1. Introduction
As a clean, efficient, and low-carbon energy source, efficient CBM extraction is vital for optimizing the global energy structure, mitigating the energy crisis, and reducing carbon emissions [
1,
2,
3]. Currently, hydraulic fracturing is a mainstream technique for the commercial extraction of low-permeability coalbed methane. By constructing complex fracture networks in coal seams, this technology can effectively improve coal seam permeability, thereby promoting the desorption and migration of coalbed methane [
4]. In this process, the efficacy of fracturing fluids is pivotal to fracture propagation, proppant transport, and ultimate reservoir stimulation [
5].
Water-based fracturing fluids are extensively employed in CBM extraction due to their low cost, eco-friendliness, and favorable rheological properties [
6,
7,
8]. Crosslinking agents, as one of the core additives in water-based fracturing fluids, can form crosslinked networks with thickening agents (such as hydroxypropyl guar gum, polyacrylamide and its derivatives, etc.), significantly improving the viscosity and viscosity stability of the fracturing fluid [
9,
10]. With CBM extraction advancing into deep, high-temperature, and complex geological zones, higher demands are placed on crosslinker performance. However, existing crosslinker systems still have notable limitations [
11].
Traditional organic boron crosslinkers form stable crosslinked structures at moderate temperatures (<90 °C) via coordination between borate ions and polymer hydroxyl groups. They offer advantages like controllable crosslinking rates and low cost and were widely used in early CBM fracturing [
12,
13,
14,
15,
16]. Zhang et al. [
13] developed a polyol-complexed organic boron crosslinker that enhanced the thermal tolerance of guar gum fracturing fluids to a certain degree, allowing them to sustain favorable rheological stability at approximately 120 °C. However, above 140 °C, the coordination bond between borate and hydroxyl groups lacks thermal stability and is prone to breakage, destroying the crosslinked network and causing a sharp drop in viscosity [
17,
18]. Additionally, organic boron crosslinkers are sensitive to system pH. Their performance is significantly impaired in strongly acidic or alkaline environments, limiting use in complex formations [
19].
Zirconium-based crosslinkers show promise in high-temperature fracturing fluids due to the strong coordination between Zr
4+ ions and polymer carboxyl groups, becoming a research focus [
20,
21]. Fan et al. [
22] synthesized a zirconium lactate crosslinker that, by optimizing ligand structure, enhanced crosslinking between Zr
4+ and partially hydrolyzed polyacrylamide (HPAM), enabling the fluid to retain a specific viscosity even at 200 °C, demonstrating excellent high-temperature resistance. However, under high shear rates (e.g., 510 s
−1), the crosslinked network is easily damaged by mechanical forces, with a low viscosity recovery rate (~65%) [
22]. This is because the single Zr
4+-carboxyl coordination bond is prone to irreversible breakage under strong shear, and broken bonds struggle to re-form a stable network quickly, impairing performance in high-shear zones like perforations [
23].
The integration of nanomaterials offers a novel approach to enhancing crosslinker performance [
24]. Li et al. [
25] used silicon dioxide nanoparticles to achieve a high loading capacity of organotitanium per unit area, strengthening the crosslinked network structure and improving the shear stability of the fracturing fluid. The viscosity retention rate at 120 °C increased by ~25% compared to traditional systems [
26]. However, nanoparticles tend to agglomerate in solutions due to high surface energy, leading to poor dispersibility in crosslinked systems and uneven networks [
22]. At high temperatures (>150 °C), stress concentration around agglomerates can cause local gel collapse, severely affecting overall fluid performance and stability [
27].
Moreover, existing crosslinker systems lack optimization in crosslinker–thickener ratios [
28]. Numerous studies indicate that crosslinking density stands as one of the critical factors influencing fracturing fluid performance [
29]. Hui et al. [
23] noted that when the crosslinker-to-HPAM ratio is at a critical value, the system forms the most stable three-dimensional crosslinked network, yielding optimal overall fluid performance [
22]. Excessive crosslinking causes polymer over-crosslinking (“self-crosslinking”), reducing fluidity and viscosity. Insufficient dosage results in an incomplete network, failing to effectively enhance viscosity and proppant-carrying capacity [
30]. In field operations, complex conditions make precise ratio control difficult. Small errors can cause significant performance fluctuations, impacting results [
28].
This study aims to address challenges faced by traditional fracturing fluids in high-temperature, high-shear environments and reservoir damage by fabricating a new Zr-N-SiO2-modified water-based crosslinker and systematically evaluating its performance in CBM fracturing. Specifically, it explores the following: (1) synthesis and structural characterization of Zr-N-SiO2 crosslinkers; (2) the temperature resistance and rheological properties of fracturing fluid systems under different temperatures and shear conditions, as well as gel breakage time; (3) mechanisms by which the Zr-N-SiO2 hybrid crosslinked network achieves excellent thermal and shear stability. Ultimately, it seeks to provide a high-performance, low-damage, eco-friendly water-based fracturing fluid system for efficient CBM extraction, supporting unconventional oil and gas development with significant theoretical and practical value.
