Comparative Removal of Hexavalent Chromium from Aqueous Solution Using Plant-Derived and Industrial Zirconia Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
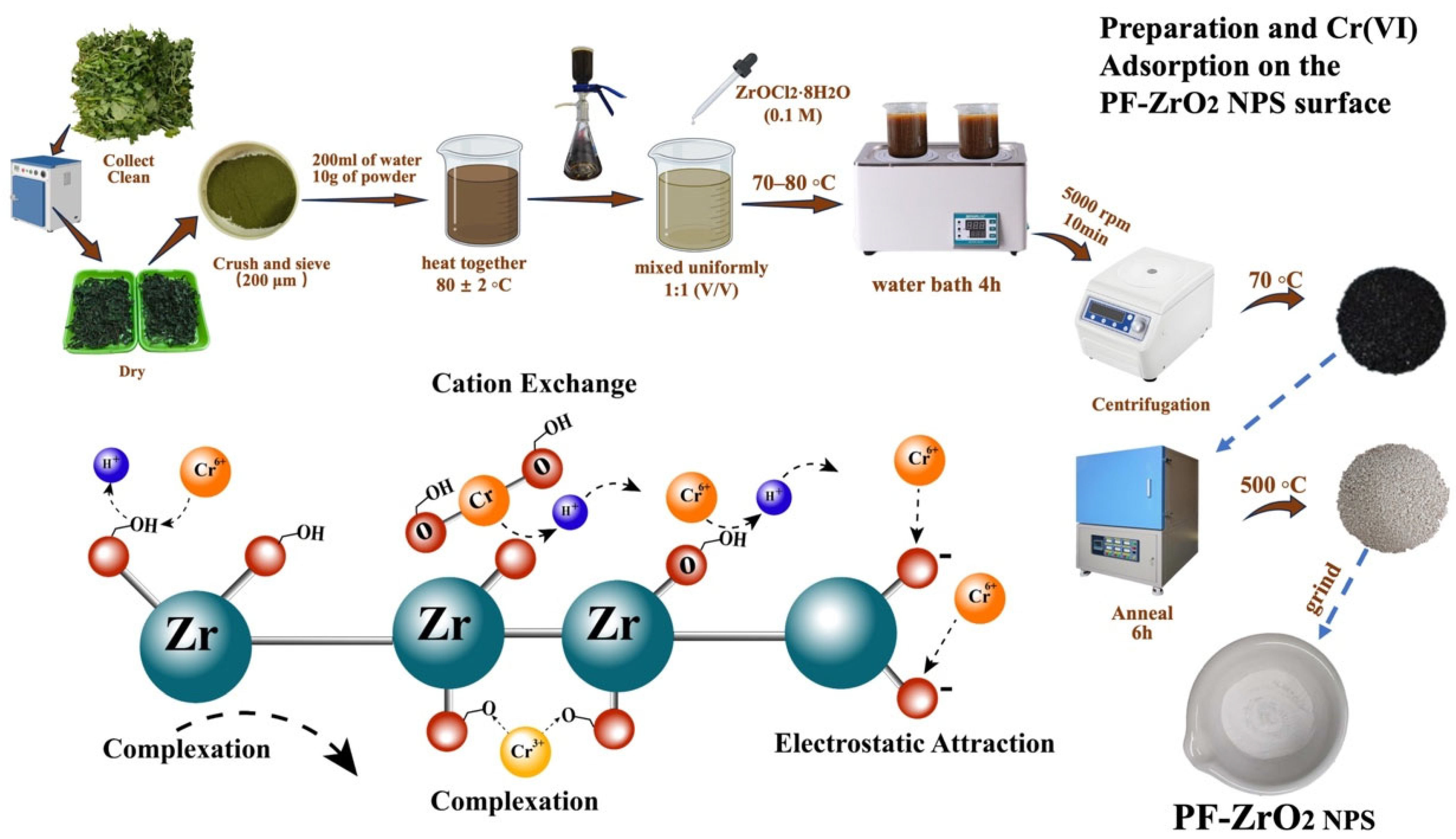
2.2. Synthesis of Plant Fabricated Zirconium Oxychloride Nanoparticles (PF-ZrO2 NPs) via Sonchus Asper Aqueous Extract
2.3. Characterization of NPs
2.4. Determination of Cr(VI) Concentration
2.5. Adsorption Studies
- ⬥
- (mg·g−1) is the amount of Cr(VI) adsorbed at time (min),
- ⬥
- (mg·g−1) is the equilibrium adsorption capacity,
- ⬥
- (min−1) is the rate constant of the pseudo-first-order model.
- ⬥
- (g·mg−1·min−1) is the rate constant of the pseudo-second-order model,
- ⬥
- : same as above,
- ⬥
- : contact time (min).
