1. Introduction
Because several pharmaceuticals or their degradation products exhibit significant persistence throughout the water and wastewater treatment process, their presence in the water cycle is receiving increasing attention [
1]. Specifically, iodinated radiocontrast agents are very hydrophilic and poorly removed in wastewater treatment plants [
1]. They cannot be eliminated by most chemical and biological wastewater treatment processes, making them potentially problematic. Consequently, elevated levels of iodinated radiocontrast agents and iodinated conversion products have been retrieved in wastewater and drinking water streams [
1,
2,
3].
The utilization of contrast media has meaningfully augmented due to the recent rapid growth in medical imaging applications. Hospitals often use iodinated contrast agents for soft tissue imaging. Diatrizoic acid (DTZA) is the most commonly reported contrast agent in the aquatic environment [
4,
5]. All stakeholders, from the DTZA manufacturer to the drinking water user, must work together to solve the DTZA problem in the water system as a whole. DTZA is rarely removed by sorption to excess sludge due to its high polarity. As a result, DTZA has been found in large quantities in surface water, wastewater treatment plant effluent, groundwater, and even potable water.
Recently, in reaction to the growing concern about the presence of DTZA in water, a number of technologies have been evaluated for the elimination of this drug [
5]. However, some of these have been shown to be ineffective in achieving the desired drug removal. Liquid surfactant membrane (LSM), also known as emulsion liquid membrane, have been shown to be highly successful in removing pharmaceuticals from water.
Treatment of aqueous effluents by extraction using LSM is an advanced separation process that works well [
6,
7,
8,
9,
10,
11,
12,
13]. It is founded on the ability of a substance to permeate a membrane (organic solution). This recognized method is particularly effective in recovering, concentrating, and eliminating various contaminants or valuable solutes. This technology is an advanced separation process because of its fast kinetics, simple design, ease of usage, and potential for membrane reuse for subsequent disposal.
At the heart of the LSM approach is the liquid emulsion. Typically, there are two insoluble liquids in an emulsion. The first one is the stripping aqueous liquid, which can be a salt, basic, or acidic solution. The second phase, the organic liquid, is the membrane and contains a selective carrier and a surfactant solubilized in a diluent, or it can be just a surfactant solubilized in a diluent. The type of permeation is also determined by the composition of the organic membrane. Each type of contaminant has a unique LSM technique. The chemical and physical properties of the species have a significant influence on the composition of the liquid membrane. A surfactant was used to encapsulate the internal stripping phase, and ultrasound or a high-speed stirrer was used to ensure proper dispersion of extremely thin droplets inside the organic membrane [
6]. The subsequent water-in-oil (W/O) emulsion was then gently mixed and dispersed as globules in the external liquid.
In the LSM method, one or more substances are then selectively transferred from the external solution to the inner phase (stripping solution) across the organic phase (membrane) to achieve removal. Consequently, permeate phenomena occur at the water–oil–water interfaces (while the water–oil interface subsequently flows across the receiving water-oil contact).
To the best of our knowledge, an advanced separation technology, namely LSM, has not been used to remove DTZA from water. The purpose of this investigation was to examine the eliminetaion of DTZA from water using LSM. The LSM system comprises Aliquat 336 as extractant, Span 80 as emulsifier, kerosene as diluent, and KCl as internal solution. The impact of operating factors affecting the extraction of DTZA from water by LSM, namely surfactant concentration, carrier concentration, pH of the external solution, nature of base in the feed solution, concentration of the stripping phase, nature of stripping solution, emulsion/external solution volume ratio, internal solution/organic phase volume ratio, mixing rate, nature of diluent, emulsification time, emulsification rate, and initial DTZA concentration, was examined. Additionally, the extraction of DTZA from different real water matrices (natural mineral water, tap water, and seawater) was studied.
2. Materials and Methods
2.1. Materials
Stock solutions of diatrizoic acid (abbreviation: DTZA; synonyms: amidotrizoic acid and urogranoic acid; chemical formula: C11H9I3N2O4, molecular mass: 613.91 g/mol, CAS: 117-96-4) were produced by solubilizing analytical grade DTZA (acquired from Sigma-Adlrich) in ultrapure water. Ultrapure water is an ultra-high-quality water produced using a Milli-Q system. It has a resistivity of 18.2 MΩ·cm and a total organic carbon content below 5 ppb. External contaminated solutions were made by mixing a basic solution with an aqueous solution of the appropriate concentration of DTZA. Ultrapure water was used to dilute DTZA-contaminated solutions from a calculated volume of DTZA stock solution to a predetermined concentration.
