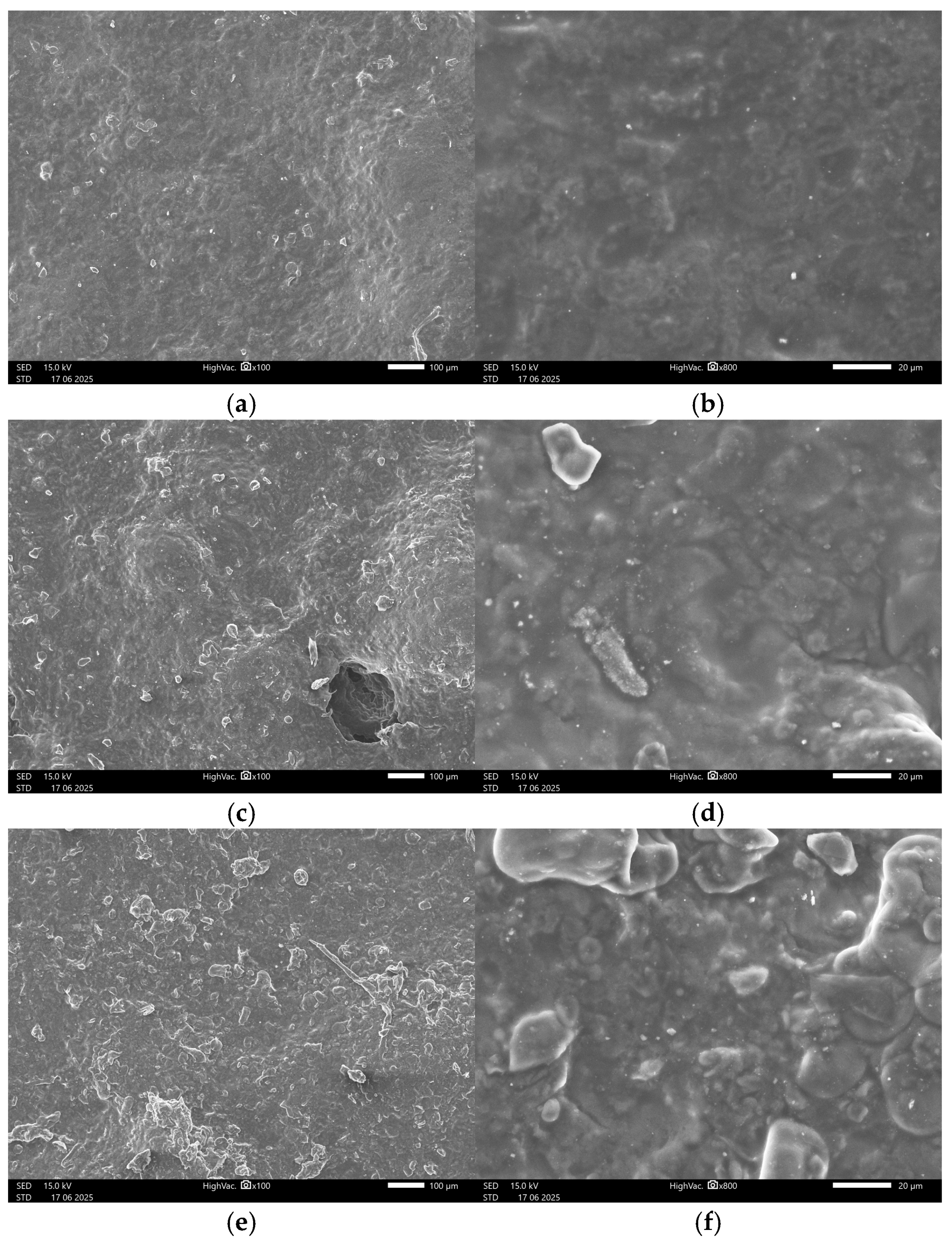
References
- Ahmad, A.M.; Sipra, H.M.; Hafsa, H. Biodegradable Films: Sustainable Solutions for Food Packaging Applications. Cukurova Univ. J. Nat. Appl. Sci. 2024, 3, 65–78. [Google Scholar] [CrossRef]
- Samuel, H.S.; Ekpan, F.-D.M.; Ori, M.O. Biodegradable, Recyclable, and Renewable Polymers as Alternatives to Traditional Petroleum-based Plastics. Asian J. Environ. Res. 2024, 1, 152–165. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, R.; Zhu, Y.; Lin, Q. Applications of biodegradable materials in food packaging: A review. Alex. Eng. J. 2024, 91, 70–83. [Google Scholar] [CrossRef]
- Flury, M.; Narayan, R. Biodegradable plastic as an integral part of the solution to plastic waste pollution of the environment. Green Sustain. Chem. 2021, 30, 100490. [Google Scholar] [CrossRef]
- Panou, A.A.; Karabagias, I.K. Biodegradable Packaging Materials for Foods Preservation: Sources, Advantages, Limitations, and Future Perspectives. Coatings 2023, 13, 1176. [Google Scholar] [CrossRef]
- Islam, S.; Islam, M.; Islam, K.N.; Sobuz, H.R. Biodegradable and Bio-Based Environmentally Friendly Polymers. Encycl. Mater. Plast. Polym. 2022, 2, 820–836. [Google Scholar] [CrossRef]
- Bastioli, C. Biodegradable Materials: State of Art and Future Perspectives; Springer: Berlin/Heidelberg, Germany, 1998; pp. 103–121. [Google Scholar] [CrossRef]
- Kothawade, S.; Padwal, V. Advancements in Biodegradable Polymers: Sustainable Solutions for Environmental Challenges. Curr. Green Chem. 2025, 12, 272–290. [Google Scholar] [CrossRef]
