Abstract
Antrodia cinnamomea is a fungus endemic to Taiwan that exhibits various medicinal properties, including anti-cancer and anti-inflammatory effects, many of which stem from its unique triterpenoids. Studies on A. cinnamomea generally focus on the red phenotype, while only a handful of studies on its naturally-occurring mutant white phenotype exist. This study investigated the effect of two different culture types (solid-state dish culture and submerged flask culture) and three carbon sources (glucose, maltose, and sucrose) on the mycelial dry weight and triterpenoid content of red (AC) and white (W) strains of A. cinnamomea. The concentrations of eight key triterpenoid compounds were also determined to compare triterpenoid profiles. Biomass accumulation under solid-state culture was more than two-fold for the W strain than for the AC strain. In submerged culture mycelial biomass was not significantly different between strains. Total triterpenoid content was 29%, 88%, and 134% greater in the AC strain than the W strain under submerged culture with glucose, maltose, and sucrose, respectively. Similarly, triterpenoid content of solid-state-cultured fungus increased by 10% with glucose and 53% with maltose. Although both strains responded similarly to each carbon source with regards to mycelial dry weight, their triterpenoid profiles differed in solid-state culture. Five of the eight key compounds were detected in the solid-state culture of the AC strain (antcin A, antcin B, antcin C, antcin K, and DMMB) and four were detected in the submerged culture (antcin A, antcin B, antcin K, and DMMB). The concentration of individual triterpenoid compounds was up to 100 times greater in the solid-state culture than in the submerged culture. None of the eight key compounds were found in the W strain. This study indicates that the triterpenoid profile of in vitro cultured white A. cinnamomea differs from red strains, and that further investigation of their metabolomic profiles is required.
1. Introduction
Antrodia cinnamomea, synonym of Taiwanofungus camphoratus, is a parasitic fungus that grows only on the trunk of the tree Cinnamomum kanehirae, a species endemic to Taiwan [1]. It is a well-known traditional medicinal fungus in Taiwan and is consumed as a functional food or used in the production of health supplements. Over 160 chemical compounds have been extracted and identified from A. cinnamomea [2], among which the triterpenoid compounds are considered to have the greatest biological activity [3]. Triterpenoids are a class of chemical compounds composed of three terpene units with the molecular formula C30H48 and consist of six isoprene units. These are produced via the mevalonate pathway from acetyl-CoA, and A. cinnamomea uses the resulting lanosterol to produce two of the main classes of triterpenoids: lanostanes and ergostanes [4]. Of these, antcins (ergostane-type triterpenoids) are unique to A. cinnamomea, and A. salmonea (synonymous with Taiwanofungus salmoneus) [2] and their medicinal properties include anti-cancer [5], anti-oxidative [6], and anti-inflammatory effects [7].
The therapeutic effects of A. cinnamomea have caused an increase in demand for the fungus. However, the fruiting body has a very slow growth rate in the wild, leading to overharvesting and the destruction of many endangered C. kanehirae trees [1]. In response, the Taiwanese government has placed prohibitions and restrictions on the harvesting of C. kanehirae, further increasing demand [8]. As a result of this, efforts to culture the fungus in vitro have been made.
Submerged culture is an alternative method of cultivating A. cinnamomea in vitro for commercial use, allowing for greater production of mycelium within a smaller area alongside reduced possibility of contamination [3]. However, studies have shown that mycelia produced by submerged culture show drastically reduced triterpenoid content compared to wild-harvested fruiting bodies (basidiomata) [2]. Alternatively, solid culture has also been used to produce A. cinnamomea mycelia for therapeutic purposes. Solid culture presents an advantage over submerged culture in that the formation of fruiting bodies is possible [9]. Lin et al. [10] found that abrasion of hyphae with cotton swabs was able to induce the production of fruiting bodies in A. cinnamomea grown on agar plates and found that the chemical profiles of plate-grown mycelia and fruiting bodies differed; although it has also been shown that the induction of fruiting bodies is inconsistent [11].
In addition to the culture method, fungal growth and secondary metabolite content also differs according to environmental conditions [12]. The carbon source present in culture media is one of the major factors affecting fungal growth. Carbohydrates—including monosaccharides, disaccharides, and polysaccharides—are the main energy source of fungi, and their availability influences fungal growth-rate, metabolism, and reproduction. The presence of monosaccharides causes a tendency toward asexual reproduction in fungi, whereas the presence of disaccharides and polysaccharides leads to increased sexual reproduction [13]. As sexual reproduction (fruiting body formation) has been linked to increased triterpenoid content [10], different carbon sources (and their effects on reproduction) may also alter triterpenoid content and composition. Furthermore, while studies have investigated the impact of carbon source on the accumulation of mycelial biomass of A. cinnamomea [14,15,16,17,18]; few have examined the effect of carbon source on triterpenoid production.
The color of A. cinnamomea typically ranges from yellow to a dark reddish-brown, but a white variant of the fungus also exists in the wild. This variant is rare and said to be more medicinally potent, making it more desirable and thus expensive, but has not been extensively studied [19]. Chung et al. [20] found that the phytomic similarity index values for common triterpenoids of variants of red A. cinnamomea were similar, while that of the white variant was notably different, suggesting that the white variant may have different therapeutic value. However, another study found that the concentrations of most of the 10 major triterpenoid compounds found in white A. cinnamomea were lower than that of the common variant, contrary to the beliefs of traditional Chinese medicine practitioners [21]. Furthermore, Liu [22] observed that the growth of different A. cinnamomea variants (including a white variant) did not respond in the same way to different carbon sources—the carbohydrate that resulted in the greatest mycelial dry weight was different among variants. However, the differences in triterpenoid content between red and white A. cinnamomea in response to different carbon sources has not yet been examined.
This study examines the effect of different culture methods and carbon sources on the triterpenoid content of two A. cinnamomea variants: a red variant and a white variant.
2. Materials and Methods
2.1. A. cinnamomea Strains and Preparation
Two A. cinnamomea strains previously studied by Liu [22] known as “A1” and “W” were obtained. A1 (hereafter referred to as AC) is a A. cinnamomea variant with orange hyphae, while W is a variant with white hyphae. Both were obtained from Da-Shan Farm in Zhushan Township, Nantou County, Taiwan. Using distilled water, spores were washed from the basidiomata onto plates and diluted 10 times. Next, 1 µL of this suspension was taken and observed under a microscope to ascertain the spore count per microlitre. This dilution was continued until only one spore remained per microlitre. The monospore was inoculated onto lysogeny broth agar (LB) and incubated for 24 h at 27 °C. After 24 h, the LB agar media was cut into 1 × 1 mm2 squares containing hyphae for inoculation onto malt extract agar (MEA) for 1 month at 27 °C.