Tricaprylmethylammonium chloride (Aliquat 336) and Span 80 (sorbitan monooleate) employed as extractant and emulsifier, respectively, were procured from Merck.
The rest of the compounds used in this work were all procured from Sigma-Aldrich and were of analytical grade.
2.2. Methods
The permeation of DTZA through a surfactant liquid membrane includes three processes: preparing the W/O emulsion, extracting the substrate from the contaminated phase by contact with the emulsion, and settling of the emulsion to separate it from the contaminated solution.
Figure 1 shows a diagram illustrating the complete ELM process steps.
The required amount of sulfate salt (Na2SO4) or chloride salt (CaCl2, NaCl, or KCl) solutions were dissolved in pure water to create inner aqueous phases. To produce the membrane (organic solution), a proper weight of Span 80 emulsifier was dissolved in kerosene. The W/O emulsion was obtained by combining the membrane solution with the internal aqueous phase utilizing an Ultra-Turrax T18 high-speed homogenizer–disperser (IKA) for a planned period. The stripping aqueous solution/organic phase volume ratio varied from 1/2 to 2/1.
A fixed quantity of the produced emulsion was placed in 250 mL of DTZA-contaminated aqueous phase in a thermostatically controlled container connected to a mechanical agitator. A four-blade stirrer with a 45° pitch (5 cm diameter) was used. To prepare the W/O/W multiple emulsions, the contents of the vessel were agitated at diverse rates for different contact times to disperse the emulsion in the contaminated phase. At various time ranges, a regular sampling of the contaminated phase was picked up. The concentration of the contaminant in the external phases was calculated using a Lightwave II UV-visible spectrophotometer adjusted at the wavelength that corresponded to the higher optical density of the contaminant tested (239 nm).
In the present work, the parameters that influenced the removal of DTZA from water were investigated, including the emulsifier dosage (1–9% (w/w)), carrier dosage (0.6–1.0% (w/w)), pH of the contaminated solution (6.1–11.3), nature of base in the feed solution (NH4OH, KOH, NaOH, Na2CO3), concentration of the stripping solution (0.2–0.8 N), nature of stripping solution (KCl, NaCl, CaCl2, Na2SO4), emulsion/contaminated solution volume ratio (5/250–60/250), organic phase/internal solution volume ratio (1/2–2/1), agitation rate (100–400 rpm), nature of diluent (n-heptane, kerosene and n-hexane), emulsification period (1–8 min), emulsification degree (11,500–24,000 rpm), initial DTZA concentration (5–100 mg/L), and several water matrices (ultrapure water, tap water, natural mineral water, and seawater).
3. Results and Discussion
3.1. Extraction Mechanism
Figure 2 shows the mechanism of DTZA transfer by LSM using tricaprylmethylammonium chloride (Aliquat 336) as an extractant. DTZA has a pKa of 3.40 [
14]. At pH < 3.40, DTZA exists in a neutral, protonated state, making it highly soluble in the aqueous phase. However, since protonated DTZA has a negligible affinity for the membrane phase carrier, extraction does not occur. This behavior is consistent with the carrier-mediated transport mechanism, which requires the deprotonated form of DTZA to form an extractable complex.
DTZA in its deprotonated form is transported by a driving force derived from the concentration gradient of other anionic counterions between both aqueous solutions, since Aliquat 336 is a cationic carrier. In systems using aliphatic diluents, Aliquat 336 may form as a third phase, also known as a second organic phase. Nevertheless, this phenomenon was not noticed in the present work.
In the current LSM configuration, the extractant Aliquat 336 acts as a mobile carrier that selectively forms complexes with the target solute, DTZA, at the external aqueous/membrane interface. This complexation step is rapid and reversible, and the resulting neutral or ion-paired complex is soluble in the organic membrane phase. Unlike passive diffusion, carrier-mediated transport in SLM can move solutes against their concentration gradient. This is possible because the driving force is the difference in solute concentration and the overall chemical potential gradient created by the high concentration of counterions (Cl− from KCl) in the internal phase. This gradient shifts the equilibrium of the complexation/decomplexation reactions. Additionally, continuous regeneration of the carrier at the stripping interface maintains low solute-carrier complex activity on the internal side. This sustains net flux toward the internal phase, even when the free solute concentration exceeds that in the external phase.