2. Experimental Section
2.1. Materials
Sodium silicate (analytical reagent, AR), sodium hydroxide (NaOH, analytical reagent, AR), γ-aminopropyltrimethoxysilane (analytical reagent, AR), and zirconium oxychloride (analytical reagent, AR) were purchased from Shanghai Aladdin Co., Ltd. (Shanghai, China) Hydrochloric acid (35.5 wt%) and xylene (analytical reagent, AR) were obtained from Shanghai Sinopharm Co., Ltd. (Shanghai, China) Ammonium persulfate ((NH4)2S2O8, 99% purity) was supplied by Shanghai Merck Co., Ltd. (Shanghai, China) Anhydrous ethanol was purchased from Hunan Huihong Reagent Co., Ltd. (Changsha, China) Anionic polyacrylamide (HPAM, 95% purity, hydrolysis degree 35–40%, molecular weight 15,000) was obtained from Shanghai An naiji Co., Ltd. (Shanghai, China) Deionized water was prepared in the laboratory
2.2. Preparation of Zr-N-SiO2
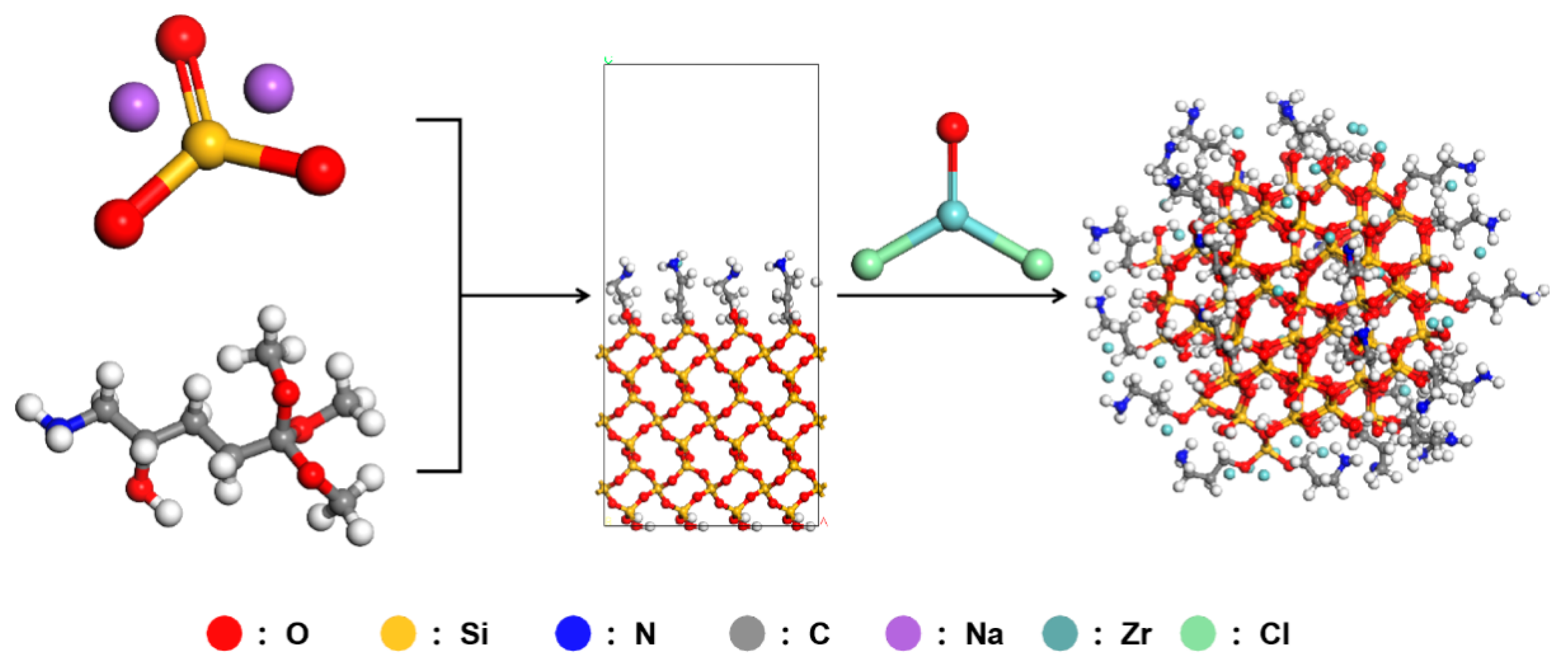
First, 50 g of a 35 wt% sodium silicate solution was prepared and transferred to a four-necked flask containing 100 mL of a deionized water–anhydrous ethanol mixture (volume ratio 4:6) along with 5 g of KH-551. Simultaneously, hydrochloric acid solution was slowly added dropwise to adjust the system pH to 4–5, and the reaction was incubated at 80 °C for 4 h. After washing, nano-silica particles with hydroxyl- and amino-rich surfaces were obtained.
In the subsequent step for preparing the Zr-N-SiO
2 crosslinker, 5 g of zirconium oxychloride, 15 g of the previously prepared nano-silica particles, and 50 mL of xylene were sequentially added to a 100 mL flask. After accurately weighing and adding the above materials, the system was stirred until uniformly mixed. The pH of the system was adjusted to an acidic range using hydrochloric acid solution, followed by a reaction at 100 °C for 5 h. After the reaction, the white solid was separated by vacuum filtration using a 0.22 μm polytetrafluoroethylene (PTFE) filter membrane, utilizing the low surface tension of xylene (28.8 mN/m) to minimize filter cake entrainment. Subsequently, the filter cake was ultrasonically dispersed and washed with 50 mL of anhydrous ethanol for 5 min, repeated three times, using the miscibility of ethanol and xylene to displace residual organic solvents. Finally, the product was dried in a vacuum oven at 60 °C and −0.1 MPa for 12 h; the low boiling point (138 °C) and high volatility of xylene ensured the solvent residual rate was controlled below 0.1%. The resulting white granular solid product was named Zr-N-SiO
2 (nano-zirconium-doped silica) crosslinker (yield 92%). The synthesis process and chemical characterization are shown in
Figure 1.
2.3. Preparation of Zr-N-SiO2 Fracturing Fluid
In accordance with the standard SY/T 5107-2016 [
31] Evaluation Methods for Performance of Water-Based Fracturing Fluids, 0.3 g of HPAM was weighed and added to 99.7 g of deionized water to prepare a 0.3% HPAM solution. A high-speed stirrer was used to rapidly stir the deionized water, while the weighed HPAM was slowly added. After uniform dissolution, the 0.3% HPAM solution was placed in an oven and swollen at a constant temperature of 35 °C for 2 h. The swollen HPAM solution was subjected to high-speed stirring, and 0.02~1.0 wt% of Zr-N-SiO
2 crosslinker was weighed and slowly added dropwise to the stirring HPAM solution. Stirring continued until the vortex liquid surface bulged and climbed the stirrer rod, at which point crosslinking was deemed successful. The resulting water-based fracturing fluid was named Zr-N-SiO
2 fracturing fluid.
2.4. Characterization and Testing
2.4.1. Structural Characterization of Zr-N-SiO2 Nano-Crosslinker
The characterization of Zr-N-SiO2 nano-crosslinker involves the analysis of morphology, particle size, and element distribution: its microstructure was observed using a scanning electron microscope (SEM, Japan Hitachi SU8220, Hitachi High-Tech Corporation, Minato-ku, Tokyo) to clarify the morphological characteristics. Particle size data were determined by a nanoparticle size and zeta potential analyzer (United Kingdom Zetasizer Nano ZS, Malvern Panalytical Ltd, Malvern, UK). Element distribution was obtained through combined analysis using a scanning electron microscope (SEM, Hitachi SU8220) and an X-ray energy dispersive spectrometer (EDS, Japan Hitachi Zeiss Quantax75).
2.4.2. Rheological Performance Test
A high-temperature rheometer was used to conduct rheological experiments on the Zr-N-SiO2 fracturing fluid to obtain its rheological parameters. In the experiments, constant shear and variable shear tests were performed on the prepared fracturing fluid at shear rates of 170 s−1 and 510 s−1, respectively, thereby obtaining the rheological characteristic curves of the fracturing fluid. Meanwhile, different temperatures (30 °C, 60 °C, 90 °C, 120 °C, 150 °C, 180 °C) were set to test the performance of the Zr-N-SiO2 fracturing fluid. In addition, ammonium persulfate was used as the gel breaker to systematically investigate the gel-breaking performance of the fracturing fluid under different breaker contents.
