Development and Validation of a Simultaneous HPLC Stability-Indicating Method for Atorvastatin and Apigenin in a Novel SMEDDS Formulation Using Quality by Design (QbD) Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation and Program Software
2.3. Design of Experiments (DoE) Software
2.4. Preparation of Stock Solution, Standard Calibration Solution, and Quality Control Solutions
2.4.1. Preparation for ATV–API SMEDDS
2.4.2. Sample Preparation (Assay)
2.5. Wavelength Selection
2.6. Method Optimization
2.7. Method Development by QbD
2.7.1. Analytical Performance Objectives
2.7.2. Risk Parameters Assessment
2.7.3. Determine the Quality Target Product Profile (QTPP)
2.7.4. Determine Critical Quality Attributes (CQAs)
2.7.5. Determine Critical Method Parameters (CMPs)
2.7.6. DoE Based on Risk Assessment Method
2.7.7. Analysis of Experimental Results and Determination of Optimal Method Conditions
2.8. Method Validation
2.8.1. Specificity
2.8.2. System Suitability
2.8.3. Linearity
2.8.4. Accuracy
2.8.5. Precision
2.8.6. Determining LOD and LOQ
2.8.7. Robustness
2.9. Stress Degradation Study
2.9.1. Acid and Alkaline Hydrolysis
2.9.2. Oxidative Degradation Study
2.9.3. Photolytic Degradation Study
2.9.4. Thermal Degradation Study
3. Statistical Analysis
4. Results
4.1. Wavelength Detection
4.2. Risk Assessment
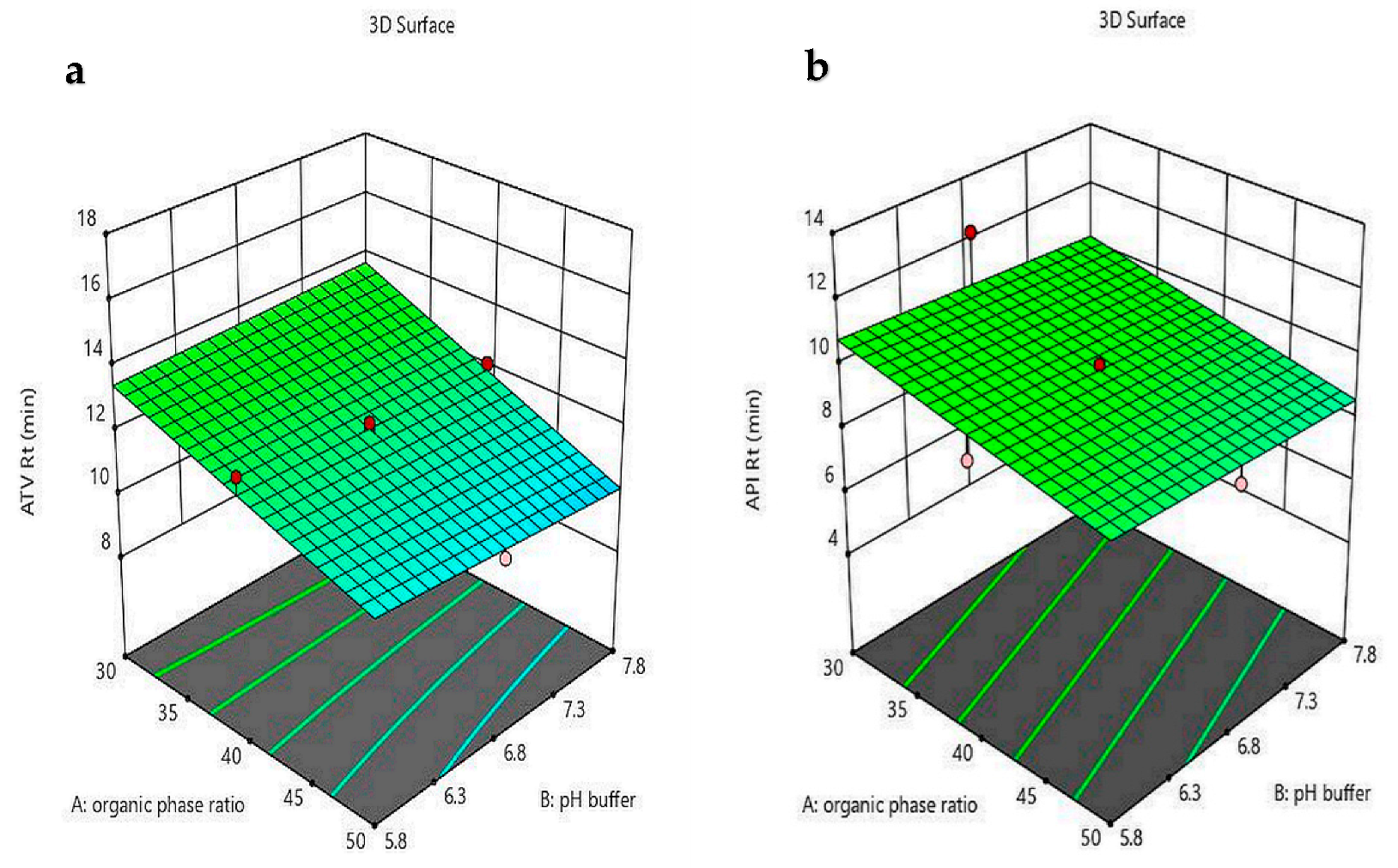
4.3. Method Development and Optimization
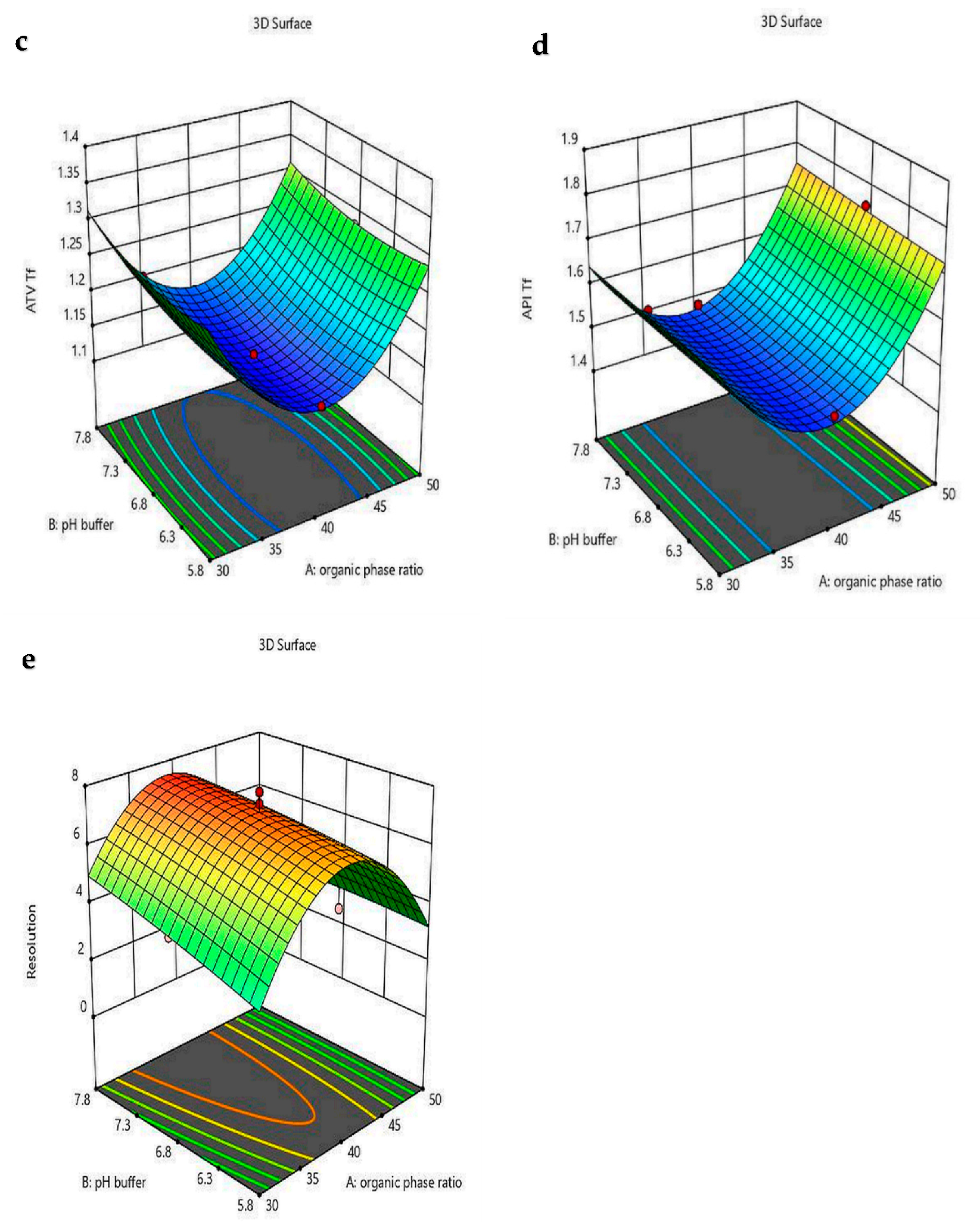
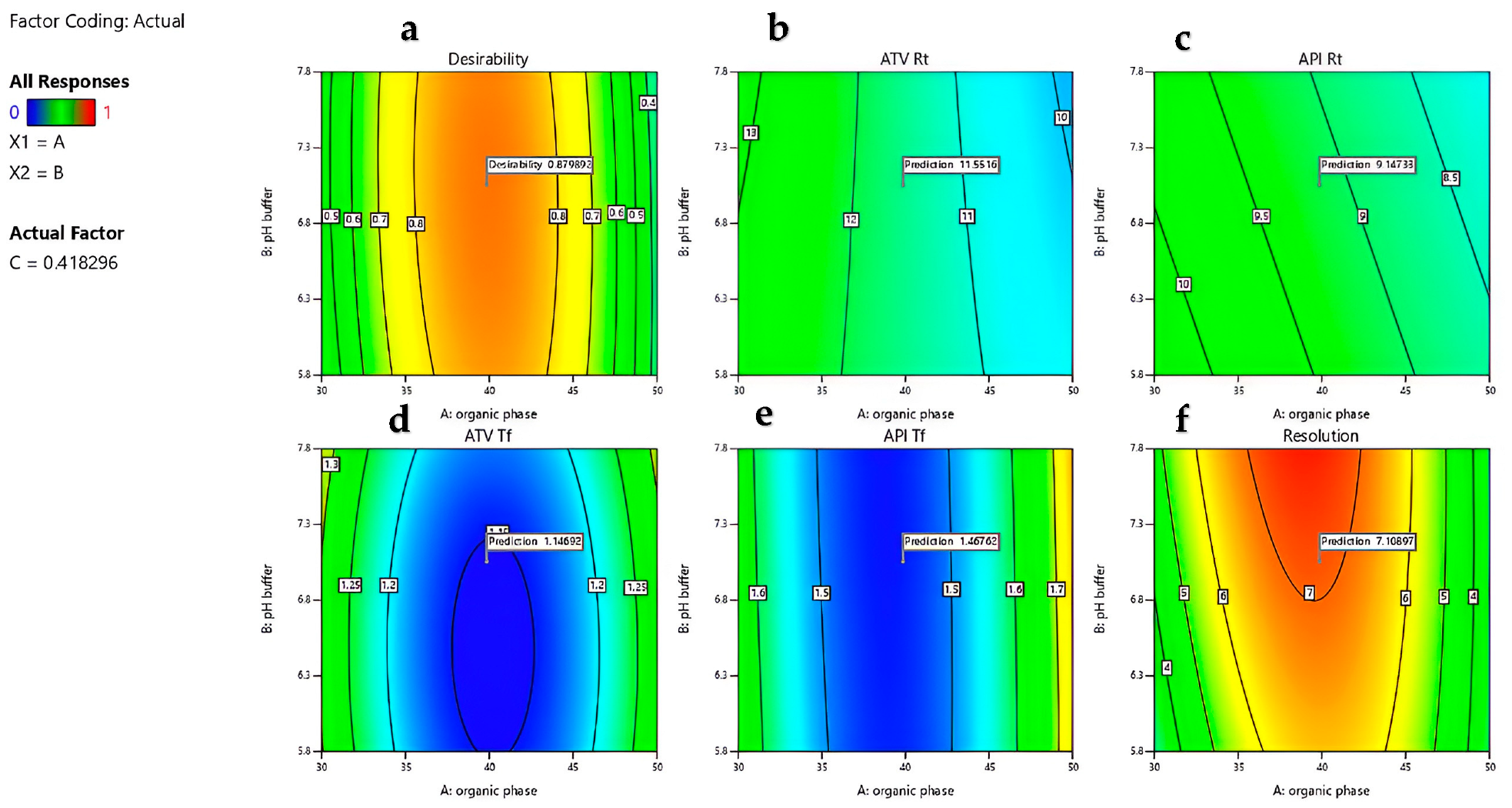
4.4. Optimizing the Risk Assessment Method by CCD
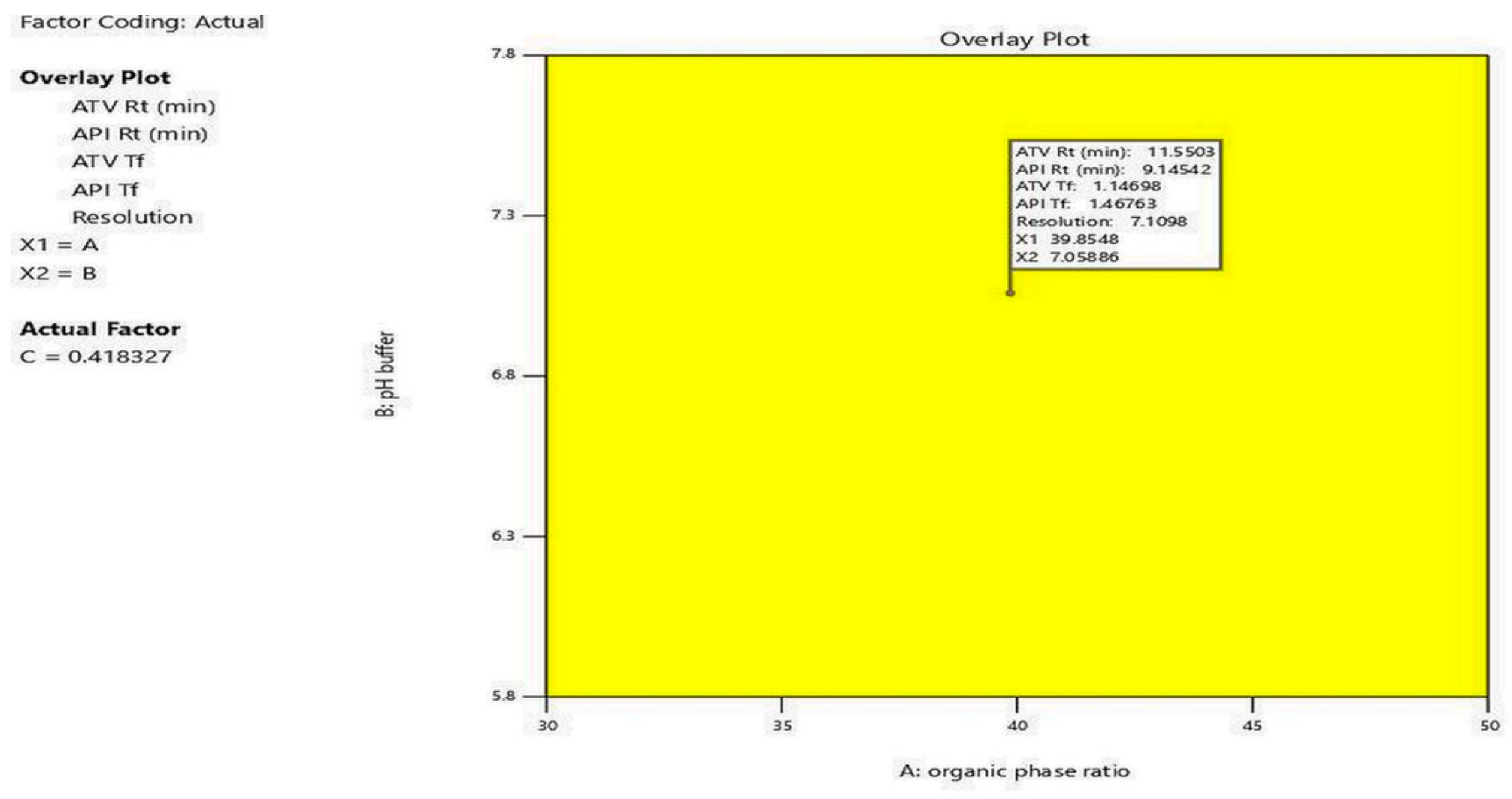
4.5. Optimization and Desirability Function
4.6. Method Validation
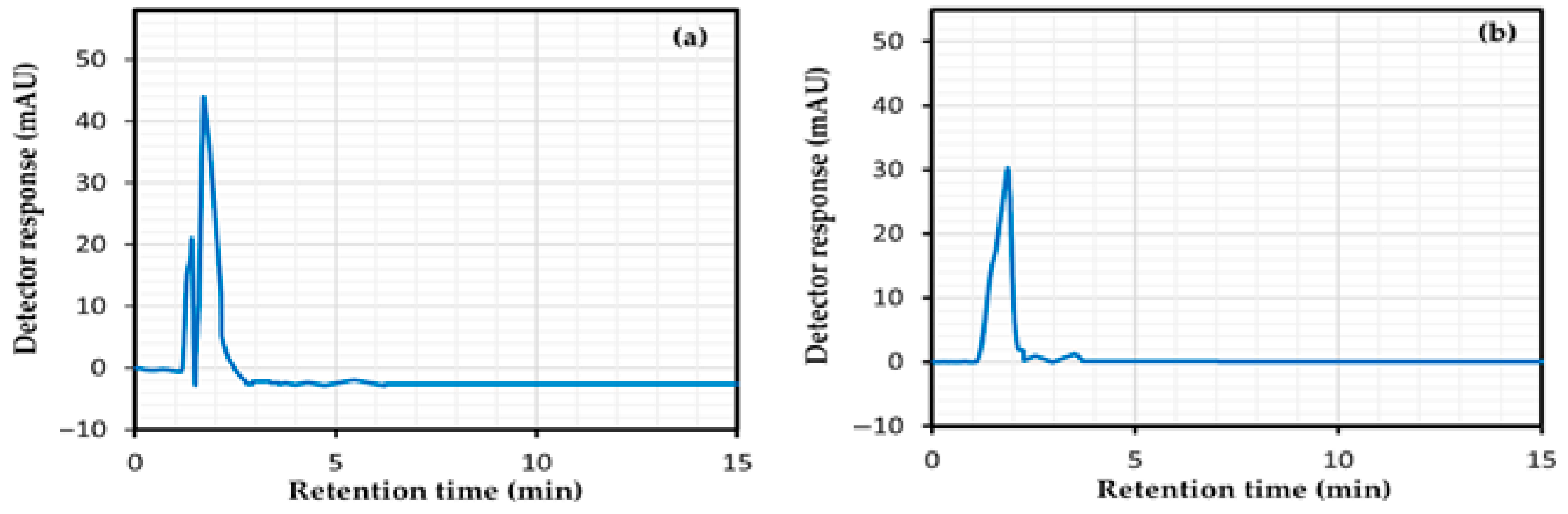
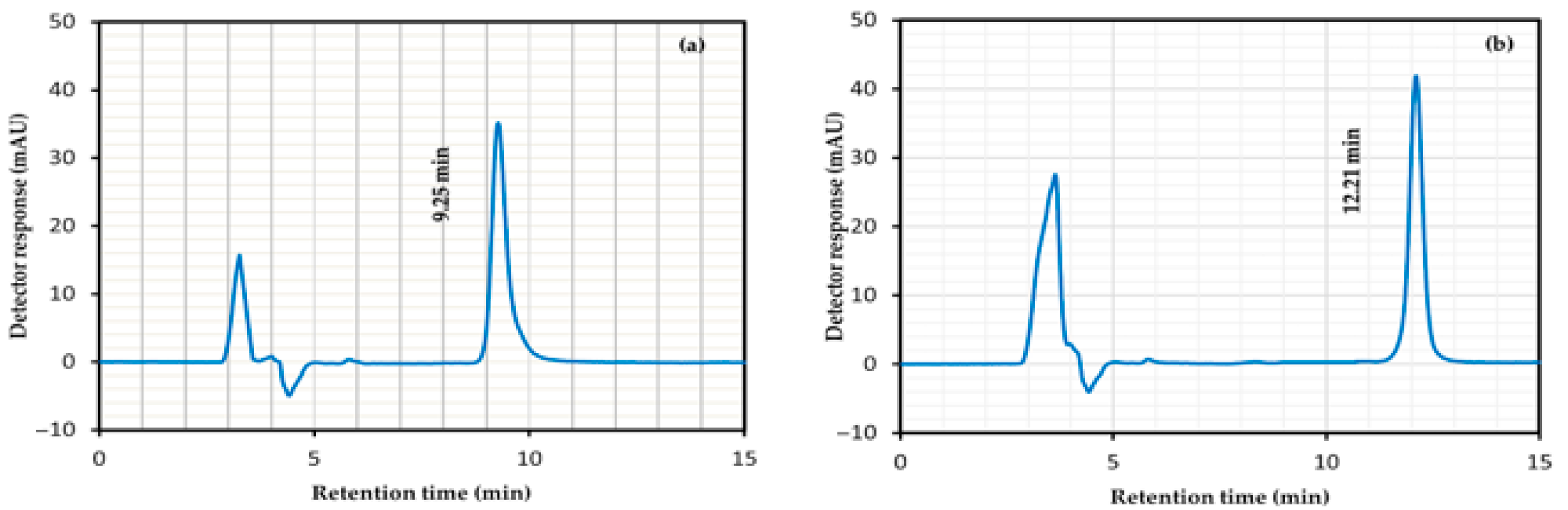
4.6.1. System Suitability
4.6.2. Linearity and Calibration Curve
4.6.3. LOD and LOQ
4.6.4. Accuracy
4.6.5. Precision
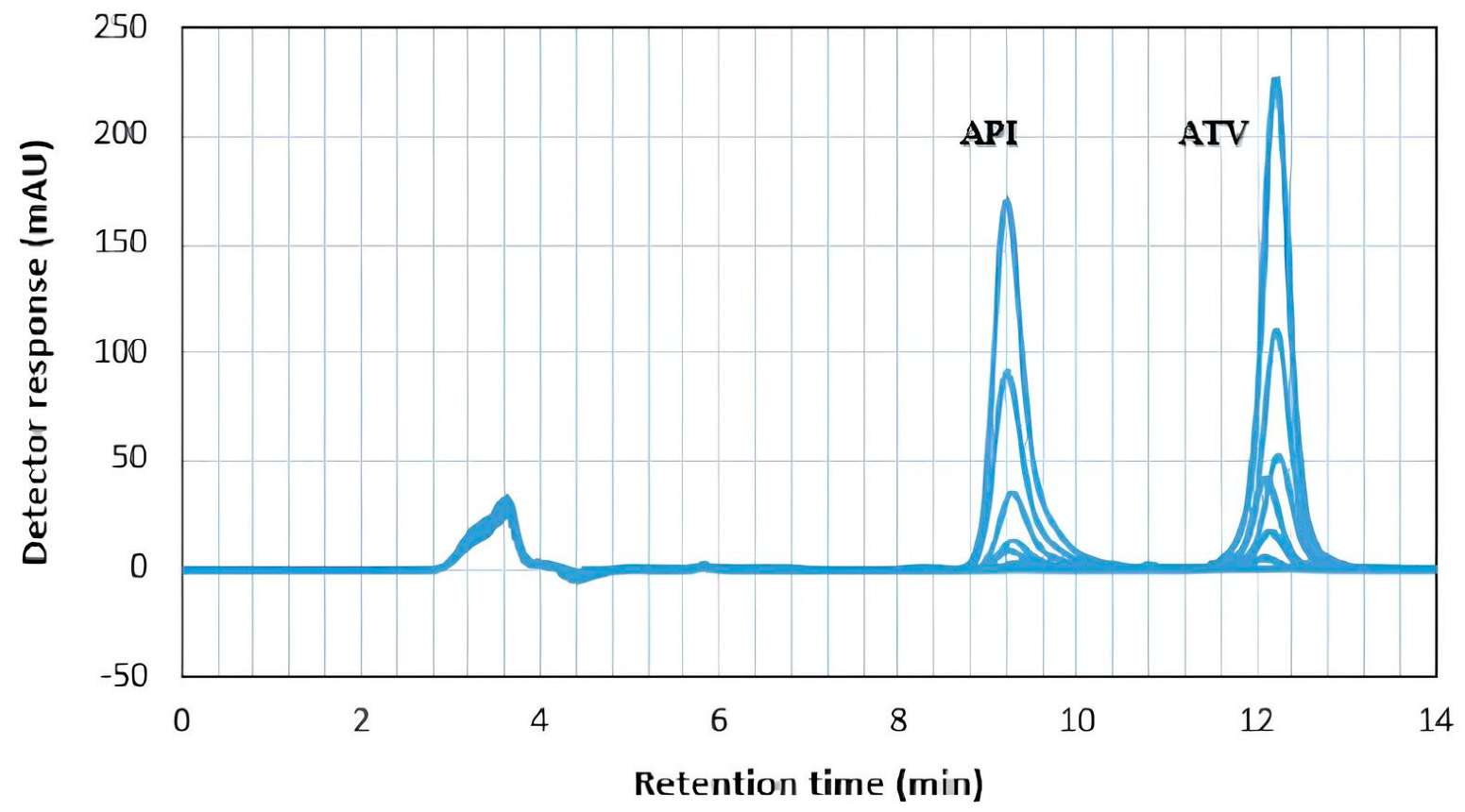
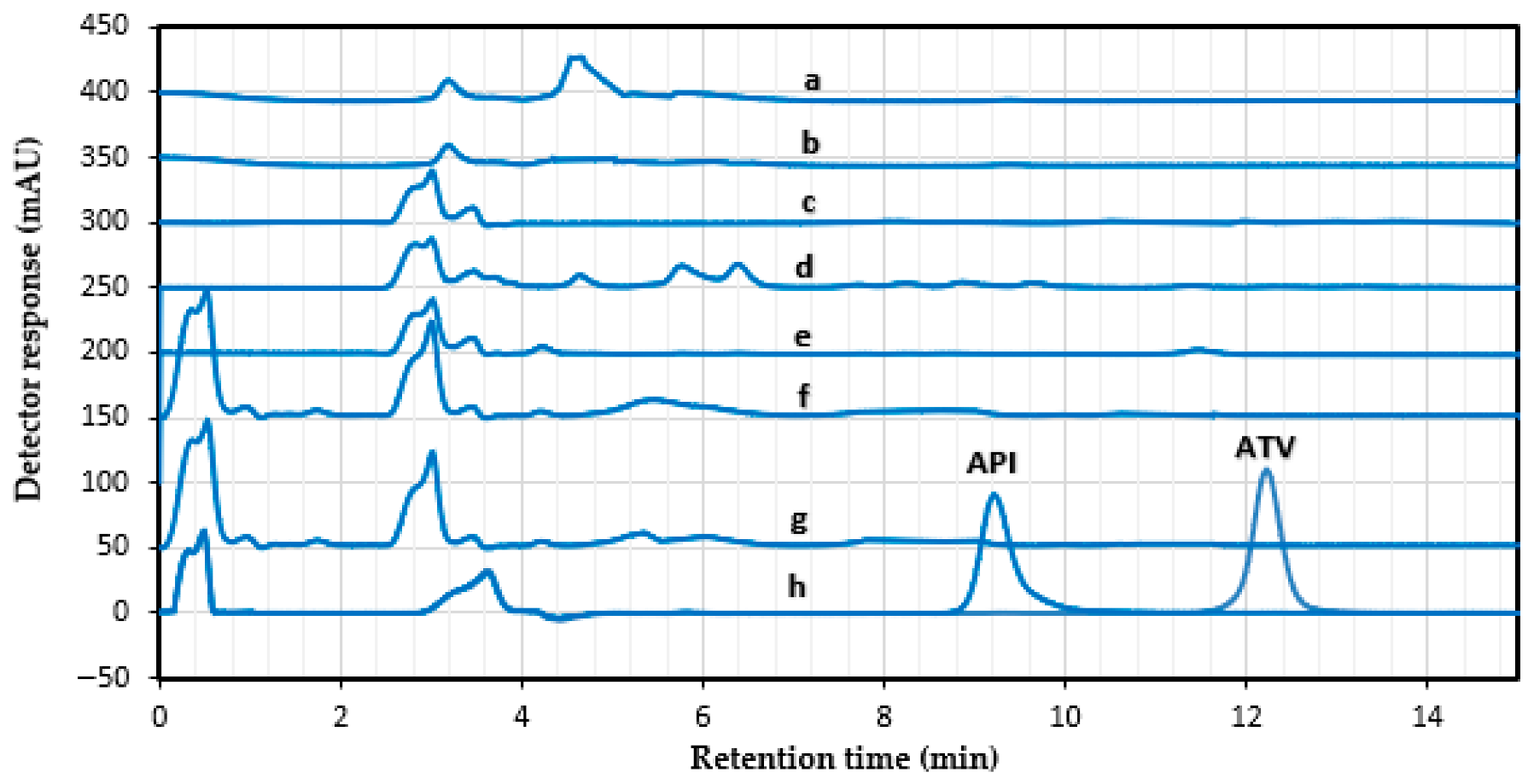
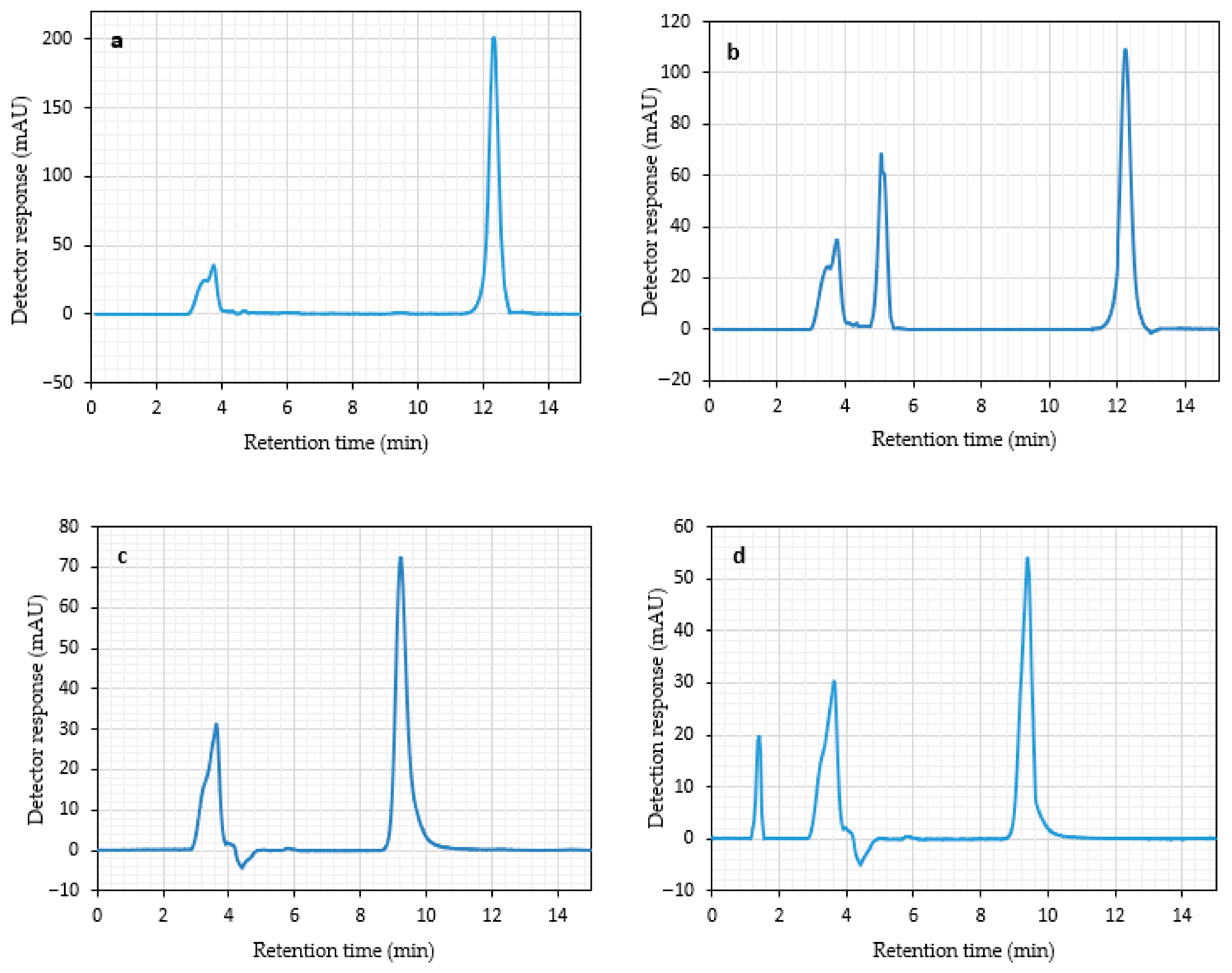
4.7. Specificity Study
4.8. Robustness Study
4.9. Assay of ATV–API in Tablet
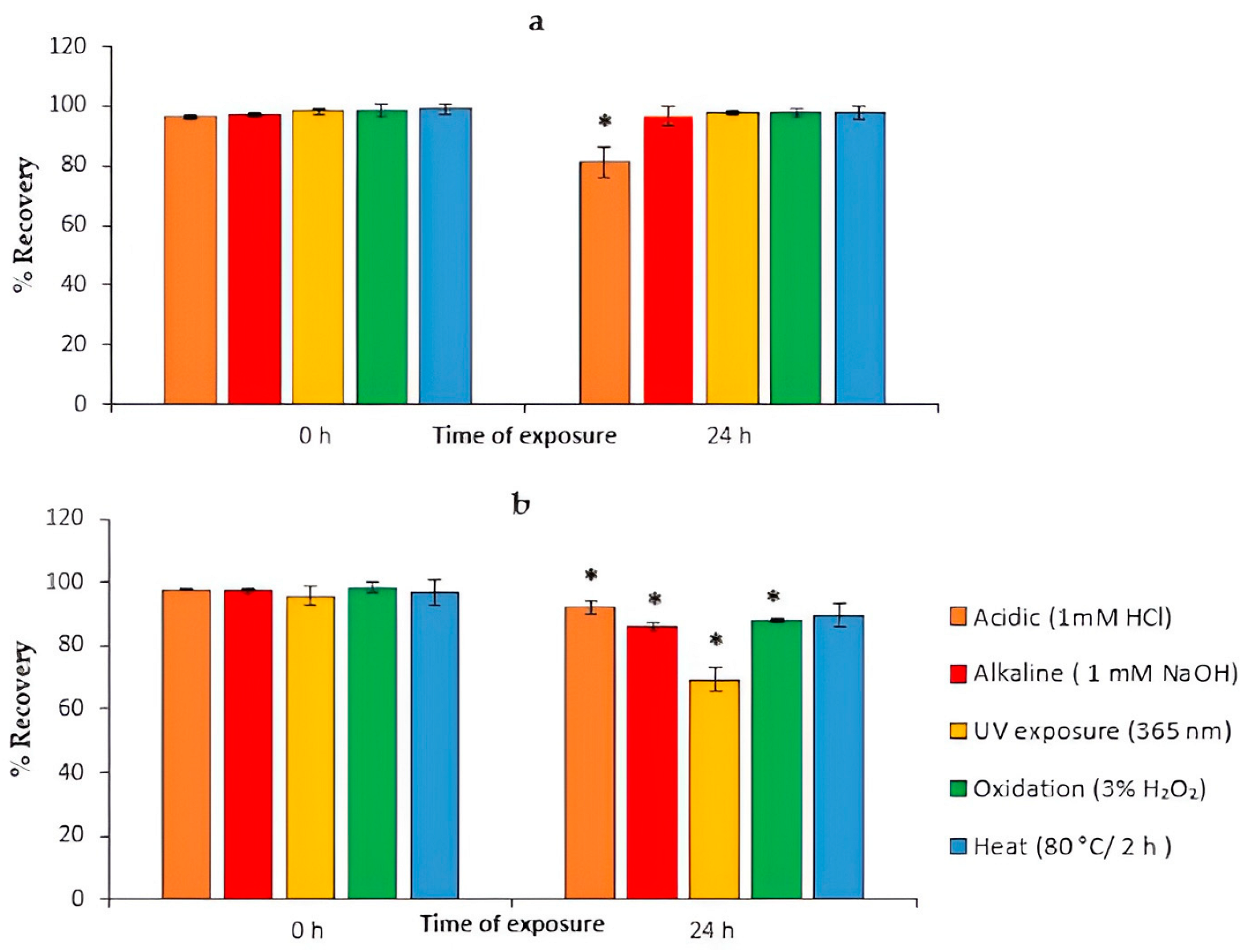
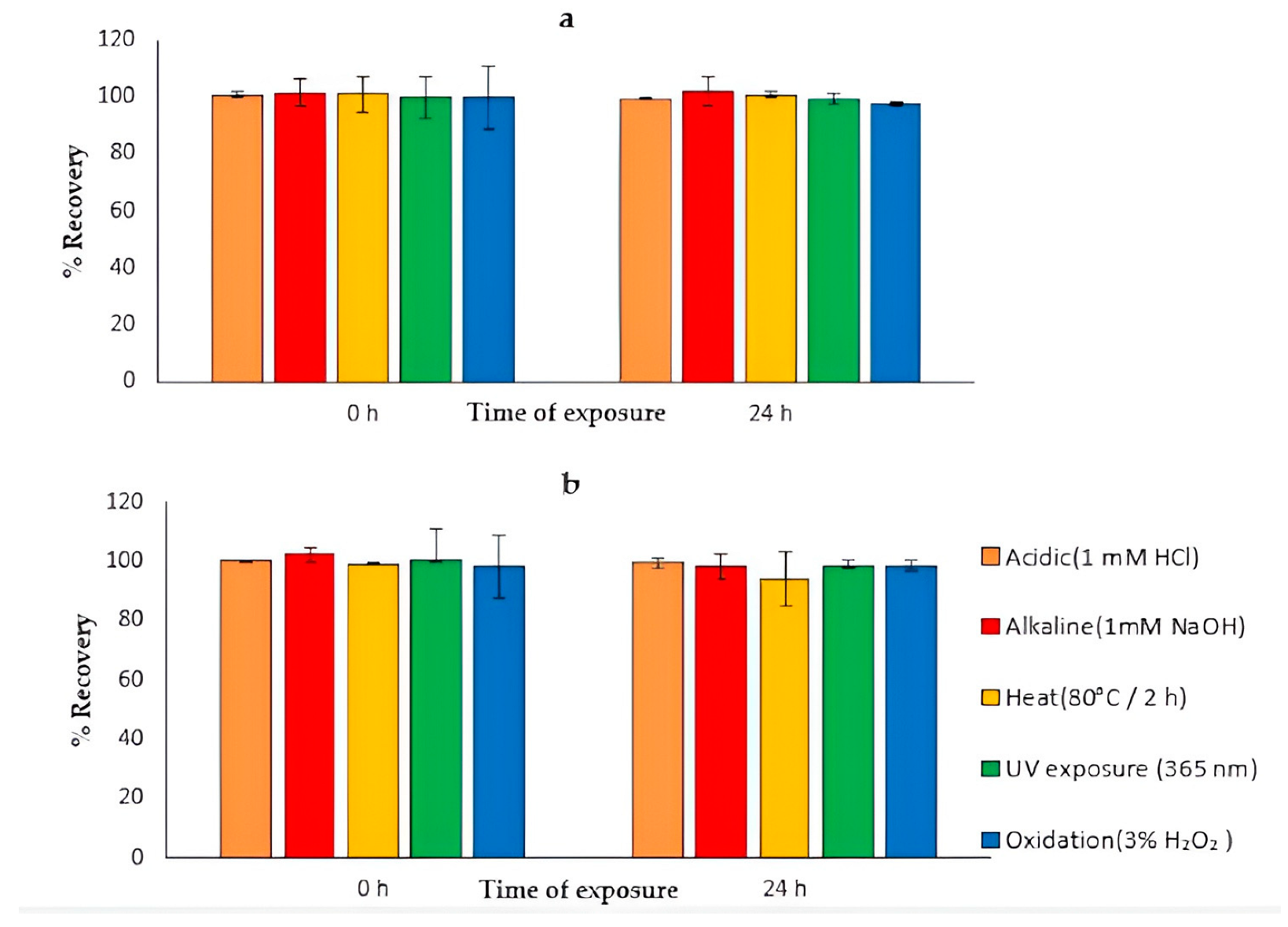
4.10. Stress Degradation Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elmowafy, M.; Ibrahim, H.M.; Ahmed, M.A.; Shalaby, K.; Salama, A.; Hefesha, H. Atorvastatin-Loaded Nanostructured Lipid Carriers (NLCs): Strategy to Overcome Oral Delivery Drawbacks. Drug Deliv. 2017, 24, 932–941. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Ghannad, F.; Zamani, M.; Andalib, S.; Danafar, H. Anti-Inflammatory Effect of Rosuvastatin Using Diblock Amphiphilic Copolymer: Synthesis, Characterization, in Vitro and in Vivo Study. J. Biomater. Appl. 2019, 34, 229–238. [Google Scholar] [CrossRef]
