Advancements in Functional Endophytic Bacterium-Assisted Phytoremediation of PHC-Contaminated Soils: A Review
Abstract
1. Introduction
2. Plant Functional Endophytic Bacteria
2.1. Types and Functions of Endophytic Bacteria
2.2. Sources and Distribution of Functional Endogenous Bacteria
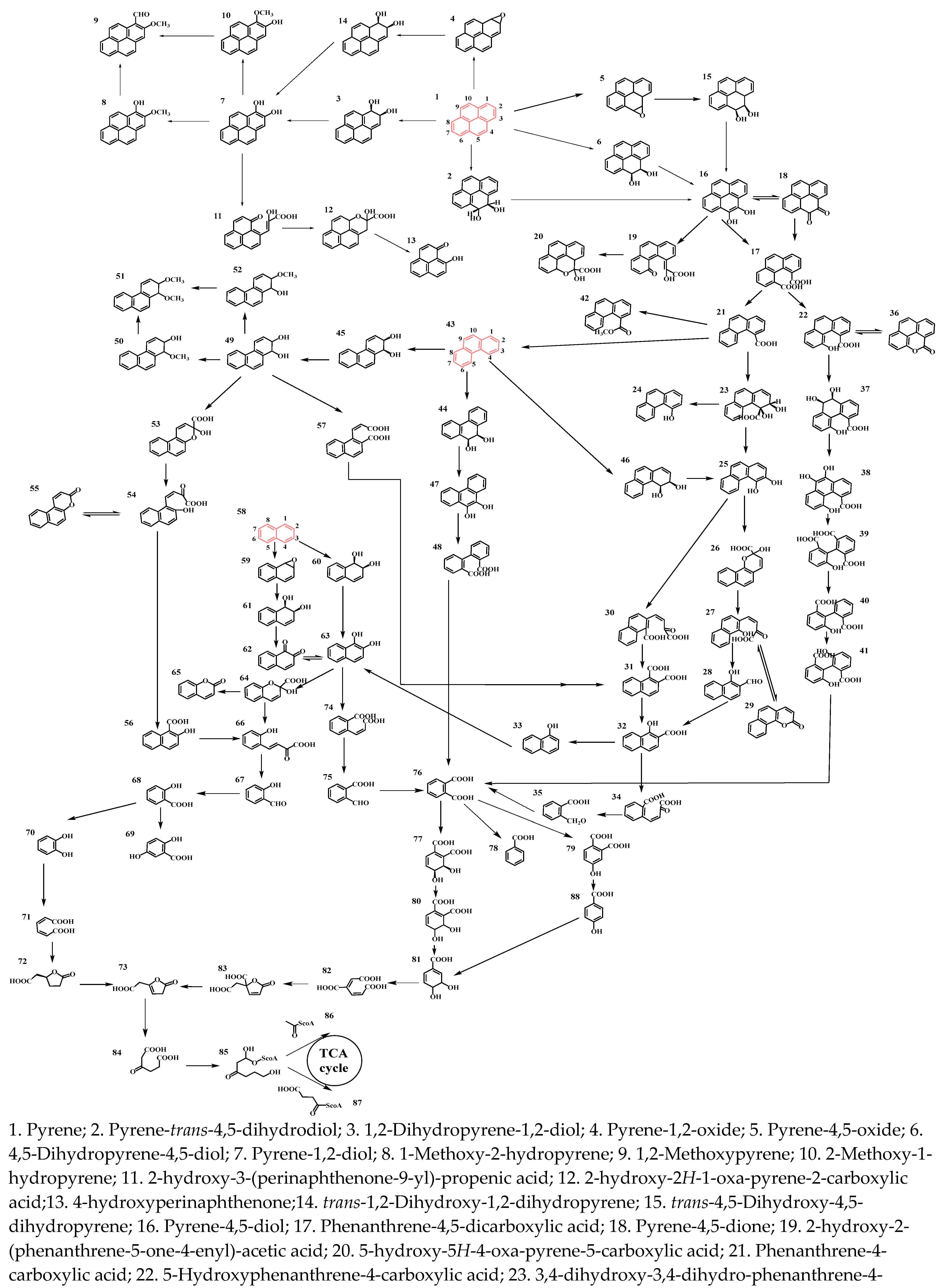
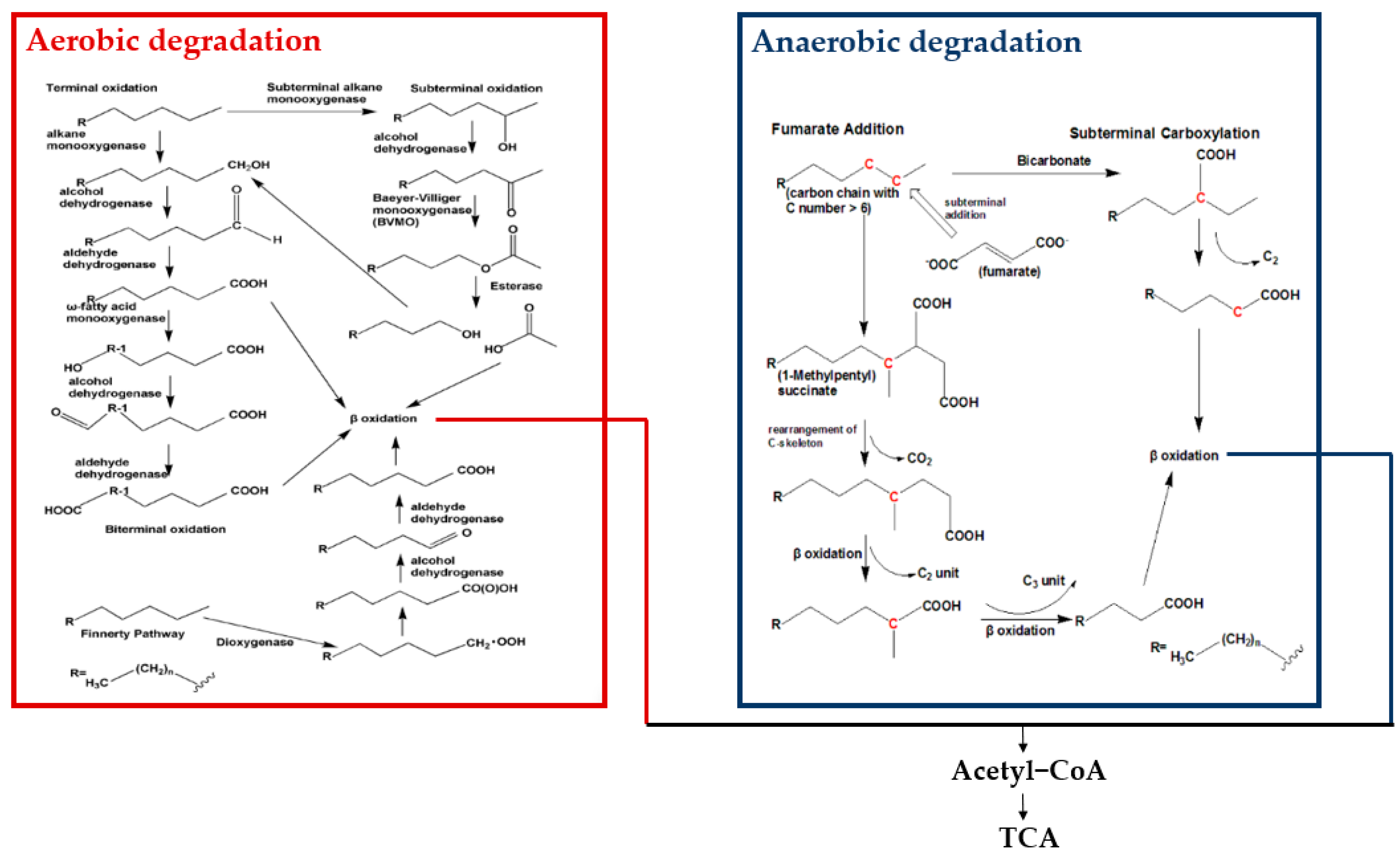
3. Molecular Mechanisms of PHC Degradation by Functional Endophytic Bacteria
4. Synergistic Mechanism of Functional Endophytic Bacteria
4.1. Direct Degradation or Co-Metabolism of PHC in Plants
4.2. Growth Promotion and Enhanced Remediation Efficiency in Restored Plants
4.3. Generation of Biosurfactants to Enhance Bioavailability of PHC
4.4. HGT Increases the Abundance and Activity of PHC-Degrading Bacteria
5. Prospects
- Isolating endophytic bacterial resources from woody plants; evaluating the efficacy of endophytic bacterium-assisted phytoremediation of PHC-contaminated soil by woody plants; and exploring the remediation potential of deep-rooted woody plant systems for deep-layer PHC pollutants in soil.
