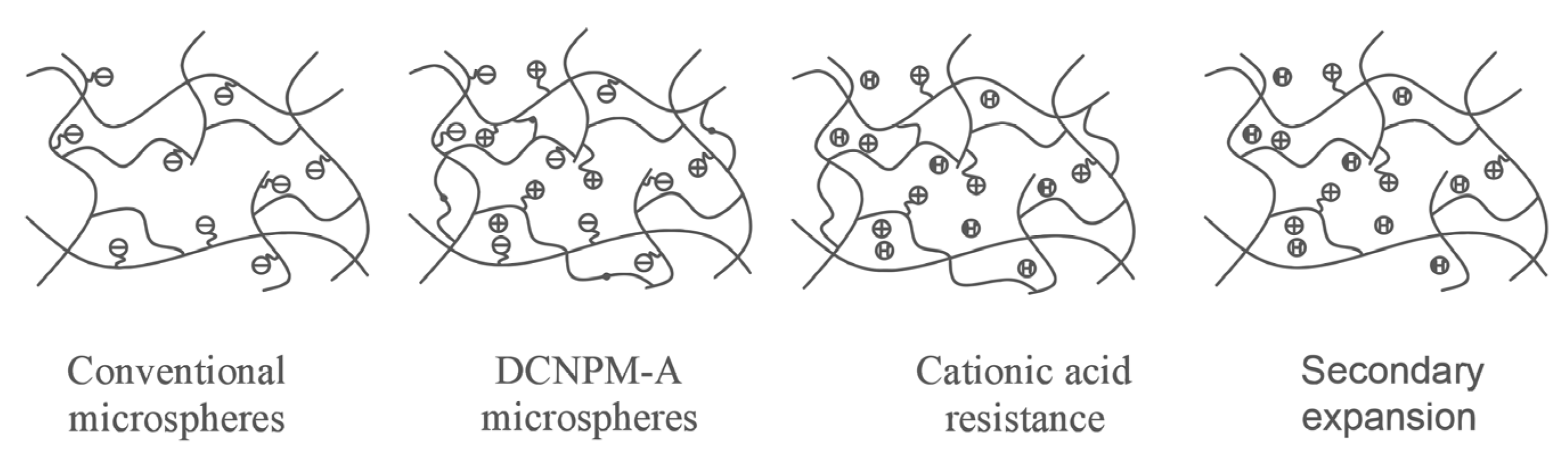
1. Introduction
Because of its good miscibility with crude oil and ease of injection into reservoirs, CO
2 can be used as an efficient oil displacement agent for developing low-permeability reservoirs. However, as reservoir fractures develop, CO
2 entering the fractures may cause serious fingering phenomena, reduce the affected volume, and cause serious gas channeling problems, thereby reducing the oil displacement effect [
1,
2]. In order to solve the problem of gas channeling in low-permeability reservoirs, water alternating gas (WAG) technology [
3,
4], CO
2 thickening technology [
5,
6], CO
2 foam technology [
7], thickened foam plugging technology [
8,
9], and gel plugging technology [
10,
11] are mainly used to solve the problem [
12]. However, most of these plugging technologies are used in shallow formations and have little effect on plugging control and channeling prevention in deep reservoirs. Therefore, as polymer microspheres and gel particles that can be used for deep plugging control and channeling prevention, they show great advantages. After injecting polymer microspheres into the formation, they can selectively enter high-permeability channels and aggregate to block, thereby guiding subsequent displacement media to shift towards unaffected areas [
13]. Under pressurized conditions, these microspheres can migrate deeper into the formation through elastic deformation, achieving step-by-step sealing. However, existing microspheres have problems such as rapid expansion and degradation of mechanical strength when exposed to water, making it difficult to maintain long-term stable deep plugging effects. Especially in the long-term CO
2 flooding process, conventional polymer microspheres generally suffer from decreased expansion performance or even failure due to the influence of the acidic environment.
In recent years, considerable efforts have been devoted to improving the CO
2 resistance of particle-based conformance control agents. Two typical approaches have attracted particular attention. The first is the development of delayed-swelling polymer microspheres, which remain minimally swollen during injection and undergo secondary expansion when exposed to reservoir temperature or acidic CO
2 conditions. This mechanism enhances deep migration and targeted sealing performance; however, existing systems often suffer from reduced long-term stability in supercritical CO
2 environments and limited swelling ratios under high-salinity conditions [
14]. The second approach involves the design of recrosslinkable CO
2-resistant preformed particle gels, which can further crosslink after placement to improve structural strength and plugging durability. While this method shows improved tolerance to CO
2-rich environments, the relatively large particle size constrains their migration in low-permeability formations, and the synthesis cost remains high [
15]. Despite these advances, both strategies still face challenges in simultaneously achieving deep injectivity, acid/thermal resistance, and long-term structural stability. Therefore, improving the acid resistance of polymer microspheres in a CO
2 environment and maintaining stable sealing ability in deep formations has become an important direction for current and future scientific research and development.
In order to achieve the deep sealing effect of polymer microspheres, the “double crosslinking” method can be used, which means that polymer microspheres enter the formation with a lower swelling ratio. After reaching the designated position of the formation, they fully expand to seal the formation [
16,
17]. “Double crosslinking” is a technique in which stable and unstable crosslinking agents are simultaneously added to polymer microspheres. Double crosslinking microspheres are slowly decomposed by temperature or pH changes, resulting in a decrease in crosslinking density and an increase in swelling performance. Therefore, double crosslinking technology can be used to make polymer microspheres have low swelling and small particle size in shallow formations, and to reduce crosslinking density after deep formation to achieve secondary expansion and effective sealing of deep layers. Yang et al. [
18] developed a high salt-resistant gel system composed of amphiphilic polymer and organic chromium, which has a plugging efficiency of more than 95% for the formation, showing excellent plugging performance, and can effectively adjust the formation heterogeneity. Zhou et al. [
19] designed a new type of gel based on the inclusion of amphiphilic polymer and cyclodextrin polymer. The system not only showed good shear stability and long-term stability, but also achieved a plugging efficiency of 96.94%. Zhu Qiangjuan et al. [
20] prepared double crosslinked microspheres with delayed swelling characteristics by combining stable crosslinker N, N-methylenebisacrylamide (MBA) and a temperature-sensitive ester unstable crosslinker. The microsphere has a low swelling rate at formation temperatures below 60 °C, but upon entering high-temperature deep formations, ester bond breakage triggers secondary expansion, thereby achieving deep sealing. Yuan Cheng et al. [
21] synthesized an ester-based unstable crosslinking agent LC using dimethylaminoethyl acrylate, 1,4-dibromobutane, and phenothiazine as raw materials. Ester groups can regulate the hydrolysis rate of LC, and further combined with MBA prepared double crosslinked nanospheres, LC exhibits controllable slow swelling behavior in weakly acidic environments, effectively achieving delayed expansion function.
The double crosslinking technology regulates the crosslinking density of microspheres by adjusting the addition ratio of unstable crosslinking agents, thereby achieving the effect of delayed swelling. This method, due to its strong controllability and easy operation, has become one of the most mature and promising technologies for achieving secondary expansion of microspheres. Therefore, this paper develops a new type of double crosslinked microsphere and characterizes it, focusing especially on its performance and acid resistance mechanism under acidic conditions. The sealing effect of the microsphere is verified and optimized through experimental testing of the sealing performance of rock cracks.
2. Development and Characterization of Double Crosslinked Nanospheres
In this chapter, a new type of microsphere was successfully prepared by inverse micro-lotion polymerization [
22,
23] and systematically characterized by zeta (Malvern Instruments, Malvern, UK) potential test, SEM (JEOL, Tokyo, Japan) microscopic observation, EDS (JEOL, Tokyo, Japan) element analysis, and FTIR (Thermo Fisher Scientific, Waltham, MA, USA) spectrum characterization. In addition, to further investigate the mechanism of action of the double crosslinked structure, the influence of different crosslinking agents on the swelling behavior of microspheres was examined.
2.1. Development of Double Crosslinked Nanospheres
- (1)
Materials and Reagents
The chemical reagents and instruments required for this experiment are shown in
Table 1 and
Table 2.
- (2)
Synthesis and preparation of double crosslinked nanospheres
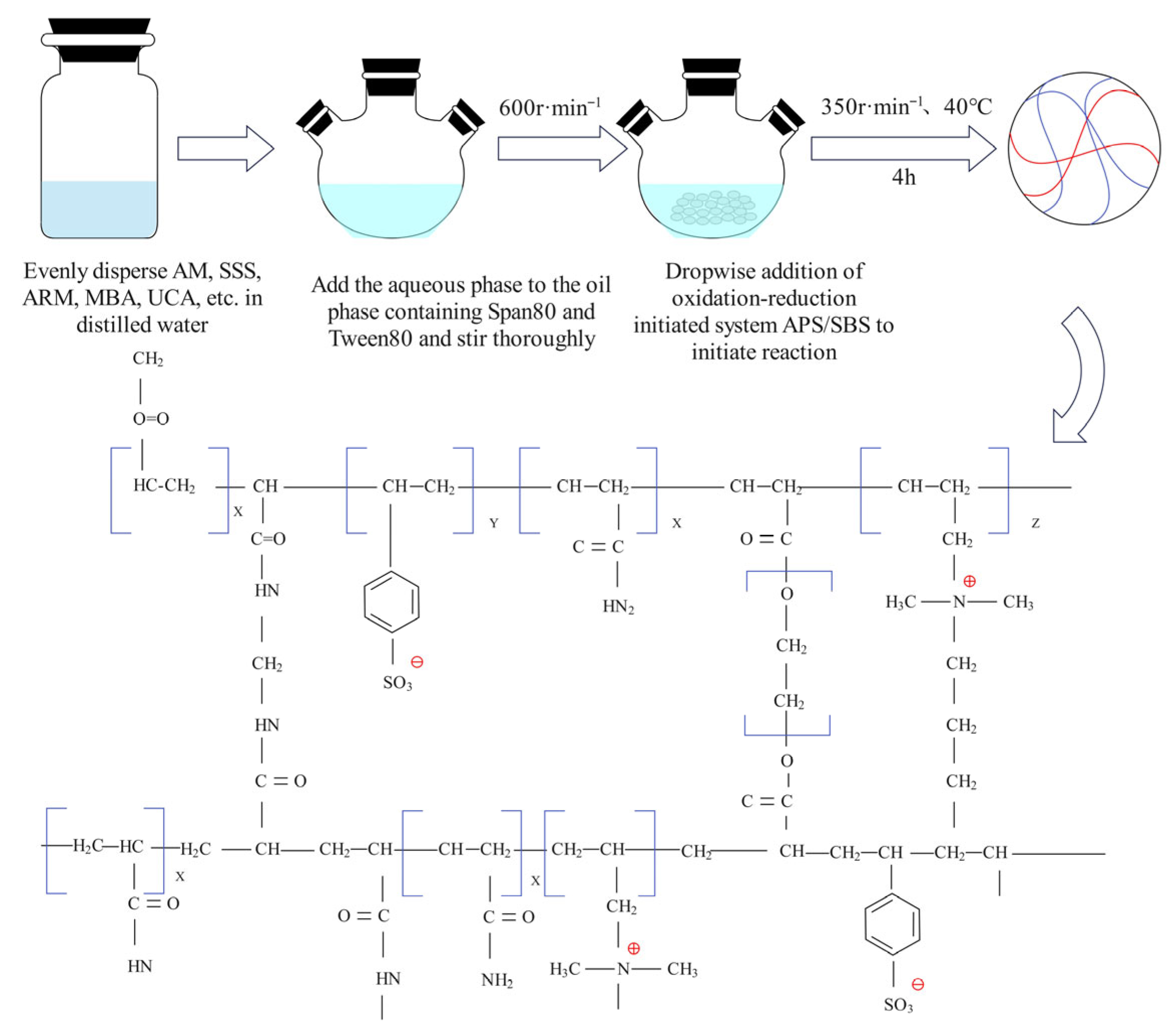
In this experiment, DCNPM-A microspheres were prepared by inverse microemulsion polymerization (
Figure 1). Firstly, 2.283 g of ARM aqueous solution and 0.4 g of UCA aqueous solution were accurately weighed and dissolved in an appropriate amount of deionized water (control the total mass of the aqueous phase to 14.175 g), and then 11.641 g of AM, 0.685 g of SSS, and 0.185 g of MBA were dissolved in turn. After each test, the agent was completely dissolved, and then continued to stir for 5 min. Then, connect the dissolved water to nitrogen for 10 min and sonicate for 10 min to remove oxygen for later use. Next, prepare the oil phase by dissolving 12.7 g of Span80 and 5.42 g of Tween80 in 53.52 g of white oil, stirring to dissolve, passing nitrogen to remove oxygen, and transferring to a four-necked flask. Under stirring at 600 rpm and nitrogen protection, the aqueous phase is dropped into the oil phase at a rate of 1–2 drops per second. After the system becomes clear, the speed is reduced to 350 rpm and maintained in a 40 °C water bath. Then, ammonium persulfate and sodium bisulfite solutions are sequentially added dropwise to initiate the polymerization reaction for 4 h. After the reaction is complete, wash 3–5 times with anhydrous ethanol to remove the oil phase, dry at 50 °C for 24 h, grind and sieve, and finally obtain the solid product of DCNPM-A microspheres.