- Kaith, B.S.; Mittal, H.; Jindal, R.; Maiti, M.; Kalia, S. Environment Benevolent Biodegradable Polymers: Synthesis, Biodegradability, and Applications. In Cellulose Fibers: Bio- and Nano-Polymer Composites; Kalia, S., Kaith, B., Kaur, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Ocieczek, A. Comparison of sorption properties of semolina and farina. Acta Agrophysica 2007, 9, 135–145. [Google Scholar]
- Lacourse, N.L.; Altieri, P.A. Biodegradable Shaped Products and the Method of Preparation Thereof. 1989. Available online: https://patents.google.com/patent/US5043196A/en (accessed on 20 August 2025).
- Brunatti, A.C.S.; Matos, A.d.L.; de Oliveira, W.A.; de Rossi, P.H.S.; Oshiiwa, M. Farinhas em foco: Estudo das propriedades e aplicações na indústria alimentícia. Desarro. Local Sosten. 2024, 17, e3384. [Google Scholar] [CrossRef]
- Peressini, D.; Sensidoni, A.; Pollini, C.M.; de Cindio, B. Rheology of wheat doughs for fresh pasta production: Influence of semolina-flour blends and salt content. J. Texture Stud. 2000, 31, 163–182. [Google Scholar] [CrossRef]
- Fanari, F.; Desogus, F.; Scano, E.A.; Carboni, G.; Grosso, M. The Rheological Properties of Semolina Doughs: Influence of the Relative Amount of Ingredients. Chem. Eng. Trans. 2019, 76, 703–708. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Alias, A.K.; Ariffin, F.; Mahmud, S. Physico-mechanical and microstructural properties of semolina flour films as influenced by different sorbitol/glycerol concentrations. Int. J. Food Prop. 2018, 21, 983–995. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Xu, X.; Sun, Q.; Li, M.; Wang, Y.; Xie, F.-J. Wheat Flour-Based Edible Films: Effect of Gluten on the Rheological Properties, Structure, and Film Characteristics. Int. J. Mol. Sci. 2022, 23, 11668. [Google Scholar] [CrossRef]
