Microparticle Production of Mefenamic Acid Using the Continuous Antisolvent Sonocrystallization Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
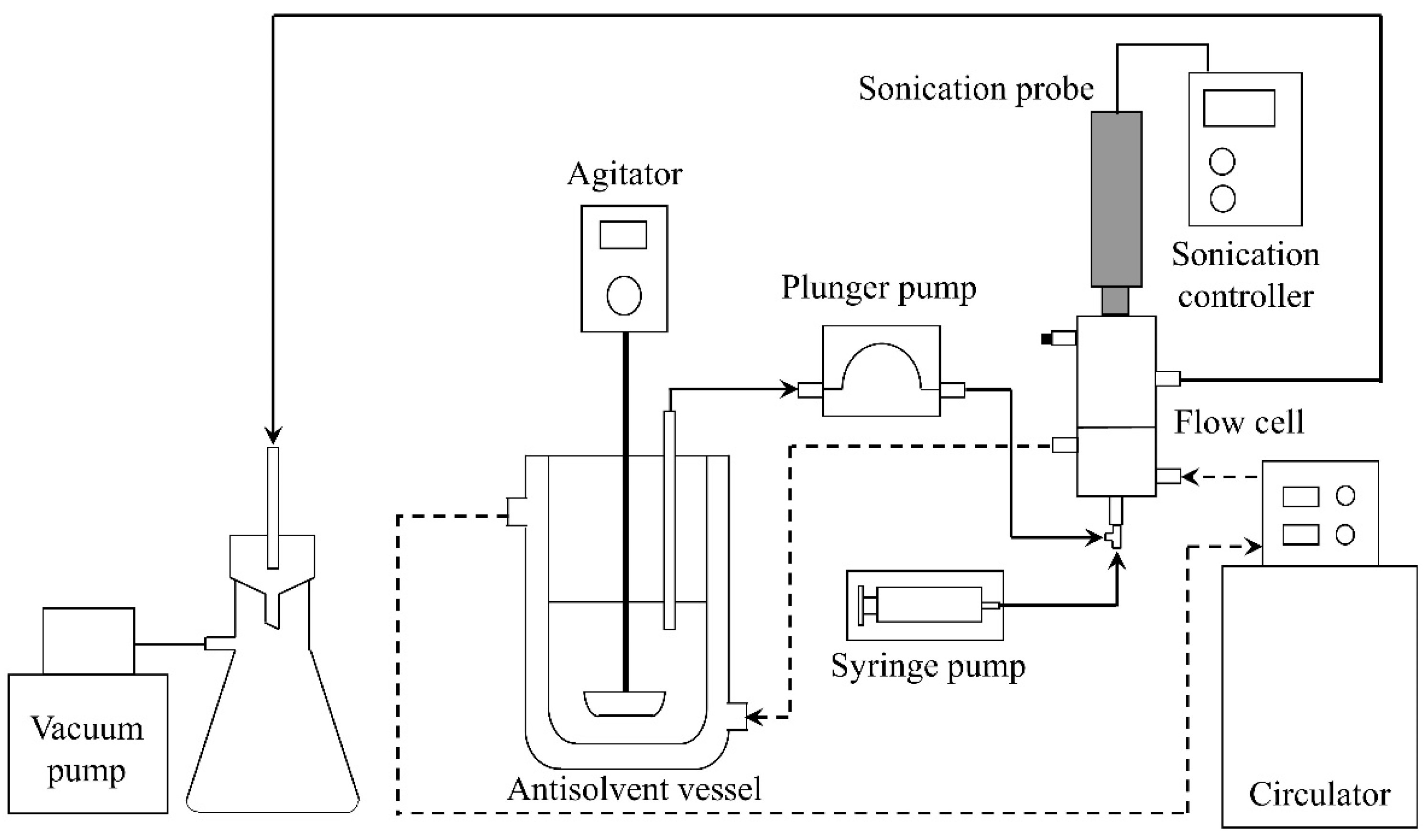
2.2. Experimental Apparatus
2.3. Characterization
3. Results and Discussion
3.1. The Effect of Operating Parameters
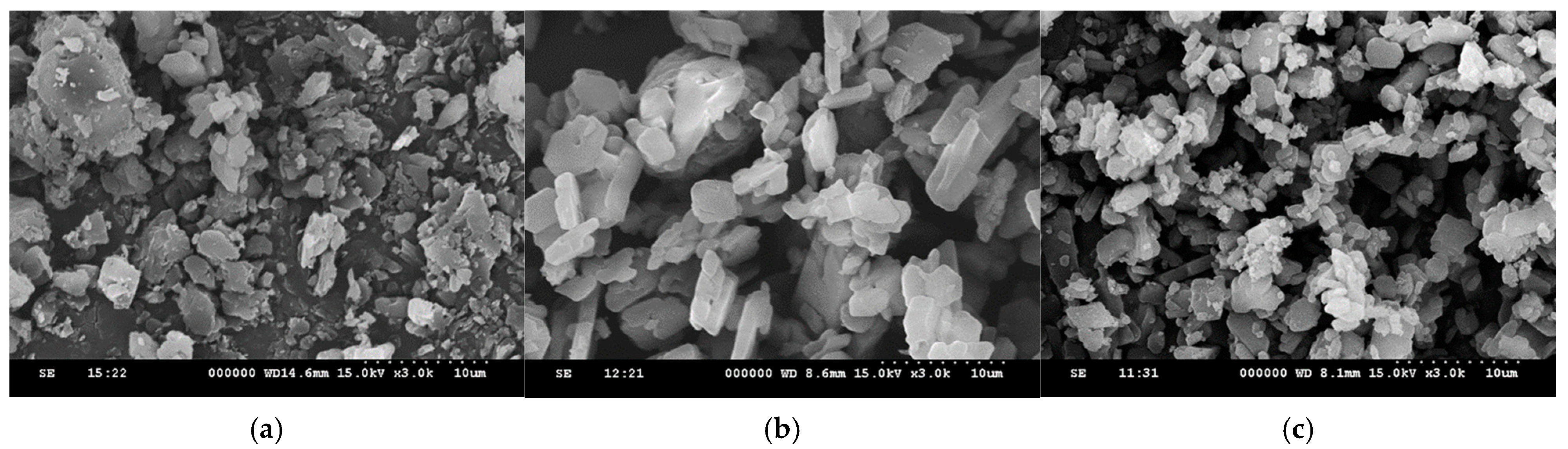
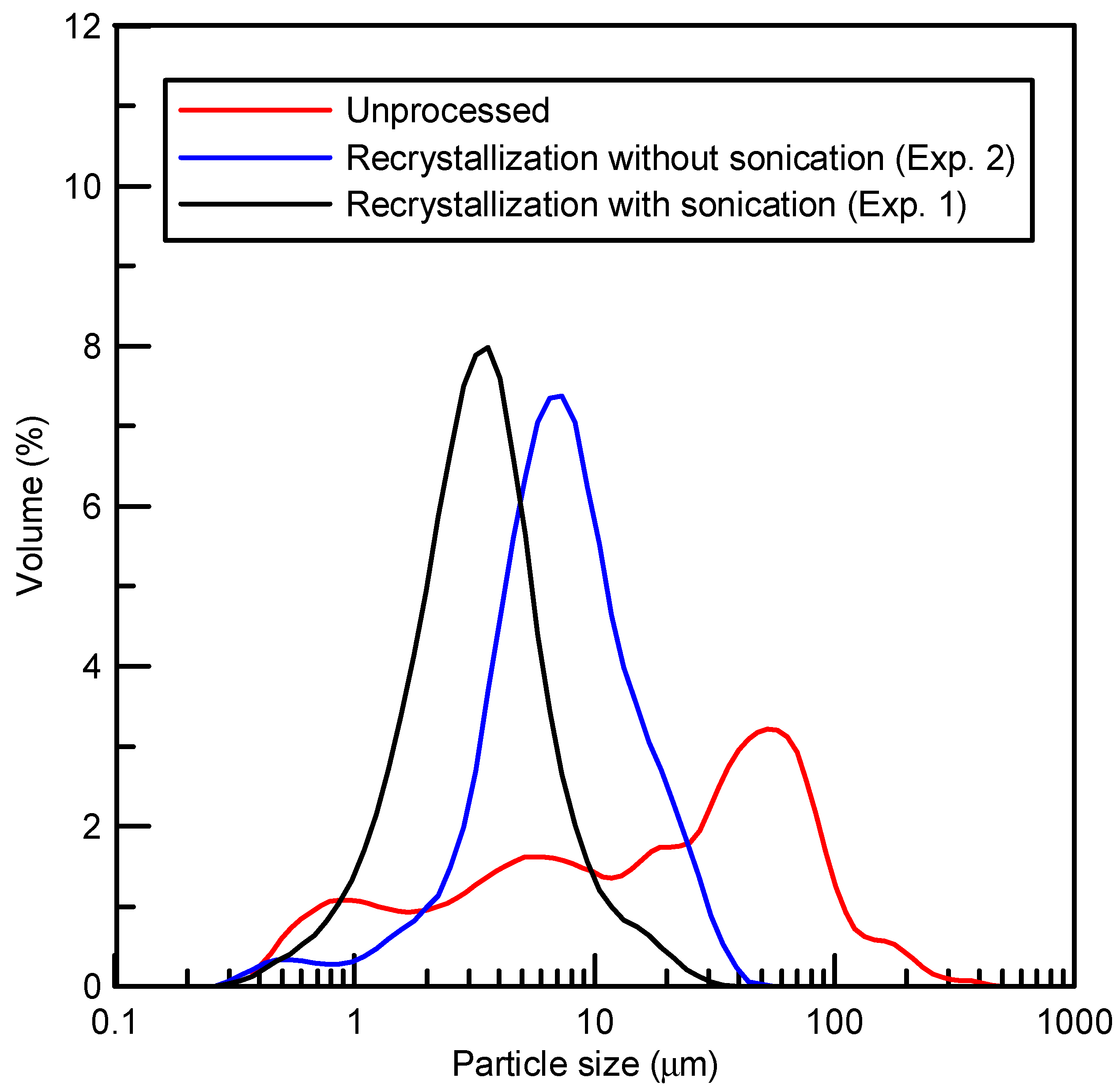
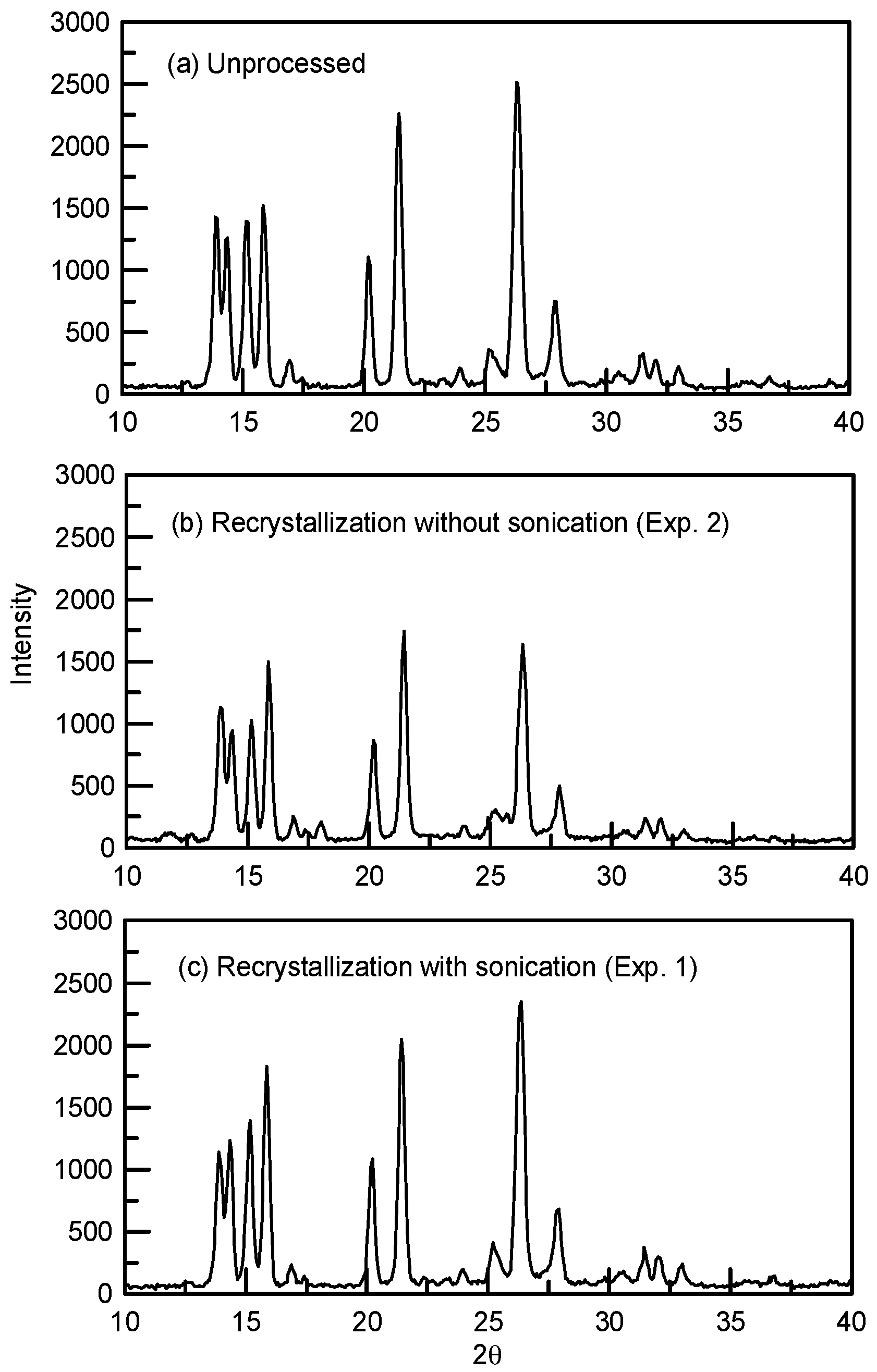
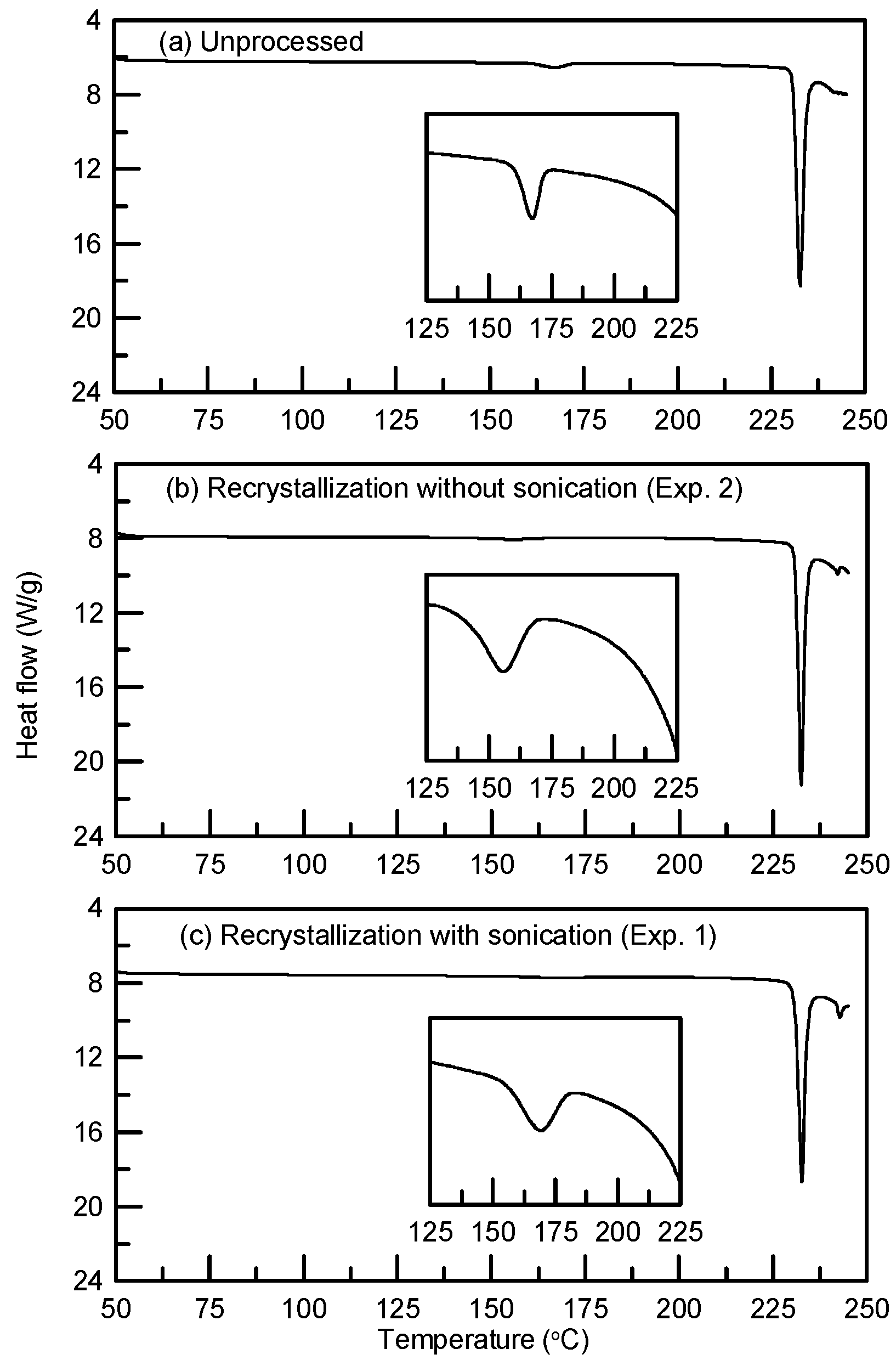
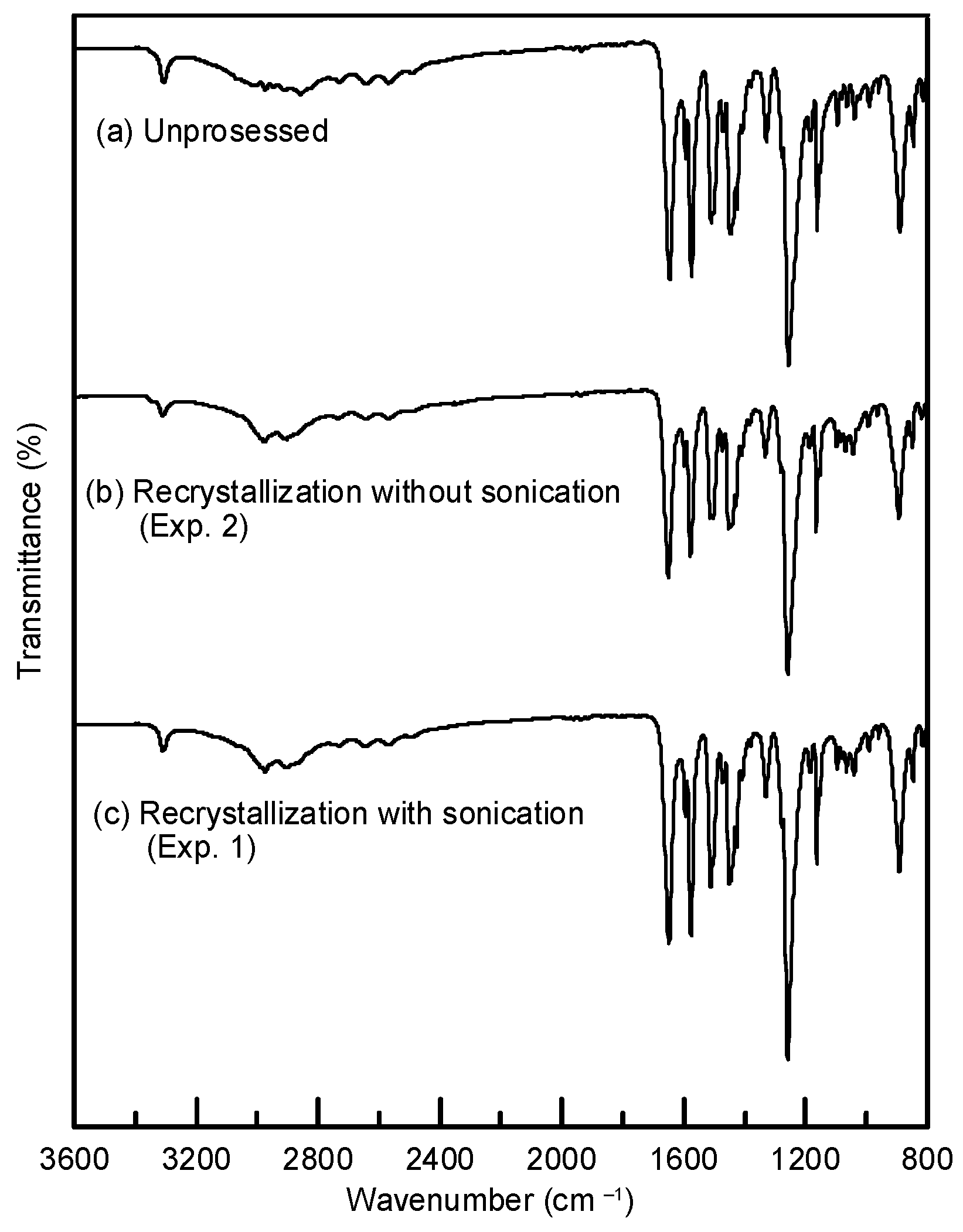
3.2. The Solid-State Property Comparison
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Steenweg, C.; Kufner, A.C.; Habicht, J.; Wohlgemuth, K. Towards continuous primary manufacturing processes—Particle design through combined crystallization and particle isolation. Processes 2021, 9, 2187. [Google Scholar] [CrossRef]
- Kufner, A.C.; Rix, M.; Wohlgemuth, K. Modeling of continuous slug flow cooling crystallization towards pharmaceutical applications. Processes 2023, 11, 2637. [Google Scholar] [CrossRef]
- Gao, Z.; Rohani, S.; Gong, J.; Wang, J. Recent developments in the crystallization process: Toward the pharmaceutical industry. Engineering 2017, 3, 343–353. [Google Scholar] [CrossRef]
- Kuo, P.H.; Zhang, B.C.; Su, C.S.; Liu, J.J.; Sheu, M.T. Application of two-level factorial design to investigate the effect of process parameters on the sonocrystallization of sulfathiazole. J. Cryst. Growth 2017, 471, 8–14. [Google Scholar] [CrossRef]
