Characteristics of Growth and Metabolism for Methane-Oxidizing Bacterial Communities in Different Coal Samples
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Preparation of Coal Samples
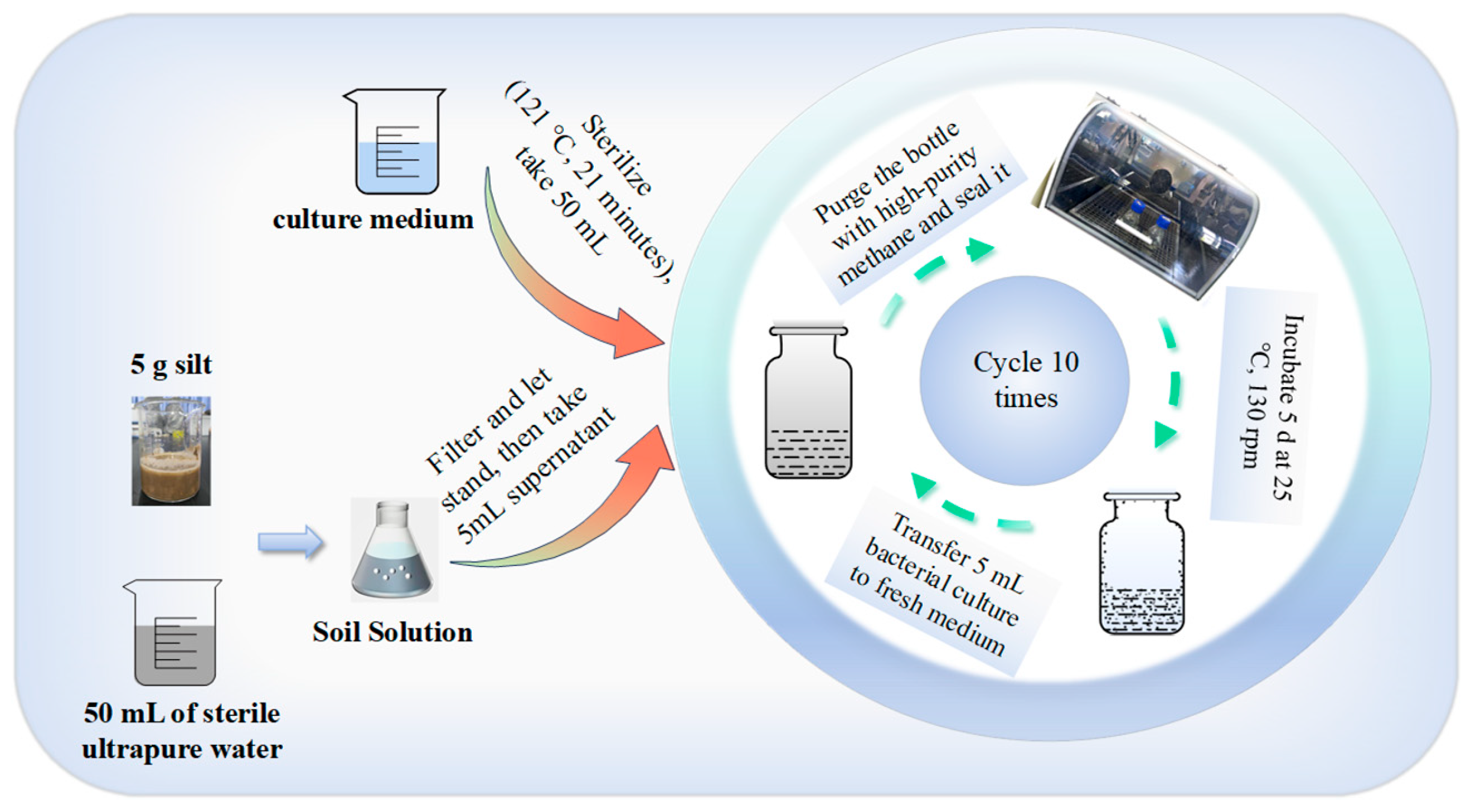
2.2. Cultivation of Microorganisms
| Massive Element Solution | Trace Element Solution | ||
|---|---|---|---|
| Reagents | Ratios/(g·L−1) | Reagents | Ratios/(g·L−1) |
| KNO3 | 1.000 | CuSO4·5H2O | 0.030 |
| MgSO4·7H2O | 1.000 | FeSO4·7H2O | 0.200 |
| Na2HPO4·12H2O | 0.717 | MnSO4·4H2O | 0.003 |
| KH2PO4 | 0.272 | ZnSO4·7H2O | 0.010 |
| NH4Cl | 0.250 | Na2EDTA | 0.500 |
| CaCl2·6H2O | 0.200 | NaMoO4·2H2O | 0.003 |
| NiCl·2H2O | 0.002 | ||
| Massive element solution: Trace element solution = 1000:1 (the volume ratio) | |||
2.3. Analytical Testing Methods
2.3.1. Methane Degradation Test by Microbial Communities
2.3.2. Liquid Phase Inorganic Ion Concentration Test
2.3.3. Amino Acid Test
2.3.4. Untargeted Metabolomics Test
3. Experimental Results and Analysis
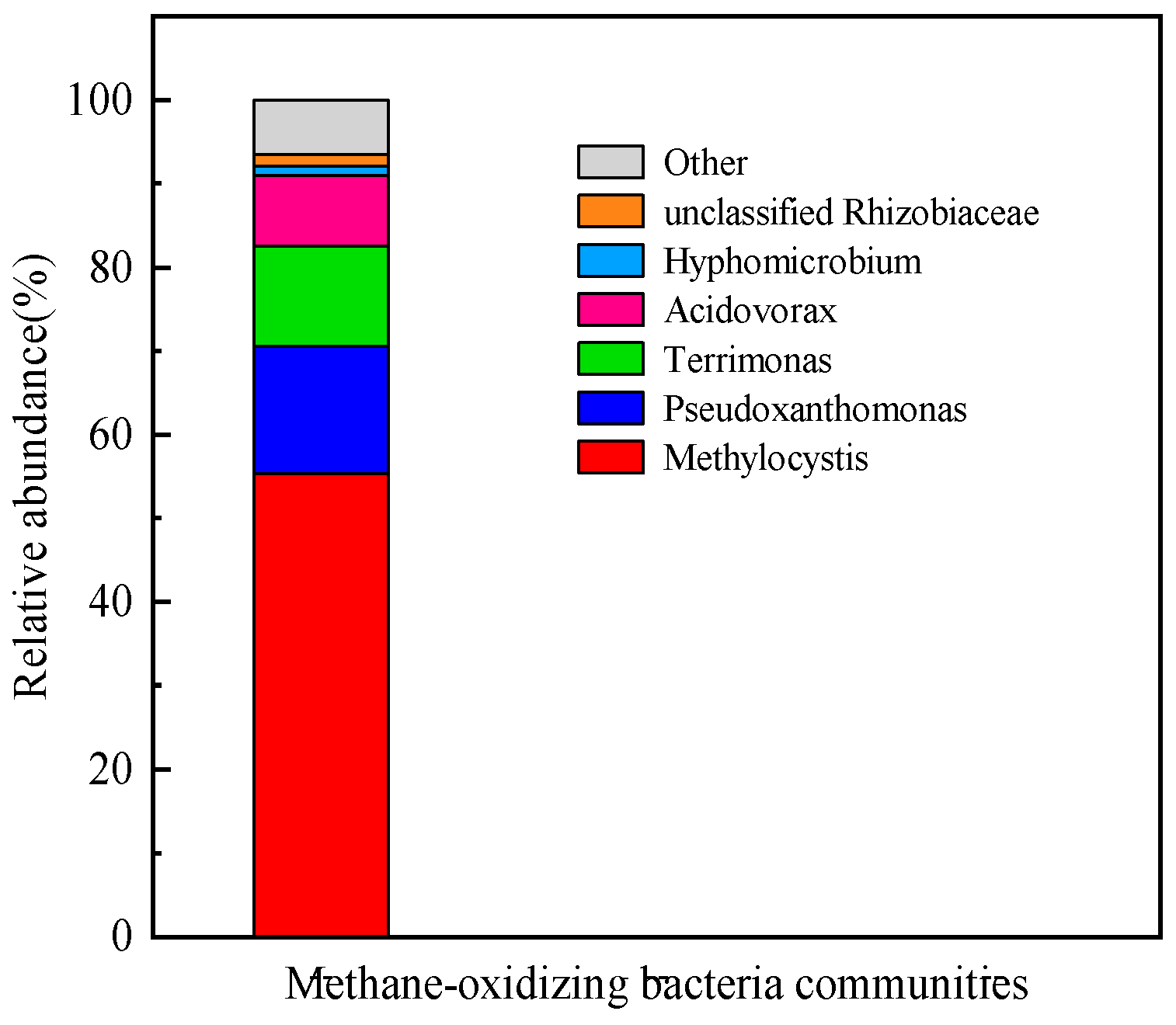
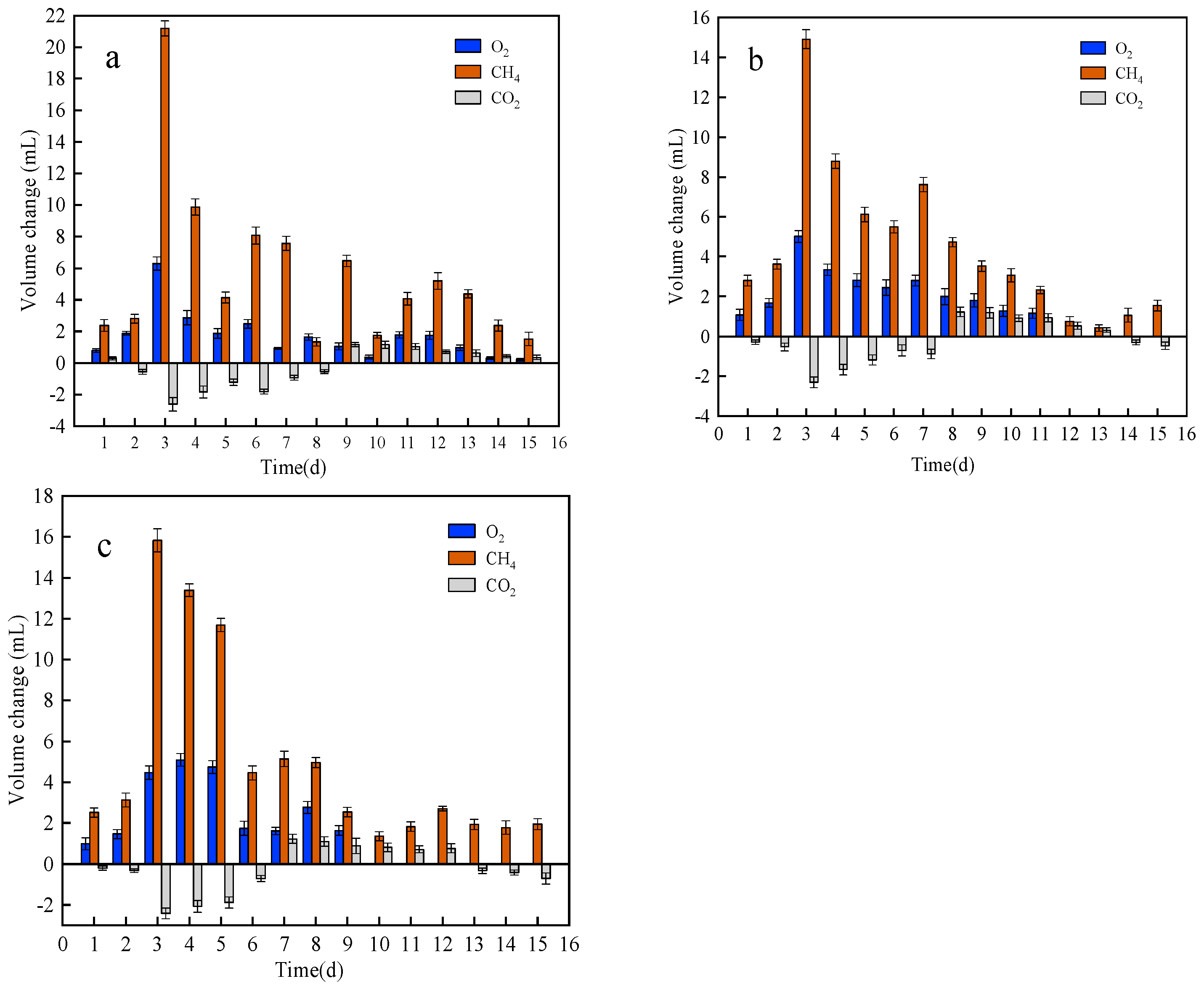
3.1. Effect of Different Coal Samples on the Methane Degradation Performance of Bacterial Colonies
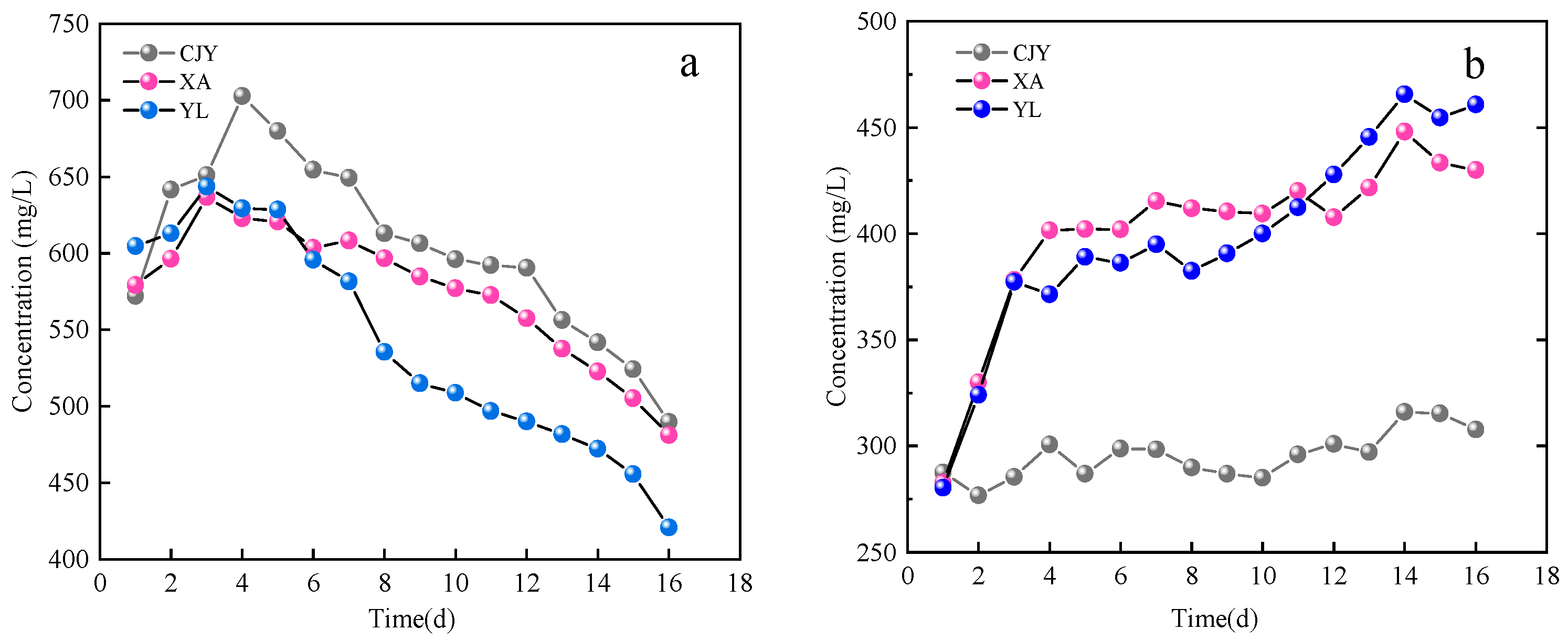
3.2. The Changes in Liquid-Phase Inorganic Ion Concentration
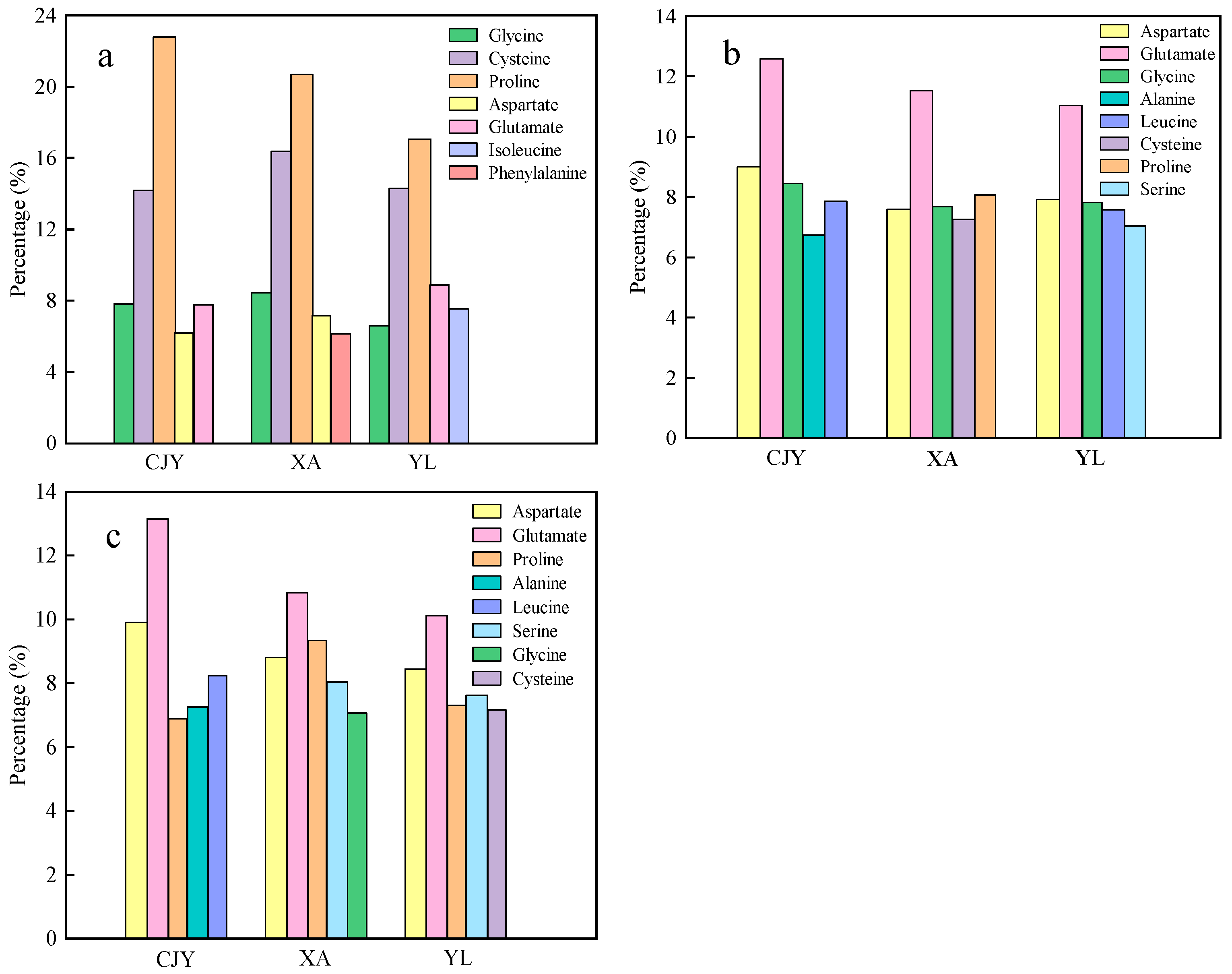
3.3. Changes in Dominant Amino Acids in Microbial Solutions
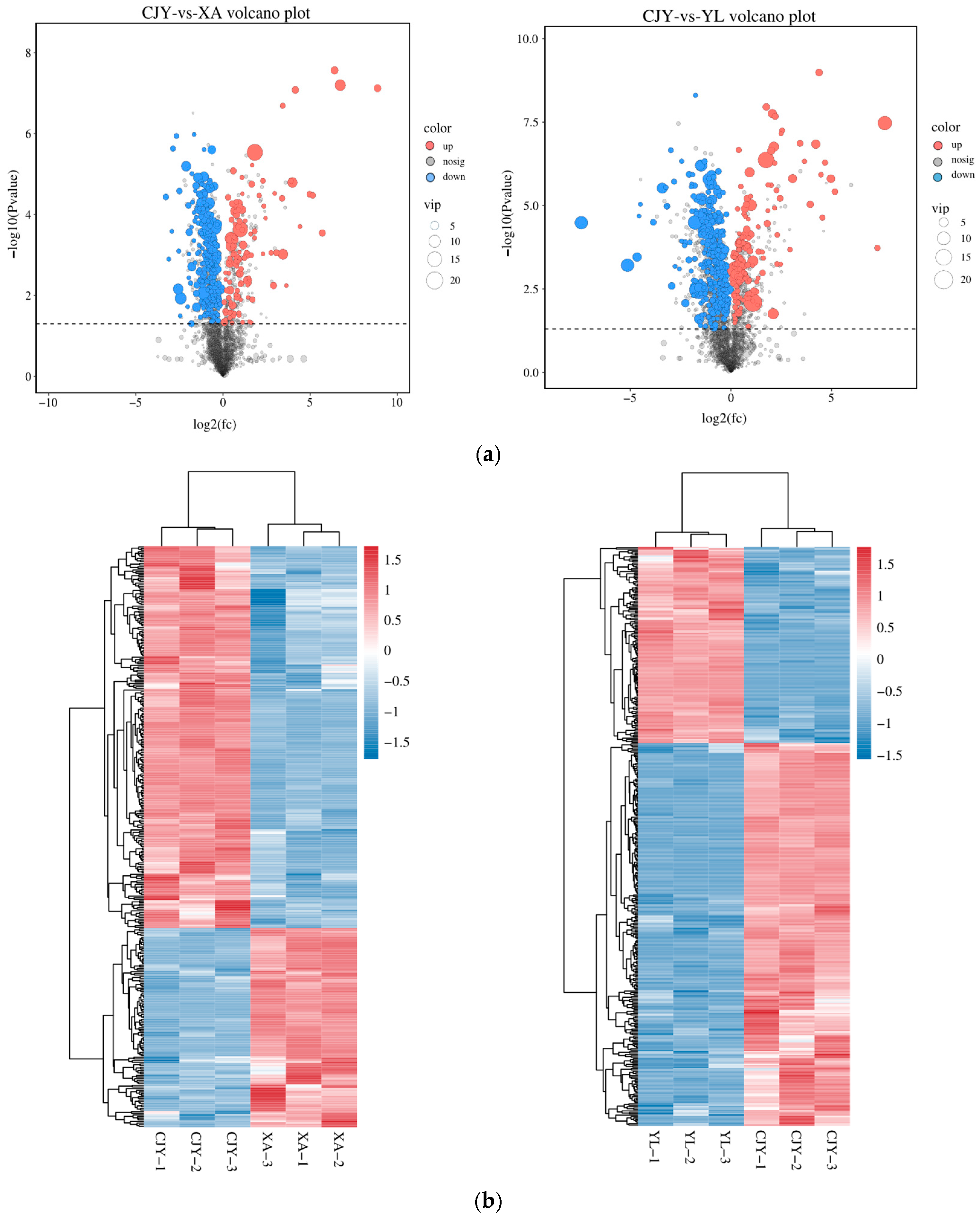
3.4. Identification and Analysis of Differential Metabolites in Microbial Communities
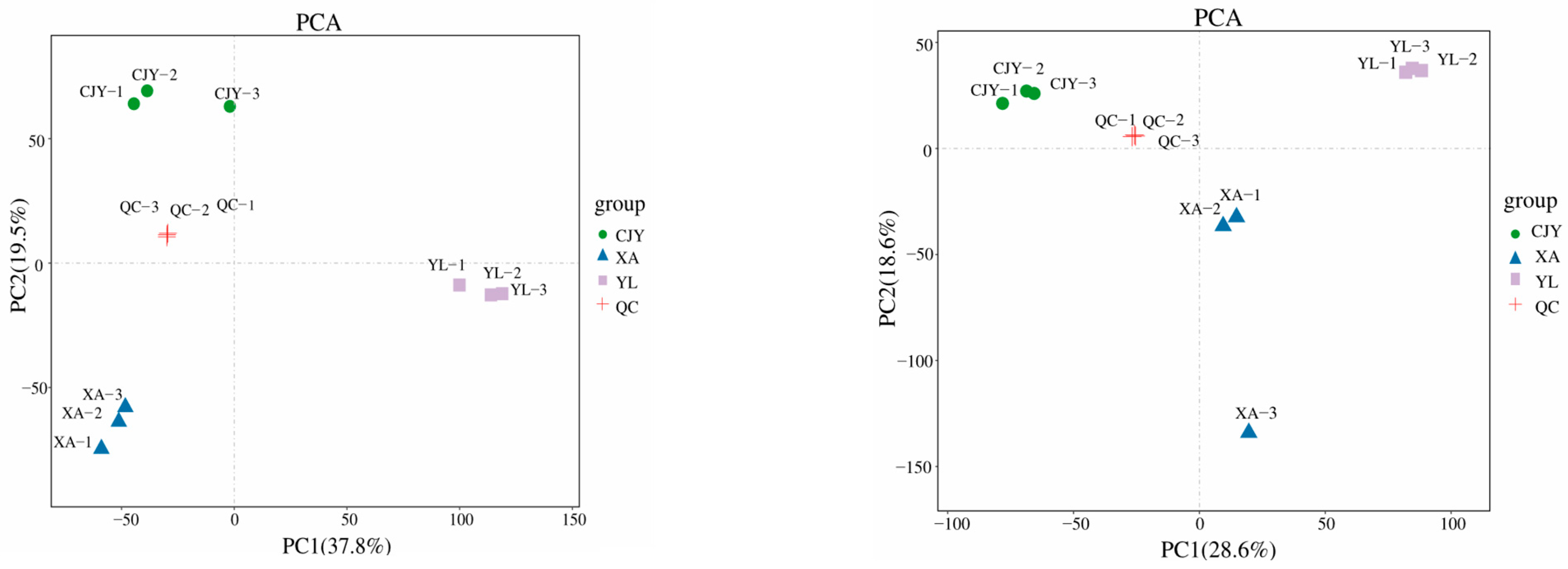
3.4.1. Multivariate Statistical Analysis
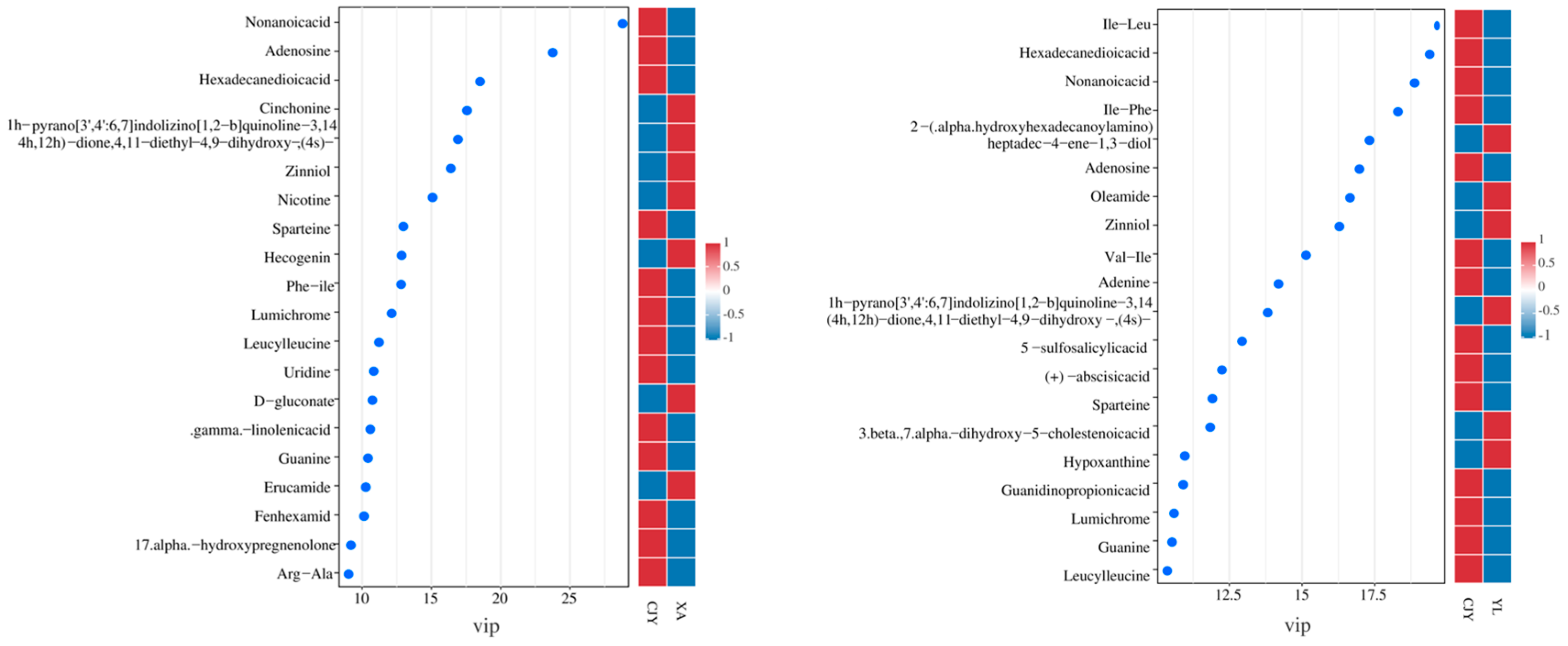
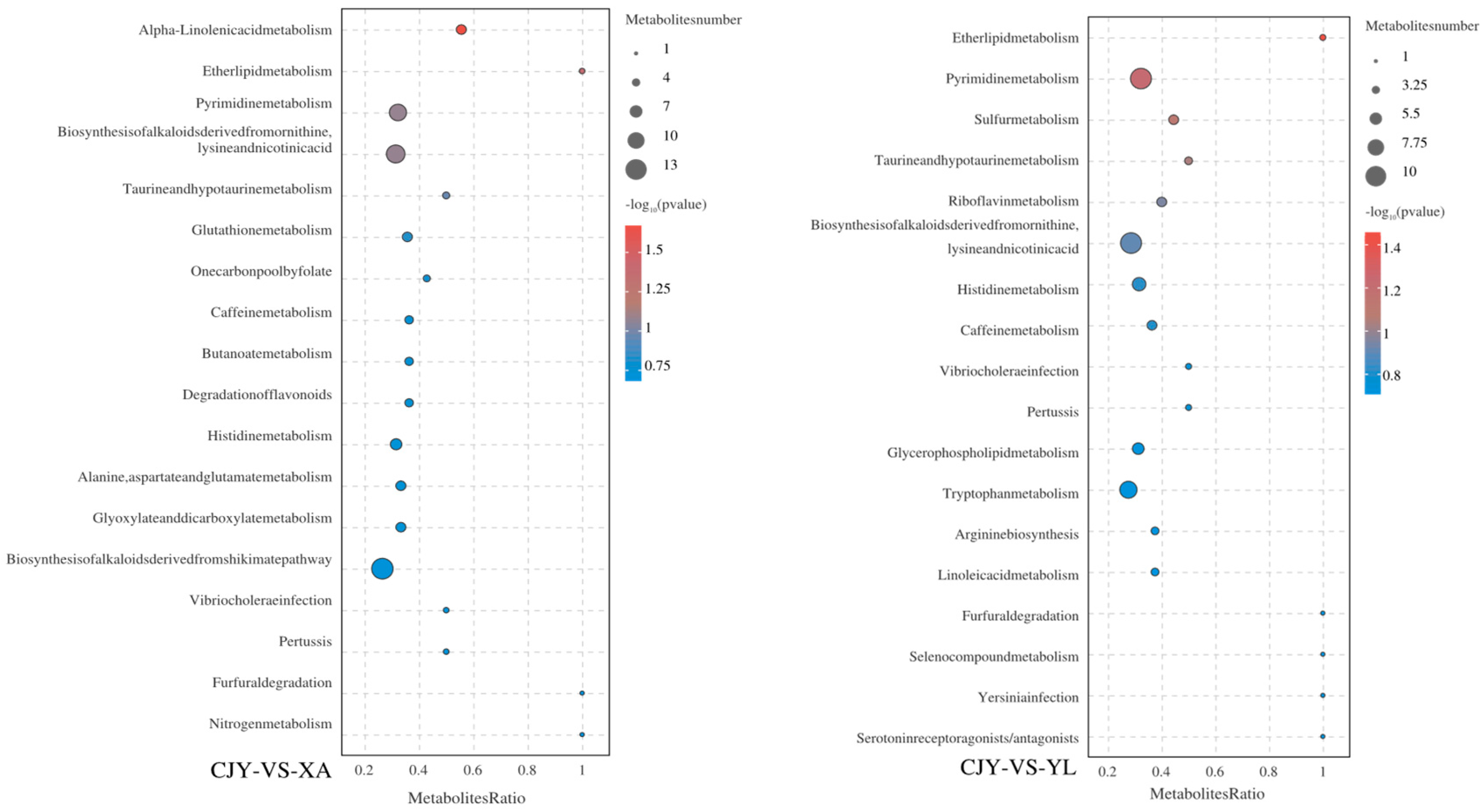
3.4.2. Identification and Analysis of Differential Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAH | Polycyclic Aromatic Hydrocarbons |
| EPS | Extracellular Polymeric Substances |
| Mad | Moisture, air dried basis |
| Ad | Air-dry basis |
| Vdaf | Volatile matter, dry ash-free basis |
| FCdaf | Fixed Carbon, dry ash-free basis |
| PCA | Principal Components Analysis |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| VIP | Variable Importance in Projection |
| FC | Fold Change |
| p | p-value |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| DMSO | Dimethyl Sulfoxide |