2.2. A. cinnamomea Culture
The AC (Figure 1) and W (Figure 2) strains were cultured on media with three carbohydrate sources: glucose, maltose, and sucrose. The culture methods included solid-state culture on MEA and submerged culture in malt extract broth (MEB) in a 250 mL flask. Both MEA and MEB contained 1% malt extract, 1% peptone, 1% agar, and 1% carbohydrate (glucose, maltose or sucrose), with a pH of 5.5. The media were sterilized in an autoclave (TOMIN, New Taipei City, Taiwan) at 121 °C for 25 min, whereas the carbohydrate sources were sterilized via being passed through a 45 µm filter. A. cinnamomea was cultured in a growth chamber at 27 °C for 1 month, and the submerged culture was stirred at 120 rpm. The samples were harvested and dried in an oven at 45 °C for 14 days and their weight was determined.
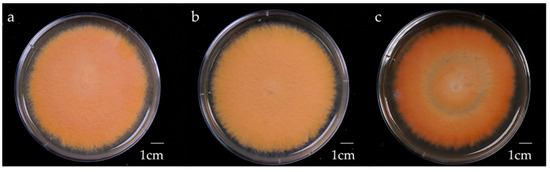
Figure 1.
Morphology of 4-week-old AC strain Antrodia cinnamomea solid-state culture on malt extract broth containing 1% (a) glucose, (b) maltose, (c) sucrose.
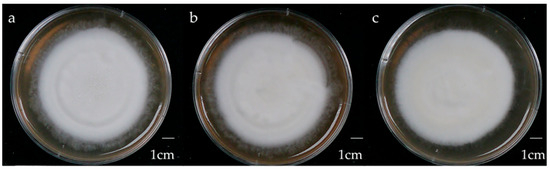
Figure 2.
Morphology of 4-week-old W strain Antrodia cinnamomea solid-state culture on malt extract broth containing 1% (a) glucose, (b) maltose, (c) sucrose.
2.3. Triterpenoid Extraction and Analysis
Triterpenoid analysis was performed according to Chen et al. [23]—0.1 g of sample was extracted in 5 mL of 90% ethanol under ultrasonication for 30 min, followed by centrifugation at 5000 rpm for 15 min. The supernatant was then removed and 0.5 mL was taken and dried at 80 °C in an oven for 2 weeks. After drying, 0.2 mL of 5% (w/v) vanillin acetic acid and 0.08 mL of perchloric acid were reacted at 60 °C with ultrasonic oscillations for 20 min until the colour of the sample was homogenous. After homogenization, acetic acid was used to dilute the solution five times. Next, the total triterpenoid content of the sample was determined through spectrophotometry at 550 nm.
Dried mycelial samples from the 1% glucose media were reconstituted in 100% methanol at a concentration of 1 mg/mL. These solutions were then filtered through a 0.22 μm membrane prior to analysis. Standards of the eight triterpenoids—antcin A, antcin B, antcin C, antcin H, antcin K, dehydroeburioic acid (DEA), dehydrosulphurenic acid (DSA), and 2,4-dimethoxy-6-methylbenzene-1,3-diol (DMMB)—were isolated and purified by Professor Chi-I Chang of the Department of Biotechnology, National Pingtung University of Science and Technology. Calibration curves with a linear range of 0.01–1 ng/μL for the antcin compounds and DEA and 0.05–5 ng/μL for DSA and DMMB were established for quantification purposes.
The concentrations of individual triterpenoids were determined through liquid chromatography-mass spectrometry (LC-MS). Liquid chromatography was performed using a Prominence UFLC System (SHIMADZU, Tokyo, Japan) equipped with a Synchronis C18 (150 × 2.1 mm, 5 µm, Thermo Scientific) and a TSQ Quantum Access MAX triple stage quadrupole mass spectrometer (Thermo Scientific, Waltham, MA, USA) with a heated-electrospray ionisation (H-ESI) source. Triterpenoids were detected under MS/MS conditions with the equipment running in multiple reaction monitoring (MRM) mode. The sample volume used for triterpenoid quantification was 5 µL.
Liquid chromatography was performed under the following conditions. The mobile phase consisted of (A) 5% acetonitrile/0.1% formic acid and (B) 95% acetonitrile/0.1% formic acid. A flow rate of 0.35 mL/min was used and a linear gradient was applied as follows: 0–3 min (A 80% isocratic), 3–6 min (A 80% to 60%), 6–9 min (A 60% to 50%), 9–11 min (A 50% isocratic), 11–14 min (A 50% to 30%), 14–18 min (A 30% to 20%), 18–23 min (A 20% isocratic), 23–25 min (A 20% to 80%), 25–30 min (A 80% isocratic). The flow rate was 0.35 mL/min.
For mass spectrometry, antcins A, B, C, H, K, DEA, and DSA were analyzed in negative ion mode with a column temperature of 25 °C, spray voltage of 4000 V, sheath gas pressure of 40 arb, ion sweep gas pressure of 0, aux gas pressure of 5 arb, capillary temperature of 320 °C, tube lens offset of 124 V, skimmer offset of 0, and a collision pressure of 0. DMMB concentration was determined in positive ion mode under the same conditions with the exception of a spray voltage of 3500 V. Table S1 and Figure S1 (Supplementary Material) show the MRM conditions of the MS analysis and the standard peaks of the eight key triterpenoid compounds, respectively.
2.4. Statistical Analysis
All the experiments were performed in triplicate to verify their reproducibility. The data were analysed using Statistical Product and Service Solution (SPSS; version 22). Analysis of variance (ANOVA) was used to compare means and Duncan’s multiple range tests were used for post-hoc analysis (p < 0.05).
3. Results
3.1. Effect of Culture Method and Carbohydrate Source on Biomass
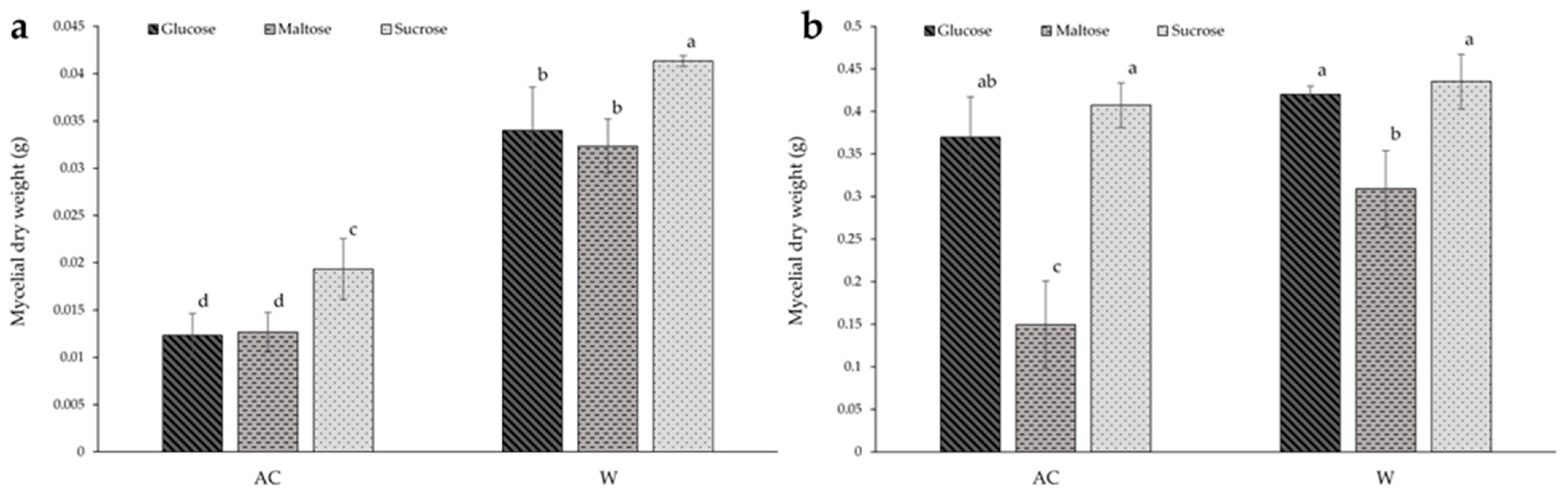
In solid-state culture (Figure 3a), the mycelial dry weight of the W strain was significantly greater under all conditions compared to the AC strain, with the dry weight of the W strain being more than double that of the AC strain when compared to the same carbohydrate culture. Of all carbon sources, sucrose was found to result in significantly greater biomass for both strains, increasing by more than 25% for both strains. Within both strains, there was no significant difference between culture with glucose or maltose.