- Jiang, M.; Braatz, R.D. Designs of continuous-flow pharmaceutical crystallizers: Developments and practice. Cryst. Eng. Comm. 2019, 21, 3534–3551. [Google Scholar] [CrossRef]
- Kumar, R.; Thakur, A.K.; Kali, G.; Pitchaiah, K.C.; Arya, R.K.; Kulabhi, A. Particle preparation of pharmaceutical compounds using supercritical antisolvent process: Current status and future perspectives. Drug Deliv. Transl. Res. 2022, 13, 946–965. [Google Scholar] [CrossRef]
- Khudaida, S.H.; Yen, Y.T.; Su, C.S. Cocrystal screening of anticancer drug p-toluenesulfonamide and preparation by supercritical antisolvent process. J. Supercrit. Fluids 2024, 204, 106106. [Google Scholar] [CrossRef]
- Muhammad, S.A.F.S.; Oubani, H.; Abbas, A.; Chan, H.K.; Kwok, P.C.L.; Dehghani, F. The Production of dry powder by sonocrystallisation for inhalation drug delivery. Powder Technol. 2013, 246, 337–344. [Google Scholar] [CrossRef]
- Prasad, R.; Dalvi, S.V. Sonocrystallization: Monitoring and controlling crystallization using ultrasound. Chem. Eng. Sci. 2020, 226, 115911. [Google Scholar] [CrossRef]
- Jang, J.; Kim, W.S.; Seo, T.S.; Park, B.J. Over a decade of progress: Crystallization in microfluidic systems. Chem. Eng. J. 2024, 495, 153657. [Google Scholar] [CrossRef]