- Employing integrated multi-omics technologies to analyze the interaction network among endophytic bacteria, plants, and PHC pollutants; elucidating the molecular regulatory mechanisms underlying synergistic remediation by endophytic bacteria and host plants; constructing a metabolic network model for plant-endophytic bacterial interactions; and developing “Engineered Bacterial–Plant Remediation Systems”.
- Exploring the efficiency and stability of horizontal gene transfer in enhancing microbial community function within PHC-contaminated environments; monitoring microbial community succession and soil microecological changes during remediation; and assessing the ecological risks of gene diffusion.
- Constructing multifunctional microbial consortia that combine degradation, plant growth promotion, and production of surface-active agents; investigating microbial interaction networks and functional complementarity mechanisms; and developing efficient and stable functional endophytic bacterial agents. Optimizing agent delivery technologies (e.g., nanocarriers, biochar immobilization, and seed coating) to improve the colonization efficiency and environmental adaptability of endophytic bacteria.
- Directionally developing efficient remediation technology systems utilizing endophytic bacteria in synergy with plants for PHC-contaminated sites under different soil types, pollutant concentrations, climatic conditions, and other factors; and enhancing the engineering application potential of remediation technologies.
- Strengthening the development of a biosafety and ecological risk regulatory framework. Conducting systematic assessments of the survival, dispersal capacity, and potential ecological impacts of exogenous and engineered bacterial agents in the environment, and establishing an environmental behavior tracking system based on molecular monitoring technologies. Developing environmental safety standards and application guidelines for the use of endogenous bacterial agents to prevent ecological risks associated with horizontal gene transfer.
- Conducting a full life-cycle cost–benefit analysis to optimize techno-economic feasibility. Overcoming bottlenecks such as high mass-production costs, stringent storage requirements, and unstable field colonization effects of bacterial agents, and develop low-cost, long-acting microbial remediation materials. Exploring integrated “remediation–energy–agriculture” models, such as the resource utilization of phytoremediation biomass, to enhance the techno-economic sustainability of the technology.
- Promoting multi-stakeholder collaboration and policy support to facilitate technology integration and demonstration. Establishing a cooperative platform involving research institutions, enterprises, and regulatory agencies, and developing guidance documents and application standards for endophytic bacterial phytoremediation technology. Selecting typical petroleum-contaminated sites for long-term engineering demonstrations to validate their applicability and stability under real-world conditions, thereby supporting the standardization and large-scale application of the technology.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PHC | Petroleum Hydrocarbon |
| ACC | 1-Aminocyclopropane-1-Carboxylic Acid |
| PGP | Plant Growth Promotion |
| PBS | Production of Biosurfactant |
| PYR | Pyrene |
| BaP | Benzo[a]pyrene |
| ACY | Acenaphthylene |
| ACN | Acenaphthene |
| PHE | Phenanthrene |
| FLA | Fluoranthene |
| FLE | Fluorene |
| ANT | Anthracene |
| NAP | Naphthalene |
| TOL | Toluene |
| PAHs | Polyaromatic Hydrocarbons |
| TCA | Tricarboxylic Acid |
| HGT | Horizontal Gene Transfer |
| IAA | Indole-3-Acetic Acid |
| ISR | Induced Systemic Resistance |
| HCN | Hydrogen Cyanide |
| MGEs | Mobile Genetic Elements |
| ICEs | Integrative and Conjugative Elements |
| GIs | Genomic Islands |
References
- Li, Y.Q.; Chen, H.M.; Li, W.; Xi, B.; Huang, C. A novel immobilized bacteria consortium enhanced remediation efficiency of PAHs in soil: Insights into key removal mechanism and main driving factor. J. Hazard. Mater. 2025, 486, 137144. [Google Scholar] [CrossRef]
- Karishma, S.; Saravanan, A.; Deivayanai, V.C.; Ajithkumar, U.; Yaashikaa, P.R.; Vickram, A.S. Emerging strategies for enhancing microbial degradation of petroleum hydrocarbons: Prospects and challenges. Bioresour. Technol. Rep. 2024, 26, 101866. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, R.X.; Song, J.T.; Ren, Y.; Luo, X.; Li, Y.; Li, X.; Li, T.; Wang, X.; Zhou, Q. Combined phyto-microbial-electrochemical system enhanced the removal of petroleum hydrocarbons from soil: A profundity remediation strategy. J. Hazard. Mater. 2021, 420, 126592. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.F.; Mukkaram, E.; Alam, C.S.; Khan, M.I.; Zhao, B.; Liqun, C.; Salim, M.A.; Naveed, M.; Khan, N.; Núñez-Delgado, A.; et al. Phytotoxicity of petroleum hydrocarbons: Sources, impacts and remediation strategies. Environ. Res. 2021, 197, 111031. [Google Scholar] [CrossRef]
- Cary, T.J.; Rylott, E.L.; Zhang, L.; Routsong, R.M.; Palazzo, A.J.; Strand, S.E.; Bruce, N.C. Field trial demonstrating phytoremediation of the military explosive RDX by XplA/XplB-expressing switchgrass. Nat. Biotechnol. 2021, 39, 1216–1219. [Google Scholar] [CrossRef]
- Liu, C.J.; Deng, S.G.; Hu, C.Y.; Gao, P.; Khan, E.; Yu, C.P.; Ma, L.Q. Applications of bioremediation and phytoremediation in contaminated soils and waters: CREST publications during 2018–2022. Crit. Rev. Environ. Sci. Technol. 2023, 53, 723–732. [Google Scholar] [CrossRef]
- Wang, J.Y.; Aghajani Delavar, M. Techno-economic analysis of phytoremediation: A strategic rethinking. Sci. Total Environ. 2023, 902, 165949. [Google Scholar] [CrossRef] [PubMed]
- Devendrapandi, G.; Liu, X.H.; Balu, R.; Ayyamperumal, R.; Valan Arasu, M.; Lavanya, M.; Minnam Reddy, V.R.; Kim, W.K.; Karthika, P.C. Innovative remediation strategies for persistent organic pollutants in soil and water: A comprehensive review. Environ. Res. 2024, 249, 118404. [Google Scholar] [CrossRef]
