The Effect of Exogenous N-Acylated-L-Homoserine Lactones on the Remediation of Chromium-Contaminated Soil by Shewanella purefaciens
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Microbial Preparation
2.2. Soil Samples
2.3. Identification of AHLs in the Microbial Reduction of Cr(VI)
2.4. The Influence of AHLs on the Microbial Reduction of Cr(VI)
2.5. Analytical Methods
3. Results and Discussion
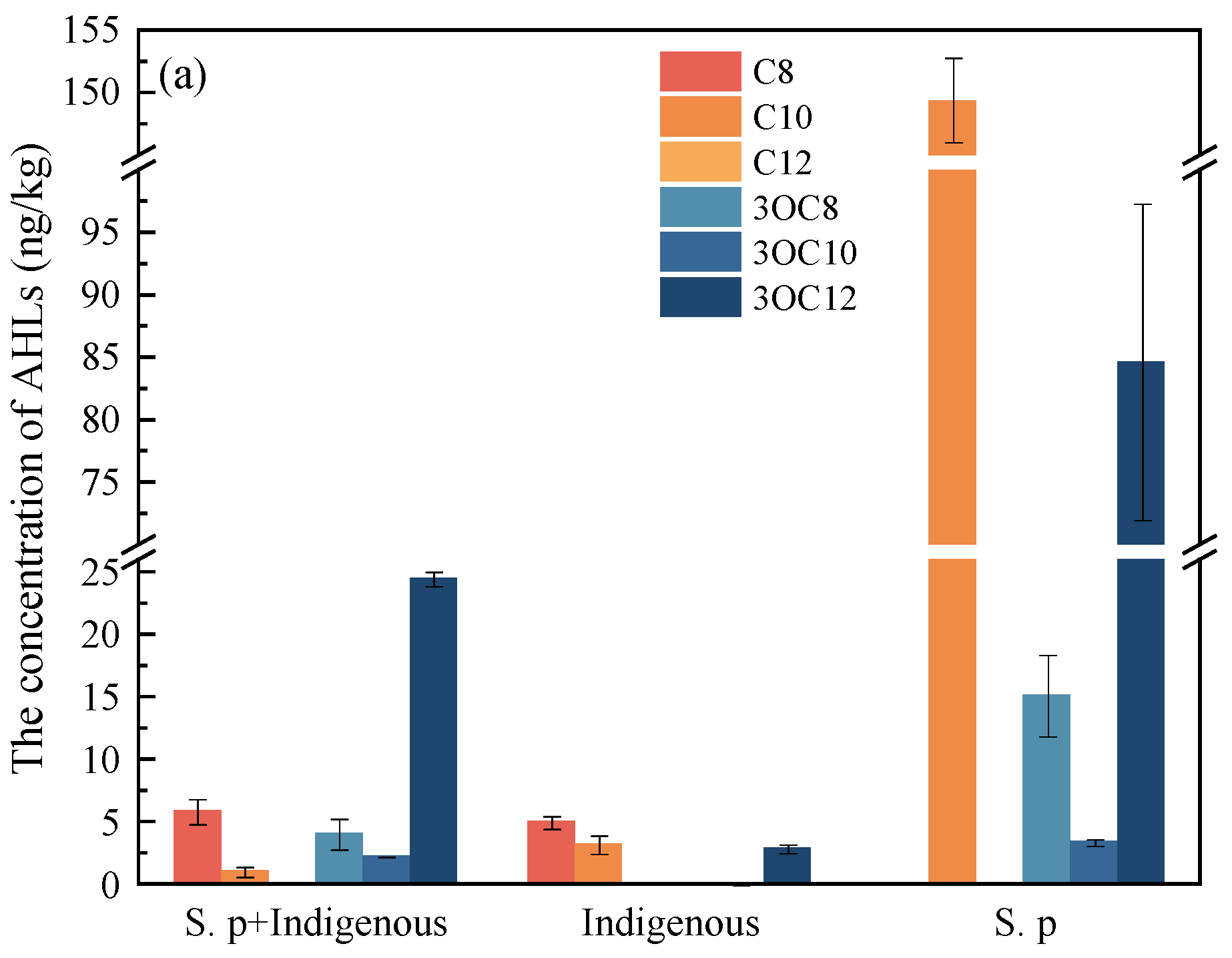
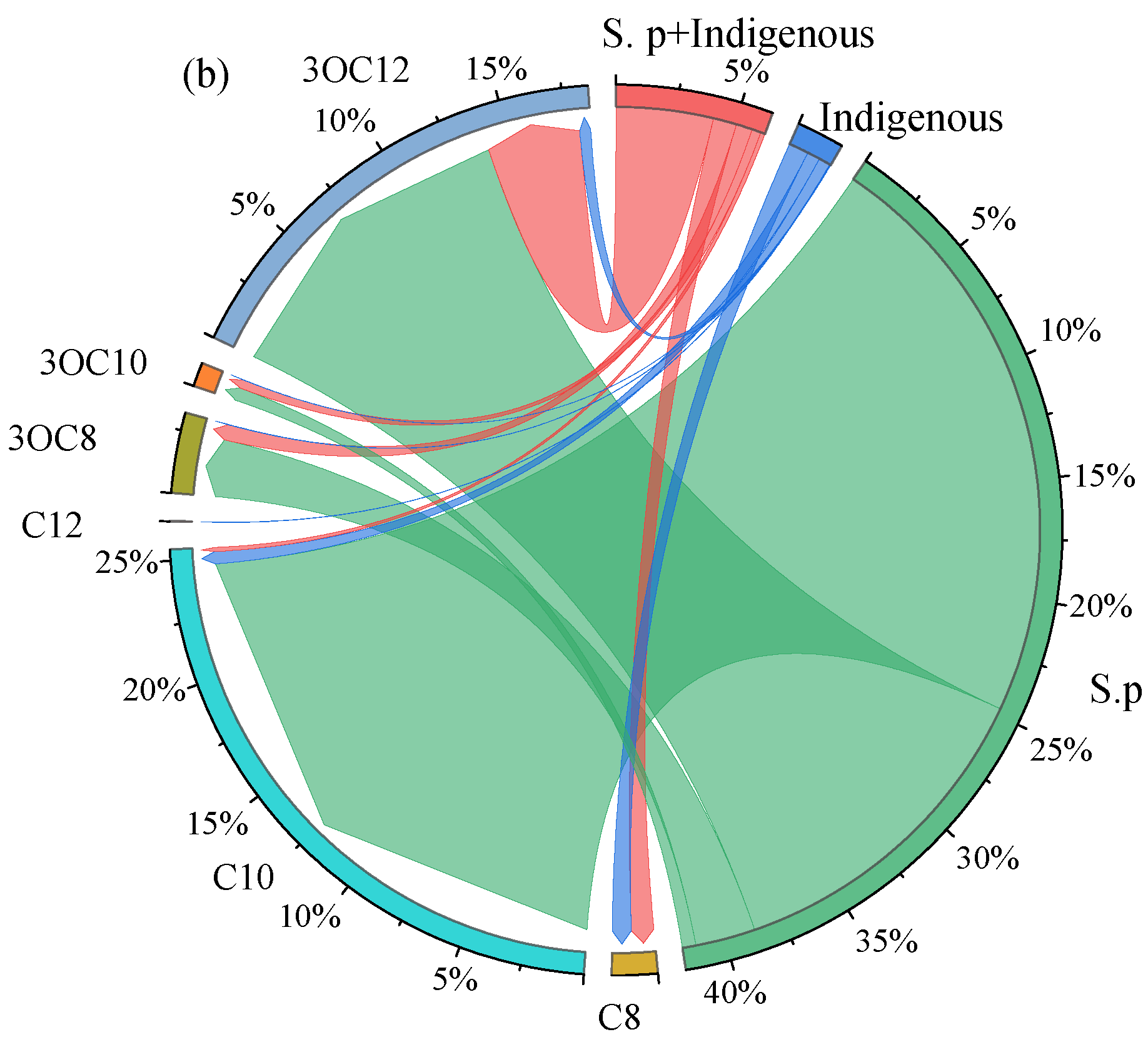
3.1. The Concentration of AHLs in the Cr(VI) Microbial Reduction
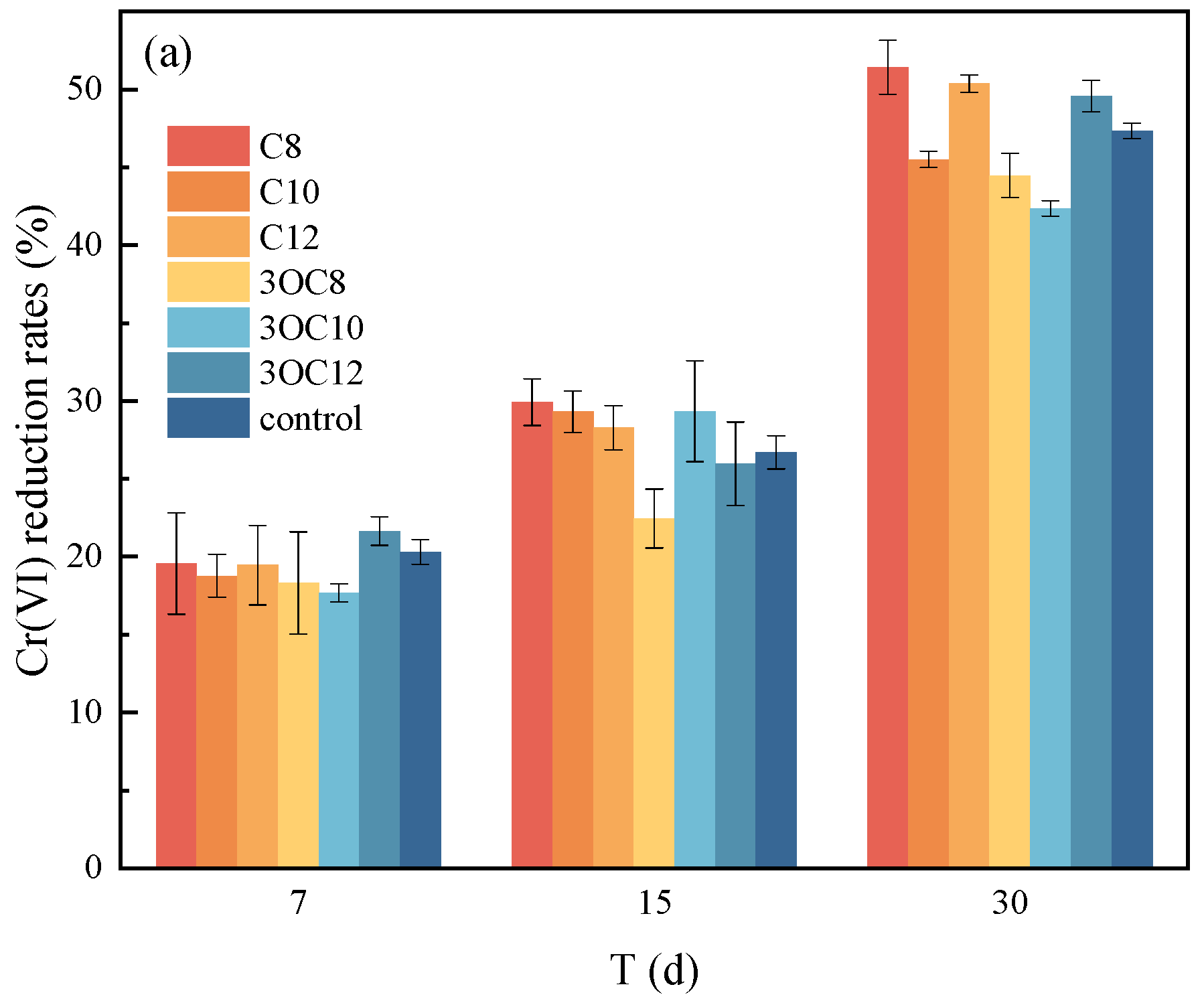
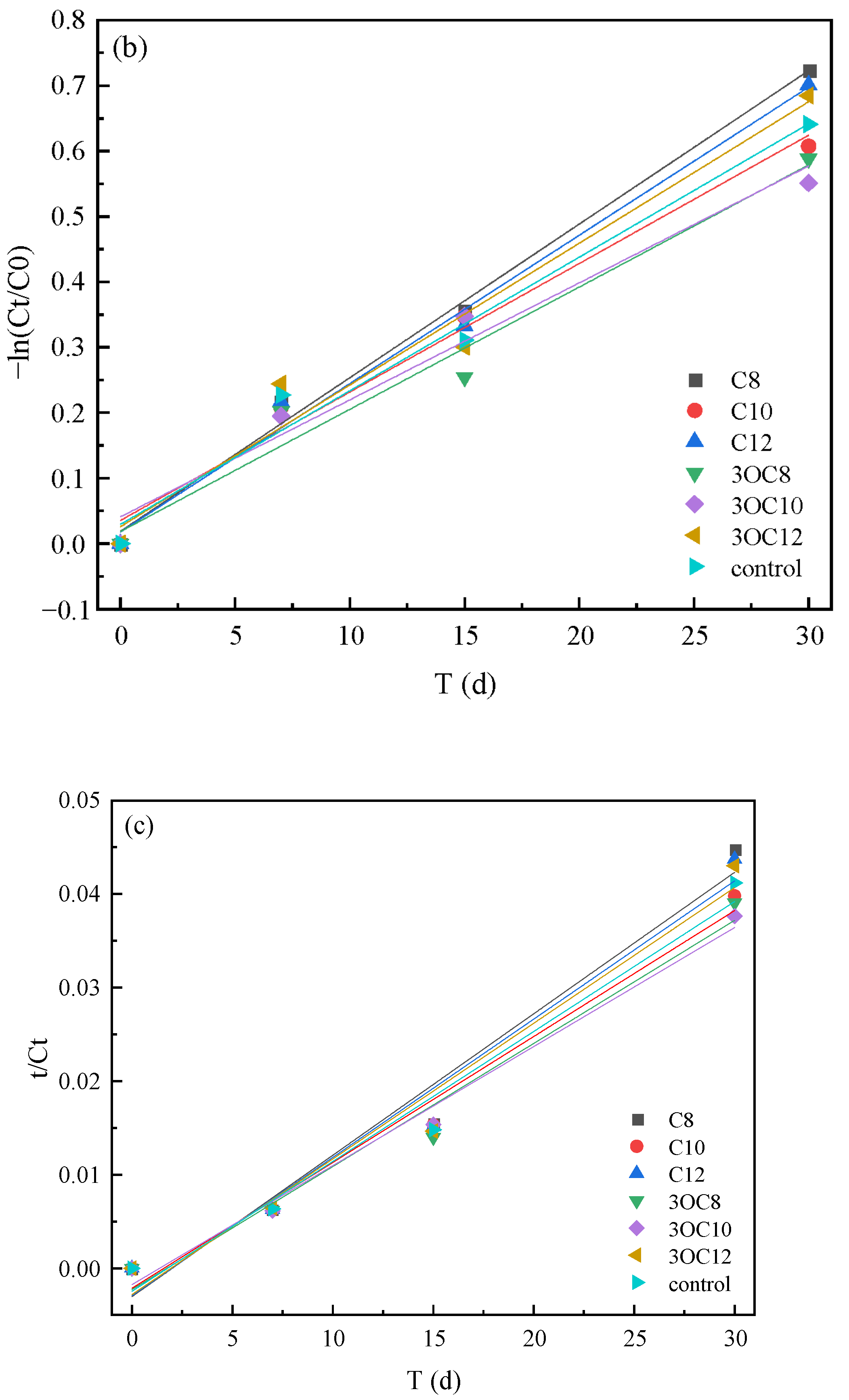
3.2. The Effect of AHLs on Cr(VI) Microbial Reduction
3.2.1. Cr(VI) Reduction by Indigenous Microorganisms
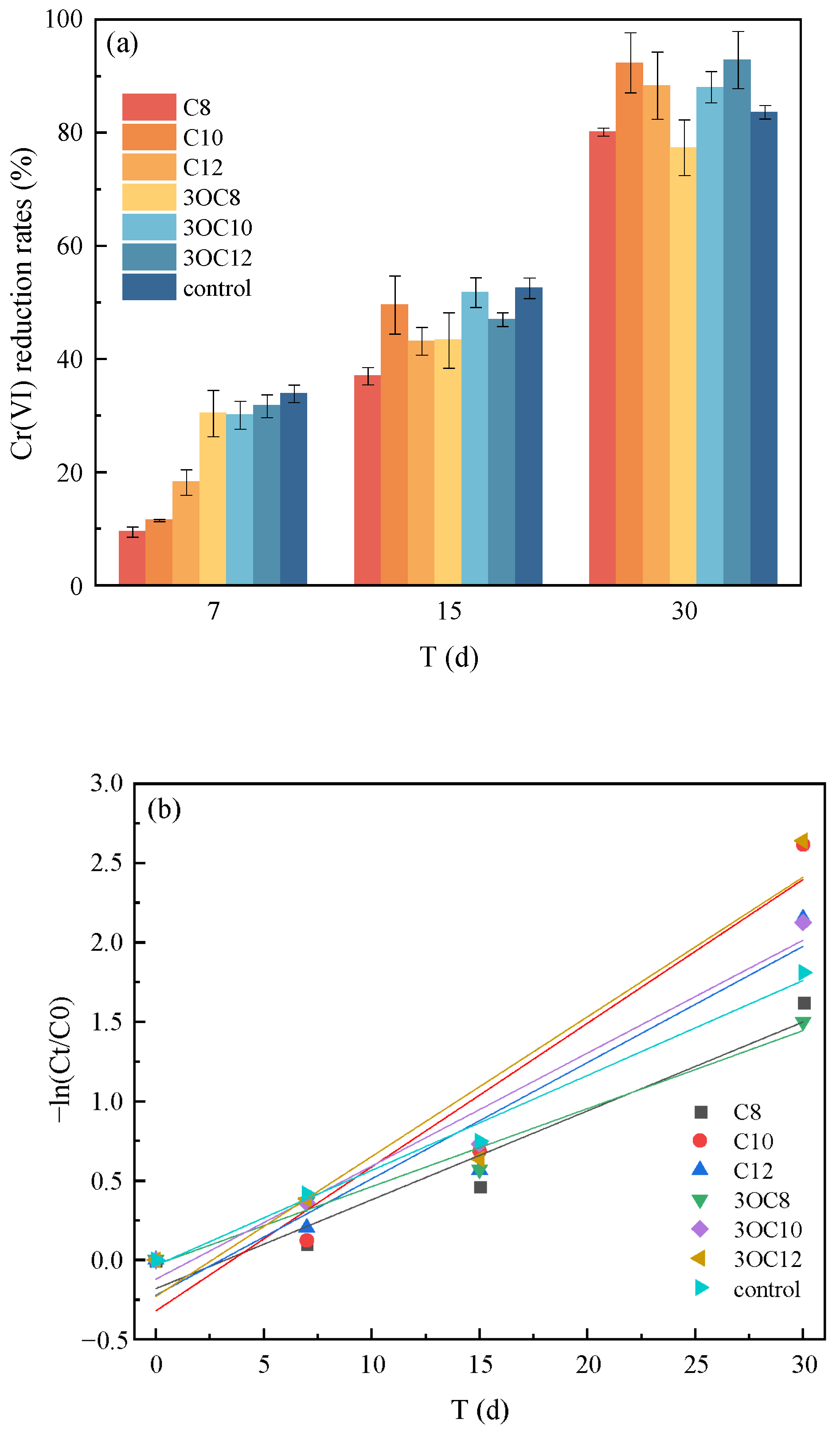
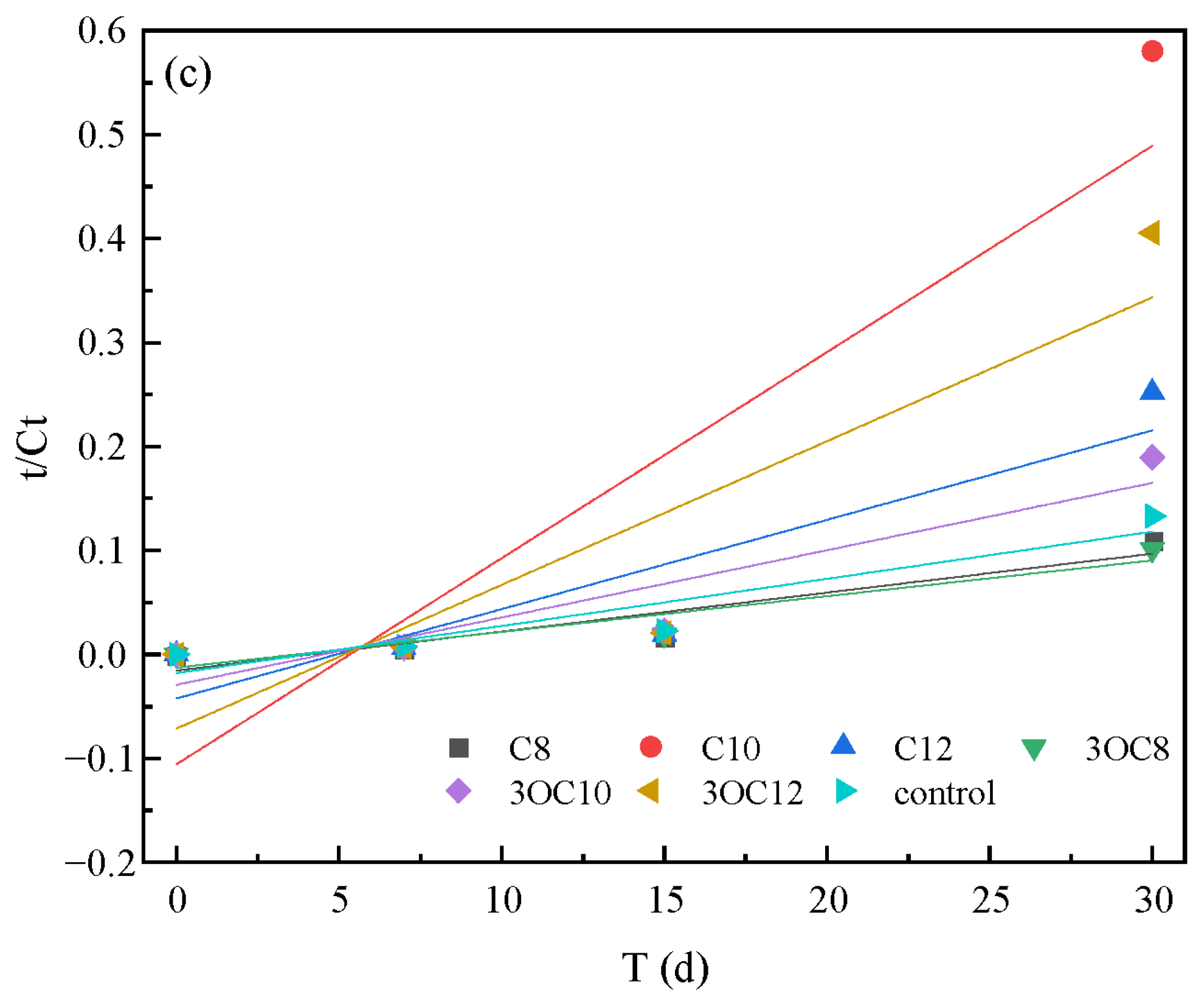
3.2.2. Cr(VI) Reduction by S. putrefaciens in Soil
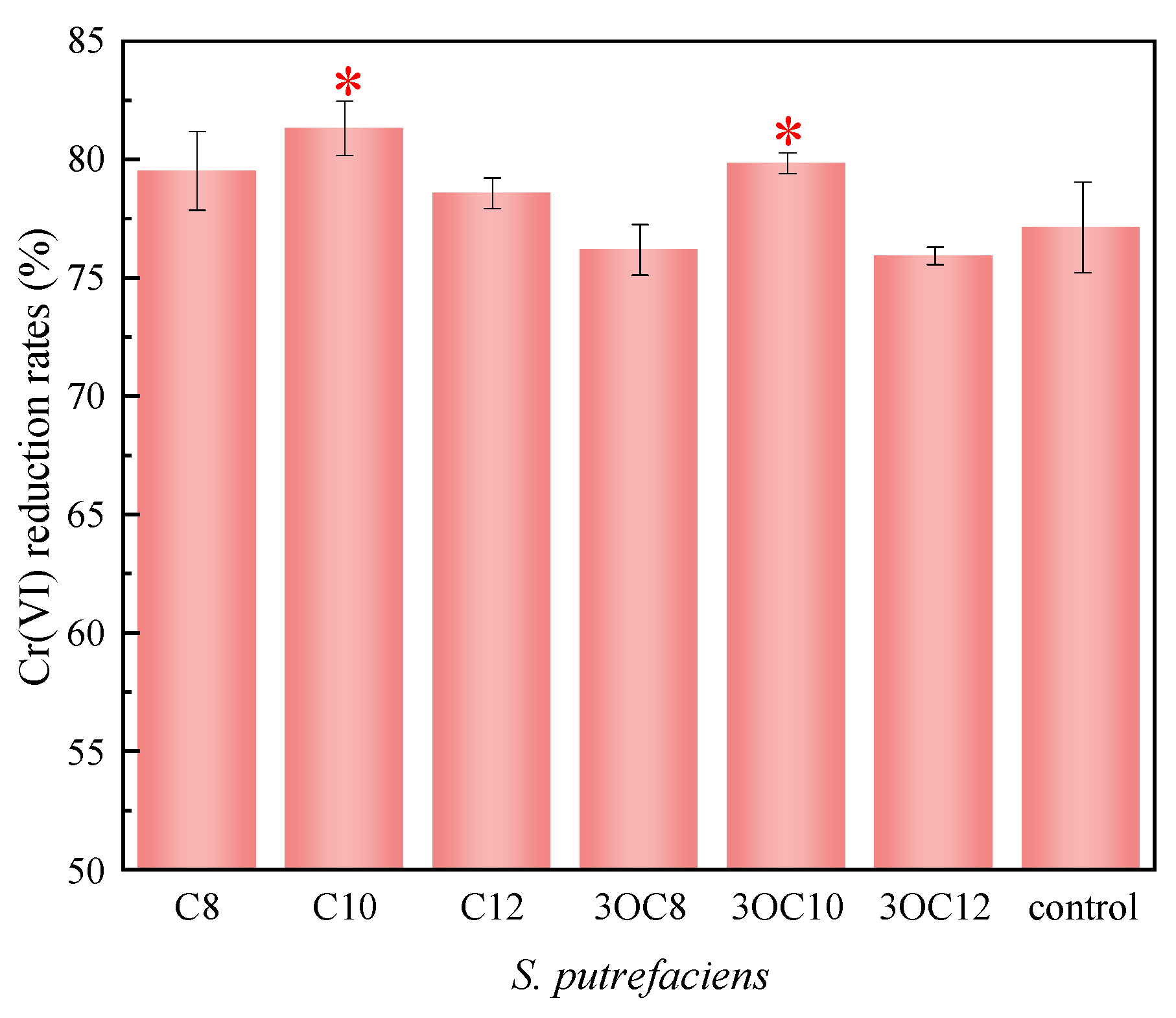
3.2.3. Cr(VI) Reduction by S. putrefaciens in Water
3.3. The Role of AHLs Played in the Remediation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, Y.; Sun, H.; Gao, B.; Dang, J.; Zhang, M.; Li, M.; Dong, J.; Wu, H.; Zhang, J.; Guo, Z. Enhanced Reduction of Cr(VI) in Iron-Carbon Micro-Electrolysis Constructed Wetlands: Mechanisms of Iron Cycle and Microbial Interactions. Chem. Eng. J. 2022, 439, 135742. [Google Scholar] [CrossRef]
- Ma, L.; Du, Y.; Chen, S.; Du, D.; Ye, H.; Zhang, T.C. Highly Efficient Removal of Cr(VI) from Aqueous Solution by Pinecone Biochar Supported Nanoscale Zero-Valent Iron Coupling with Shewanella oneidensis MR-1. Chemosphere 2022, 287, 132184. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, B.; Qiu, R.; Lai, C.; Jiang, Y.; He, C.; Guo, J. Microbial Chromate Reduction Coupled to Anaerobic Oxidation of Elemental Sulfur or Zerovalent Iron. Environ. Sci. Technol. 2019, 53, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wu, S.; Zhou, Z.; Wang, G. Microbial Cd(II) and Cr(VI) Resistance Mechanisms and Application in Bioremediation. J. Hazard. Mater. 2021, 401, 123685. [Google Scholar] [CrossRef]
- Gomathy, M.; Sabarinathan, K.G.; Subramaian, K.S.; Sivashankari Devi, T.; Ananthi, K.; Kalaiselvi, P.; Jeyshree, M. Microbial Remediation of Chromium. In Microbial Metabolism of Metals and Metalloids; Hurst, C.J., Ed.; Springer International Publishing: Cham, Swizterland, 2022; pp. 255–278. ISBN 978-3-030-97185-4. [Google Scholar]
- Aarthy, M.; Rajesh, T.; Thirunavoukkarasu, M. Critical Review on Microbial Fuel Cells for Concomitant Reduction of Hexavalent Chromium and Bioelectricity Generation. J. Chem. Technol. Biotechnol. 2020, 95, 1298–1307. [Google Scholar] [CrossRef]
- Tan, H.; Wang, C.; Zeng, G.; Luo, Y.; Li, H.; Xu, H. Bioreduction and Biosorption of Cr(VI) by a Novel Bacillus Sp. CRB-B1 Strain. J. Hazard. Mater. 2020, 386, 121628. [Google Scholar] [CrossRef]
- Cologgi, D.L.; Lampa-Pastirk, S.; Speers, A.M.; Kelly, S.D.; Reguera, G. Extracellular Reduction of Uranium via Geobacter Conductive Pili as a Protective Cellular Mechanism. Proc. Natl. Acad. Sci. USA 2011, 108, 15248–15252. [Google Scholar] [CrossRef]