- Sun, X.; Garetz, B.A.; Myerson, A.S. Supersaturation and polarization dependence of polymorph control in the nonphotochemical laser-induced nucleation (NPLIN) of aqueous glycine solutions. Crystal Growth Des. 2006, 6, 684–689. [Google Scholar] [CrossRef]
- Zhang, H.; Du, S.; Wang, Y.; Xue, F. Prevention of crystal agglomeration: Mechanisms, factors, and impact of additives. Crystals 2024, 14, 676. [Google Scholar] [CrossRef]
- Yadav, J.; Srivastava, A.; Patel, S.A. Analysis of thermal characteristics of batch cooling sonocrystallization: Effect on crystal attributes. Cryst. Res. Technol. 2023, 58, 2200156. [Google Scholar] [CrossRef]
- Sharma, A.; Gogate, P.R. Improvements in crystallization of mefenamic acid using ultrasonic bath operating at two frequencies. Chem. Eng. Process. Process Intensif. 2020, 147, 107768. [Google Scholar] [CrossRef]
- Awari, H.D.; Sabnis, S.S.; Gogate, P.R. Improved crystallization of ampicillin trihydrate based on the use of ultrasound. Ind. Eng. Chem. Res. 2022, 61, 2538–2547. [Google Scholar] [CrossRef]
- Jordens, J.; Gielen, B.; Xiouras, C.; Hussain, M.N.; Stefanidis, G.D.; Thomassen, L.C.J.; Braeken, L.; Van Gerven, T. Sonocrystallisation: Observations, theories and guidelines. Chem. Eng. Process. Process Intensif. 2019, 139, 130–154. [Google Scholar] [CrossRef]
