Optimization of Milling Process Parameters for Waste Plum Stones for Their Sustainable Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of the Untreated Plum Stone Sample
3.1.1. Elemental Analysis of PS-U Sample
3.1.2. XRD Analysis of PS-U Sample
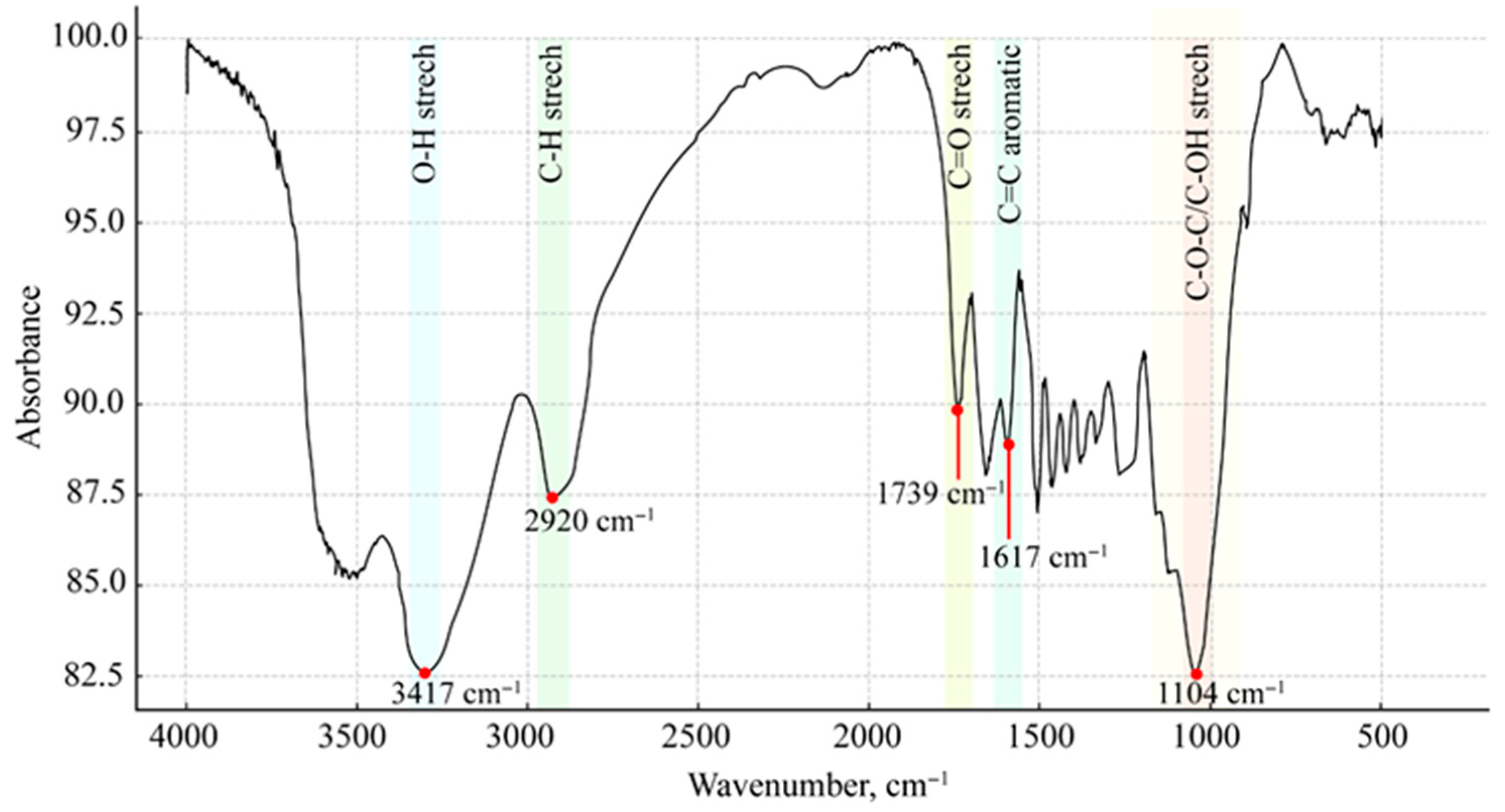
3.1.3. FTIR Analysis of PS-U Sample
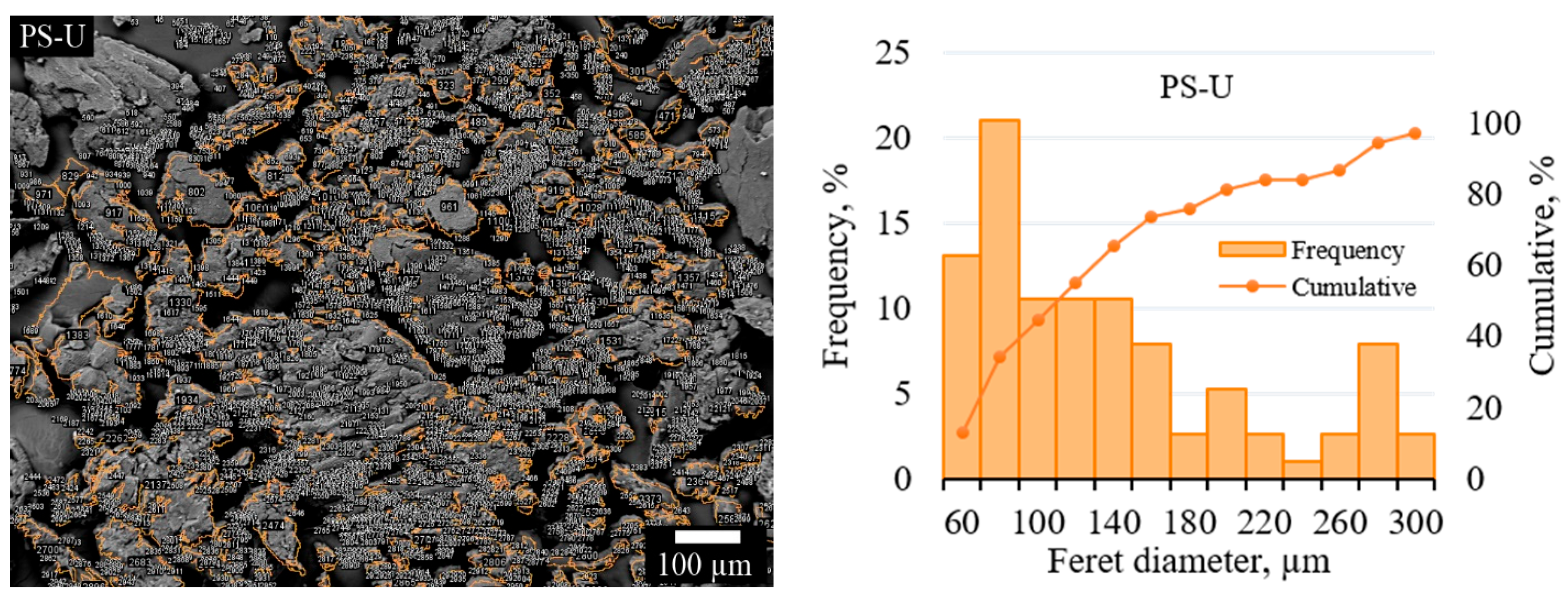
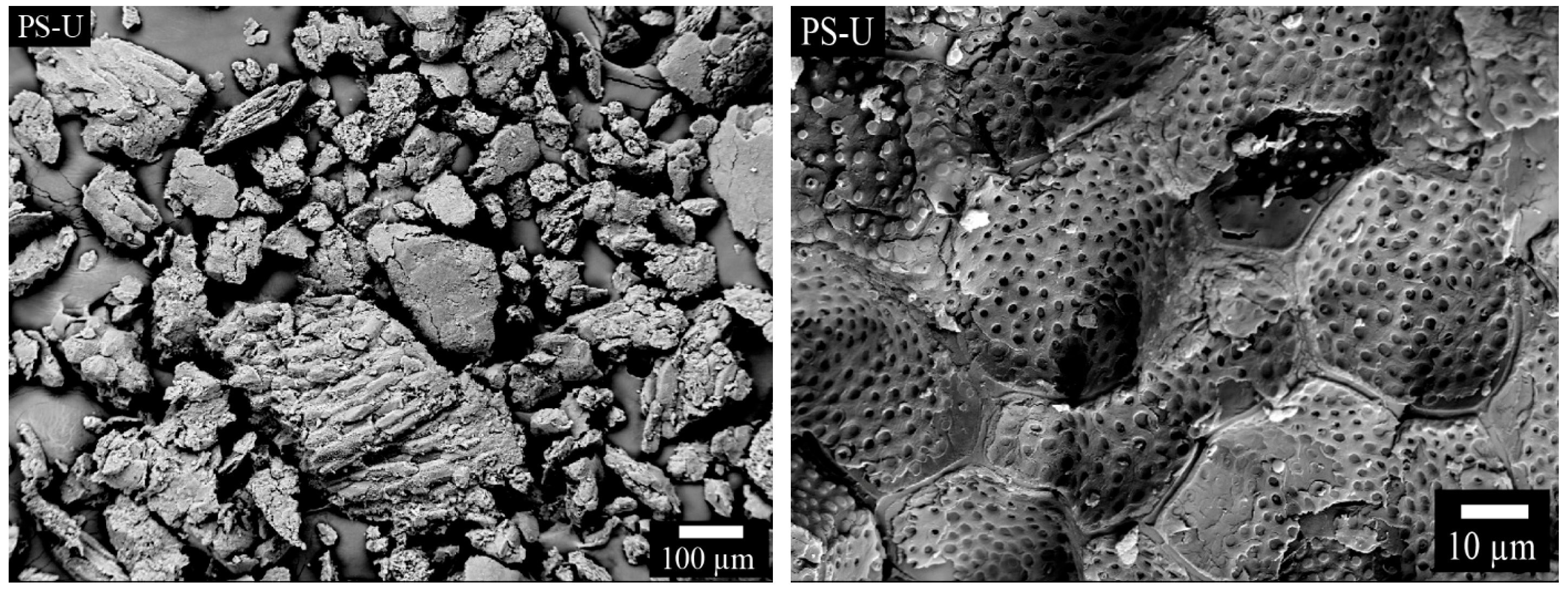
3.1.4. SEM Anaysis and Particle Size Distribution of PS-U Sample
3.2. Characterization of the Milled Plum Stone Samples
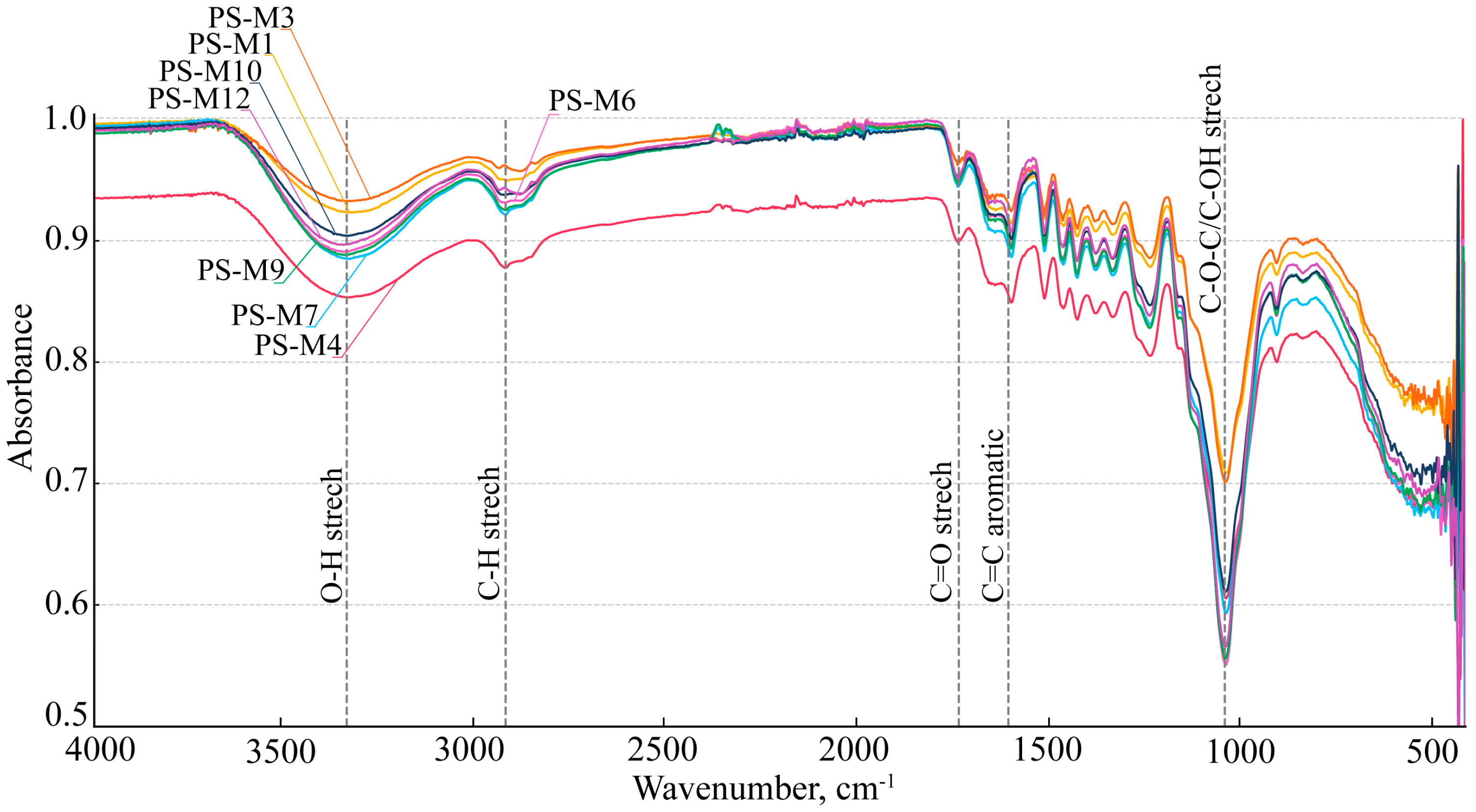
3.2.1. FTIR Analysis of the Milled Plum Stone Samples
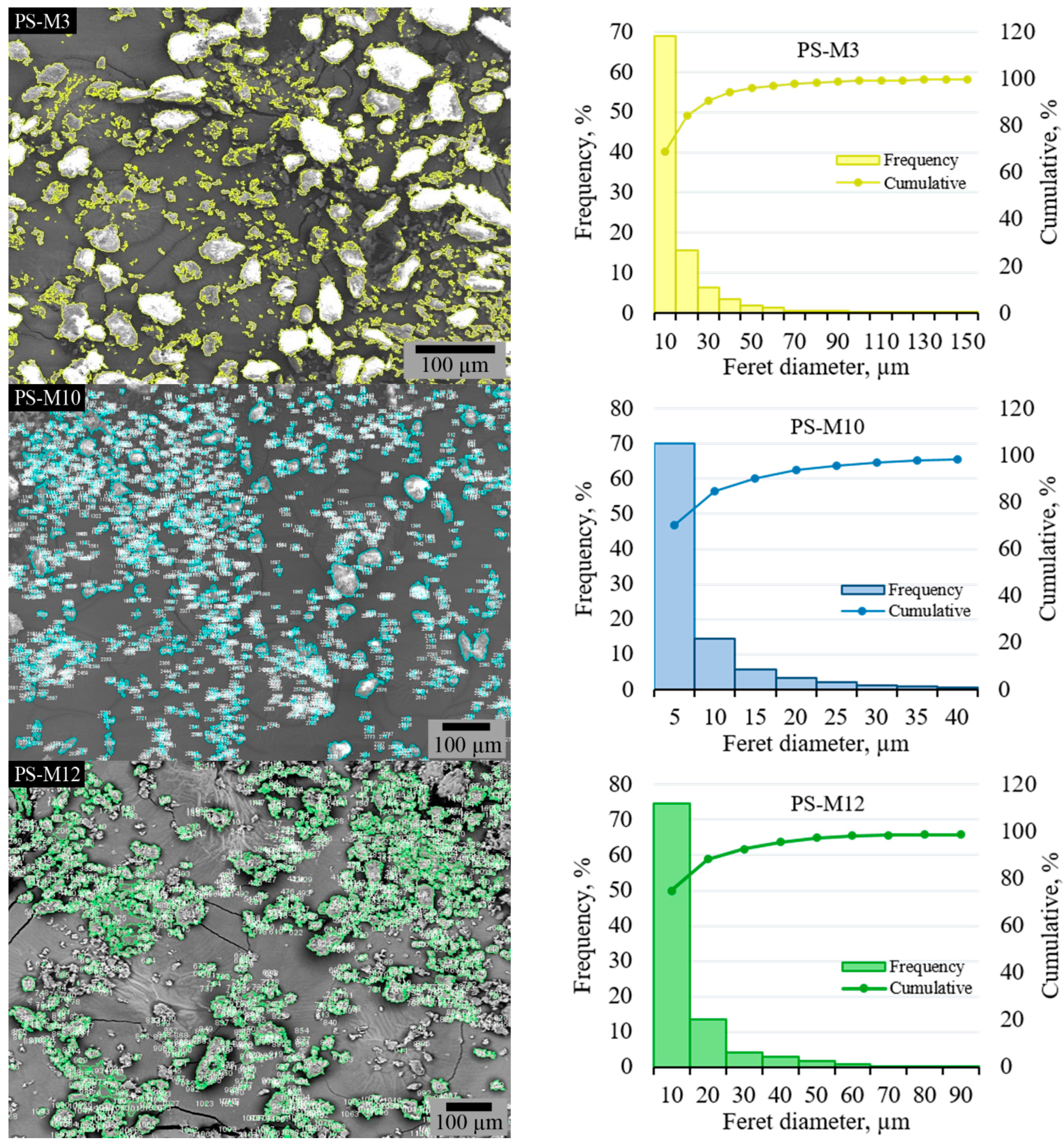
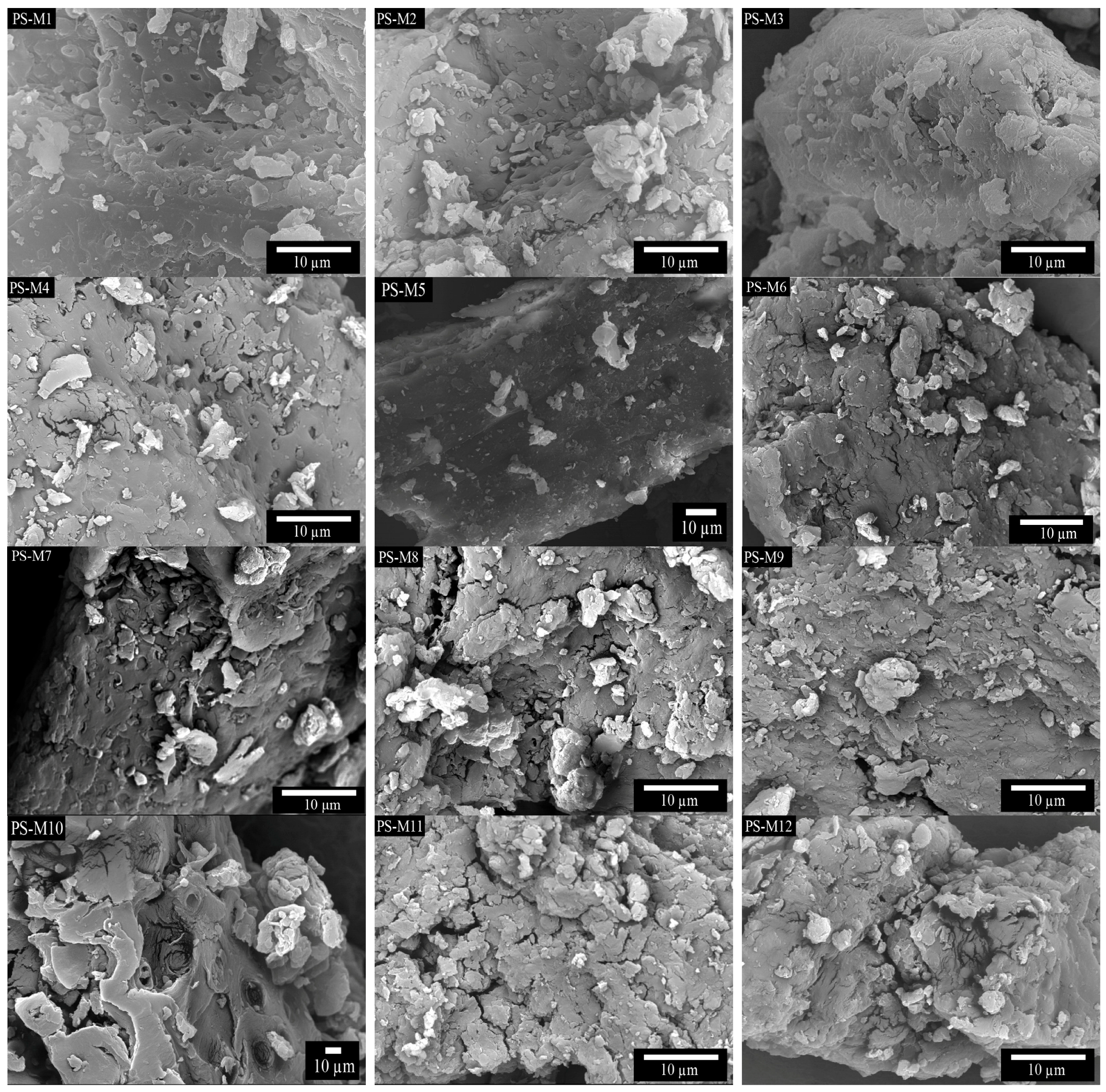
3.2.2. SEM Anaysis and Particle Size Distribution of the PS-M Samples
3.3. Adsorption Performance of Selected Milled Plum Stone Samples
3.3.1. Adsorption Efficiency
3.3.2. Adsorption Isotherms
3.3.3. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 May 2025).
- Milatović, D.; Zec, G.; Đurović, D.; Boškov, Đ. Production and processing of plum in Serbia. Acta Agric. Serb. 2020, 25, 59–63. [Google Scholar] [CrossRef]
- Usmonova, Z.T.; Salikhanova, D.S. Change of functional groups and structure in the content of plum seed, carbonization, and activated carbon depending on temperature. Univ. Tech. Sci. 2024, 7, 17935. Available online: https://7universum.com/ru/tech/archive/item/17935 (accessed on 1 May 2025). [CrossRef]