2.6. Desorption Studies
3. Results and Discussion
3.1. Characterization of Nanoparticles
3.2. Batch Adsorption Experiment
3.2.1. Effect of pH
3.2.2. Effect of Metal Ion Concentration
3.2.3. Effect of Dose
3.2.4. Effect of Contact Time
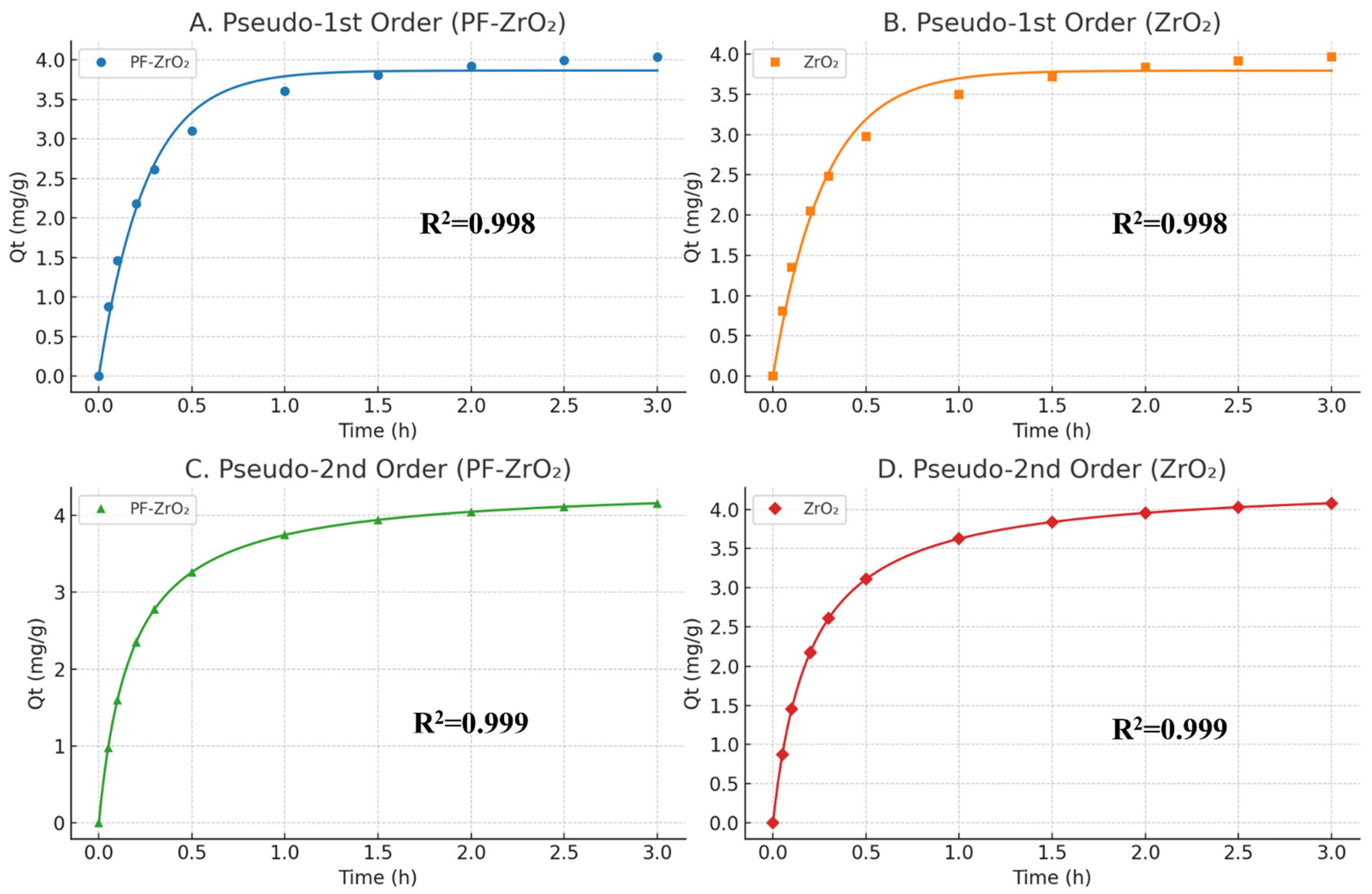
3.2.5. Adsorption Kinetics
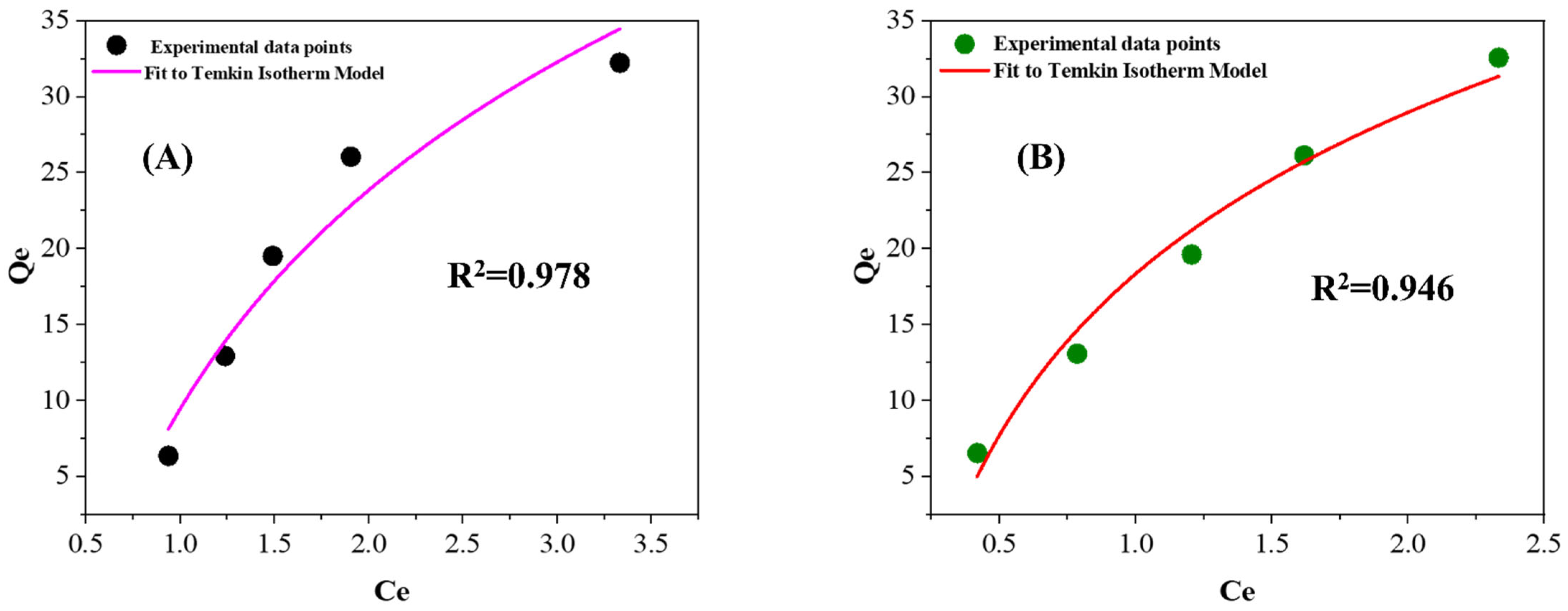
3.2.6. Adsorption Isotherms
3.3. Desorption Efficiency of Nanoparticles
3.4. Adsorption Mechanism
4. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jaffari, Z.H.; Hong, J.; Park, K.Y. A Systematic Review of Innovations in Tannery Solid Waste Treatment: A Viable Solution for the Circular Economy. Sci. Total Environ. 2024, 948, 174848. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. Chromium. In Encyclopedia of Human Nutrition, 4th ed.; Academic Press: New York, NY, USA, 2023; Volumes 1–4, ISBN 9780323908160. [Google Scholar]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium Bioaccumulation and Its Impacts on Plants: An Overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Sirajuddin; Kakakhel, L.; Lutfullah, G.; Bhanger, M.I.; Shah, A.; Niaz, A. Electrolytic Recovery of Chromium Salts from Tannery Wastewater. J. Hazard. Mater. 2007, 148, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.A.; Agustina, L. The Potential Application of Photocatalytic Processes in the Processing of Wastewater in the Leather Industry: A Review. IOP Conf. Ser. Earth Environ. Sci. 2023, 1253, 012025. [Google Scholar] [CrossRef]
- Nur-E-Alam, M.; Mia, M.A.S.; Ahmad, F.; Rahman, M.M. An Overview of Chromium Removal Techniques from Tannery Effluent. Appl. Water Sci. 2020, 10, 205. [Google Scholar] [CrossRef]
- Anjum, A.; Mazari, S.A.; Hashmi, Z.; Jatoi, A.S.; Abro, R.; Bhutto, A.W.; Mubarak, N.M.; Dehghani, M.H.; Karri, R.R.; Mahvi, A.H.; et al. A Review of Novel Green Adsorbents as a Sustainable Alternative for the Remediation of Chromium (VI) from Water Environments. Heliyon 2023, 9, e15575. [Google Scholar] [CrossRef]
- Das, P.K. Toxicological Implications of Hexavalent Chromium in Humans. Adv. Clin. Toxicol. 2022, 7, 1–4. [Google Scholar] [CrossRef]
- Munir, N.; Jahangeer, M.; Bouyahya, A.; El Omari, N.; Ghchime, R.; Balahbib, A.; Aboulaghras, S.; Mahmood, Z.; Akram, M.; Shah, S.M.A.; et al. Heavy Metal Contamination of Natural Foods Is a Serious Health Issue: A Review. Sustainability 2022, 14, 161. [Google Scholar] [CrossRef]
- Kamila, S.; Shaw, P.; Islam, S.; Chattopadhyay, A. Ecotoxicology of Hexavalent Chromium in Fish: An Updated Review. Sci. Total Environ. 2023, 890, 164395. [Google Scholar] [CrossRef]
