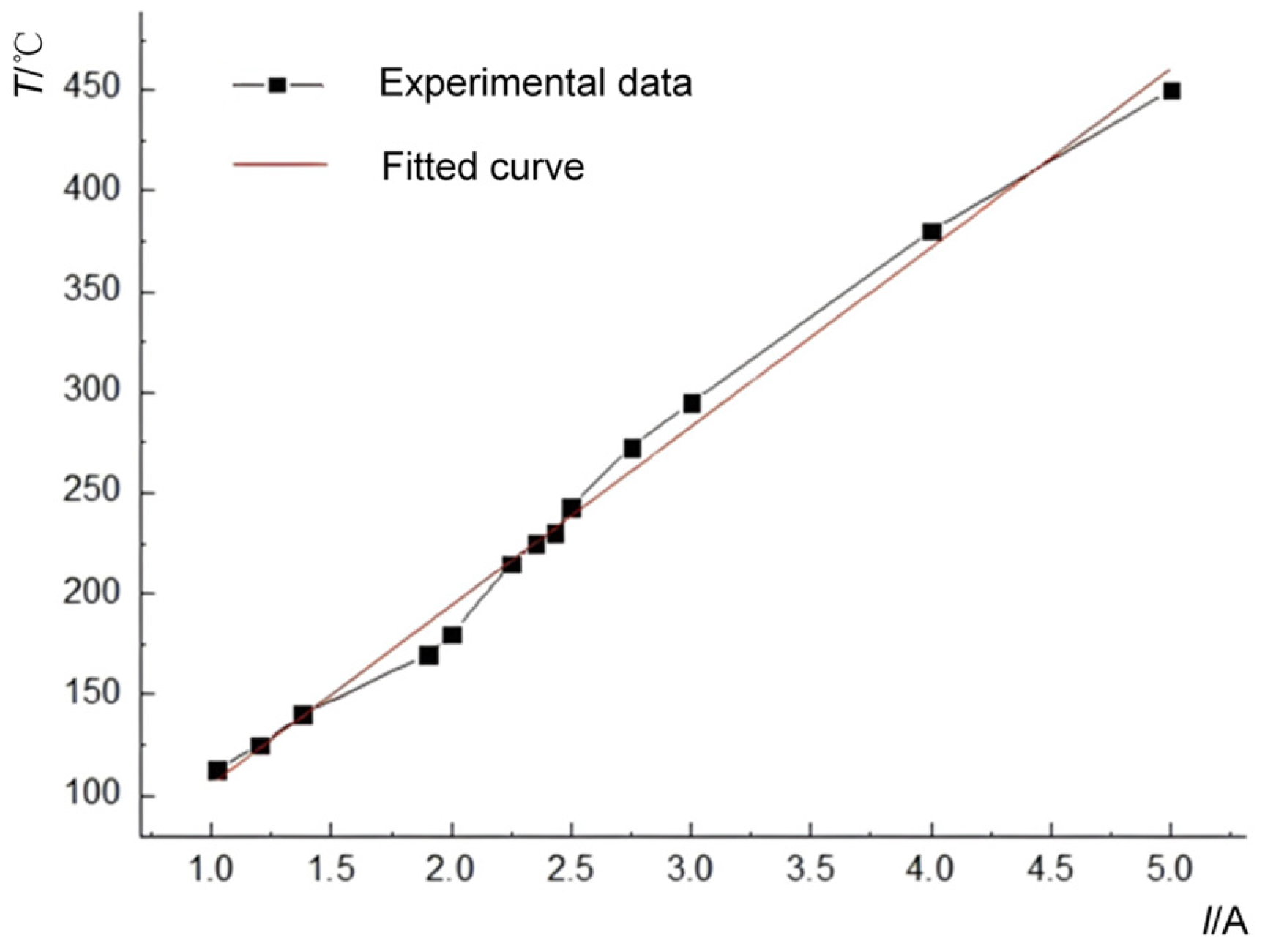
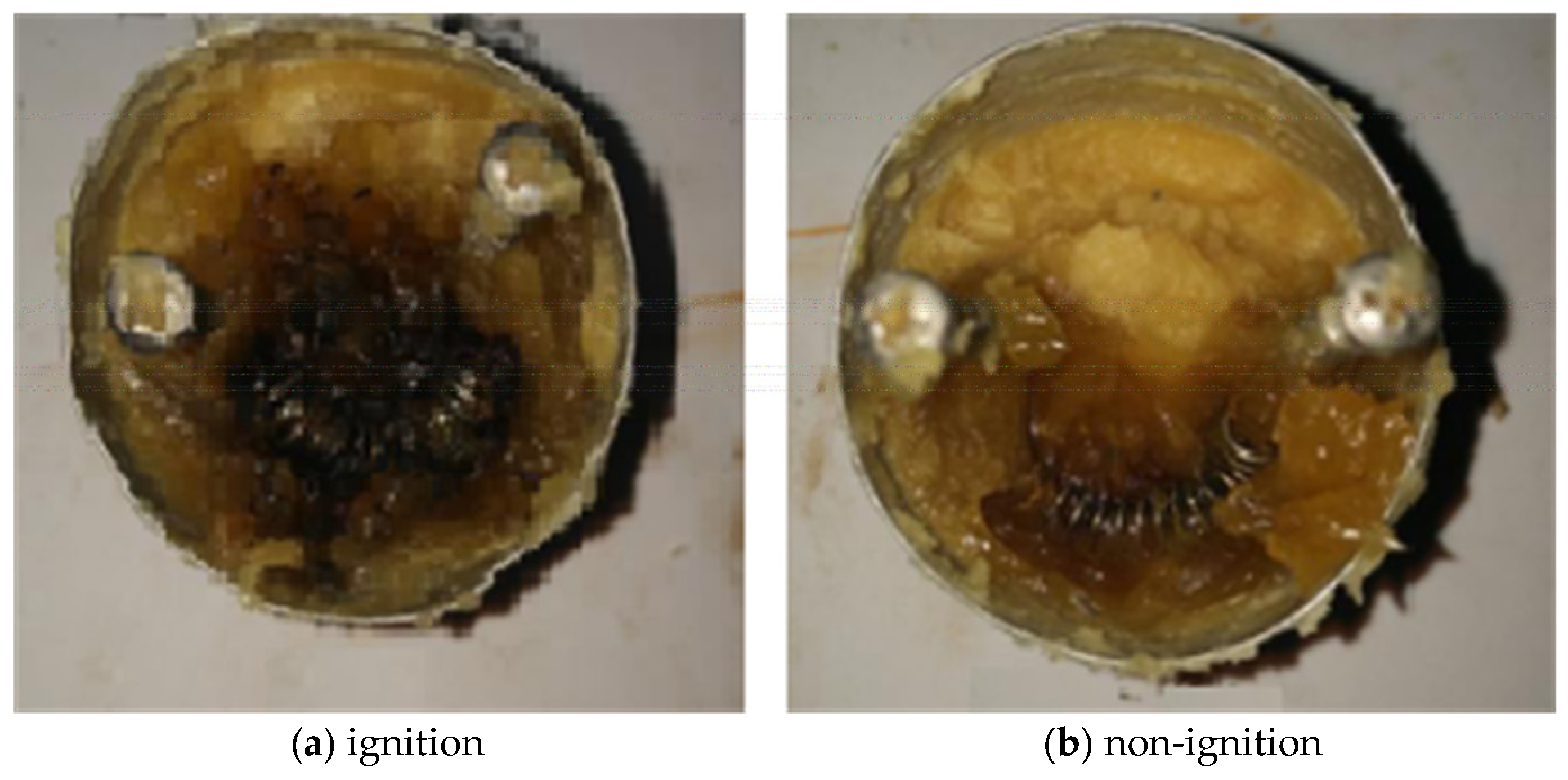
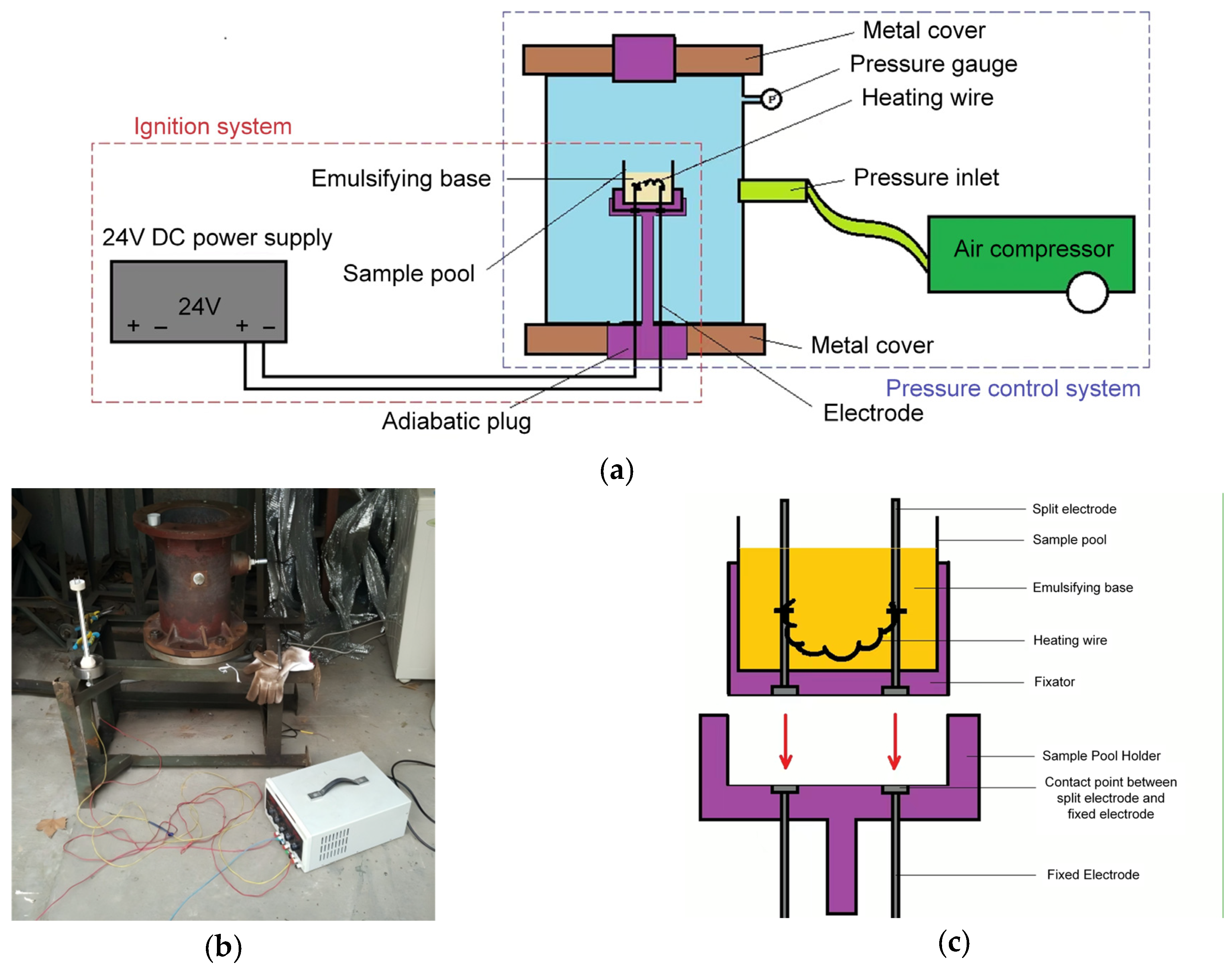
3.1.1. Impact of Ambient Pressure on Critical Hot-Spot Temperature
During the exploratory experimental phase, it was observed that the ignition delay time of the emulsion matrix was approximately 3 min when the nichrome heating filament reached ≈260 °C, whereas the delay increased to ≈10 min at ≈180 °C. Following the experimental protocol, trials with currents exceeding 1.5 A (IL > 1.5 A) were classified as high-temperature heating experiments, while those with currents below 1.5 A (IL < 1.5 A) were designated low-temperature heating experiments. To systematically investigate the pressure-dependent mechanisms influencing critical hot-spot temperature, both low-temperature and high-temperature heating experiments were conducted under controlled ambient pressure conditions.