- Oso, S.; Fuchs, F.; Übermuth, C.; Zander, L.; Daunaraviciute, S.; Remus, D.M.; Stötzel, I.; Wüst, M.; Schreiber, L.; Remus-Emsermann, M.N.P. Biosurfactants Produced by Phyllosphere-Colonizing Pseudomonads Impact Diesel Degradation but Not Colonization of Leaves of Gnotobiotic Arabidopsis thaliana. Appl. Environ. Microbiol. 2021, 87, e00091-21. [Google Scholar] [CrossRef]
- Tiwari, P.; Kang, S.; Bae, H. Plant-endophyte associations: Rich yet under-explored sources of novel bioactive molecules and applications. Microbiol. Res. 2023, 266, 127241. [Google Scholar] [CrossRef] [PubMed]
- Faoro, H.; Rene Menegazzo, R.; Battistoni, F.; Gyaneshwar, P.; do Amaral, F.P.; Taulé, C.; Rausch, S.; Gonçalves Galvão, P.; de los Santos, C.; Mitra, S.; et al. The oil-contaminated soil diazotroph Azoarcus olearius DQS-4T is genetically and phenotypically similar to the model grass endophyte Azoarcus sp. BH72. Environ. Microbiol. Rep. 2017, 9, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Karaś, M.A.; Wdowiak-Wróbel, S.; Marek-Kozaczuk, M.; Sokolowski, W.; Melianchuk, K.; Komaniecka, I. Assessment of phenanthrene degradation potential by plant-growth-promoting endophytic strain Pseudomonas chlororaphis 23aP isolated from Chamaecytisus albus (Hacq.) Rothm. Molecules 2023, 28, 7851. [Google Scholar] [CrossRef]
- Kukla, M.; Płociniczak, T.; Piotrowska-Seget, Z. Diversity of endophytic bacteria in Lolium perenne and their potential to degrade petroleum hydrocarbons and promote plant growth. Chemosphere 2014, 117, 40–46. [Google Scholar] [CrossRef]
- Wu, T.; Xu, J.; Xie, W.J.; Yao, Z.G.; Yang, H.J.; Sun, C.L.; Li, X.B. Pseudomonas aeruginosa L10: A Hydrocarbon-Degrading, Biosurfactant-Producing, and Plant-Growth-Promoting Endophytic Bacterium Isolated From a Reed (Phragmites australis). Front. Microbiol. 2018, 9, 01087. [Google Scholar] [CrossRef]
- Yousaf, S.; Afzal, M.; Reichenauer, T.G.; Brady, C.L.; Sessitsch, A. Hydrocarbon degradation, plant colonization and gene expression of alkane degradation genes by endophytic Enterobacter ludwigii strains. Environ. Pollut. 2011, 159, 2675–2683. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Byrski, A.; Chlebek, D.; Prach, M.; Płociniczak, T. A deeper insight into the phytoremediation of soil polluted with petroleum hydrocarbons supported by the Enterobacter ludwigii ZCR5 strain. Appl. Soil Ecol. 2023, 181, 104651. [Google Scholar] [CrossRef]
- Yang, J.; Gu, Y.J.; Chen, Z.G.; Song, Y.; Sun, F.F.; Liu, J.; Waigi, M.G. Colonization and performance of a pyrene-degrading bacterium Mycolicibacterium sp. Pyr9 on root surfaces of white clover. Chemosphere 2021, 263, 127918. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Schippers, B.; Bakker, P.A.H.M. Proposed elimination of the term Endorhizosphere. Phytopathology 1992, 82, 726–727. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Fortin, N.; Mihoc, A.; Wisse, G.; Labelle, S.; Beaumier, D.; Ouellette, D.; Roy, R.; Whyte, L.G.; Banks, M.K.; et al. Selection of specific endophytic bacterial genotypes by plants in response to soil contamination. Appl. Environ. Microbiol. 2001, 67, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Afzal, M.; Imran, A.; Khan, Q.M. Bacterial rhizosphere and endosphere populations associated with grasses and trees to be used for phytoremediation of crude oil contaminated soil. Bull. Environ. Contam. Toxicol. 2015, 94, 314–320. [Google Scholar] [CrossRef]
- Luo, P.H.; Tang, Y.K.; Lu, J.H.; Jiang, L.; Huang, Y.T.; Jiang, Q.M.; Chen, X.M.; Qin, T.F.; Shiels, H.A. Diesel degradation capability and environmental robustness of strain Pseudomonas aeruginosa WS02. J. Environ. Manag. 2024, 351, 119937. [Google Scholar] [CrossRef]
- Zhao, H.; Gu, Y.J.; Liu, X.Y.; Liu, J.; Waigi, M.G. Reducing phenanthrene contamination in Trifolium repens L. with root-associated phenanthrene-degrading bacterium Diaphorobacter sp. Phe15. Front. Microbiol. 2021, 12, 792698. [Google Scholar] [CrossRef]
- Guan, C.F.; Fu, W.T.; Zhang, X.G.; Li, Z.M.; Zhu, Y.L.; Chen, F.Y.; Ji, J.; Wang, G.; Gao, X.P. Enhanced phytoremediation efficiency of PHE-contaminated soil by rape (Brassica napus L.) assisted with PHE-degradable PGPR through modulating rhizobacterial communities. Ind. Crops Prod. 2023, 202, 117057. [Google Scholar] [CrossRef]
- Wu, T.; Li, X.b.; Xu, J.; Liu, L.X.; Ren, L.-l.; Dong, B.; Li, W.; Xie, W.-j.; Yao, Z.-g.; Chen, Q.-f.; et al. Diversity and functional characteristics of endophytic bacteria from two grass species growing on an oil-contaminated site in the Yellow River Delta, China. Sci. Total Environ. 2021, 767, 144340. [Google Scholar] [CrossRef]
- Biswas, S.; Jayaram, S.; Philip, I.; Balasubramanian, B.; Pappuswamy, M.; Barceló, D.; Chelliapan, S.; Kamyab, H.; Sarojini, S.; Vasseghian, Y. Appraisal of the potential of endophytic bacterium Bacillus amyloliquefaciens from Alternanthera philoxeroides: A triple approach to heavy metal bioremediation, diesel biodegradation, and biosurfactant production. J. Environ. Chem. Eng. 2024, 12, 113454. [Google Scholar] [CrossRef]
- Wang, X.; Lin, C.B.; Wang, D.Q.; Zhu, X.Z.; Zhao, H.Y.; Lyu, B.T. Colonization performance and pyrene degradation characteristics of Stenotrophomonas maltophilis PX1. Chin. J. Appl. Ecol. 2022, 33, 2547–2556. (In Chinese) [Google Scholar] [CrossRef]
- Sun, K.; Liu, J.; Li, X.; Ling, W. Isolation, identification, and performance of two pyrene-degrading endophytic bacteria. Acta Ecol. Sin. 2014, 34, 853–861. (In Chinese) [Google Scholar] [CrossRef]
- Marchut-Mikolajczyk, O.; Drożdżyński, P.; Struszczyk-Świta, K. Biodegradation of slop oil by endophytic Bacillus cereus EN18 coupled with lipase from Rhizomucor miehei (Palatase®). Chemosphere 2020, 250, 126203. [Google Scholar] [CrossRef]