- Dołżyńska, M.; Obidziński, S.; Piekut, J.; Yildiz, G. The utilization of plum stones for pellet production and investigation of post-combustion flue gas emissions. Energies 2020, 13, 5107. [Google Scholar] [CrossRef]
- Giri, D.D.; Jha, J.M.; Tiwari, A.K.; Srivastava, N. Java plum and amaltash seed biomass based bio-adsorbents for synthetic wastewater treatment. Environ. Pollut. 2021, 280, 116890. [Google Scholar] [CrossRef]
- Katnić, Đ.; Marinović-Cincović, M.; Porobić, S.J.; Vujčić, I.; Šaponjić, A.; Sikirić, B.; Živojinović, D. Characterization and kinetics of thermal decomposition behavior of plum and fig pomace biomass. J. Clean. Prod. 2022, 352, 131637. [Google Scholar] [CrossRef]
- Rubangakene, N.O.; Elkady, M.; Elwardany, A.; Fujii, M.; Sekiguchi, H.; Shokry, H. Novel nano-biosorbent materials from thermal catalytic degradation of green pea waste for cationic and anionic dye decolorization. Biomass Convers. Biorefin. 2023, 13, 14873–14888. [Google Scholar] [CrossRef]
- Voća, N.; Bilandžija, N.; Jurišić, V.; Matin, A.; Krička, T.; Sedak, I. Proximate, Ultimate, and Energy Values Analysis of Plum Biomass By-products Case Study: Croatia’s Potential. J. Agric. Sci. Technol. 2016, 18, 1655–1666. [Google Scholar]
