Mineral-Based Magnesium Extraction Technologies: Current and Future Practices
Abstract
1. Introduction
2. Magnesium-Bearing Mineral Sources
2.1. Oxide and Hydroxide Minerals
2.2. Carbonate Group Minerals
2.3. Silicate Group Minerals
2.4. Evaporate Halides
3. Magnesium Extraction Processes
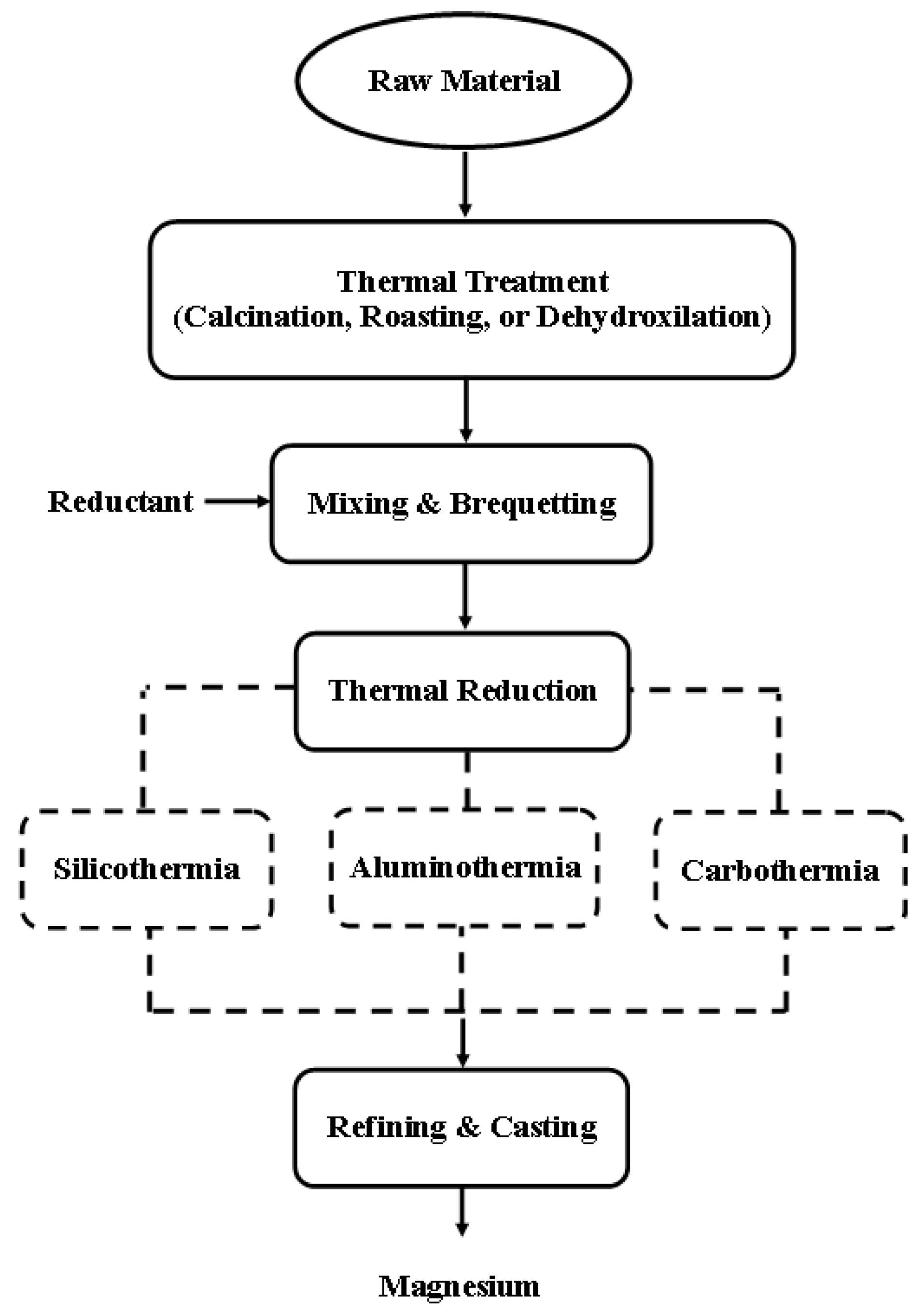
3.1. Pyrometallurgy
3.2. Hydrometallurgy
- Ammonium chloride leaching: Aqueous NH4Cl can selectively extract magnesium from Mg(OH)2-containing solids, resulting in an aqueous MgCl2 solution while simultaneously regenerating NH3. The primary reaction involved in this process is as follows [149]:
- Organic acid leaching: Dissolving MgO with organic acids offers benefits such as mild reaction conditions and a lower environmental impact compared to inorganic acids. Organic acids like acetic acid react with MgO, producing soluble magnesium salts and water. For instance, acetic acid can gradually dissolve magnesium from MgO, forming magnesium acetate (Equation (12)).
- CO2 leaching: Magnesia derived from calcination often contains complex oxide impurities, such as SiO2, Al2O3, Fe2O3, and CaO, which complicate the refinement of magnesium products. To overcome this issue, a CO2 leaching technique has been developed where MgO reacts with aqueous CO2 to form soluble magnesium bicarbonate (Mg(HCO3)2) [153,154,155]. This method selectively dissolves magnesia, leaving impurities behind, and produces a solution that can be decomposed through heating or aeration to form basic magnesium carbonate. This carbonate is then further processed to yield high-purity magnesia [150].
- HCl leaching: Dolomite and magnesite, both magnesium-rich carbonate minerals, are widely used as feedstocks for magnesium extraction via hydrometallurgical processes [22]. One common method is the hydro-magnesium process, which involves direct acid leaching with hydrochloric acid (Figure 3) [32,33,159,160]. In this process, magnesite or dolomite reacts with HCl to form magnesium chloride, carbon dioxide, and water, as shown in Equations (19) and (20).
- Salt roasting–water leaching The (NH4)2SO4 roasting–water leaching process efficiently enhances magnesium recovery from magnesite, achieving a maximum extraction rate of 98.7% at 475 °C. The process follows a mixed chemical–diffusion control mechanism and allows cyclic use of reagents, offering a promising alternative for magnesite processing [161].
- Organic acid leaching: Several studies have examined the leaching of magnesium-rich carbonates using various organic acids, including citric acid [162], acetic acid [151,163,164,165,166], lactic acid [167], formic acid [168], succinic acid [169], and gluconic acid [170,171]. Organic acids offer high selectivity but limited dissolution power, making them most effective for carbonaceous compounds. They operate under mildly acidic conditions (pH 3–5), which reduces CO2 pressure and frothing issues commonly encountered with inorganic acids during large-scale processing. Moreover, organic acids tend to cause less corrosion in industrial systems. However, their effectiveness decreases with more refractory minerals, and they are unsuitable for high-temperature applications due to their low boiling points and thermal decomposition [164]. Calcined carbonates leach faster with organic acids than raw carbonates, due to the increased reactivity of magnesium oxide [151]. The reaction between magnesite and a generic organic acid (HR) can be represented schematically as follows:
- Mechanical pretreatment: Activates by means of physical methods the serpentine surface to improve its leachability by removing the surface layer of silica (SiO2), which can hinder the dissolution of magnesium within the mineral. This layer of silica acts as a barrier, limiting solvent access to the mineral’s interior. Techniques such as grinding, stirring, ultrasonic treatment, and microwave irradiation are applied prior to leaching to overcome this barrier [176,185,186,187].
- Chemical pretreatment: To reduce costs and energy consumption in the leaching of layered aluminosilicates, researchers are exploring chemical activation as a cost-effective alternative to thermal and mechanical methods. One promising approach involves using additive materials such as sodium fluoride or calcium fluoride. These fluoride ions react with aluminum in hydrosilicates, aluminosilicates, and laterites to form soluble complexes. This reaction lowers the activation energy required for leaching, thereby improving magnesium dissolution [195,196].
- HCl leaching: The recovery of magnesium from serpentine using hydrochloric acid (HCl) has been extensively studied as a method for extracting magnesium from Mg-rich silicate ores [34,159,197,198,199,200,201,202]. This process involves leaching serpentine with hydrochloric acid to produce magnesium chloride hexahydrate (MgCl2·6H2O). Atmospheric chloride leaching offers several benefits, including lower capital costs, reduced reagent usage, and improved residue handling characteristics such as better settling and filtration [203].