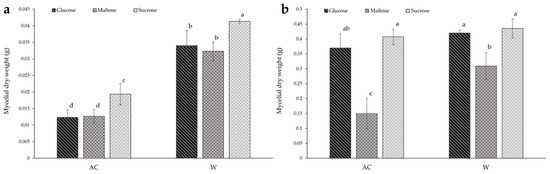
Figure 3.
The effect of carbon source on the biomass of red (AC) and white (W) Antrodia cinnamomea grown in (a) solid-state culture and (b) submerged culture for one month. Values shown are the mean ± standard deviation of three replicates. Significance was determined using two-way ANOVA with Duncan’s multiple range test for post-hoc analysis. Letters indicate significant difference (p < 0.05).
Figure 3b shows that there were no significant differences in biomass between the two strains when grown with glucose or sucrose in submerged culture. However, the culture with maltose as the carbon source resulted in double the mycelial dry weight for the W strain compared to the AC strain.
3.2. Effect of Culture Method and Carbohydrate Source on Total Triterpenoid Content
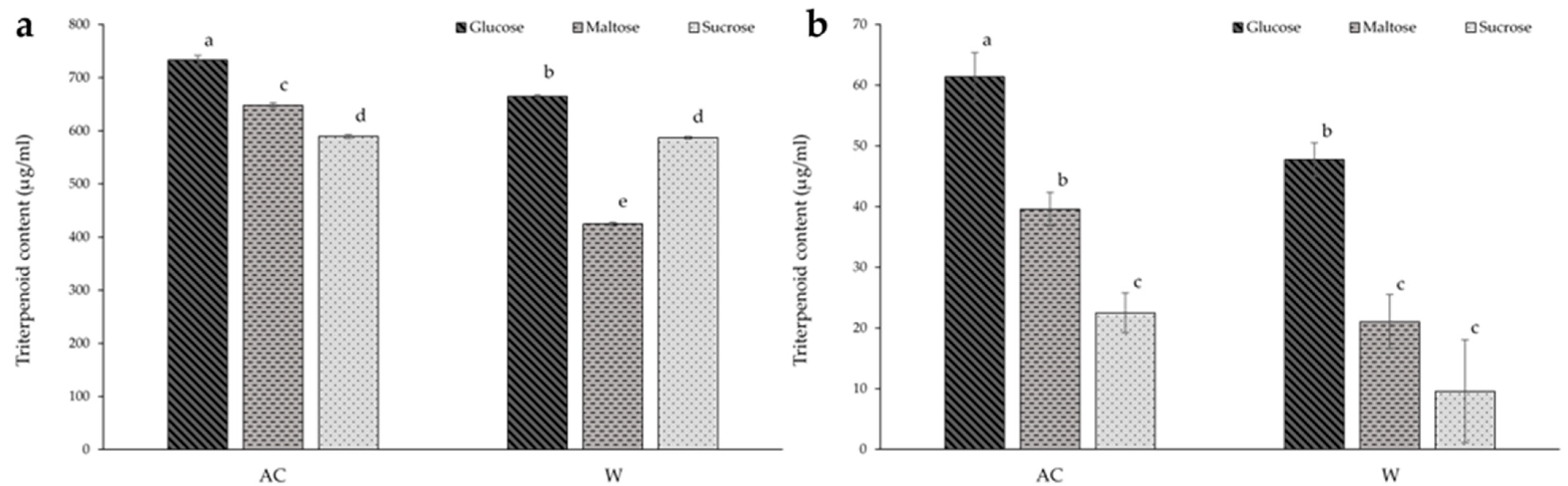
The effect of different carbohydrates on the triterpenoid content of A. cinnamomea from solid-state culture is shown in Figure 4a. The AC strain grown with glucose media resulted in the greatest total triterpenoid content overall (13% higher than the maltose media and 24% higher the sucrose media), while glucose media also showed the highest triterpenoid content among the W strain conditions—13% and 56% larger than the maltose and sucrose medias, respectively. The lowest triterpenoid content overall was found in the W strain culture cultivated on maltose media. Furthermore, there was no significant difference in triterpenoid content between both strains when sucrose was the carbon source.
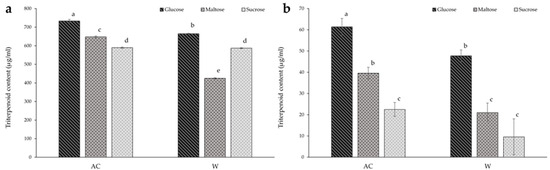
Figure 4.
The effect of carbon source on the triterpenoid content of red (AC) and white (W) Antrodia cinnamomea grown in (a) solid-state culture and (b) submerged culture for one month. Values shown are the mean ± standard deviation of three replicates. Significance was determined using two-way ANOVA with Duncan’s multiple range test for post-hoc analysis. Letters indicate significant difference (p < 0.05).
As with solid-state culture, media containing glucose resulted in the highest triterpenoid content in both strains in the submerged culture, with the glucose-cultured AC strain containing the highest triterpenoid content overall (Figure 4b). Total triterpenoid content of mycelia of the AC strain grown on glucose media were 2.7 times that of those grown on sucrose media, and 1.6 times that of the maltose media. Furthermore, cultivation of the AC strain on maltose media increased triterpenoid content by 75% in comparison to the sucrose media. With regards to the W strain, triterpenoid content of the samples from glucose media were five-fold that of the sucrose media, and 2.3 times that of the maltose media. When comparing the two strains, the triterpenoid content of the W strain samples from submerged culture were 22% and 47% lower when cultivated on glucose and maltose media, respectively.