- Assefa, H.; Singh, S.; Olu, F.E.; Dhanjal, D.S.; Mani, D.; Khan, N.A.; Singh, J.; Ramamurthy, P.C. Advances in Adsorption Technologies for Hexavalent Chromium Removal: Mechanisms, Materials, and Optimization Strategies. Desalin. Water Treat. 2024, 319, 100576. [Google Scholar] [CrossRef]
- Kushwaha, B.K.; Singh, V.P. Glutathione and Hydrogen Sulfide Are Required for Sulfur-Mediated Mitigation of Cr(VI) Toxicity in Tomato, Pea and Brinjal Seedlings. Physiol. Plant. 2020, 168, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Tamez, C.; Hernandez, R.; Parsons, J.G. Removal of Cu (II) and Pb (II) from Aqueous Solution Using Engineered Iron Oxide Nanoparticles. Microchem. J. 2016, 125, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.F.; Nouri, A.; Ang, W.L.; Mahmoudi, E.; Mohammad, A.W.; Benamor, A.; Ba-Abbad, M. The Emergence of Multifunctional Adsorbents and Their Role in Environmental Remediation. J. Environ. Chem. Eng. 2021, 9, 104793. [Google Scholar] [CrossRef]
- Tamayo-Meza, P.A.; Silva-Rivera, U.S.; Flores-Herrera, L.A.; Rodríguez-Alvarado, L.W.; Pérez-Cruz, J.H.; Rivera-López, J.E. Analysis of the Extreme Equilibrium Conditions of an Internal Cavity Located inside a Flat Metal Plate Subjected to an Internal Pressure P. Materials 2020, 13, 2043. [Google Scholar] [CrossRef]
- Qin, H.; Kuang, T.; Li, Q.; Yue, X.; Gao, H.; Liu, F.; Yi, Y. Stress Concentration Induced by the Crystal Orientation in the Transient-Liquid-Phase Bonded Joint of Single-Crystalline Ni3Al. Materials 2019, 12, 2765. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Zhang, M.; Ge, M.; Wang, J.; Tang, Y.; Zhang, Y.; Mi, J.; Cai, W.; Lai, Y.; et al. Rational Design of Electrospun Nanofibers for Gas Purification: Principles, Opportunities, and Challenges. Chem. Eng. J. 2022, 446, 137099. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Jasiński, J.; Mazurak, A.; Stonio, B.; Majkusiak, B. Investigation of Electrical Properties of the Al/SiO2/N++-Si Resistive Switching Structures by Means of Static, Admittance, and Impedance Spectroscopy Measurements. Materials 2021, 14, 6042. [Google Scholar] [CrossRef]
- Chakravarty, R.; Shukla, R.; Ram, R.; Tyagi, A.K.; Dash, A.; Venkatesh, M. Practicality of Tetragonal Nano-Zirconia as a Prospective Sorbent in the Preparation of 99Mo/99mTc Generator for Biomedical Applications. Chromatographia 2010, 72, 875–884. [Google Scholar] [CrossRef]
- Fathima, J.B.; Pugazhendhi, A.; Venis, R. Synthesis and Characterization of ZrO2 Nanoparticles-Antimicrobial Activity and Their Prospective Role in Dental Care. Microb. Pathog. 2017, 110, 245–251. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Yun, Y.S. Green Fabrication of Zirconia Nano-Chains Using Novel Curcuma longa Tuber Extract. Mater. Lett. 2013, 98, 242–245. [Google Scholar] [CrossRef]