- Giordano, L.; Marras, F.; Mangiaracina, R.; Perego, A. Mapping Emerging Circular-Economy Technologies: A Topic Modeling Analysis. PLoS ONE 2024, 19, e0312709. [Google Scholar] [CrossRef]
- Doczekalska, B.; Bartkowiak, M.; Kuśmierek, K.; Świątkowski, A. Activated carbons from plum stones as efficient adsorbents for the removal of phenol and bisphenol A from aqueous solutions. Desalin. Water Treat. 2023, 306, 51–62. [Google Scholar] [CrossRef]
- Salikhanova, D.; Usmonova, Z.; Sobirjonova, S.; Ubaydullaeva, N.; Karabayeva, M. Obtainment of activated carbon by processing plum stones and using them in industrial wastewater purification. E3S Web Conf. 2024, 486, 01017. [Google Scholar] [CrossRef]
- Odobašić, A.; Ahmetović, M.; Šestan, I.; Salihović, E.; Karić, A. Plum pits as natural sorbents for removal of lead ions. Technol. Acta 2021, 14, 33–37. [Google Scholar] [CrossRef]
- Miladinović, M.; Pavlović, S.; Banković-Ilić, I.; Kostić, M.; Stamenković, O.; Veljković, V. Utilization of waste plum stones as a source of oil and catalyst for biodiesel production. Hem. Ind. 2023, 77, 39–52. [Google Scholar] [CrossRef]