- Karthik, C.; Ramkumar, V.S.; Pugazhendhi, A.; Gopalakrishnan, K.; Arulselvi, P.I. Biosorption and Biotransformation of Cr(VI) by Novel Cellulosimicrobium funkei Strain AR6. J. Taiwan Inst. Chem. Eng. 2017, 70, 282–290. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Nie, K.; Cao, K.; Liao, Q.; Si, M.; Yang, Z.; Yang, W. Synergistic Effect of Sulfidated Nano Zerovalent Iron and Proton-Buffering Montmorillonite in Reductive Immobilization of Alkaline Cr(VI)-Contaminated Soil. Chemosphere 2023, 321, 138132. [Google Scholar] [CrossRef]
- Meng, Y.; Zhao, Z.; Burgos, W.D.; Li, Y.; Zhang, B.; Wang, Y.; Liu, W.; Sun, L.; Lin, L.; Luan, F. Iron(III) Minerals and Anthraquinone-2,6-Disulfonate (AQDS) Synergistically Enhance Bioreduction of Hexavalent Chromium by Shewanella Oneidensis MR-1. Sci. Total Environ. 2018, 640–641, 591–598. [Google Scholar] [CrossRef]
- Zou, D.; Tong, J.; Feng, C.; Wang, Y.; Li, X.; Zheng, X.; Wang, X.; Liu, Y. Synthesis of Biochar@α-Fe2O3@Shewanella Loihica Complex for Remediation of Soil Contaminated by Hexavalent Chromium: Optimization of Conditions and Mechanism. Chemosphere 2022, 303, 134858. [Google Scholar] [CrossRef]
- Yang, C.; Wei, J.; Li, Y.; Peng, Y.; Wu, X.; Zhang, X.; Ding, Y.; Lu, S.; Feng, C.; Huang, C. Riboflavin-Mediated Reactivation of Aged Nano Zero-Valent Iron by Shewanella oneidensis MR-1 for Sustained Cr(VI) Detoxification. Bioresour. Technol. 2025, 436, 133006. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.; Wu, Y.; Yuan, Y.; Du, Y. Enhanced Bio-Reduction of Cr(VI) Using Shewanella Putrefaciens CN32 Mediated by Fe(III) Minerals and Riboflavin Synergistically. Biodegradation 2025, 36, 25. [Google Scholar] [CrossRef]