- Simitchiev, A.; Dushkova, M.; Toshkov, N.; Dobrev, G.; Koleva, A.; Nenov, V. Rheological characteristics of extrudates from corn semolina enriched with tapioca flour. Emir. J. Food Agric. 2016, 28, 560–565. [Google Scholar] [CrossRef]
- Marti, A.; Cecchini, C.; D’Egidio, M.G.; Dreisoerner, J.; Pagani, M.A. Characterization of Durum Wheat Semolina by Means of a Rapid Shear-Based Method. Cereal Chem. 2014, 91, 542–547. [Google Scholar] [CrossRef]
- Otey, F.H.; Westhoff, R.P. Biodegradable starch-based blown films. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 592–595. [Google Scholar] [CrossRef]
- Abbate, C.; Scavo, A.; Pesce, G.R.; Fontanazza, S.; Restuccia, A.; Mauromicale, G. Soil Bioplastic Mulches for Agroecosystem Sustainability: A Comprehensive Review. Agriculture 2023, 13, 197. [Google Scholar] [CrossRef]
- Jayakody, M.; Kaushani, K.; Vanniarachchy, M. Edible Seaweed-Based Biodegradable Films and Coatings for Food and Nutraceutical Applications. In Algal Functional Foods and Nutraceuticals: Benefits, Opportunities, and Challenges; Bentham Science: Sharjah, United Arab Emirates, 2022. [Google Scholar] [CrossRef]
- Shi, B.; Funk, S.A.; Wang, J.H.; Wideman, G.J.; Kaufman, R.T.; Topolkaraev, V.A. Biodegradable and Renewable. Film. Patent WO-2010070469-A3, 18 December 2008. [Google Scholar]