The mechanistic nature of Aliquat 336-mediated transport in this LSM system (
Figure 2) can be illustrated by the following steps:
- 1.
Interfacial complexation: At the external interface, Aliquat 336 binds to the solute to form a membrane-soluble complex. This step is driven by chemical affinity and is not limited by the diffusivity of free DTZA in the aqueous phase.
- 2.
Membrane phase diffusion: The uncharged carrier-solute complex (charges added for clarity and then removed to avoid confusion, as suggested by the reviewer) diffuses through the organic membrane phase. The diffusion coefficient is governed by the membrane phase’s viscosity and microstructure.
- 3.
Interfacial decomplexation/stripping: At the internal interface, the complex encounters a stripping agent (Cl− from KCl), which displaces the solute from the carrier. The carrier then regenerates and diffuses back toward the external interface to repeat the cycle.
The re-extraction of the extractant molecules to the external phase side is caused by the KCl existing in the stripping aqueous solution. The extractant is regenerated and the chloride anion is released when it reaches the external solution side.
The observed transport rates and their dependence on membrane thickness, carrier concentration, and stirring speed are consistent with a diffusion-controlled, carrier-mediated membrane mechanism. Net transport occurs when the internal free solute concentration exceeds the external concentration. This confirms that the process is driven by the coupled reaction–diffusion cycle of the carrier rather than by the simple diffusion of free solutes governed by Fick’s law.
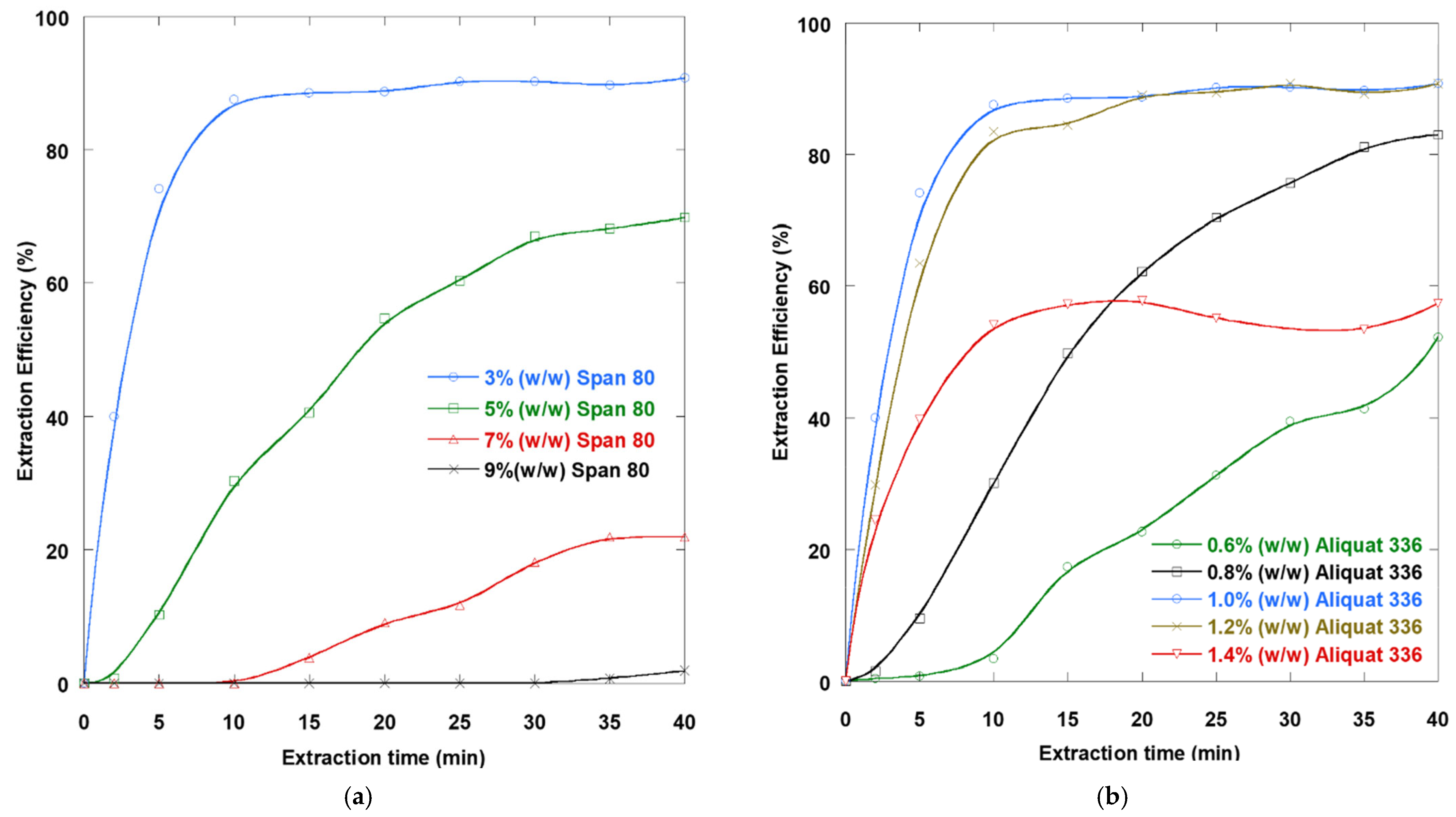
3.2. Influence of Surfactant Dosage
Determining the impact of emulsifier dosage on the performance of DTZA elimination by LSM is essential in this process.
Figure 3a illustrates the evolution in the amount of DTZA removed from the contaminated solution for different Span 80 dosages in the interval from 1 to 9% (
w/
w). The removal percentage was maximum at 3% (
w/
w) emulsifier concentration. To prevent emulsion leakage, the surfactant was added to the liquid organic membrane as an emulsifier. It functions as a physical barrier separating the internal solution and the contaminated solution. Because low surfactant levels lead to inadequate membrane interface coverage, an emulsifier concentration of 1% (
w/
w) failed to produce a stable emulsion. Augmenting the emulsifier dosage to 3% (
w/
w) decreased the membrane surface tension and resulted in small globules, thus increasing the contact area. Higher surfactant concentrations of 5, 7, and 9% (
