Transition Metal Exchanged β Zeolites: CoM/β (M = Zn, Ce, and Cu) as Oxygen Electrode in Alkaline Media
Abstract
1. Introduction
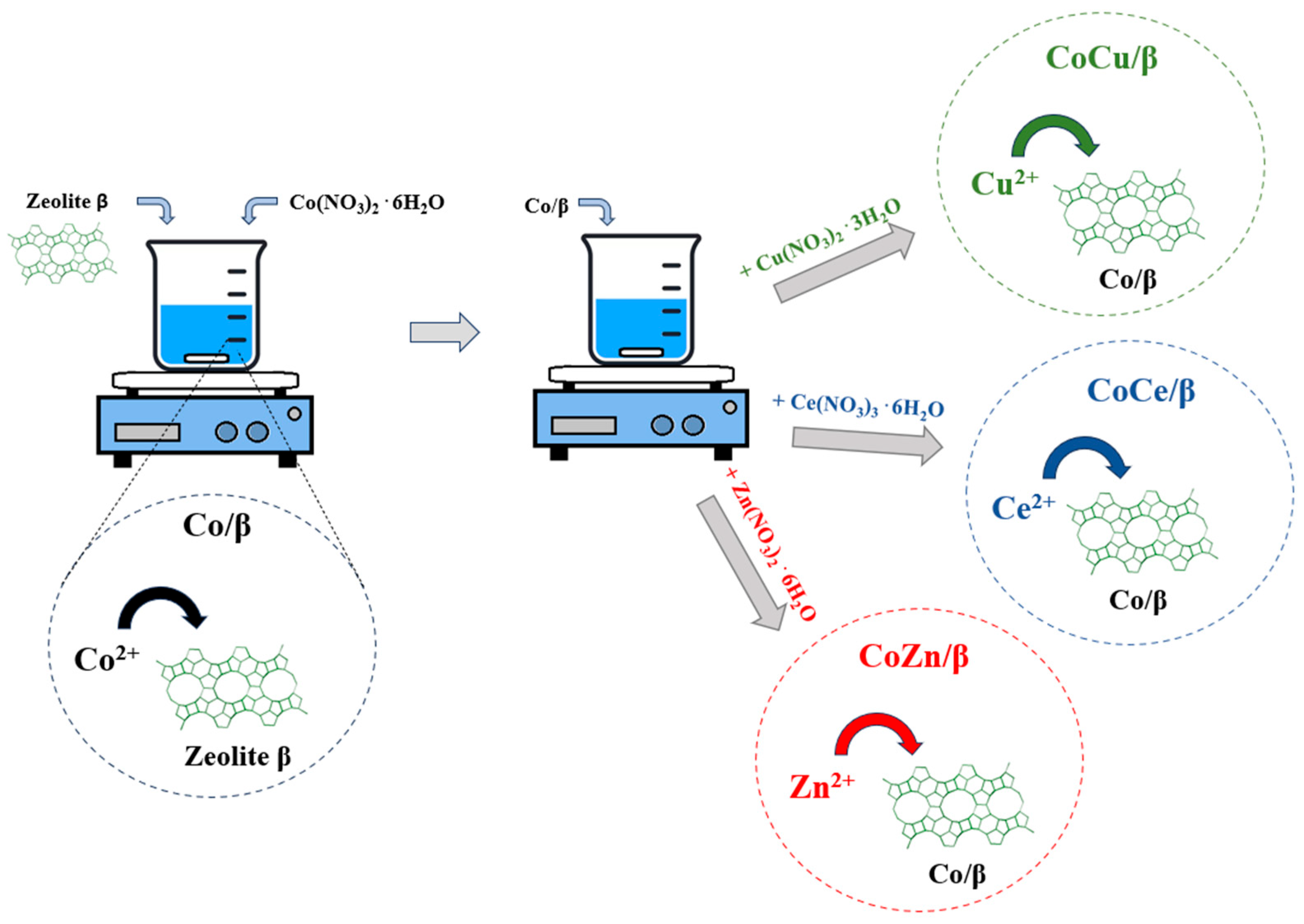
2. Materials and Methods
3. Results
3.1. Characterization of CoM/β (M = Zn, Ce, and Cu) Electrocatalyst
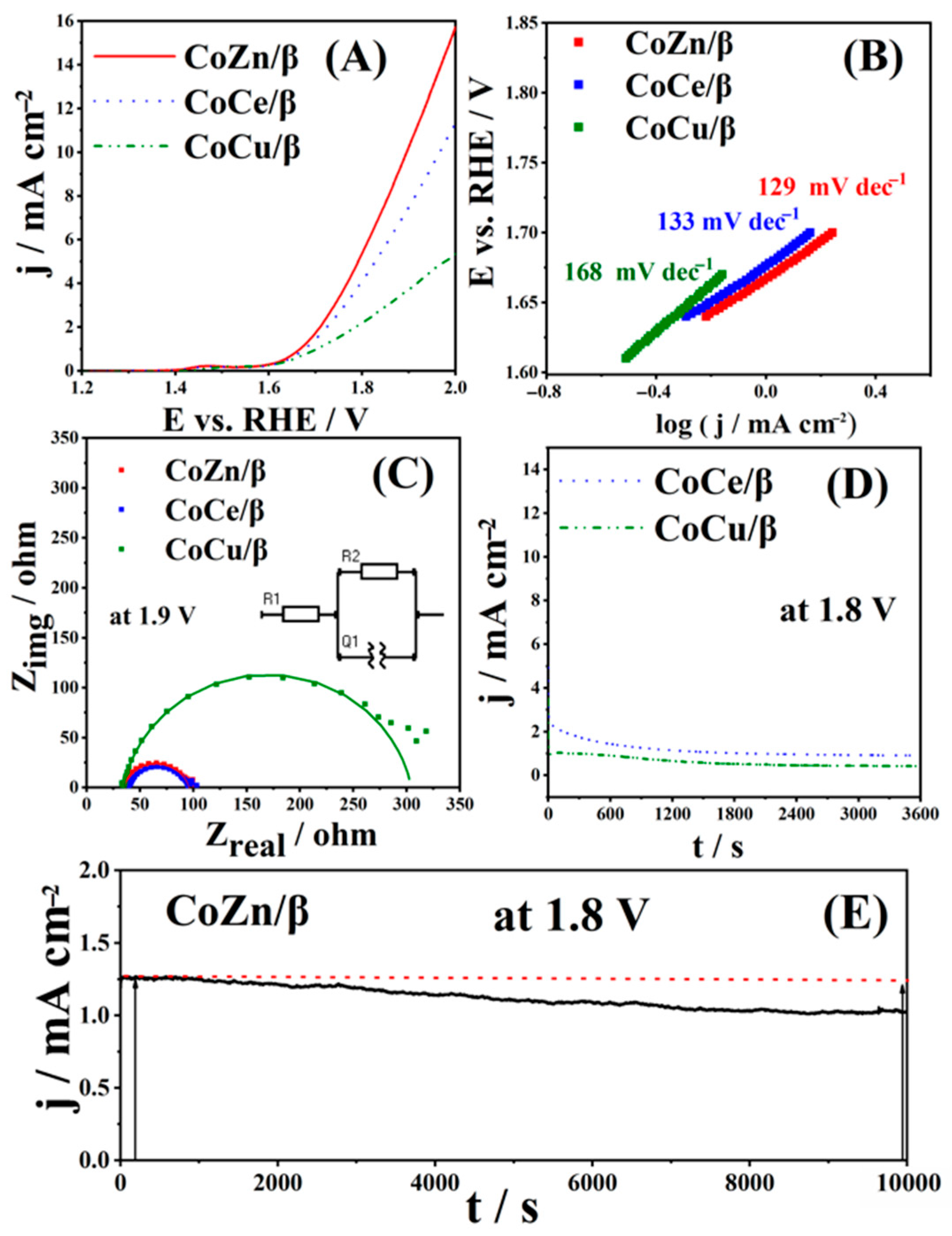
3.2. OER Study of CoM/β Zeolites
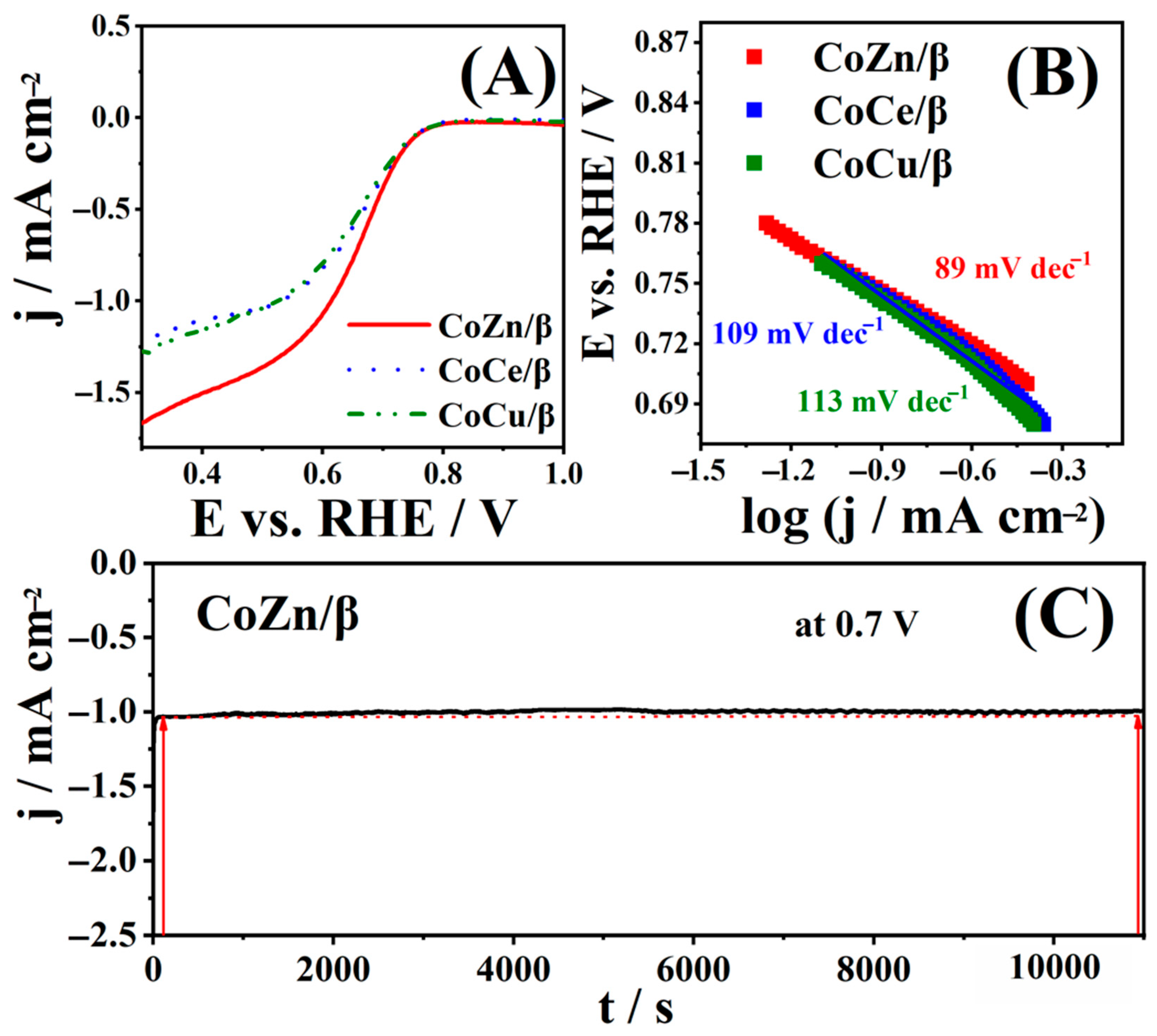
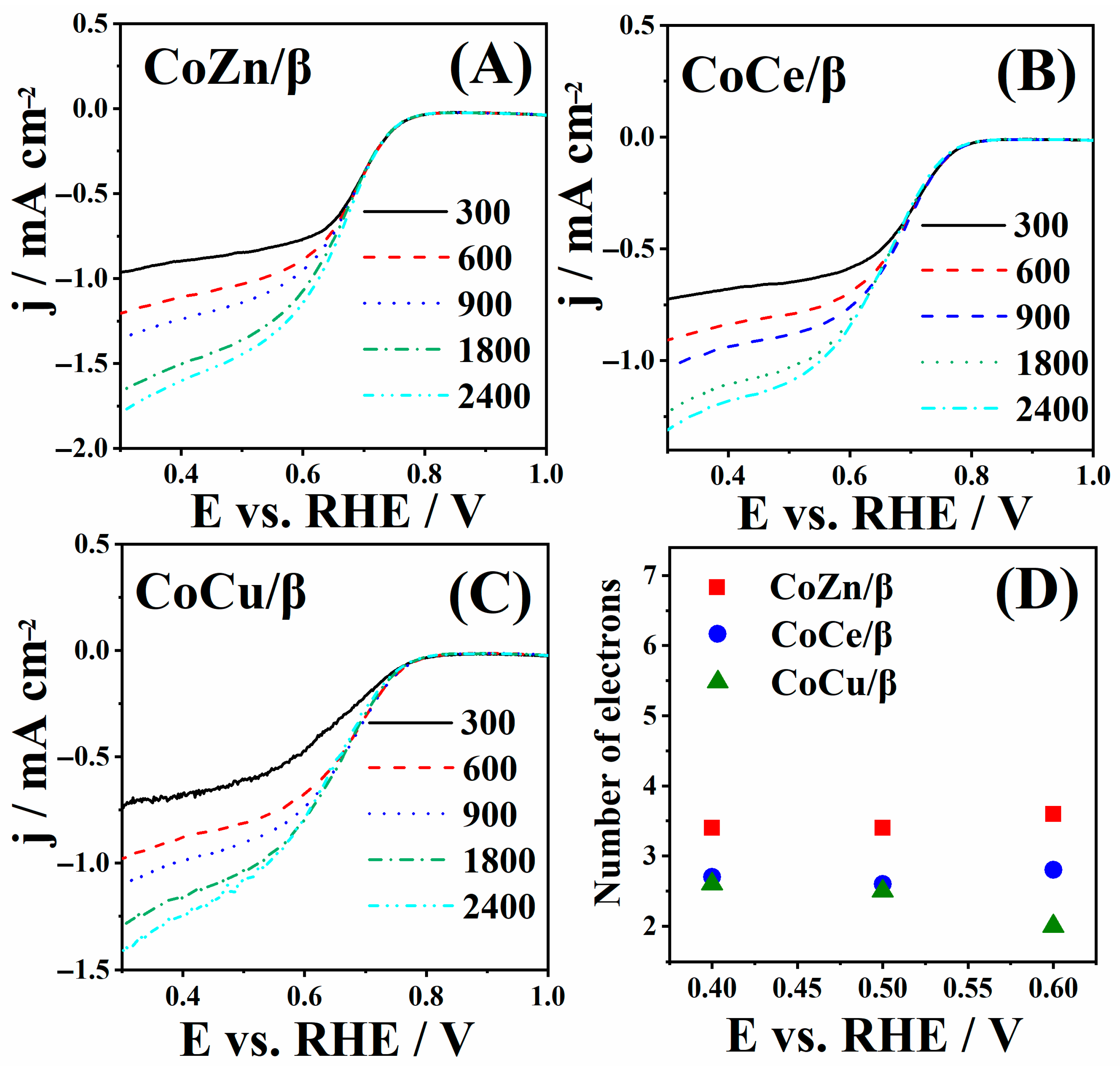
3.3. ORR Study of CoM/β Zeolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Zhang, D.; Xu, K.; Qian, B.; Chen, K.; Xue, D. Ir Cluster/Fe2O3 Heterostructures on Carbon Nanotubes Boosting Oxygen Evolution/Reduction Reactions for Flexible Zn-Air Battery. Nano Mater. Sci. 2025; in press. [Google Scholar] [CrossRef]
- Milikić, J.; Martins, M.; Dobrota, A.S.; Bozkurt, G.; Soylu, G.S.P.; Yurtcan, A.B.; Skorodumova, N.V.; Pašti, I.A.; Šljukić, B.; Santos, D.M.F. A Pt/MnV2O6 Nanocomposite for the Borohydride Oxidation Reaction. J. Energy Chem. 2021, 55, 428–436. [Google Scholar] [CrossRef]
- Milikić, J.; Tapia, A.; Stamenović, U.; Vodnik, V.; Otoničar, M.; Škapin, S.; Santos, D.M.F.; Šljukić, B. High-Performance Metal (Au, Cu)–Polypyrrole Nanocomposites for Electrochemical Borohydride Oxidation in Fuel Cell Applications. Int. J. Hydrogen Energy 2022, 47, 36990–37001. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, R.; Ai, Z.; Liu, H.; Wei, C.; Song, Y.; Dirican, M.; Zhang, X.; Ma, C.; Shi, J. Hollow Co3O4-x Nanoparticles Decorated N-Doped Porous Carbon Prepared by One-Step Pyrolysis as an Efficient ORR Electrocatalyst for Rechargeable Zn-Air Batteries. Carbon 2021, 181, 87–98. [Google Scholar] [CrossRef]
- Shen, M.; Hu, W.; Duan, C.; Li, J.; Ding, S.; Zhang, L.; Zhu, J.; Ni, Y. Cellulose Nanofibers Carbon Aerogel Based Single-Cobalt-Atom Catalyst for High-Efficiency Oxygen Reduction and Zinc-Air Battery. J. Colloid Interface Sci. 2023, 629, 778–785. [Google Scholar] [CrossRef]
- Niu, W.J.; Yan, Y.Y.; Li, R.J.; Zhao, W.W.; Chen, J.L.; Liu, M.J.; Gu, B.; Liu, W.W.; Chueh, Y.L. Identifying the Impact of Fe Nanoparticles Encapsulated by Nitrogen-Doped Carbon to Fe Single Atom Sites for Boosting Oxygen Reduction Reaction toward Zn-Air Batteries. Chem. Eng. J. 2023, 456, 140858. [Google Scholar] [CrossRef]
- Ling, W.; Wang, H.; Chen, Z.; Ji, Z.; Wang, J.; Wei, J.; Huang, Y. Intrinsic Structure Modification of Electrode Materials for Aqueous Metal-Ion and Metal-Air Batteries. Adv. Funct. Mater. 2021, 31, 2006855. [Google Scholar] [CrossRef]
- Wang, R.; Meng, Z.; Yan, X.; Tian, T.; Lei, M.; Pashameah, R.A.; Abo-Dief, H.M.; Algadi, H.; Huang, N.; Guo, Z.; et al. Tellurium Intervened Fe-N Codoped Carbon for Improved Oxygen Reduction Reaction and High-Performance Zn-Air Batteries. J. Mater. Sci. Technol. 2023, 137, 215–222. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Niu, J.; Liu, Y.; Bridges, D.; Liu, X.; Pooran, J.; Zhang, Y.; Hu, A. Recent Progress of Metal-Air Batteries—A Mini Review. Appl. Sci. 2019, 9, 2787. [Google Scholar] [CrossRef]
- Li, H.; Shu, X.; Tong, P.; Zhang, J.; An, P.; Lv, Z.; Tian, H.; Zhang, J.; Xia, H. Fe–Ni Alloy Nanoclusters Anchored on Carbon Aerogels as High-Efficiency Oxygen Electrocatalysts in Rechargeable Zn–Air Batteries. Small 2021, 17, e2102002. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Segre, C.U.; Pour, G.S.; Vazquez, M.; Benard, L. Aqueous Air Cathodes and Catalysts for Metal-Air Batteries. Curr. Opin. Electrochem. 2023, 38, 101246. [Google Scholar] [CrossRef]