- Sulfuric acid leaching Several studies [35,36,172,179,186,209,210] have proposed that the H2SO4-leaching process is an effective method for extracting magnesium from magnesium-bearing silicate minerals. The extraction of magnesium from magnesium silicates using sulfuric acid involves the following reactions [37,172,211]:
- Organic acid leaching: The leaching of silicate minerals, particularly those in the serpentine group, has been investigated using various organic acids such as formic acid [182,214], acetic acid [39,215,216], oxalic acid [216,217], citric acid [217,218], succinic acid [216], lactic acid [214], and EDTA [217]. Research indicates that the presence of organic ligands markedly improves the dissolution rate of serpentine in mildly acidic conditions [217,218]. This is thought to be due to the adsorption of organic ligands, which form surface complexes and create precursors that detach from the mineral surface. Additionally, it is believed that a negatively charged ligand binds to positively charged hydrated Mg sites on the surface [40,218,219].
- Ammonium salt leaching: Researchers have also used ammonia and its soluble salts, such as ammonium chloride (NH4Cl), ammonium sulfate ((NH4)2SO4), and ammonium bisulfate (NH4HSO4), to dissolve magnesium from silicates [222,223]. Ammonium chloride (NH4Cl) is an effective lixiviant for extracting magnesium from silicates because it produces a relatively pure and easily refined MgCl2-rich solution. Its use is advantageous in leaching processes, as it selectively dissolves magnesium from silicates while leaving other impurities behind. Ammonia released during the process can impede mineral dissolution, so removing NH3 is necessary for smooth reaction progress. The generated ammonia can then be utilized to precipitate magnesium hydroxide (Mg(OH)2) from the MgCl2-rich solution [223].
3.3. Electrometallurgy
4. Conclusions and Future Research Priorities
Funding
Acknowledgments
Conflicts of Interest
References
- Loganathan, P.; Naidu, G.; Vigneswaran, S. Mining valuable minerals from seawater: A critical review. Environ. Sci. Water Res. Technol. 2016, 3, 37–53. [Google Scholar] [CrossRef]
- La Corte, D.; Vassallo, F.; Cipollina, A.; Turek, M.; Tamburini, A.; Micale, G. A novel ionic exchange membrane crystallizer to recover magnesium hydroxide from seawater and industrial brines. Membranes 2020, 10, 303. [Google Scholar] [CrossRef]
- Sano, Y.; Hao, Y.; Kuwahara, F. Development of an electrolysis based system to continuously recover magnesium from seawater. Heliyon 2018, 4, e00923. [Google Scholar] [CrossRef]
- Tripp, T.G. Production of Magnesium from Great Salt Lake, Utah USA. Nat. Resour. Environ. Issues 2009, 15, 10. [Google Scholar]
- Bhatti, A.S.; Dollimore, D.; Dyer, A. Magnesia from seawater: A review. Clay Miner. 1984, 19, 865–875. [Google Scholar] [CrossRef]
- Bardi, U. Extracting minerals from seawater: An energy analysis. Sustainability 2010, 2, 980–992. [Google Scholar] [CrossRef]
- Cipollina, A.; Bevacqua, M.; Dolcimascolo, P.; Tamburini, A.; Brucato, A.; Glade, H.; Buether, L.; Micale, G. Reactive crystallisation process for magnesium recovery from concentrated brines. Desalination Water Treat. 2014, 55, 2377–2388. [Google Scholar] [CrossRef]
- Chu, S.; Yang, E.H.; Unluer, C. Chemical synthesis of magnesium oxide (MgO) from brine towards minimal energy consumption. Desalination 2023, 556, 116594. [Google Scholar] [CrossRef]
- Sharma, K.; Akther, N.; Choo, Y.; Zhang, P.; Matsuyama, H.; Shon, H.K.; Naidu, G. Positively charged nanofiltration membranes for enhancing magnesium separation from seawater. Desalination 2023, 568, 117026. [Google Scholar] [CrossRef]
- Turek, M.; Gnot, W. Precipitation of Magnesium Hydroxide from Brine. Ind. Eng. Chem. Res. 1995, 34, 244–250. [Google Scholar] [CrossRef]
- Estefan, S.; Awadalla, F.; Yousef, A. Process technology for recovery of magnesia from brines. Powder Technol. 1980, 27, 233–240. [Google Scholar] [CrossRef]
- Schwochau, K. Extraction of metals from sea water. In Inorganic Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; pp. 91–133. [Google Scholar] [CrossRef]
- Wang, Q.; Nakouzi, E.; Ryan, E.A.; Subban, C.V. Flow-assisted selective mineral extraction from seawater. Environ. Sci. Technol. Lett. 2022, 9, 645–649. [Google Scholar] [CrossRef]
- Tran, K.T.; Han, K.S.; Kim, S.J.; Kim, M.J.; Tran, T. Recovery of magnesium from Uyuni salar brine as hydrated magnesium carbonate. Hydrometallurgy 2016, 160, 106–114. [Google Scholar] [CrossRef]
- Tran, K.T.; Van Luong, T.; An, J.-W.; Kang, D.-J.; Kim, M.-J.; Tran, T. Recovery of magnesium from Uyuni salar brine as high purity magnesium oxalate. Hydrometallurgy 2013, 138, 93–99. [Google Scholar] [CrossRef]
- Abdel-Aal, H.K. Magnesium: From Resources to Production; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Ahmed, M.; Arakel, A.; Hoey, D.; Thumarukudy, M.R.; Goosen, M.F.; Al-Haddabi, M.; Al-Belushi, A. Feasibility of salt production from inland RO desalination plant reject brine: A case study. Desalination 2003, 158, 109–117. [Google Scholar] [CrossRef]
- Shahmansouri, A.; Min, J.; Jin, L.; Bellona, C. Feasibility of extracting valuable minerals from desalination concentrate: A comprehensive literature review. J. Clean. Prod. 2015, 100, 4–16. [Google Scholar] [CrossRef]
- Mahmud, N.; Alvarez, D.V.F.; Ibrahim, M.H.; El-Naas, M.H.; Esposito, D.V. Magnesium recovery from desalination reject brine as pretreatment for membraneless electrolysis. Desalination 2022, 525, 115489. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Cao, J. Efficient magnesium recovery from seawater desalination brine via CO2 mineralization to synthesize hydromagnesite for uranium extraction. Desalination 2023, 559, 116629. [Google Scholar] [CrossRef]
- Hajbi, F.; Hammi, H.; M’nIf, A. Reuse of RO desalination plant reject brine. J. Phase Equilibria Diffus. 2010, 31, 341–347. [Google Scholar] [CrossRef]
- Fontana, D.; Forte, F.; Pietrantonio, M.; Pucciarmati, S.; Marcoaldi, C. Magnesium recovery from seawater desalination brines: A technical review. Environ. Dev. Sustain. 2022, 25, 13733–13754. [Google Scholar] [CrossRef]
- Birnhack, L.; Lahav, O. A new post-treatment process for attaining Ca2+, Mg2+, SO42− and alkalinity criteria in desalinated water. Water Res. 2007, 41, 3989–3997. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Ehara, R.; Itoi, S.; Goto, T. Ion-exchange membrane electrodialytic salt production using brine discharged from a reverse osmosis seawater desalination plant. J. Membr. Sci. 2003, 222, 71–86. [Google Scholar] [CrossRef]
- Kim, D.H. A review of desalting process techniques and economic analysis of the recovery of salts from retentates. Desalination 2011, 270, 1–8. [Google Scholar] [CrossRef]
- Dong, H.; Unluer, C.; Yang, E.-H.; Al-Tabbaa, A. Recovery of reactive MgO from reject brine via the addition of NaOH. Desalination 2018, 429, 88–95. [Google Scholar] [CrossRef]
- Dong, H.; Unluer, C.; Yang, E.-H.; Al-Tabbaa, A. Synthesis of reactive MgO from reject brine via the addition of NH4OH. Hydrometallurgy 2017, 169, 165–172. [Google Scholar] [CrossRef]