w/
w) resulted in lower extraction efficiencies, especially at 9% (
w/
w). This may result from increasing the emulsion viscosity and membrane thickness, increasing the mass transport resistance by the existence of excess surfactant at the organic membrane-water phase interface, and thus reducing the transport of DTZA into the internal solution. In addition, it was noted that at an emulsifier dosage of 9% (
w/
w), swelling occurs, resulting in a reduction in removal efficiency. Consequently, 3% (
w/
w) Span 80 was chosen as the most appropriate emulsifier dosage.
3.3. Influence of Extractant Dosage
Figure 3b illustrates the impact of Aliquat 336 extractant concentration on the elimination of DTZA by LSM. As the extractant dosage augmented from 0.6 to 1.0% (
w/
w), the removal percentage also increased. Substrate transfer is facilitated by the increased extractant concentration at the contaminated solution–W/O emulsion contact. The amount of DTZA extracted is reduced when the carrier dosage is increased above a given threshold (1%
w/
w). Since a very high carrier dosage in the organic membrane causes a corresponding increase in viscosity and larger globules, there is no benefit. A concentration of Aliquat 336 of 1% (
w/
w) in the liquid membrane was found to be the optimum extractant concentration.
3.4. Influence of Contaminated Phase Initial pH
The removal of DTZA was performed at various initial pH of the polluted phase in order to minimize the issue of membrane swelling and to study the essential function that the initial pH of the contaminated solution plays in the transport of the substrate in the LSM technique. The results of the extraction studies performed at diverse initial pH of the polluted phase in the interval 6.0–11.3 were represented in
Figure 4a. This figure illustrates that the initial pH of the contaminated phase has a considerable effect on the permeation performance. As the initial pH augmented from 6.0 to 10, the DTZA removal increased and then decreased at pH 11.3. At pH 10, both maximum removal and maximum transport were achieved. At pH 11.3, the properties of the emulsifier are reduced, engendering the emulsion to become unstable and the removal percentage to decline. It has been found that in the absence of ammonia, swelling phenomena occur, leading to emulsion breakdown and diminished removal percentage. The primary force behind the movement of water from the contaminated phase to the inner stripping solution is the change in osmotic pressure between the two aqueous solutions. Hence, an optimum initial pH of 10 was chosen for the feed solution.