- Wang, H.; Pang, Y.; Mo, Z.; Wang, X.; Ren, J.; Wang, R. Performance Evaluation of Functionalized Carbon Aerogel as Oxygen Reduction Reaction Electrocatalyst in Zinc-Air Cell. J. Power Sources 2021, 511, 230458. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, Z.; Ge, X.; Yang, S.; Peng, Y.; Kang, Z.; Liu, Z.; Lee, J.Y.; Zhao, D. A Metal-Free ORR/OER Bifunctional Electrocatalyst Derived from Metal-Organic Frameworks for Rechargeable Zn-Air Batteries. Carbon 2017, 111, 641–650. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, X.; Chen, Y.; Tian, M.; Yang, T.; Zhang, J.; Gao, S. Ordered Mesoporous Carbon Fiber Bundles with High-Density and Accessible Fe-NX Active Sites as Efficient ORR Catalysts for Zn-Air Batteries. Chin. Chem. Lett. 2023, 34, 108142. [Google Scholar] [CrossRef]
- Sun, J.; Wang, N.; Qiu, Z.; Xing, L.; Du, L. Recent Progress of Non-Noble Metal Catalysts for Oxygen Electrode in Zn-Air Batteries: A Mini Review. Catalysts 2022, 12, 843. [Google Scholar] [CrossRef]
- Ding, F.; Liu, H.; Jiang, X.; Jiang, Y.; Cheng, J.; Tu, Y.; Xiao, W.; Li, C.; Yan, X. Bimetallic Zeolite Imidazolium Framework Derived Multiphase Co/HNC as PH-Universal Catalysts with Efficient Oxygen Reduction Performance for Microbial Fuel Cells. Electrochim. Acta 2023, 438, 141548. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhu, Z.; Zheng, L.; Qiu, H.; Fang, L.; Zheng, L.; Gao, J.; Zhu, G. Surface Modified FeCoZn-ZIF Derived Polyhedral Electrocatalysts for ORR under Alkaline and Acidic Conditions. J. Solid State Chem. 2024, 336, 124768. [Google Scholar] [CrossRef]
- Gamal, S.; Kospa, D.A.; Kaid, M.M.; El-Hakam, S.A.; Ahmed, A.I.; Ibrahim, A.A. Fe-Co Spinel Oxides Supported UiO-66-NH2 derived Zirconia/ N-Dopped Porous Hollow Carbon as an Efficient Oxygen Reduction Reaction Electrocatalyst. J. Environ. Chem. Eng. 2023, 11, 109359. [Google Scholar] [CrossRef]
- Jeon, Y.; Kwon, O.; Ji, Y.; Jeon, O.S.; Lee, C.; Shul, Y. Development of Micro-Tubular Perovskite Cathode Catalyst with Bi-Functionality on ORR / OER for Metal-Air Battery Applications. Korean Chem. Eng. Res. 2019, 57, 425–431. [Google Scholar] [CrossRef]
- Dong, J.; Jiao, Q.; Wang, H.; Wang, H.; Ren, Y. ke Encapsulated Fe3C Boosted Electrocatalytic Performance for Oxygen Reduction Reaction of N-Doped Carbon Nanotube. Heliyon 2025, 11, e40862. [Google Scholar] [CrossRef]
- Kuk, Y.; Ahmed, S.; Sun, H.J.; Shim, J.; Park, G. Synthesis of Porous Carbon-Coated Cobalt Catalyst through Pyrolyzing Metal–Organic Framework and Their Bifunctional OER/ORR Catalytic Activity for Zn-Air Rechargeable Batteries. Bull. Korean Chem. Soc. 2020, 41, 310–316. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.; Qi, S.; Li, W.; Zhao, M. Bifunctional HER/OER or OER/ORR Catalytic Activity of Two-Dimensional TM3(HITP)2 with TM = Fe − Zn. J. Phys. Chem. C 2020, 124, 9350–9359. [Google Scholar] [CrossRef]
- Wu, R.; Li, Y. Multi-Level Architecture Optimization of MOF-Templated Co-Based Nanoparticles Embedded in Hollow N—Doped Carbon Polyhedra for Efficient OER and ORR. ACS Catal. 2018, 8, 7879–7888. [Google Scholar] [CrossRef]
- Ostadhossein, A.; Guo, J.; Simeski, F.; Ihme, M. Functionalization of 2D Materials for Enhancing OER/ORR Catalytic Activity in Li-Oxygen Batteries. Commun. Chem. 2019, 2, 95. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Yan, J.; Wu, Z.; Jin, W. MOF-Derived Two-Dimensional N-Doped Carbon Nanosheets Coupled with Co–Fe–P–Se as Efficient Bifunctional OER/ORR Catalysts. Nanoscale 2019, 11, 20144–20150. [Google Scholar] [CrossRef]