3. Results and Discussion
3.1. Structural Analysis of Zr-N-SiO2 Nano-Crosslinker
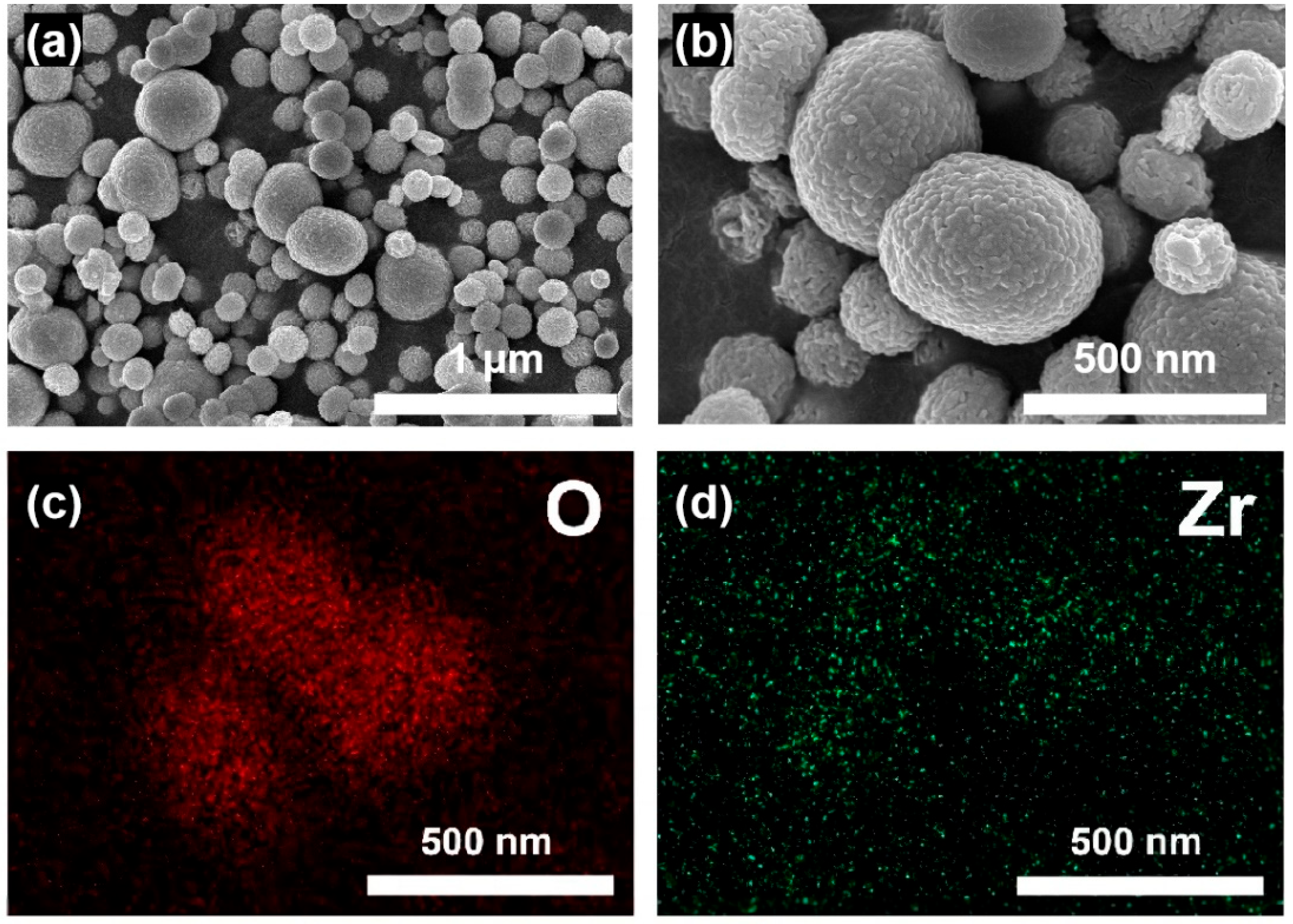
In this study, scanning electron microscopy (SEM) was used to observe the microstructure of the material, and energy dispersive spectroscopy (EDS) was employed to confirm the elemental distribution of the sample, thereby clarifying the surface morphology and zirconium ion loading state of the Zr-N-SiO
2 nano-crosslinker (
Figure 2).
Figure 2a,b clearly show the uniform and regular spherical or near-spherical nanoparticle microstructure of the Zr-N-SiO
2 nano-crosslinker. These particles are all at the nanoscale, and no obvious agglomeration was observed in the images. Their excellent dispersibility may be attributed to the surface modification process. During preparation, we modified the surface of nano-silica using an amino-containing silane coupling agent (e.g., γ-aminopropyltrimethoxysilane). The modification process introduces amino functional groups on the particle surface to increase steric hindrance between nanoparticles, thereby effectively inhibiting the agglomeration caused by high specific surface area and surface energy; the EDS elemental mapping in
Figure 2d further confirms that zirconium has been successfully loaded onto the surface of silica nanospheres. Furthermore, Fourier transform infrared (FTIR) spectroscopy analysis (
Figure S1) further confirmed the successful preparation of the Zr-N-SiO
2 crosslinker from the perspective of functional groups: the absorption peak at 1764 cm
−1 is assigned to the deformation vibration of —NH groups, the absorption peaks around 935 cm
−1 and 1169 cm
−1 correspond to the stretching vibrations of Si-O-Si bonds, and the broad absorption peaks in the range of 3075~3556 cm
−1 are characteristic of —OH groups, which confirm the successful modification of nano-SiO
2 by γ-aminopropyltrimethoxysilane; the absorption peak at 598 cm
−1 arises from the stretching vibration of Zr-O bonds, indicating the successful doping of Zr ions into nano-SiO
2.
This well-dispersed nanoparticle morphology is crucial for materials used as fracturing fluid crosslinkers. Uniformly dispersed nanoparticles can provide a large number of active crosslinking sites in HPAM polymer solutions, ensuring the uniform progress of crosslinking reactions. This avoids “over-crosslinking” caused by excessive local crosslinker concentration and uneven gel networks, thereby forming a stronger, more stable, and more uniform three-dimensional crosslinked network. Therefore, the excellent dispersibility shown in the SEM images is an important prerequisite for the Zr-N-SiO
2 crosslinker to endow the fracturing fluid with excellent rheological properties and long-term stability. As shown in
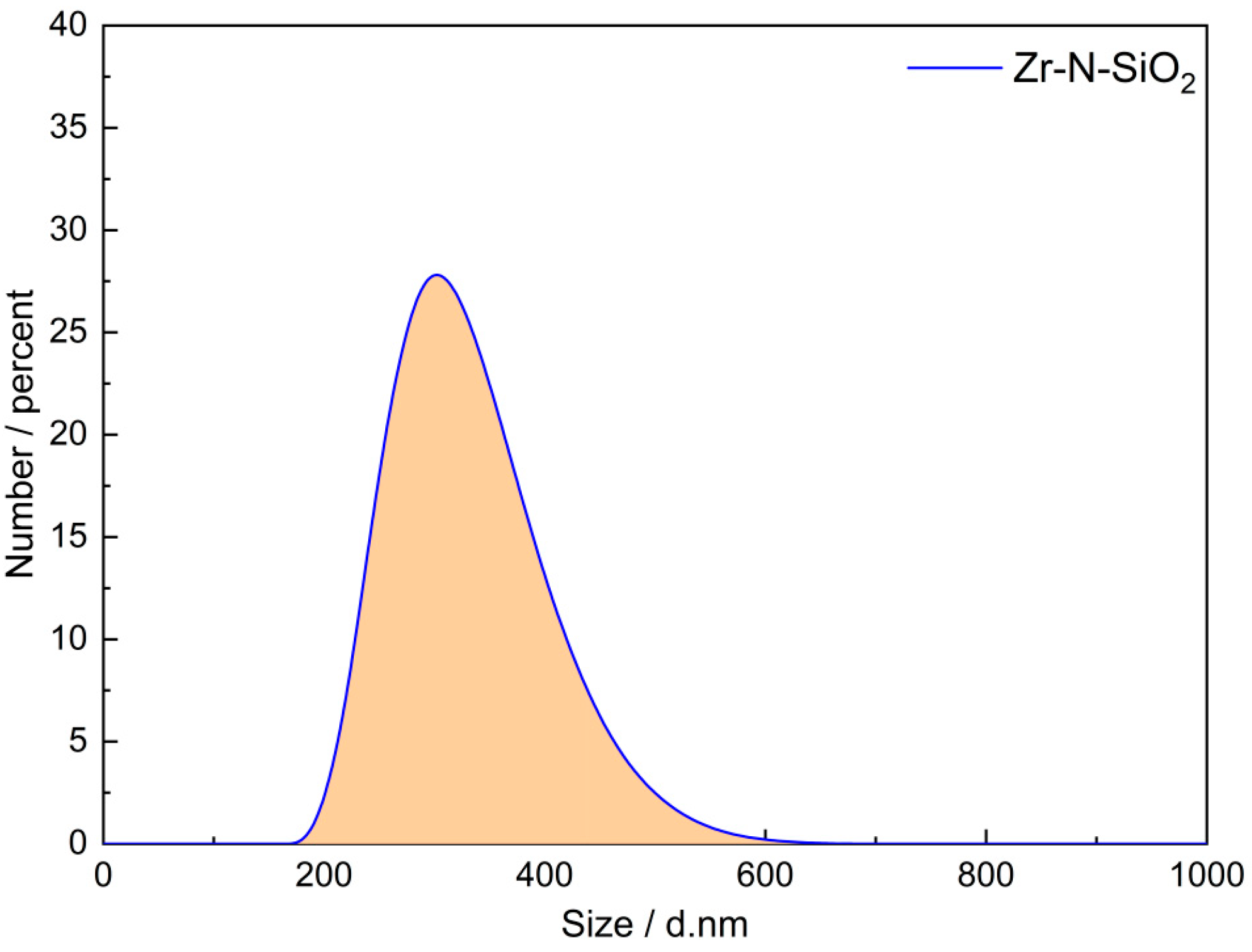
Figure 3, the particle size of the Zr-N-SiO
2 nano-crosslinking agent we prepared ranges from 200 to 600 nm, with an average particle size of approximately 300 nm. The small particle size enables more sites for crosslinking with HPAM in a limited space. Although Zr-N-SiO
2 is an inorganic nanoparticle (with core components of SiO
2 and ZrO
2), it has a controllable particle size (average 300 nm,
Figure 3) and a density (2.2 g/cm