2.2. Factors Affecting the Preparation of Double Crosslinked Nanospheres
This section focuses on the influence of key parameters such as reaction temperature, reaction time, initiator dosage, and crosslinking agent dosage on the initial particle size and swelling performance of DCNPMA microspheres. By systematically investigating the mechanisms of each factor and optimizing the synthesis process conditions, the aim is to prepare double crosslinked nanospheres with the smallest initial particle size and optimal swelling performance.
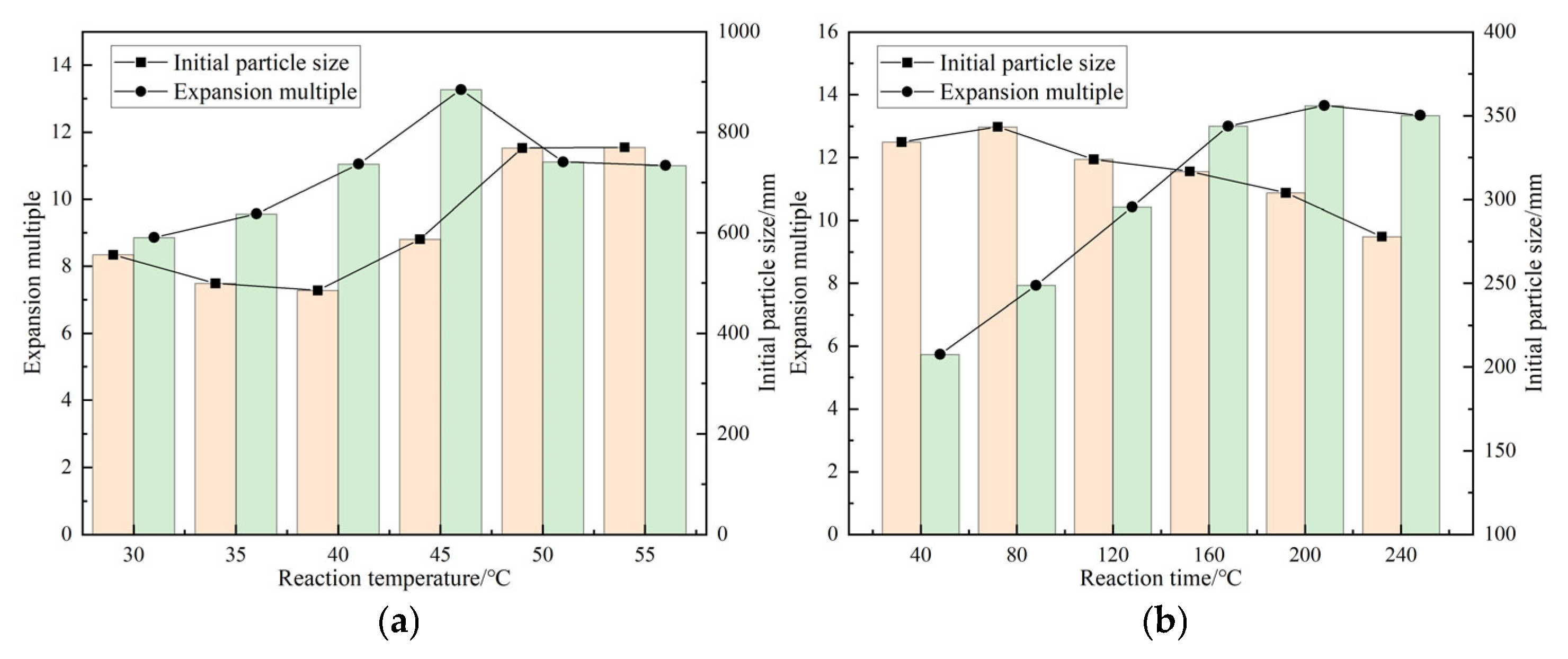
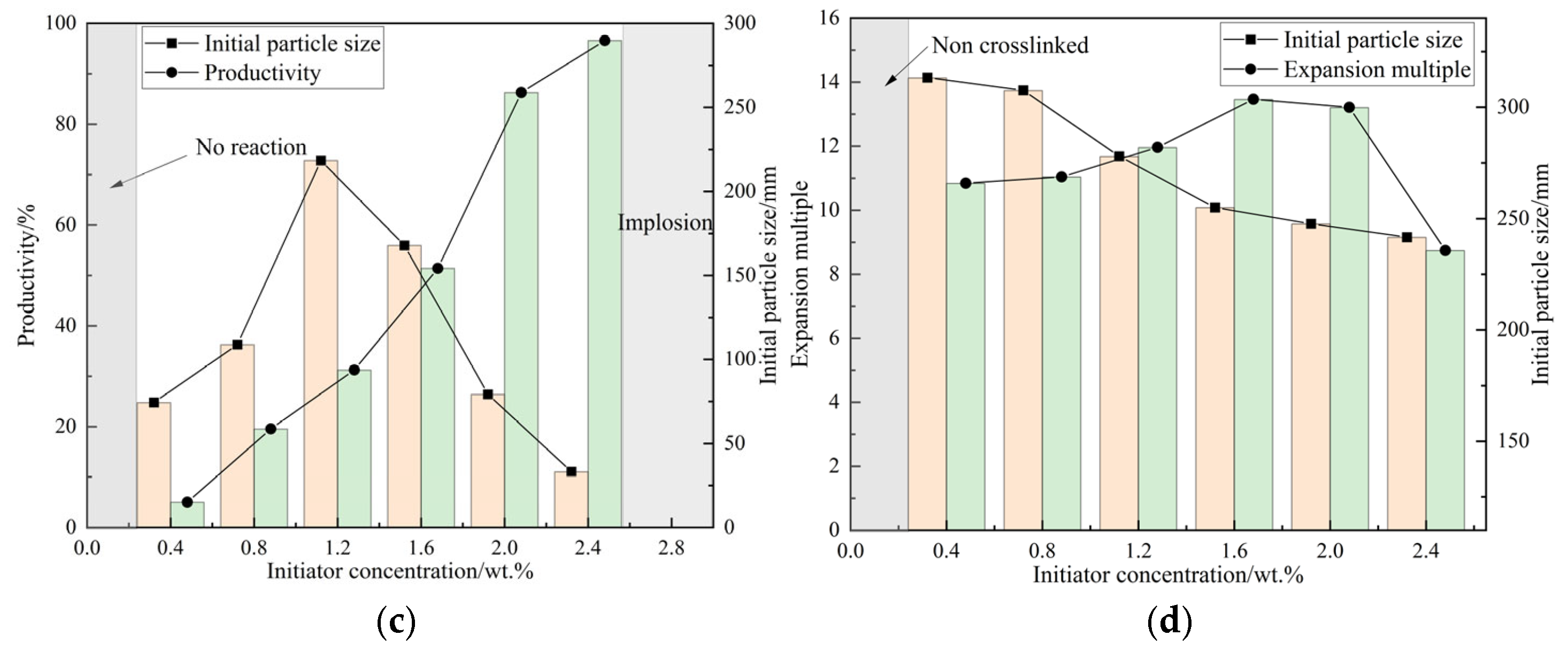
As shown in
Figure 2, in the study of optimizing the synthesis process of DCNPM-A microspheres, experimental results showed that reaction parameters have a significant impact on the performance of microspheres. Under the conditions of fixed initiator (2.0 wt.%) and crosslinking agent (1.5 wt.%) dosage and 4 h reaction time, the initial particle size of microspheres showed a trend of first decreasing and then increasing with the increase in reaction temperature (reaching the minimum value at 40 °C), while the expansion ratio first increased and then decreased (reaching the maximum value at 45 °C). Taking the average, 43 °C was determined as the optimal reaction temperature. At this temperature, the effect of reaction time (0–4 h) was examined, and it was found that the initial particle size first increased and then decreased, and the expansion ratio rapidly increased before stabilizing. Finally, 4 h was determined as the optimal reaction time. The optimization experiment of initiator dosage (0.4–2.4 wt.%) showed that the initial particle size first increased and then decreased, and the yield continued to increase. 2.4 wt.% was determined as the optimal dosage. The study on the dosage of crosslinking agent showed that within the range of 0.4–1.6 wt.%, as the concentration increased, the initial particle size decreased from 310 nm to 250 nm, and the expansion performance increased from 10.5 times to 13.4 times. After exceeding 1.6 wt.%, the expansion performance decreased to 8.4 times; therefore, the optimal amount of crosslinking agent was determined to be 1.6 wt.%. Through system optimization, the optimal synthesis conditions were determined to be a reaction temperature of 43 °C, a time of 4 h, an initiator of 2.4 wt.%, and a crosslinking agent of 1.6 wt.%. At this time, the microspheres had a small initial particle size (248 nm) and optimal swelling performance (13.4 times), ensuring both the integrity of the crosslinking network and maintaining good swelling ability.