- Paulraj, A.; Kiros, Y.; Göthelid, M.; Johansson, M. NiFeOx as a Bifunctional Electrocatalyst for Oxygen Reduction (OR) and Evolution (OE) Reaction in Alkaline Media. Catalysts 2018, 8, 328. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Lv, F.; Fan, Q.; Zhao, Y.Q.; Zhang, Q.; Wang, W.; Cheng, F.; Xi, P.; Guo, S. NiO/CoN Porous Nanowires as Efficient Bifunctional Catalysts for Zn-Air Batteries. ACS Nano 2017, 11, 2275–2283. [Google Scholar] [CrossRef]
- Wei, L.; Karahan, H.E.; Zhai, S.; Liu, H.; Chen, X.; Zhou, Z.; Lei, Y.; Liu, Z.; Chen, Y. Amorphous Bimetallic Oxide–Graphene Hybrids as Bifunctional Oxygen Electrocatalysts for Rechargeable Zn–Air Batteries. Adv. Mater. 2017, 29, 1701410. [Google Scholar] [CrossRef]
- Kreider, M.E.; Burke Stevens, M. Material Changes in Electrocatalysis: An In Situ/Operando Focus on the Dynamics of Cobalt-Based Oxygen Reduction and Evolution Catalysts. ChemElectroChem 2022, 10, e202200958. [Google Scholar] [CrossRef]
- Zhong, H.; Alberto Estudillo-Wong, L.; Gao, Y.; Feng, Y.; Alonso-Vante, N. Oxygen Vacancies Engineering by Coordinating Oxygen-Buffering CeO2 with CoOx Nanorods as Efficient Bifunctional Oxygen Electrode Electrocatalyst. J. Energy Chem. 2021, 59, 615–625. [Google Scholar] [CrossRef]
- Wang, H.F.; Tang, C.; Wang, B.; Li, B.Q.; Zhang, Q. Bifunctional Transition Metal Hydroxysulfides: Room-Temperature Sulfurization and Their Applications in Zn–Air Batteries. Adv. Mater. 2017, 29, 1702327. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, D.; Park, J.; Lee, W.; Park, J.; Kang, S.J.; Kim, Y.; Ryu, J. Tetraruthenium Polyoxometalate as an Atom-Efficient Bifunctional Oxygen Evolution Reaction/Oxygen Reduction Reaction Catalyst and Its Application in Seawater Batteries. ACS Appl. Mater. Interfaces 2020, 12, 32689–32697. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; You, S.; Li, X.; Zhang, Y.; Yu, Y.; Li, Q.; Wang, F.; Ren, N.; Zou, J. In Situ Immobilization of Copper Oxide Thin-Layer on Zeolitic Imidazolate Framework-67-Derived Cobalt Oxide@nitrogen-Doped Carbon with Multi-Level Architecture and Versatile Active Sites for Enhancing Oxygen Evolution/Reduction Reactions. J. Power Sources 2020, 478, 228707. [Google Scholar] [CrossRef]
- Zhou, H.; Ban, J.; Shen, Y.; Ning, Y.; Zhang, S.; Liu, F.; Cao, G.; Shao, G.; Silva, S.R.P.; Hu, J. Strategies to Maximize the Oxygen Evolution Reaction in Layered Double Hydroxides by Electronic Defect Engineering. eScience 2025, 2, 100380. [Google Scholar] [CrossRef]
- Tüysüz, H. Alkaline Water Electrolysis for Green Hydrogen Production. Acc. Chem. Res. 2024, 57, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Kempler, P.A.; Boettcher, S.W. Electrolyte Engineering for Advanced Alkaline Water Electrolysis. ECS Meet. Abstr. 2025, MA2025-01, 1950. [Google Scholar] [CrossRef]
- Magnier, L.; Cossard, G.; Martin, V.; Pascal, C.; Roche, V.; Sibert, E.; Shchedrina, I.; Bousquet, R.; Parry, V.; Chatenet, M. Fe–Ni-Based Alloys as Highly Active and Low-Cost Oxygen Evolution Reaction Catalyst in Alkaline Media. Nat. Mater. 2024, 23, 252–261. [Google Scholar] [CrossRef]