- Marchut-Mikolajczyk, O.; Drożdżyński, P.; Pietrzyk, D.; Antczak, T. Biosurfactant production and hydrocarbon degradation activity of endophytic bacteria isolated from Chelidonium majus L. Microb. Cell Fact. 2018, 17, 171–179. [Google Scholar] [CrossRef]
- Bisht, S.; Pandey, P.; Kaur, G.; Aggarwal, H.; Sood, A.; Sharma, S.; Kumar, V.; Bisht, N.S. Utilization of endophytic strain Bacillus sp. SBER3 for biodegradation of polyaromatic hydrocarbons (PAH) in soil model system. Eur. J. Soil Biol. 2014, 60, 67–76. [Google Scholar] [CrossRef]
- Lumactud, R.; Shen, S.Y.; Lau, M.; Fulthorpe, R. Bacterial endophytes isolated from plants in natural oil seep soils with chronic hydrocarbon contamination. Front. Microbiol. 2016, 7, 755. [Google Scholar] [CrossRef]
- Sheng, X.F.; Chen, X.B.; He, L.Y. Characteristics of an endophytic pyrene-degrading bacterium of Enterobacter sp. 12J1 from Allium macrostemon Bunge. Int. Biodeterior. Biodegrad. 2008, 62, 88–95. [Google Scholar] [CrossRef]
- Tao, J.Y.; Hong, Y.J.; Chen, X.M.; Liu, W.T.; Zhu, X.Z.; Miao, Y.H. Isolation, identification and PAH-degrading performance of an endophytic bacterium Enterobacter sp. PRd5. J. Ecol. Rural Environ. 2019, 35, 83–90. (In Chinese) [Google Scholar] [CrossRef]
- Mitter, E.K.; Kataoka, R.; de Freitas, J.R.; Germida, J.J. Potential use of endophytic root bacteria and host plants to degrade hydrocarbons. Int. J. Phytoremediation 2019, 21, 928–938. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Sun, K.; Sheng, Y.; Gu, Y.; Gao, Y. Colonization on root surface by a phenanthrene-degrading endophytic bacterium and its application for reducing plant phenanthrene contamination. PLoS ONE 2014, 9, e108249. [Google Scholar] [CrossRef]
- Lu, L.; Chai, Q.W.; He, S.Y.; Yang, C.P.; Zhang, D. Effects and mechanisms of phytoalexins on the removal of polycyclic aromatic hydrocarbons (PAHs) by an endophytic bacterium isolated from ryegrass. Environ. Pollut. 2019, 253, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Sessitsch, A. The inoculation method affects colonization and performance of bacterial inoculant strains in the phytoremediation of soil contaminated with diesel oil. Int. J. Phytoremediation 2012, 14, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, S.; Ripka, K.; Reichenauer, T.G.; Andria, V.; Afzal, M.; Sessitsch, A. Hydrocarbon degradation and plant colonization by selected bacterial strains isolated from Italian ryegrass and birdsfoot trefoil. J. Appl. Microbiol. 2010, 109, 1389–1401. [Google Scholar] [CrossRef]
- Sun, K.; Liu, J.; Gao, Y.Z.; Jin, L.; Gu, Y.; Wang, W.Q. Isolation, plant colonization potential, and phenanthrene degradation performance of the endophytic bacterium Pseudomonas sp. Ph6-gfp. Sci. Rep. 2014, 4, 5462. [Google Scholar] [CrossRef]
- Germaine, K.J.; Keogh, E.; Ryan, D.; Dowling, D.N. Bacterial endophyte-mediated naphthalene phytoprotection and phytoremediation. FEMS Microbiol. Lett. 2009, 296, 226–234. [Google Scholar] [CrossRef]
- Khan, Z.; Roman, D.; Kintz, T.; delas Alas, M.; Yap, R.; Doty, S. Degradation, phytoprotection and phytoremediation of phenanthrene by endophyte Pseudomonas putida, PD1. Environ. Sci. Technol. 2014, 48, 12221–12228. [Google Scholar] [CrossRef]
- Iqbal, A.; Arshad, M.; Karthikeyan, R.; Gentry, T.J.; Rashid, J.; Ahmed, I.; Schwab, A.P. Diesel degrading bacterial endophytes with plant growth promoting potential isolated from a petroleum storage facility. 3 Biotech 2019, 9, 35. [Google Scholar] [CrossRef]
- Wu, T.; Xu, J.; Guo, W.H.; Xia, J.B.; Li, X.B.; Wang, R.Q. Isolation and characterization of a halotolerant, hydrocarbon-degrading endophytic bacterium from halobiotic reeds (Phragmites australis) growing in petroleum-contaminated soils. Sci. Adv. Mater. 2019, 11, 189–195. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Ni, X.; Waigi, M.; Liu, J.; Sun, K.; Gao, Y.Z. Biodegradation of mixed PAHs by PAH-degrading endophytic bacteria. Int. J. Environ. Res. Public Health 2016, 13, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Liu, X.Y.; Wang, Q.; Chen, X.P.; Li, H.B.; Wei, J.; Xu, G. Diesel degradation potential of endophytic bacteria isolated from Scirpus triqueter. Int. Biodeterior. Biodegrad. 2014, 87, 99–105. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Wang, W.Q.; Crowley, D.E.; Sun, K.; Hao, S.P.; Waigi, M.G.; Gao, Y.Z. The endophytic bacterium Serratia sp. PW7 degrades pyrene in wheat. Environ. Sci. Pollut. Res. 2017, 24, 6648–6656. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ma, Z.; Li, S.Y.; Waigi, M.G.; Jiang, J.D.; Liu, J.; Ling, W.T. Colonization of polycyclic aromatic hydrocarbon-degrading bacteria on roots reduces the risk of PAH contamination in vegetables. Environ. Int. 2019, 132, 105081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M. A Preliminary Study on the Mechanismsinvolved in Reducing Plant Phenanthrenepollution Level by Phenanthrenedegrading Bacterium RS2 on Root Surfaces. Master’s Thesis, The College of Resources and Environmental Sciences, Nanjing Agricultural University, Nanjing, China, 2019. (In Chinese). [Google Scholar]
- Baoune, H.; Aparicio, J.D.; Pucci, G.; Ould El Hadj-Khelil, A.; Polti, M.A. Bioremediation of petroleum-contaminated soils using Streptomyces sp. Hlh1. J. Soils Sediments 2019, 19, 2222–2230. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Hnamte, L.; Vanlallawmzuali; Kumar, A.; Yadav, M.K.; Zothanpuia; Singh, P.K. An updated view of bacterial endophytes as antimicrobial agents against plant and human pathogens. Curr. Res. Microb. Sci. 2024, 7, 100241. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.-J. What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Front. Plant Sci. 2018, 9, 00024. [Google Scholar] [CrossRef]
- Pal, G.; Saxena, S.; Kumar, K.; Verma, A.; Kumar, D.; Shukla, P.; Pandey, A.; Verma, S.K. Seed endophytic bacterium Bacillus velezensis and its lipopeptides acts as elicitors of defense responses against Fusarium verticillioides in maize seedlings. Plant Soil 2023, 492, 109–124. [Google Scholar] [CrossRef]