- (1)
Low-current heating experiments with electric heating wire (IL < 1.5 A)
This study first investigated the formation of low-temperature hot-spots within the emulsion matrix. Low-current heating was implemented using a nichrome filament, and exploratory experiments established the current excitation thresholds at
IU = 4 A (upper limit) and
IL = 1 A (lower limit). Subsequent experiments were conducted under controlled ambient pressures of 1 atm (101.325 kPa), 2 atm (202.650 kPa), and 3 atm (303.975 kPa) with a fixed electrification duration of 10 min. The ignition response results under varying current excitation levels are summarized in
Table 4. The experimental setup in this study involves placing a semi-enclosed sample chamber inside a sealed container, with internal heating applied to the sample within the chamber. Considering the potential for heat dissipation from the semi-enclosed sample chamber into the sealed container, the observed behavior may differ from the general principle in fundamental combustion physics where increased pressure enhances ignition probability.
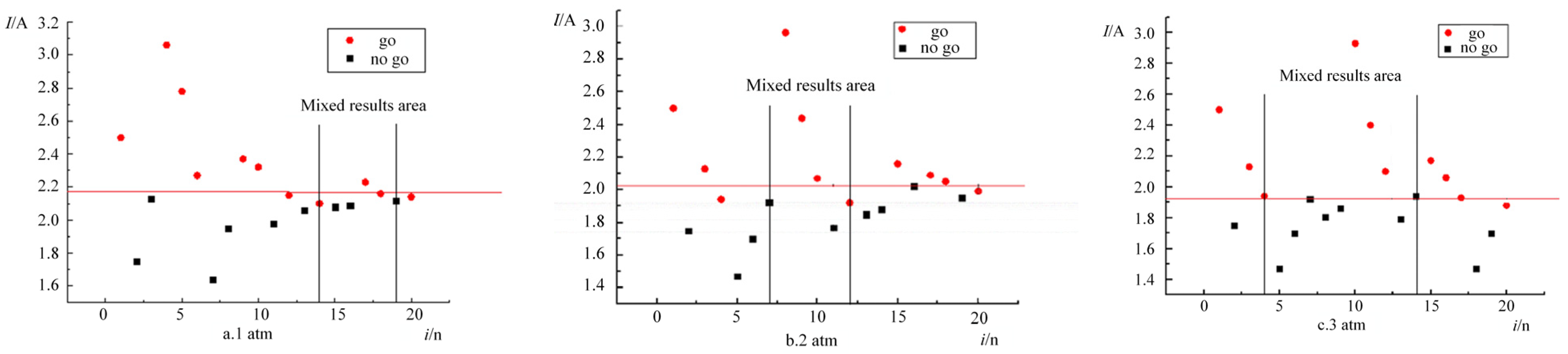
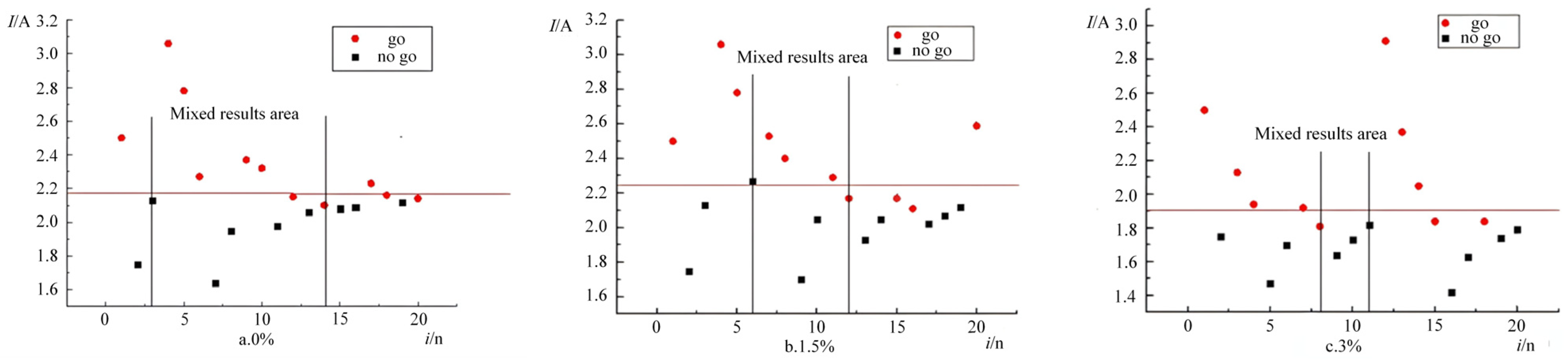
The ignition response distributions under three ambient pressures (1 atm [101.325 kPa], 2 atm [202.650 kPa], and 3 atm [303.975 kPa]) are shown in
Figure 4. Following the Langley reliability criterion, experimental validity was confirmed by observing mixed-response regions (where non-ignition stimuli exceeded ignition stimuli). At 1 atm, Trial 14 (
I = 2.10 A) ignited, while Trial 19 (
I = 2.12 A) failed. At 2 atm, Trial 7 (
I = 1.92 A) showed no ignition, but Trial 12 (
I = 1.92 A) succeeded. At 3 atm, Trial 4 (
I = 1.94 A) ignited, whereas Trial 14 (
I = 1.94 A) did not. The consistent emergence of mixed-response zones across all pressures validates the statistical reliability of the experimental results.