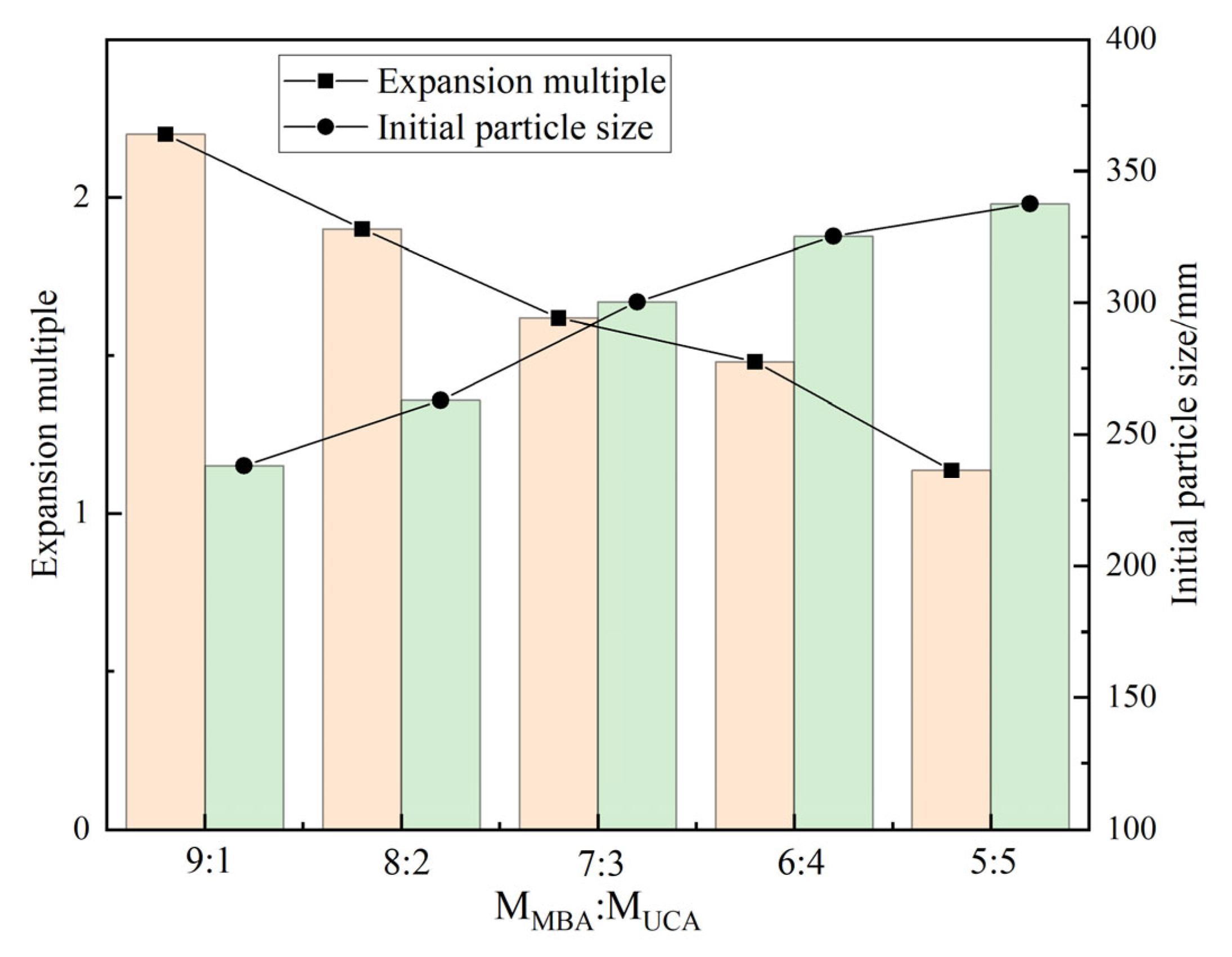
In addition, the influence of MBA/UCA mass ratio on the properties of DCNPM-A double crosslinked microspheres was systematically studied under the conditions of a reaction temperature of 42 °C, a reaction time of 4 h, and a total crosslinking agent dosage of 2.4 wt.% and 1.6 wt.% initiators.
Figure 3 shows that as the content of MBA/UCA increases (0–50%), the initial particle size of microspheres increases from 228 nm to 342 nm, and the expansion ratio decreases from 2.2 times to 1.1 times. Taking into account the secondary expansion characteristics and particle size control, the optimal ratio of MBA:UCA = 9:1 is determined. At this time, the microspheres have both a small initial particle size (228 nm) and good temperature-responsive expansion performance.
2.3. Characterization of Double Crosslinked Nanospheres
- (1)
Surface electrical properties and initial particle size
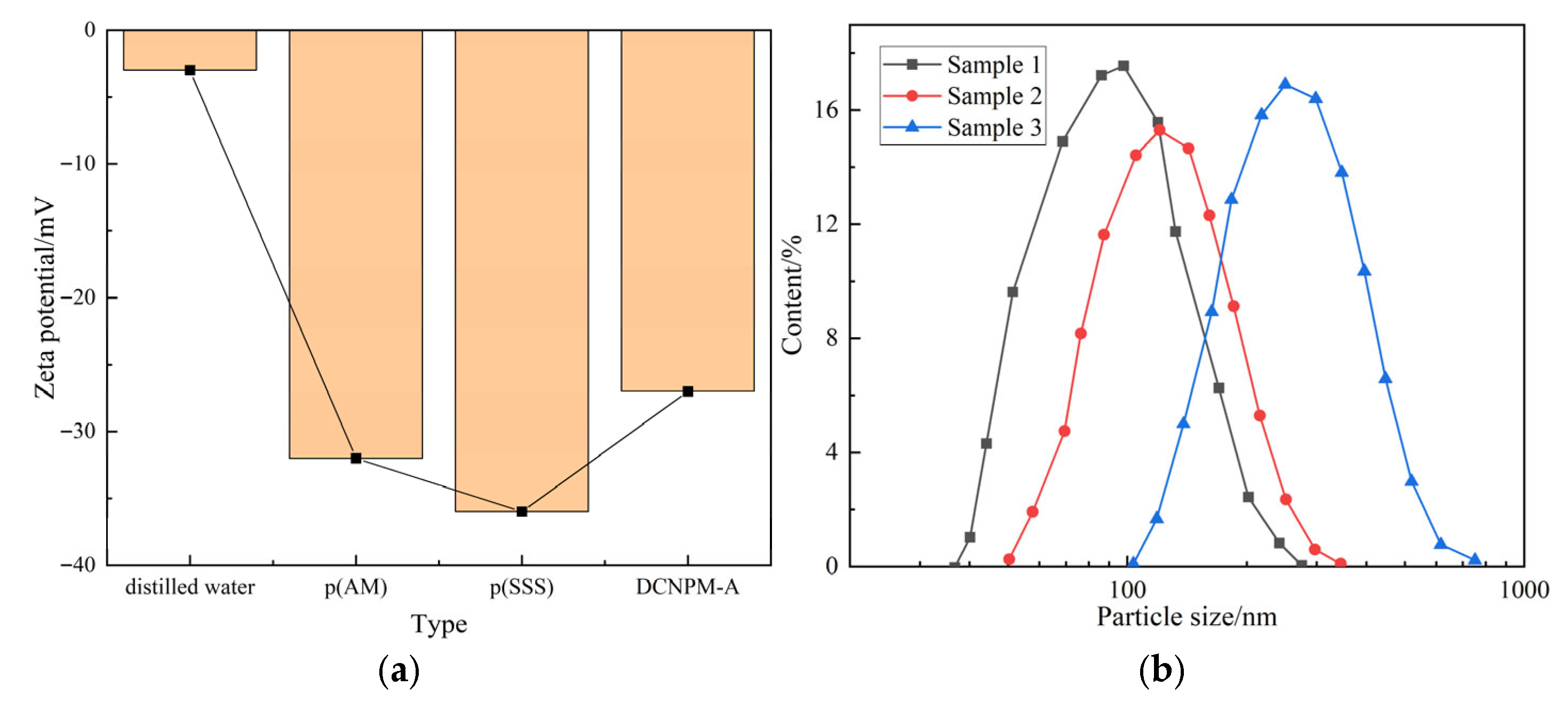
The copolymerization reaction between various functional monomers and DCNPM-A microspheres was verified by Zeta potential testing. As shown in
Figure 4, the pure water potential was −3.29 mV, and the pure polyacrylamide microspheres P (AM) showed −32.6 mV due to amide hydrolysis. After introducing the sulfonic acid monomer SSS, the P (AM-SSS) potential decreased to −36.3 mV. However, the DCNPM-A microspheres with cationic monomer ARM added had a potential recovery of −28.2 mV due to neutralization of positively charged CN
+ groups. Particle size analysis showed that DCNPM-A microspheres were monodisperse, mainly distributed in the range of 90–450 nm, with an average particle size of 227.2 nm.