- Banerjee, S.; van der Heijden, M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2022, 21, 6–20. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G. How plants recruit their microbiome? New insights into beneficial interactions. J. Adv. Res. 2022, 40, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Aswani, R.; Jishma, P.; Radhakrishnan, E.K. Endophytic bacteria from the medicinal plants and their potential applications. In Microbial Endophytes, 2nd ed.; Kumar, A., Singh, V.K., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 15–36. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda Mdel, C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Phillips, L.; Germida, J.; Farrell, R.; Greer, C. Hydrocarbon degradation potential and activity of endophytic bacteria associated with prairie plants. Soil Biol. Biochem. 2008, 40, 3054–3064. [Google Scholar] [CrossRef]
- Taghavi, S.; Weyens, N.; Vangronsveld, J.; van der Lelie, D. Improved phytoremediation of organic contaminants through engineering of bacterial endophytes of trees. In Endophytes of Forest Trees: Biology and Applications; Pirttilä, A.M., Frank, A.C., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 80, pp. 205–216. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M. Bacterial endophytes: The endophytic niche, its occupants, and its utility. In Plant-Associated Bacteria; Gnanamanickam, S.S., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 155–194. [Google Scholar] [CrossRef]
- Yousaf, S.; Andria, V.; Reichenauer, T.G.; Smalla, K.; Sessitsch, A. Phylogenetic and functional diversity of alkane degrading bacteria associated with Italian ryegrass (Lolium multiflorum) and Birdsfoot trefoil (Lotus corniculatus) in a petroleum oil-contaminated environment. J. Hazard. Mater. 2010, 184, 523–532. [Google Scholar] [CrossRef]
- Duan, Z.Y.; Gao, N.Z.; Zhang, G.C.; Wang, J.; Ling, W.T. Inoculation of endophytic bacteria for the abatement of the phenanthreneaccumulation in vegetable subcellular fraction:Efficiency and mechanism. Acta Sci. Circumstantiae 2021, 41, 1529–1537. (In Chinese) [Google Scholar] [CrossRef]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Kuffner, M.; Sessitsch, A. Soil type affects plant colonization, activity and catabolic gene expression of inoculated bacterial strains during phytoremediation of diesel. J. Hazard. Mater. 2011, 186, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Andria, V.; Reichenauer, T.G.; Sessitsch, A. Expression of alkane monooxygenase (alkB) genes by plant-associated bacteria in the rhizosphere and endosphere of Italian ryegrass (Lolium multiflorum L.) grown in diesel contaminated soil. Environ. Pollut. 2009, 157, 3347–3350. [Google Scholar] [CrossRef]
- Afzal, M.; Khan, S.; Iqbal, S.; Mirza, M.S.; Khan, Q.M. Inoculation method affects colonization and activity of Burkholderia phytofirmans PsJN during phytoremediation of diesel-contaminated soil. Int. Biodeterior. Biodegrad. 2013, 85, 331–336. [Google Scholar] [CrossRef]
- Zhou, N.; Guo, H.J.; Liu, Q.X.; Zhang, Z.T.; Sun, J.; Wang, H. Bioaugmentation of polycyclic aromatic hydrocarbon (PAH)-contaminated soil with the nitrate-reducing bacterium PheN7 under anaerobic condition. J. Hazard. Mater. 2022, 439, 129643. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.Y.; Lu, J.C.; Liu, S.C.; Chen, M.Y.; Ma, Y.L. Biodegradation of C18 n-alkane by biosurfactant-producing Pseudomonas aeruginosa TJM4 and its genomic analysis. J. Environ. Chem. Eng. 2025, 13, 115590. [Google Scholar] [CrossRef]
- Liu, X.L.; Cui, Q.F.; Yang, Z.M.; Wei, S.P.; Zhang, Q. Microbial degradation and molecular mechanism of medium and long-chain alkanes in petroleum: A review. Microbiol. China 2023, 50, 1559–1575. (In Chinese) [Google Scholar] [CrossRef]
- Ji, Y.; Mao, G.; Wang, Y.; Bartlam, M. Structural insights into diversity and n-alkane biodegradation mechanisms of alkane hydroxylases. Front. Microbiol. 2013, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Qaria, M.A.; Xu, Q.; Chen, Z.D. The role of microorganisms in petroleum degradation: Current development and prospects. Sci. Total Environ. 2023, 865, 161112. [Google Scholar] [CrossRef]
- Jing, J.W.; Wang, T.T.; Guo, X.Y.; Huang, P.F.; Li, C.; Qu, Y.Y. Construction and application of petroleum-degrading bacterial agents: Community composition, lyophilization technology, and degradation mechanism. J. Environ. Chem. Eng. 2024, 12, 114904. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Fuchs, G.; Boll, M.; Heider, J. Microbial degradation of aromatic compounds—From one strategy to four. Nat. Rev. Microbiol. 2011, 9, 803–816. [Google Scholar] [CrossRef]
- Seo, J.-S.; Keum, Y.-S.; Li, Q.X. Bacterial degradation of aromatic compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Xu, J.G.; Hua, T.Y.; Liu, M.M.; Yan, W. Combined transcriptome and metabolome analysis reveal the dual catabolic pathways of naphthalene in Pseudomonas putida ND6. J. Environ. Chem. Eng. 2025, 13, 115632. [Google Scholar] [CrossRef]
- Li, J.L.; Peng, W.L.; Yin, X.Q.; Wang, X.Z.; Liu, Z.X.; Liu, Q.C.; Deng, Z.X.; Lin, S.J.; Liang, R.B. Identification of an efficient phenanthrene-degrading Pseudarthrobacter sp. L1SW and characterization of its metabolites and catabolic pathway. J. Hazard. Mater. 2024, 465, 133138. [Google Scholar] [CrossRef]
- Sun, S.S.; Wang, H.Z.; Chen, Y.Z.; Lou, J.; Wu, L.S.; Xu, J.M. Salicylate and phthalate pathways contributed differently on phenanthrene and pyrene degradations in Mycobacterium sp. WY10. J. Hazard. Mater. 2019, 364, 509–518. [Google Scholar] [CrossRef]