- Chang, C.H.; Hsieh, C.M.; Su, C.S. Particle size and crystal habit modification of active pharmaceutical ingredient using cooling sonocrystallization: A case study of probenecid. Cryst. Res. Technol. 2021, 56, 2000182. [Google Scholar] [CrossRef]
- Lin, S.; Khudaida, S.H.; Su, C.S. Particle size and crystal habit modification of ammonium perchlorate using cooling sonocrystallization process. Cryst. Res. Technol. 2024, 59, 2400163. [Google Scholar] [CrossRef]
- SeethaLekshm, S.; Guru Row, T.N. Conformational polymorphism in a non-steroidal anti-inflammatory drug, Mefenamic acid. Cryst. Growth Des. 2012, 12, 4283–4289. [Google Scholar] [CrossRef]
- Orehek, J.; Teslić, D.; Likozar, B. Continuous crystallization processes in pharmaceutical manufacturing: A review. Org. Process Res. Dev. 2021, 25, 16–42. [Google Scholar] [CrossRef]
- Furuta, M.; Mukai, K.; Cork, D.; Mae, K. Continuous crystallization using a sonicated tubular system for controlling particle size in an API manufacturing process. Chem. Eng. Process. Process Intensif. 2016, 102, 210–218. [Google Scholar] [CrossRef]
- Hadiwinoto, G.D.; Kwok, P.C.L.; Tong, H.H.Y.; Wong, S.N.; Chow, S.F.; Lakerveld, R. Integrated continuous plug-flow crystallization and spray drying of pharmaceuticals for dry powder inhalation. Ind. Eng. Chem. Res. 2019, 58, 16843–16857. [Google Scholar] [CrossRef]
- Schmalenberg, M.; Weick, L.K.; Kockmann, N. Nucleation in continuous flow cooling sonocrystallization for coiled capillary crystallizers. J. Flow Chem. 2021, 11, 303–319. [Google Scholar] [CrossRef]