- Mohammad, A.; El-Naas, M.; Al-Marzouqi, A.; Suleiman, M.; Al Musharfy, M. Optimization of magnesium recovery from reject brine for reuse in desalination post-treatment. J. Water Process. Eng. 2019, 31, 100810. [Google Scholar] [CrossRef]
- Wu, J.-H.; Xiao, Y.; Yang, X.-S.; Xu, D.-H.; Zhang, Z.-Y.; Zhong, Y.-J.; Wang, X.-L. Leaching kinetics for magnesium extraction from phosphate rock in the nitric acid method. Miner. Eng. 2022, 189, 107894. [Google Scholar] [CrossRef]
- González, Y.; Navarra, A.; Jeldres, R.I.; Toro, N. Hydrometallurgical processing of magnesium minerals—A review. Hydrometallurgy 2021, 201, 105573. [Google Scholar] [CrossRef]
- Jiao, F.; Li, W.; Xue, K.; Yang, C.; Qin, W. Recovery of chromium and magnesium from spent magnesia-chrome refractories by acid leaching combined with alkali precipitation and evaporation. Sep. Purif. Technol. 2019, 227, 115705. [Google Scholar] [CrossRef]
- Özdemir, M.; Çakır, D.; Kıpçak, I. Magnesium recovery from magnesite tailings by acid leaching and production of magnesium chloride hexahydrate from leaching solution by evaporation. Int. J. Miner. Process. 2009, 93, 209–212. [Google Scholar] [CrossRef]
- Royani, A.; Sulistiyono, E.; Prasetiyo, A.B.; Subagja, R. Extraction of magnesium from calcined dolomite ore using hydrochloric acid leaching. AIP Conf. Proc. 2018, 1964, 020017. [Google Scholar] [CrossRef]
- Fedoročková, A.; Hreus, M.; Raschman, P.; Sučik, G. Dissolution of magnesium from calcined serpentinite in hydrochloric acid. Miner. Eng. 2012, 32, 1–4. [Google Scholar] [CrossRef]
- McDonald, R.G.; Whittington, B.I. Atmospheric acid leaching of nickel laterites review. Part I. Chloride and bio-technologies. Hydrometallurgy 2008, 91, 35–55. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S.; Zafiratos, I.G. Beneficiation of a Greek serpentinic nickeliferous ore Part II. Sulphuric acid heap and agitation leaching. Hydrometallurgy 2004, 74, 267–275. [Google Scholar] [CrossRef]
- Gao, F.; Huang, Z.; Li, H.; Li, X.; Wang, K.; Hamza, M.F.; Wei, Y.; Fujita, T. Recovery of magnesium from ferronickel slag to prepare hydrated magnesium sulfate by hydrometallurgy method. J. Clean. Prod. 2021, 303, 127049. [Google Scholar] [CrossRef]
- Yang, X.; Gao, L.; Wu, Y.; Chen, Y.; Tong, L. Extraction of magnesium and nickel from nickel-rich serpentine with sulfation roasting and water leaching. Metals 2022, 12, 318. [Google Scholar] [CrossRef]
- Teir, S.; Revitzer, H.; Eloneva, S.; Fogelholm, C.-J.; Zevenhoven, R. Dissolution of natural serpentinite in mineral and organic acids. Int. J. Miner. Process. 2007, 83, 36–46. [Google Scholar] [CrossRef]
- Wogelius, R.A.; Walther, J.V. Olivine dissolution at 25 °C: Effects of pH, CO2, and organic acids. Geochim. Cosmochim. Acta 1991, 55, 943–954. [Google Scholar] [CrossRef]
- Velema, R.; Bullen, J.; Visser, J.; Bruining, J. Magnesium from solution mining of bischofite layers and carnallite layers. In Proceedings of the ECMOR XII-12th European Conference on the Mathematics of Oil Recovery, Oxford, UK, 6–9 September 2010; European Association of Geoscientists & Engineers: Kosterijland, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Murase, K.; Nishikawa, K.-I.; Ozaki, T.; Machida, K.-I.; Adachi, G.-Y.; Suda, T. Recovery of vanadium, nickel and magnesium from a fly ash of bitumen-in-water emulsion by chlorination and chemical transport. J. Alloys Compd. 1998, 264, 151–156. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.-A.; Lv, G.-Z.; Dou, Z.-H.; Zhang, W.-G.; Niu, L.-P.; Zhang, Z.-M. Kinetics of magnesium and calcium extraction from fly ash by carbochlorination. JOM 2019, 71, 2798–2805. [Google Scholar] [CrossRef]
- Geng, W.; Zou, J.; Niu, Q.; Lin, Y.; Liu, H.; Jing, Y.; Yang, C. Recovery of magnesium from flue gas desulfurization wastewater using thermomorphic hydrophilicity amines. Sep. Purif. Technol. 2023, 316, 123776. [Google Scholar] [CrossRef]
- Watanabe, A.; Yamamoto, K.; Orikasa, Y.; Masese, T.; Mori, T.; Uchiyama, T.; Matsunaga, T.; Uchimoto, Y. Reaction mechanism of electrochemical insertion/extraction of magnesium ions in olivine-type FePO4. Solid State Ion. 2020, 349, 115311. [Google Scholar] [CrossRef]
- Moyo, L.; Simate, G.; Mamvura, T. Magnesium recovery from ferrochrome slag: Kinetics and possible use in a circular economy. Heliyon 2022, 8, e12176. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.-H.; Lee, H.-Y.; Gyan-Barimah, C.; Yu, J.-H.; Yu, J.-S. Magnesium: Properties and rich chemistry for new material synthesis and energy applications. Chem. Soc. Rev. 2023, 52, 2145–2192. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.A. Magnesium casting technology for structural applications. J. Magnes. Alloys 2013, 1, 2–22. [Google Scholar] [CrossRef]
- Jayasathyakawin, S.; Ravichandran, M.; Baskar, N.; Chairman, C.A.; Balasundaram, R. Mechanical properties and applications of Magnesium alloy—Review. Mater. Today Proc. 2020, 27, 909–913. [Google Scholar] [CrossRef]
- Gupta, M.; Sharon, N.M.L. Magnesium, Magnesium Alloys, and Magnesium Composites; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Sankaranarayanan, S.; Gupta, M. Review on mechanical properties of magnesium (nano)composites developed using energy efficient microwaves. Powder Met. 2015, 58, 183–192. [Google Scholar] [CrossRef]
- Landkof, B. Magnesium applications in aerospace and electronic industries. In Magnesium Alloys and Their Applications; Wiley: Hoboken, NJ, USA, 2000; pp. 168–172. [Google Scholar] [CrossRef]
- Monteiro, W.A. The influence of alloy element on magnesium for electronic devices applications—A review. Light Met. Alloys Appl. 2014, 12, 229. [Google Scholar] [CrossRef]
- Kurzynowski, T.; Pawlak, A.; Smolina, I. The potential of SLM technology for processing magnesium alloys in aerospace industry. Arch. Civ. Mech. Eng. 2020, 20, 23. [Google Scholar] [CrossRef]
- Blawert, C.; Hort, N.; Kainer, K.U. Automotive applications of magnesium and its alloys. Trans. Indian Inst. Met 2004, 57, 397–408. [Google Scholar]
- Friedrich, H.; Schumann, S. Research for a “new age of magnesium” in the automotive industry. J. Mech. Work. Technol. 2001, 117, 276–281. [Google Scholar] [CrossRef]
- Kulekci, M.K. Magnesium and its alloys applications in automotive industry. Int. J. Adv. Manuf. Technol. 2008, 39, 851–865. [Google Scholar] [CrossRef]
- Czerwinski, F. Controlling the ignition and flammability of magnesium for aerospace applications. Corros. Sci. 2014, 86, 1–16. [Google Scholar] [CrossRef]
- Kumar, D.S.; Sasanka, C.T.; Ravindra, K.; Suman, K. Magnesium and Its Alloys in Automotive Applications—A Review. Am. J. Mater. Sci. Technol. 2015, 4, 12–30. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, N. The promise of magnesium based materials in aerospace sector. Int. J. Aeronaut. Sci. Aerosp. Res. 2017, 4, 141–149. [Google Scholar] [CrossRef]
- Ercetin, A.; Özgün, Ö.; Aslantaş, K.; Der, O.; Yalçın, B.; Şimşir, E.; Aamir, M. Microstructural and mechanical behavior investigations of Nb-reinforced Mg–Sn–Al–Zn–Mn matrix magnesium composites. Metals 2023, 13, 1097. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, B.; Panigrahi, S. Investigation into machining performance of microstructurally engineered in-situ particle reinforced magnesium matrix composite. J. Magnes. Alloys 2023, 11, 916–935. [Google Scholar] [CrossRef]