| DMSO2 | Dimethyl Sulfone |
References
- Zhou, Y.; Zhang, R.; Tian, K.; Zhao, S.; Shi, H.; Gong, W.; Lei, Q. Characteristics of the Methanotroph Used in Coalbed Methane Emission Reduction: Methane Oxidation Efficiency and Coal Wettability. Fuel 2023, 349, 128596. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Y.; Yu, H.; Wang, S.; Ren, T. Experimental Investigation of the Use of Methanotrophs for the Degradation of Low-Concentration Methane. Arab. J. Geosci. 2021, 14, 685. [Google Scholar] [CrossRef]
- Mimura, K.; Harada, M.; Sumiyoshi, S.; Toya, G.; Takagi, M.; Fujita, E.; Takata, A.; Tatetsu, S. Long-Term Effects of Carbon Monoxide Poisoning at Miike Coal Mine: A 33-Year Follow-up Study. Undersea Hyperb. Med. 2023, 50, 111–143. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Ming, T.; Wu, Y.; Wang, C.; Chen, Q.; Li, W.; Mu, L.; de Richter, R.; Yuan, Y. Numerical Analysis of Solar Chimney Power Plant Integrated with CH4 Photocatalytic Reactors for Fighting Global Warming under Ambient Crosswind. Renew. Energy 2022, 201, 678–690. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Y.; Bodelier, P.L.E.; Conrad, R.; Jia, Z. Conventional Methanotrophs Are Responsible for Atmospheric Methane Oxidation in Paddy Soils. Nat. Commun. 2016, 7, 11728. [Google Scholar] [CrossRef]
- Joon, U.K.; Sun, M.K.; Yun, K.K.; Yun, H.J. A Study on Optimum Ventilation System in the Deep Coal Mine. ResearchGate 2025, 25, 186–198. [Google Scholar]
- Zhang, C.; Li, Y. Optimization of Directional Long Boreholes Unloading Gas Extraction Process and Application Research. Appl. Sci. 2025, 15, 230. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, K.; Kang, M.; Zhao, W.; Wei, G.; Yue, J.; Yao, H. Plugging Methods for Underground Gas Extraction Boreholes in Coal Seams: A Review of Processes, Challenges and Strategies. Gas Sci. Eng. 2024, 122, 205225. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Deng, B.; Li, M.; Zhang, D. Field Investigation of Hydraulic Fracturing in Coal Seams and Its Enhancement for Methane Extraction in the Southeast Sichuan Basin, China. Energies 2018, 11, 3451. [Google Scholar] [CrossRef]