3) slightly higher than that of water. Its settling velocity in static water is approximately 0.5 mm/min, making it unlikely to suspend and migrate for a long time. Meanwhile, the amino functional groups on its surface enable efficient removal via coagulation precipitation (e.g., adding polyaluminum chloride); laboratory simulation experiments on flowback fluid treatment show that after the coagulation–filtration process, the removal rate of Zr-N-SiO
2 in water reaches 98.5%, with a residual concentration < 0.1 mg/L, which meets the limit requirements for “specific items of centralized drinking water sources” specified in the
Environmental Quality Standard for Surface Water (GB 3838-2002) [
32].
3.2. Fracturing Fluid Crosslinking Agent–Thickening Agent Ratio Analysis
As demonstrated in previous studies, there is a close correlation between the mass ratio of the crosslinking agent (Zr-N-SiO
2) to the thickening agent (HPAM) and the performance of the fracturing fluid [
21]. As demonstrated in the accompanying figure, under constant temperature (30 °C) and constant shear rate (170 s
−1) conditions, the concentrations of Zr-N-SiO
2 and HPAM were examined in isolation. As illustrated in
Figure 4a, when the Zr-N-SiO
2 crosslinking agent concentration is fixed at 0.4 wt%, the critical association concentration of HPAM is determined by varying the HPAM dosage. This confirms the HPAM dosage in the fracturing fluid. It is evident from
Figure 4a that the viscosity of the fracturing fluid increases in proportion to the HPAM concentration. When the HPAM dosage was 0.26 wt%, the first “tangent intersection” appeared in the system viscosity tangent, primarily attributed to the transition of HPAM from intramolecular crosslinking to intermolecular crosslinking. Consequently, the growth trend of system viscosity decelerated. When the HPAM dosage was 0.4 wt%, the second “tangent intersection” appeared, indicating the transition of HPAM from intermolecular crosslinking to gelation.
In the context of HPAM thickener concentration levels of 0.4 wt%, temperature settings of 30 °C, and shear rates of 170 s
−1, the growth rate of fracturing fluid viscosity exhibited a tendency to increase rapidly, followed by a subsequent gradual rise in conjunction with the augmentation of the mass fraction of Zr-N-SiO
2 crosslinking agent, as depicted in
Figure 4b. At low concentrations (0.02–0.6 wt%), with relatively low crosslinker content, increasing concentration introduces more Zr
4+ coordination sites, which “bridge” adjacent HPAM chains to form a 3D network, significantly boosting viscosity. Here, crosslinkers are highly utilized, driving gel network formation. At 0.8 wt% crosslinker, viscosity change slows, indicating optimal crosslinking degree and gel strength [
22,
25]. This ratio balances Zr-N-SiO
2 nanoparticles with HPAM carboxyl groups, enabling each particle to efficiently bind multiple HPAM segments, forming a tight, stable network with high viscosity and strong proppant-carrying capacity. Above 0.8 wt%, viscosity decreases due to “over-crosslinking”: excess nanoparticles preferentially coordinate with different carboxyls on the same HPAM chain, causing chain curling into microgel agglomerates, restricting intermolecular crosslinking, and reducing efficiency via particle agglomeration.
Thus, the optimal formulation is 0.8 wt% Zr-N-SiO2 + 0.4 wt% HPAM, ensuring sand-carrying capacity while avoiding viscosity loss from over-crosslinking.