- Hidayat, A.F.; Fakih, T.M.; Darma, G.C.E.; Choesrina, R. In silico coformer screening for mefenamic acid cocrystallization. J. Info. Kesehatan 2024, 22, 182–189. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, H.; Zhao, Y.; Ma, Z. Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs. Pharmaceutics 2018, 10, 74. [Google Scholar] [CrossRef]
- Dixit, M.; Kini, A.G.; Kulkarni, P.K. Enhancing the dissolution of polymorphs I and II of mefenamic acid by spray drying. Turk J. Pharm. Sci. 2012, 9, 13–26. [Google Scholar]
- Iwasaki, T.; Takahara, M.; Sonoda, R.; Watano, S. Dry grinding of mefenamic acid particles for enhancement of its water dissolution rate. Part. Part. Syst. Charact. 2007, 24, 236–241. [Google Scholar] [CrossRef]
- Abdul Mudalip, S.K.; Abu Bakar, M.R.; Jamal, P.; Adam, F. Solubility and dissolution thermodynamic data of mefenamic acid crystals in different classes of organic solvents. J. Chem. Eng. Data 2013, 58, 3447–3452. [Google Scholar] [CrossRef]
- Cesur, S.; Gokbel, S. Crystallization of mefenamic acid and polymorphs. Cryst. Res. Technol. 2008, 43, 720. [Google Scholar] [CrossRef]