- Limbri, H.; Gunawan, C.; Thomas, T.; Smith, A.; Scott, J.; Rosche, B. Coal-Packed Methane Biofilter for Mitigation of Green House Gas Emissions from Coal Mine Ventilation Air. PLoS ONE 2014, 9, e94641. [Google Scholar] [CrossRef]
- Han, Y.; Xu, L.; Zhang, R.; Lv, J.; Yang, F.; Ma, C. Study on Methane Degradation by Microbial Agents Based on Chelating Wetting Agent Carriers. Sci. Rep. 2024, 14, 15420. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Sun, X.; Du, H.; Chen, D.; Wang, Y. Exploring the Effects of Different Methane and Oxygen Concentrations on the Methane-Oxidizing Bacteria Mixed Community. J. Environ. Eng. 2023, 149, 04023081. [Google Scholar] [CrossRef]
- Liu, D.; Yang, Y.; Ai, J.; Li, Y.; Xing, Y.; Li, J. Research on Microbial Structures, Functions and Metabolic Pathways in an Advanced Denitrification System Coupled with Aerobic Methane Oxidation Based on Metagenomics. Bioresour. Technol. 2021, 332, 125047. [Google Scholar] [CrossRef]
- Jung, G.; Rhee, S.; Han, Y.; Kim, S. Genomic and Physiological Properties of a Facultative Methane-Oxidizing Bacterial Strain of Methylocystis Sp. from a Wetland. Microorganisms 2020, 8, 1719. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, H. First Evidence for Anaerobic Oxidation of Methane Process in Landfill Cover Soils: Activity and Responsible Microorganisms. Sci. Total Environ. 2022, 841, 156790. [Google Scholar] [CrossRef]
- Asakawa, S. Ecology of Methanogenic and Methane-Oxidizing Microorganisms in Paddy Soil Ecosystem. Soil Sci. Plant Nutr. 2021, 67, 520–526. [Google Scholar] [CrossRef]
- Paul, B.G.; Ding, H.; Bagby, S.C.; Kellermann, M.Y.; Redmond, M.C.; Andersen, G.L.; Valentine, D.L. Methane-Oxidizing Bacteria Shunt Carbon to Microbial Mats at a Marine Hydrocarbon Seep. Front. Microbiol. 2017, 8, 186. [Google Scholar] [CrossRef]
- Yu, Z.; Chistoserdova, L. Communal Metabolism of Methane and the Rare Earth Element Switch. J. Bacteriol. 2017, 199, e00328-17. [Google Scholar] [CrossRef]
- Jiang, L.; Chu, Y.; Zhang, X.; Wang, J.; He, X.; Liu, C.; Chen, T.; He, R. Characterization of Anaerobic Oxidation of Methane and Microbial Community in Landfills with Aeration. Environ. Res. 2022, 214, 114102. [Google Scholar] [CrossRef]