3.5. Influence of the Nature of Base in the Contaminated Phase
One of the most important components of an effective LSM method is considered to be the selection of a suitable base in the aqueous external solution. The impact of ammonia, sodium hydroxide, sodium carbonate, and potassium hydroxide (pH 10) in the feed solution on the permeation percentages was explored. The results obtained, as presented in
Figure 4b, reveal that the removal percentages show the following trend: ammonia > potassium hydroxide > sodium hydroxide > sodium carbonate. As a result, ammonia was selected to be the optimal base and was employed to adjust the initial pH of the contaminated phase in the subsequent tests.
3.6. Influence of Type and Concentration of Stripping Solution
Figure 5a depicts the impact of varying the dosage of KCl in the stripping solution on the removal of DTZA, ranging from 0.2 to 0.8 N. This figure shows that the use of 0.3 N KCl results in the highest removal percentage of DTZA. When the concentration of KCl was changed from 0.2 to 0.3 N, the permeation efficiency was found to increase. The lower extraction efficiency was the result of insufficient stripping reagent at the lower concentration (0.2 N) to remove DTZA from the organic phase. The stripping procedure was retarded, resulting in DTZA exhaustion of the membrane. The process of DTZA removal from the organic membrane can be improved by augmenting the KCl content. However, the degree of DTZA removal diminished when the KCl dosage in the internal solution was changed from 0.3 to 0.8N. It was predictable that the addition of more KCl to the stripping solution would diminish the difference in density and raise the emulsion viscosity. Droplet size increased with increasing viscosity of the emulsion. In addition, the volume of the stripping solution increased due to the disparity in ionic strength between the contaminated solution and the internal solution, resulting in excessive leakage of the emulsion. Droplet size increased with increasing W/O emulsion viscosity. Therefore, the optimal stripping solution concentration was determined to be 0.3 N KCl.
In the LSM technique, the removal of the substrate from the contaminated phase to the inner stripping solution is critically dependent on the stripping reaction at the organic membrane–inner solution interface. Other chloride salts (sodium chloride and calcium chloride) and a sulfate salt (sodium sulfate) have also been investigated, although the stripping solution in the initial tests was potassium chloride. At the organic membrane-stripping phase interface, the impact of stripping solution composition on an effective stripping process was investigated.
Figure 5b shows the results of a study investigating the effect of adding 0.3 N of different internal solutions on the removal of DTZA. The removal percentages were found to have the following tendency: KCl > CaCl
2 > NaCl. In the case of Na
2SO
4, there is no removal of the drug by the process. Therefore, it was decided that KCl solution would be the best stripping agent.
3.7. Influence of Internal Solution/Organic Phase Volume Ratio
An important factor in evaluating the success of the LSM process is the internal solution/organic phase volume ratio. This ratio was varied from 1/2 to 2/1 to examine its impact on the extraction of DTZA by the LSM technique. The results were presented in
Figure 6a. The membrane thinness and viscosity of W/O emulsion are high at low volume ratios because of the comparatively high organic portion. Furthermore, a low volume ratio implies a reduced availability of the stripping reagent for the substrate stripping process. The extraction percentage and the transport rate of DTZA increase as the volume ratio is increased from 1/2 to 1/1. This is due to the fact that a higher volume fraction of stripping solution changes the internal droplet dimension distribution to bigger dimensions and reduces the membrane phase thickness, which in turn conducts to improved mass transfer. Consequently, the ability of the membrane to allow more substrate penetration is increased. The inner solution/membrane solution volume ratio can only increase so far before the droplet size becomes unmanageable. The rate and effectiveness of removal is reduced by increasing the volume ratio of stripping solution to membrane beyond a ratio of 1/1. This can be the result of both a rise in the diameter of inner droplet and an increase in emulsion viscosity. The interfacial exchange zone between the external solution and the emulsion is reduced as the droplet diameter increases, which reduces the removal percentage. In addition, the amount of membrane is unsatisfactory to entirely enfold the stripping phase at larger volume ratios. Therefore, the ideal stripping solution/membrane phase volume ratio was determined to be 1/1.