By substituting the current excitation levels into Equations (4) and (5), the mean values and standard deviations of the current stimuli under ambient pressures of 1 atm (101.325 kPa), 2 atm (202.650 kPa), and 3 atm (303.975 kPa) were computed and are summarized in
Table 5.
By substituting the mean values and standard deviations of current stimuli under three pressure conditions (1 atm [101.325 kPa], 2 atm [202.650 kPa], and 3 atm [303.975 kPa]) into Equation (8), the critical current intensity and current thresholds corresponding to 50% and 99% ignition probabilities were calculated. Using the calibrated current-temperature correlation, the resultant nichrome filament temperatures were derived and are tabulated in
Table 6.
When the electric heating wire heats the emulsified matrix at a lower temperature, the ignition current (Icig) is 1.38 A with a corresponding ignition temperature (Tcig) of 140 °C under the ambient pressure of 1 atm. At elevated ambient pressures, these parameters exhibit a decreasing trend: Icig decreases to 1.20 A with Tcig = 125 °C at 2 atm, and Icig further declines to 1.08 A with Tcig = 115 °C at 3 atm. The critical hot-spot temperature exhibits a significant decrease with increasing ambient pressure. This phenomenon arises from the acceleration of the decomposition reaction kinetics in the emulsified matrix under elevated pressure conditions. The applied pressure enhances heat generation rates by accelerating exothermic reactions, thereby lowering the minimum hot-spot temperature required for the matrix to reach its ignition threshold.
- (2)
High-temperature heating experiments with electric heating wire (IL > 1.5 A)
When elevated-temperature hot spots develop within the emulsified matrix (i.e., under conditions of high electric heating wire temperatures), a significant reduction in the ignition delay period is observed. Consequently, a standardized heating duration of 3 min was adopted for all experimental trials. Through preliminary exploratory testing, the upper and lower bounds of current intensity were empirically determined to be
IU = 3 A and
IL = 2 A, respectively. The experimental protocol utilized the Langlie method, with the resulting ignition response characteristics quantitatively summarized in
Table 7.
The ignition response distribution of the emulsified matrix under three distinct pressure conditions (1 atm, 2 atm, and 3 atm) is presented in
Figure 5. As illustrated, the transition to mixed-result regimes occurs at the 15th, 11th, and 8th experimental trials for 1 atm, 2 atm, and 3 atm conditions, respectively. This systematic shift in transitional thresholds with increasing pressure confirms the statistical validity and experimental reliability of the test methodology.
The mean values and standard deviations of the emulsified matrix stimulus parameters under 1 atm, 2 atm, and 3 atm ambient pressures were determined through maximum likelihood estimation (MLE), with quantitative results detailed in
Table 8.
Following advanced data processing, the corresponding current values for specified ignition probabilities of the emulsified matrix at 1 atm, 2 atm, and 3 atm ambient pressures were derived. Subsequent calibration against reference standards yielded the associated electric heating wire temperatures, as systematically presented in
Table 9.