- Janik, W.; Nowotarski, M.; Shyntum, D.Y.; Banaś, A.; Krukiewicz, K.; Kudla, S.; Dudek, G. Antibacterial and Biodegradable Polysaccharide-Based Films for Food Packaging Applications: Comparative Study. Materials 2022, 15, 3236. [Google Scholar] [CrossRef]
- Bambaeero, A.; Bazargan-Lari, R.; Vafa, E.; Memarzadeh, R. Electrophoretic deposition of a schiff base natural chitosan, reinforced with polyvinyl alcohol (PVA) and silver nanoparticles/silver-doped bioactive glass nanocomposite, onto anodized pure titanium implants. Mater. Chem. Phys. 2025, 345, 131294. [Google Scholar] [CrossRef]
- Pu, S.; Ma, H.; Deng, D.; Xue, S.; Zhu, R.; Zhou, Y.; Xiong, X. Isolation, identification, and characterization of an Aspergillus niger bioflocculant-producing strain using potato starch wastewater as nutrilite and its application. PLoS ONE 2018, 13, e0190236. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Qian, Y.; Su, W.; Shi, L.; Wang, E.; Yu, A.; Zheng, J.; Lu, Y. Degradation of agricultural polyethylene film by greater wax moth (Galleria mellonella) larvae and screening of involved gut bacteria. Ecotoxicol. Environ. Saf. 2025, 303, 118841. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Agate, S.; Williams, A.; Dougherty, J.; Velev, O.D.; Pal, L. Polymer Color Intelligence: Effect of Materials, Instruments, and Measurement Techniques—A Review. ACS Omega 2023, 8, 23257–23270. [Google Scholar] [CrossRef] [PubMed]
- Murawski, T.T.; Żołek-Tryznowska, Z.; Szałapak, J. Impact of Substrate Type on the Properties of Cast Biodegradable Starch-Based Films. Processes 2025, 13, 1197. [Google Scholar] [CrossRef]