- Xu, K.; Yan, Z.; Tao, C.; Wang, F.; Zheng, X.; Ma, Y.; Sun, Y.; Zheng, Y.; Jia, Z. A Novel Bioprospecting Strategy via 13C-Based High-Throughput Probing of Active Methylotrophs Inhabiting Oil Reservoir Surface Soil. Sci. Total Environ. 2024, 924, 171686. [Google Scholar] [CrossRef]
- Ding, J.; Lu, Y.; Fu, L.; Ding, Z.; Mu, Y.; Cheng, S.H.; Zeng, R.J. Decoupling of DAMO Archaea from DAMO Bacteria in a Methane Driven Microbial Fuel Cell. Water Res. 2017, 110, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Duan, C.; Luo, M.; Xing, X. Enrichment and Characteristics of Mixed Methane-Oxidizing Bacteria from a Chinese Coal Mine. Appl. Microbiol. Biotechnol. 2016, 100, 10331–10341. [Google Scholar] [CrossRef] [PubMed]
- Pytlak, A.; Sparkes, R.; Goraj, W.; Szafranek-Nakonieczna, A.; Banach, A.; Akhmetkaliyeva, S.; Slowakiewicz, M. Methanotroph-Derived Bacteriohopanepolyol Signatures in Sediments Covering Miocene Brown Coal Deposits. Int. J. Coal Geol. 2021, 242, 103759. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Q.; Wang, Y.; Chen, D.; Zhang, J.; Yue, Z.; Lyu, J. Research on Coal Mine Gas Microbial Degradation Device Based on Charcoal Filling Material. ACS Omega 2025, 10, 28932–28943. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, R.; Tian, K.; Ma, H.; Zhao, S.; Gong, W.; Duan, C.; Chu, S. Adsorption Characteristics of Methanotrophic Bacteria in Coalbed Methane Emission Reduction. Chem. Eng. J. 2025, 519, 164825. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Y.; Lin, H.; Liu, C. Improving Vanadium Extraction from Stone Coal via Combination of Blank Roasting and Bioleaching by ARTP-Mutated Bacillus Mucilaginosus. Trans. Nonferrous Met. Soc. China 2019, 29, 849–858. [Google Scholar] [CrossRef]
- Li, Q.; Dong, F.; Dai, Q.; Zhang, C.; Yu, L. Surface Properties of PM2.5 Calcite Fine Particulate Matter in the Presence of Same Size Bacterial Cells and Exocellular Polymeric Substances (EPS) of Bacillus Mucitaginosus. Environ. Sci. Pollut. Res. 2018, 25, 22429–22436. [Google Scholar] [CrossRef]
- Gataraddi, S.; Aladakatti, R.H.; Patil, S.J.; Sadashiv, S.O. Microbe–Mineral Interactions: Environmental Pollution and Management. In Biotechnology for Environmental Sustainability; Springer: Singapore, 2025. [Google Scholar]