- da Silva, A.F.V.; Fagundes, A.P.; Macuvele, D.L.P.; de Carvalho, E.F.U.; Durazzo, M.; Padoin, N.; Soares, C.; Riella, H.G. Green Synthesis of Zirconia Nanoparticles Based on Euclea natalensis Plant Extract: Optimization of Reaction Conditions and Evaluation of Adsorptive Properties. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 583, 123915. [Google Scholar] [CrossRef]
- Cho, M.S.; Kim, J.H.; Kim, C.S.; Mejías, J.A.; Kim, S.C. Sow Thistle Chloroplast Genomes: Insights into the Plastome Evolution and Relationship of Two Weedy Species, Sonchus asper and Sonchus oleraceus (Asteraceae). Genes 2019, 10, 881. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M.; Salerno, G.; Caneva, G. Food, Flavouring and Feed Plant Traditions in the Tyrrhenian Sector of Basilicata, Italy. J. Ethnobiol. Ethnomed. 2006, 2, 37. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.R.; Sahreen, S. Protective Effect of Sonchus asper Extracts against Experimentally Induced Lung Injuries in Rats: A Novel Study. Exp. Toxicol. Pathol. 2012, 64, 725–731. [Google Scholar] [CrossRef]
- Al-nayili, A.; Idan, A.H. Environmentally Friendly Production, Characterization, and Utilization of ZrO2 Nanoparticles for the Adsorption of Amoxicillin in Water Solutions. J. Mol. Liq. 2023, 389, 122875. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Yang, X.; Wang, X.; Lu, L. Preparation of ZrO2-Al2O3 Composite Membranes by Sol-Gel Process and Their Characterization. Mater. Sci. Eng. A 2004, 367, 243–247. [Google Scholar] [CrossRef]
- Chen, S.; Yin, Y.; Wang, D.; Liu, Y.; Wang, X. Structures, Growth Modes and Spectroscopic Properties of Small Zirconia Clusters. J. Cryst. Growth 2005, 282, 498–505. [Google Scholar] [CrossRef]
- Amin, F.; Fozia; Khattak, B.; Alotaibi, A.; Qasim, M.; Ahmad, I.; Ullah, R.; Bourhia, M.; Gul, A.; Zahoor, S.; et al. Green Synthesis of Copper Oxide Nanoparticles Using Aerva javanica Leaf Extract and Their Characterization and Investigation of In Vitro Antimicrobial Potential and Cytotoxic Activities. Evid.-Based Complement. Altern. Med. 2021, 2021, 5589703. [Google Scholar] [CrossRef]
- Pantò, F.; Dahrouch, Z.; Saha, A.; Patanè, S.; Santangelo, S.; Triolo, C. Photocatalytic Degradation of Methylene Blue Dye by Porous Zinc Oxide Nanofibers Prepared via Electrospinning: When Defects Become Merits. Appl. Surf. Sci. 2021, 557, 149830. [Google Scholar] [CrossRef]
- Cha, T.G.; Baker, B.A.; Salgado, J.; Bates, C.J.; Chen, K.H.; Chang, A.C.; Akatay, M.C.; Han, J.H.; Strano, M.S.; Choi, J.H. Understanding Oligonucleotide-Templated Nanocrystals: Growth Mechanisms and Surface Properties. ACS Nano 2012, 6, 8136–8143. [Google Scholar] [CrossRef]