- Sirbu, E.-E.; Dinita, A.; Tănase, M.; Portoacă, A.-I.; Bondarev, A.; Enascuta, C.-E.; Calin, C. Influence of Plasticizers Concentration on Thermal, Mechanical, and Physicochemical Properties on Starch Films. Processes 2024, 12, 2021. [Google Scholar] [CrossRef]
- Chantawee, K.; Riyajan, S.-A. Effect of Glycerol on the Physical Properties of Carboxylated Styrene-Butadiene Rubber/Cassava Starch Blend Films. J. Polym. Environ. 2019, 27, 50–60. [Google Scholar] [CrossRef]
- Żołek-Tryznowska, Z.; Cichy, Ł. Glycerol Derivatives as a Modern Plasticizers for Starch Films. In Proceedings of the 9th International Symposium on Graphic Engineering and Design, Cluj-Napoca, Romania, 19 November 2018; pp. 217–222. [Google Scholar] [CrossRef]
- Medina, I.; Scholl, S.; Rädle, M. Film Thickness and Glycerol Concentration Mapping of Falling Films Based on Fluorescence and Near-Infrared Technique. Micromachines 2022, 13, 2184. [Google Scholar] [CrossRef]
- Fahrullah, F.; Basriani, B.; Anita, C.; Febryanti, F.; Fitri, F. Modification of Protein-Based Edible Film Characteristics with Different Glycerol Concentrations: A Study on Thickness, Gelation, and Microstructure. J. Biol. Trop. 2024, 24, 952–960. [Google Scholar] [CrossRef]
- Maria, V.D.; Bernal, C.; Francois, N.J. Development of Biodegradable Films Based on Chitosan/Glycerol Blends Suitable for Biomedical Applications. J. Tissue Sci. Eng. 2016, 7, 187. [Google Scholar] [CrossRef]

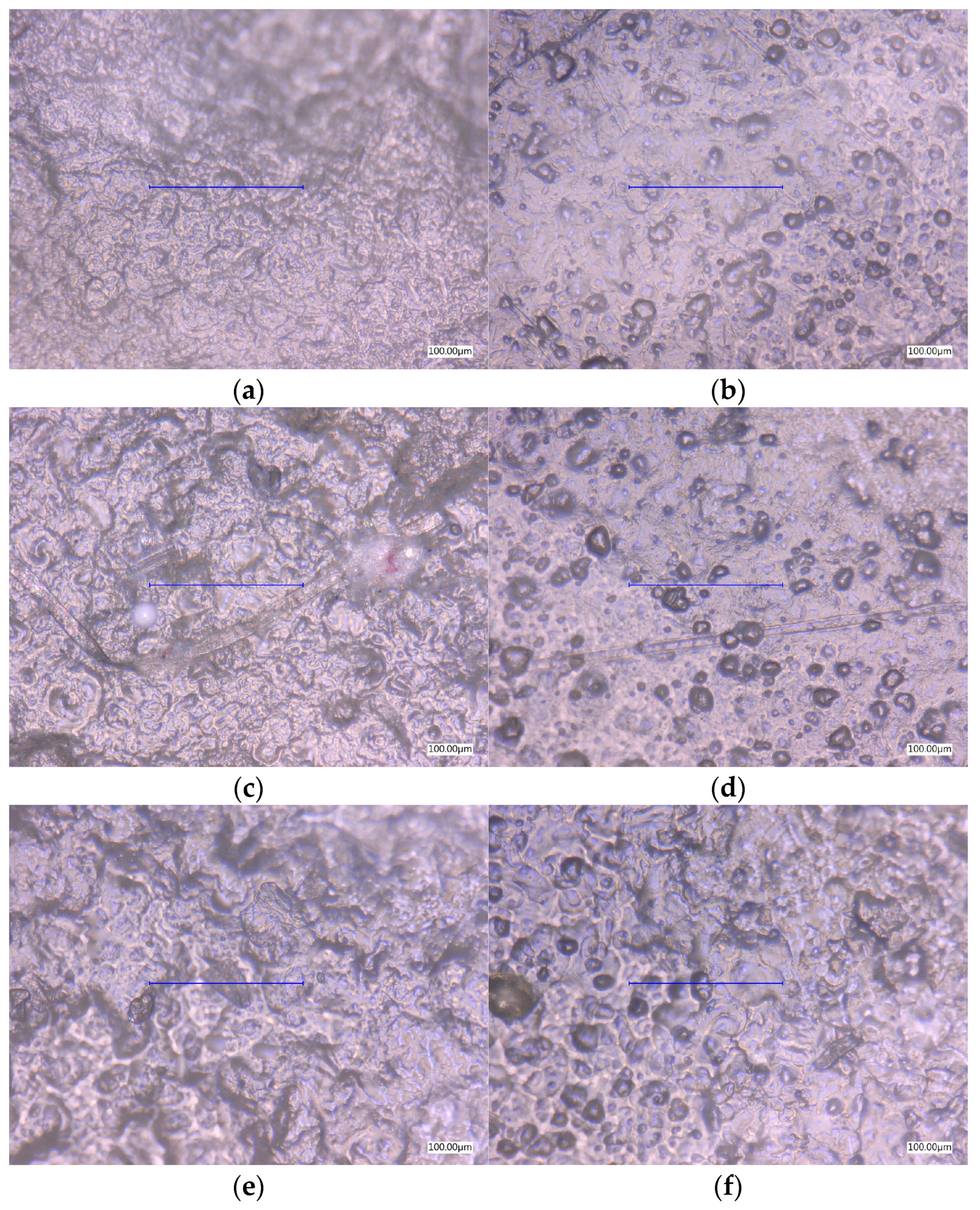
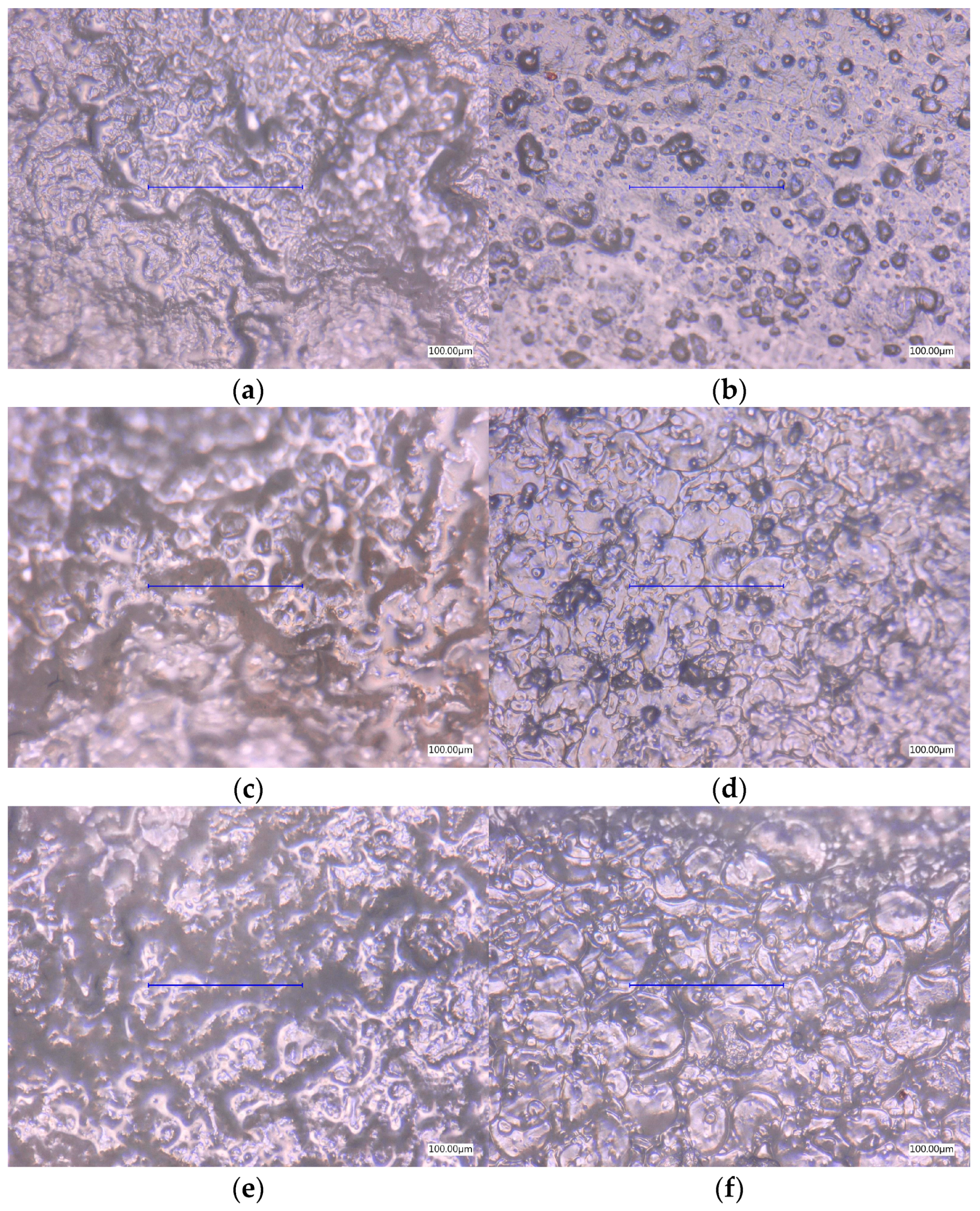
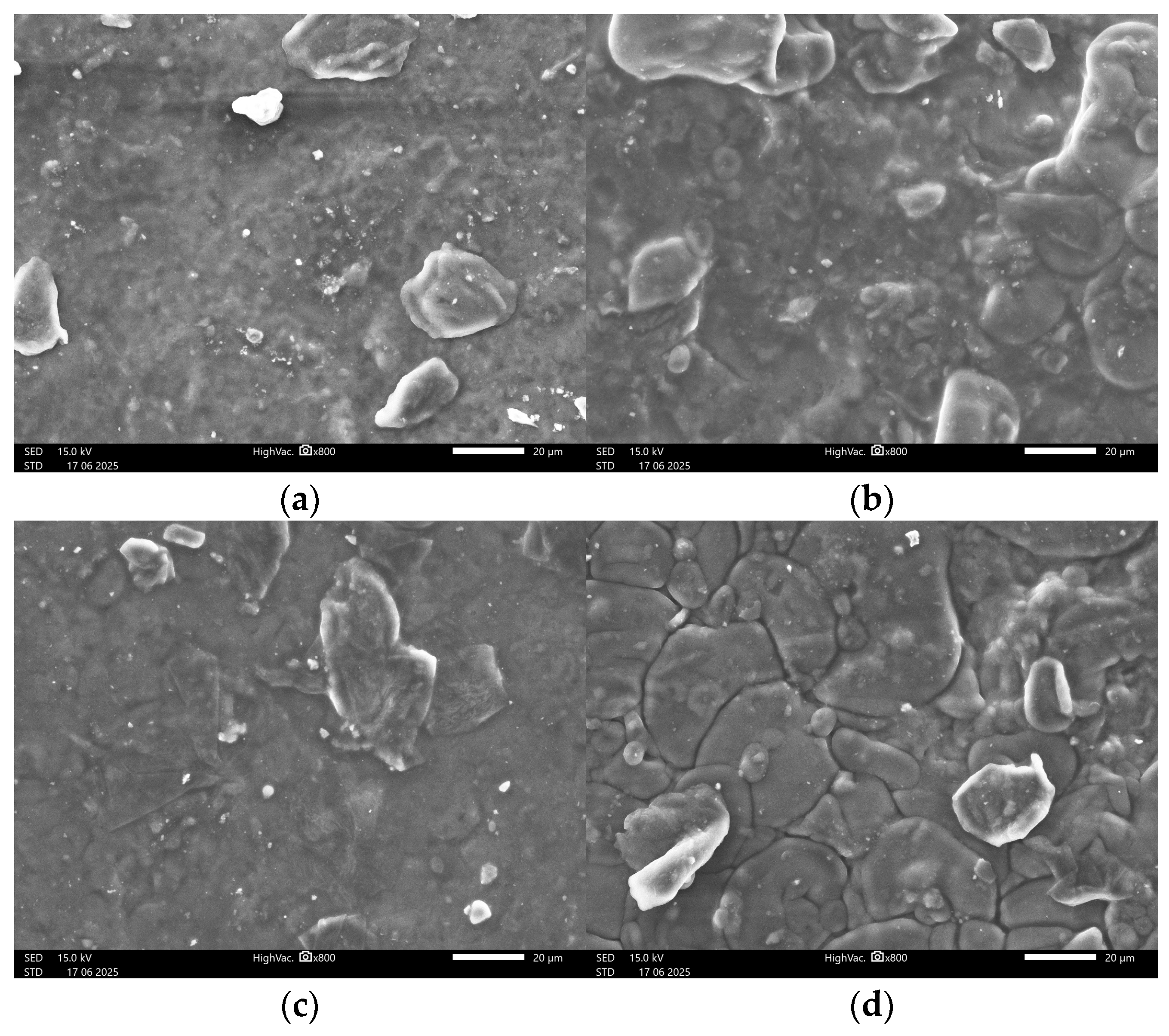