- David, Y.; Baylon, M.G.; Pamidimarri, S.D.V.N.; Baritugo, K.-A.; Chae, C.G.; Kim, Y.J.; Kim, T.W.; Kim, M.-S.; Na, J.G.; Park, S.J. Screening of Microorganisms Able to Degrade Low-Rank Coal in Aerobic Conditions: Potential Coal Biosolubilization Mediators from Coal to Biochemicals. Biotechnol. Bioprocess Eng. 2017, 22, 178–185. [Google Scholar] [CrossRef]
- Ma, S.; Zong, Q. Exploration and Application of Microbial Method to Enhance the Effect of Hydraulic Fracturing on Coal Seam Permeability Enhancement and Gas Extraction. Energy Explor. Exploit. 2024, 42, 837–855. [Google Scholar] [CrossRef]
- Liu, C.; Ma, S.; Wang, X.; Ou, Y.; Du, H. Biodegradation of Organic Compounds in the Coal Gangue by Bacillus Sp. into Humic Acid. Biodegradation 2023, 34, 125–138. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Gao, Y.; Tan, X.; Sun, Y.; Chen, W. Effects of Coal Mining Activities on the Changes in Microbial Community and Geochemical Characteristics in Different Functional Zones of a Deep Underground Coal Mine. Water 2024, 16, 1836. [Google Scholar] [CrossRef]
- Zhu, Q.; Ruan, M.; Hu, Z.; Miao, K.; Ye, C. The Relationship between Acid Production and the Microbial Community of Newly Produced Coal Gangue in the Early Oxidation Stage. Microorganisms 2023, 11, 2626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mo, Q.; Huang, Z.; Sabar, M.A.; Medunic, G.; Ivosevic, T.; He, H.; Urynowicz, M.; Liu, F.-J.; Guo, H.; et al. Contaminants from a Former Croatian Coal Sludge Dictate the Structure of Microbiota in the Estuarine (Rasa Bay) Sediment and Soil. Front. Microbiol. 2023, 14, 1126612. [Google Scholar]
- Lian, Z.; Yang, Z.; Song, W.; Sun, M.; Gan, Y.; Bai, X. Characteristics of EPS from Pseudomonas Aeruginosa and Alcaligenes Faecalis under Cd(II) Stress: Changes in Chemical Components and Adsorption Performance. Environ. Sci. Pollut. Res. 2022, 29, 75883–75895. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Edwards, A.C. Influence of Sorption on the Biological Utilization of Two Simple Carbon Substrates. Soil Biol. Biochem. 1998, 30, 1895–1902. [Google Scholar] [CrossRef]
- DIAB, S.; Shilo, M. Effect of Adhesion to Particles on the Survival and Activity of Nitrosomonas sp. and Nitrobacter sp. Arch. Microbiol. 1988, 150, 387–393. [Google Scholar]