- Pérez Merchán, A.M.; Rodríguez Carballo, G.; Torres Olea, B.; García Sancho, C.; Maireles Torres, P.J.; Mérida Robles, J.; Moreno Tost, R. Recent advances in mechanochemical pretreatment of lignocellulosic biomass. Energies 2022, 15, 5948. [Google Scholar] [CrossRef]
- Konan, D.; Koffi, E.; Ndao, A.; Peterson, E.C.; Rodrigue, D.; Adjallé, K. An overview of extrusion as a pretreatment method of lignocellulosic biomass. Energies 2022, 15, 3002. [Google Scholar] [CrossRef]
- Di Domenico, G.; Cioccolo, E.; Bianchini, L.; Venanzi, R.; Colantoni, A.; Picchio, R.; Cozzolino, L.; Di Stefano, V. A Systematic Review of Mechanical Pretreatment Techniques of Wood Biomass for Bioenergy. Energies 2025, 18, 3294. [Google Scholar] [CrossRef]
- Gallego-García, M.; Hernández-Serrano, A.; Salazar, A.; Urbano, B.; García-Sánchez, C. Recent advances on physical technologies for the pretreatment of lignocellulosic biomass: Milling, extrusion, ultrasound, and microwave methods. Renew. Sustain. Energy Rev. 2023, 167, 112720. [Google Scholar]
- Sitotaw, Y.W.; Habtu, N.G.; Nunes, S.P.; Van Gerven, T. Mechanical pretreatment of lignocellulosic biomass: Milling as an initial step to enhance enzymatic hydrolysis. iScience 2022, 25, 104610. [Google Scholar] [CrossRef]