| Entry | Farina or Semolina (g) | Glycerol (g) | Glycerol (%) |
|---|---|---|---|
| 1 | 40 | 10 | 25 |
| 2 | 40 | 20 | 50 |
| 3 | 40 | 30 | 75 |
| Sample | Direction | σM [MPa] | Uncertainty [MPa] | εtM [%] | Uncertainty [%] |
|---|---|---|---|---|---|
| Farina 50 | Along | 0.95 | 0.13 | 84 | 11 |
| Across | 1.08 | 0.09 | 100 | 4.7 | |
| Farina 75 | Along | 0.73 | 0.73 | 110 | 110 |
| Across | 0.73 | 0.03 | 95 | 5.8 | |
| Semolina 25 | Along | 17.1 | 0.7 | 5.1 | 0.4 |
| Across | 13.9 | 0.774 | 7.5 | 1.1 | |
| Semolina 50 | Along | 1.04 | 0.097 | 89 | 6.8 |
| Across | 1.81 | 0.69 | 72 | 9.7 | |
| Semolina 75 | Along | 0.226 | 0.0391 | 38 | 6.9 |
| Across | 0.168 | 0.0448 | 38 | 7.8 |
| Sample | [mm] | s [mm] |
|---|---|---|
| Farina 25 | 0.2361 | 0.0204 |
| Farina 50 | 0.1967 | 0.0415 |
| Farina 75 | 0.4646 | 0.0201 |
| Semolina 25 | 0.3767 | 0.0576 |
| Semolina 50 | 0.3922 | 0.0457 |
| Semolina 75 | 0.4466 | 0.0592 |
| Side | Concentration | SFE [mJ/m2] | Dispersive [mJ/m2] | Polar [mJ/m2] |
|---|---|---|---|---|
| Up | 25 | 38.07 | 36.34 | 1.73 |
| 50 | 41.76 | 41.2 | 0.56 | |
| 75 | 46.88 | 42.34 | 4.54 | |
| Down | 25 | 46.92 | 41.32 | 5.6 |
| 50 | 63.44 | 40.1 | 23.34 | |
| 75 | 61.53 | 41.33 | 20.2 |
| Side | Concentration | SFE [mJ/m2] | Dispersive [mJ/m2] | Polar [mJ/m2] |
|---|---|---|---|---|
| Up | 25 | 39.82 | 39.78 | 0.04 |
| 50 | 55.83 | 40.42 | 15.41 | |
| 75 | 49.01 | 36.41 | 12.6 | |
| Down | 25 | 47.2 | 41.44 | 5.76 |
| 50 | 65.08 | 37.77 | 27.31 | |
| 75 | 63.28 | 35.47 | 27.81 |