- Wielinga, B.; Mizuba, M.M.; Hansel, C.M.; Fendorf, S. Iron Promoted Reduction of Chromate by Dissimilatory Iron-Reducing Bacteria. Environ. Sci. Technol. 2001, 35, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum Sensing Signal–Response Systems in Gram-Negative Bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and Promise of Bacterial Quorum Sensing Research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial Volatile Organic Compounds in Intra-Kingdom and Inter-Kingdom Interactions. Nat. Rev. Microbiol. 2021, 19, 391–404. [Google Scholar] [CrossRef]
- Huang, J.; Shi, Y.; Zeng, G.; Gu, Y.; Chen, G.; Shi, L.; Hu, Y.; Tang, B.; Zhou, J. Acyl-Homoserine Lactone-Based Quorum Sensing and Quorum Quenching Hold Promise to Determine the Performance of Biological Wastewater Treatments: An Overview. Chemosphere 2016, 157, 137–151. [Google Scholar] [CrossRef]
- Shrout, J.D.; Nerenberg, R. Monitoring Bacterial Twitter: Does Quorum Sensing Determine the Behavior of Water and Wastewater Treatment Biofilms? Environ. Sci. Technol. 2012, 46, 1995–2005. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, X.; Zheng, J.; Zou, Y.; Qiu, S.; Dai, Y. AHL-Mediated Quorum Sensing to Regulate Bacterial Substance and Energy Metabolism: A Review. Microbiol. Res. 2022, 262, 127102. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Liang, J.; Li, Y.; Zhang, J.; Fang, W.; Zhang, P.; Zhang, G.; Hao Ngo, H. Improvement of Anaerobic Digestion Containing Sulfur with Conductive Materials: Focusing on Recent Advances and Internal Biological Mechanisms. Chem. Eng. J. 2023, 472, 144867. [Google Scholar] [CrossRef]