- Seo, J.S.; Keum, Y.S.; Hu, Y.T.; Lee, S.E.; Li, Q.X. Phenanthrene degradation in Arthrobacter sp. P1-1: Initial 1,2-, 3,4- and 9,10-dioxygenation, and meta- and ortho-cleavages of naphthalene-1,2-diol after its formation from naphthalene-1,2-dicarboxylic acid and hydroxyl naphthoic acids. Chemosphere 2006, 65, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.Y.; Okoye, C.O.; Lu, G.; Jiang, H.; Wu, Y.; Wang, Y.; Li, X.; Jiang, J. Whole-genome sequence analysis reveals phenanthrene and pyrene degradation pathways in newly isolated bacteria Klebsiella michiganensis EF4 and Klebsiella oxytoca ETN19. Microbiol. Res. 2023, 273, 127410. [Google Scholar] [CrossRef]
- Kweon, O.; Kim, S.-J.; Holland, R.D.; Chen, H.; Kim, D.-W.; Gao, Y.; Yu, L.-R.; Baek, S.; Baek, D.-H.; Ahn, H.; et al. Polycyclic aromatic hydrocarbon metabolic network in Mycobacterium vanbaalenii PYR-1. J. Bacteriol. 2011, 193, 4326–4337. [Google Scholar] [CrossRef]
- Lin, C.B.; Zhang, F.Y.; Chen, R.; Lin, S.P.; Jiao, P.Y.; Ma, Y.J.; Zhu, X.Z.; Lv, B.T. Potential of a novel endophytic diazotrophic Serratia sp. Wed4 for pyrene biodegradation. Int. Biodeterior. Biodegrad. 2024, 186, 105705. [Google Scholar] [CrossRef]
- Calderón-Preciado, D.; Renault, Q.; Matamoros, V.; Cañameras, N.; Bayona, J.M. Uptake of organic emergent contaminants in spath and lettuce: An in vitro experiment. J. Agric. Food Chem. 2012, 60, 2000–2007. [Google Scholar] [CrossRef]
- Bittsánszky, A.; Gullner, G.; Gyulai, G.; Komives, T. A case study: Uptake and accumulation of persistent organic pollutants in Cucurbitaceae species. In Organic Xenobiotics and Plants: From Mode of Action to Ecophysiology, 4th ed.; Schröder, P., Collins, C.D., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 77–85. [Google Scholar] [CrossRef]
- Wu, H.J.; Song, Q.W.; Zheng, J.; Yu, W.H.; Zhang, K.F.; Lin, S.J.; Liang, R.B. Function genes in microorganisms capable of degrading petroleum hydrocarbon. Microbiol. China 2020, 47, 3355–3368. (In Chinese) [Google Scholar] [CrossRef]
- Mitter, B.; Petric, A.; Shin, M.W.; Chain, P.S.G.; Hauberg-Lotte, L.; Reinhold-Hurek, B.; Nowak, J.; Sessitsch, A. Comparative genome analysis of Burkholderia phytofirmans PsJN reveals a wide spectrum of endophytic lifestyles based on interaction strategies with host plants. Front. Plant Sci. 2013, 4, 120. [Google Scholar] [CrossRef]
- Gao, Y.C.; Du, J.H.; Bahar, M.M.; Wang, H.; Subashchandrabose, S.; Duan, L.; Yang, X.; Megharaj, M.; Zhao, Q.Q.; Zhang, W.; et al. Metagenomics analysis identifies nitrogen metabolic pathway in bioremediation of diesel contaminated soil. Chemosphere 2021, 271, 129566. [Google Scholar] [CrossRef]
- Wu, M.L.; Ma, C.; Wang, D.; Liu, H.; Zhu, C.C.; Xu, H.N. Nutrient drip irrigation for refractory hydrocarbon removal and microbial community shift in a historically petroleum-contaminated soil. Sci. Total Environ. 2020, 713, 136331. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Q.; Zhang, X.L.; Yan, C.H.; Zhou, R.; Li, J.H.; Liu, S.Q.; Wang, Z.; Zhou, J.; Zhu, L.; Jia, H. Novel Insights into the Promoted Accumulation of Nitro-Polycyclic Aromatic Hydrocarbons in the Roots of Legume Plants. Environ. Sci. Technol. 2024, 58, 2058–2068. [Google Scholar] [CrossRef] [PubMed]
- Kour, D.; Rana, K.L.; Kaur, T.; Yadav, N.; Yadav, A.N.; Kumar, M.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and -mobilizing microbes: A review. Pedosphere 2021, 31, 43–75. [Google Scholar] [CrossRef]
- Khan, S.; Afzal, M.; Iqbal, S.; Khan, Q.M. Plant–bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 2013, 90, 1317–1332. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Li, X.Z.; Peng, D.L.; Zhang, Y.; Ju, D.; Guan, C.F. Klebsiella sp. PD3, a phenanthrene (PHE)-degrading strain with plant growth promoting properties enhances the PHE degradation and stress tolerance in rice plants. Ecotoxicol. Environ. Saf. 2020, 201, 110804. [Google Scholar] [CrossRef]
- Oukala, N.; Aissat, K.; Pastor, V. Bacterial endophytes: The hidden actor in plant immune responses against biotic stress. Plants 2021, 10, 1012. [Google Scholar] [CrossRef]
- Muhammad, M.; Wahab, A.; Waheed, A.; Mohamed, H.I.; Hakeem, K.R.; Li, L.; Li, W.-J. Harnessing bacterial endophytes for environmental resilience and agricultural sustainability. J. Environ. Manag. 2024, 368, 122201. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hijri, M.; Bhowmik, A.; Elkelish, A.; Hassan, S.E.-D. Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: An overview. Plants 2021, 10, 935. [Google Scholar] [CrossRef]
- Boraschi, D.; Alijagic, A.; Auguste, M.; Barbero, F.; Ferrari, E.; Hernadi, S.; Mayall, C.; Michelini, S.; Navarro Pacheco, N.I.; Prinelli, A.; et al. Addressing nanomaterial immunosafety by evaluating innate immunity across living species. Small 2020, 16, 2000598. [Google Scholar] [CrossRef]
- Naskar, S.; Roy, C.; Ghosh, S.; Mukhopadhyay, A.; Hazarika, L.K.; Chaudhuri, R.K.; Roy, S.; Chakraborti, D. Elicitation of biomolecules as host defense arsenals during insect attacks on tea plants (Camellia sinensis (L.) Kuntze) Appl. Microbiol. Biotechnol. 2021, 105, 7187–7199. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Feng, L.Y.; Jiang, X.P.; Huang, Y.N.; Wen, D.D.; Fu, T.Y.; Fu, R.B. Petroleum hydrocarbon-contaminated soil bioremediation assisted by isolated bacterial consortium and sophorolipid. Environ. Pollut. 2021, 273, 116476. [Google Scholar] [CrossRef]
- Ahmad, Z.; Zhang, X.; Imran, M.; Zhong, H.; Andleeb, S.; Zulekha, R.; Liu, G.; Ahmad, I.; Coulon, F. Production, functional stability, and effect of rhamnolipid biosurfactant from Klebsiella sp. on phenanthrene degradation in various medium systems. Ecotoxicol. Environ. Saf. 2021, 207, 111514. [Google Scholar] [CrossRef]
- Sharuddin, S.S.N.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ismail, N.I. Potential bifunctional rhizobacteria from crude oil sludge for hydrocarbon degradation and biosurfactant production. Process Saf. Environ. Prot. 2021, 155, 108–121. [Google Scholar] [CrossRef]
- Zhang, K.C.; Tao, W.Y.; Lin, J.Z.; Wang, W.D.; Li, S. Production of the biosurfactant serrawettin W1 by Serratia marcescens S-1 improves hydrocarbon degradation. Bioprocess Biosyst. Eng. 2021, 44, 2541–2552. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Saravanan, V. Biosurfactants-types, sources and applications. Res. J. Microbiol. 2015, 10, 181–192. [Google Scholar] [CrossRef]