3.3. Effect of Culture Method on Triterpenoid Profiles
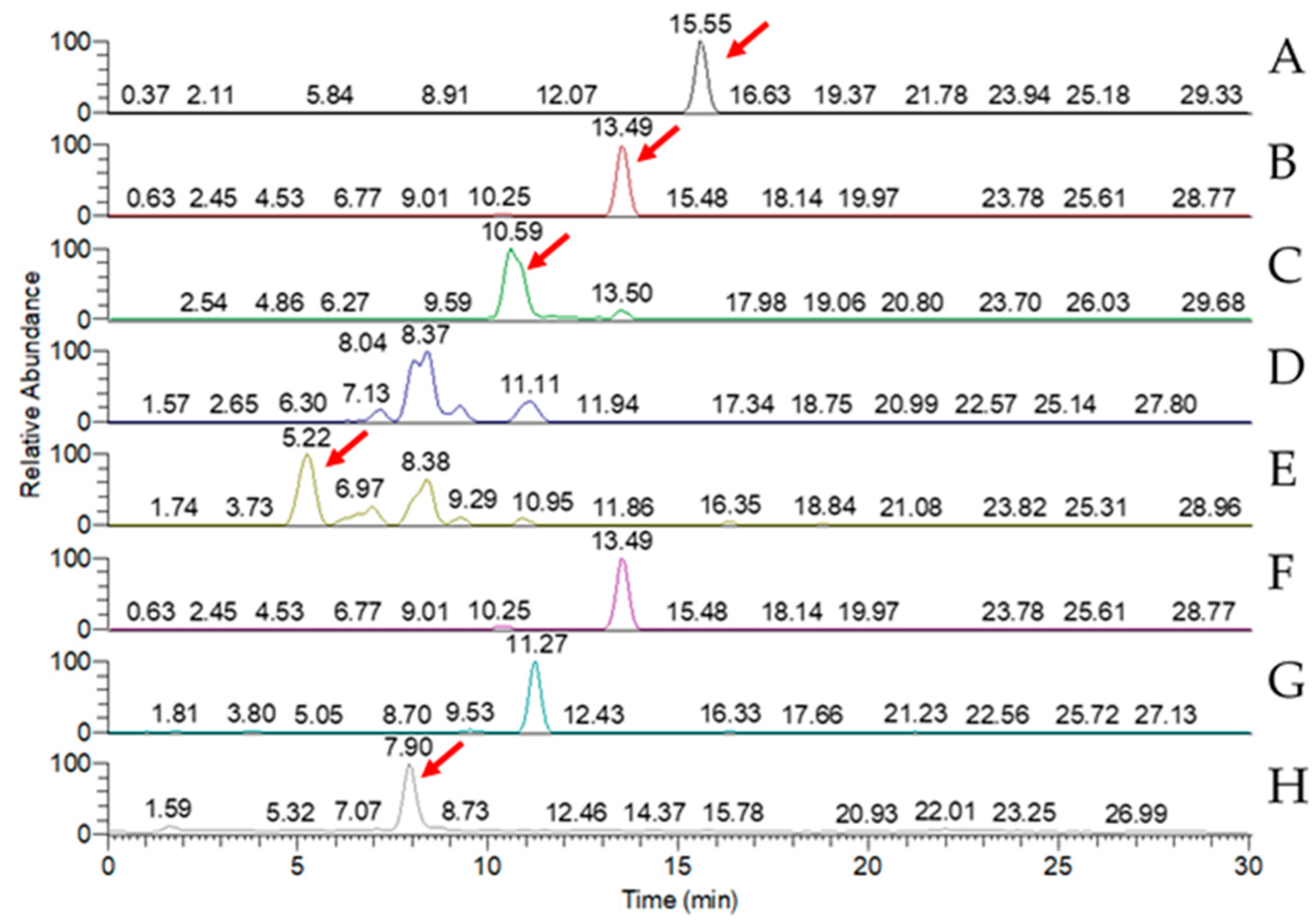
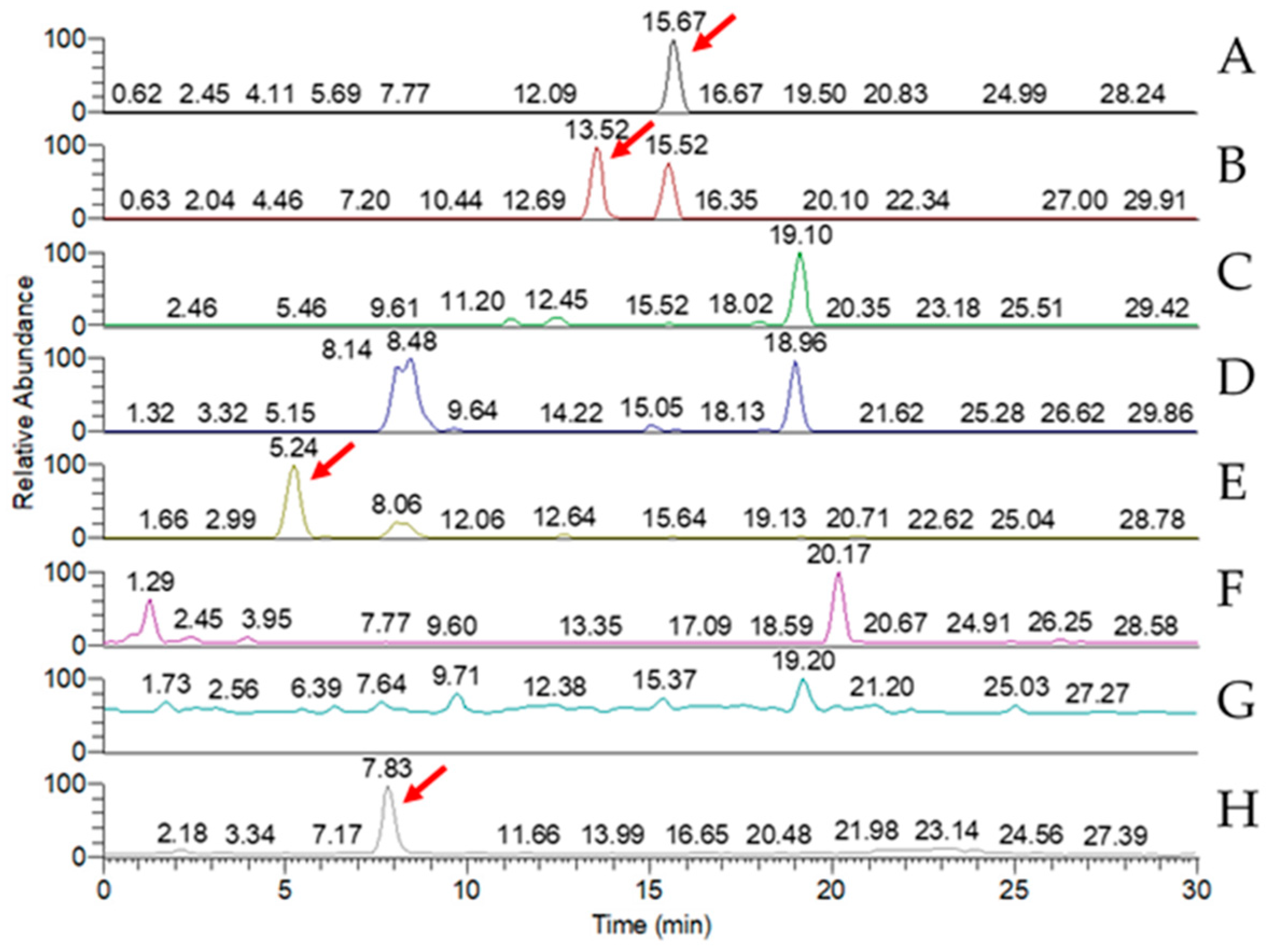
LC-MS analysis of the triterpenoid extract of the AC strain cultured in solid-state culture with 1% glucose (Figure 5) showed that, of the eight key triterpenoids, five were detected in the extract: antcin A, antcin B, antcin C, antcin K, and DMMB. In submerged culture, extract of the AC strain showed the presence of four of the key compounds: antcin A, antcin B, antcin K, and DMMB (Figure 6). None of the eight triterpenoids were detected in the W strain samples for either culture method.