- Bumajdad, A.; Nazeer, A.A.; Al Sagheer, F.; Nahar, S.; Zaki, M.I. Controlled Synthesis of ZrO2 Nanoparticles with Tailored Size, Morphology and Crystal Phases via Organic/Inorganic Hybrid Films. Sci. Rep. 2018, 8, 3695. [Google Scholar] [CrossRef]
- Madkikar, P.; Wang, X.; Mittermeier, T.; Monteverde Videla, A.H.A.; Denk, C.; Specchia, S.; Gasteiger, H.A.; Piana, M. Synthesis Optimization of Carbon-Supported ZrO2 Nanoparticles from Different Organometallic Precursors. J. Nanostruct. Chem. 2017, 7, 133–147. [Google Scholar] [CrossRef]
- Febriantini, D.; Purnamasari; Liandi, A.R.; Usman; Yulizar, Y. A Comprehensive Study on the Influence of Single and Multiple Phytochemicals in Facilitating Green Synthesis of ZrO2 Nanoparticles. Nano-Struct. Nano-Objects 2024, 39, 101303. [Google Scholar] [CrossRef]
- Luo, M.; Jiang, X.; Liu, Y.; Liu, Y.; Yu, H.; Niu, Y.; Meng, X.; Wang, L.; Niu, Y. Enhanced Adsorption Complexation of Biochar by Nitrogen-Containing Functional Groups. J. Environ. Chem. Eng. 2023, 11, 111194. [Google Scholar] [CrossRef]
- Zeng, G.; Li, G.; Wu, Y.; Tang, C.; Yuan, W.; Zhong, H.; Deng, N.; Liu, Q.; Liu, J.; Ouyang, K.; et al. Preparation of High Specific Surface Area Zn/Al/Zr LDH@HTCC for Enrichment and Recovery of Phosphorus from Water. Surf. Interfaces 2023, 42, 103330. [Google Scholar] [CrossRef]
- Hijazi, B.U.; Faraj, M.; Mhanna, R.; El-Dakdouki, M.H. Biosynthesis of Silver Nanoparticles as a Reliable Alternative for the Catalytic Degradation of Organic Dyes and Antibacterial Applications. Curr. Res. Green Sustain. Chem. 2024, 8, 100408. [Google Scholar] [CrossRef]
- Daulay, A.; Andriayani; Marpongahtun; Gea, S. Synthesis and Application of Silicon Nanoparticles Prepared from Rice Husk for Lithium-Ion Batteries. Case Stud. Chem. Environ. Eng. 2022, 6, 100256. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Islam, A.; Rahman, M.M.; Asiri, A.M.; Khaleque, M.A.; Sheikh, M.C. Introducing an Amine Functionalized Novel Conjugate Material for Toxic Nitrite Detection and Adsorption from Wastewater. J. Clean. Prod. 2019, 228, 778–785. [Google Scholar] [CrossRef]
- Yao, Y.; Mi, N.; He, C.; Zhang, Y.; Yin, L.; Li, J.; Wang, W.; Yang, S.; He, H.; Li, S.; et al. A Novel Colloid Composited with Polyacrylate and Nano Ferrous Sulfide and Its Efficiency and Mechanism of Removal of Cr(VI) from Water. J. Hazard. Mater. 2020, 399, 123082. [Google Scholar] [CrossRef]