- Parthiban, G.; Parthiban, T.; Ravi, R.; Saraswathy, V.; Palaniswamy, N.; Sivan, V. Cathodic protection of steel in concrete using magnesium alloy anode. Corros. Sci. 2008, 50, 3329–3335. [Google Scholar] [CrossRef]
- Pathak, S.S.; Mendon, S.K.; Blanton, M.D.; Rawlins, J.W. Magnesium-based sacrificial anode cathodic protection coatings (Mg-rich primers) for aluminum alloys. Metals 2012, 2, 353–376. [Google Scholar] [CrossRef]
- Cain, T.; Melia, M.; Fitz-Gerald, J.; Scully, J. Evaluation of the potential range for sacrificial mg anodes for the cathodic protection of mg alloy AZ31B-H24. Corrosion 2017, 73, 544–562. [Google Scholar] [CrossRef]
- Zidane, N.; Albrimi, Y.; Addi, A.; Douch, J.; Souto, R.; Hamdani, M. Evaluation of the corrosion of AZ31 magnesium alloy used as sacrificial anode for cathodic protection of hot-water tank storage containing chloride. Int. J. Electrochem. Sci. 2018, 13, 29–44. [Google Scholar] [CrossRef]
- Yan, L.; Song, G.-L.; Zheng, D. Magnesium alloy anode as a smart corrosivity detector and intelligent sacrificial anode protector for reinforced concrete. Corros. Sci. 2019, 155, 13–28. [Google Scholar] [CrossRef]
- Aksel, C.; Riley, F.L. Magnesia–spinel (MgAl2O4) refractory ceramic composites. In Ceramic-Matrix Composites; Elsevier: Amsterdam, The Netherlands, 2006; pp. 359–399. [Google Scholar] [CrossRef]
- Landy, R.A. Magnesia refractories. In Refractories Handbook; Then Crc Press/Taylor and Francis: Boca Raton, FL, USA, 2004; Mechanical Engineering-New York and Basel-Marcel Dekker; Volume 178, p. 109. [Google Scholar]
- Hojamberdiev, M.; Arifov, P.; Tadjiev, K.; Xu, Y.-H. Processing of refractory materials using various magnesium sources derived from Zinelbulak talc-magnesite. Int. J. Miner. Met. Mater. 2011, 18, 105–114. [Google Scholar] [CrossRef]
- Sarkar, R. Refractory applications of magnesium aluminate spinel. Interceram Refract. Man. 2010, 1, 11–14. [Google Scholar]
- Zhang, Y.; Yan, X.; Wang, L.; Sun, W. Forsterite refractory preparation using magnesium resources from salt lake brines. Miner. Eng. 2023, 203, 108333. [Google Scholar] [CrossRef]
- Hornak, J. Synthesis, properties, and selected technical applications of magnesium oxide nanoparticles: A Review. Int. J. Mol. Sci. 2021, 22, 12752. [Google Scholar] [CrossRef]
- Yang, J.; Cui, F.; Lee, I.S. Surface modifications of magnesium alloys for biomedical applications. Ann. Biomed. Eng. 2011, 39, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Poinern, G.E.J.; Brundavanam, S.; Fawcett, D. Biomedical magnesium alloys: A review of Material properties, surface modifications and potential as a biodegradable orthopaedic implant. Am. J. Biomed. Eng. 2012, 2, 218–240. [Google Scholar] [CrossRef]
- Riaz, U.; Shabib, I.; Haider, W. The current trends of Mg alloys in biomedical applications—A review. J. Biomed. Mater. Res. Part B 2018, 107, 1970–1996. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; Weissman, I.; Gofer, Y.; Levi, E. Nonaqueous magnesium electrochemistry and its application in secondary batteries. Chem. Rec. 2003, 3, 61–73. [Google Scholar] [CrossRef]
- Shah, R.; Mittal, V.; Matsil, E.; Rosenkranz, A. Magnesium-ion batteries for electric vehicles: Current trends and future perspectives. Adv. Mech. Eng. 2021, 13, 16878140211003398. [Google Scholar] [CrossRef]
- Aghion, E.; Bronfin, B.; Eliezer, D. The role of the magnesium industry in protecting the environment. J. Mech. Work. Technol. 2001, 117, 381–385. [Google Scholar] [CrossRef]
- Huie, M.M.; Bock, D.C.; Takeuchi, E.S.; Marschilok, A.C.; Takeuchi, K.J. Cathode materials for magnesium and magnesium-ion based batteries. Coord. Chem. Rev. 2015, 287, 15–27. [Google Scholar] [CrossRef]
- Muldoon, J.; Bucur, C.B.; Gregory, T. Quest for nonaqueous multivalent secondary batteries: Magnesium and beyond. Chem. Rev. 2014, 114, 11683–11720. [Google Scholar] [CrossRef]
- Friedrich, H. Magnesium Technology; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Mazzotti, M.; Abanades, J.C.; Allam, R.; Lackner, K.S.; Meunier, F.; Rubin, E.; Sanchez, J.C.; Yogo, K.; Zevenhoven, R. Mineral carbonation and industrial uses of carbon dioxide. In IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Pronost, J.; Beaudoin, G.; Tremblay, J.; Larachi, F.; Duchesne, J.; Hébert, R.; Constantin, M. Carbon sequestration kinetic and storage capacity of ultramafic mining waste. Environ. Sci. Technol. 2011, 45, 9413–9420. [Google Scholar] [CrossRef]
- Hasib-Ur-Rahman, M.; Larachi, F. CO2 capture in alkanolamine-rtil blends via carbamate crystallization: Route to efficient regeneration. Environ. Sci. Technol. 2012, 46, 11443–11450. [Google Scholar] [CrossRef]
- Hasib-Ur-Rahman, M.; Siaj, M.; Larachi, F. CO2 capture in alkanolamine/room-temperature ionic liquid emulsions: A viable approach with carbamate crystallization and curbed corrosion behavior. Int. J. Greenh. Gas Control 2012, 6, 246–252. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Beaudoin, G.; Molson, J. Dynamics of carbon dioxide uptake in chrysotile mining residues—Effect of mineralogy and liquid saturation. Int. J. Greenh. Gas Control 2013, 12, 124–135. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Molson, J.; Beaudoin, G. Accurate and direct quantification of native brucite in serpentine ores—New methodology and implications for CO2 sequestration by mining residues. Thermochim. Acta 2013, 566, 281–291. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Beaudoin, G.; Molson, J. CO2 Sequestration in Chrysotile Mining Residues Implication of Watering and Passivation under Environmental Conditions. Ind. Eng. Chem. Res. 2012, 51, 8726–8734. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Zarandi, A.E.; Larachi, F.; Beaudoin, G.; Plante, B.; Sciortino, M. Multivariate study of the dynamics of CO2 reaction with brucite-rich ultramafic mine tailings. Int. J. Greenh. Gas Control 2016, 52, 110–119. [Google Scholar] [CrossRef]
- Zarandi, A.E.; Larachi, F.; Beaudoin, G.; Plante, B.; Sciortino, M. Ambient mineral carbonation of different lithologies of mafic to ultramafic mining wastes/tailings—A comparative study. Int. J. Greenh. Gas Control 2017, 63, 392–400. [Google Scholar] [CrossRef]
- Zarandi, A.E.; Larachi, F.; Beaudoin, G.; Plante, B.; Sciortino, M. Nesquehonite as a carbon sink in ambient mineral carbonation of ultramafic mining wastes. Chem. Eng. J. 2017, 314, 160–168. [Google Scholar] [CrossRef]
- Beaudoin, G.; Nowamooz, A.; Assima, G.P.; Lechat, K.; Gras, A.; Entezari, A.; Kandji, E.H.B.; Awoh, A.-S.; Horswill, M.; Turcotte, S.; et al. Passive mineral carbonation of Mg-rich mine wastes by atmospheric CO2. Energy Procedia 2017, 114, 6083–6086. [Google Scholar] [CrossRef]
- Larachi, F.; Aksenova, D.; Yousefi, B.; Maldague, X.; Beaudoin, G. Thermochemical monitoring of brucite carbonation using passive infrared thermography. Chem. Eng. Process.-Process. Intensif. 2018, 130, 43–52. [Google Scholar] [CrossRef]