- Lu, F.; Zhou, M.; Zhou, Y.; Zeng, X. First-Row Transition Metal Based Catalysts for the Oxygen Evolution Reaction under Alkaline Conditions: Basic Principles and Recent Advances. Small 2017, 13, 1701931. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Wang, Y.; Xu, X. N-Doped CuCo2S4 with Bifunctional ORR/OER Activities as Cathode Material in Zn-Air Battery. Inorg. Chem. Commun. 2025, 181, 115223. [Google Scholar] [CrossRef]
- Jithul, K.P.; Tamilarasi, B.; Pandey, J. In-Situ Growth of γ-Mn2O3 on Activated Carbon Cloth for Enhanced Bifunctional Electrocatalysis of ORR and OER. Mater. Chem. Phys. 2025, 341, 130955. [Google Scholar] [CrossRef]
- Wang, R.; Su, W.; Kang, Z.; Guo, S.; Pan, J. High-Efficient OER/ORR Bifunctional Electrocatalyst Based on Single Transition-Metal Anchored Graphynes: Key Descriptors under Acceptance-Backdonation Mechanism Framework. Appl. Surf. Sci. 2025, 690, 162482. [Google Scholar] [CrossRef]
- Liang, W.; Shi, J.; Qin, Z.; Cai, J.; He, Y.; Li, J. Fe-Nx Sites Coupled with Co2P Nanoparticles to Boost the ORR/OER Bifunctional Catalytic Performance. J. Alloys Compd. 2025, 1026, 180455. [Google Scholar] [CrossRef]
- Du, Y.; Zhong, Z.; Shi, Z.; Zhou, L.; Pan, S.; Xu, X.; Liu, Y.; Xiong, D.; Wang, K. Dual-Ligand Engineered CoNi Alloy/N-Doped Carbon Nanotubes Bifunctional ORR/OER Electrocatalyst for Long-Lifespan Rechargeable Zn-Air Batteries. J. Colloid Interface Sci. 2025, 683, 631–640. [Google Scholar] [CrossRef]
- Mooste, M.; Ahmed, Z.; Kapitulskis, P.; Ivanov, R.; Treshchalov, A.; Piirsoo, H.M.; Kikas, A.; Kisand, V.; Kukli, K.; Hussainova, I.; et al. Bifunctional Oxygen Electrocatalyst Based on Fe, Co, and Nitrogen Co-Doped Graphene-Coated Alumina Nanofibers for Zn-Air Battery Air Electrode. Appl. Surf. Sci. 2024, 660, 160024. [Google Scholar] [CrossRef]
- Milikić, J.; Nastasić, A.; Knežević, S.; Rakočević, L.; Stojadinović, S.; Stanković, D.; Šljukić, B. Efficient Nano-Size ZnM/rGO (M = Ni, Cu, and Fe) Electrocatalysts for Oxygen Electrode Reactions in Alkaline Media. Int. J. Hydrogen Energy 2025, 97, 247–258. [Google Scholar] [CrossRef]
- Milikić, J.; Knežević, S.; Stojadinović, S.; Alsaiari, M.; Harraz, F.A.; Santos, D.M.F.; Šljukić, B. Facile Synthesis of Low-Cost Copper-Silver and Cobalt-Silver Alloy Nanoparticles on Reduced Graphene Oxide as Efficient Electrocatalysts for Oxygen Reduction Reaction in Alkaline Media. Nanomaterials 2022, 12, 2657. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, B.; Ocon, J.D. Oxygen Electrocatalysis in Chemical Energy Conversion and Storage Technologies. Curr. Appl. Phys. 2013, 13, 309–321. [Google Scholar] [CrossRef]
- Nernprom, K.; Sanetuntikul, J.; Saejio, A.; Pitipuech, N.; Wichianwat, K.; Chanlek, N.; Poompipatpong, C.; Chanunpanich, N.; Ketpang, K. Water Hyacinth Root Derived Hybrid Metal Oxides/Nitrogen Doped Porous Carbon as an Efficient Non-Precious Metal Oxygen Reduction Reaction Electrocatalyst in Alkaline Media. Int. J. Hydrogen Energy 2024, 50, 1549–1558. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, R.; Shao, X.; Dai, B.; Ma, L.; Yang, J.; Shi, J.; Zhang, X.; Ma, C.; Jin, Z. Co/CoO Hetero-Nanoparticles Incorporated into Lignin-Derived Carbon Nanofibers as a Self-Supported Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Batteries. J. Colloid Interface Sci. 2025, 682, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, X.; Yue, D.; Zheng, F.; Li, Q.; Ma, Z.; Wang, H. MnS Doping Regulating Co Active Sites on Fibrous Cobalt Nitride as Bifunctional Oxygen Electrocatalyst for High-Performance Zn-Air Battery. J. Alloys Compd. 2024, 1005, 176153. [Google Scholar] [CrossRef]