- (2)
Microscopic morphology and elemental composition
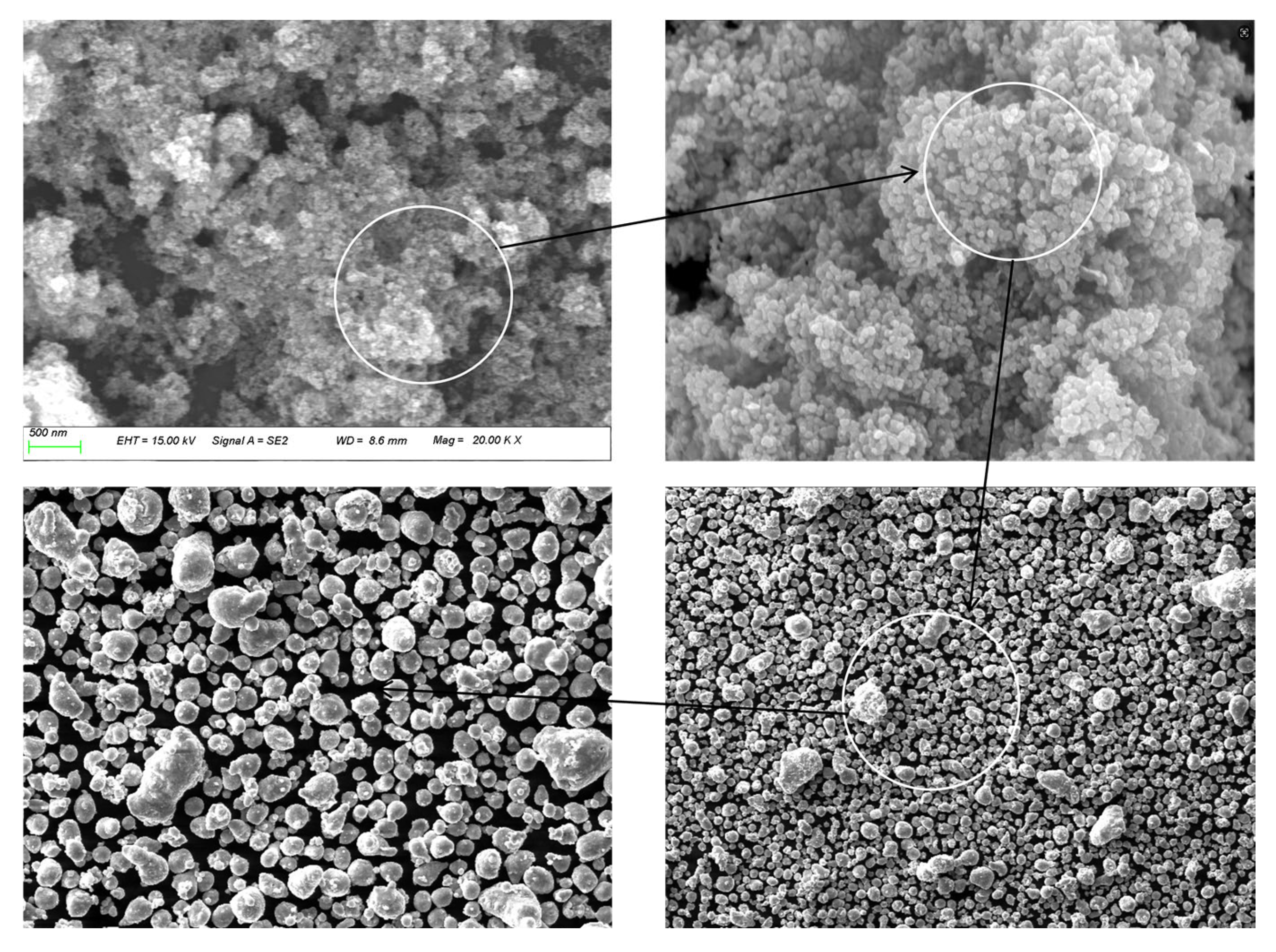
To verify the successful synthesis of DCNPM-A microspheres, their morphology was characterized using scanning electron microscopy (SEM), and their elemental composition was analyzed by EDS spectroscopy. The SEM observation results show that DCNPM-A microspheres exhibit a uniformly dispersed, regular spherical structure with a particle size distribution in the range of 150–400 nm (
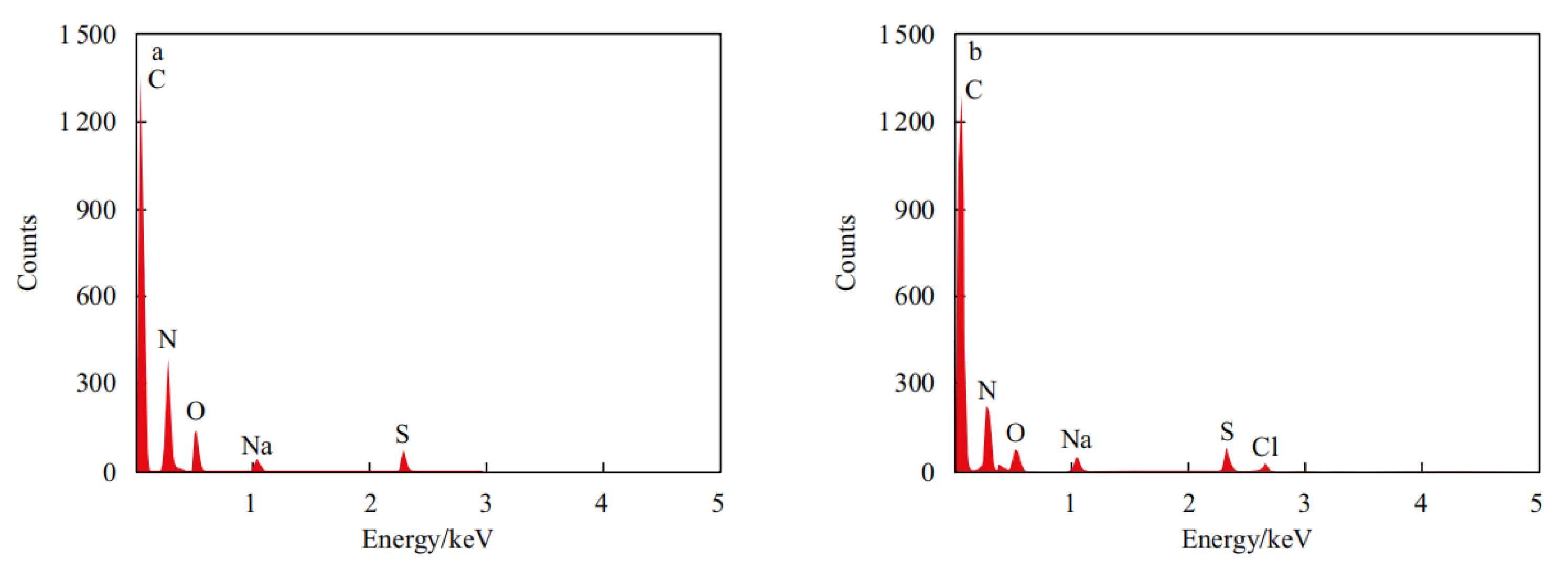
Figure 5). EDS spectroscopy analysis detected characteristic elements such as C, N, O, S, Na, and Cl in the microspheres. The N and Cl elements were derived from the cationic monomer ARM, while the S and Na elements were derived from the temperature and salt-resistant monomer SSS. However, ordinary nanospheres did not contain CL elements (
Figure 6). These results confirm the successful preparation of DCNPM-A microspheres.
In order to further compare and analyze the microstructure of AM monomer and DCNPM-A microspheres, infrared spectroscopy experiments were conducted.
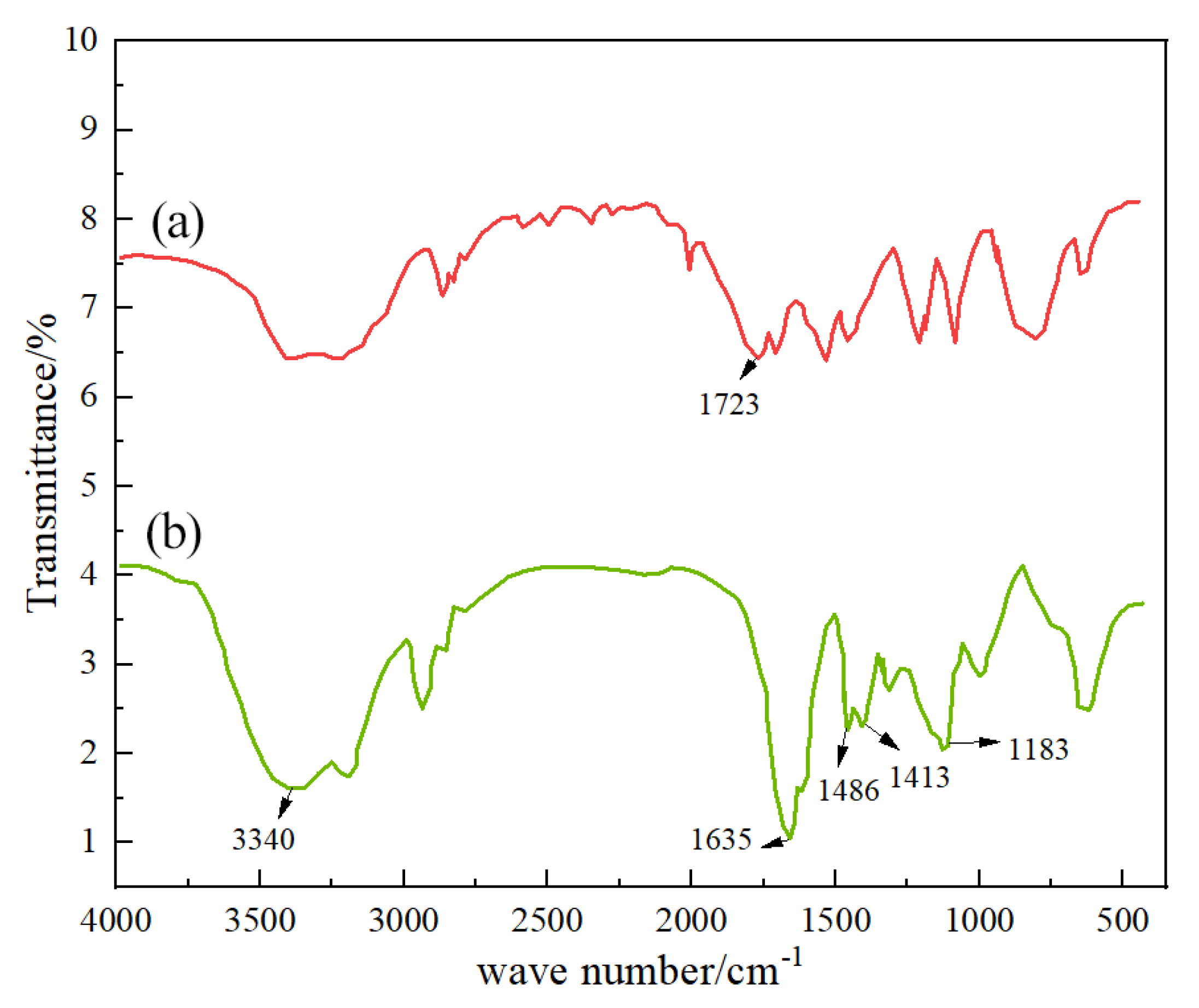
Figure 7 shows the infrared spectra of the AM monomer and DCNPM-A microspheres.
Infrared spectroscopy analysis showed that DCNPM-A microspheres successfully achieved copolymerization of functional monomers. Specifically, the characteristic peak of the C=C double bond of AM monomer at 1723 cm−1 in the curve (a) completely disappears in the curve (b), confirming that all polymerizable double bonds have participated in the reaction. (b) The characteristic peaks at 3340 cm−1 (N-H in -CONH2) and 1635 cm−1 (C=O) in the curve confirm the successful introduction of AM. At the same time, the -SO3- vibration peak at 1183 cm−1 and the benzene ring characteristic peak at 1486 cm−1 confirmed the involvement of SSS monomer, while the C-N+ vibration peak at 1413 cm−1 verified the effective polymerization of ARM monomer. The joint appearance of these characteristic peaks confirms that both the temperature and salt-resistant monomer SSS and the acid-resistant monomer ARM have been successfully integrated into the microsphere structure.
3. Performance and Acid Resistance Mechanism Analysis of Double Crosslinked Nanospheres
In response to the technical bottleneck of insufficient expansion performance of conventional microspheres in CO2-driven acidic reservoirs, this study focuses on investigating the expansion characteristics, stability, and rheological behavior of DCNPM-A microspheres in different environments, in order to optimize the microsphere dispersion formula suitable for acidic reservoirs and deeply analyze its acid resistance mechanism. Meanwhile, by precisely regulating the combination of functional monomers, two characteristic microsphere systems were constructed, namely dual crosslinked DCNPM-A containing ARM and UCA, and single crosslinked SCNPM-A containing only ARM.