- Du, Q.; Mu, Q.; Wu, G. Metagenomic and Bioanalytical Insights into Quorum Sensing of Methanogens in Anaerobic Digestion Systems with or without the Addition of Conductive Filter. Sci. Total Environ. 2021, 763, 144509. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, I.; Bolognini, M.; Ricci, J.; Bini, E.; Vetriani, C. From Deep-Sea Volcanoes to Human Pathogens: A Conserved Quorum-Sensing Signal in Epsilonproteobacteria. ISME J. 2015, 9, 1222–1234. [Google Scholar] [CrossRef]
- Jing, X.; Liu, X.; Deng, C.; Chen, S.; Zhou, S. Chemical Signals Stimulate Geobacter Soli Biofilm Formation and Electroactivity. Biosens. Bioelectron. 2019, 127, 1–9. [Google Scholar] [CrossRef]
- Chen, S.; Jing, X.; Tang, J.; Fang, Y.; Zhou, S. Quorum Sensing Signals Enhance the Electrochemical Activity and Energy Recovery of Mixed-Culture Electroactive Biofilms. Biosens. Bioelectron. 2017, 97, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-L.; Xu, Q.; Sun, J.-D.; Li, C.-R.; Yang, Q.-W.; Li, B.; Zhang, X.-Y.; Zhou, J.; Yong, X.-Y. Quorum Sensing Signals Improve the Power Performance and Chlortetracycline Degradation Efficiency of Mixed-Culture Electroactive Biofilms. iScience 2022, 25, 104299. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.-C.; Wu, X.-Y.; Sun, J.-Z.; Cao, Y.-X.; Song, H. Engineering Quorum Sensing Signaling of Pseudomonas for Enhanced Wastewater Treatment and Electricity Harvest: A Review. Chemosphere 2015, 140, 18–25. [Google Scholar] [CrossRef]
- Edel, M.; Sturm, G.; Sturm-Richter, K.; Wagner, M.; Ducassou, J.N.; Couté, Y.; Horn, H.; Gescher, J. Extracellular Riboflavin Induces Anaerobic Biofilm Formation in Shewanella Oneidensis. Biotechnol. Biofuels 2021, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Paquete, C.M.; Rosenbaum, M.A.; Bañeras, L.; Rotaru, A.-E.; Puig, S. Let’s Chat: Communication between Electroactive Microorganisms. Bioresour. Technol. 2022, 347, 126705. [Google Scholar] [CrossRef]