The experimental data obtained under three-minute high-temperature hot-spot heating conditions revealed minimal variation in critical ignition parameters across different ambient pressures. Specifically, the critical ignition current (Icig) measured 2.15 A (Tcig = 205 °C) at 1 atm, 2.13 A (Tcig = 202 °C) at 2 atm, and 2.14 A (Tcig = 203 °C) at 3 atm, demonstrating negligible pressure dependence of the critical hot-spot temperature under elevated thermal loading conditions. Analysis indicates that during high-temperature heating, the electric heating wire’s temperature is significantly higher than that of the emulsified matrix, enabling rapid heating of the matrix to its ignition temperature. Additionally, the temperature rise in the emulsified matrix induced by increasing ambient pressure is much slower compared to heating by the high-temperature electric wire. Therefore, under high-temperature hot-spot conditions, ambient pressure exerts minimal influence on the critical hot-spot temperature of the emulsified matrix.
3.1.2. Influence of Gas Bubbles on Critical Hot-Spot Temperature
During the preparation of emulsified matrices, air bubbles often become entrained, which increases the sensitivity of the matrix and adversely affects the thermal safety of the emulsification process. Since controlling the amount of intentionally added bubbles is challenging, this study employs expanded perlite as a bubble carrier. The dosage of expanded perlite can be precisely controlled, enabling systematic investigation of how bubble content influences the critical hot-spot temperature of the emulsified matrix. Using the same calculation method as described in the previous section, the critical hot-spot temperatures of the emulsified matrix were determined under an ambient pressure of 1 atm for expanded perlite contents of 0%, 1.5%, and 3%, respectively. In this experiment, only low-temperature hot-spot scenarios were considered. The influence of expanded perlite on the ignition response of the emulsified matrix is presented in
Table 10.
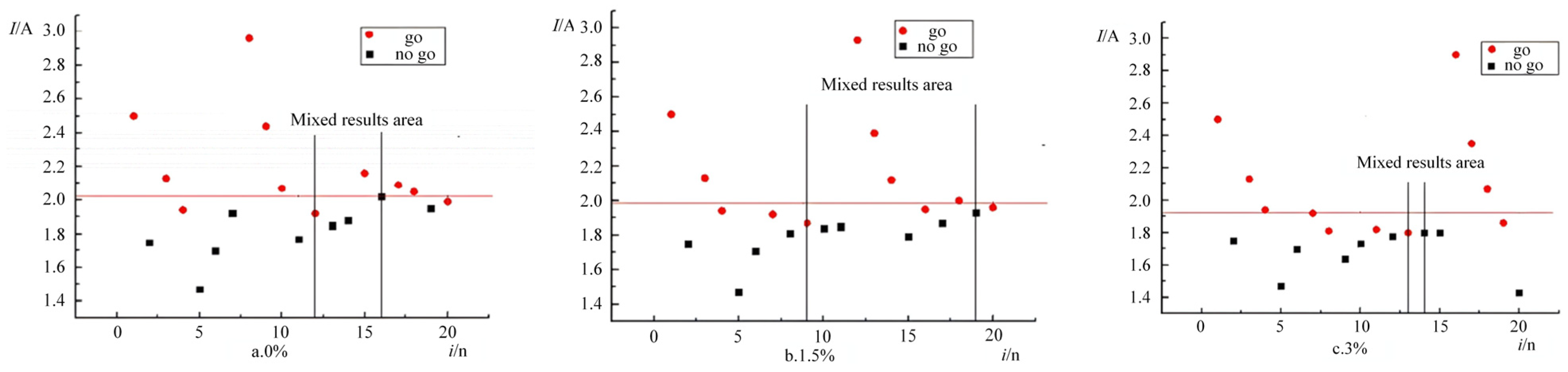
The ignition response distribution of the emulsified matrix at 1 atm, obtained from the experiment, is shown in
Figure 6. When the expanded perlite contents were 0%, 1.5%, and 3%, the emulsified matrix samples exhibited mixed-result zones in the 14th, 12th, and 11th experiments, respectively, demonstrating the reliability and validity of the experimental results. The data were processed using the maximum likelihood estimation method, and the means and standard deviations of the ignition currents for the emulsified matrices containing 0%, 1.5%, and 3% expanded perlite are presented in
Table 11.
The current values corresponding to specified ignition probabilities and their associated heating wire temperatures for emulsified matrices containing 0%, 1.5%, and 3% expanded perlite at 1 atm ambient pressure were determined through maximum likelihood estimation (MLE), with complete quantitative results presented in
Table 12.