3.1. Factors Affecting the Expansion Performance of Double Crosslinked Nanospheres
In the acidic CO
2 flooding reservoir environment, conventional polymer microspheres often exhibit poor swelling performance, and their performance is easily affected by various formation factors such as hydration time, temperature, mineralization degree, and pH value. Therefore, this study compared and analyzed the expansion behavior of DCNPM-A microspheres with different functional microspheres under acidic conditions, aiming to elucidate the excellent acid resistance mechanism of DCNPM-A microspheres. The analysis results are shown in
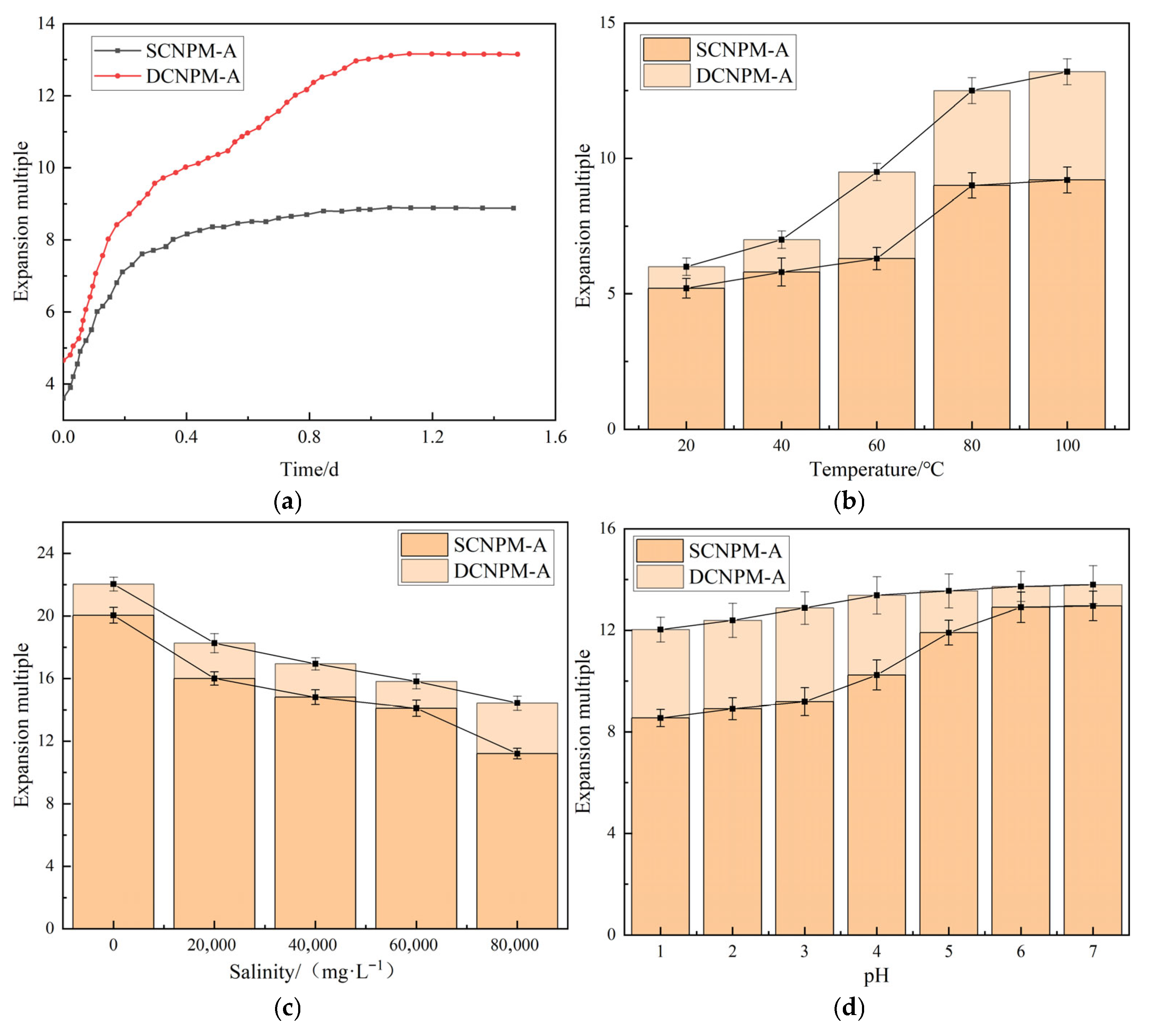
Figure 8.
DCNPM-A microspheres exhibit excellent swelling performance and environmental adaptability in CO2-driven acidic reservoirs through the design of unstable crosslinking agents (UCA). Experimental data shows that under acidic conditions (pH = 2–4), the final expansion ratio of the microsphere reaches 13.5 times (traditional SCNPM-A only 8.7 times), and the equilibrium time is extended to 1 day (traditional 0.5 days), exhibiting a unique gradient expansion characteristic, namely rapid expansion from 0 to 0.17 days, slowing down expansion rate from 0.17 to 0.5 days, and then accelerating to equilibrium. The temperature response test shows that the expansion ratio of microspheres increases with temperature in the range of 20–100 °C, and a significant secondary expansion phenomenon occurs around 70 °C, with a significant increase in expansion rate. In terms of mineralization adaptability, the expansion ratio sharply decreases from 22 to 18 in low salt environments (<20,000 mg·L−1). The decrease in the range of medium salt (20,000–60,000 mg·L−1) slowed down, gradually decreasing from 18 to 15.8. Under high salt conditions (>60,000 mg·L−1), DCNPM-A microspheres still maintain excellent expansion performance of 15.8, and even under high mineralization conditions of 80,000 mg·L−1, DCNPM-A microspheres maintain an excellent expansion ability of about 14 times. The acid resistance test confirmed that compared with traditional DCNPM microspheres, DCNPM-A exhibits significant advantages in acidic environments, and its expansion performance is almost unaffected by pH value, while the expansion ability of conventional microspheres significantly decreases under acidic conditions. These advantages stem from the gradient decomposition mechanism of UCA, salt-resistant group modification, and introduction of acidic stabilizing groups, enabling DCNPM-A microspheres to achieve intelligent response of “small particle size injection deep expansion plugging”, effectively solving the technical difficulties of conventional microspheres in acidic reservoirs.
3.2. Stability Analysis of Double Crosslinked Nanospheres
- (1)
Thermal stability analysis
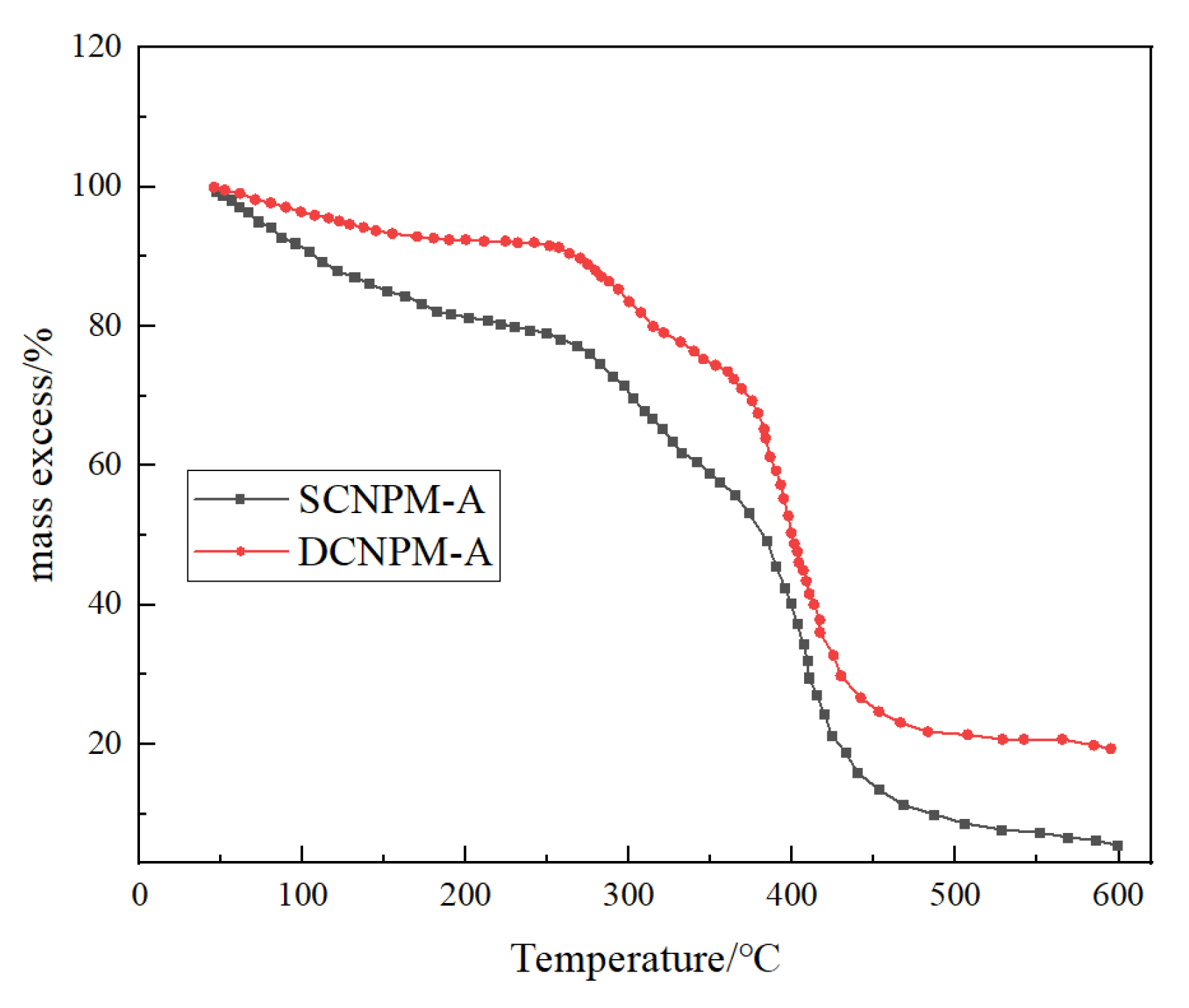
The results of thermogravimetric analysis (TGA) indicate that DCNPM-A microspheres have significantly improved thermal stability, as evidenced by the data presented in
Figure 9. The thermal degradation process exhibits typical three-stage characteristics, including the main loss of bound water and UCA crosslinking agent decomposition products in the 40–250 °C stage. Thermal decomposition of side groups (-NH
2, -COO
−, -SO
3−, and benzene ring) occurs at temperatures between 250 and 450 °C. The 450–600 °C stage is characterized by main chain fracture and three-dimensional network collapse. It is worth noting that the final residual carbon rate of DCNPM-A microspheres reached 18.53%, which is more than three times higher than that of SCNPM microspheres (5.6%). This excellent temperature resistance enables DCNPM-A microspheres to meet the application requirements of high-temperature oil reservoirs.