- Bu, D.X.; Griffin, G.; Lichtman, A.H. Mechanisms for the Anti-Inflammatory Effects of Statins. Curr. Opin. Lipidol. 2011, 22, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Yeom, D.W.; Son, H.Y.; Kim, J.H.; Kim, S.R.; Lee, S.G.; Song, S.H.; Chae, B.R.; Choi, Y.W. Development of a Solidified Self-Microemulsifying Drug Delivery System (S-SMEDDS) for Atorvastatin Calcium with Improved Dissolution and Bioavailability. Int. J. Pharm. 2016, 506, 302–311. [Google Scholar] [CrossRef]
- Li, B.; Robinson, D.H.; Birt, D.F. Evaluation of Properties of Apigenin and [G-3H]Apigenin and Analytic Method Development. J. Pharm. Sci. 1997, 86, 721–725. [Google Scholar] [CrossRef]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y.H. Health Functionality of Apigenin: A Review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Dutta, D.; Chakraborty, A.; Mukherjee, B.; Gupta, S. Aptamer-Conjugated Apigenin Nanoparticles to Target Colorectal Carcinoma: A Promising Safe Alternative of Colorectal Cancer Chemotherapy. ACS Appl. Bio Mater. 2018, 1, 1538–1556. [Google Scholar] [CrossRef] [PubMed]
- El Shoubaky, G.A.; Abdel-Daim, M.M.; Mansour, H.; Salem, E.A. Isolation and Identification of a Flavone Apigenin from Marine Red Alga Acanthophora Spicifera with Antinociceptive and Anti-Inflammatory Activities. J. Exp. Neurosci. 2016, 10, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Palko-Łabuz, A.; Środa-Pomianek, K.; Wesołowska, O.; Kostrzewa-Susłow, E.; Uryga, A.; Michalak, K. MDR Reversal and Pro-Apoptotic Effects of Statins and Statins Combined with Flavonoids in Colon Cancer Cells. Biomed. Pharmacother. 2019, 109, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Zaafan, M.A.; Zaki, H.F.; El-Brairy, A.I.; Kenawy, S.A. Protective Effects of Atorvastatin and Quercetin on Isoprenaline-Induced Myocardial Infarction in Rats. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 35–41. [Google Scholar] [CrossRef]
- Dash, R.; Biswal, J.; Yadav, M.; Sharma, T.; Mohapatra, S.; Prusty, S.K. Novel Atorvastatin-Curcumin Conjugate Nanogel, a Selective COX2 Inhibitor with Enhanced Biopharmaceutical Profile: Design, Synthesis, in Silico, in Vitro, and in Vivo Investigation. J. Drug Deliv. Sci. Technol. 2023, 81, 104211. [Google Scholar] [CrossRef]
- Assi, R.A.; Abdulbaqi, I.M.; Ming, T.S.; Yee, C.S.; Wahab, H.A.; Asif, S.M.; Darwis, Y. Liquid and Solid Self-Emulsifying Drug Delivery Systems (Sedds) as Carriers for the Oral Delivery of Azithromycin: Optimization, in Vitro Characterization and Stability Assessment. Pharmaceutics 2020, 12, 1052. [Google Scholar] [CrossRef]