3.8. Influence of Emulsion/Feed Solution Volume Ratio
The emulsion/aqueous feed phase volume ratio regulates mass transfer and is a key parameter in determining the success of the LSM process.
Figure 6b illustrates the results of a study of the impact of emulsion/aqueous feed phase volume ratio on the permeation efficiency of DTZA. This figure shows how increasing the emulsion/aqueous feed solution volume ratio from 5/250 to 20/250 increased the removal percentage. This is because, as the quantity of emulsion in the contaminated phase rises, the reachable globules and the interfacial zone per unit volume of the contaminated solution rise, resulting in improved removal efficiency and DTZA transfer rate. At an emulsion/aqueous feed solution volume ratio of 40/250, almost the same permeation efficiency of DTZA was obtained as that at a ratio of 20/250, with problems in distributing the emulsion in the contaminated solution. When the ratio reached 60/250, the augmented quantity of the more viscous emulsion produced inadequate solution mixing and a reduction in extraction. As the volume ratio increases, the globule dimension rises and the membrane densifies, making it more difficult for DTZA to be transported. As a result, an emulsion/feed phase volume ratio of 20/250 was determined to be the optimal in order to guarantee appropriate dispersion of the emulsion in the contaminated solution.
3.9. Influence of Mixing Rate
An important parameter affecting the rate of mass transport by LSM is the agitation rate. Mixing speeds ranging from 100 to 400 rpm were used to sparsify the emulsion in the contaminated phase and to obtain the best possible DTZA removal rate during the LSM technique. The results were shown in
Figure 7a. The larger emulsion globules formed at the lower agitating rate (100 rpm) reduced the area available for mass transfer, resulting in a low removal rate. The mixing rate was augmented from 100 to 250 rpm in order to remove the DTZA from the contaminated solution at a faster rate throughout the process. This may be because increasing the mixing intensity reduces both the thickness of the membrane and the size of the globules. Smaller emulsion globules provide better dispersion. Simultaneously, the permeation percentage and the transfer rate are higher because there is a greater amount of accessible interfacial area between the liquid membrane and the contaminated solution. However, the removal percentage decreased when the mixing rate was changed from 250 to 400 rpm. In addition to the decrease in removal percentage, increasing the mixing rate beyond a certain point also disturbs the stability of the emulsion and causes instability. Additionally, there is an upper limit to the mixing rate due to shear-provoked breakage of delicate emulsion droplets at the impeller tip or collision with the contactor wall. Therefore, it has been suggested that the best mixing rate is 250 rpm.
3.10. Influence of Diluent
The impact of organic diluents on the effectiveness of an LSM technique was critical. Tests were conducted using kerosene, n-heptane, and n-hexane to explore the influence of diluents on the elimination of DTZA from water (
Figure 7b). The trend for DTZA removal was kerosene > n-heptane > n-hexane. Kerosene had the highest density (0.8000 g/mL), viscosity (1.64 cP), and surface tension (23 dyn/cm), followed by n-heptane (density = 0.6837 g/mL, viscosity = 0.42 cP, surface tension = 20.30 dyn/cm), then n-hexane (density = 0.6594 g/mL, viscosity = 0.31 cP, surface tension = 17.91 dyn/cm). It is noteworthy that the worst removal result was obtained when n-hexane was used. This is due to its lower density, viscosity, and surface tension, which disturb the permeability and thickness of the membrane and ultimately reducing the transfer rate. Kerosene shows the best results as the ideal diluent due to its harmonious values of density, viscosity and surface tension. It can be extremely difficult to make a definitive judgment on the influence of the diluent as the possible significance of the interactions between diluent and surfactant, diluent and extractant, or any recombination of these. DTZA was further extracted by LSM using kerosene as a diluent.