- Keen, G.A.; Prosser, J.I. The Surface Growth and Activity of Nitrobacter. Microb. Ecol. 1988, 15, 21–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Zhang, B.; Zhang, W.; Shen, B. Interannual Variation and Chemical Characterization of Major Water-Soluble Inorganic Ions in Snow across Northwest China. Front. Earth Sci. 2023, 11, 1099178. [Google Scholar] [CrossRef]
- Reis, P.C.J.; Tsuji, J.M.; Weiblen, C.; Schiff, S.L.; Scott, M.; Stein, L.Y.; Neufeld, J.D. Enigmatic Persistence of Aerobic Methanotrophs in Oxygen-Limiting Freshwater Habitats. ISME J. 2024, 18, wrae041. [Google Scholar] [CrossRef]
- Rout, P.G.; Mohanty, A.K.; Pradhan, N.; Biswal, S.K.; Behera, S.K. Study on the Reaction Mechanism of Oxidative Microbial Desulfurization of Organic Sulfur-Rich Coal. Geomicrobiol. J. 2022, 39, 210–218. [Google Scholar] [CrossRef]
- Ai, C.; Xue, S.; Liu, C.; Wang, S. Optimization of Experimental Conditions and Study of Desulfurization Mechanism of Rhodococcus erythropolis for Organic Sulfur Removal from Coal. J. Environ. Chem. Eng. 2025, 13, 116103. [Google Scholar] [CrossRef]
- Gotz, F.; Longnecker, K.; Soule, M.C.K.; Becker, K.W.; McNichol, J.; Kujawinski, E.B.; Sievert, S.M. Targeted Metabolomics Reveals Proline as a Major Osmolyte in the Chemolithoautotroph Sulfurimonas Denitrificans. MicrobiologyOpen 2018, 7, e586. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, Y.; Shen, Y.; Yang, K.; Cai, X.; Zhang, B.; Liu, Z.; Zheng, Y. Microbial Production of Sulfur-Containing Amino Acids Using Metabolically Engineered Escherichia coli. Biotechnol. Adv. 2024, 73, 108353. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, J.; Yuan, H.; Niu, Y.; Sun, J.; Tian, Q.; Wu, Y.; Yu, J.; Tang, Z.; Xiao, X.; et al. Exogenous Application of ALA Enhanced Sugar, Acid and Aroma Qualities in Tomato Fruit. Front. Plant Sci. 2023, 14, 1323048. [Google Scholar] [CrossRef]
- McDonald, I.R.; Murrell, J.C. The Methanol Dehydrogenase Structural Gene mxaF and Its Use as a Functional Gene Probe for Methanotrophs and Methylotrophs. Appl. Environ. Microbiol. 1997, 63, 3218–3224. [Google Scholar] [CrossRef]