- Laghari, M.; Darwis, Y.; Memon, A.H.; Khan, A.A.; Abdulbaqi, I.M.T.; Assi, R.A. Nanoformulations and Clinical Trial Candidates as Probably Effective and Safe Therapy for Tuberculosis. Trop. J. Pharm. Res. 2016, 15, 201–211. [Google Scholar] [CrossRef]
- Assi, R.A.; Abdulbaqi, I.M.; Yee, C.S. The Evaluation of Drug Delivery Nanocarrier Development and Pharmacological Briefing for Metabolic-Associated Fatty Liver Disease (MAFLD): An Update. Pharmaceuticals 2021, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.D.; Van Speybroeck, M.; Barillaro, V.; Martens, J.; Annaert, P.; Augustijns, P.; Van Humbeeck, J.; Vermant, J.; Van den Mooter, G. Formulate-Ability of Ten Compounds with Different Physicochemical Profiles in SMEDDS. Eur. J. Pharm. Sci. 2009, 38, 479–488. [Google Scholar] [CrossRef]
- Kaushik, D.; Verma, R. A Comprehensive Text Book on Self-Emulsifying Drug Delivery Systems. In A Comprehensive Text Book on Self-Emulsifying Drug Delivery Systems; Kaushik, D., Verm, R., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2021; pp. 1–195. [Google Scholar] [CrossRef]
- Dokania, S.; Joshi, A.K. Self-Microemulsifying Drug Delivery System (SMEDDS)–Challenges and Road Ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mehta, I. Surface Stabilized Atorvastatin Nanocrystals with Improved Bioavailability, Safety and Antihyperlipidemic Potential. Sci. Rep. 2019, 9, 16105. [Google Scholar] [CrossRef]
- Varshosaz, J.; Masoudi, S.; Mehdikhani, M.; Beni, B.H.; Farsaei, S. Atorvastatin Lipid Nanocapsules and Gold Nanoparticles Embedded in Injectable Thermo-gelling Hydrogel Scaffold Containing Adipose Tissue Extracellular Matrix For myocardial tissue regeneration. IET Nanobiotechnol. 2019, 13, 933–941. [Google Scholar] [CrossRef]
- Haiss, M.A.; Maraie, N.K. Utilization of Ultrasonication Technique for the Preparation of Apigenin Nanocrystals. Int. J. Drug Deliv. Technol. 2021, 11, 964–973. [Google Scholar]
- Kazmi, I.; Al-Abbasi, F.A.; Imam, S.S.; Afzal, M.; Nadeem, M.S.; Altayb, H.N.; Alshehri, S. Formulation and Evaluation of Apigenin-Loaded Hybrid Nanoparticles. Pharmaceutics 2022, 14, 783. [Google Scholar] [CrossRef]
- Alshabrawy, A.; Mostafa, A.; Abotaleb, N. Sensitive Spectrophotometric Determination of Atorvastatin in Pharmaceutical Formulation by Ion Pair Complexation with Pararosaniline Hydrochloride. J. Adv. Pharm. Res. 2017, 1, 193–200. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and Characterization of an Apigenin-Phospholipid Phytosome (APLC) for Improved Solubility, in Vivo Bioavailability, and Antioxidant Potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef]