- Wawrzaszek, B.; Charmas, B.; Jedynak, K. Impact of Mechanochemical Activation (MChA) on Characteristics and Dye Adsorption Behavior of Sawdust-Based Biocarbons. Materials 2024, 17, 4458. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, Y.; Borrion, A.; Li, J.; Li, X. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 259, 117494. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, Z.; Li, D.; Wu, M. Effects of ball milling processes on the microstructure and rheological properties of microcrystalline cellulose as a sustainable polymer additive. Materials 2018, 11, 1057. [Google Scholar] [CrossRef]
- Bian, R.; Li, L.; Ji, C.; Wang, Z. Effects of different mechanical treatments on structural changes of lignocellulosic waste biomass and subsequent Cu(II) removal kinetics. Arab. J. Chem. 2020, 13, 556–566. [Google Scholar] [CrossRef]
- Kim, H.; Park, Y.; Lee, J. Engineering porosity and active sites in peach pit-derived carbon materials for high-performance sodium storage. J. Energy Storage 2025, 65, 116215. [Google Scholar] [CrossRef]
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Biomass feedstock pre-processing—Part 1: Pre-treatment. In Biomass Conversion; InTech: Rijeka, Croatia, 2011; pp. 1–18. [Google Scholar] [CrossRef]
- Lopičić, Z.R.; Milojković, J.V.; Šoštarić, T.D.; Petrović, M.D.; Mihajlović, M.L.; Lačnjevac, Č.M.; Stojanović, M.D. Influence of pH value on Cu(II) biosorption by lignocellulose peach shell waste material. Hem. Ind. 2013, 67, 287–296. [Google Scholar] [CrossRef]
- Šoštarić, T.; Petrović, M.; Stojanović, J.; Marković, M.; Avdalović, M.; Hosseini-Bandegharaei, A.-B.; Lopičić, Z. Structural changes of waste biomass induced by alkaline treatment. Biomass Convers. Biorefin. 2020, 12, 1051–1064. [Google Scholar] [CrossRef]
- Pap, S.; Šoštarić, T.D.; Radonić, J.; Maletić, S.; Igić, S.; Turk Sekulić, M. Utilization of fruit processing industry waste as green activated carbon for the treatment of heavy metals and chlorophenols contaminated water. J. Clean. Prod. 2017, 162, 958–972. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Núñez-Decap, M.; Wechsler-Pizarro, A.; Vidal-Vega, M. Mechanical, physical, thermal and morphological properties of polypropylene composite materials developed with particles of peach and cherry stones. Sustain. Mater. Technol. 2021, 29, e00300. [Google Scholar] [CrossRef]
- ASTM D2866-94; Standard Test Method for Total Ash Content of Activated Carbon. ASTM International: West Conshohocken, PA, USA, 1999.
- ASTM D2867-04; Standard Test Methods for Moisture Content of Activated Carbon. ASTM International: West Conshohocken, PA, USA, 2004.