| Sample | Surface | Sa [µm] | Sz [µm] | Sq [µm] | Ssk [µm] | Sku [µm] | Sp [µm] | Sv [µm] | Area Size [µm2] |
|---|---|---|---|---|---|---|---|---|---|
| Farina 25 | Bottom | 1.15 | 8.32 | 1.43 | −0.73 | 3.32 | 3.31 | 5.01 | 86,251.58 |
| Farina 50 | Bottom | 4.15 | 26.27 | 5.02 | 0.15 | 2.35 | 14.06 | 12.21 | 219,435.57 |
| Farina 75 | Bottom | 4.88 | 24.00 | 5.74 | 0.34 | 2.14 | 14.28 | 9.71 | 216,146.84 |
| Semolina 25 | Bottom | 3.09 | 18.80 | 3.75 | −0.59 | 2.65 | 6.77 | 12.02 | 236,273.00 |
| Semolina 50 | Bottom | 4.09 | 19.44 | 4.76 | 0.68 | 2.23 | 12.05 | 7.39 | 190,221.24 |
| Semolina 75 | Bottom | 15.61 | 67.65 | 18.36 | −0.27 | 2.06 | 29.60 | 38.05 | 227,487.94 |
| Farina 25 | Top | 6.34 | 34.65 | 7.52 | −0.15 | 2.12 | 16.86 | 17.79 | 192,593.52 |
| Farina 50 | Top | 5.59 | 29.85 | 6.84 | 0.74 | 2.76 | 19.14 | 10.71 | 222,282.77 |
| Farina 75 | Top | 2.87 | 23.96 | 3.70 | 0.63 | 3.67 | 15.86 | 8.10 | 227,060.74 |
| Semolina 25 | Top | 5.41 | 45.24 | 7.31 | 1.16 | 5.70 | 32.05 | 13.19 | 222,621.67 |
| Semolina 50 | Top | 10.39 | 46.82 | 11.95 | −0.17 | 1.82 | 22.88 | 23.93 | 195,662.68 |
| Semolina 75 | Top | 11.00 | 47.39 | 12.56 | 0.55 | 2.06 | 27.51 | 19.88 | 214,698.17 |
| Sample | Substrate | L* | a* | b* | D |
|---|---|---|---|---|---|
| White background | 101.740 | 0.520 | −7.420 | −0.020 | |
| Black Background | 37.680 | 1.480 | 1.960 | 0.990 | |
| Farina 25 | On white | 90.724 | −0.360 | −0.454 | 0.112 |
| Farina 50 | On white | 96.318 | 0.154 | −2.240 | 0.042 |
| Farina 75 | On white | 90.394 | 0.040 | −0.944 | 0.128 |
| Semolina 25 | On white | 96.376 | −0.306 | 1.310 | 0.044 |