- Norhasyima, R.; Mahlia, T. Advances in CO2 utilization technology: A patent landscape review. J. CO2 Util. 2018, 26, 323–335. [Google Scholar] [CrossRef]
- Yadav, S. Carbon storage by mineral carbonation and industrial applications of CO2. Mater. Sci. Energy Technol. 2020, 3, 494–500. [Google Scholar] [CrossRef]
- Kim, C.; Yoo, C.-J.; Oh, H.-S.; Min, B.K.; Lee, U. Review of carbon dioxide utilization technologies and their potential for industrial application. J. CO2 Util. 2022, 65, 102239. [Google Scholar] [CrossRef]
- Hanifa, M.; Agarwal, R.; Sharma, U.; Thapliyal, P.; Singh, L. A review on CO2 capture and sequestration in the construction industry: Emerging approaches and commercialised technologies. J. CO2 Util. 2022, 67, 102292. [Google Scholar] [CrossRef]
- Kramer, D.A. Magnesium and magnesium alloys. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2000; pp. 1–55. [Google Scholar] [CrossRef]
- Kipouros, G.J.; Sadoway, D.R. The chemistry and electrochemistry of magnesium production. Adv. Molten Salt Chem. 1987, 6, 127–209. [Google Scholar]
- Sharma, R.A. A new electrolytic magnesium production process. JOM 1996, 48, 39–43. [Google Scholar] [CrossRef]
- Choi, M.S.; Lee, C.K.; Lee, G.G.; Cho, S.K.; Jung, J.Y. Technology of molten salt electrolysis of magnesium chloride. Mater. Sci. Forum 2010, 654–656, 799–802. [Google Scholar] [CrossRef]
- Shekhovtsov, G.; Shchegolev, V.; Devyatkin, V.; Tatakin, A.; Zabelin, I. Magnesium electrolytic production process. In Essential Readings in Magnesium Technology; Springer International Publishing: Cham, Switzerland, 2016; pp. 97–100. [Google Scholar] [CrossRef]
- Thayer, R.L.; Neelameggham, R. Improving the electrolytic process magnesium production. JOM 2001, 53, 15–17. [Google Scholar] [CrossRef]
- Toguri, J.M.; Pidgeon, L.M. High-temperature studies of metallurgical processes: Part I. The thermal reduction of magnesium oxide with silicon. Can. J. Chem. 1961, 39, 540–547. [Google Scholar] [CrossRef]
- Yang, C.-B.; Tian, Y.; Qu, T.; Yang, B.; Xu, B.-Q.; Dai, Y.-N. Production of magnesium during carbothermal reduction of magnesium oxide by differential condensation of magnesium and alkali vapours. J. Magnes. Alloys 2013, 1, 323–329. [Google Scholar] [CrossRef]
- Ramachandran, M.; Reddy, R.G. Direct reduction of magnesium oxide to magnesium using thermal plasma technology. Min. Met. Explor. 2015, 32, 30–37. [Google Scholar] [CrossRef]
- Jeoung, H.-J.; Lee, T.-H.; Lee, J.-Y.; Yi, K.-W.; Kang, J. Production of high-purity Mg metal from dolomite through novel molten salt electrolysis and vacuum distillation. J. Magnes. Alloys 2023, 11, 1308–1320. [Google Scholar] [CrossRef]
- Jeoung, H.-J.; Lee, T.-H.; Yi, K.-W.; Kang, J. Review on Electrolytic Processes for Magnesium Metal Production: Industrial and Innovative Processes. Mater. Trans. 2025, 66, 823–839. [Google Scholar] [CrossRef]
- Wulandari, W.; Brooks, G.; Rhamdhani, M.; Monaghan, B. Magnesium: Current and Alternative Production Routes. In Proceedings of the Chemeca, Adelaide, Australi, 26–29 September 2010. [Google Scholar]
- Simandl, G.J.; Paradis, S.; Irvine, M. Brucite–industrial mineral with a future. Geosci. Can. 2007, 34, 57–64. [Google Scholar]
- Duke, J.M. Ultramafic-Hosted Asbestos. In Geology of Canadian Mineral Deposit Types; Geological Society of America, Inc.: Boulder, CO, USA, 1995. [Google Scholar] [CrossRef]
- Lee, W.J.; Fanelli, M.F.; Cava, N.; Wyllie, P.J. Calciocarbonatite and magnesiocarbonatite rocks and magmas represented in the system CaO-MgO-CO2-H2O at 0.2 GPa. Miner. Pet. 2000, 68, 225–256. [Google Scholar] [CrossRef]
- Ross, M.; Nolan, R.P. History of asbestos discovery and use and asbestos-related disease in context with the occurrence of asbestos within ophiolite complexes. In Ophiolite Concept and the Evolution of Geological Thought; Special Papers; Geological Society of America: Boulder, CO, USA, 2003; pp. 447–470. [Google Scholar] [CrossRef]
- Simandl, G.J.; Schultes, H.; Simandl, J.; Paradis, S. Magnesium-raw materials, metal extraction and economics-global picture. In Proceedings of the Ninth Biennial SGA Meeting; Irish Association for Economic Geology: Dublin, Ireland, 2007. [Google Scholar]
- Christini, R.A.; Rolles, R.; Bowman, K.A.; Ballain, M.D. Thermal Reduction Process for Production of Magnesium. U.S. Patent 4,478,637, 23 October 1984. [Google Scholar]
- Wu, H.; Zhao, P.; Jing, M.; Li, J.; Chen, T. Magnesium production by a coupled electric and thermal field. Vacuum 2021, 183, 109822. [Google Scholar] [CrossRef]
- Guo, J.; Li, X.; Zhang, T.; Han, J.; Geng, J.; Wang, Y. Comparison of extraction behavior of magnesium from magnesite/magnesia by aluminothermic process in flowing argon. J. Sustain. Met. 2022, 8, 1756–1768. [Google Scholar] [CrossRef]
- Wadsley, M.W. Magnesium metal by the Heggie–Iolaire process. Magnes. Technol. 2000, 2000, 65–70. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Z.; Wang, Y.; Wang, D. Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum. Green Process. Synth. 2020, 9, 164–170. [Google Scholar] [CrossRef]
- Hidayat, T.; Siregar, M.Y.; Santoso, I.; Zulhan, Z. The effects of reductant and additive on the magnesium extraction from calcined dolomite via metallothermic reduction under vacuum condition. Vacuum 2022, 202, 111196. [Google Scholar] [CrossRef]
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2014, 119, 2239–2248. [Google Scholar] [CrossRef]
- Buğdayci, M.; Turan, A.; Alkan, M.; Yücel, O. Magnesium Production from Calcined Dolomite via the Pidgeon Process. In Magnesium and Its Alloys; CRC Press: Boca Raton, FL, USA, 2019; pp. 47–56. [Google Scholar]
- Rizley, J.H.; Høy-Petersen, N. Magnesium Processing. 2022. Available online: https://www.britannica.com/technology/magnesium-processing (accessed on 15 October 2022).
- Zhang, X.; Zhao, W.; Zhang, Y.; Jegatheesan, V. A review of resource recovery from seawater desalination brine. Rev. Environ. Sci. Bio/Technol. 2021, 20, 333–361. [Google Scholar] [CrossRef]
- Li, Z.; Mercken, J.; Li, X.; Riaño, S.; Binnemans, K. Efficient and sustainable removal of magnesium from brines for lithium/magnesium separation using binary extractants. ACS Sustain. Chem. Eng. 2019, 7, 19225–19234. [Google Scholar] [CrossRef]
- Ahmad, K.S.; Nawaz, M.; Jaffri, S.B. Role of renewable energy and nanotechnology in sustainable desalination of water: Mini review. Int. J. Environ. Anal. Chem. 2020, 102, 7700–7719. [Google Scholar] [CrossRef]
- Drioli, E.; Curcio, E.; Criscuoli, A.; Di Profio, G. Integrated system for recovery of CaCO3, NaCl and MgSO4·7H2O from nanofiltration retentate. J. Membr. Sci. 2004, 239, 27–38. [Google Scholar] [CrossRef]
- Kumar, M.; Badruzzaman, M.; Adham, S.; Oppenheimer, J. Beneficial phosphate recovery from reverse osmosis (RO) concentrate of an integrated membrane system using polymeric ligand exchanger (PLE). Water Res. 2007, 41, 2211–2219. [Google Scholar] [CrossRef]
- Davis, T.A. Zero Discharge Seawater Desalination: Integrating the Production of Freshwater; US Department of the Interior, Bureau of Reclamation, Technical Service Center, Environmental Resources Team, Water Treatment Engineering and Research Group: Denver, CO, USA, 2006.