- Silva, B.; Figueiredo, H.; Quintelas, C.; Neves, I.C.; Tavares, T. Zeolites as Supports for the Biorecovery of Hexavalent and Trivalent Chromium. Microporous Mesoporous Mater. 2008, 116, 555–560. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, L.; Chen, A.; Shang, C.; Lei, M.; He, K.; Luo, S.; Shao, J.; Zeng, Q. Chitosan-Stabilized FeS Magnetic Composites for Chromium Removal: Characterization, Performance, Mechanism, and Stability. Carbohydr. Polym. 2019, 214, 276–285. [Google Scholar] [CrossRef]
- Qi, Z.; Zhong, S.; Huang, X.; Xu, Y.; Zhang, H.; Shi, B. Concentration Division for Adsorption Coefficient Prediction Using Machine Learning with Abraham Descriptors: Data-Splitting Approach Comparison and Critical Factors Identification. Carbon 2024, 230, 100949. [Google Scholar] [CrossRef]
- Qi, L.; Qin, W. Unveiling the Fast Adsorption and Desorption of Heavy Metals on/off Nanoplastics by Real-Time in-Situ Potentiometric Sensing. Sci. Total Environ. 2024, 943, 173789. [Google Scholar] [CrossRef]
- Ehrampoush, M.H.; Miria, M.; Salmani, M.H.; Mahvi, A.H. Cadmium Removal from Aqueous Solution by Green Synthesis Iron Oxide Nanoparticles with Tangerine Peel Extract. J. Environ. Health Sci. Eng. 2015, 13, 84. [Google Scholar] [CrossRef]
- Luo, M.; Lin, H.; Li, B.; Dong, Y.; He, Y.; Wang, L. A Novel Modification of Lignin on Corncob-Based Biochar to Enhance Removal of Cadmium from Water. Bioresour. Technol. 2018, 259, 312–318. [Google Scholar] [CrossRef]
- Rahmawati, I.; Pratama, A.W.; Amalia, R.; Wahab, I.A.; Qolbi, N.S.; Putri, B.A.; Fachri, B.A.; Palupi, B.; Fitriana, M.R.; Reza, M.; et al. Solvothermal Synthesis of Sugarcane Bagasse-Bentonite-Magnetite Nanocomposite for Efficient Cr(VI) Removal from Electroplating Wastewater: Kinetics and Isotherm Investigations. Case Stud. Chem. Environ. Eng. 2024, 10, 100949. [Google Scholar] [CrossRef]
- Yang, M.; Cai, X.; Chen, X.; Guan, S.; Yan, K.; An, L.; Xing, J. Shape Recovery Aerogels from Wheat Straw-Based Cellulose Nanofibrils for Dynamic Removal of Cr(VI). Cellulose 2023, 30, 5777–5793. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Mohamed, F.M.; Alsohaimi, I.H.; Khalifa, R.E. Fabrication of Novel Valorized Ecofriendly Olive Seed Residue/Anthracite/Chitosan Composite for Removal of Cr(VI): Kinetics, Isotherms and Thermodynamics Modeling. Cellulose 2021, 28, 7165–7183. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Khan, T.A.; Ali, I. Zirconium Oxide-Coated Sand Based Batch and Column Adsorptive Removal of Arsenic from Water: Isotherm, Kinetic and Thermodynamic Studies. Egypt. J. Pet. 2017, 26, 553–563. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Maschio, L.J.; da Silva, R.E.; da Silva, M.L.C.P. Adsorption of Cr(VI) from Aqueous Solution by Hydrous Zirconium Oxide. J. Hazard. Mater. 