3.3. Shear Resistance of Zr-N-SiO2 Fracturing Fluid
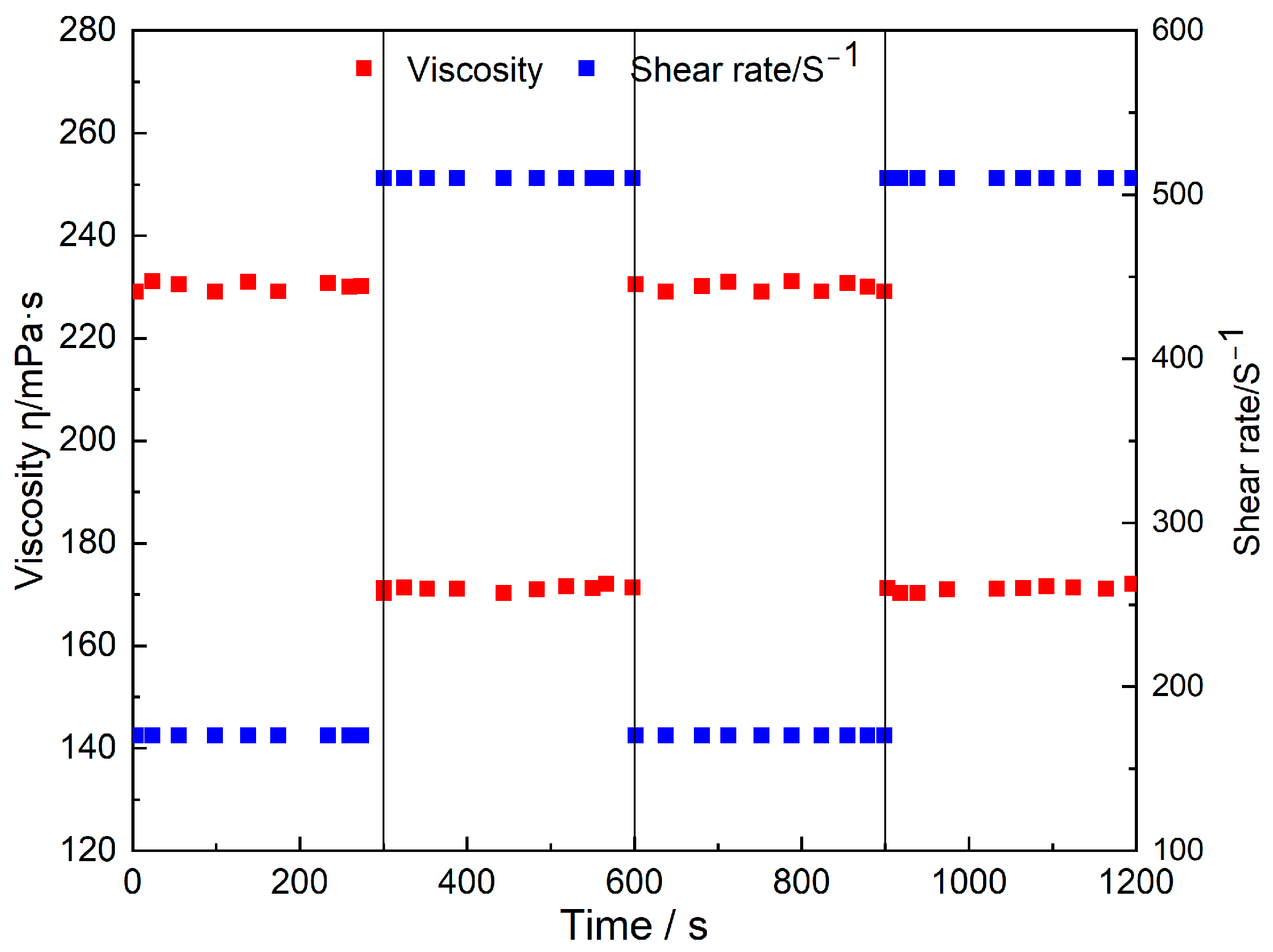
Fracturing fluids experience varying shear rate environments in wellbores, perforation holes, and fractures during actual construction. Therefore, evaluating the viscosity response and recovery ability of fracturing fluids under dynamic shearing is a key indicator to measure their sand-carrying stability and proppant placement efficiency in fractures. In this study, cyclic shear recovery tests were performed on the prepared Zr-N-SiO
2 fracturing fluid at 30 °C (
Figure 5).
As clearly observed in
Figure 5, the fracturing fluid exhibits excellent dynamic response and rapid recovery characteristics during multiple cycles of alternating shear rates (170 s
−1 and 510 s
−1). When the shear rate increases from a low shear rate (170 s
−1) to a high shear rate (510 s
−1), the viscosity of the fracturing fluid decreases from 229 mPa·s to 172 mPa·s; when the shear rate returns to a low value, its viscosity can quickly rise back to near the initial level in a very short time. This situation can be found by carefully observing the viscosity curve and shear rate. Throughout the test, the viscosity change curve shows a stable cyclic pattern, indicating that the gel network structure does not suffer significant permanent damage under repeated shearing.
This excellent dynamic shear recovery performance can be explained in depth by the unique crosslinking mechanism of the fracturing fluid. The system is not formed by irreversible covalent bonds but relies on coordination bonds formed between Zr4+ ions on Zr-N-SiO2 nanoparticles and carboxyl groups (-COO−) on HPAM polymer chains. This coordination bond has typical dynamic reversibility. Under high-speed shear force, some crosslinking coordination bonds break due to mechanical stress, causing HPAM molecular chains to orient along the shear direction, and the gel network structure temporarily disintegrates, which macroscopically manifests as a significant decrease in viscosity, which is beneficial for the fluid to pass through high-shear areas (such as perforation holes). When the fluid enters the main fracture and the shear rate decreases, the broken coordination bonds can quickly re-form under the action of molecular thermal motion and the strong affinity between Zr4+ and -COO− groups, allowing the gel network to be rapidly reconstructed. It is worth emphasizing that as a nanoscale crosslinker, Zr-N-SiO2’s multi-site crosslinking characteristics further enhance the toughness and recovery ability of the gel network. Compared with small-molecule crosslinkers, nanoparticles can crosslink with multiple HPAM segments at the same time, forming a more stable three-dimensional network structure. The existence of these multiple connection points ensures that the gel network can still maintain a certain degree of connection even after some crosslinking bonds are broken, avoiding complete collapse of the system and providing a structural guarantee for rapid viscosity recovery. This excellent cyclic shear recovery ability ensures the construction stability of the fracturing fluid in complex formations, which can effectively prevent proppant settlement due to instantaneous viscosity reduction, thereby ensuring uniform placement of proppant in fractures, which is a key technical advantage for efficient CBM extraction.
3.4. Analysis of Rheological Properties of Zr-N-SiO2 Fracturing Fluid
To deeply analyze the structural characteristics of the prepared Zr-N-SiO2 fracturing fluid from the perspective of micro-rheology, viscoelasticity tests were performed, and the results are shown in the figures. Viscoelastic data are key indicators for evaluating the strength, stability, and dynamic response behavior of crosslinked systems.
The storage modulus (G′) reflects the ability of the HPAM crosslinked network in the fracturing fluid to store and release elastic energy, representing its elastic (solid-like) behavior, and the loss modulus (G’’) reflects the energy dissipation trend of the HPAM crosslinked network in the fracturing fluid during flow, representing its viscous (liquid-like) behavior.
Figure 6a shows the variation trends of the above two moduli with angular frequency at different temperatures. It can be clearly observed from the figure that G′ is consistently greater than G′′ across the entire test frequency range, indicating that the Zr-N-SiO
2 fracturing fluid is not a simple viscous fluid but a crosslinked gel system with significant elastic characteristics. G′ > G′′ is a classic criterion for judging the formation of a stable crosslinked network (i.e., a “true gel”) [
25]. In the low-frequency region, both G′ and G’’ change relatively gently, and the value of G′ is higher than that of G’’. This result confirms that the prepared Zr-N-SiO
2 fracturing fluid has an excellent viscoelastic crosslinked network. The elasticity of this network mainly stems from the conformational elasticity generated by HPAM polymer chains through physical anchoring and chemical coordination with the nano-crosslinker Zr-N-SiO
2, a structure that can effectively resist external stress and provide strong suspension force for proppants. This result is highly consistent with the macroscopic shear recovery performance observed in
Figure 5: when the shear rate changes, the gel network undergoes partial disintegration (G′′ increases), but due to its dominant strong elastic recovery force (G′ prevails), the network can rapidly reconstruct once the shear force weakens, macroscopically manifesting as rapid viscosity recovery. Additionally, G′ remains greater than G′′ at 180 °C, indicating that the system can still maintain a robust three-dimensional crosslinked network at high temperatures.