The experimental results show that the critical hot-spot temperature Tcig of the emulsified matrix without expanded perlite is 140 °C. In contrast, for emulsified matrices containing 1.5% and 3% expanded perlite, the critical hot-spot temperatures Tcig are 140 °C and 110 °C, respectively. Overall, the critical hot-spot temperature decreases as the amount of expanded perlite increases. This indicates that, within the scope of this study, higher bubble content correlates with reduced safety in the production of the emulsified matrix. The analysis suggests that as bubble content increases, the sensitivity of the emulsified matrix gradually rises, reducing the heat required for ignition and consequently decreasing the critical hot-spot temperature.
3.1.3. Coupled Effects of Gas Bubbles and Ambient Pressure on Critical Hot-Spot Temperature
The previous two parts of this study investigated the influence laws of single factors, namely ambient pressure and expanded perlite content, on the critical ignition temperature of the emulsified matrix. This section focuses on analyzing the variation in the critical hot-spot temperature under the combined effects of both factors (expanded perlite content and ambient pressure). When the ambient pressure was 2 atm or 3 atm, the influence of expanded perlite content on the critical hot-spot temperature was systematically studied. Specifically,
Table 13 presents the experimental results for the case when the ambient pressure was maintained at 2 atm.
- (1)
Experimental Investigation on the Influence of Expanded Perlite at 2 atm Ambient Pressure
Table 13.
Ignition response of emulsified matrix containing expanded perlite with different percentages at 2 atm.
Table 13.
Ignition response of emulsified matrix containing expanded perlite with different percentages at 2 atm.
| i | 0% | 1.5% | 3% |
|---|
| I/A | pi | I/A | pi | I/A | pi |
|---|
| 1 | 2.50 | 1 | 2.50 | 1 | 2.50 | 1 |
| 2 | 1.75 | 0 | 1.75 | 0 | 1.75 | 0 |
| 3 | 2.13 | 1 | 2.13 | 1 | 2.13 | 1 |
| 4 | 1.94 | 1 | 1.94 | 1 | 1.94 | 1 |
| 5 | 1.47 | 0 | 1.47 | 0 | 1.47 | 0 |
| 6 | 1.70 | 0 | 1.71 | 0 | 1.70 | 0 |
| 7 | 1.92 | 0 | 1.92 | 1 | 1.92 | 1 |
| 8 | 2.96 | 1 | 1.81 | 0 | 1.81 | 1 |
| 9 | 2.44 | 1 | 1.87 | 1 | 1.64 | 0 |
| 10 | 2.07 | 1 | 1.84 | 0 | 1.73 | 0 |
| 11 | 1.77 | 0 | 1.85 | 0 | 1.82 | 1 |
| 12 | 1.92 | 1 | 2.93 | 1 | 1.78 | 0 |
| 13 | 1.85 | 0 | 2.39 | 1 | 1.80 | 1 |
| 14 | 1.88 | 0 | 2.12 | 1 | 1.80 | 0 |
| 15 | 2.16 | 1 | 1.79 | 0 | 1.80 | 0 |
| 16 | 2.02 | 0 | 1.95 | 1 | 2.90 | 1 |
| 17 | 2.09 | 1 | 1.87 | 0 | 2.35 | 1 |
| 18 | 2.05 | 1 | 2.00 | 1 | 2.07 | 1 |
| 19 | 1.95 | 0 | 1.93 | 0 | 1.86 | 1 |
| 20 | 1.99 | 1 | 1.96 | 1 | 1.43 | 0 |
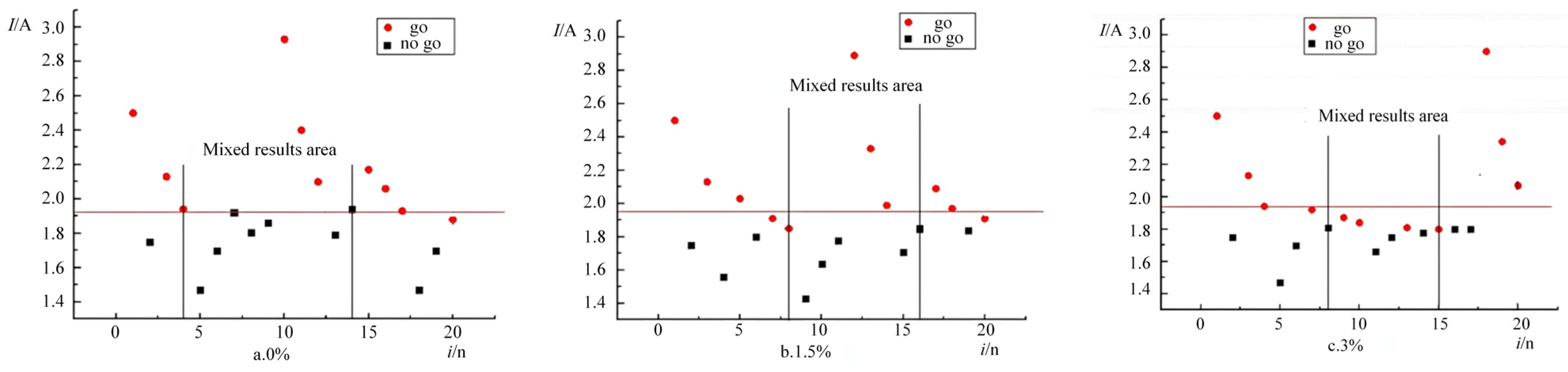
When the ambient pressure was maintained at 2 atm, the ignition response distributions of the emulsified matrices containing 0%, 1.5%, and 3% expanded perlite are shown in
Figure 7. The results demonstrate that the emulsified matrix samples with 0%, 1.5%, and 3% expanded perlite exhibited mixed-result zones in the 16th, 19th, and 14th experiments, respectively, confirming the validity of the experimental data.