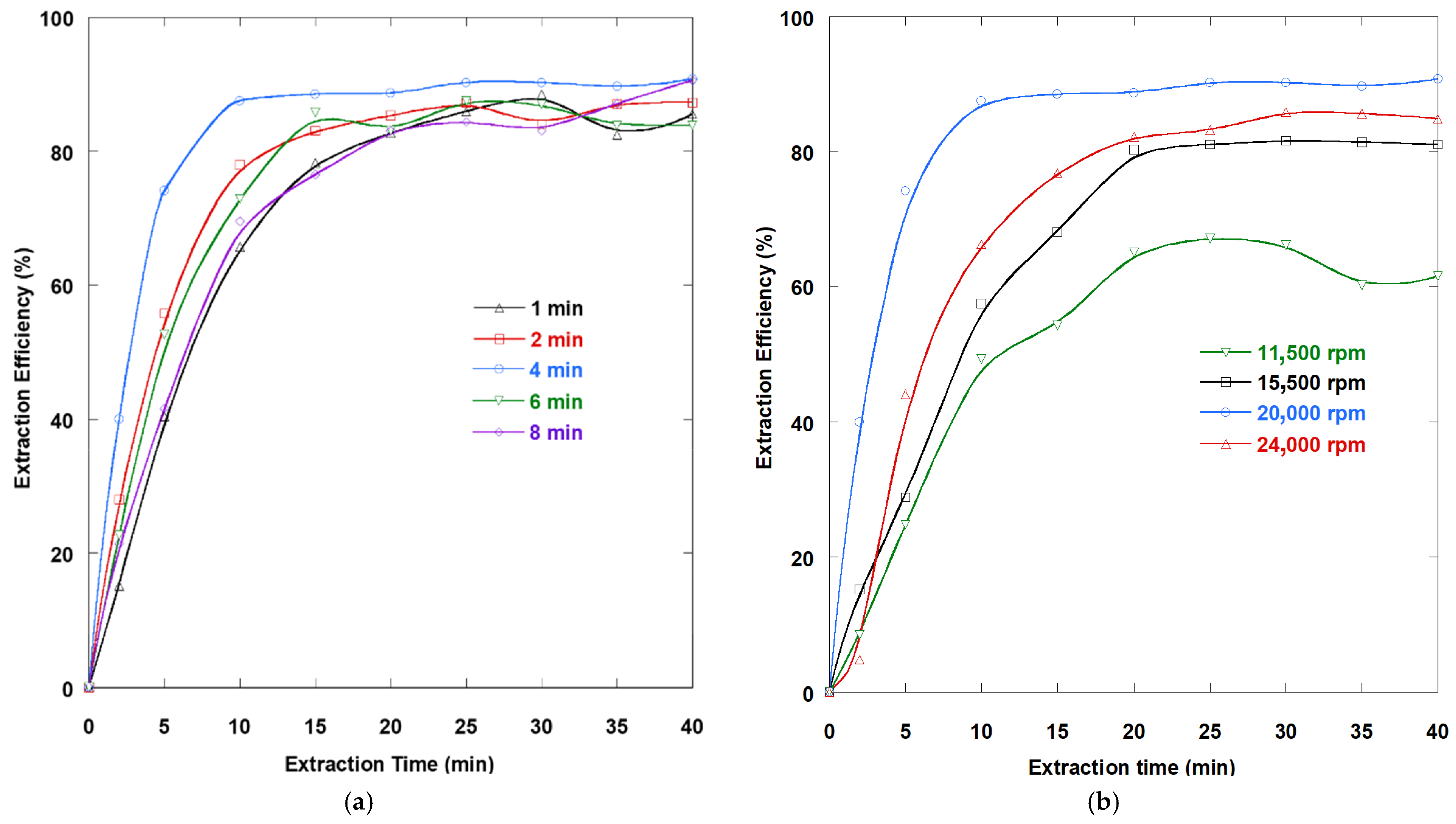
3.11. Influence of Emulsification Rate and Time
The tests were conducted by changing the emulsification period in the range from 1 to 8 min at an emulsification rate of 20,000 rpm.
Figure 8a illustrates how the period of the emulsification process affects the ability of the process to remove DTZA from aqueous solution. Because of the great dimension of the stripping solution droplet, which facilitates quicker coalescence phenomena in a lesser time and reduces the mass transport rate as a result of a reduced interfacial area, the extraction effectiveness of DTZA was low for inadequate emulsification times (1 and 2 min). After 4 min of emulsification, a higher extraction efficiency was achieved, indicating that the steady state had been attained. This is because the uniformity of the dispersed solution has enhanced and the dimension of the aqueous stripping phase droplets containing the stripping substance has shrunk. Further increases in emulsification time yielded lower DTZA extraction. It was found that when the emulsification period exceeded 4 min, the removal percentage decreased due to extreme internal shearing, which produces a high number of smaller droplets per unit volume and indorses their coalescence. Therefore, 4 min was preferred as the emulsification period for additional investigation in this work.