- García-Contreras, R.; Nuñez-López, L.; Jasso-Chávez, R.; Kwan, B.W.; Belmont, J.A.; Rangel-Vega, A.; Maeda, T.; Wood, T.K. Quorum Sensing Enhancement of the Stress Response Promotes Resistance to Quorum Quenching and Prevents Social Cheating. ISME J. 2015, 9, 115–125. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Harrison, J.J.; Ceri, H.; Turner, R.J. Multimetal Resistance and Tolerance in Microbial Biofilms. Nat. Rev. Microbiol. 2007, 5, 928–938. [Google Scholar] [CrossRef]
- Xie, X.; Yin, S.; Zhang, X.; Tian, Q.; Zeng, Y.; Zhang, X. Boron-Dependent Autoinducer-2-Mediated Quorum Sensing Stimulates the Cr(VI) Reduction of Leucobacter chromiireducens CD49. J. Environ. Manag. 2025, 375, 124290. [Google Scholar] [CrossRef]
- Qu, C.; Tang, J.; Liu, J.; Wang, W.; Song, F.; Cheng, S.; Tang, X.; Tang, C.-J. Quorum Sensing-Enhanced Electron Transfer in Anammox Consortia: A Mechanism for Improved Resistance to Variable-Valence Heavy Metals. J. Hazard. Mater. 2025, 487, 137130. [Google Scholar] [CrossRef] [PubMed]
- Ryden, J.C.; Syers, J.K.; Tillman, R.W. Inorganic Anion Sorption and Interactions with Phosphate Sorption by Hydrous Ferric Oxide Gel. J. Soil Sci. 1987, 38, 211–217. [Google Scholar] [CrossRef]
- Li, Y.-C.; Zhu, J.-R. Role of N-Acyl Homoserine Lactone (AHL)-Based Quorum Sensing (QS) in Aerobic Sludge Granulation. Appl. Microbiol. Biotechnol. 2014, 98, 7623–7632. [Google Scholar] [CrossRef]