- Turek, M. Dual-purpose desalination-salt production electrodialysis. Desalination 2003, 153, 377–381. [Google Scholar] [CrossRef]
- Lehmann, O.; Nir, O.; Kuflik, M.; Lahav, O. Recovery of high-purity magnesium solutions from RO brines by adsorption of Mg(OH)2(s) on Fe3O4 micro-particles and magnetic solids separation. Chem. Eng. J. 2014, 235, 37–45. [Google Scholar] [CrossRef]
- Epstein, J.A. Utilization of the dead sea minerals (a review). Hydrometallurgy 1976, 2, 1–10. [Google Scholar] [CrossRef]
- Nelson, K.H.; Thompson, T.G. Deposition of Salts from Sea Water by Frigid Concentration; Technical Report #29; University of Washington, Department of Oceanography: Seattle, WA, USA, 1954. [Google Scholar]
- Ravizky, A.; Nadav, N. Salt production by the evaporation of SWRO brine in Eilat: A success story. Desalination 2007, 205, 374–379. [Google Scholar] [CrossRef]
- Fattah, K.P.; Sinno, S.; Atabay, S.; Khan, Z.; Al-Dawood, Z.; Yasser, A.K.; Temam, R. Impact of magnesium sources for phosphate recovery and/or removal from waste. Energies 2022, 15, 4585. [Google Scholar] [CrossRef]
- Vassallo, F.; La Corte, D.; Cipollina, A.; Tamburini, A.; Micale, G. High purity recovery of magnesium and calcium hydroxides from waste brines. Chem. Eng. Trans. 2021, 86, 931–936. [Google Scholar] [CrossRef]
- Ahmad, M.; Garudachari, B.; Al-Wazzan, Y.; Kumar, R.; Thomas, J.P. Mineral extraction from seawater reverse osmosis brine of Gulf seawater. Desalination Water Treat. 2019, 144, 45–56. [Google Scholar] [CrossRef]
- Casas, S.; Aladjem, C.; Larrotcha, E.; Gibert, O.; Valderrama, C.; Cortina, J.L. Valorisation of Ca and Mg by-products from mining and seawater desalination brines for water treatment applications. J. Chem. Technol. Biotechnol. 2014, 89, 872–883. [Google Scholar] [CrossRef]
- Singh, I.; Hay, R.; Celik, K. Recovery and direct carbonation of brucite from desalination reject brine for use as a construction material. Cem. Concr. Res. 2022, 152, 106673. [Google Scholar] [CrossRef]
- Mohammadesmaeili, F.; Badr, M.K.; Abbaszadegan, M.; Fox, P. Byproduct recovery from reclaimed water reverse osmosis concentrate using lime and soda-ash treatment. Water Environ. Res. 2010, 82, 342–350. [Google Scholar] [CrossRef]
- Morgante, C.; Vassallo, F.; Battaglia, G.; Cipollina, A.; Vicari, F.; Tamburini, A.; Micale, G. Influence of operational strategies for the recovery of magnesium hydroxide from brines at a pilot scale. Ind. Eng. Chem. Res. 2022, 61, 15355–15368. [Google Scholar] [CrossRef] [PubMed]
- Sorour, M.H.; Hani, H.A.; Shaalan, H.F.; Al-Bazedi, G.A. Schemes for salt recovery from seawater and RO brines using chemical precipitation. Desalination Water Treat. 2014, 55, 2398–2407. [Google Scholar] [CrossRef]
- Guo, R.-T.; Pan, W.-G.; Zhang, X.-B.; Xu, H.-J.; Ren, J.-X. Dissolution rate of magnesium hydrate for wet flue gas desulfurization. Fuel 2011, 90, 7–10. [Google Scholar] [CrossRef]
- Azizi, D.; Larachi, F. Surface speciation of brucite dissolution in aqueous mineral carbonation: Insights from density-functional theory simulations. J. Phys. Chem. A 2019, 123, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Wang, T.; Guo, R.; Unluer, C. Assessment of the properties and environmental impact of carbonated reactive magnesia containing industrial waste. Thermochim. Acta 2021, 706, 179051. [Google Scholar] [CrossRef]
- Arce, G.L.A.F.; Soares Neto, T.G.; Ávila, I.; Luna, C.M.R.; Carvalho, J.A. Leaching optimization of mining wastes with lizardite and brucite contents for use in indirect mineral carbonation through the pH swing method. J. Clean. Prod. 2017, 141, 1324–1336. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Y.; Li, Z. Enhanced hydromagnesite process for CO2 sequestration by desilication of serpentine ore in NaOH solution. Ind. Eng. Chem. Res. 2020, 59, 11370–11380. [Google Scholar] [CrossRef]
- Sheila, D.; Sankaran, C.; Khangaonkar, P. Studies on the extraction of magnesia from low grade magnesites by carbon dioxide pressure leaching of hydrated magnesia. Miner. Eng. 1991, 4, 79–88. [Google Scholar] [CrossRef]
- Dönmez, B.; Demir, F.; Laçin, O. Leaching kinetics of calcined magnesite in acetic acid solutions. J. Ind. Eng. Chem. 2009, 15, 865–869. [Google Scholar] [CrossRef]
- Liu, J.; Yin, W.; Wang, Y.; Han, H.; Yang, B.; Yin, X.; Yao, J.; Zhang, Z.; Sun, H. Green 2-hydroxybenzoic acid-ASDA-Na4 synergistic system induces morphology-controlled anhydrous MgCO3 synthesis via magnesium leaching and carbonation from brucite solid waste. Chem. Eng. J. 2025, 520, 166026. [Google Scholar] [CrossRef]
- Canterford, J.H.; Moorrees, C. Magnesia from magnesite by calcinations/carbonic acid leaching: Precipitation from solution and final production recovery. Bull. Proc.—Australas. Inst. Min. Metall. 1985, 290, 67–70. [Google Scholar]
- Zhu, G.; Zhang, H.; Zhao, Y. A novel recirculating reactor for the leaching of magnesia by CO2. Hydrometallurgy 2008, 92, 141–147. [Google Scholar] [CrossRef]
- Mesci, A.K.; Sevim, F. Dissolution of magnesia in aqueous carbon dioxide by ultrasound. Int. J. Miner. Process. 2006, 79, 83–88. [Google Scholar] [CrossRef]
- Pasquier, L.-C.; Mercier, G.; Blais, J.-F.; Cecchi, E.; Kentish, S. Reaction mechanism for the aqueous-phase mineral carbonation of heat-activated serpentine at low temperatures and pressures in flue gas conditions. Environ. Sci. Technol. 2014, 48, 5163–5170. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Conway, W.; Burns, R.; McCann, N.; Maeder, M. Comprehensive study of the hydration and dehydration reactions of carbon dioxide in aqueous solution. J. Phys. Chem. A 2009, 114, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Demir, F.; Dönmez, B.; Çolak, S. Leaching kinetics of magnesite in citric acid solutions. J. Chem. Eng. Jpn. 2003, 36, 683–688. [Google Scholar] [CrossRef]
- Nagamori, M.; Boivin, J.-A. Technico-economic simulation for the hcl-leaching of hybrid serpentine and magnesite feeds. Can. Met. Q. 2001, 40, 47–60. [Google Scholar] [CrossRef]
- Raschman, P.; Fedoročková, A. Study of inhibiting effect of acid concentration on the dissolution rate of magnesium oxide during the leaching of dead-burned magnesite. Hydrometallurgy 2004, 71, 403–412. [Google Scholar] [CrossRef]
- Shen, X.-Y.; Huang, Y.-X.; Shao, H.-M.; Liu, Y.; Gu, H.-M.; Zhai, Y.-C. Improvement of utilization efficiency of magnesite by (NH4)2SO4 roasting–water leaching process. Trans. Nonferrous Met. Soc. China 2023, 33, 576–583. [Google Scholar] [CrossRef]
- Demir, F.; Dönmez, B. The determination of the optimum conditions upon the leaching performance of calcined magnesite. Heliyon 2019, 5, e02830. [Google Scholar] [CrossRef] [PubMed]
- Economou, E.D.; Vaimakis, T.C.; Papamichael, E.M. The kinetics of dissolution of the carbonate minerals of phosphate ores using dilute acetic acid solutions: The case of pH range from 3.96 to 6.40. J. Colloid Interface Sci. 2002, 245, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Laçin, O.; Dönmez, B.; Demir, F. Dissolution kinetics of natural magnesite in acetic acid solutions. Int. J. Miner. Process. 2005, 75, 91–99. [Google Scholar] [CrossRef]
- Demir, F.; Dönmez, B. Optimization of the dissolution of magnesite in citric acid solutions. Int. J. Miner. Process. 2008, 87, 60–64. [Google Scholar] [CrossRef]
- Raza, N.; Raza, W.; Asif, M. Reaction Kinetics of magnesite ore in dilute ethanoic acid. Russ. J. Non-Ferr. Met. 2016, 57, 308–315. [Google Scholar] [CrossRef]
- Bakan, F.; Laçin, O.; Bayrak, B.; Saraç, H. Dissolution kinetics of natural magnesite in lactic acid solutions. Int. J. Miner. Process. 2006, 80, 27–34. [Google Scholar] [CrossRef]