- El-Zailik, A.; Cheung, L.K.; Wang, Y.; Sherman, V.; Chow, D.S.L. Simultaneous LC-MS/MS Analysis of Simvastatin, Atorvastatin, Rosuvastatin and Their Active Metabolites for Plasma Samples of Obese Patients Underwent Gastric Bypass Surgery. J. Pharm. Biomed. Anal. 2019, 164, 258–267. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Lv, Z.; Yu, M. LC-MS/MS Determination of Apigenin in Rat Plasma and Application to Pharmacokinetic Study. Curr. Pharm. Biotechnol. 2021, 22, 274–280. [Google Scholar] [CrossRef]
- Karaźniewicz-Łada, M.; Wójtowski, J.A.; Główka, F.; Danków, R.; Pikul, J.; Gryszczyńska, A.; Foksowicz-Flaczyk, J.; Mikołajczak, P.Ł. Application of UPLC-MS/MS Method for Analysis of Apigenin, Apigenin 7-Glucoside and Chlorogenic Acid in Goat Serum. Chromatographia 2023, 86, 401–411. [Google Scholar] [CrossRef]
- Patil, U.P.; Gandhi, S.V.; Sengar, M.R.; Rajmane, V.S.; Gandhi, S.V. A Validated Densitometric Method for Analysis of Telmisartan and Atorvastatin Calcium in Fixed Dose Combination. J. Chil. Chem. Soc. 2010, 55, 94–96. [Google Scholar] [CrossRef]
- Hwang, K.-M.; Park, S.-A.; Kim, J.-Y.; Park, C.-W.; Rhee, Y.-S.; Park, E.-S. Formulation and in Vitro Evaluation of Self-microemulsifying Drug Delivery System Containing Fixed-Dose Combination of Atorvastatin and Ezetimibe. Chem. Pharm. Bull. 2015, 63, 423–430. [Google Scholar] [CrossRef]
- Gomathy, S.; Narenderan, S.T.; Meyyanathan, S.N.; Gowramma, B. Development and Validation of Hplc Method for the Simultaneous Estimation of Apigenin and Luteolin in Commercial Formulation. J. Crit. Rev. 2020, 7, 4785–4790. [Google Scholar]
- Shetti, P.; Jalalpure, S.S. A Single Robust Stability-Indicating RP-HPLC Analytical Tool for Apigenin Quantification in Bulk Powder and in Nanoliposomes: A Novel Approach. Futur. J. Pharm. Sci. 2021, 7, 122. [Google Scholar] [CrossRef]
- Prajapati, P.B.; Bagul, N.; Kalyankar, G. Implementation of DoE and Risk-Based Enhanced Analytical Quality by Design Approach to Stability-Indicating RP-HPLC Method for Stability Study of Bosutinib. J. AOAC Int. 2021, 104, 1742–1753. [Google Scholar] [CrossRef]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel Solid Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) for Oral Delivery of Olmesartan Medoxomil: Design, Formulation, Pharmacokinetic and Bioavailability Evaluation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef]
- Bonde, S.; Bonde, C.G.; Prabhakar, B. Quality by Design Based Development and Validation of HPLC Method for Simultaneous Estimation of Paclitaxel and Vinorelbine Tartrate in Dual Drug Loaded Liposomes. Microchem. J. 2019, 149, 103982. [Google Scholar] [CrossRef]
- Ganorkar, A.V.; Gupta, K.R. Analytical Quality by Design: A Mini Review. Biomed. J. Sci. Tech. Res. 2017, 1, 1555–1559. [Google Scholar] [CrossRef]