- Li, X.J.; Zhao, Q.; Wang, X.; Li, Y.T.; Zhou, Q.X. Surfactants selectively reallocated the bacterial distribution in soil bioelectrochemical remediation of petroleum hydrocarbons. J. Hazard. Mater. 2018, 344, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraz, A.; Sumbal, S.; Qin, Y.; Faqir, Y.; Zveushe, O.K.; Zhou, L.; Zhang, W.; Li, J.; Lv, Z.; Han, Y.; et al. Bioaugmentation-assisted phytoremediation of petroleum hydrocarbon-contaminated soils. J. Environ. Chem. Eng. 2025, 13, 115895. [Google Scholar] [CrossRef]
- Jha, P.; Panwar, J.; Jha, P.N. Secondary plant metabolites and root exudates: Guiding tools for polychlorinated biphenyl biodegradation. Int. J. Environ. Sci. Technol. 2014, 12, 789–802. [Google Scholar] [CrossRef]
- French, K.E.; Zhou, Z.R.; Terry, N. Horizontal ‘gene drives’ harness indigenous bacteria for bioremediation. Sci. Rep. 2020, 10, 15091. [Google Scholar] [CrossRef]
- Mohapatra, B.; Phale, P.S. Microbial degradation of naphthalene and substituted naphthalenes: Metabolic diversity and genomic insight for bioremediation. Front Bioeng. Biotech 2021, 9, 602445. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhandari, G.; Bhatt, K.; Maithani, D.; Mishra, S.; Gangola, S.; Bhatt, R.; Huang, Y.; Chen, S. Plasmid-mediated catabolism for the removal of xenobiotics from the environment. J. Hazard. Mater. 2021, 420, 126618. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Selvarajan, R.; Abia, A.L.K.; Matambo, T. Medium-chain alkane biodegradation and its link to some unifying attributes of alkB genes diversity. Sci. Total Environ. 2023, 877, 162951. [Google Scholar] [CrossRef] [PubMed]
- Sessitsch, A.; Hardoim, P.; Döring, J.; Weilharter, A.; Krause, A.; Woyke, T.; Mitter, B.; Hauberg-Lotte, L.; Friedrich, F.; Rahalkar, M.; et al. Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant. Microbe Interact. 2012, 25, 28–36. [Google Scholar] [CrossRef]
- Billane, K.; Harrison, E.; Cameron, D.; Brockhurst, M.A. Why do plasmids manipulate the expression of bacterial phenotypes? Philos. Trans. R. Soc. B 2021, 377, 20200461. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, W. Genome analysis of naphthalene-degrading Pseudomonas sp. AS1 harboring the megaplasmid pAS1. J. Microbiol. Biotechnol. 2018, 28, 330–337. [Google Scholar] [CrossRef]
- Boronin, A.M.; Kosheleva, I.A. The role of catabolic plasmids in biodegradation of petroleum hydrocarbons. In Current Environmental Issues and Challenges, 9th ed.; Cao, G., Orrù, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 159–168. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.S.; Du, D.; Wang, D.; Yan, W. Conjugative transfer of megaplasmids pND6–1 and pND6–2 enhancing naphthalene degradation in aqueous environment: Characterization and bioaugmentation prospects. Appl. Microbiol. Biotechnol. 2020, 104, 861–871. [Google Scholar] [CrossRef]
- Martínez-Lavanchy, P.M.; Müller, C.; Nijenhuis, I.; Kappelmeyer, U.; Buffing, M.; McPherson, K.; Heipieper, H.J. High stability and fast recovery of expression of the TOL plasmid-carried toluene catabolism genes of Pseudomonas putida mt-2 under conditions of oxygen limitation and oscillation. Appl. Environ. Microbiol. 2010, 76, 6715–6723. [Google Scholar] [CrossRef]
- Kasak, L.; Horak, R.; Nurk, A.; Talvik, K.; Kivisaar, M. Regulation of the catechol 1,2-dioxygenase- and phenol monooxygenase-encoding pheBA operon in Pseudomonas putida PaW85. J. Bacteriol. 1993, 175, 8038–8042. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, Y.; Jeon, C.O. Biodegradation of naphthalene, BTEX, and aliphatic hydrocarbons by Paraburkholderia aromaticivorans BN5 isolated from petroleum-contaminated soil. Sci. Rep. 2019, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Muneeswari, R.; Iyappan, S.; Swathi, K.V.; Vinu, R.; Ramani, K.; Sekaran, G. Biocatalytic lipoprotein bioamphiphile induced treatment of recalcitrant hydrocarbons in petroleum refinery oil sludge through transposon technology. J. Hazard. Mater. 2022, 431, 128520. [Google Scholar] [CrossRef] [PubMed]
- Bellanger, X.; Payot, S.; Leblond-Bourget, N.; Guédon, G. Conjugative and mobilizable genomic islands in bacteria: Evolution and diversity. FEMS Microbiol. Rev. 2014, 38, 720–760. [Google Scholar] [CrossRef]
- Dobrindt, U.; Hochhut, B.; Hentschel, U.; Hacker, J. Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2004, 2, 414–424. [Google Scholar] [CrossRef]

| Bacterial Strains | Host Plants | Characterization | Cultivation Environment | Pollutants and Initial Concentration | Degradation Efficiency | References |
|---|---|---|---|---|---|---|
| Acinetobacter sp. BRSI56 | Brachiaria mutica | plant growth promotion (PGP), production of biosurfactant (PBS) | liquid | Crude, 2% (w/v) | 78% (7 d) | [20] |
| Acinetobacter sp. BJ03 | Conyza canadensis | - | liquid | PYR, 50 mg/L | 65.0% (15 d) | [27] |
| Bacillus amyloliquefaciens MEBAphL4 | Alternanthera philoxeroides | PBS | liquid | Diesel, 2% (v/v) | 56.46% (42 d) | [25] |
| Bacillus cereus EN18 | Chelidonium majus | - | liquid | Diesel, 5% (v/v) | 45.5% (14 d) | [28] |
| Bacillus pumilus 2A | Chelidonium majus L. | PGP, PBS | liquid | Diesel, 5% (v/v) | 98% (10 d) | [29] |
| Bacillus sp. SBER3 | Populus deltoides | PGP | liquid | ANT, 466 mg/L; naphthalene (NAP), 332 mg/L | ANT, 83.4% (6 d); NAP, 75.1% (6 d) | [30] |
| Brevundimonas nasdae 210 | Solidago canadensis | - | liquid | TOL, 1 mM; NAP, 1 mM | TOL, 53%; NAP, 39% (7 d) | [31] |
| Chryseobacterium sp. 127 | Dactylis glomerata | - | liquid | TOL, 1 mM; NAP, 1 mM | TOL, 53%; NAP, 41% (7 d) | [31] |
| Curtobacterium flaccumflaciens 153 | Dactylis glomerata | - | liquid | TOL, 1 mM; NAP, 1 mM | TOL, 21%; NAP, 13% (7 d) | [31] |
| Diaphorobacter sp. Phe15 | Eleusine indica L. Gaertn. | PGP | soil | PHE, 100 mg/kg | 39% (40 d) | [22] |