Figure 8b illustrates the effect of emulsification speeds in the interval from 11,500 to 24,000 rpm on DTZA extraction for 4 min of emulsification time. Removal efficiency increases as the emulsification speed rises from 11,500 to 20,000 rpm. When efficient emulsification causes the internal phase to spread into the membrane in small droplets, good dispersion occurs. As these droplets reduce in size, they take more time to coalesce. This allows for high emulsion stability. Additionally, the dimension of the inner phase droplets diminishes as the emulsification rate increases, which increases the surface area accessible for extraction. At the higher emulsification rate (24,000), drug removal decreased significantly, and emulsion stability was also affected. Therefore, the optimal value was selected for its higher extraction yield, which was achieved at an emulsification rate of 20,000 rpm.
3.12. Influence of DTZA Initial Concentration
The impact of initial DTZA concentration varying from 5 to 100 mg/L on the extent of removal was examined. The outcomes were reported in
Figure 9a after an extraction period of 40 min. As the substrate dosage increases from 5 to 10 mg/L, the extraction effectiveness augments. With the augmentation in initial DTZA concentration, the concentration gradient (driving force) increased. The stripping solution droplets located in the peripheral zones of the emulsion globules remove most of the drug molecules diffusing into the globules when the initial DTZA concentration is low. The removal percentage decreased insignificantly at solute concentrations greater than 10 mg/L. At high concentrations of DTZA in the external solution, the inner droplets in the peripheral zone of the emulsion reach saturation more quickly. The substrate must penetrate further into the globule before it is removed because the peripheral droplets quickly run out of DTZA as the initial concentration increases. Therefore, the diffusion path lengths increase in proportion to the increase in initial DTZA concentration. In general, a good removal percentage was attained for initial DTZA concentrations ranging from 10 to 100 mg/L, which is a real advantage for the decontamination of water polluted with this drug by the LSM process.
3.13. Removal of DTZA from Natural Water Matrix
The extraction percentage of DTZA was investigated by dissolving the contaminant in seawater, tap water and natural mineral water. 10 mg/L DTZA was used as the initial concentration for the experiments. The major properties of the natural mineral water, tap water, and seawater were consigned in
Table 1. The results were shown in
Figure 9b. The extraction of DTZA from distilled water was consistently greater than those observed in the natural water matrices. Almost the same elimination percentage was achieved in natural mineral water and tap water, with a slight advantage in removal rate for natural mineral water. This is due to the fact that the chloride ion content in tap water is moderately greater than in natural mineral water. In contrast, no removal was achieved in seawater due to the very higher concentration of chloride anions, which inhibited the release of chloride anion exchanged by the mobile carrier from the membrane to the contaminated aqueous solution. Therefore, the LSM treatment technique offers a fascinating enhanced separation method for the extraction of DTZA from waters with low concentration of chloride ions.
4. Conclusions
In this study, an emulsion (W/O) was implemented for the extraction of DTZA from aqueous phase by an emulsified liquid membrane formed of an emulsifier (Span 80), an extractant (Aliquat 336) and a diluent (kerosene). The effect of experimental parameters on DTZA removal was investigated. A fully stabilized emulsion with an excellent removal efficiency of 90.8% of DTZA in water was obtained for an emulsifier dosage of 3% (w/w), an extractant dosage of 1.0% (w/w), a pH of the contaminated solution of 10 using NH4OH, a concentration of the inner solution of 0. 3 N KCl, an emulsion/external solution volume ratio of 20/250, an internal solution/organic phase volume ratio of 1/1, a mixing rate of 250 rpm, an emulsification time of 4 min, and an emulsification degree of 20,000 rpm. The developed LSM system was able to treat water contaminated with DTZA over a concentration range of 5 to 100 mg/L. Even in natural water matrices, DTZA can be successfully removed from waters using the LSM decontamination process, an advanced separation method, except in seawater due to the huge concentration of chloride anions that inhibit the transport by carrier.