- Kim, H.J.; Yeo, S.-D. Liquid antisolvent crystallization of griseofulvin from organic solutions. Chem. Eng. Res. Des. 2015, 97, 68. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Yen, S.K.; Hu, W.S.; Huang, Y.Z.; Yang, T.M.; Su, C.S. Sonocrystallization case studies of salicylamide particle size reduction and isoniazid derivative synthesis and crystallization. Crystals 2018, 8, 249. [Google Scholar] [CrossRef]
- Kaneko, S.; Yamagami, Y.; Tochihara, H.; Hirasawa, I. Effect of supersaturation on crystal size and number of crystals produced in antisolvent crystallization. J. Chem. Eng. Jpn. 2002, 35, 1219–1223. [Google Scholar] [CrossRef]
- Khudaida, S.H.; Chen, Y.M.; Zheng, Y.F.; Hsieh, C.M.; Su, C.S. Solid solubility measurement of haloperidol in supercritical carbon dioxide and nanonization using the rapid expansion of supercritical solutions process. J. Supercrit. Fluids 2023, 192, 105785. [Google Scholar] [CrossRef]
- Park, M.W.; Yeo, S.-D. Antisolvent crystallization of roxithromycin and the effect of ultrasound. Sep. Sci. Technol. 2010, 45, 1402–1410. [Google Scholar] [CrossRef]
- Hatkar, U.N.; Gogate, P.R. Process intensification of anti-solvent crystallization of salicylic acid using ultrasonic irradiations. Chem. Eng. Process. Process Intensif. 2012, 57–58, 16–24. [Google Scholar] [CrossRef]
- Panchagnula, R.; Sundaramurthy, P.; Pillai, O.; Agrawal, S.; Raj, Y.A. Solid-state characterization of mefenamic acid. J. Pharm Sci. 2004, 93, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Su, C.S.; Tang, M.; Chen, Y.P. Recrystallization of pharmaceuticals using the batch supercritical anti-solvent process. Chem. Eng. Process. Process Intensif. 2009, 48, 92–100. [Google Scholar] [CrossRef]