- Choi, J.H.; Chun, H.; Kim, D.W.; Kabiraz, M.K.; Kim, J.; Kim, J.; Kim, K.H.; Wang, B.; Jeong, H.M.; Choi, S., II; et al. Zeolitic Imidazolate Framework-Derived Bifunctional CoO-Mn3O4 Heterostructure Cathode Enhancing Oxygen Reduction/Evolution via Dynamic O-Vacancy Formation and Healing for High-Performance Zn-Air Batteries. Energy Storage Mater. 2025, 75, 104040. [Google Scholar] [CrossRef]
- Ma, C.; Zhu, B.; Wang, Y.; Ma, S.; Shi, J.; Zhang, X.; Song, Y. Porous Carbon Nanosheets Integrated with Graphene-Wrapped CoO and CoNx as Efficient Bifunctional Oxygen Electrocatalysts for Rechargeable Zinc-Air Batteries. J. Colloid Interface Sci. 2025, 685, 793–803. [Google Scholar] [CrossRef]
- Zheng, H.; Lin, L.; Chen, Z.; Yang, T.C.; Wang, H.; Jiang, Z.; Bao, C.; Yang, C.M.; Lai, N.C. Carbon Doped Cobalt Nanoparticles Encapsulated in Graphitic Carbon Shells: Efficient Bifunctional Oxygen Electrocatalysts for Ultrastable Zn-Air Batteries. J. Colloid Interface Sci. 2025, 686, 624–633. [Google Scholar] [CrossRef]
- Jović, A.; Milikić, J.; Bajuk-Bogdanović, D.; Milojević-Rakić, M.; Vasiljević, B.N.; Krstić, J.; Cvjetićanin, N.; Šljukić, B. 12-Phosphotungstic Acid Supported on BEA Zeolite Composite with Carbonized Polyaniline for Electroanalytical Sensing of Phenols in Environmental Samples. J. Electrochem. Soc. 2018, 165, H1013–H1020. [Google Scholar] [CrossRef]
- Nedić Vasiljević, B.; Takić, M.; Mijailović, N.R.; Janošević Ležaić, A.; Jevremović, A.; Uskoković-Marković, S.; Milojević-Rakić, M.; Bajuk-Bogdanović, D. Phenolics over Zeolites and Related Materials—Biomedical and Environmental Applications. Antioxidants 2024, 13, 1548. [Google Scholar] [CrossRef]
- Smeets, P.J.; Woertink, J.S.; Sels, B.F.; Solomon, E.I.; Schoonheydt, R.A. Transition-Metal Ions in Zeolites: Coordination and Activation of Oxygen. Inorg. Chem. 2010, 49, 3573–3583. [Google Scholar] [CrossRef]
- Lawton, J.A.; Lawton, S.L.; Leonowicz, M.E.; Rubin, M.K. The Framework Topology of Zeolite MCM-22. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1995; Volume 98, pp. 250–251. [Google Scholar]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th Revised ed.; Elsevier Science: Amsterdam, The Netherlands, 2007; ISBN 9780080554341. [Google Scholar]
- Esquivel, D.; Cruz-Cabeza, A.J.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Transition Metal Exchanged β Zeolites: Characterization of the Metal State and Catalytic Application in the Methanol Conversion to Hydrocarbons. Microporous Mesoporous Mater. 2013, 179, 30–39. [Google Scholar] [CrossRef]
- Wu, C.; Tang, Y.; Zou, A.; Li, J.; Meng, H.; Gao, F.; Wu, J.; Wang, X. Recommended Electrochemical Measurement Protocol for Oxygen Evolution Reaction. DeCarbon 2025, 8, 100108. [Google Scholar] [CrossRef]
- Newsam, J.M.; Treacy, M.M.J.J.; Koetsier, W.T.; De Gruyter, C.B. Structural Characterization of Zeolite Beta. Proc. R. Soc. Lond. A Math. Phys. Sci. 1988, 420, 375–405. [Google Scholar] [CrossRef]
- Fu, L.; Gong, J.; Li, H.; Xiao, J.; Lv, B.; Wu, X.; Huang, Z.; Zhou, Z.; Jing, G. Modulating Acidity in Nickel-Modified H-β Zeolite Catalyzes Low-Energy Regeneration of CO2-captured Amine Solution. Sep. Purif. Technol. 2025, 361, 131046. [Google Scholar] [CrossRef]