The means and mean square deviations of the stimulating currents for the emulsified matrices containing 0%, 1.5%, and 3% expanded perlite under an ambient pressure of 2 atm were determined using the maximum likelihood estimation method, as summarized in
Table 14. Subsequent calculations revealed the current values corresponding to different ignition probabilities, along with the associated heating wire temperatures, for the same emulsified matrices under 2 atm ambient pressure. These results are compiled in
Table 15.
As shown in
Table 15, under an ambient pressure of 2 atm, the critical current intensity
Icig of the emulsified matrix without expanded perlite is 2.03 A, with the corresponding critical hot-spot temperature
Tcig being 180 °C. For the emulsified matrix containing 1.5% expanded perlite, the critical current intensity
Icig decreases to 1.19 A, and the critical hot-spot temperature
Tcig reduces to 120 °C. When the expanded perlite content increases to 3%, the critical current intensity
Icig further lowers to 1.02 A, while
Tcig slightly decreases to 113 °C. The experimental results indicate that, at 2 atm ambient pressure, the critical hot-spot temperature still decreases with increasing bubble content in the emulsified matrix, but the rate of decline slows as the bubble content rises.
- (2)
Experimental Investigation of Expanded Perlite Effects under 3 atm Ambient Pressure
Table 16 presents the ignition response characteristics of the emulsified matrix containing expanded perlite at different percentages under 3 atm ambient pressure. The ignition response distributions of the emulsified matrices with varying expanded perlite contents at 3 atm are presented in
Figure 8. As shown in the figure, the emulsified matrix samples containing 0%, 1.5%, and 3% expanded perlite exhibited mixed-result zones in the 14th, 16th, and 15th experiments, respectively. These results validate the experimental data.
Using the maximum likelihood estimation method, the influence of expanded perlite content on the mean and variance of the stimulating current in the emulsified matrix under 3 atm ambient pressure was analyzed, as summarized in
Table 17. The calculated current values corresponding to different ignition probabilities, along with the associated heating wire temperatures, for the emulsified matrices with varying expanded perlite contents are presented in
Table 18.
As shown in
Table 18, under an ambient pressure of 3 atm, the critical current intensity
Icig of the emulsified matrix without expanded perlite is 1.08 A (corresponding to
Tcig of 115 °C) when the ignition probability is 1%. When the expanded perlite content increases to 1.5% and 3%, the critical current intensity
Icig remains unchanged at 1.09 A, with the corresponding
Tcig also maintaining a constant value of 116 °C. The experimental results demonstrate that, the critical hot-spot temperature does not decrease with increasing bubble content in the emulsified matrix.