- Crowther, G.J.; Kosaly, G.; Lidstrom, M.E. Formate as the Main Branch Point for Methylotrophic Metabolism in Methylobacterium Extorquens AM1. J. Bacteriol. 2008, 190, 5057–5062. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, L.; Tuyishirne, P.; Zheng, P.; Sun, J. Synthetic Methylotrophy: A Practical Solution for Methanol-Based Biomanufacturing. Trends Biotechnol. 2020, 38, 650–666. [Google Scholar] [CrossRef]
- Aroca, A.; Benito, J.M.; Gotor, C.; Romero, L.C. Persulfidation Proteome Reveals the Regulation of Protein Function by Hydrogen Sulfide in Diverse Biological Processes in Arabidopsis. J. Exp. Bot. 2017, 68, 4915–4927. [Google Scholar] [CrossRef]
- Kawano, Y.; Onishi, F.; Shiroyama, M.; Miura, M.; Tanaka, N.; Oshiro, S.; Nonaka, G.; Nakanishi, T.; Ohtsu, I. Improved Fermentative L-Cysteine Overproduction by Enhancing a Newly Identified Thiosulfate Assimilation Pathway in Escherichia coli. Appl. Microbiol. Biotechnol. 2017, 101, 6879–6889. [Google Scholar] [CrossRef]
- Feng, S.; Yang, H.; Wang, W. System-Level Understanding of the Potential Acid-Tolerance Components of Acidithiobacillus Thiooxidans ZJJN-3 under Extreme Acid Stress. Extremophiles 2015, 19, 1029–1039. [Google Scholar] [CrossRef]
- Luan, Y.; Xu, Y.; Guo, Z.; Yin, Y.; Wang, Q.; Zhang, F.; Xiao, Y.; Liu, C.; Jiang, S. Enhanced Nitrogen Removal in Immersed Rotating Self-Aerated Biofilm Reactor: Nitrogen Removal Pathway and Microbial Mechanism. Bioresour. Technol. 2023, 385, 129426. [Google Scholar] [CrossRef]
- Blohm, A.; Kumar, S.; Knebl, A.; Herrmann, M.; Kuesel, K.; Popp, J.; Frosch, T. Activity and Electron Donor Preference of Two Denitrifying Bacterial Strains Identified by Raman Gas Spectroscopy. Anal. Bioanal. Chem. 2022, 414, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, X.; Li, Q.; Xu, D.; Li, J.; Hou, W.-T.; Chen, Y.; Jiang, Y.-L.; Zhou, C.-Z. Allosteric Regulation of Nitrate Transporter NRT via the Signaling Protein PII. Proc. Natl. Acad. Sci. USA 2024, 121, e2318320121. [Google Scholar] [CrossRef] [PubMed]
- Martineau, C.; Mauffrey, F.; Villemur, R. Comparative Analysis of Denitrifying Activities of Hyphomicrobium Nitrativorans, Hyphomicrobium Denitrificans, and Hyphomicrobium Zavarzinii. Appl. Environ. Microbiol. 2015, 81, 5003–5014. [Google Scholar] [CrossRef]