- Cunha, V.R.R.; Izumi, C.M.S.; Petersen, P.A.D.; Magalhães, A.; Temperini, M.L.A.; Petrilli, H.M.; Constantino, V.R.L. Mefenamic acid anti-Inflammatory drug: Probing its polymorphs by vibrational (IR and Raman) and solid-state NMR spectroscopies. J. Phys. Chem. B 2014, 118, 4333. [Google Scholar] [CrossRef]
| Compound | Mefenamic Acid |
|---|---|
| IUPAC name | 2-(2,3-Dimethylphenyl) aminobenzoic acid |
| Structure | 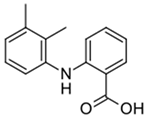 |
| Formula | C15H15NO2 |
| CAS No. | 61-68-7 |
| Mw | 241.28 |
| Tm (°C) | 230–231 (a) |
| Aqueous solubility | 20 mg/L (at 30 °C) (a) |
| Supplier | Tokyo Chemical Industry |
| Product number | M1782 |
| Purity (%) | >98.0 |
| Exp. No. | Operating Parameters | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Intensity (%) | Temp. (°C) | Fantisolvent (mL/min) | Fsolution (mL/min) | Conc. (a) (mg/mL) | Recovery (%) | Mean Size (μm) | Span (-) | |
| Unprocessed | --- | --- | --- | --- | --- | --- | 33.4 ± 2.77 | 4.6 |
| 1 | 30 | 35 | 10 | 2.5 | 16 | 79.5 | 3.0 ± 0.17 | 1.9 |
| 2 (b) | 0 | 35 | 10 | 2.5 | 16 | 75.7 | 6.4 ± 0.25 | 2.1 |
| 3 | 10 | 35 | 10 | 2.5 | 16 | 86.8 | 3.1 ± 0.14 | 1.8 |