Figure 6b is used to determine the viscoelasticity of the fracturing fluid and the yield point of the crosslinked network structure and can evaluate its structural stability under different stress environments. This figure shows the trend relationship between G’ and G’’. G′ and G′′ remain essentially constant at low strain, and the Zr-N-SiO
2 fracturing fluid exhibits a high yield strain, benefiting from its unique nano-crosslinking mechanism: Zr-N-SiO
2 nanoparticles, as multi-site crosslinking cores, coordinate with multiple carboxyl groups on HPAM chains to form a more stable network than small-molecule crosslinkers. This multi-site anchoring endows the gel network with higher toughness and deformation resistance when subjected to external stress, ensuring that its gel structure remains intact even in complex stress environments such as high-intensity fluid shear and proppant particle collision in wellbores and fractures, thus guaranteeing stable transport of proppants.
3.5. Temperature Resistance and Shear Resistance
Deep coalbed methane reservoirs are typically characterized by high-temperature and high-shear stress environments, requiring fracturing fluids to maintain stable high viscosity throughout the construction period (usually several hours) to ensure effective transport of proppants to deep fractures.
Figure 7a shows that the viscosity curves of fracturing fluids exhibit shear thinning behavior as the shear rate increases. This behavior is crucial in coalbed methane extraction: it ensures that the fracturing fluid maintains low viscosity when passing through wellbores and high-shear surface equipment, thereby reducing pumping resistance and energy consumption. Further analysis indicates that as the ambient temperature rises from 30 °C (room temperature) to 180 °C, the viscosity of the fracturing fluid decreases with increasing temperature. This is because higher temperatures enhance the activity of polymer chains, leading to an increase in molecular chain disturbance angles and disturbance indices. It is worth noting that even at high temperatures of 180 °C, the viscosity remains significantly higher than that of the base fluid. This demonstrates that the prepared Zr-N-SiO
2 nano-crosslinker has excellent high-temperature resistance and can still maintain crosslinking under high-temperature conditions. This confirms the system’s excellent temperature resistance, attributed to the inorganic nano-framework of Zr-N-SiO
2 crosslinkers (which exhibit superior thermal stability) and the stable coordination bonds between Zr
4+ and HPAM (which are resistant to cleavage or degradation at high temperatures). This heat resistance ensures sufficient sand-carrying capacity in deep high-temperature formations, representing a key advantage over traditional organic crosslinker systems.
Figure 7b results indicate that viscosity remains highly stable with almost no attenuation over the 600 s test period, fully demonstrating the crosslinked system’s exceptional resistance to mechanical shear degradation. Shear stability is a critical indicator of fracturing fluid reliability: traditional polymer chains are prone to mechanical scission under continuous high shear, leading to molecular weight reduction and permanent viscosity loss. In contrast, the crosslinked network of the prepared fluid is centered on Zr-N-SiO
2 nanoparticles, which act as robust multi-site crosslinking cores to effectively anchor HPAM chains within the spatial network. Under sustained shear, although coordination bonds may undergo dynamic breakage and reformation, the physical stability of the nanoparticle framework prevents network destruction, ensuring long-term stability of macroscopic viscosity. These characteristics collectively guarantee the fluid’s reliability in deep high-shear and high-temperature environments, providing strong fluid support for efficient coalbed methane extraction.
3.6. Analysis of Long-Term High-Temperature Stability of Fracturing Fluid
Deep geological reservoirs typically exhibit extremely high temperatures, imposing strict requirements on the high-temperature stability of fracturing fluids. Such fluids must not only resist high temperatures but also maintain viscosity over an extended period to ensure sand-carrying capacity throughout the construction process.
The results in
Figure 8a show that under the condition of 90 °C, after the fracturing fluid is heated for 18 min and stabilized at 90 °C, its viscosity begins to maintain a relatively stable state, with a very small degree of viscosity attenuation, indicating a robust crosslinked network formed by Zr-N-SiO
2 and HPAM. While 90 °C poses a challenge for many traditional crosslinked systems (e.g., guar gum), it falls within the optimal performance range of the developed fluid, where crosslinking bonds are not significantly affected by thermal degradation, fully demonstrating excellent temperature resistance. The results in
Figure 8b show that during the temperature rise to 120 °C, the initial viscosity of the fracturing fluid remains consistent with that at 90 °C, and after the temperature stabilizes at 120 °C, its viscosity attenuation is still very slow. This confirms that even at 120 °C, the crosslinked network can effectively resist thermal damage. The inorganic framework of the nano-crosslinker and stable Zr-O coordination bonds maintain structural integrity at this temperature, ensuring continuous sand-carrying capacity, making it suitable for medium–high-temperature reservoirs. At 150 °C (
Figure 8c), viscosity attenuation became more pronounced (approximately 60 mPa·s), with reduced initial viscosity and a steeper curve slope, indicating compromised network stability under high temperatures. Increased thermal energy caused partial weak crosslinks to break, leading to gradual viscosity decline. Notably, viscosity decays slowly rather than failing instantly, indicating the crosslinked structure retains effective working capacity. At an extreme 180 °C (
Figure 8d), viscosity decreased rapidly and significantly, suggesting the system approached its thermal stability limit. Intense heat not only accelerated coordination bond cleavage but may also induce thermal degradation of the HPAM main chain, causing a sharp drop in molecular weight and rapid collapse of the gel network, though viscosity remained at approximately 140 mPa·s.
In summary, the Zr-N-SiO
2 fracturing fluid exhibits excellent high-temperature stability: it maintains stable viscosity for a long time below 120 °C, still has a working window at 150 °C, and retains a viscosity of ~140 mPa·s at 180 °C. Its performance stems from the physical stability of the inorganic nano-framework and the strong Zr
4+-HPAM coordination bonds, making it an ideal choice for fracturing operations in medium-temperature, high-temperature, and even some ultra-high-temperature coalbed methane reservoirs. This is further confirmed by comparative tests with commercial organic zirconium crosslinkers (
Figure S2): under the temperature and shear resistance conditions of 0–150 °C and 170 s
−1, the Zr-N-SiO
2 crosslinker shows significantly better high-temperature resistance with its viscosity stably maintained at around 150 mPa·s, while the viscosity of the commercial organic zirconium crosslinker drops below the requirement of ≥50 mPa·s specified in the SY/T 5107-2016 standard after only about 40 min at 150 °C. Further proppant-carrying performance tests have also verified its advantages in engineering applications: using 20/40 mesh ceramsite (density: 2.65 g/cm
3) as the standard proppant, the settling behavior of ceramsite in the fracturing fluid was determined by the static suspension method, and the proppant settling velocity and sand bed concentration of the Zr-N-SiO
2 crosslinked system and the commercial organic zirconium system were compared under the conditions of 170 s
−1 shear rate and 150 °C. The results (
Table S1) showed that due to the stable viscosity of approximately 150 mPa·s maintained in the system of this study, the proppant settling velocity was only 0.8 mm/min (the settling velocity of the commercial system reached 3.2 mm/min when its viscosity dropped to 50 mPa·s), and the sand bed concentration (proppant volume fraction) was 150% higher than that of the commercial system. This result is directly related to the aforementioned stable high-temperature viscosity performance, confirming that the stable high viscosity can effectively delay proppant settling, meeting the field requirements for long-distance proppant transport in deep well fracturing.