- Chirra, S.; Siliveri, S.; Adepu, A.K.; Goskula, S.; Gujjula, S.R.; Narayanan, V. Pd-KIT-6: Synthesis of a Novel Three-Dimensional Mesoporous Catalyst and Studies on Its Enhanced Catalytic Applications. J. Porous Mater. 2019, 26, 1667–1677. [Google Scholar] [CrossRef]
- Mendes, A.N.; Matynia, A.; Toullec, A.; Capela, S.; Ribeiro, M.F.; Henriques, C.; Da Costa, P. Potential Synergic Effect between MOR and BEA Zeolites in NOx SCR with Methane: A Dual Bed Design Approach. Appl. Catal. A Gen. 2015, 506, 246–253. [Google Scholar] [CrossRef]
- Qiu, B.; Zhang, Y.Y.; Zhang, Y.Y. A Stable Zinc Zeolite Catalyst for Dehydrogenation of Ethane to Aromatics and Ethylene. Catal. Lett. 2022, 152, 1372–1385. [Google Scholar] [CrossRef]
- Landripet, I.; Puškarić, A.; Robić, M.; Bronić, J. Fine Tuning of Hierarchical Zeolite Beta Acid Sites Strength. Crystals 2023, 14, 53. [Google Scholar] [CrossRef]
- Giordanino, F.; Vennestrøm, P.N.R.; Lundegaard, L.F.; Stappen, F.N.; Mossin, S.; Beato, P.; Bordiga, S.; Lamberti, C. Characterization of Cu-Exchanged SSZ-13: A Comparative FTIR, UV-Vis, and EPR Study with Cu-ZSM-5 and Cu-β with Similar Si/Al and Cu/Al Ratios. Dalt. Trans. 2013, 42, 12741–12761. [Google Scholar] [CrossRef]
- Klingan, K.; Ringleb, F.; Zaharieva, I.; Heidkamp, J.; Chernev, P.; Gonzalez-Flores, D.; Risch, M.; Fischer, A.; Dau, H. Water Oxidation by Amorphous Cobalt-Based Oxides: Volume Activity and Proton Transfer to Electrolyte Bases. ChemSusChem 2014, 7, 1301–1310. [Google Scholar] [CrossRef]
- Stevens, M.B.; Enman, L.J.; Batchellor, A.S.; Cosby, M.R.; Vise, A.E.; Trang, C.D.M.; Boettcher, S.W. Measurement Techniques for the Study of Thin Film Heterogeneous Water Oxidation Electrocatalysts. Chem. Mater. 2017, 29, 120–140. [Google Scholar] [CrossRef]
- Milikić, J.; Stojanović, S.; Rondović, K.; Damjanović-Vasilić, L.; Rac, V.; Šljukić, B. CoM-ZSM5 (M = Zn and Ni) Zeolites for an Oxygen Evolution Reaction in Alkaline Media. Processes 2024, 12, 907. [Google Scholar] [CrossRef]
- Charoen-amornkitt, P.; Pholauyphon, W.; Suzuki, T.; Tsushima, S. An Approach to Unify Capacitance Measurements of Electric Double Layer Capacitors Using Sinusoidal Potential Scan. J. Energy Storage 2023, 66, 107522. [Google Scholar] [CrossRef]
- Al-Sharif, M.S.; Arunachalam, P.; Abiti, T.; Amer, M.S.; Al-Shalwi, M.; Ghanem, M.A. Mesoporous Cobalt Phosphate Electrocatalyst Prepared Using Liquid Crystal Template for Methanol Oxidation Reaction in Alkaline Solution. Arab. J. Chem. 2020, 13, 2873–2882. [Google Scholar] [CrossRef]
- Milikić, J.; Stojanović, S.; Damjanović-Vasilić, L.; Vasilić, R.; Šljukić, B. Efficient Bifunctional Cerium-Zeolite Electrocatalysts for Oxygen Evolution and Oxygen Reduction Reactions in Alkaline Media. Synth. Met. 2023, 292, 117231. [Google Scholar] [CrossRef]
- Milikić, J.; Stojanović, S.; Damjanović-Vasilić, L.; Vasilić, R.; Rakočević, L.; Lazarević, S.; Šljukić, B. Porous Cerium-Zeolite Bifunctional ORR/OER Electrocatalysts in Alkaline Media. J. Electroanal. Chem. 2023, 944, 117668. [Google Scholar] [CrossRef]
- Xi, W.; Shen, M.; Yin, X.; Gao, B.; He, L.; Chen, Y.; Lin, B. Molten-Salt Confined Synthesis of Nitrogen-Doped Carbon Nanosheets Supported Co3O4 Nanoparticles as a Superior Oxygen Electrocatalyst for Rechargeable Zn-Air Battery. J. Power Sources 2023, 560, 232692. [Google Scholar] [CrossRef]