- Raza, N.; Zafar, Z.I.; Najam-Ul-Haq, M. Utilization of formic acid solutions in leaching reaction kinetics of natural magnesite ores. Hydrometallurgy 2014, 149, 183–188. [Google Scholar] [CrossRef]
- Raza, N.; Zafar, Z.I.; Haq, N.U.; Kumar, R. Leaching of natural magnesite ore in succinic acid solutions. Int. J. Miner. Process. 2015, 139, 25–30. [Google Scholar] [CrossRef]
- Bayrak, B.; Lacin, O.; Bakan, F.; Sarac, H. Investigation of dissolution kinetics of natural magnesite in gluconic acid solutions. Chem. Eng. J. 2006, 117, 109–115. [Google Scholar] [CrossRef]
- Bayrak, B.; Laçin, O.; Saraç, H. Kinetic study on the leaching of calcined magnesite in gluconic acid solutions. J. Ind. Eng. Chem. 2010, 16, 479–484. [Google Scholar] [CrossRef]
- Yoo, K.; Kim, B.-S.; Kim, M.-S.; Lee, J.-C.; Jeong, J. Dissolution of magnesium from serpentine mineral in sulfuric acid solution. Mater. Trans. 2009, 50, 1225–1230. [Google Scholar] [CrossRef]
- Ivanova, T.K.; Kremenetskaya, I.P.; Marchevskaya, V.V.; Slukovskaya, M.V.; Drogobuzhskaya, S.V. Magnesium Silicate Binding Materials Formed from Heat-Treated Serpentine-Group Minerals and Aqueous Solutions: Structural Features, Acid-Neutralizing Capacity, and Strength Properties. Materials 2022, 15, 8785. [Google Scholar] [CrossRef]
- Scott, A.; Oze, C.; Shah, V.; Yang, N.; Shanks, B.; Cheeseman, C.; Marshall, A.; Watson, M. Transformation of abundant magnesium silicate minerals for enhanced CO2 sequestration. Commun. Earth Environ. 2021, 2, 25. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Wu, L.; Tong, L.; Zhu, J. Recovery of Mg from H2SO4 Leaching Solution of Serpentine to Precipitation of High-Purity Mg(OH)2 and 4MgCO3·Mg(OH)2·4H2O. Minerals 2023, 13, 318. [Google Scholar] [CrossRef]
- Dlugogorski, B.Z.; Balucan, R.D. Dehydroxylation of serpentine minerals: Implications for mineral carbonation. Renew. Sustain. Energy Rev. 2014, 31, 353–367. [Google Scholar] [CrossRef]
- Luce, R.W.; Bartlett, R.W.; A Parks, G. Dissolution kinetics of magnesium silicates. Geochim. Cosmochim. Acta 1972, 36, 35–50. [Google Scholar] [CrossRef]
- Tebbiche, I.; Pasquier, L.-C.; Mercier, G.; Blais, J.-F.; Kentish, S. Thermally activated serpentine leaching under flue gas conditions in a bubble column reactor operated at ambient pressure and temperature. Hydrometallurgy 2020, 195, 105391. [Google Scholar] [CrossRef]
- Abouel-Leef, M.; Abeidu, A.E.M.; Mahdy, A.E.F. Utilization of serpentine ore for production of magnesium sulphate. World J. Eng. Pure Appl. Sci. 2012, 2, 31. [Google Scholar]
- Brindley, G.W.; Hayami, R. Mechanism of formation of forsterite and enstatite from serpentine. Miner. Mag. J. Miner. Soc. 1965, 35, 189–195. [Google Scholar] [CrossRef]
- Raschman, P.; Fedoročková, A.; Sučik, G. Thermal activation of serpentine prior to acid leaching. Hydrometallurgy 2013, 139, 149–153. [Google Scholar] [CrossRef]
- Ghoorah, M.; Dlugogorski, B.Z.; Oskierski, H.C.; Kennedy, E.M. Study of thermally conditioned and weak acid-treated serpentinites for mineralisation of carbon dioxide. Miner. Eng. 2014, 59, 17–30. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Meinhold, R.H. Thermal reactions of chrysotile revisited: A 29Si and 25Mg MAS NMR study. Am. Mineral. 1994, 79, 43–50. [Google Scholar]
- Zulumyan, N.O.; Isaakyan, A.R.; Oganesyan, Z.G. A new promising method for processing of serpentinites. Russ. J. Appl. Chem. 2007, 80, 1020–1022. [Google Scholar] [CrossRef]
- Park, A.-H.A.; Fan, L.-S. CO2 mineral sequestration: Physically activated dissolution of serpentine and pH swing process. Chem. Eng. Sci. 2004, 59, 5241–5247. [Google Scholar] [CrossRef]
- Kim, D.-J.; Sohn, J.-S.; Ahn, J.-G.; Chung, H.-S. Extraction of metals from mechanically milled serpentine. Geosystem Eng. 2008, 11, 25–28. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Mechanical activation of magnesium silicates for mineral carbonation, a review. Miner. Eng. 2018, 128, 69–83. [Google Scholar] [CrossRef]
- Suquet, H. Effects of dry grinding and leaching on the crystal structure of chrysotile. Clays Clay Miner. 1989, 37, 439–445. [Google Scholar] [CrossRef]
- Kim, D.-J.; Chung, H.-S. Effect of grinding on the structure and chemical extraction of metals from serpentine. Part. Sci. Technol. 2002, 20, 159–168. [Google Scholar] [CrossRef]
- Yang, H.; Du, C.; Hu, Y.; Jin, S.; Yang, W.; Tang, A.; Avvakumov, E. Preparation of porous material from talc by mechanochemical treatment and subsequent leaching. Appl. Clay Sci. 2006, 31, 290–297. [Google Scholar] [CrossRef]
- Brindley, G.W.; Hayami, R. Kinetics and Mechanisms of Dehydration and Recrystallization of Serpentine—I. In Clays and Clay Minerals (National Conference on Clays and Clay Minerals); Cambridge University Press & Assessment: Cambridge, UK, 1963. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, J.L.; Franco, F.; Ramírez-Valle, V.; Pérez-Maqueda, L.A. Modification of the thermal dehydroxylation of antigorite by ultrasound treatment. J. Therm. Anal. Calorim. 2005, 82, 769–774. [Google Scholar] [CrossRef]
- Franco, F.; Pérez-Maqueda, L.A.; Ramírez-Valle, V.; Pérez-Rodríiguez, J.L. Spectroscopic study of the dehydroxylation process of a sonicated antigorite. Eur. J. Miner. 2006, 18, 257–264. [Google Scholar] [CrossRef]
- Forster, J.; Maham, Y.; Bobicki, E. Microwave heating of magnesium silicate minerals. Powder Technol. 2018, 339, 1–7. [Google Scholar] [CrossRef]
- Liu, W.; Peng, X.; Liu, W.; Zhang, N.; Wang, X. A cost-effective approach to recycle serpentine tailings: Destruction of stable layered structure and solvent displacement crystallization. Int. J. Min. Sci. Technol. 2022, 32, 595–603. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Liu, T.; Huang, J.; Xue, N. Vanadium extraction from black shale: Enhanced leaching due to fluoride addition. Hydrometallurgy 2019, 187, 141–148. [Google Scholar] [CrossRef]
- Dutrizac, J.; Chen, T.; White, C. Fundamentals of serpentine leaching in hydrochloric acid media. Magnes. Technol. 2000, 2000, 40–51. [Google Scholar]
- Kirichenko, D.V.; Tolkachev, V.A. Studying the recovery of magnesium from serpentinite with a hydrochloric acid solution. Russ. J. Non-Ferr. Met. 2013, 54, 18–21. [Google Scholar] [CrossRef]
- Daval, D.; Hellmann, R.; Martinez, I.; Gangloff, S.; Guyot, F. Lizardite serpentine dissolution kinetics as a function of pH and temperature, including effects of elevated pCO2. Chem. Geol. 2013, 351, 245–256. [Google Scholar] [CrossRef]
- Taubert, L. Hydrochloric attack of serpentinites: Mg2+ leaching from serpentinites. Magnes. Res. 2000, 13, 167–173. [Google Scholar] [PubMed]
- Beglaryan, H.; Isahakyan, A.; Zulumyan, N.; Melikyan, S.; Terzyan, A. A study of magnesium dissolution from serpentinites composed of different serpentine group minerals. Miner. Eng. 2023, 201, 108171. [Google Scholar] [CrossRef]
- Vieira, K.R.; Arce, G.L.; Luna, C.M.; Facio, V.O.; A Carvalho, J.; Neto, T.G.S.; Ávila, I. Understanding the acid dissolution of Serpentinites (Tailings and waste rock) for use in indirect mineral carbonation. S. Afr. J. Chem. Eng. 2022, 40, 154–164. [Google Scholar] [CrossRef]
- McDonald, R.; Whittington, B. Atmospheric acid leaching of nickel laterites review. Part II. Chloride and bio-technologies. Hydrometallurgy 2008, 91, 56–69. [Google Scholar] [CrossRef]
- Ficara, P.; Chin, E.; Walker, T.; Laroche, D.; Palumbo, E.; Celik, C. Magnola: A novel commercial process for the primary production of magnesium. CIM Bull. 1998, 91, 75–80. [Google Scholar]
- El-Sayed, D.; Ismail, A.K.; El-Hosiny, F.I. Magnesium Chloride Crystals with Studying Mechanism and Leaching Kinetics of Serpentinite Ore by Hydrochloric Acid. Trans. Indian Inst. Met. 2023, 76, 1439–1446. [Google Scholar] [CrossRef]