3.7. Analysis of the Controllability of Zr-N-SiO2 Fracturing Fluid Gel Breakdown
The controllability of gel breakage in fracturing fluids is one of the key indicators for evaluating their efficiency and environmental performance in practical applications [
33,
34]. After coalbed methane fracturing operations, the recovered fracturing fluid must be able to achieve maximum gel breakage to minimize damage to the underground environment and ensure smooth flow of underground oil and gas. To assess the controllability of gel breakdown in Zr-N-SiO
2 fracturing fluid, this study added 0.02–0.10 wt% (NH
4)
2S
2O
8 to the stably crosslinked fracturing fluid and observed changes in fluid viscosity with gel-breaking agent concentration and gel breakdown time.
As demonstrated in
Figure 7b, the viscosity of the fracturing fluid without the incorporation of a gel breaker exhibits a notable stability during the shear process. However, upon the addition of (NH
4)
2S
2O
8, there is a rapid and significant decrease in the viscosity of the fracturing fluid over time. Furthermore, the rate of viscosity decrease exhibits a positive correlation with the amount of gel breaker added, as illustrated in
Figure 9. When the (NH
4)
2S
2O
8 concentration is below 0.06 wt%, the viscosity of the fracturing fluid does not decrease to below the industry standard requirement of 5 mPa·s within 8 h (480 min). This finding suggests that, at these concentrations, the gel-breaking reaction rate is inadequate to achieve effective gel breaking within an acceptable construction timeframe. When the (NH
4)
2S
2O
8 concentration exceeds 0.08 wt%, the viscosity of the fracturing fluid decreases to 2.91 mPa·s within 480 min, far below the national standard requirement of 5.0 mPa·s. When the (NH
4)
2S
2O
8 concentration is 0.10 wt%, the gel-breaking rate accelerates further, with the viscosity dropping to 2.762 mPa·s within 400 min.
The controllability of the fracturing fluid’s gel-breaking performance stems from the precise oxidative degradation of the crosslinked network by ammonium persulfate, and the gel-breaking experiments strictly controlled the temperature at 120–180 °C (simulating different deep well fracturing temperature environments); the decomposition rate of ammonium persulfate and the generation efficiency of sulfate radical anions (SO4−) are significantly temperature-dependent: the decomposition half-life shortens by approximately 50% for every 20 °C increase in temperature, directly determining the gel-breaking reaction kinetics. Specifically, (NH4)2S2O8 decomposes at 120–180 °C to generate strongly oxidizing species such as SO4−·, which attack the C–C bonds and amide groups of HPAM molecular chains, triggering main chain scission, reducing molecular weight, and disrupting the crosslinked network, macroscopically manifested as a sharp viscosity drop. Within the set temperature range, when the (NH4)2S2O8 concentration is below 0.08 wt%, even at the maximum temperature of 180 °C, the sulfate radical generation is insufficient (<0.005 mmol·L−1·min−1), failing to rapidly destroy the stable three-dimensional crosslinked network constructed by Zr-N-SiO2, resulting in low gel-breaking efficiency. When the concentration is ≥0.08 wt%, temperature and concentration exhibit a synergistic effect; for example, at 150 °C, the radical generation rate corresponding to this concentration reaches 0.012 mmol·L−1·min−1, enabling the system viscosity to drop from 150 mPa·s to below 5 mPa·s within 30 min, while at 120 °C with the same concentration, 60 min are required to achieve the same gel-breaking effect. Therefore, precise control of both ammonium persulfate dosage and reaction temperature allows for dual accurate regulation of the gel-breaking reaction rate.
3.8. Mechanism Verification of Zr-N-SiO2 Fracturing Fluid
The formation mechanism of coordination bonds between HPAM ligands and zirconium ions is as follows: the anionic groups in the ligands achieve coordination chelation by occupying the coordination sites of zirconium ions. During the crosslinking reaction, the carboxylate ions dissociated from the HPAM thickener competitively combine with the zirconium ions on the surface of Zr-N-SiO2, promoting the gradual release of coordination sites of zirconium ions on the surface of Zr-N-SiO2, thereby effectively regulating the crosslinking rate between Zr-N-SiO2 nano-crosslinkers and HPAM. Based on the above reaction mechanism, the preparation process and core principles of Zr-N-SiO2 nano-crosslinkers can be elaborated in detail as follows:
- (1)
- (2)
Hydrolysis reaction under acidic conditions
- (3)
Formation of hydroxyl bridge bonds (μ-OH)
Through hydroxyl bridging (olation reaction), two [Zr(OH)(OH
2)
5]
3+ condense to form a dinuclear structure. In this step, water molecules are released, and stable Zr-OH-Zr bridge bonds are formed:
Based on existing data, molecular models of Zr-N-SiO
2 nanocluster crosslinkers and HPAM thickeners were constructed using the Materials Studio (MS) software package, followed by energy minimization, structural optimization, and molecular dynamics simulations. The silica unit cell was obtained from the American Mineral Crystal Structure Database (AMCSD) and optimized using the COMPASS force field. The optimized silica unit cell was then used to construct nanoclusters, resulting in Zr-N-SiO
2 nanocluster crosslinkers with a diameter of 20.00 Å (
Figure 10).
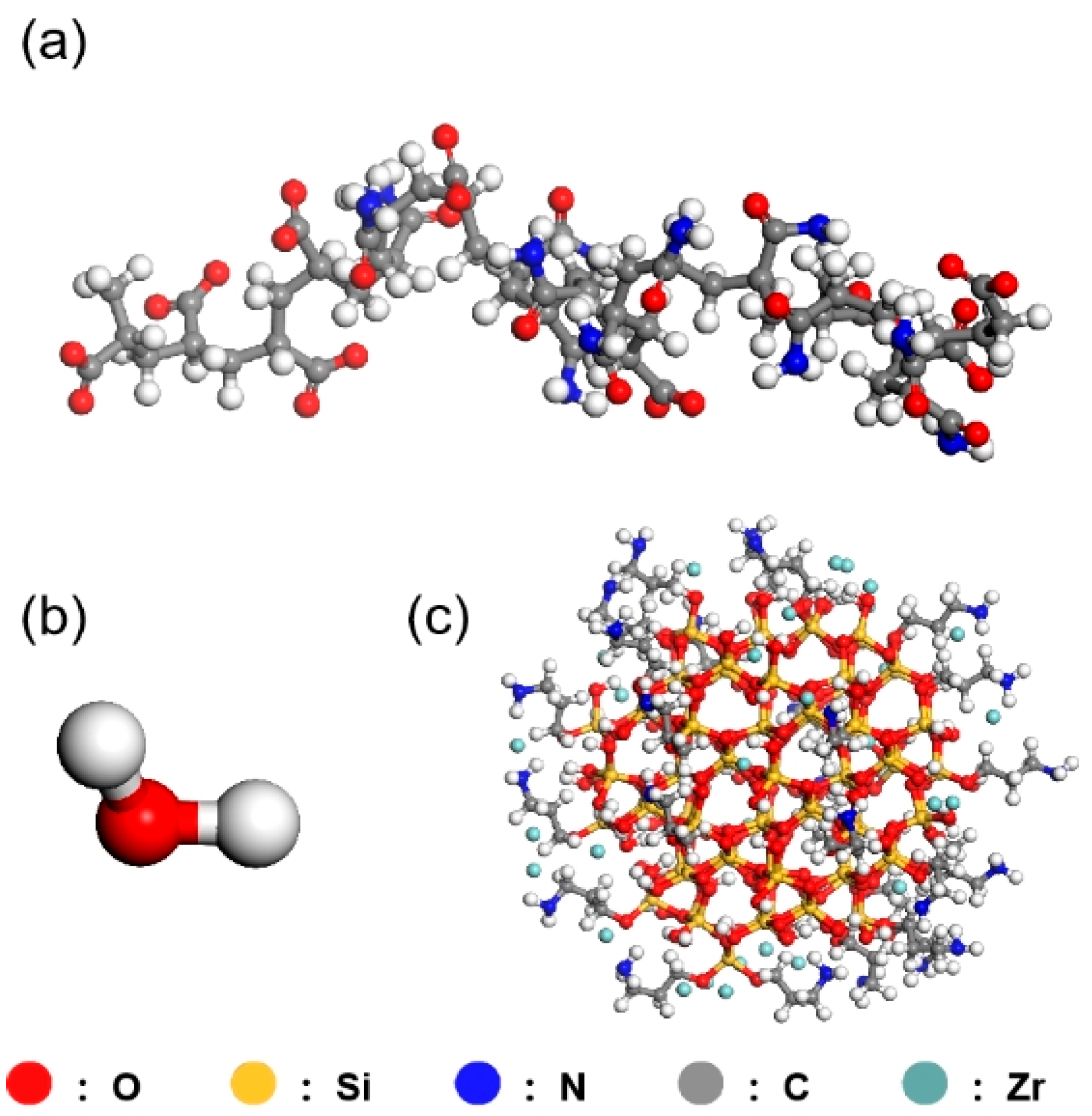
To investigate the interaction between the HPAM thickener and Zr-N-SiO
2 nanocluster crosslinker in an aqueous environment, molecular dynamics simulations were performed using Materials Studio 2023 software: 10 HPAM chains, 1 Zr-N-SiO