- (2)
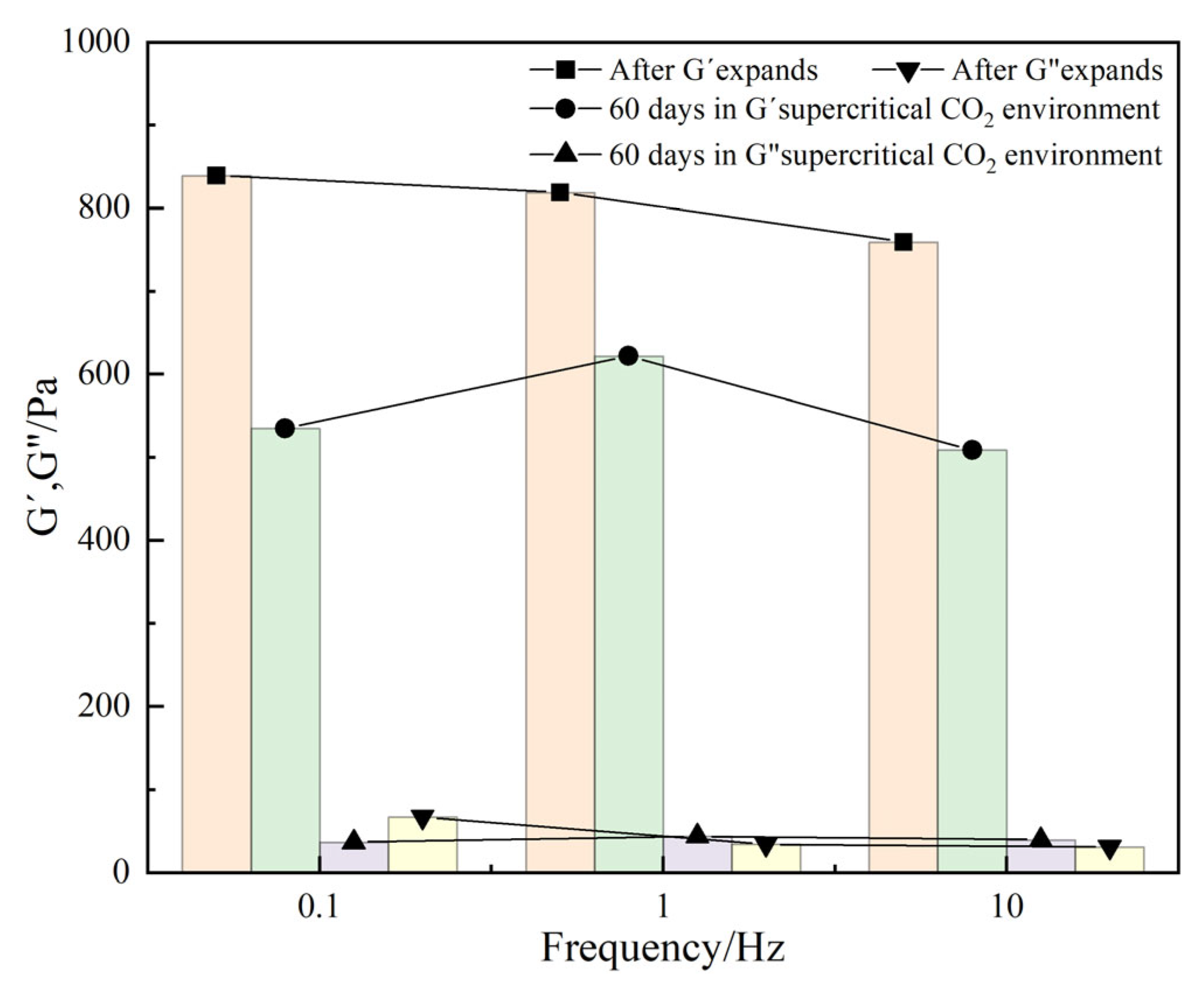
Long-term stability analysis in a supercritical CO2 environment
Rheological tests show that DCNPM-A microspheres exhibit typical elastic characteristics (G′ > G″) after water absorption and expansion, as shown in
Figure 10. After 60 days of exposure to a supercritical CO
2 (8 MPa, 80 °C) environment, the storage modulus G′ decreased while the loss modulus G′ slightly increased. This is mainly due to the long-term acid corrosion of supercritical CO
2, which led to partial degradation of the microsphere network structure. The high-pressure environment promoted the full dissolution of CO
2 and reached an equilibrium state, enhancing the acidic corrosion effect. The experiment confirmed that DCNPM-A microspheres can maintain structural stability for more than 60 days in a supercritical CO
2 environment, demonstrating excellent resistance to supercritical CO
2.
3.3. Rheological Properties Analysis of Double Crosslinked Nanospheres
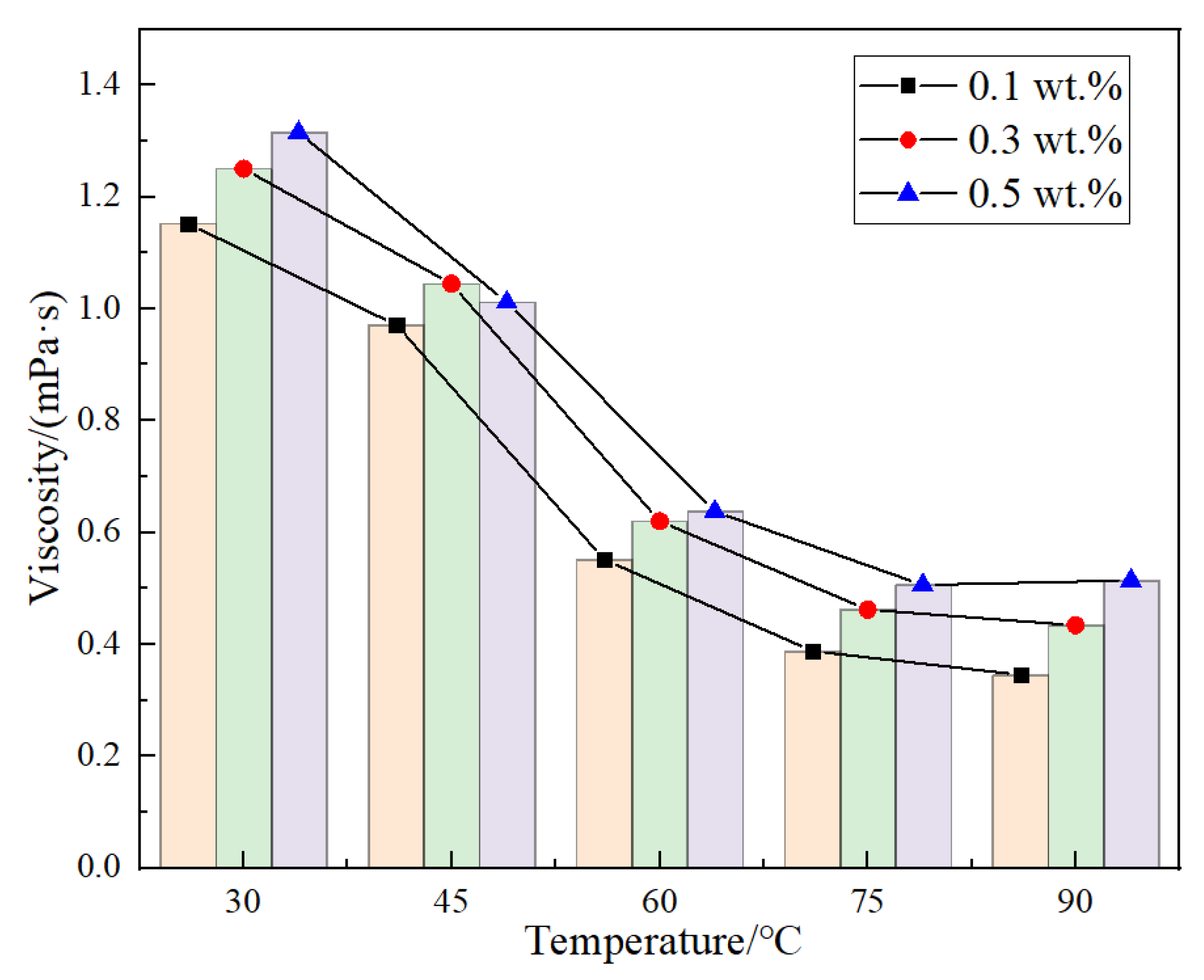
The study on the viscosity characteristics of DCNPM-A microsphere dispersion showed that within the temperature range of 20–90 °C, the viscosity of the three concentration systems remained in a low viscosity state below 1.4 mPa·s (
Figure 11), demonstrating good injection performance. The viscosity temperature curve exhibits three-stage characteristics, including a gradual decrease in viscosity in the 30–45 °C range, reflecting the initial hydration process of microspheres. The viscosity rapidly decreases in the range of 45–60 °C, corresponding to the dispersion enhancement effect dominated by molecular thermal motion. The temperature range of 60–90 °C tends to stabilize, indicating that the microspheres have completed secondary expansion. This intelligent rheological property endows DCNPM-A dispersion with excellent injectability and deep migration capability.
3.4. Acid-Resistant Mechanism of Double Crosslinked Nanospheres
DCNPM-A microspheres achieve acid resistance through a unique charge regulation mechanism. In a weak alkaline environment, the amide groups on the microsphere molecular chain hydrolyze to produce carboxylate ions, which form an “internal salt bond” structure with the cationic monomer ARM (
Figure 12). When in an acidic environment, the dissociation of the internal salt bond produces a dual effect: on the one hand, carboxylate ions combine with H
+ to form carboxyl groups, and on the other hand, the released cationic groups maintain charge balance. This dynamic charge recombination forms an osmotic pressure difference inside and outside the microspheres, which not only offsets the adverse effects of the acidic environment on hydration ability but also promotes water absorption and expansion. This intelligent response mechanism enables DCNPM-A microspheres to exhibit better swelling performance than conventional microspheres under acidic conditions.
After reaching the target layer, the unique thermal response mechanism of DCNPM-A microspheres is activated. When the formation temperature reaches a critical value, the unstable crosslinking agent UCA inside the microspheres selectively fractures, resulting in a decrease in the density of the crosslinking network. This intelligent structural change significantly improves the water absorption and expansion ability of microspheres and enhances acid resistance through the release of carboxylate groups. This in situ structural reorganization enables microspheres to achieve secondary expansion in acidic environments, effectively solving the technical problem of insufficient expansion performance of conventional microspheres in acidic reservoirs.
4. The Sealing Effect of Double Crosslinked Nanospheres on Fractured Rock Cores
The injection performance of microspheres directly affects the operating load of ground equipment and the effectiveness of deep displacement control. Excellent injectability not only reduces equipment loss but also ensures effective entry of microspheres into deep formations to achieve sealing function. This study focuses on examining the injection characteristics of DCNPM-A microspheres and systematically analyzing their compatibility with formation permeability.
4.1. Matching Relationship Between DCNPM-A Microspheres and Permeability
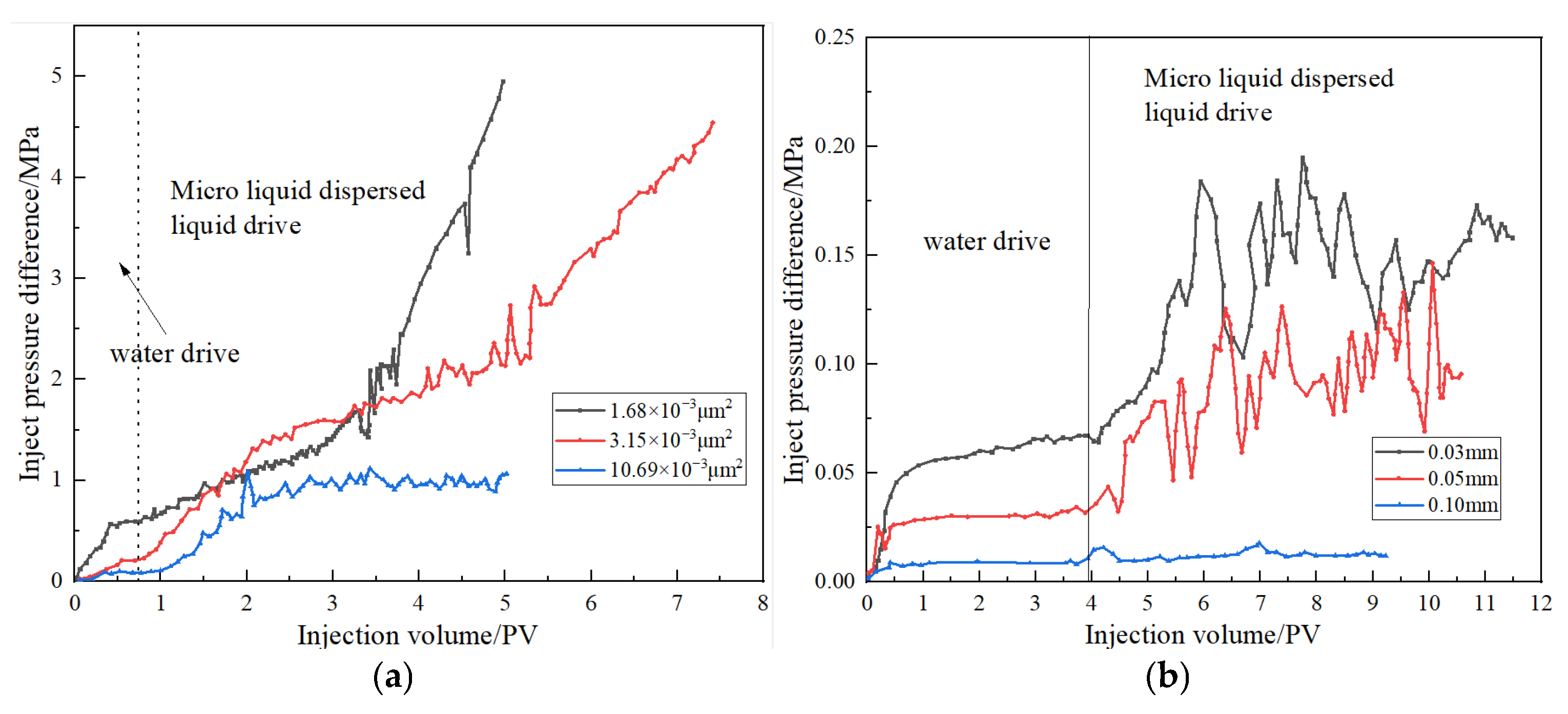
This study evaluated the injection performance of DCNPM-A microspheres through core displacement experiments. The matrix core injection experiment used water phase permeability cores with permeability of 1.68, 3.15, and 10.69 × 10−3 μm2, respectively. The fracture core injection experiment used matrix permeability cores with permeability of 10 × 10−3 μm2 and fracture widths of 0.10, 0.05, and 0.03 mm, respectively. The fracture core permeability cores with permeability of (849.26, 70.77, 28.31) × 10−3 μm2 were tested.