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [PubMed]
- Bozecka, A.; Bozecki, P.; Sanak-Rydlewska, S. Removal of Pb(II) and Cd(II) ions from aqueous solutions with selected organic wastes. Physicochem. Probl. Miner. Process. 2016, 52, 380–396. [Google Scholar] [CrossRef]
- Bożęcka, A.; Bożęcki, P.; Sanak-Rydlewska, S. Study of chemical surface structure of natural sorbents used for removing of Pb2+ ions from model aqueous solutions (Part II). Arch. Min. Sci. 2014, 59, 217–223. [Google Scholar] [CrossRef]
- Gala, A.; Sanak-Rydlewska, S. A Comparison of Pb2+ Sorption from Aqueous Solutions on Walnut Shells and Plum Stones. Pol. J. Environ. Stud. 2011, 20, 877–883. [Google Scholar]
- Marković, D.; Bojić, D.; Bojić, A.; Nikolić, G. The biosorption potential of waste biomass young fruit walnuts for lead ions: Kinetic and equilibrium study. Hem. Ind. 2016, 70, 243–255. [Google Scholar] [CrossRef]
- Arroub, H.H.; El Harfi, A. Preparation and characterization of carbons based on date stones: Application to the adsorption of Zn2+ and Cu2+ ions. Appl. J. Environ. Eng. Sci. 2019, 5, 131–143. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Li, J.; Salamh, Y.; Al-Laqtah, N.; Walker, G.; Ahmad, M.N. Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent. J. Hazard. Mater. 2010, 176, 510–520. [Google Scholar] [CrossRef]
- Mikoda, B.; Klojzy-Karczmarczyk, B.; Mazurkiewicz, M.; Bąk, A. Air pollution control and flue gas desulfurization residues from Polish copper smelting facility as adsorbents of Pb (II) and Cu (II) from aqueous solutions. Environ. Sci. Pollut. Res. 2018, 25, 31520–31534. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Wu, C.; Gu, S. Ginkgo biloba L. shells-based adsorbent for the removal of Cu2+ and Cd2+ from aqueous solution: Kinetics, isotherm, thermodynamics and mechanisms. J. Mol. Liq. 2017, 241, 603–611. [Google Scholar] [CrossRef]
- Areco, M.M.; Saleh-Medina, L.; Trinelli, M.A.; Marco-Brown, J.L.; dos Santos Afonso, M. Adsorption of Cu(II), Zn(II), Cd(II) and Pb(II) by dead Avena fatua biomass and the effect of these metals on their growth. Colloids Surf. B Biointerfaces 2013, 110, 305–312. [Google Scholar] [CrossRef]
- Zhao, J.; Boada, R.; Cibin, G.; Palet, C. Enhancement of selective adsorption of Cr species via modification of pine biomass. Sci. Total Environ. 2021, 756, 143816. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, M.; Marciniak, M.; Gęca, M.; Herda, K.; Pietrzak, R.; Nowicki, P. Activated Biocarbons Obtained from Plant Biomass as Adsorbents of Heavy Metal Ions. Materials 2022, 15, 5856. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Oh, S. Adsorption of As and Pb by stone powder/chitosan/maghemite composite beads (SCM beads): Kinetics and column study. Processes 2023, 11, 581. [Google Scholar] [CrossRef]
- Yıldız, T.D. Waste management costs (WMC) of mining companies in Turkey: Can waste recovery help meeting these costs? Resour. Policy 2020, 68, 101706. [Google Scholar] [CrossRef]
- Kulczycka, J.; Góralczyk, M.; Włodarczyk, B. Cost of waste management versus competitiveness of mining: The example of non-ferrous metal industry. Miner. Energy-Raw Mater. Rep. 2003, 18, 10–15. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, M.; Zhang, J.; Zhao, L. Effects of particle size distribution on the physicochemical, functional, and structural properties of alfalfa leaf powder. Agriculture 2024, 14, 634. [Google Scholar] [CrossRef]





| Sample Name | Milling Time (h) | Milling Speed (rpm) | Ball-to-Powder Ratio |
|---|---|---|---|
| PS-U | / | / | / |
| PS-M1 | 1 | 250 | 10:1 |
| PS-M2 | 2 | 250 | 10:1 |
| PS-M3 | 3 | 250 | 10:1 |
| PS-M4 | 1 | 250 | 20:1 |
| PS-M5 | 2 | 250 | 20:1 |
| PS-M6 | 3 | 250 | 20:1 |
| PS-M7 | 1 | 500 | 10:1 |
| PS-M8 | 2 | 500 | 10:1 |
| PS-M9 | 3 | 500 | 10:1 |
| PS-M10 | 1 | 500 | 20:1 |
| PS-M11 | 2 | 500 | 20:1 |
| PS-M12 | 3 | 500 | 20:1 |