- Ungwiwatkul, S.; Jaikua, M.; Prasertboonyai, K.; Thana, P.; Tamman, A.; Matra, K. Plasma-Activated Municipal Wastewater (PAMW): Revolutionizing Municipal Wastewater into High-Value Liquid Fertilizer for Duckweed Cultivation through Air Plasma Treatment. Appl. Sci. Eng. Prog. 2025, 18, 7791. [Google Scholar] [CrossRef]
- Xuan, Y.; Mai, Y.; Xu, Y.; Zheng, J.; He, Z.; Shu, L.; Cao, Y. Enhanced Microbial Nitrification-Denitrification Processes in a Subtropical Metropolitan River Network. Water Res. 2022, 222, 118857. [Google Scholar] [CrossRef]
- Ouyang, L.; Thamdrup, B.; Trimmer, M. Coupled Nitrification and N2 Gas Production as a Cryptic Process in Oxic Riverbeds. Nat. Commun. 2021, 12, 1217. [Google Scholar] [CrossRef]


| Coal Sample | Industrial Analysis (%) | |||
|---|---|---|---|---|
| Mad | Ad | Vdaf | FCdaf | |
| XA | 1.21 | 5.53 | 36.87 | 55.06 |
| YL | 0.96 | 11.29 | 24.85 | 58.34 |
| Sample | Number | OTUs | Shannon | Chao | Ace | Simpson | Shannoneven |
|---|---|---|---|---|---|---|---|
| original solution | 81,649.00 | 221.00 | 1.64 | 227.16 | 237.59 | 0.35 | 0.30 |
| Pair | R2X (cum) | R2Y (cum) | Q2 (cum) |
|---|---|---|---|
| CJY-vs.-XA.POS | 0.658 | 0.999 | 0.958 |
| CJY-vs.-XA.NEG | 0.695 | 0.996 | 0.923 |
| CJY-vs.-YL.POS | 0.698 | 1 | 0.988 |
| CJY-vs.-YL.NEG | 0.763 | 0.991 | 0.932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Zhang, R.; Zhou, Y.; Tian, K.; Gong, W.; Duan, C.; Chu, S. Characteristics of Growth and Metabolism for Methane-Oxidizing Bacterial Communities in Different Coal Samples. Processes 2025, 13, 2884. https://doi.org/10.3390/pr13092884
Ma H, Zhang R, Zhou Y, Tian K, Gong W, Duan C, Chu S. Characteristics of Growth and Metabolism for Methane-Oxidizing Bacterial Communities in Different Coal Samples. Processes. 2025; 13(9):2884. https://doi.org/10.3390/pr13092884
Chicago/Turabian StyleMa, Haojuan, Ruilin Zhang, Yinbo Zhou, Kunyun Tian, Weidong Gong, Chaosheng Duan, and Shihai Chu. 2025. "Characteristics of Growth and Metabolism for Methane-Oxidizing Bacterial Communities in Different Coal Samples" Processes 13, no. 9: 2884. https://doi.org/10.3390/pr13092884
APA StyleMa, H., Zhang, R., Zhou, Y., Tian, K., Gong, W., Duan, C., & Chu, S. (2025). Characteristics of Growth and Metabolism for Methane-Oxidizing Bacterial Communities in Different Coal Samples. Processes, 13(9), 2884. https://doi.org/10.3390/pr13092884