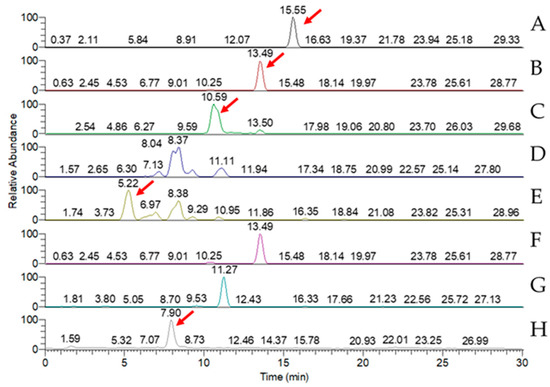
Figure 5.
MRM chromatogram of eight key triterpenoid compounds of red Antrodia cinnamomea grown in solid-state culture for one month. (A) Antcin A; (B) antcin B; (C) antcin C; (D) antcin H; (E) antcin K; (F) dehydroeburicoic acid; (G) dehydrosulphurenic acid; (H) 2,4-dimethoxy-6-methylbenzene-1,3-diol. Red arrows identify peaks indicating the presence of the triterpenoid.
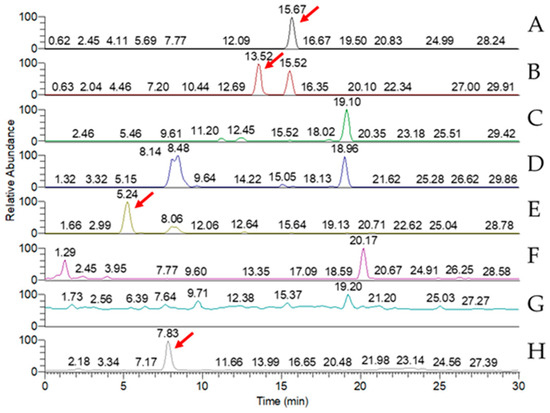
Figure 6.
MRM chromatogram of eight key triterpenoid compounds of red Antrodia cinnamomea grown in submerged culture for one month. (A) Antcin A; (B) antcin B; (C) antcin C; (D) antcin H; (E) antcin K; (F) dehydroeburicoic acid; (G) dehydrosulphurenic acid; (H) 2,4-dimethoxy-6-methylbenzene-1,3-diol. Red arrows identify peaks indicating the presence of the triterpenoid.
Table 1 shows the concentrations of eight key triterpenoids detected in the glucose-cultured AC strain from MRM chromatography. Mycelia of solid-state and submerged culture contained similar concentrations of DMMB, with the concentration from submerged-culture AC being marginally larger. However, the concentration of antcins A, B, and K were magnitudes greater in the solid-state culture sample than in the submerged-culture sample (5 times in the case of antcin K, 20 times for antcin A, and over 100 times for antcin B). Of all the key compounds, the antcin B concentration in the solid-state culture was by far the highest at 3.58 ± 0.19 µg/g, followed by antcin C at 1.09 ± 0.01 µg/g.

Table 1.
Concentrations of detected key triterpenoid compounds of red Antrodia cinnamomea grown identified from MRM chromatograms.
4. Discussion
When comparing the two culture methods, in concurrence with the existing literature, mycelia yield was lower in the solid-state culture than the submerged culture (Figure 3) [24,25]. This is theorised to be due to increased aeration in the liquid culture as it is continuously stirred, dispersing oxygen throughout the mixture, a critical factor affecting protein production of basidiomycete fungi such as A. cinnamomea [26,27,28].
Contrastingly, it was also observed that the increase in mycelial dry weight from submerged culture was proportionate to the decrease in triterpenoid content—an approximately 10-fold difference (Figure 3 and Figure 4). In a review on solid-state culture, Barrios-González and Tarragó-Castellanos [29] suggested that increased secondary metabolite production in solid-state culture compared to submerged culture is due to three main factors: direct air contact, stimulation from the supporting substrate, and water availability. The presence of a solid substrate for hyphae to anchor to and provide structure, as well as contact with the air may therefore induce greater triterpenoid production in A. cinnamomea cultured with solid-state culture over submerged culture.
Furthermore, secondary metabolite production is usually initiated during the secondary growth stage (idiophase)—rather than the growth phase (trophophase)—which is usually initiated by the depletion of nutrients [30]. The concentration gradients present in solid culture also facilitate this. Nutrients must be taken up and transported from the penetrative hyphae on the inner layer of the solid substrate to the aerial hyphae on the outer layer of the mycelium via diffusion in the cytoplasm [31]. This process is slower than direct uptake from the nutrient solution the mycelia are suspended in when grown with submerged culture. As a result, mycelia in submerged culture are saturated with nutrients, and so do not enter idiophase until the sugar concentration in the entire solution is reduced, whereas there is a constant nutritional gradient between the outermost and innermost hyphae in solid culture, inducing the synthesis of secondary metabolites [32]. Consequently, it is possible that fungi grown in submerged culture deplete local nutrients more slowly, and thus require a longer cultivation time to accumulate secondary metabolites.
This indicates an antagonistic relationship between mycelial and secondary metabolite yield. Submerged culture is able to produce more product volume, but suffers in terms of quality. Conversely, solid-state culture produces a higher quality product, but results in reduced biomass production. Commercial growers must therefore evaluate the benefits and drawbacks of each culture method.