- Tambare, R.S.; Shahi, S.R.; Gurumukhi, V.C.; Kakade, S.M.; Tapadiya, G.G. Quality by Design (QbD) Based Development and Validation of RP-HPLC Method for Buserelin Acetate in Polymeric Nanoparticles: Release Study. Heliyon 2024, 10, e39172. [Google Scholar] [CrossRef]
- Jayagopal, B.; Murugesh, S. QbD-Mediated RP-UPLC Method Development Invoking an FMEA-Based Risk Assessment to Estimate Nintedanib Degradation Products and Their Pathways. Arab. J. Chem. 2020, 13, 7087–7103. [Google Scholar] [CrossRef]
- Patel, K.; Shah, U.A.; Patel, C.N. Box–Behnken Design-Assisted Optimization of RP-HPLC Method for the Estimation of Evogliptin Tartrate by Analytical Quality by Design. Futur. J. Pharm. Sci. 2023, 9, 57. [Google Scholar] [CrossRef]
- Bairagi, A.; Kothrukar, R.; Chikhale, H.; Kosanam, S.; Borse, L. AQbD-Novel Strategy for Analytical Methods. Futur. J. Pharm. Sci. 2024, 10, 138. [Google Scholar] [CrossRef]
- Goud, V.M.; Sirisha, P.; Rachana, K.N.; Godela, R.; Prasad, B.D. QbD Approach for Analysis of Tirzepatide in Its Bulk and Marketed Formulation by Stability Indicating RP-HPLC. Int. J. Pharm. Qual. Assur. 2023, 14, 404–409. [Google Scholar] [CrossRef]
- Dinç-Zor, Ş.; Dönmez, Ö.A.; Aşçı, B.; Pingo, E. Chemometric Optimization of an HPLC Method for the Simultaneous Analysis of a Multi Component Drug Product by the Help of Central Composite Design. Microchem. J. 2020, 152, 104322. [Google Scholar] [CrossRef]
- Chandarana, C. Analytical Quality by Design: A Review for Chromatography. Biomed. J. Sci. Tech. Res. 2022, 42, 33714–33732. [Google Scholar] [CrossRef]
- Eldin, A.; Shalaby, A.; El-Tohamy, M. Development and Validation of a HPLC Method for the Determination of Montelukast and Its Degradation Products in Pharmaceutical Formulation Using An. Acta Pharm. Sci. 2011, 53, 45–56. [Google Scholar]
- Patel, K.Y.; Dedania, Z.R.; Dedania, R.R.; Patel, U. QbD Approach to HPLC Method Development and Validation of Ceftriaxone Sodium. Futur. J. Pharm. Sci. 2021, 7, 141. [Google Scholar] [CrossRef]
- Ali, H.S.M.; Hanafy, A.F.; Bafail, R.; Alrbyawi, H.; Almaghrabi, M.; Alahmadi, Y.M.; El Achy, S. Locally Acting Budesonide-Loaded Solid Self-Microemulsifying Drug Delivery Systems (SMEDDS) for Distal Ulcerative Colitis. Int. J. Nanomed. 2024, 19, 11819–11846. [Google Scholar] [CrossRef]
- Cerrahoğlu, E.; Kayan, A.; Bingöl, D. New Inorganic–Organic Hybrid Materials and Their Oxides for Removal of Heavy Metal Ions: Response Surface Methodology Approach. J. Inorg. Organomet. Polym. Mater. 2017, 27, 427–435. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Q2(R2) Validation of Analytical Procedures—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 17 July 2024).