| Enterobacter ludwigii ZCR5 | Zea mays | PGP, PBS | soil | Crude, 17,500 mg/kg | 30.62% (28 d) | [16] |
| Enterobacter sp. 12J1 | Allium macrostemon Bunge | PGP, PBS | liquid | PYR, 5 mg/L | 83.8% (7 d) | [32] |
| Enterobacter sp. PRd5 | Ophiopogon japonicus | - | liquid | PYR, 50 mg/L; NAP, 500 mg/L; fluorene (FLE), 100 mg/L; PHE, 50 mg/L; FLA, 50 mg/L; BaP, 10 mg/L | PYR, 41.4~50.6% (10 d); NAP, FLE, PHE mixed hydrocarbons, 95.0% (7 d); FLA, 35.9% (10 d); BaP, 17.4% (10 d) | [33] |
| Enterobacter cloacae LCRI86 | Lecucaena leucocephala | PGP, PBS | liquid | Crude, 2% (w/v) | 72% (7 d) | [20] |
| Flavobacterium sp. EA2-30 | - | PGP | soil | Diesel, 10,000 mg/kg | 63.4% (65 d) | [34] |
| Kocuria sp. BJ05 | Trifolium pratense L. | - | liquid | PYR, 50 mg/L | 53.3% (15 d) | [27] |
| Massilia sp. Pn2 | Alopecurus aequalis Sobol. | - | liquid | NAP, 100 mg/L; ACN, 100 mg/L; ANT, 50 mg/L; PHE, 50 mg/L; PYR, 20 mg/L; BaP, 10 mg/L | NAP, 95.8% (48 h); ACN, 97.3% (48 h); ANT, 27.8% (72 h); PHE, 99.6% (72 h); PYR, 67.6% (14 d); BaP, 2.5% (14 d) | [35] |
| Methylobacterium extorquens C1 | Lolium perenne | - | liquid | ACY, 3.5 mg/L; PHE, 1.0 mg/L | ACY, 52.5% (3 d); PHE, 43.8% (3 d) | [36] |
| Microbacterium foliorum 117 | Dactylis glomerata | - | liquid | TOL, 1 mM; NAP, 1 mM | TOL, 53%; NAP, 41% (7 d) | [31] |
| Mycolicibacterium sp. Pyr9 | Eleusine indica L. Gaertn. | PGP | liquid | PYR, 50 mg/L; ACY, 100 mg/L; ACN, 100 mg/L; PHE, 100 mg/L; ANT, 100 mg/L; FLA, 50 mg/L; BaP, 10 mg/L | PYR, 98% (8 d); ACY, 93% (4 d); ACN, 88% (4 d); PHE, 100% (4 d); ANT, 31.5% (4 d); FLA, 9.9% (14 d); BaP, 23.2% (14 d) | [17] |
| Pantoea sp. ITSI10 | Lolium multiflorum var. Taurus | - | soil | Diesel, 7.5 g/kg | 69.2% (93 d) | [37] |
| Diesel, 10 g/kg | 48.5% (155 d) | [38] | ||||
| Pantoea sp. EA4-40 | - | PGP | soil | Diesel, 10,000 mg/kg | 60.1% (65 d) | [34] |
| Plantibacter flavus 259 | Achillea millefolium | - | liquid | TOL, 1 mM; NAP, 1 mM | TOL, 53%; NAP, 39% (7 d) | [31] |
| Plantibacter flavus 279 | Achillea millefolium | - | liquid | TOL, 1 mM; NAP, 1 mM | TOL, 53%; NAP, 37% (7 d) | [31] |
| Pseudomonas chlororaphis 23aP | Chamaecytisus albus | PGP, PBS | liquid | PHE, 50, 100, 200, 500 ppm | - | [12] |
| Pseudomonas aeruginosa L10 | Phragmites australis | PGP, PBS | liquid | Diesel, 5 g/L; NAP, 200 mg/L; PHE, 200 mg/L; PYR, 200 mg/L | Diesel, 79.3% (7 d); NAP, 79.7% (10 d); PHE, 71.6% (10 d); PYR, 34.7% (10 d) | [14] |
| Pseudomonas aeruginosa WS02 | Myriophyllum verticillatum | PBS | liquid | Diesel, 8400 mg/L | C10–C14, 100% (14 d); C15–C22, 90% (14 d) | [21] |
| Pseudomonas sp. Ph6-gfp | Trifolium pratense L | - | liquid | PHE, 50 mg/L | 85% (15 d) | [39] |
| Pseudomonas sp. MixRI75 | Lolium multiflorum var. Taurus | - | soil | Diesel, 7.5 g/kg | 53% (93 d) | [37] |
| Pseudomonas putida VM1441 | - | - | soil | NAP, 220~280 mg/kg | 68% (14 d) | [40] |
| Pseudomonas putida PD1 | Populus | PGP | soil | PHE, 100 mg/kg | 65% (30 d) | [41] |
| Pseudomonas sp. J10 | Echinochloa crus-galli | PGP | liquid | Diesel, 1.0% (v/v) | 69% (4 d) | [42] |
| Pseudomonas stutzeri Z11 | Phragmites australis | - | liquid | Diesel, 3000 mg/L | 72.1% (7 d) | [43] |
| Pseudomonas sp. P3 | Trifolium pretense | - | liquid | NAP, 100 mg/L; FLE, 100 mg/L; PHE, 100 mg/L; PYR, 100 mg/L | NAP, 95.3%; FLE, 87.9%; PHE, 90.4%; PYR, 6.9% (7 d) | [44] |
| Pseudomonas aeruginosa BRRI54 | Brachiaria mutica | PGP, PBS | liquid | Crude, 2% (w/v) | 71% (7 d) | [20] |
| Pseudomonas sp. J4AJ | Scirpus triqueter | - | liquid | Diesel, 6000 mg/L | 42.55% (7 d) | [45] |
| Pseudomonas sp. ITRI15 | Lolium multiflorum var. Taurus | - | soil | Diesel, 10 g/kg | 38.6% (90 d) | [38] |
| Serratia sp. DLN5 | Festuca arundinacea Schreb. | PGP | soil | PHE, 100 mg/kg | 82.5% (40 d) | [23] |
| Serratia sp. PW7 | Plantago asiatica | - | liquid | PYR, 40 mg/L | 51.2% (14 d) | [46] |
| Sphingobium sp. RS1-gfp | Plantago depressa Willd | - | liquid | PHE, 100 mg/L | 97% (48 h) | [47] |
| Sphingobium sp. RS2 | Conyza Canadensis L.Cronq. | PGP | liquid | PHE, 100 mg/L | 99% (72 h) | [48] |
| Stenotrophomonas maltophilia PX1 | Eleusine indica | PGP | liquid | NAP, 100 mg/L; PHE, 50 mg/L; PYR, 20 mg/L; FLA, 20 mg/L; BaP, 10 mg/L | NAP, 100% (7 d); PHE, 72.6% (10 d); PYR, 50.7% (10 d); FLA, 31.9% (10 d); BaP, 12.9% (10 d) | [26] |
| Stenotrophomonas sp. EA1-17 | - | PGP | soil | Diesel, 10,000 mg/kg | 63.6% (65 d) | [34] |
| Stenotrophomonas sp. P1 | Conyza canadensis | - | liquid | NAP, 100 mg/L; FLE, 100 mg/L; PHE, 100 mg/L; PYR, 100 mg/L; BaP, 10 mg/L | NAP, 98%; FLE, 83.1%; PHE, 87.8%; PYR, 14.4%; BaP, 1.6% (7 d) | [44] |
| Streptomyces sp. Hlh1 | Zea mays | PGP, PBS | soil | Petroleum, 5% (w/w) | 51% (28 d) | [49] |
| Xanthomonas gardneri 209 | Solidago canadensis | - | liquid | TOL, 1 mM; NAP, 1 mM | TOL, 49%; NAP, 40% (7 d) | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Xu, J.; Wu, Y.; Bao, J.; Wang, H.; Liu, L.; Zhang, J.; Li, J.; Wu, T. Advancements in Functional Endophytic Bacterium-Assisted Phytoremediation of PHC-Contaminated Soils: A Review. Processes 2025, 13, 2954. https://doi.org/10.3390/pr13092954
Qiao Y, Xu J, Wu Y, Bao J, Wang H, Liu L, Zhang J, Li J, Wu T. Advancements in Functional Endophytic Bacterium-Assisted Phytoremediation of PHC-Contaminated Soils: A Review. Processes. 2025; 13(9):2954. https://doi.org/10.3390/pr13092954
Chicago/Turabian StyleQiao, Yuyan, Jie Xu, Yichun Wu, Jianfeng Bao, Haifeng Wang, Longxiang Liu, Jiqiang Zhang, Jian Li, and Tao Wu. 2025. "Advancements in Functional Endophytic Bacterium-Assisted Phytoremediation of PHC-Contaminated Soils: A Review" Processes 13, no. 9: 2954. https://doi.org/10.3390/pr13092954
APA StyleQiao, Y., Xu, J., Wu, Y., Bao, J., Wang, H., Liu, L., Zhang, J., Li, J., & Wu, T. (2025). Advancements in Functional Endophytic Bacterium-Assisted Phytoremediation of PHC-Contaminated Soils: A Review. Processes, 13(9), 2954. https://doi.org/10.3390/pr13092954