- Li, X.; Fekete, A.; Englmann, M.; Götz, C.; Rothballer, M.; Frommberger, M.; Buddrus, K.; Fekete, J.; Cai, C.; Schröder, P.; et al. Development and Application of a Method for the Analysis of N-Acylhomoserine Lactones by Solid-Phase Extraction and Ultra High Pressure Liquid Chromatography. J. Chromatogr. A 2006, 1134, 186–193. [Google Scholar] [CrossRef]
- Hu, H.; He, J.; Liu, J.; Yu, H.; Zhang, J. Biofilm Activity and Sludge Characteristics Affected by Exogenous N-Acyl Homoserine Lactones in Biofilm Reactors. Bioresour. Technol. 2016, 211, 339–347. [Google Scholar] [CrossRef]
- Wang, S.; Payne, G.F.; Bentley, W.E. Quorum Sensing Communication: Molecularly Connecting Cells, Their Neighbors, and Even Devices. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 447–468. [Google Scholar] [CrossRef]
- Zheng, X.; Tong, J.; Zhou, S.; Liu, Y.; Liu, G.; Zou, D. Remediation of Hexavalent Chromium Contaminated Soils by Stimulating Indigenous Microorganisms: Optimization, Community Succession and Applicability. J. Environ. Manag. 2024, 372, 123222. [Google Scholar] [CrossRef]
| Soil Type | Loam Soil |
|---|---|
| pH b | 7.36 ± 0.06 |
| Moisture content (%) a | 18.55% ± 0.43 |
| Soil organic matter (g/kg) b | 9.75 ± 1.39 |
| Cr(VI) concentration (mg/kg) b | 1383.06 ± 42.26 |
| AHLs | Time (d) | Pseudo-First-Order | Pseudo-Second-Order | |||
|---|---|---|---|---|---|---|
| k1 (d−1) a | R2 b | k2 (d−1) a | R2 b | |||
| S. putrefaciens | C8 | 0–30 | 0.0560 ± 0.0010 | 0.91 | 0.0038 ± 0.0009 | 0.82 |
| C10 | 0.0906 ± 0.0177 | 0.89 | 0.0198 ± 0.0070 | 0.70 | ||
| C12 | 0.0733 ± 0.0136 | 0.90 | 0.0086 ± 0.0028 | 0.74 | ||
| 3OC8 | 0.0492 ± 0.0051 | 0.97 | 0.0034 ± 0.0008 | 0.85 | ||
| 3OC10 | 0.0712 ± 0.0086 | 0.96 | 0.0065 ± 0.0019 | 0.78 | ||