The results of matrix injectability show that for cores with permeability ≤ 3.15 × 10
−3 μm
2, the injection pressure continues to increase, while in cores with permeability of 10.69 × 10
−3 μm
2, the injection pressure first increases and then stabilizes at around 1.066 MPa, accompanied by periodic fluctuations, as clearly illustrated in
Figure 13. This characteristic pressure response reveals the dynamic migration mechanism of DCNPM-A microspheres in low-permeability reservoirs (approximately 10 × 10
−3 μm
2), where the leading edge of the microspheres undergoes a cyclic process of “migration plugging” during propulsion, forming a typical fluctuating pressure curve. The fracture core experiment showed that the pressure change in microsphere flooding was weak in the 0.10 mm wide fracture, indicating that it was difficult to effectively retain. But when the seam width is reduced to 0.05 mm and 0.03 mm, the microspheres can achieve temporary sealing, confirming their excellent sealing ability for narrow cracks. Especially under the condition of a seam width of 0.03 mm, microspheres can form effective temporary blockages even in an incompletely expanded state through adsorption retention and aggregation bridging mechanisms, demonstrating optimal sealing performance. These results systematically reveal the differential sealing behavior of DCNPM-A microspheres in low-permeability reservoirs and fractures of different scales.
4.2. Matching Relationship Between DCNPM-A Microsphere Plugging Rate and Permeability
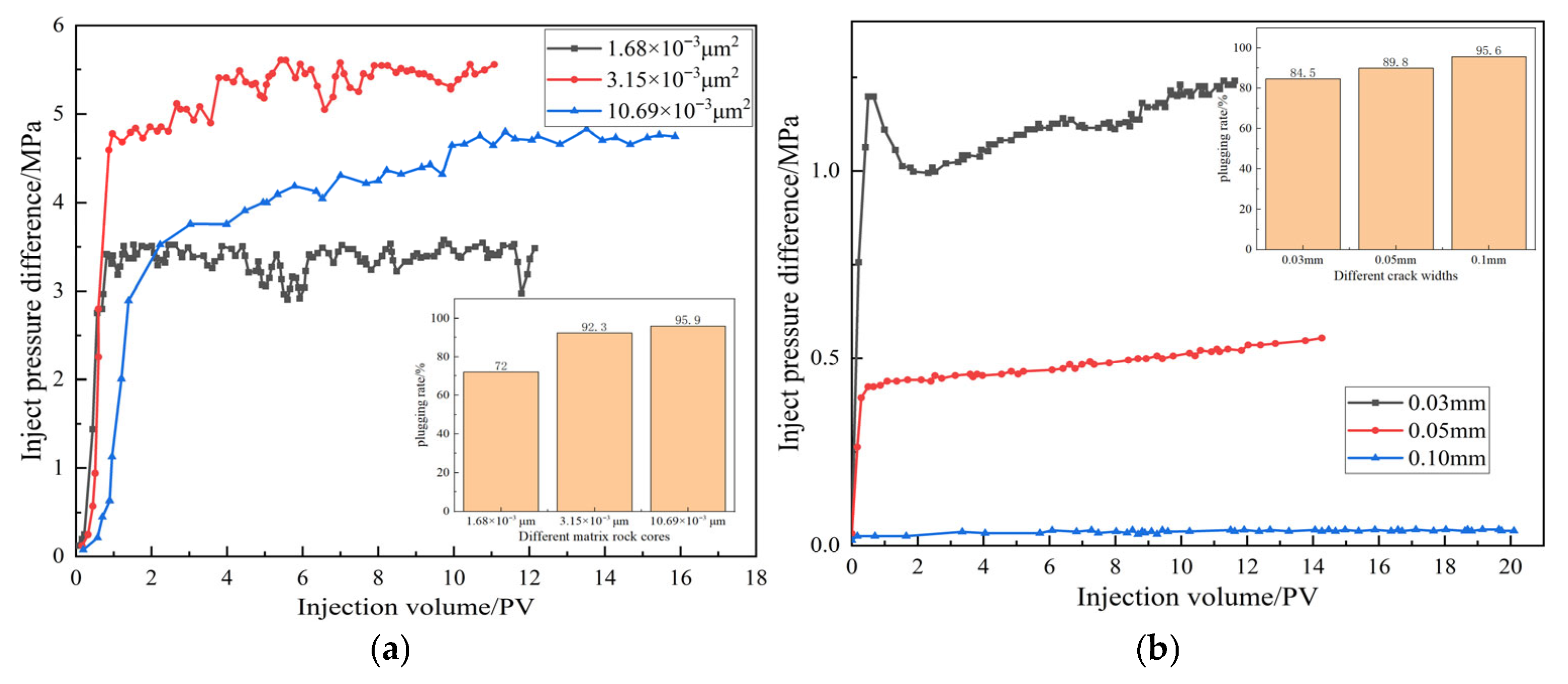
This section mainly explores the sealing rate of rock cores with different permeability after sufficient swelling of microspheres, following injection experiments, and explores their resistance to erosion.
Figure 14 indicates that the sealing performance of DCNPM-A microspheres is closely related to the core permeability and fracture width. In the matrix core, microspheres exhibit significant permeability dependence. In low-permeability cores (1.68 × 10
−3 μm
2), microspheres mainly block at the injection end and are easily broken by water-driven shear, with a blocking rate of only 72%. In the moderately permeable core (3.15 × 10
−3 μm
2), microspheres can fully enter the matrix and achieve a sealing rate of 92.3% through adsorption and retention. In the high permeability core (10.69 × 10
−3 μm
2), microspheres can penetrate deep into the matrix and fully swell, preferentially blocking large pore throats and forcing water flow to turn, achieving the optimal sealing effect (95.9%). In the crack system, microspheres exhibit dynamic sealing characteristics, namely, it is difficult to effectively retain in a 0.1 mm wide crack, the sealing rate increases to 89.8% in a 0.05 mm crack, and shows a “breakthrough re-sealing” phenomenon in a 0.03 mm narrow crack, with a final sealing rate as high as 95.6%. The experimental results indicate that when the DCNPM-A microspheres achieve a high plugging rate of over 95%, the optimal conditions for their application are that for the matrix core, the permeability needs to reach 10 × 10
−3 μm
2. For fractured rock cores, the crack width should be controlled at 0.03 mm.
4.3. Optimization of Injection Parameters for DCNPM-A Microspheres
The goal of this section is to improve the efficiency and sealing effect of supercritical CO2 oil displacement. Based on the results of previous matrix core experiments (optimal matching permeability of 10 × 10−3 μm2, optimal crack width of 0.03 mm), the injection parameters of DCNPM-A microsphere dispersion are systematically optimized to provide an ideal experimental model for profile control operations in supercritical CO2 oil reservoirs.
- (1)
Injection volume
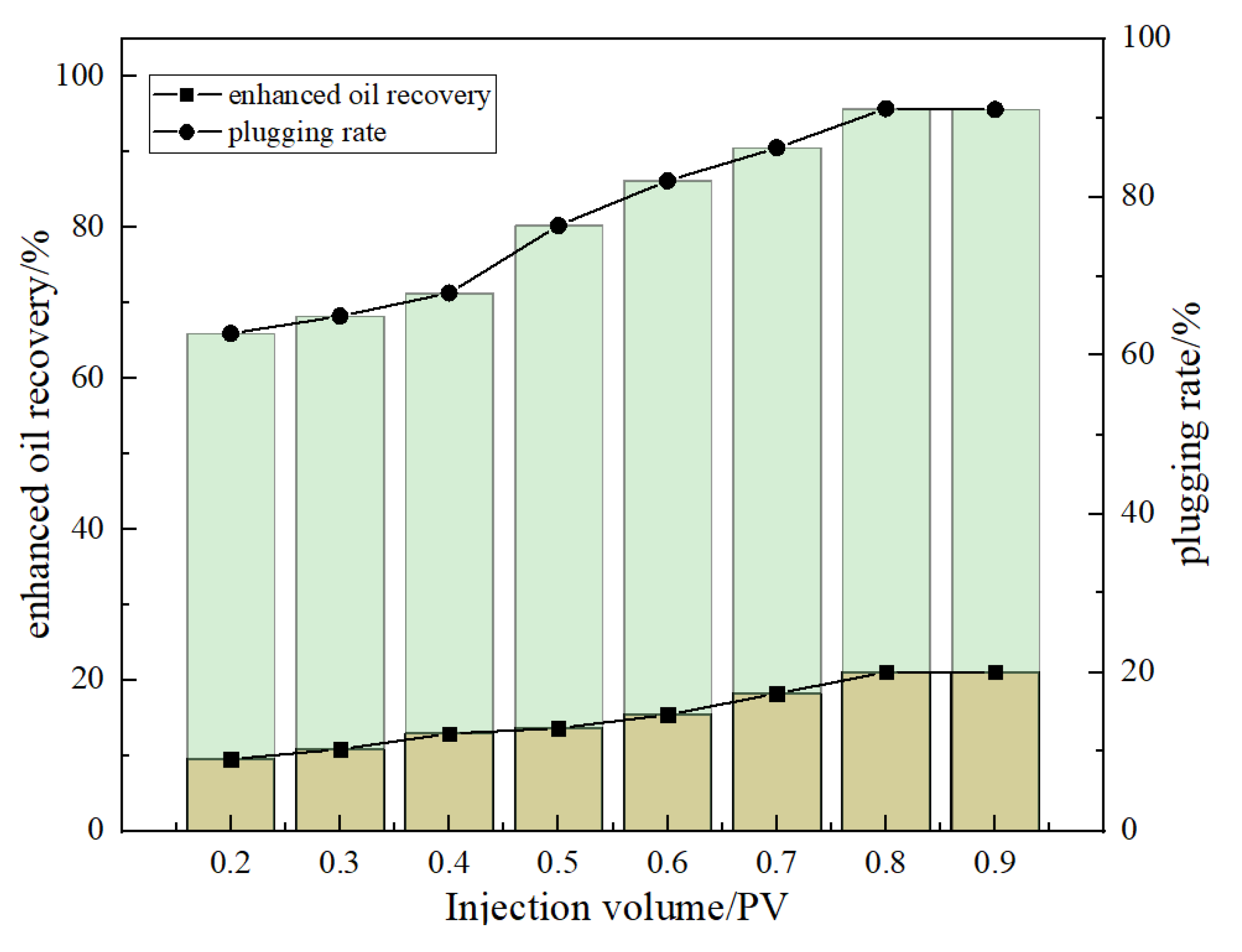
Figure 15 shows that the injection number of DCNPM-A microspheres has a significant impact on the sealing effect. When the injection volume increased from 0.4 PV to 0.8 PV, the plugging rate increased from 71.28% to 95.70%, and the recovery rate increased from 12.9% to 21.03%. Taking into account the sealing effect and economy, it is recommended that the optimal injection volume of DCNPM-A microspheres be 0.8 PV.