However, some these drawbacks may be ameliorated by the selection of a beneficial carbon source. In the solid culture condition, sucrose resulted in the greatest biomass (Figure 3). This is contrary to the existing literature, which shows that glucose stimulates greater increases in A. cinnamomea biomass than sucrose in solid-state culture [14,22]. Glucose and sucrose were equally effective at promoting mycelial growth in the submerged culture. However similar studies find that either sucrose [16,17] or glucose [15,18,33] result in greater mycelial biomass. Previous research has also noted that other sugars were superior to both glucose and sucrose in increasing A. cinnamomea growth, including galactose [14,22], xylose [1,17], and fructose [18,33]. These differences may be due to variability between strains, and some research has already shown that A. cinnamomea strains respond differently to individual sugars [19,22]. Indeed, this was also observed within the present study, as although the growth of both strains responded similarly to submerged culture (Figure 3b), the W strain accumulated significantly greater mycelial dry weight in solid-state culture across all carbon sources than the AC strain (Figure 3a). Typically, white cinnamomea phenotypes have lower growth than red phenotypes, but this observation is not universal for all white and red strains [19,22].
Regardless, the preference of A. cinnamomea for certain sugars may be due to its ecology. As a parasitic fungus, A. cinnamomea relies on its host tree C. kanehirae to supply it with carbohydrates, within which sucrose, glucose, and fructose are the most common forms of soluble carbohydrates [34]. Additionally, the addition of water-soluble wood extracts of Cinnamomum camphora (a relative of C. kanehirae) resulted in hyphal growth significantly greater than extracts of C. kanehirae [35]. In a subsequent study, analysis of the crude polysaccharide content of this extract of C. camphora revealed the presence of galactose and glucose, in addition to high concentrations of mannose and galactosamine [36]. This suggests that these are some of the most abundant carbohydrates available in the natural environment of A. cinnamomea, and that they are a common energy source for the fungus in the wild. Further investigation into other carbohydrates or carbohydrate combinations that assist in simulating its natural environment may be warranted, possibly improving yield.
Carbon source also had an important effect on medicinal value—triterpenoid content was significantly higher when glucose was used as the carbon source in the cultivation media for both solid-state and submerged culture (Figure 4). This finding is supported by both Chang et al. [15] and Shu et al. [1] who found that glucose induced greater triterpenoid production than when cultured with sucrose or xylose, respectively. In A. cinnamomea, triterpenoids are synthesised via the mevalonic pathway, and a number of genes regulating this production have been identified [37]. The initial reaction in the mevalonic pathway is the generation of acetoacetyl-CoA from acetyl-CoA (Noushahi et al. 2022) [38], with acetyl-CoA itself a product of the decarboxylation of pyruvate, which in turn is generated from glycolysis—the initial substrate of this metabolic pathway being glucose [39]. An abundance of glucose in the environment may therefore result in increased production of triterpenoid precursors, resulting in the observed increase in triterpenoid content.
While the red phenotype of A. cinnamomea is the most ubiquitous, uncommon phenotypes of different colours exist in the wild and Chinese medicine practitioners claim that these variants—the white variant in particular—are more medicinally potent than its common red-coloured counterpart [21]. In contrast, the total triterpenoid content of the AC strain was significantly greater at its peak (when glucose was used as the carbohydrate source) in both solid-state and submerged culture than the W strain (Figure 4). Analysis of both wild grown [40] and in vitro cultured [19] A. cinnamomea have shown lower concentrations of triterpenoids in white strains than red ones, as was observed in this research. While both the AC and W strains responded similarly to the different carbon sources with regards to mycelial growth they responded differently in terms of triterpenoid content, particularly in the solid-state culture (Figure 4a). The use of sucrose as the carbon source resulted in the lowest triterpenoid content in the AC strain, while in the W strain it resulted in the second highest. This suggests that the while the primary metabolism of the strains is similar their secondary metabolism differs. Analysis of the triterpenoid profiles of the two strains provides evidence for this difference. None of the eight key triterpenoids were detected in the W strain, while in the AC strain, five and four were present in the solid-state cultured and submerged cultured fungus, respectively (Table 1). A total of 44 triterpenoid compounds have been identified in A. cinnamomea [4,41], and the W strain therefore likely contains compounds other than those considered key triterpenoids.
This inference is also well supported—differences in the triterpenoid profiles of red and white A. cinnamomea strains have been previously investigated. Chung et al. [20] noted that while the phytomic similarity indices of three red A. cinnamomea strains were similar, the index of a white strain was significantly different. Likewise, Chen et al. [6] observed that no antcin I was detected in white mycelia before four weeks of growth, while the same was not true for red mycelia. Additionally, the concentrations of some triterpenoids—DSA, eburicoic acid, and DEA—were at some time points greater in the white strain. The triterpenoid profiles of A. cinnamomea also appear to vary widely between strains, even among those cultured on the same media, with large differences in the content of fruiting bodies in particular [41]. Consequently, the selection of strains that produce the desired secondary metabolites is vital with regards to the production of medicinal material.
Ergostane-type triterpenoids (such as antcins) are connected to the formation of basidiomata, and are not produced in mycelia [42,43,44]. This is usually thought to be an advantage of solid-state culture as basidomata, and therefore antcins, can be produced [9]. Nonetheless, in this study three ergostanes were detected in submerged culture (antcins A, B, and K) suggesting that some degree of basidiomatal formation is possible in submerged culture. However, their concentrations were very low, and further research should focus on improving yield. While the findings of this study overall indicate that solid-state culture is superior for the production of triterpenoids, submerged culture may have some advantages. A. cinnamomea production in submerged culture usually takes around 14 days [45], whereas solid-state dish cultures require anywhere from six weeks [9,11,46], to three months [47]. Moreover, the use of repeated batch fermentation—when a portion of one culture is used to inoculate future batches—also has the potential to improve commercial cultivation, allowing for continuous cultivation and peak triterpenoid content observed after only seven days [45]. Submerged culture may therefore also be a reliable source of medicinally-valuable A. cinnamomea, in spite of its low triterpenoid yield, as submerged culture has greater scalability compared with solid-state culture in petri dishes as the size of the culture vessel can be increased [26].
5. Conclusions
This study investigated the effect of carbon source and culture method on the growth and triterpenoid content of red and white variants of A. cinnamomea. Sucrose and glucose were found to be the best carbon sources for production of mycelial biomass, while glucose was the most effective at inducing triterpenoid synthesis. This study also established that ergostane-type triterpenoids could be produced in submerged culture, albeit at a lower concentration to solid-state culture. Further research should further examine the triterpenoid profile of the white-phenotype A. cinnamomea and investigate methods of amplifying the triterpenoid content of submerge-cultured fungi.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13092732/s1, Figure S1: MRM chromatogram of the standard peaks of eight key triterpenoid compounds of Antrodia cinnamomea. (A) antcin A; (B) antcin B; (C) antcin C; (D) antcin H; (E) antcin K; (F) dehydroeburioic acid; (G) dehydrosulphurenic acid; (H) 2,4-dimethoxy-6-methylbenzene-1,3-diol; Table S1: Multiple Reaction Monitoring conditions of the Mass Spectrometry analysis of eight key triterpenoids of Antrodia cinnamomea.