- Aminu, N.; Chan, S.Y.; Khan, N.H.; Farhan, A.B.; Umar, M.N.; Toh, S.M. A Simple Stability-Indicating HPLC Method for Simultaneous Analysis of Paracetamol and Caffeine and Its Application to Determinations in Fixed-Dose Combination Tablet Dosage Form. Acta Chromatogr. 2019, 31, 85–91. [Google Scholar] [CrossRef]
- Fouad, M.A.; Elsabour, S.A.; Elkady, E.F.; Elshazly, H.M. Design of Experiment (DOE), Multiple Response Optimization and Utilizing the Desirability Function in the Simultaneous HPLC Separation of Five Antihypertensive Drugs. J. Iran. Chem. Soc. 2022, 19, 269–282. [Google Scholar] [CrossRef]
- Vial, J.; Jardy, A. Experimental Comparison of the Different Approaches to Estimate LOD and LOQ of an HPLC Method. Anal. Chem. 1999, 71, 2672–2677. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Loh, G.O.K. A Simple (HPLC–UV) Method for the Quantification of Colchicine in Bulk and Ethosomal Gel Nano-Formulation and its Validation. Int. J. Pharm. Pharm. Sci. 2017, 9, 72. [Google Scholar] [CrossRef]
- Assi, R.A.; Darwis, Y.; Abdulbaqi, I.M.; Asif, S.M. Development and Validation of a Stability-Indicating RP-HPLC Method for the Detection and Quantification of Azithromycin in Bulk, and Self-Emulsifying Drug Delivery System (SEDDs) Formulation. J. Appl. Pharm. Sci. 2017, 7, 20–29. [Google Scholar] [CrossRef]
- Talele, G.S.; Porwal, P.K. Development of Validated Bioanalytical HPLC-UV Method for Simultaneous Estimation of Amlodipine and Atorvastatin in Rat Plasma. Indian J. Pharm. Sci. 2015, 77, 742–750. [Google Scholar] [CrossRef]
- Chalikwar, S.S.; Surana, S.J.; Goyal, S.N.; Chaturvedi, K.K.; Dangre, P.V. Solid Self-Microemulsifying Nutraceutical Delivery System for Hesperidin Using Quality by Design: Assessment of Biopharmaceutical Attributes and Shelf-Life. J. Microencapsul. 2021, 38, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Gurumukhi, V.C.; Bari, S.B. Quality by Design (QbD)–Based Fabrication of Atazanavir-Loaded Nanostructured Lipid Carriers for Lymph Targeting: Bioavailability Enhancement Using Chylomicron Flow Block Model and Toxicity Studies. Drug Deliv. Transl. Res. 2022, 12, 1230–1252. [Google Scholar] [CrossRef]
- Kogawa, A.C.; Pires, A.E.D.T.; Salgado, H.R.N. Atorvastatin: A Review of Analytical Methods for Pharmaceutical Quality Control and Monitoring. J. AOAC Int. 2019, 102, 801–809. [Google Scholar] [CrossRef]
- Rajagopal, S.S.; Vazhayil, B.K.; Varghese, L.; Nanjaian, M. Development and Validation of RP-HPLC Method for Simultaneous Determination of Apigenin and Luteolin in Ethanol Extract of Clerodendrum serratum (Linn.) Leaves. Asian J. Appl. Sci. 2017, 5, 52–60. [Google Scholar]
- Hinge, M.A.; Patel, D. Optimization of HPLC Method Using Central Composite Design for Estimation of Torsemide and Eplerenone in Tablet Dosage Form. Braz. J. Pharm. Sci. 2022, 58, e20219. [Google Scholar] [CrossRef]
- United States Pharmacopeia; United States Pharmacopeial Convention. General Chapter <621> Chromatography. In United States Pharmacopeia 43–National Formulary 38 (USP 43–NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- Seshachalam, U.; Kothapally, C.B. HPLC Analysis for Simultaneous Determination of Atorvastatin and Ezetimibe in Pharmaceutical Formulations. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 714–721. [Google Scholar] [CrossRef]
- Mustafa, G.; Azeem, A.; Ahmad, F.J.; Khan, Z.I.; Shakeel, F.; Talegaonkar, S. Stability-Indicating RP-HPLC Method for Analysis of Atorvastatin in Bulk Drug, Marketed Tablet and Nanoemulsion Formulation. J. Chil. Chem. Soc. 2010, 55, 184–188. [Google Scholar] [CrossRef]
- Shabir, G.A. Validation of High-Performance Liquid Chromatography Methods for Pharmaceutical Analysis: Understanding the Differences and Similarities between Validation Requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. J. Chromatogr. A 2003, 987, 57–66. [Google Scholar] [CrossRef]
- Bakshi, M.; Singh, S. Development of Validated Stability-Indicating Assay Methods—Critical Review. J. Pharm. Biomed. Anal. 2002, 28, 1011–1040. [Google Scholar] [CrossRef] [PubMed]
- Bkhaitan, M.M.; Mirza, A.Z. Stability-Indicating HPLC-DAD Method for Simultaneous Determination of Atorvastatin, Irbesartan, and Amlodipine in Bulk and Pharmaceutical Preparations. Bull. Korean Chem. Soc. 2015, 36, 2230–2237. [Google Scholar] [CrossRef]
- Kumar Mantri, S.; Pashikanti, S.; Ramana Murthy, K.V. Development and Characterization of Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) of Atorvastatin Calcium. Curr. Drug Deliv. 2012, 9, 182–196. [Google Scholar] [CrossRef]
- Mohammadi, A.; Rezanour, N.; Ansari Dogaheh, M.; Ghorbani Bidkorbeh, F.; Hashem, M.; Walker, R.B. A Stability-Indicating High Performance Liquid Chromatographic (HPLC) Assay for the Simultaneous Determination of Atorvastatin and Amlodipine in Commercial Tablets. J. Chromatogr. B 2007, 846, 215–221. [Google Scholar] [CrossRef]
- Goel, A.; Baboota, S.; Sahni, J.K.; Srinivas, K.S.; Gupta, R.S.; Gupta, A.; Semwal, V.P.; Ali, J. Development and Validation of Stability-Indicating Assay Method by UPLC for a Fixed Dose Combination of Atorvastatin and Ezetimibe. J. Chromatogr. Sci. 2013, 51, 222–228. [Google Scholar] [CrossRef]
- Chaudhari, B.G.; Patel, N.M.; Shah, P.B. Stability Indicating RP-HPLC Method for Simultaneous Determination of Atorvastatin and Amlodipine from Their Combination Drug Products. Chem. Pharm. Bull. 2007, 55, 241–246. [Google Scholar] [CrossRef]