- (2)
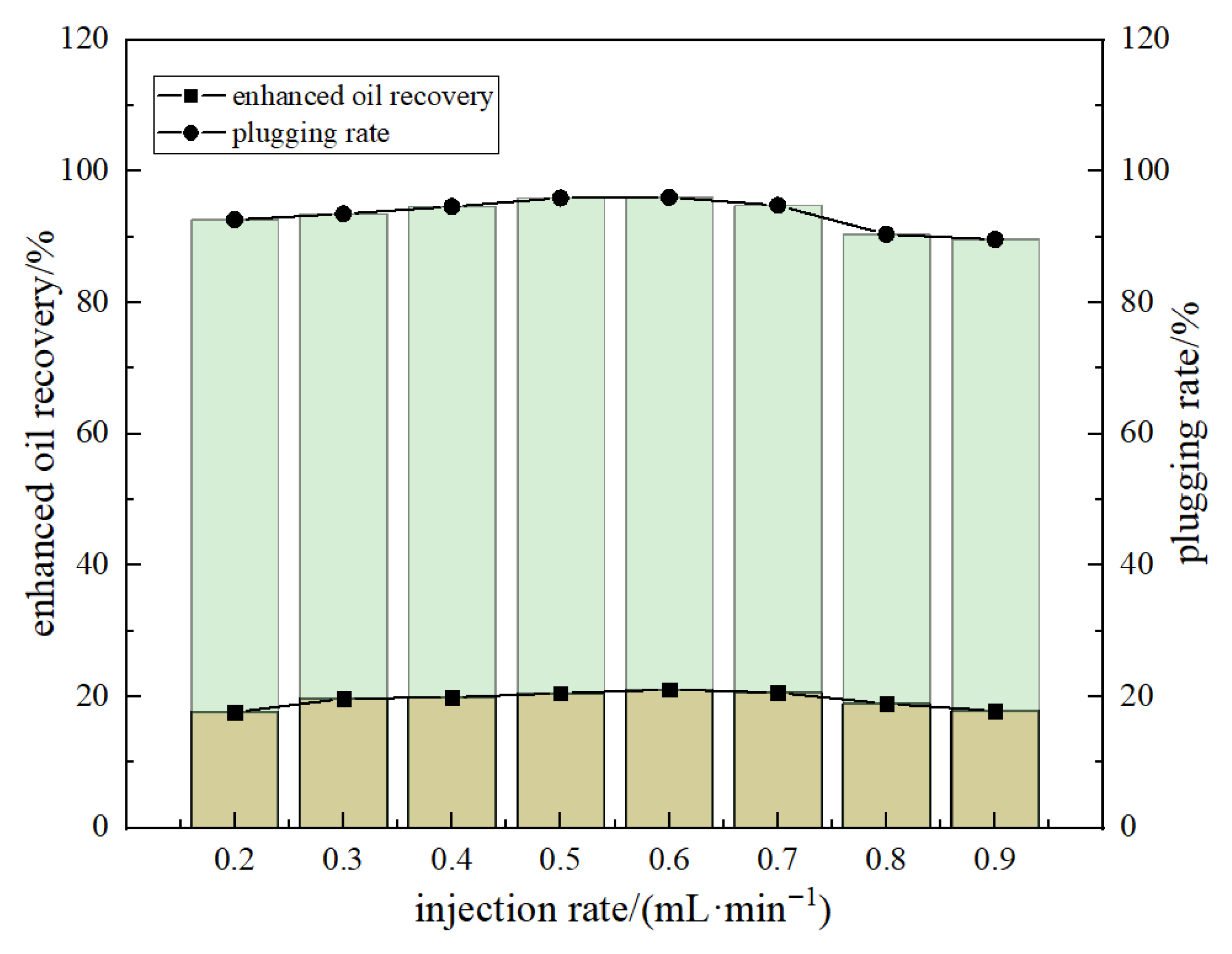
Injection rate
Figure 16 indicates that the injection rate of DCNPM-A microspheres has a significant impact on their sealing performance. When the injection rate increased from 0.1 mL·min
−1 to 0.5 mL·min
−1, both the plugging rate and CO
2 recovery rate showed an upward trend, mainly due to the faster flow rate promoting the migration of microspheres towards the deep cracks, forming more effective deep plugging. However, when the speed was further increased to 0.7 mL·min
−1, the excessively high flow rate resulted in a decrease in the retention of microspheres and a decrease in the sealing effect. Specifically, at an optimized flow rate of 0.5 mL·min
−1, microspheres can achieve optimal equilibrium. In this state, it ensures sufficient deep plugging strength (with the highest plugging rate) and significantly improves reservoir heterogeneity (with the most significant increase in CO
2 drive recovery rate). Therefore, under the condition of 0.8 PV injection volume, it is recommended to use 0.5 mL·min
−1 as the optimal injection rate for DCNPM-A microspheres.
- (3)
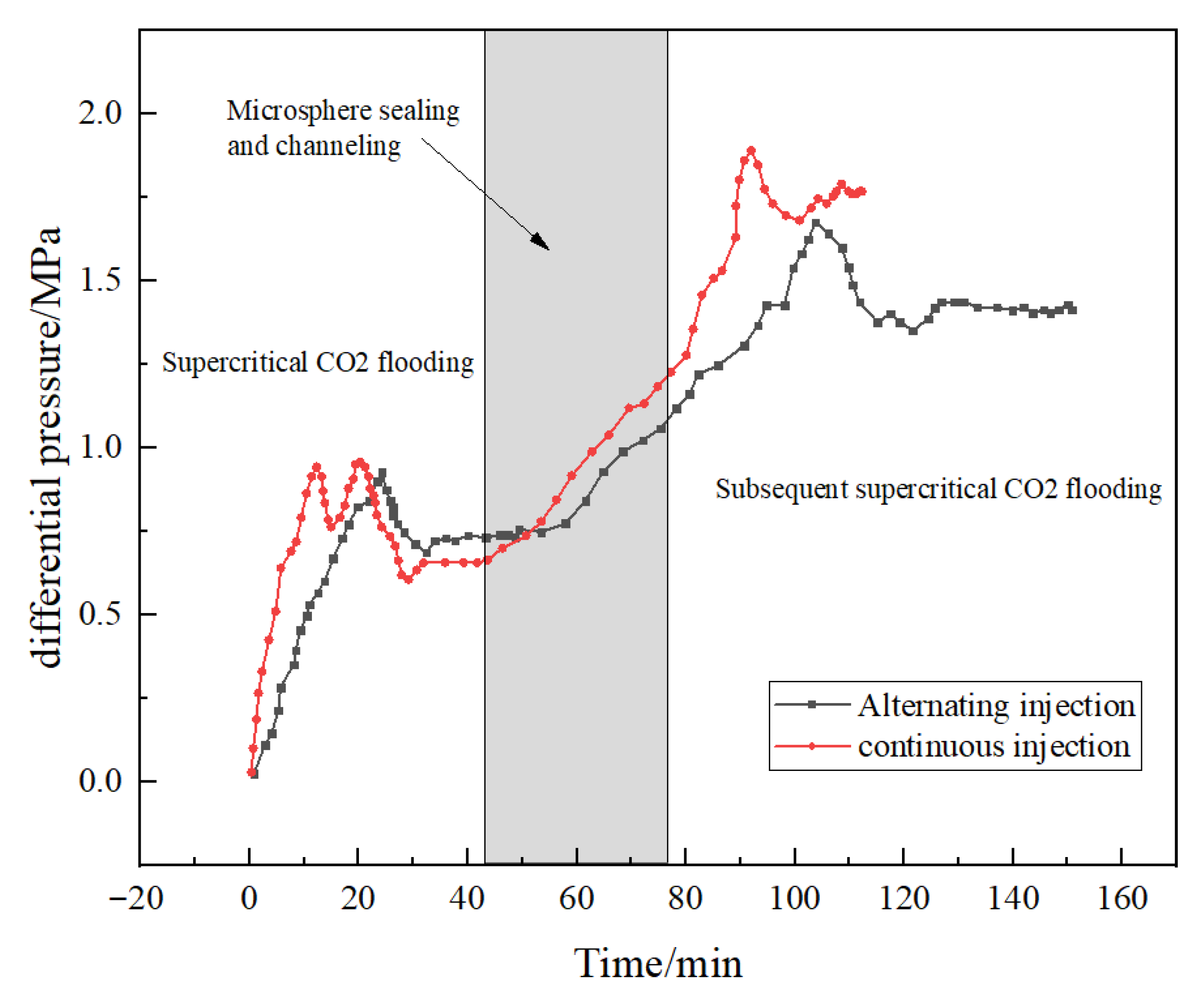
Injection method
Figure 17 indicates that the optimal injection parameter combination for DCNPM-A microspheres is 0.8 PV injection volume, 0.5 mL·min
−1 injection rate, and a continuous injection method. Compared with the alternating injection method, the continuous injection scheme can better promote the efficient enrichment and sufficient swelling of microspheres in fractures, thereby significantly improving the sealing strength and anti-erosion performance. Experimental data confirms that the continuous injection method can better adapt to the sealing requirements under complex geological conditions while maintaining long-term stable sealing effects.
Moreover, attention should be given to the interactions between chemical agents and mineral phases during CO
2 injection, which can alter permeability and thereby affect the transport and retention behavior of microspheres [
24]. While porosity reduction due to precipitation may enhance the plugging efficiency of the microspheres, excessive mineral deposition could also compete with microsphere swelling, potentially influencing the overall blocking performance.
4.4. Application Implementation Potential
The inverse microemulsion polymerization method applied in this study is a mature technique widely used in industrial polymer production, and the synthesis parameters employed here (temperature, initiator dosage, and crosslinker ratios) can be readily scaled up with conventional equipment. Laboratory core flooding experiments further demonstrated that the microsphere dispersions remain at a very low viscosity (<1.4 mPa·s), ensuring excellent injectivity. Effective migration was observed even in matrix cores with permeability as low as 10 × 10−3 μm2, suggesting that the system can adapt well to heterogeneous reservoirs.
In addition to favorable injectivity, the microspheres exhibited strong durability, maintaining stability for more than 60 days under supercritical CO2 conditions, thereby supporting their potential for long-term field application. Nevertheless, possible interactions with reservoir brine chemistry, such as scaling and mineral precipitation, merit further investigation. Based on the optimized laboratory conditions, we recommend pilot tests in high-temperature, CO2-rich reservoirs using an injection volume of 0.8 PV, an injection rate of 0.5 mL·min−1, and a continuous injection strategy. These findings provide a solid foundation for advancing the system from laboratory evaluation to practical reservoir deployment.