Author Contributions
Conceptualisation, P.L. and S.Y.M.; methodology, P.L. and S.Y.M.; formal analysis S.Y.M., P.L. and C.R.S.; investigation, S.Y.M.; writing—original draft preparation, S.Y.M. and C.R.S.; writing—review and editing, P.L.; supervision, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We would like to thank Kun Cheng Liu for kindly providing both the red and white strains of A. cinnamomea.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shu, C.H.; Wu, H.J.; Ko, Y.H.; Lin, W.H.; Jaiswal, R. Effects of Red Light and Addition of Monoterpenes and Tangerine oil on the Production of Biomass and Triterpenoids of Antrodia cinnamomea in Submerged Cultures. J. Taiwan Inst. Chem. Eng. 2016, 67, 140–147. [Google Scholar] [CrossRef]
- Senthil Kumar, K.J.; Gokila Vani, M.; Chen, C.Y.; Hsiao, W.W.; Li, J.; Lin, Z.X.; Chu, F.H.; Yen, G.C.; Wang, S.Y. A Mechanistic and Empirical Review of Antcins, A New Class of Phytosterols of Formosan Fungi Origin. J. Food Drug Anal. 2020, 28, 38–59. [Google Scholar] [CrossRef]
- Ma, T.W.; Lai, Y.T.; Chen, L.T.; Yang, F.C. The Cultivation Strategy of Enhancing Triterpenoid Production in Submerged Cultures of Antrodia cinnamomea by Adding Monoterpenes. J. Taiwan Inst. Chem. Eng. 2016, 58, 210–218. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Yuan, X.L.; Luo, Y.N.; Luo, M.N.; Zheng, Y. Effects of Culture Mechanism of Cinnamomum kanehirae and C. camphora on the Expression of Genes Related to Terpene Biosynthesis in Antrodia cinnamomea. Mycobiology 2022, 50, 121–131. [Google Scholar] [CrossRef]
- Ma, T.W.; Chiu, M.Y.; Yang, F.C. Enhancement of Triterpenoids Formation of Antrodia cinnamomea in thin Stillage-submerged Culture by Shifting the Shaking Rate and Adding Soybean Oil. Asian J. Biotechnol. Bioresour. Technol. 2018, 4, 1–13. [Google Scholar] [CrossRef]
- Chen, Y.T.; Shen, Y.C.; Chang, M.C.; Lu, M.K. Precursor-Feeding Strategy on the Triterpenoid Production and Anti-Inflammatory Activity of Antrodia cinnamomea. Process Biochem. 2016, 51, 941–949. [Google Scholar] [CrossRef]
- Tien, A.J.; Chien, C.Y.; Chen, Y.H.; Lin, L.C.; Chien, C.T. Fruiting Bodies of Antrodia cinnamomea and Its Active Triterpenoid, Antcin K, Ameliorates N-Nitrosodiethylamine-Induced Hepatic Inflammation, Fibrosis and Carcinogenesis in Rats. Am. J. Chin. Med. 2017, 45, 173–198. [Google Scholar] [CrossRef]
- Zhao, S.S.; Leung, K.S. Quality Evaluation of Mycelial Antrodia camphorata using High-Performance Liquid Chromatography (HPLC) Coupled with Diode Array Detector and Mass Spectrometry (DAD-MS). Chin. Med. 2010, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Wang, W.R. Basidiomatal Formation of Antrodia cinnamomea on Artificial Agar Media. Bot. Bull. Acad. Sin. 2005, 46, 151–154. [Google Scholar]
- Lin, J.Y.; Wu, T.Z.; Chou, J.C. In Vitro Induction of Fruiting Body in Antrodia cinnamomea-A Medicinally Important Fungus. Bot. Stud. 2006, 47, 267–272. [Google Scholar]
- Chu, Y.C.; Yang, R.M.; Chang, T.T.; Chou, J.C. Fructification of Antrodia cinnamomea Was Strain Dependent in Malt Extract Media and Involved Specific Gene Expression. J. Agric. Food Chem. 2010, 58, 257–261. [Google Scholar] [CrossRef]
- He, Y.C.; He, K.Z.; Pu, Q.; Li, J.; Zhao, Z.J. Optimization of Cultivating Conditions for Triterpenoids Production from Antrodia cinnamomea. Indian J. Microbiol. 2012, 52, 648–653. [Google Scholar] [CrossRef]
- Moore-Landecker, E. Effects of Medium Composition and Light on Formation of Apothecia and Sclerotia by Pyronema domesticum. Can. J. Bot. 1987, 65, 2276–2279. [Google Scholar] [CrossRef]
- Lee, I.H.; Chen, C.T.; Chen, H.C.; Hsu, W.C.; Lu, M.K. Sugar Flux in Response to Carbohydrate-Feeding of Cultured Antrodia camphorata, a Recently Described Medicinal Fungus in Taiwan. J. Chin. Med. 2002, 13, 21–31. [Google Scholar] [CrossRef]
- Chang, C.Y.; Lee, C.L.; Pan, T.M. Statistical Optimization of Medium Components for the Production of Antrodia cinnamomea AC0623 in Submerged Cultures. Appl. Microbiol. Biotechnol. 2006, 72, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.C.; Huang, H.C.; Yang, M.J. The Influence of Environmental Conditions on the Mycelial Growth of Antrodia cinnamomea in Submerged Cultures. Enzym. Microb. Technol. 2003, 33, 395–402. [Google Scholar] [CrossRef]
- Lin, E.S.; Wang, C.C.; Sung, S.C. Cultivating Conditions Influence Lipase Production by the Edible Basidiomycete Antrodia cinnamomea in Submerged Culture. Enzym. Microb. Technol. 2006, 39, 98–102. [Google Scholar] [CrossRef]
- Geng, Y.; He, Z.; Lu, Z.M.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Antrodia camphorata ATCC 200183 Sporulates Asexually in Submerged Culture. Appl. Microbiol. Biotechnol. 2013, 97, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L. Chemical Variation of White Antrodia cinnamomea from Regular Antrodia cinnamomea; National Dong Hwa University: Hualien, Taiwan, 2013. [Google Scholar]
- Chung, C.H.; Yeh, S.C.; Tseng, H.C.; Siu, M.L.; Lee, K.T. Chemical Quality Evaluation of Antrodia cinnamomea Fruiting Bodies using Phytomics Similarity Index Analysis. J. Food Drug Anal. 2016, 24, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Ho, Y.P.; Chou, J.C. Phenologic Variation of Major Triterpenoids in Regular and White Antrodia cinnamomea. Bot. Stud. 2016, 57, 33. [Google Scholar] [CrossRef]
- Liu, K.C. The Effect of Different Solid Plate Medium Culture on Mycelium Growth and Polysaccharides Content of White Antrodia cinnamomea; National Pingtung University of Science and Technology: Pingtung, Taiwan, 2019. [Google Scholar]
- Chen, Y.; Xie, M.Y.; Gong, X.F. Microwave-assisted extraction used for the isolation of total triterpenoid saponins from. J. Food Eng. 2007, 81, 162–170. [Google Scholar] [CrossRef]