| 3OC12 | 0.0881 ± 0.0177 | 0.89 | 0.0138 ± 0.0048 | 0.71 | ||
| control | 0.0598 ± 0.0043 | 0.98 | 0.0045 ± 0.0012 | 0.83 | ||
| control | C8 | 0.0235 ± 0.0013 | 0.99 | 0.0015 ± 0.0002 | 0.96 | |
| C10 | 0.0196 ± 0.0018 | 0.98 | 0.0014 ± 0.0001 | 0.97 | ||
| C12 | 0.0227 ± 0.0016 | 0.99 | 0.0015 ± 0.0002 | 0.96 | ||
| 3OC8 | 0.0187 ± 0.0023 | 0.96 | 0.0013 ± 0.0001 | 0.96 | ||
| 3OC10 | 0.0179 ± 0.0022 | 0.96 | 0.0013 ± 0.0001 | 0.98 | ||
| 3OC12 | 0.0216 ± 0.0028 | 0.95 | 0.0015 ± 0.0002 | 0.95 | ||
| control | 0.0204 ± 0.0021 | 0.97 | 0.0014 ± 0.0002 | 0.96 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Zheng, C.; Zhou, S.; Zou, D. The Effect of Exogenous N-Acylated-L-Homoserine Lactones on the Remediation of Chromium-Contaminated Soil by Shewanella purefaciens. Processes 2025, 13, 2931. https://doi.org/10.3390/pr13092931
Zheng X, Zheng C, Zhou S, Zou D. The Effect of Exogenous N-Acylated-L-Homoserine Lactones on the Remediation of Chromium-Contaminated Soil by Shewanella purefaciens. Processes. 2025; 13(9):2931. https://doi.org/10.3390/pr13092931
Chicago/Turabian StyleZheng, Xusheng, Chenglong Zheng, Shufang Zhou, and Dexun Zou. 2025. "The Effect of Exogenous N-Acylated-L-Homoserine Lactones on the Remediation of Chromium-Contaminated Soil by Shewanella purefaciens" Processes 13, no. 9: 2931. https://doi.org/10.3390/pr13092931
APA StyleZheng, X., Zheng, C., Zhou, S., & Zou, D. (2025). The Effect of Exogenous N-Acylated-L-Homoserine Lactones on the Remediation of Chromium-Contaminated Soil by Shewanella purefaciens. Processes, 13(9), 2931. https://doi.org/10.3390/pr13092931