| Parameter | Value | Method | Literature Range [8,10,13,27,29] | Comment |
|---|---|---|---|---|
| Moisture | 5.21% | ASTM D2867-04 [30] | 6–10% | Low moisture, good for storage |
| pH | 6.06 | pH meter | 5.5–6.5 | Slightly acidic environment |
| Carbon (C) | 64.47% | Vario EL III C, H, N, S/O Elemental Analyzer (Elementar, Germany) | 50–65% | High organic matter content |
| Nitrogen (N) | 2.40% | 1.0–2.5% | Approx. 15% crude protein (N × 6.25), within the typical range | |
| Hydrogen (H) | 7.64% | 6–8% | Typical for plant biomass | |
| Sulfur (S) | 0.04% | 0.02–0.1% | Low sulfur content, nontoxic level | |
| Ash | 2.3% | ASTM [31] D2866-94 | 1–3% | Slightly above average, typical mineral content |
| Lignin | 35.7% | Klason, Kürschner–Hoffer methods | 30–40% | Typical for lignocellulosic biomass |
| Cellulose | 12.9% | 10–15% | Within reported values | |
| Hemicellulose | 30.1% | 20–35% | On the higher side |
| Peak No. | Wavenumber Center (cm−1) | FWHM (cm−1) | Area (a.u. × cm−1) | Relative Share (%) |
|---|---|---|---|---|
| 1 | 1695.76 | 30.33 | 69.081 | 1.8 |
| 2 | 1538.58 | 57.73 | 429.896 | 11.4 |
| 3 | 1480.19 | 28.15 | 211.376 | 5.6 |
| 4 | 1440.31 | 29.79 | 179.343 | 4.8 |
| 5 | 1393.60 | 44.25 | 337.625 | 9.0 |
| 6 | 1350.61 | 35.76 | 218.766 | 5.8 |
| 7 | 1293.29 | 71.92 | 800.572 | 21.3 |
| 8 | 1176.84 | 98.77 | 1508.935 | 40.2 |
| Langmuir Isotherm | Freundlich Isotherm | ||||||
|---|---|---|---|---|---|---|---|
| qmax | KL | R2 | KF | 1/nF | R2 | ||
| Pb2+ | PS-M1 | 19.792 | 0.138 | 0.9909 | 5.349 | 0.331 | 0.9645 |
| PS-M10 | 22.849 | 0.361 | 0.9981 | 13.277 | 0.292 | 0.9951 | |
| PS-M12 | 20.566 | 0.230 | 0.9997 | 6.806 | 0.314 | 0.9812 | |
| Cu2+ | PS-M1 | 8.513 | 0.359 | 0.9899 | 3.696 | 0.196 | 0.9638 |
| PS-M10 | 11.207 | 0.219 | 0.9998 | 3.682 | 0.261 | 0.9574 | |
| PS-M12 | 10.268 | 0.234 | 0.9962 | 3.527 | 0.250 | 0.9537 | |
| PFO | PSO | ||||||
|---|---|---|---|---|---|---|---|
| k1, 1/h | q1, mg/g | R2 | k2, g/mg/h | q2, mg/g | R2 | ||
| Pb2+ | PS-M1 | 3.886 | 14.741 | 0.9967 | 0.822 | 15.063 | 0.9999 |
| PS-M10 | 5.260 | 18.690 | 0.9993 | 1.221 | 18.961 | 0.9999 | |
| PS-M12 | 3.655 | 16.015 | 0.9985 | 0.616 | 16.466 | 0.9991 | |
| Cu2+ | PS-M1 | 2.481 | 7.614 | 0.9678 | 0.530 | 8.102 | 0.9914 |
| PS-M10 | 2.604 | 8.896 | 0.9542 | 0.446 | 9.523 | 0.9962 | |
| PS-M12 | 2.429 | 8.254 | 0.9510 | 0.432 | 8.870 | 0.9919 | |
| Parameter | F Value a,b | p Value b |
|---|---|---|
| Feret diameters max | 15,850.49 | <0.00001 |
| Feret diameters min | 950.65 | <0.00001 |
| Feret diameters median | 554.19 | <0.00001 |
| Feret diameters average | 1744.55 | <0.00001 |
| FTIR 3335 cm−1 | 17,730.37 | <0.00001 |
| FTIR 2881 cm−1 | 20,613.47 | <0.00001 |
| FTIR 1733 cm−1 | 145.86 | <0.00001 |
| FTIR 1558 cm−1 | 460.83 | <0.00001 |
| FTIR 1030 cm−1 | 2261.20 | <0.00001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajić, N.; Radovanović, D.; Đokić, J.; Jelić, I.; Jevtić, S.; Sokić, K.; Štulović, M. Optimization of Milling Process Parameters for Waste Plum Stones for Their Sustainable Application. Processes 2025, 13, 2759. https://doi.org/10.3390/pr13092759
Gajić N, Radovanović D, Đokić J, Jelić I, Jevtić S, Sokić K, Štulović M. Optimization of Milling Process Parameters for Waste Plum Stones for Their Sustainable Application. Processes. 2025; 13(9):2759. https://doi.org/10.3390/pr13092759
Chicago/Turabian StyleGajić, Nataša, Dragana Radovanović, Jovana Đokić, Ivana Jelić, Sanja Jevtić, Katarina Sokić, and Marija Štulović. 2025. "Optimization of Milling Process Parameters for Waste Plum Stones for Their Sustainable Application" Processes 13, no. 9: 2759. https://doi.org/10.3390/pr13092759
APA StyleGajić, N., Radovanović, D., Đokić, J., Jelić, I., Jevtić, S., Sokić, K., & Štulović, M. (2025). Optimization of Milling Process Parameters for Waste Plum Stones for Their Sustainable Application. Processes, 13(9), 2759. https://doi.org/10.3390/pr13092759