- Vukkum, P.; Moses Babu, J.; Muralikrishna, R. Stress Degradation Behavior of Atorvastatin Calcium and Development of a Suitable Stability-Indicating LC Method for the Determination of Atorvastatin, Its Related Impurities, and Its Degradation Products. Sci. Pharm. 2012, 81, 93–114. [Google Scholar] [CrossRef]
- Liu, W.N.; Shi, J.; Fu, Y.; Zhao, X.H. The Stability and Activity Changes of Apigenin and Luteolin in Human Cervical Cancer Hela Cells in Response to Heat Treatment and Fe2+/Cu2+ Addition. Foods 2019, 8, 346. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Meyyanathan, S.N.; Byran, G. Stability-Indicating Reverse-Phase High-Performance Liquid Chromatography Method for the Simultaneous Quantification of Apigenin and Luteolin from Achillea Millefolium Linn. Asian J. Pharm. Clin. Res. 2019, 12, 169–172. [Google Scholar] [CrossRef]
- Bradwell, J.; Hurd, M.; Pangloli, P.; McClure, A.; Dia, V.P. Storage Stability of Sorghum Phenolic Extracts’ Flavones Luteolin and Apigenin. LWT 2018, 97, 787–793. [Google Scholar] [CrossRef]
- Narang, A.S.; Delmarre, D.; Gao, D. Stable Drug Encapsulation in Micelles and Microemulsions. Int. J. Pharm. 2007, 345, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Salaw, A. Self-Emulsifying Drug Delivery Systems: A Novel Approach To Deliver Drugs. Drug Deliv. 2022, 29, 1811–1823. [Google Scholar] [CrossRef]
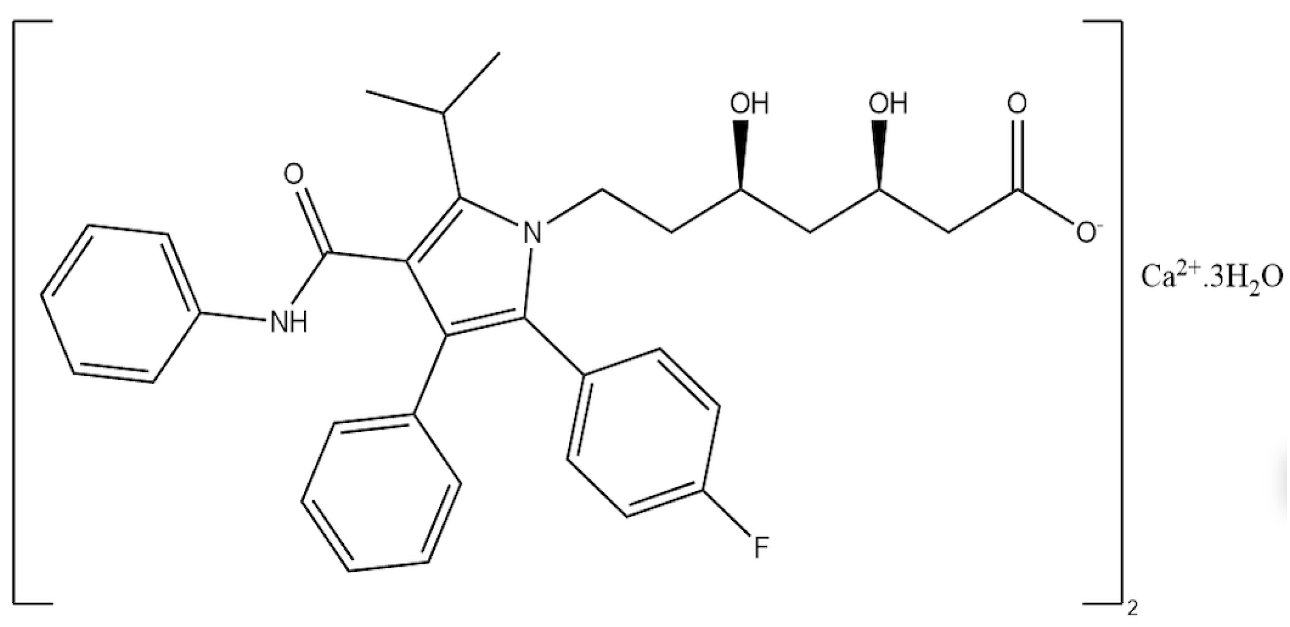
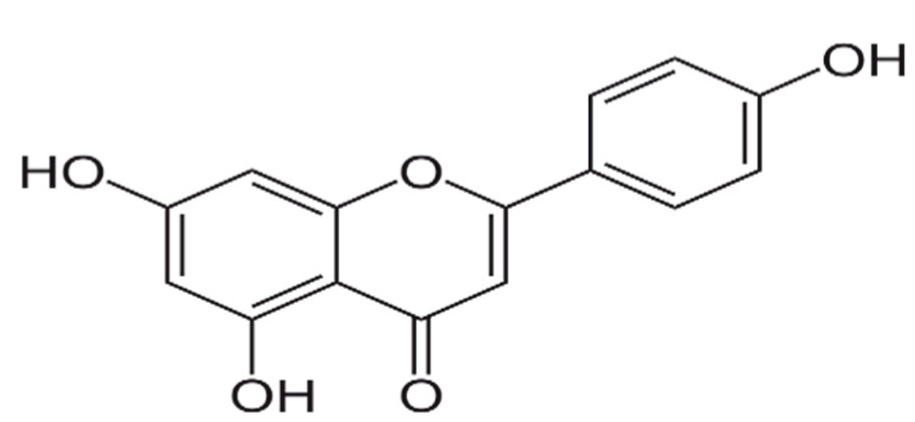
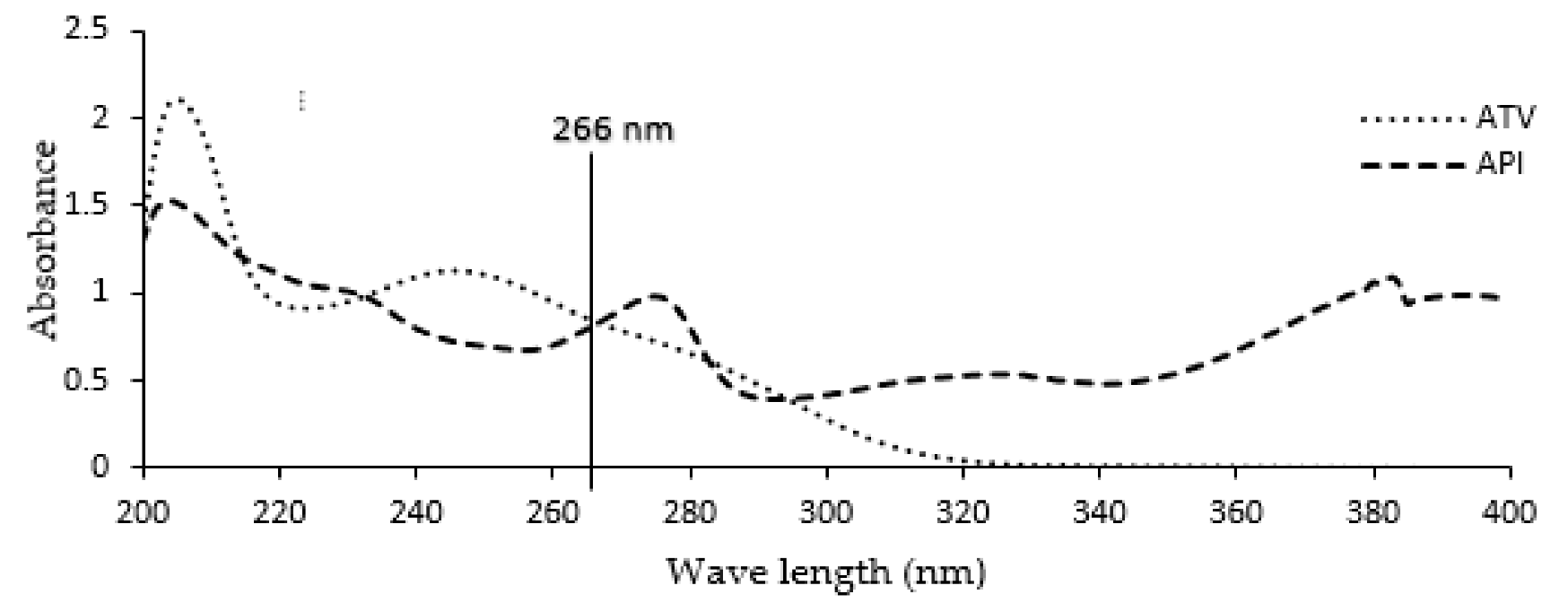
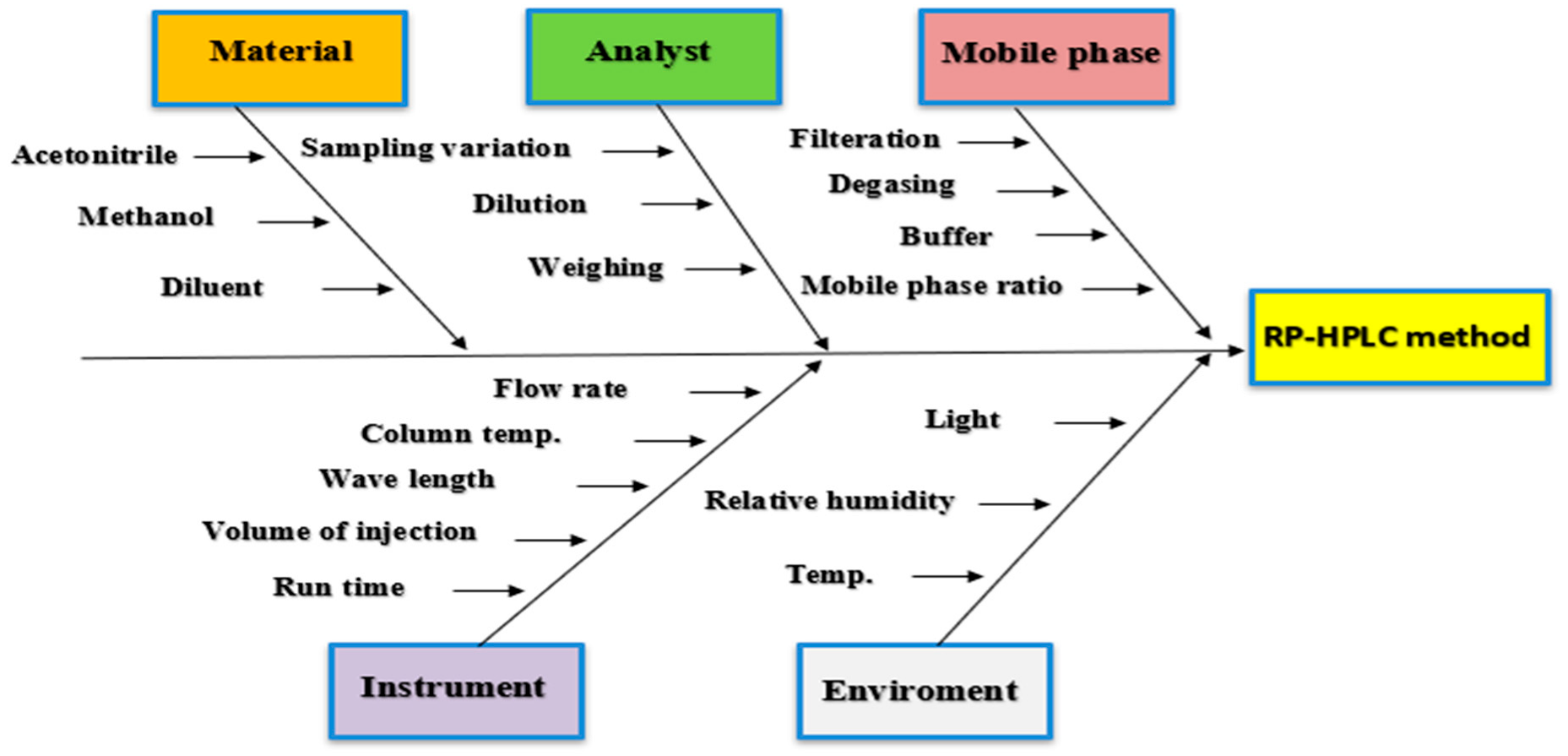
| Variables | Levels | ||
|---|---|---|---|
| Independent Variables | Low | Medium | High |
| 30 | 40 | 50 |
| 5.8 | 6.8 | 7.8 |
| 0.3 | 0.4 | 0.5 |
| Response | Risk Affecting Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| QTPP | Solvent | Ratio of Mobile Phase | pH of Buffer System | Wavelength | Flow Rate | Run Time | Oven Temperature | Volume of Injection |
| Tailing factor (Tf) | L | H | H | M | L | L | M | L |
| Retention time (Rt) | L | M | L | L | H | L | L | L |
| Resolution | M | H | H | L | L | L | L | L |
| Theoretical plate number (TPN) | L | M | M | L | L | L | L | L |
| STD | RUN | Organic Phase Ratio | pH | Flow Rate | ATV Rt (min) | API Rt (min) | ATV Tf | API Tf | Resolution |
|---|---|---|---|---|---|---|---|---|---|
| 6 | 1 | 50 | 5.8 | 0.5 | 8.23 | 5.98 | 1.31 | 1.81 | 1.88 |
| 4 | 2 | 50 | 7.8 | 0.3 | 11.76 | 11.9 | 1.35 | 1.77 | 1.65 |
| 5 | 3 | 30 | 5.8 | 0.5 | 9.2 | 7.88 | 1.34 | 1.66 | 0.4 |
| 2 | 4 | 50 | 5.8 | 0.3 | 13.4 | 12.8 | 1.31 | 1.66 | 1.65 |
| 13 | 5 | 40 | 6.8 | 0.3 | 14.12 | 12.13 | 1.14 | 1.48 | 4.8 |
| 15 | 6 | 40 | 6.8 | 0.4 | 12.05 | 9.88 | 1.14 | 1.45 | 7.8 |
| 10 | 7 | 50 | 6.8 | 0.4 | 10.17 | 8.32 | 1.28 | 1.75 | 2.55 |
| 14 | 8 | 40 | 6.8 | 0.5 | 9.45 | 7.25 | 1.18 | 1.5 | 5.35 |
| 8 | 9 | 50 | 7.8 | 0.5 | 8.4 | 6.3 | 1.32 | 1.76 | 2.1 |
| 11 | 10 | 40 | 5.8 | 0.4 | 12.7 | 9.2 | 1.15 | 1.48 | 6.2 |
| 9 | 11 | 30 | 6.8 | 0.4 | 13.3 | 12.21 | 1.29 | 1.66 | 4.2 |
| 7 | 12 | 30 | 7.8 | 0.5 | 11.25 | 7.67 | 1.39 | 1.62 | 2.15 |
| 1 | 13 | 30 | 5.8 | 0.3 | 17.55 | 13.78 | 1.29 | 1.66 | 2.88 |
| 12 | 14 | 40 | 7.8 | 0.4 | 12.1 | 9.15 | 1.16 | 1.48 | 7 |
| 16 | 15 | 40 | 6.8 | 0.4 | 12.21 | 9.25 | 1.14 | 1.45 | 7.8 |
| 17 | 16 | 40 | 6.8 | 0.4 | 12.21 | 9.25 | 1.14 | 1.45 | 7.4 |
| 3 | 17 | 30 | 7.8 | 0.3 | 16.21 | 12.04 | 1.28 | 1.68 | 4.56 |
| Response | Actual Equation | p-Value | F-Value | R2 | Predicted R2 | Adjusted R2 | Adequate Precision | Lack-of-Fit p-Value |
|---|---|---|---|---|---|---|---|---|
| R1 = ATV Rt | = 49.59 − 0.2 A − 0.146 B − 94.6 C − 0.272 AB + 0.597 AC + 6.5 BC | <0.0001 | 133.47 | 0.98 | 0.93 | 0.98 | 40.132 | 0.052 |
| R2 = API Rt | = 25.79 − 0.82 A − 0.25 B − 27.57 C | <0.0001 | 51.91 | 0.92 | 0.86 | 0.9 | 21.63 | 0.16 |
| R3 = ATV Tf | = 4.21 − 0.108 A − 0.231 B − 0.85 C + 0.0012 AB − 0.023 AC + 0.0037 BC + 0.145 A2 + 0.016 B2 + 2.14 C2 | <0.0001 | 86.43 | 0.99 | 0.8 | 0.97 | 24.65 | - |
| R4 = API Tf | = 5 − 0.19 A + 0.05 B + 0.05 C + 0.001 AB + 0.025 AC − 0.027 BC + 0.22 A2 + 0.0014 B2 + 1.14 C2 | <0.0001 | 65.49 | 0.98 | 0.85 | 0.97 | 20.97 | - |
| R5 = Resolution | = − 75.59 + 2.61 A + 3.31 B − 96.31 C − 0.4 AB + 0.69 AC + 0.03 BC − 3.33 A2 − 0.1 B2 − 16.3 C2 | <0.0003 | 20.26 | 0.96 | 0.79 | 0.91 | 12.67 | 0.073 |
| Name | Goal | Lower Limit | Upper Limit | Importance |
|---|---|---|---|---|
| A: Organic phase ratio | In range | 30 | 50 | 3 |
| B: pH buffer | In range | 5.8 | 7.8 | 3 |
| C: Flow rate | In range | 0.3 | 0.5 | 3 |
| ATV Rt | Minimize | 8.23 | 17.55 | 3 |
| API Rt | In range | 5.98 | 13.78 | 3 |
| ATV Tf | Minimize | 1.14 | 1.39 | 4 |
| API Tf | Minimize | 1.45 | 1.81 | 5 |
| Resolution | Maximize | 0.4 | 7.8 | 5 |
| Organic Phase Ratio | pH Buffer | Flow Rate (mL min−1) | ATV Rt (min) | ATV Tf | API Rt (min) | API Tf | Resolution | Desirability |
|---|---|---|---|---|---|---|---|---|
| 39.86 | 7.03 | 0.41 | 11.55 | 1.14 | 9.14 | 1.46 | 7.1 | 0.88 |
| Response | ATV Predicted | API Predicted | ATV Experimental | API Experimental | Relative Prediction Error (%) ATV | Relative Prediction Error (%) API |
|---|---|---|---|---|---|---|
| Rt | 11.55 | 9.14 | 12.21 | 9.25 | 5.81 | 0.65 |
| Tf | 1.14 | 1.46 | 1.14 | 1.45 | 0 | 1.36 |
| Resolution ATV–API | 7.1 | 7.1 | 7.8 | 7.8 | 8.08 | 8.08 |
| Suitability Parameters | ATV (5 µg mL−1) | RSD% | API (5 µg mL−1) | RSD% | Accepted Limits [61] |