- Mladenović, D.; Aykut, Y.; Yurtcan, A.B.; Soylu, G.S.P.; Santos, D.M.F.; Miljanić, Š.; Šljukić, B. Optimizing Oxygen Electrode Bifunctionality with Platinum and Nickel Nanoparticle-Decorated Nitrogen-Doped Binary Metal Oxides. Processes 2024, 12, 453. [Google Scholar] [CrossRef]
- Milikić, J.; Rondović, K.; Vasilić, R.; Rac, V.; Damjanović-Vasilić, L.; Stanković, D. NiM/β Zeolites (M = Co, Cu, and Zn) as Bifunctional Oxygen Electrocatalysts. J. Electroanal. Chem. 2025, 996, 119357. [Google Scholar] [CrossRef]
- Milikić, J.; Vasić, M.; Amaral, L.; Cvjetićanin, N.; Jugović, D.; Hercigonja, R.; Šljukić, B. NiA and NiX Zeolites as Bifunctional Electrocatalysts for Water Splitting in Alkaline Media. Int. J. Hydrogen Energy 2018, 43, 18977–18991. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, H.; Luo, L. Gas Bubbles in Electrochemical Gas Evolution Reactions. Langmuir 2019, 35, 5392–5408. [Google Scholar] [CrossRef]
- Han, S.; Liu, S.; Wang, R.; Liu, X.; Bai, L.; He, Z. One-Step Electrodeposition of Nanocrystalline ZnxCo3-XO4 Films with High Activity and Stability for Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 17186–17194. [Google Scholar] [CrossRef]
- Kim, T.W.; Woo, M.A.; Regis, M.; Choi, K.-S. Electrochemical Synthesis of Spinel Type ZnCo2O4 Electrodes for Use as Oxygen Evolution Reaction Catalysts. J. Phys. Chem. Lett. 2014, 5, 2370–2374. [Google Scholar] [CrossRef]
- Ma, F.; Liu, X.; Wang, X.; Liang, J.; Huang, J.; Priest, C.; Liu, J.; Jiao, S.; Wang, T.; Wu, G.; et al. Atomically Dispersed Zn-Co-N-C Catalyst Boosting Efficient and Robust Oxygen Reduction Catalysis in Acid via Stabilizing Co-N Bonds. Fundam. Res. 2023, 3, 909–917. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Mukherjee, A.; Su, W.N.; Basu, S. Improved Bi-Functional ORR and OER Catalytic Activity of Reduced Graphene Oxide Supported ZnCo2O4 Microsphere. Int. J. Hydrogen Energy 2019, 44, 1565–1578. [Google Scholar] [CrossRef]
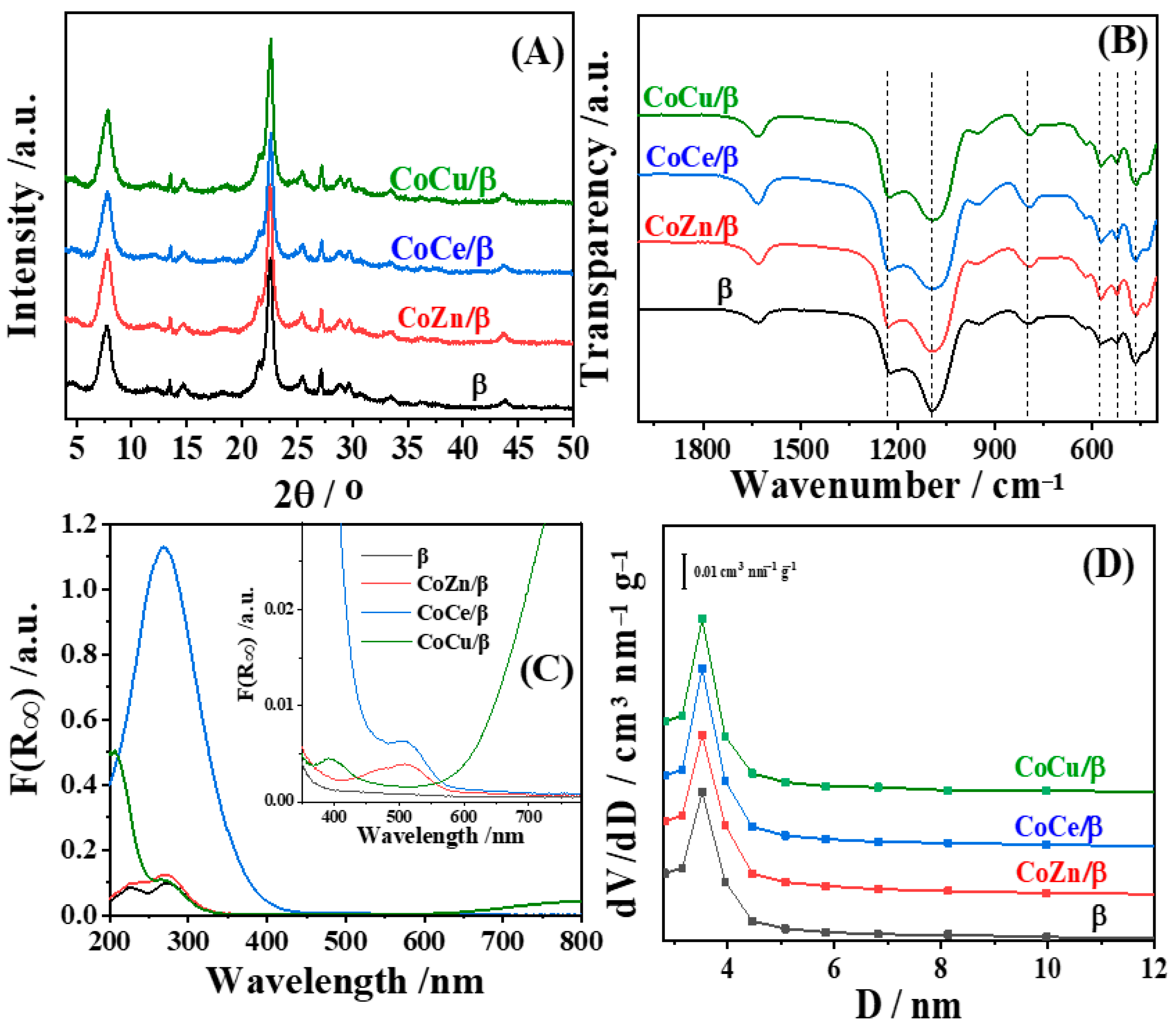
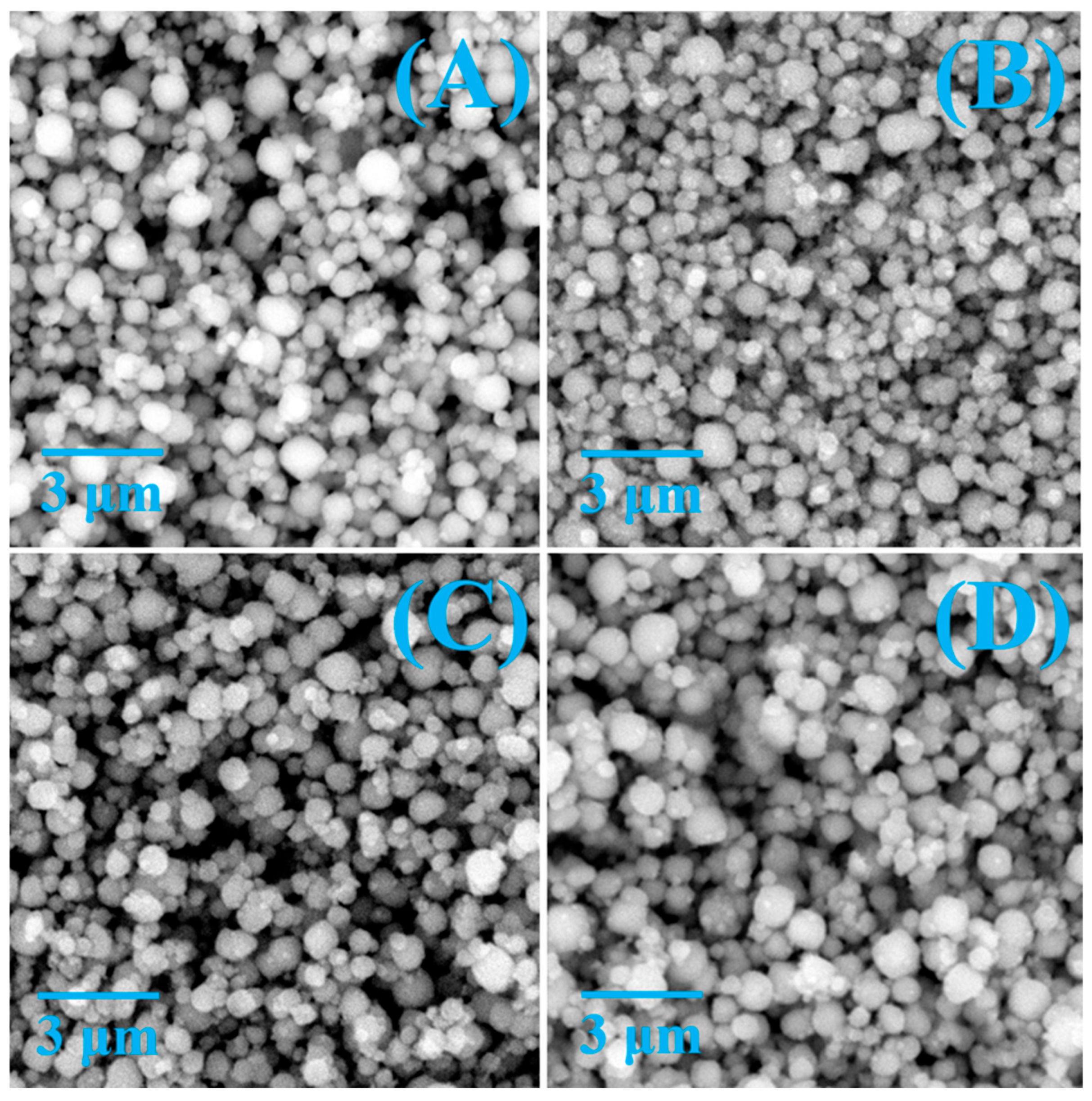
| Zeolites | SBET a, m2/g | Sexternal b, m2/g | Vmicro c, cm3/g | Vtot d, cm3/g |
| β | 638 | 132 | 0.200 | 0.361 |
| CoZn/β | 637 | 139 | 0.198 | 0.436 |
| CoCe/β | 622 | 142 | 0.191 | 0.403 |
| CoCu/β | 628 | 138 | 0.194 | 0.437 |
| Element | O | Si | Al | Co | Zn | Ce | Cu |
| wt % | |||||||
| β | 53.79 (1.57) | 43.75 (1.23) | 2.46 (0.05) | ||||
| CoZn/β | 51.16 (0.96) | 44.93 (0.71) | 2.43 (0.05) | 0.15 (0.10) | 1.34 (0.97) | ||
| CoCe/β | 49.35 (0.83) | 45.95 (0.20) | 2.43 (0.02) | 0.23 (0.29) | 2.04 (0.26) | ||
| CoCu/β | 52.15 (0.53) | 44.54 (0.44) | 2.43 (0.05) | 0.22 (0.28) | 0.66 (0.27) | ||
| OER Electrocatalysts | Electrolyte | Mass Loading/mg cm−2 | Eonset/V | Ƞonset/mV | b/mV dec−1 | j at 2 V/mA cm−2 | References |
|---|---|---|---|---|---|---|---|
| CoZn/β | 1 M KOH | 2 | 1.67 | 470 | 129 | 15.8 | This work. |
| CoCe/β | 1 M KOH | 2 | 1.67 | 470 | 133 | 11.1 | This work. |
| CoCu/β | 1 M KOH | 2 | 1.70 | 500 | 168 | 5.4 | This work. |
| NiCo/β | 1 M KOH | 2 | 1.66 | 460 | 119 | 34.2 | [77] |
| NiCu/β | 1 M KOH | 2 | 1.81 | 610 | 200 | 3.0 | [77] |
| NiZn/β | 1 M KOH | 2 | 1.63 | 430 | 171 | 19.4 | [77] |
| Co-ZSM5 | 1 M KOH | 3 | 1.61 | 410 | 269 | 6.8 | [70] |
| CoZn-ZSM5 | 1 M KOH | 3 | 1.64 | 440 | 234 | 2.3 | [70] |
| CoNi-ZSM5 | 1 M KOH | 3 | 1.61 | 410 | 134 | 9.5 | [70] |
| Ce-ZSM-5 | 1 M KOH | 3 | 1.68 | 480 | 207 | 1.6 | [74] |
| Ce-ZSM-5 cal | 1 M KOH | 3 | 1.66 | 460 | 202 | 1.7 | [74] |
| Ce-β cal | 1 M KOH | 3 | 1.61 | 410 | 114 | 7.3 | [74] |