- Fathi, H. Textbook of Hydrometallurgy; Metallurie Extractive Quebec: Quebec, QC, Canada, 1999. [Google Scholar]
- Fezei, R.; Hammi, H.; M’nif, A. Magnesium chloride precipitation from mixed salt solution using 1,4-dioxan. Chem. Eng. Res. Des. 2011, 89, 367–372. [Google Scholar] [CrossRef]
- Sarpong, B.; Shah, V.; Scott, A.; Watson, M. Extraction of Magnesium Oxide from Magnesium Silicate Minerals; Chemeca: Brisbane, Australia, 2020. [Google Scholar]
- Kosuge, K.; Shimada, K.; Tsunashima, A. Micropore formation by acid treatment of antigorite. Chem. Mater. 1995, 7, 2241–2246. [Google Scholar] [CrossRef]
- Gladikova, L.A.; Teterin, V.V.; Freidlina, R.G. Production of magnesium oxide from solutions formed by acid processing of serpentinite. Russ. J. Appl. Chem. 2008, 81, 889–891. [Google Scholar] [CrossRef]
- Fan, B.; Zhao, L.-S.; Feng, Z.-Y.; Liu, D.-P.; Yin, W.-Q.; Long, Z.-Q.; Huang, X.-W. Leaching behaviors of calcium and magnesium in ion-adsorption rare earth tailings with magnesium sulfate. Trans. Nonferrous Met. Soc. China 2021, 31, 288–296. [Google Scholar] [CrossRef]
- Kou, W.; Liu, W.; Liu, W.; Zuo, W.; Li, W. Microwave-enhanced leaching of magnesium and iron from iron-bearing serpentine tailings. Hydrometallurgy 2025, 237, 106536. [Google Scholar] [CrossRef]
- Ahmadjonov, A.; Alimov, U.; Tuychi, P.; Seitnazarov, A.; Reimov, A.; Namazov, S.; Sadullayev, S. Effect of temperature on the kinetics of the process of nitric acid decomposition of Arvaten serpentinite. IOP Conf. Ser. Earth Environ. Sci. 2023, 1142, 012034. [Google Scholar] [CrossRef]
- Ghoorah, M.; Dlugogorski, B.Z.; Balucan, R.D.; Kennedy, E.M. Selection of acid for weak acid processing of wollastonite for mineralisation of CO2. Fuel 2014, 122, 277–286. [Google Scholar] [CrossRef]
- Kakizawa, M.; Yamasaki, A.; Yanagisawa, Y. A new CO2 disposal process via artificial weathering of calcium silicate accelerated by acetic acid. Energy 2001, 26, 341–354. [Google Scholar] [CrossRef]
- Bałdyga, J.; Henczka, M.; Sokolnicka, K. Utilization of carbon dioxide by chemically accelerated mineral carbonation. Mater. Lett. 2010, 64, 702–704. [Google Scholar] [CrossRef]
- Krevor, S.C.; Lackner, K.S. Enhancing process kinetics for mineral carbon sequestration. Energy Procedia 2009, 1, 4867–4871. [Google Scholar] [CrossRef]
- Hänchen, M.; Prigiobbe, V.; Storti, G.; Seward, T.; Mazzotti, M. Dissolution kinetics of fosteritic olivine at 90–150 °C including effects of the presence of CO2. Geochim. Cosmochim. Acta 2006, 70, 4403–4416. [Google Scholar] [CrossRef]
- Park, A.-H.A.; Jadhav, R.; Fan, L.-S. CO2 mineral sequestration: Chemically enhanced aqueous carbonation of serpentine. Can. J. Chem. Eng. 2003, 81, 885–890. [Google Scholar] [CrossRef]
- Ghaderi, S.; Zhang, W. Microwave-assisted organic-acid leaching of major and critical elements from olivine: Characteristics, kinetics, and method comparison. Green Smart Min. Eng. 2025, 2, 168–183. [Google Scholar] [CrossRef]
- Grandstaff, D. The dissolution rate of forsteritic olivine from Hawaiian beach sand. In Rates of Chemical Weathering of Rocks and Minerals; Academic Press: New York, NY, USA, 1986. [Google Scholar]
- Wang, X.; Maroto-Valer, M.M. Dissolution of serpentine using recyclable ammonium salts for CO2 mineral carbonation. Fuel 2011, 90, 1229–1237. [Google Scholar] [CrossRef]
- Gao, W.; Wen, J.; Li, Z. Dissolution kinetics of magnesium from calcined serpentine in NH4Cl solution. Ind. Eng. Chem. Res. 2014, 53, 7947–7955. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, H.; Li, G.; An, S.; Ding, X.; Teng, H.H.; Zhao, L. CO2 absorption and magnesium carbonate precipitation in MgCl2–NH3–NH4Cl solutions: Implications for carbon capture and storage. Minerals 2017, 7, 172. [Google Scholar] [CrossRef]
- Romão, I.S.S. Production of Magnesium Carbonates from Serpentinites for CO2 Mineral Sequestration: Optimisation Towards Industrial Application. Doctoral Dissertation, Universidade de Coimbra (Portugal), Coimbra, Portugal, 2015. [Google Scholar]
- Sanna, A.; Wang, X.; Lacinska, A.; Styles, M.; Paulson, T.; Maroto-Valer, M.M. Enhancing Mg extraction from lizardite-rich serpentine for CO2 mineral sequestration. Miner. Eng. 2013, 49, 135–144. [Google Scholar] [CrossRef]
- Nduagu, E.I.; Highfield, J.; Chen, J.; Zevenhoven, R. Mechanisms of serpentine–ammonium sulfate reactions: Towards higher efficiencies in flux recovery and Mg extraction for CO2 mineral sequestration. RSC Adv. 2014, 4, 64494–64505. [Google Scholar] [CrossRef]
- Hänchen, M.; Prigiobbe, V.; Baciocchi, R.; Mazzotti, M. Precipitation in the Mg-carbonate system—Effects of temperature and CO2 pressure. Chem. Eng. Sci. 2008, 63, 1012–1028. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Chu, L.; Sun, H.; Peng, T.; Lu, H.; Li, M.; Zhang, Y.; Luo, L. Selective Extraction of Mg2+ from Chrysotile Asbestos Tailings via Ammonium Sulfate Roasting and Water Leaching: Process Optimization and Mechanistic Insights. J. Clean. Prod. 2025, 515, 145773. [Google Scholar] [CrossRef]





| Mineral Category | Mineral’s Name | Chemical Formula | Mg Content (wt%) | Availability |
|---|---|---|---|---|
| Oxide and hydroxide | Periclase | MgO | 60.30 | Occurs as a synthetic product; natural deposits are limited and localized (Mainly in Russia, the U.S., and China). |
| Brucite | Mg(OH)2 | 41.70 | ||
| Carbonate | Magnesite | MgCO3 | 28.83 | Extremely abundant worldwide, >50 billion tonnes with major deposits in China, Russia, and Turkey. |
| Dolomite | CaMg(CO3)2 | 13.18 | ||
| Silicate | Forsterite | Mg2SiO4 | 34.55 | Vast global abundance, >100 billion tonnes. Common in ultramafic rocks especially in ophiolite belts and metamorphic deposits, with major reserves in China, India, Iran, and the U.S. |
| Serpentine | Mg3Si2O5(OH)4 | 26.31 | ||
| Olivine | (Mg,Fe)2SiO4 | 25.37 | ||
| Enstatite | MgSiO3 | 24.21 | ||
| Talc | Mg3Si4O10(OH)2 | 19.23 | ||
| Tremolite | Ca2Mg5Si8O22(OH)2 | 14.96 | ||
| Evaporite halides | Bishofite | MgCl2·6H2O | 11.96 | Extensive in brine deposits; major reserves in Canada, Russia, and the U.S. |
| Carnallite | KMgCl3·6H2O | 8.75 |
| Thermal Reduction | Main Process | Additive | Pressure | Temperature (°C) |
|---|---|---|---|---|
| Silicothermia | -Pidgeon | -Ferrosilicon | 10 mm-Hg | 1200–1400 |
| -Magnetherm | -Ferrosilicon, Al2O3, Al | 1 atm | 1300–1700 | |
| -Bolzano | -Ferrosilicon, Al2O3 | 3 mm Hg | 1200 | |
| Aluminothermia | Heggie | Al scrap | 1 atm Ar | 1500 (arc plasma) |
| Carbothermia | Coke CaC2 | 1 atm 1 atm | 1900 1120–1140 |
| Category | Feedstocks | Main Processes | Products |
|---|---|---|---|
| Mg Metal Production | Magnesite Dolomite Brucite | Pidgeon process Electrolytic reduction | High-purity Mg Metal |
| Mg Compound Production | Seawater Brines Leaching solutions | Precipitation | MgO Mg(OH)2 MgCl2 MgSO4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taheri, B.; Larachi, F. Mineral-Based Magnesium Extraction Technologies: Current and Future Practices. Processes 2025, 13, 2945. https://doi.org/10.3390/pr13092945
Taheri B, Larachi F. Mineral-Based Magnesium Extraction Technologies: Current and Future Practices. Processes. 2025; 13(9):2945. https://doi.org/10.3390/pr13092945
Chicago/Turabian StyleTaheri, Bijan, and Faïçal Larachi. 2025. "Mineral-Based Magnesium Extraction Technologies: Current and Future Practices" Processes 13, no. 9: 2945. https://doi.org/10.3390/pr13092945
APA StyleTaheri, B., & Larachi, F. (2025). Mineral-Based Magnesium Extraction Technologies: Current and Future Practices. Processes, 13(9), 2945. https://doi.org/10.3390/pr13092945