2010, 173, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Sivashankar, R.; Sathya, A.B.; Krishnakumar, U.; Sivasubramanian, V. Synthesis of Magnetic Biocomposite for Efficient Adsorption of Azo Dye from Aqueous Solution. Ecotoxicol. Environ. Saf. 2015, 121, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Hung, D.Q.; Dinh, L.X.; Van Tung, N.; Huong, L.T.M.; Lien, N.T.; Minh, P.T.; Le, T.H. The Adsorption Kinetic and Isotherm Studies of Metal Ions (Co2+, Sr2+, Cs+) on Fe3O4 Nanoparticle of Radioactive Importance. Results Chem. 2023, 6, 101095. [Google Scholar] [CrossRef]
- Duan, J.; Chen, B.; Zhang, Y.; Cai, P.; Wang, F. Enhanced Adsorption of Cr(VI) from Aqueous Solutions by CTAB-Modified Schwertmannite: Adsorption Performance and Mechanism. Chem. Eng. Res. Des. 2024, 208, 464–474. [Google Scholar] [CrossRef]
- Lu, J.; Wu, C.; Tian, C.; Wang, L.; Li, W.; Liu, B. A Multifunctional Fertilizer for Long-Term Release and Remediation of Cr(VI)-Polluted Soil: Dialdehyde CMC Urea-Formaldehyde Grafted on NZVI@MOF(Mg)-74. Surf. Interfaces 2024, 46, 104039. [Google Scholar] [CrossRef]
- Younis, A.M.; Saleh, S.M.; Albadri, A.E.A.E.; Elkady, E.M. Enteromorpha compressa Macroalgal Biomass Nanoparticles as Eco-Friendly Biosorbents for the Efficient Removal of Harmful Metals from Aqueous Solutions. Analytica 2024, 5, 322–342. [Google Scholar] [CrossRef]
- Haruna, M.S.; Oladapo, B.H.; Mustapha, S.; Scholartica, C.E.; Tijani, J.O.; Abdulkareem, A.S. A Review of Iron-Tungstate Nanomaterials: Synthesis Methods, Physicochemical Properties, Environmental Fate and Application for Wastewater Treatment. Sustain. Chem. Environ. 2024, 5, 100074. [Google Scholar] [CrossRef]
- Premachandra, A.; McKay, Y.; McClure, M.; Sarkar, I.; Lutes, K.; Rollings-Scattergood, S.; Latulippe, D. High-Throughput Screening to Evaluate Optimum Coagulation Conditions via Colloidal Stability Analysis. Chemosphere 2023, 341, 139798. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Kumar, P.S.; Din, A.T.M.; Jalil, A.A.; Vo, D.V.N. Green Synthesis of ZrO2 Nanoparticles and Nanocomposites for Biomedical and Environmental Applications: A Review. Environ. Chem. Lett. 2022, 20, 1309–1331. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-Mediated Biosynthesis of Metallic Nanoparticles: A Review of Literature, Factors Affecting Synthesis, Characterization Techniques and Applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Xu, D.; Yang, W.; Bai, S. Application of Nanoscale Zero-Valent Iron in Hexavalent Chromium-Contaminated Soil: A Review. Nanotechnol. Rev. 2020, 9, 736–750. [Google Scholar] [CrossRef]