- Fazenda, M.L.; Seviour, R.; McNeil, B.; Harvey, L.M. Submerged Culture Fermentation of “Higher Fungi”: The Macrofungi. Adv. Appl. Microbiol. 2008, 63, 33–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Guan, Y.Y.; Hu, P.F.; Chen, L.; Xu, G.R.; Liu, L.M.; Cheung, P.C.K. Production of Bioactive Metabolites by Submerged Fermentation of the Medicinal Mushroom Antrodia cinnamomea: Recent Advances and Future Development. Crit. Rev. Biotechnol. 2019, 39, 541–554. [Google Scholar] [CrossRef]
- Gibbs, P.A.; Seviour, R.J.; Schmid, F. Growth of Filamentous Fungi in Submerged Culture: Problems and Possible Solutions. Crit. Rev. Biotechnol. 2000, 20, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Stark, B.C.; Dikshit, K.L.; Pagilla, K.R. Recent Advances in Understanding the Structure, Function, and Biotechnological Usefulness of the Hemoglobin from the bacterium Vitreoscilla. Biotechnol. Lett. 2011, 33, 1705–1714. [Google Scholar] [CrossRef]
- Wei, X.X.; Chen, G.Q. Applications of the VHb Gene for Improved Microbial Fermentation Processes. Method Enzym. 2008, 436, 273–287. [Google Scholar] [CrossRef]
- Barrios-González, J.; Tarragó-Castellanos, M.R. Solid-State Fermentation: Special Physiology of Fungi. In Fungal metabolites; Reference Series in Phytochemistry; Merillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2015; pp. 319–347. [Google Scholar] [CrossRef]
- Barrios-González, J. Solid-State Fermentation: Physiology of Solid Medium, Its Molecular Basis and Applications. Process Biochem. 2012, 47, 175–185. [Google Scholar] [CrossRef]
- Rahardjo, Y.S.P.; Tramper, J.; Rinzema, A. Modeling Conversion and Transport Phenomena in Solid-State Fermentation: A Review and Perspectives. Biotechnol. Adv. 2006, 24, 161–179. [Google Scholar] [CrossRef]
- Acuña-Argüelles, M.E.; Gutiérrez-Rojas, M.; Viniegra-González, G.; Favela-Torres, E. Production and Properties of Three Pectinolytic Activities Produced by Aspergillus niger in Submerged and Solid-State Fermentation. Appl. Microbiol. Biotechnol. 1995, 43, 808–814. [Google Scholar] [CrossRef]
- Shih, I.L.; Pan, K.; Hsieh, C.Y. Influence of Nutritional Components and Oxygen Supply on the Mycelial Growth and Bioactive Metabolites Production in Submerged Culture of Antrodia cinnamomea. Process Biochem. 2006, 41, 1129–1135. [Google Scholar] [CrossRef]
- Magel, E.; Einig, W.; Hampp, R. Carbohydrates in Trees. In Developments in Crop Science; Gupta, A.K., Kaur, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 26, pp. 317–336. [Google Scholar]
- Shen, Y.C.; Chou, C.J.; Wang, Y.H.; Chen, C.F.; Chou, Y.C.; Lu, M.K. Anti-Inflammatory Activity of the Extracts from Mycelia of Antrodia camphorata Cultured with Water-Soluble Fractions from Five Different Cinnamomum Species. FEMS Microbiol. Lett. 2004, 231, 137–143. [Google Scholar] [CrossRef]
- Hsu, F.L.; Chou, C.J.; Chang, Y.C.; Chang, T.T.; Lu, M.K. Promotion of Hyphal Growth and Underlying Chemical Changes in Antrodia camphorata by Host Factors from Cinnamomum camphora. Int. J. Food Microbiol. 2006, 106, 32–38. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Yuan, X.; Wang, J.; Yang, Y.; Zheng, Y. Transcriptome Profiling of Antrodia cinnamomea Fruiting Bodies Grown on Cinnamomum kanehirae and C. camphora Wood Substrates. Res. Square, 2023; preprint. [Google Scholar] [CrossRef]
- Noushahi, H.A.; Khan, A.H.; Noushahi, U.F.; Hussain, M.; Javed, T.; Zafar, M.; Batool, M.; Ahmed, U.; Liu, K.; Harrison, M.T.; et al. Biosynthetic Pathways of Triterpenoids and Strategies to Improve their Biosynthetic Efficiency. Plant Growth Regul. 2022, 97, 439–454. [Google Scholar] [CrossRef]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl Coenzyme A: A Central Metabolite and Second Messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- Su, C.H.; Hsieh, Y.C.; Chng, J.Y.; Lai, M.N.; Ng, L.T. Metabolomic Profiling of Different Antrodia cinnamomea Phenotypes. J. Fungi 2023, 9, 97. [Google Scholar] [CrossRef]
- Qiao, X.; Song, W.; Wang, Q.; Liu, K.D.; Zhang, Z.X.; Bo, T.; Li, R.Y.; Liang, L.N.; Tzeng, Y.M.; Guo, D.A.; et al. Comprehensive Chemical Analysis of Triterpenoids and Polysaccharides in the Medicinal Mushroom Antrodia cinnamomea. RSC Adv. 2015, 5, 47040–47052. [Google Scholar] [CrossRef]
- Chang, T.T.; Wang, W.R.; Chou, C.J. Differentiation of Mycelia and Basidiomes of Antrodia cinnamomea using Certain Chemical Components. Taiwan J. For. Sci. 2011, 26, 125–133. [Google Scholar]
- Lin, T.Y.; Chen, C.Y.; Chien, S.C.; Hsiao, W.W.; Chu, F.H.; Li, W.H.; Lin, C.C.; Shaw, J.F.; Wang, S.Y. Metabolite Profiles for Antrodia cinnamomea Fruiting Bodies Harvested at Different Culture Ages and from Different Wood Substrates. J. Agric. Food Chem. 2011, 59, 7626–7635. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.M.; Geng, Y.; Li, H.X.; Sun, Q.; Shi, J.S.; Xu, Z.H. Alpha-Terpineol Promotes Triterpenoid Production of Antrodia cinnamomea in Submerged Culture. FEMS Microbiol. Lett. 2014, 358, 36–43. [Google Scholar] [CrossRef]
- Li, H.X.; Lu, Z.M.; Geng, Y.; Gong, J.S.; Zhang, X.J.; Shi, J.S.; Xu, Z.H.; Ma, Y.H. Efficient Production of Bioactive Metabolites from Antrodia camphorata ATCC 200183 by Asexual Reproduction-Based Repeated Batch Fermentation. Bioresour. Technol. 2015, 194, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.W.; Chen, T.C.; Liu, C.H.; Wang, S.Y.; Shaw, J.F.; Chen, Y.T. Identification and Isolation of an Intermediate Metabolite with Dual Antioxidant and Anti-Proliferative Activity Present in the Fungus Antrodia cinnamomea Cultured on an Alternative Medium with Cinnamomum kanehirai Leaf Extract. Plants 2021, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Wu, T.Y.; Hsu, T.H.; Lai, M.N.; Wu, Y.C.; Ng, L.T. Chemical Composition and Chronic Toxicity of Disc-Cultured Antrodia cinnamomea Fruiting Bodies. Toxics 2022, 10, 587. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).