| Semolina 50 | On white | 96.550 | −0.310 | −0.120 | 0.044 |
| Semolina 75 | On white | 96.070 | −0.410 | −0.326 | 0.050 |
| Farina 25 | On black | 51.116 | −0.184 | 1.332 | 0.740 |
| Farina 50 | On black | 45.734 | 0.472 | 0.834 | 0.822 |
| Farina 75 | On black | 47.392 | 0.126 | 0.966 | 0.770 |
| Semolina 25 | On black | 47.716 | 0.086 | 1.794 | 0.776 |
| Semolina 50 | On black | 48.284 | 0.160 | 1.812 | 0.778 |
| Semolina 75 | On black | 49.048 | −0.136 | 1.354 | 0.776 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murawski, T.T.; Olczak, M.; Laskowski, S.M.; Żołek-Tryznowska, Z.; Szałapak, J. Effect of Glycerol Concentration on the Properties of Semolina- and Farina-Based Biodegradable Films. Processes 2025, 13, 3006. https://doi.org/10.3390/pr13093006
Murawski TT, Olczak M, Laskowski SM, Żołek-Tryznowska Z, Szałapak J. Effect of Glycerol Concentration on the Properties of Semolina- and Farina-Based Biodegradable Films. Processes. 2025; 13(9):3006. https://doi.org/10.3390/pr13093006
Chicago/Turabian StyleMurawski, Tomasz Tadeusz, Mikołaj Olczak, Szymon Mateusz Laskowski, Zuzanna Żołek-Tryznowska, and Jerzy Szałapak. 2025. "Effect of Glycerol Concentration on the Properties of Semolina- and Farina-Based Biodegradable Films" Processes 13, no. 9: 3006. https://doi.org/10.3390/pr13093006
APA StyleMurawski, T. T., Olczak, M., Laskowski, S. M., Żołek-Tryznowska, Z., & Szałapak, J. (2025). Effect of Glycerol Concentration on the Properties of Semolina- and Farina-Based Biodegradable Films. Processes, 13(9), 3006. https://doi.org/10.3390/pr13093006