- Thatyana, M.; Dube, N.P.; Kemboi, D.; Manicum, A.L.E.; Mokgalaka-Fleischmann, N.S.; Tembu, J.V. Advances in Phytonanotechnology: A Plant-Mediated Green Synthesis of Metal Nanoparticles Using Phyllanthus Plant Extracts and Their Antimicrobial and Anticancer Applications. Nanomaterials 2023, 13, 2616. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Oladzadabbasabadi, N.; Aziz, A.A.; Khaniabadi, P.M.; Al-ouqaili, M.T.S.; Jameel, M.S.; Braim, F.S.; Mehrdel, B.; Ghasemlou, M. Recent Advances of Plant-Mediated Metal Nanoparticles: Synthesis, Properties, and Emerging Applications for Wastewater Treatment. J. Environ. Chem. Eng. 2024, 12, 112345. [Google Scholar] [CrossRef]
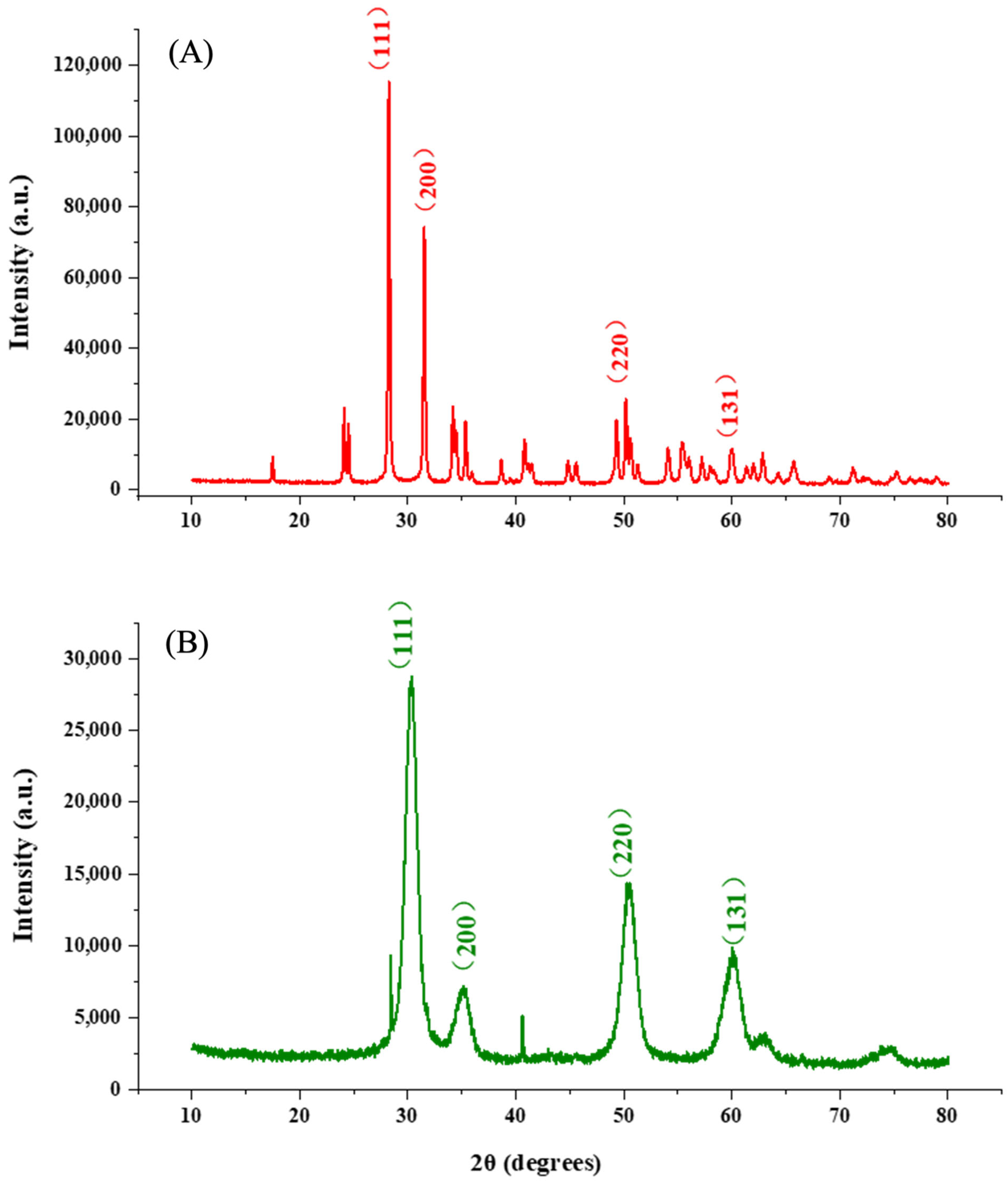

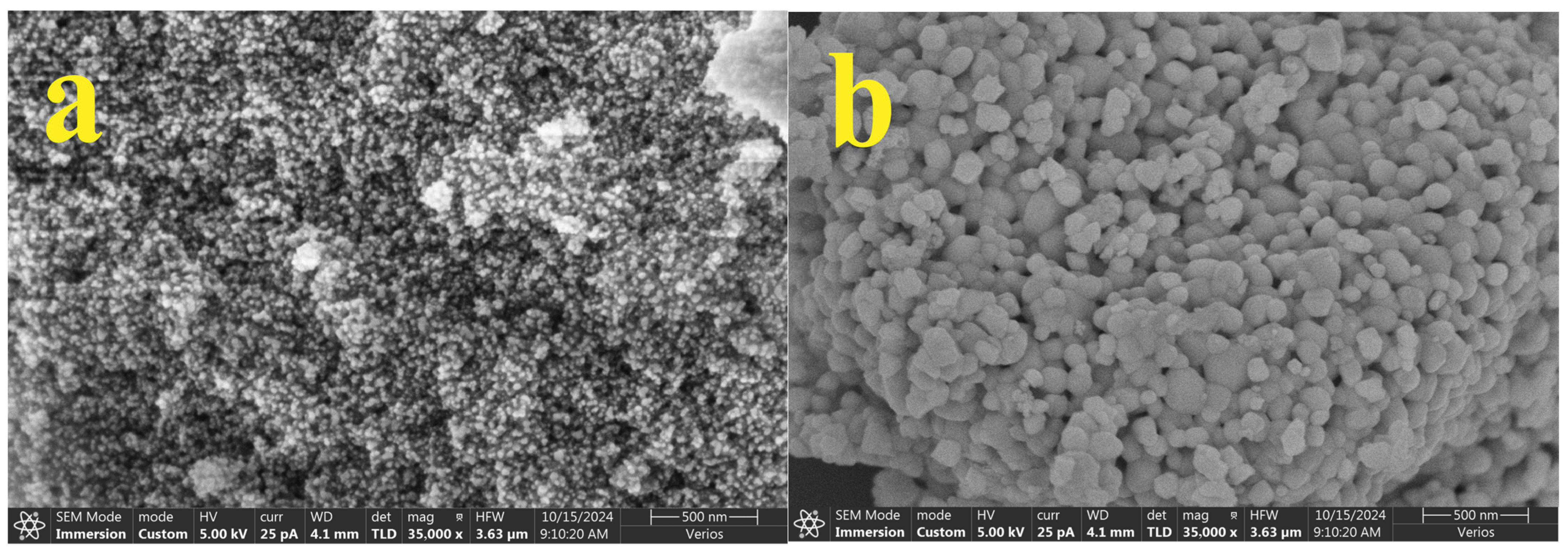
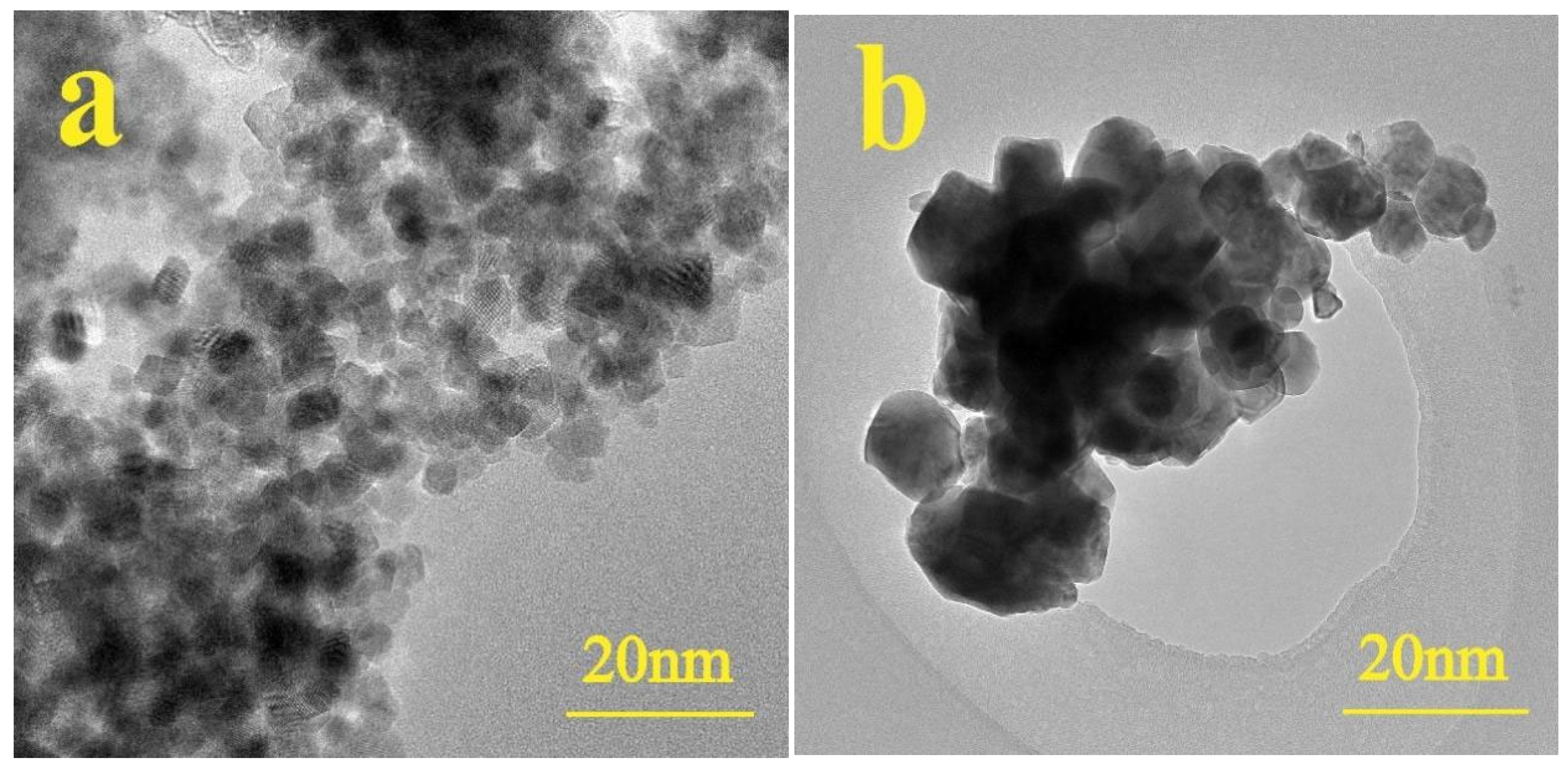
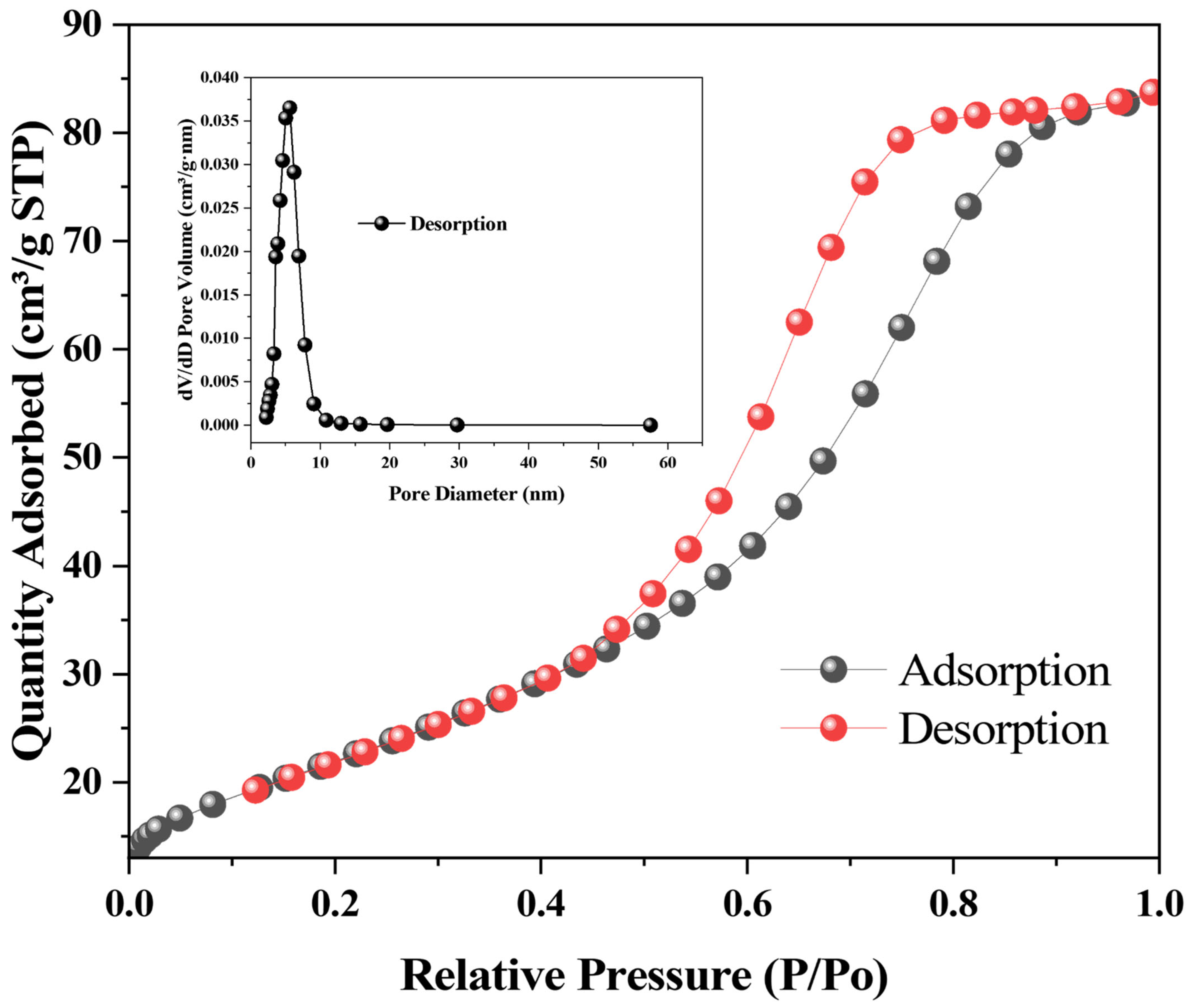

| Wavenumber (cm−1) | Assignment | Observed in |
|---|---|---|
| ~500–800 | Zr–O | Both |
| ~1380 | C–N | PF-ZrO2 |
| ~1600–1800 | C=O | PF-ZrO2 |
| ~2920 | C–H | PF-ZrO2 |
| ~3400–3500 | O–H | Both |
| PFO | PSO | |||||
|---|---|---|---|---|---|---|
| k1 (min−1) | (mg g−1) | R2 | k2 (g mg−1 min) | (mg g−1) | R2 | |
| PF-ZrO2NPs | 1.73 × 108 | 4.2295 | 0.998 | 8.10 × 1018 | 4.2295 | 0.999 |
| ZrO2NPs | 14.38437 | 4.2042 | 0.998 | 426.4825 | 4.2056 | 0.999 |
| R2 = 0.994 | ||||||
| Langmuir | Freundlich | |||||
| (mg g−1) | (L mg−1) | R2 | (L mg−1) | 1/ | R2 | |
| PF-ZrO2NPs | 142.24 | 0.1422 | 0.997 | 16.296 | 0.8530 | 0.994 |
| ZrO2NPs | 133.11 | 0.0931 | 0.845 | 11.963 | 0.8737 | 0.834 |
| Temkin | ||||||
| (L g−1) | R2 | |||||
| PF-ZrO2NPs | 1.5726 | 20.789 | 0.946 | |||
| ZrO2NPs | 3.2993 | 15.344 | 0.978 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, G.; Li, W.; Qin, F.; Dong, M.; Yue, S.; Weng, J.; Mehmood, S. Comparative Removal of Hexavalent Chromium from Aqueous Solution Using Plant-Derived and Industrial Zirconia Nanoparticles. Processes 2025, 13, 2794. https://doi.org/10.3390/pr13092794
Weng G, Li W, Qin F, Dong M, Yue S, Weng J, Mehmood S. Comparative Removal of Hexavalent Chromium from Aqueous Solution Using Plant-Derived and Industrial Zirconia Nanoparticles. Processes. 2025; 13(9):2794. https://doi.org/10.3390/pr13092794
Chicago/Turabian StyleWeng, Guojie, Weidong Li, Fengyue Qin, Menglu Dong, Shuangqi Yue, Jiechang Weng, and Sajid Mehmood. 2025. "Comparative Removal of Hexavalent Chromium from Aqueous Solution Using Plant-Derived and Industrial Zirconia Nanoparticles" Processes 13, no. 9: 2794. https://doi.org/10.3390/pr13092794
APA StyleWeng, G., Li, W., Qin, F., Dong, M., Yue, S., Weng, J., & Mehmood, S. (2025). Comparative Removal of Hexavalent Chromium from Aqueous Solution Using Plant-Derived and Industrial Zirconia Nanoparticles. Processes, 13(9), 2794. https://doi.org/10.3390/pr13092794