| Ce-Cli | 1 M KOH | 3 | 1.73 | 530 | 220 | 1.2 | [73] |
| Ce-Cli cal | 1 M KOH | 3 | 1.69 | 490 | 278 | 1.54 | [73] |
| Ce-13X | 1 M KOH | 3 | 1.67 | 470 | 280 | 2.14 | [73] |
| Ce-13X cal | 1 M KOH | 3 | 1.60 | 400 | 296 | 4.6 | [73] |
| NiA | 1 M KOH | 1 | / | / | 463 | ~13 | [78] |
| NiX | 1 M KOH | 1 | / | / | 842 | ~3 | [78] |
| CoO/CoNx-C | 0.1 M KOH | 0.5 | / | / | 180 | / | [52] |
| P-CoO/CoNx-C | 0.1 M KOH | 0.5 | / | / | 198 | / | [52] |
| MnS/CoNx | 0.1 M KOH | 0.26 | / | / | 116.9 | / | [50] |
| Co-NC | 0.1 M KOH | / | / | / | 220 | / | [75] |
| Co3O4@NCNs | 0.1 M KOH | / | / | / | 107 | / | [75] |
| Pt/Mn2O3-NiO-N (1:1) | 0.1 M KOH | 0.2 | / | / | 249 | ∼25 | [76] |
| Pt/Mn2O3-NiO- N (1:2) | 0.1 M KOH | 0.2 | / | / | 245 | ∼27 | [76] |
| Electrocatalyst | Rs (Ω) | Rct (Ω) | Qe (mF) |
|---|---|---|---|
| CoZn/β | 34.9 | 60.4 | 1.9 × 10−4 |
| CoCe/β | 39.8 | 54.6 | 1.1 × 10−4 |
| CoCu/β | 34.7 | 268.7 | 1.1 × 10−4 |
| ORR Electrocatalysts | Electrolyte | Mass Loading/mg cm−2 | Eonset/V | E1/2/V | b/mV dec−1 | n | References |
|---|---|---|---|---|---|---|---|
| CoZn/β | 1 M KOH | 2 | 0.76 | 0.66 | 89 | 3.4–3.6 | This work. |
| CoCe/β | 1 M KOH | 2 | 0.76 | 0.66 | 109 | 2.6–2.8 | This work. |
| CoCu/β | 1 M KOH | 2 | 0.76 | 0.66 | 113 | 2.0–2.6 | This work. |
| NiCo/β | 1 M KOH | 2 | 0.74 | 0.65 | 102 | 2.2–2.4 | [77] |
| NiCu/β | 1 M KOH | 2 | 0.74 | 0.66 | 91 | 2.3–2.4 | [77] |
| NiZn/β | 1 M KOH | 2 | 0.76 | 0.68 | 81 | 3.0–3.3 | [77] |
| Ce-ZSM-5 | 1 M KOH | 3 | 0.82 | 0.77 | 79 | 2.3–2.5 | [74] |
| Ce-ZSM-5 cal | 1 M KOH | 3 | 0.81 | 0.73 | 70 | 2.3–2.4 | [74] |
| Ce-β cal | 1 M KOH | 3 | 0.79 | 0.71 | 178 | 1.6–1.9 | [74] |
| Ce-Cli | 1 M KOH | 3 | 0.82 | 0.71 | 399 | 2.4–2.7 | [73] |
| Ce-Cli cal | 1 M KOH | 3 | 0.82 | 0.70 | 228 | 2.2–2.6 | [73] |
| Ce-13X | 1 M KOH | 3 | 0.82 | 0.71 | 145 | 2.3–2.7 | [73] |
| Ce-13X cal | 1 M KOH | 3 | 0.84 | 0.75 | 80 | 2.2–2.4 | [73] |
| CoO/CoNx-C | 0.1 M KOH | 0.5 | / | 0.844 | 100 | / | [52] |
| P-CoO/CoNx-C | 0.1 M KOH | 0.5 | / | 0.788 | 103 | / | [52] |
| MnO/CoNx | 0.1 M KOH | 0.26 | / | 0.748 | 100.9 | / | [50] |
| CoNx | 0.1 M KOH | 0.26 | / | 0.752 | 93.2 | / | [50] |
| Fe/Co-NGr-1 | 0.1 M KOH | 0.2 | / | 0.68 | 53 | / | [44] |
| Co3O4@NGC@CuO-0.6 | 0.1 M KOH | / | 0.96 | 0.865 | 115 | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milikić, J.; Rondović, K.; Damjanović-Vasilić, L.; Rac, V.; Vasilić, R.; Stanković, D. Transition Metal Exchanged β Zeolites: CoM/β (M = Zn, Ce, and Cu) as Oxygen Electrode in Alkaline Media. Processes 2025, 13, 2996. https://doi.org/10.3390/pr13092996
Milikić J, Rondović K, Damjanović-Vasilić L, Rac V, Vasilić R, Stanković D. Transition Metal Exchanged β Zeolites: CoM/β (M = Zn, Ce, and Cu) as Oxygen Electrode in Alkaline Media. Processes. 2025; 13(9):2996. https://doi.org/10.3390/pr13092996
Chicago/Turabian StyleMilikić, Jadranka, Katarina Rondović, Ljiljana Damjanović-Vasilić, Vladislav Rac, Rastko Vasilić, and Dalibor Stanković. 2025. "Transition Metal Exchanged β Zeolites: CoM/β (M = Zn, Ce, and Cu) as Oxygen Electrode in Alkaline Media" Processes 13, no. 9: 2996. https://doi.org/10.3390/pr13092996
APA StyleMilikić, J., Rondović, K., Damjanović-Vasilić, L., Rac, V., Vasilić, R., & Stanković, D. (2025). Transition Metal Exchanged β Zeolites: CoM/β (M = Zn, Ce, and Cu) as Oxygen Electrode in Alkaline Media. Processes, 13(9), 2996. https://doi.org/10.3390/pr13092996