| 4 | 50 | 35 | 10 | 2.5 | 16 | 82.4 | 2.8 ± 0.15 | 1.7 |
| 5 | 70 | 35 | 10 | 2.5 | 16 | 80.8 | 3.0 ± 0.13 | 1.5 |
| 6 | 30 | 25 | 10 | 2.5 | 16 | 84.8 | 2.6 ± 0.07 | 1.6 |
| 7 | 30 | 45 | 10 | 2.5 | 16 | 74.8 | 3.1 ± 0.13 | 1.8 |
| 8 | 30 | 35 | 25 | 2.5 | 16 | 82.9 | 3.0 ± 0.08 | 2.0 |
| 9 | 30 | 35 | 50 | 2.5 | 16 | 82.9 | 2.9 ± 0.13 | 2.7 |
| 10 | 30 | 35 | 10 | 1.0 | 16 | 82.2 | 3.3 ± 0.15 | 2.2 |
| 11 | 30 | 35 | 10 | 7.5 | 16 | 78.8 | 3.5 ± 0.08 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khudaida, S.H.; Lee, C.-Y.; Su, C.-S. Microparticle Production of Mefenamic Acid Using the Continuous Antisolvent Sonocrystallization Process. Processes 2025, 13, 2813. https://doi.org/10.3390/pr13092813
Khudaida SH, Lee C-Y, Su C-S. Microparticle Production of Mefenamic Acid Using the Continuous Antisolvent Sonocrystallization Process. Processes. 2025; 13(9):2813. https://doi.org/10.3390/pr13092813
Chicago/Turabian StyleKhudaida, Salal Hasan, Chia-Yi Lee, and Chie-Shaan Su. 2025. "Microparticle Production of Mefenamic Acid Using the Continuous Antisolvent Sonocrystallization Process" Processes 13, no. 9: 2813. https://doi.org/10.3390/pr13092813
APA StyleKhudaida, S. H., Lee, C.-Y., & Su, C.-S. (2025). Microparticle Production of Mefenamic Acid Using the Continuous Antisolvent Sonocrystallization Process. Processes, 13(9), 2813. https://doi.org/10.3390/pr13092813