2 nanocluster with a diameter of 20.00 angstroms, and 600 water molecules were placed in a cubic simulation box with x = y = z = 10 nm. Periodic boundary conditions were applied along the x, y, and z directions, and the COMPASSⅢ force field was used for all molecular models. The simulation first completed energy minimization via 5000 steps of the steepest descent method and 10,000 steps of the conjugate gradient method and then equilibrated for 100 ps under the NVT ensemble at 303.15 K using the Berendsen thermostat (relaxation time 0.1 ps); finally, a 200 ps production simulation was conducted with a time step of 1 fs (trajectories saved every 1 ps). The initial distribution model (
Figure 11) obtained after equilibration showed that HPAM existed in a random coil shape and Zr-N-SiO
2 was uniformly dispersed, and the model validity was verified by energy fluctuation (<5 kJ/mol) and density stability (1.00 ± 0.02 g/cm
3).
Molecular dynamics simulations were used to study the crosslinking behavior of Zr-N-SiO
2 fracturing fluids at different temperatures, essentially investigating the promotion effect of Zr-N-SiO
2 nanocluster crosslinkers on HPAM crosslinking in water under varying temperatures.
Figure 12 illustrates the structural evolution of Zr-N-SiO
2 fracturing fluids with increasing temperature: when the temperature rises from 303.15 K to 333.15 K, under zero-shear conditions, the density of the HPAM crosslinking network decreases slightly, which is related to the incomplete dissociation of Zr
4+ in the Zr-N-SiO
2 nanocluster crosslinker (
Figure 12a,b). At 363.15 K (
Figure 12c), HPAM tends to aggregate toward Zr-N-SiO
2 nanoclusters due to further dissociation of Zr
4+ and enhanced coordination chelation between carboxylate ions in HPAM and surface Zr
4+ of nanoclusters induced by elevated temperature. At 453.15 K, thermal perturbation disrupts the coordination network, leading to gradual disentanglement and dispersion of the coordination chelation network. However, due to the high stability of Zr
4+ coordination bonds, the dissociation degree of HPAM enriched near Zr-N-SiO
2 nanoclusters remains low. These molecular dynamics simulation results regarding HPAM crosslinking promoted by Zr-N-SiO
2 nanocluster crosslinkers at different temperatures further confirm the high-temperature resistance of Zr-N-SiO
2.
This study conducted molecular dynamics simulations based on the NVT ensemble (using the Berendsen temperature control method) at 303.15 K, 333.15 K, and 363.15 K, respectively, with a shear rate of 0.17 ps
−1 and a time step of 50 fs. The structural evolution is shown in
Figure 13. At 303.15 K, HPAM exhibits a certain degree of crosslinking at the initial stage of shearing. As shearing proceeds, partial dissociation of the HPAM crosslinked network is observed at 3 × 10
4 ps and 5 × 10
6 ps, showing a disordered and independent state. However, the HPAM crosslinked network near the Zr-N-SiO
2 nanoclusters remains stable. A similar phenomenon is observed at 333.15 K: the HPAM crosslinked network gradually dissociates with shearing, while the crosslinked network near the nanoclusters remains stable, with a larger stable range compared to that at 303.15 K. This is attributed to the elevated temperature promoting further dissociation of Zr
4+ and enhancing the coordination chelation between carboxylate ions in HPAM and surface Zr
4+ of the nanoclusters. When the temperature rises to 363.15 K, it is evident that the HPAM crosslinked network does not undergo extensive dissociation within 5 × 10
6 ps, maintaining overall stability. This is because the dissociation of Zr
4+ on the Zr-N-SiO
2 nanoclusters forms multi-point coordination chelation with HPAM, effectively resisting shear forces and ensuring the shear resistance of the fracturing fluid under high-temperature conditions. The above molecular dynamics shear simulation results are consistent with previous experimental conclusions, further confirming that Zr-N-SiO
2 fracturing fluid still has good proppant-carrying capacity under high-temperature conditions.
4. Conclusions
The present study successfully prepared a Zr-N-SiO2-modified water-based crosslinking agent, using silane coupling agent-modified nano-silica as the inorganic skeleton and combining zirconium ions. The crosslinking agent is composed of spherical nanoparticles with a size range of 200–600 nm and an average size of 300 nm, which are highly dispersible. The Zr-N-SiO2 nano-crosslinker has a stable reaction yield of 85–90% and cost advantages over commercial organic zirconium crosslinkers; potential challenges in large-scale synthesis such as mass transfer inhomogeneity, continuous production, and separation efficiency can be effectively addressed via combined processes including composite stirring, continuous flow reaction, and membrane filtration, exhibiting good industrialization feasibility.
Through a process of formulation optimization, it was determined that a fracturing fluid system composed of 0.8 wt% Zr-N-SiO2 and 0.4 wt% HPAM thickener exhibits optimal performance. Performance tests indicate that this fracturing fluid system exhibits outstanding comprehensive performance. Under cyclic shear conditions, viscosity can rapidly recover, demonstrating excellent dynamic shear stability. At high temperatures of 180 °C, it maintains a viscosity of over 140 mPa·s and remains stable over the long term within the 90–180 °C temperature range, offering a wide temperature operating window. The gel-breaking performance is controllable by adjusting the dosage of ammonium persulphate gel-breaker. It has been demonstrated that, at a rate of 0.08–0.10 wt%, the viscosity can be reduced to below 5 mPa·s within 400–480 min, thus meeting the post-fracturing flowback requirements. Molecular dynamics simulations further confirm that the crosslinked network formed by Zr-N-SiO2 maintains a certain degree of integrity under high-temperature and high-shear conditions, providing theoretical support for the long-term stability of the fracturing fluid.