|---|---|---|---|---|---|
| Retention time (min) | 12.21 ± 0.02 | 0.16 | 9.25 ± 0.01 | 0.11 | - |
| Tailing factor | 1.14 ± 0.01 | 0.88 | 1.45 ± 0.02 | 1.38 | ≤2 |
| Resolution | 7.83 ± 0.00 | 0.0 | 6.58 ± 0.04 | 0.61 | >2 |
| Theoretical plate number | 9445 ± 13.61 | 0.14 | 6948 ± 17.34 | 0.25 | >2000 |
| Parameter | ATV | API |
|---|---|---|
| Regression equation | y = (49,285)x + (−209.45) | y = (38,797)x + (1873.4) |
| Slope | 49,285 | 38,797 |
| y-intercept | −209.45 | 1873.4 |
| R2 | 0.9999 | 0.9998 |
| Ranges (μg mL−1) | 0.1–10 | 0.1–10 |
| LOD (μg mL−1) | 0.16 | 0.26 |
| LOQ (μg mL−1) | 0.49 | 0.81 |
| Compound | Nominated Conc. µg mL−1 | Calculated Concentration µg mL−1 (Intra-Day) | Precision (% RSD) | Calculated Concentration µg mL−1 (Inter-Day) | Precision (% RSD) |
|---|---|---|---|---|---|
| ATV | 0.5 | 0.5 ± 0.01 | 1.94 | 0.51 ± 0.00 | 0.98 |
| 5 | 5 ± 0.05 | 0.9 | 4.96 ± 0.1 | 2.02 | |
| 10 | 10.1 ± 0.07 | 0.72 | 9.97 ± 0.05 | 0.51 | |
| API | 1 | 1 ± 0.02 | 1.96 | 0.97 ± 0.02 | 2.04 |
| 5 | 5.13 ± 0.08 | 1.49 | 5.17 ± 0.11 | 2.04 | |
| 10 | 10.4 ± 0.17 | 1.62 | 10.38 ± 0.13 | 1.22 |
| Parameters | Modification Level | Peak Area (API) | % RSD | Peak Area (ATV) | % RSD | Rt (API) | % RSD | Rt (ATV) | % RSD |
|---|---|---|---|---|---|---|---|---|---|
| Wavelength detection (nm) | +2 (268 nm) | 197,817 ± 3658 | 1.85 | 242,506 ± 3151 | 1.30 | 9.11 ± 0.01 | 0.16 | 12.26 ± 0.03 | 0.24 |
| 0 (266 nm) | 203,008 ± 3281 | 1.62 | 246,038 ± 4228 | 1.72 | 9.11 ± 0.01 | 0.13 | 12.31 ± 0.04 | 0.36 | |
| −2 (264 nm) | 202,255 ± 3461 | 1.71 | 246,825 ± 3192 | 1.29 | 9.15 ± 0.05 | 0.59 | 12.31 ± 0.06 | 0.47 | |
| pH of buffer | +0.2 (7.2) | 200,237 ± 4179 | 2.09 | 248,466 ± 2487 | 1.00 | 8.86 ± 0.05 | 0.61 | 12.29 ± 0.07 | 0.55 |
| 0 (7.0) | 200,720 ± 3283 | 1.64 | 246,730 ± 2916 | 1.18 | 9.24 ± 0.04 | 0.39 | 12.23 ± 0.01 | 0.12 | |
| −0.2 (6.8) | 198,031 ± 4129 | 2.09 | 247,924 ± 2712 | 1.09 | 9.28 ± 0.06 | 0.59 | 12.25 ± 0.03 | 0.24 | |
| Flow rate (mL min−1) | +0.2 (0.43) | 205,241 ± 3547 | 1.73 | 241,799 ± 2985 | 1.23 | 8.39 ± 0.06 | 0.72 | 11.29 ± 0.1 | 0.91 |
| 0 (0.41) | 199,054 ± 3858 | 1.94 | 247,597 ± 3938 | 1.59 | 9.11 ± 0.02 | 0.25 | 12.05 ± 0.13 | 1.11 | |
| −0.2 (0.39) | 205,631 ± 2972 | 1.45 | 240,924 ± 3811 | 1.58 | 9.39 ± 0.03 | 0.28 | 12.36 ± 0.03 | 0.26 | |
| Mobile phase composition | +0.05 (0.45:0.55) | 202,010 ± 1924 | 0.95 | 247,580 ± 3829 | 1.55 | 9.02 ± 0.1 | 1.55 | 11.62 ± 0.05 | 0.44 |
| 0 (0.4:0.6) | 200,904 ± 1367 | 0.68 | 247,596 ± 3796 | 1.53 | 9.21 ± 0.06 | 0.71 | 12.25 ± 0.04 | 0.34 | |
| −0.05 (0.35:0.65) | 201,250 ± 3105 | 1.54 | 235,465 ± 2679 | 1.14 | 9.17 ± 0.33 | 0.3 | 12.22 ± 0.04 | 0.29 |
| Stress Study | Time of Exposure (h) | % Recovery (ATV) | Time of Exposure (h) | % Recovery (ATV) | Time of Exposure (h) | % Recovery (API) | Time of Exposure (h) | % Recovery (API) |
|---|---|---|---|---|---|---|---|---|
| Acidic (1 mM HCl) | 0 | 96.29 ± 0.66 | 24 | 81.33 ± 4.96 | 0 | 97.83 ± 0.35 | 24 | 92.25 ± 1.9 |
| Basic (1 mM NaOH) | 0 | 97.11 ± 0.4 | 24 | 96.46 ± 3.25 | 0 | 97.39 ± 1.02 | 24 | 86.16 ± 1.4 |
| Oxidative (3% H2O2) | 0 | 98.35 ± 2.1 | 24 | 97.67 ± 1.57 | 0 | 98.29 ± 1.76 | 24 | 88.12 ± 0.47 |
| Photolytic (UV) | 0 | 98.25 ± 1.23 | 24 | 97.94 ± 0.8 | 0 | 97.83 ± 0.37 | 24 | 69.19 ± 3.84 |
| Heat (80 °C) | 0 | 98.88 ± 1.9 | 2 | 97.62 ± 0.4 | 0 | 97.1 ± 4.08 | 2 | 89.72 ± 3.5 |
| Stress Study | Time of Exposure (h) | % Recovery (ATV) | Time of Exposure (h) | % Recovery (ATV) | Time of Exposure (h) | % Recovery (API) | Time of Exposure (h) | % Recovery (API) |
|---|---|---|---|---|---|---|---|---|
| Acidic (1 mM HCl) | 0 | 100.79 ± 1.31 | 24 | 99.22 ± 0.29 | 0 | 100.2 ± 0.34 | 24 | 99.53 ± 1.75 |
| Basic (1 mM NaOH) | 0 | 101.55 ± 5.02 | 24 | 101.99 ± 5.24 | 0 | 102.32 ± 2.49 | 24 | 98.35 ± 4.4 |
| Oxidative (3% H2O2) | 0 | 98.48 ± 11.24 | 24 | 97.7 ± 0.71 | 0 | 98.2 ± 10.83 | 24 | 98.44 ± 1.73 |
| Photolytic (UV) | 0 | 100.64 ± 9.37 | 24 | 99.37 ± 1.96 | 0 | 100.69 ± 10.09 | 24 | 98.4 ± 1.67 |
| Heat (80 °C) | 0 | 100.79 ± 1.31 | 2 | 101.28 ± 6.23 | 0 | 99.2 ± 0.44 | 2 | 93.94 ± 8.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashmar, S.A.; Abou Assi, R.; Alhijjaj, M.; Chan, S.Y. Development and Validation of a Simultaneous HPLC Stability-Indicating Method for Atorvastatin and Apigenin in a Novel SMEDDS Formulation Using Quality by Design (QbD) Approach. Processes 2025, 13, 2933. https://doi.org/10.3390/pr13092933
Kashmar SA, Abou Assi R, Alhijjaj M, Chan SY. Development and Validation of a Simultaneous HPLC Stability-Indicating Method for Atorvastatin and Apigenin in a Novel SMEDDS Formulation Using Quality by Design (QbD) Approach. Processes. 2025; 13(9):2933. https://doi.org/10.3390/pr13092933
Chicago/Turabian StyleKashmar, Sarmad Abdulabbas, Reem Abou Assi, Muqdad Alhijjaj, and Siok Yee Chan. 2025. "Development and Validation of a Simultaneous HPLC Stability-Indicating Method for Atorvastatin and Apigenin in a Novel SMEDDS Formulation Using Quality by Design (QbD) Approach" Processes 13, no. 9: 2933. https://doi.org/10.3390/pr13092933
APA StyleKashmar, S. A., Abou Assi, R., Alhijjaj, M., & Chan, S. Y. (2025). Development and Validation of a Simultaneous HPLC Stability-Indicating Method for Atorvastatin and Apigenin in a Novel SMEDDS Formulation Using Quality by Design (QbD) Approach. Processes, 13(9), 2933. https://doi.org/10.3390/pr13092933






