Abstract
In this study, Stevia rebaudiana biomass was hydrothermally carbonized (HTC) at 215 °C for 60 min with acrylic acid (AA) as a catalyst at concentrations of 0.25, 0.50, and 1.00 mol L−1. The maximum hydrochar yield (48.5%) was obtained at 0.25 mol L−1 AA, while fixed carbon contents ranged from 20.79% to 34.27%. Higher heating values (HHV) varied between 26.95 and 36.61 MJ kg−1, with the highest catalytic HHV (32.20 MJ kg−1) achieved at 1.00 mol L−1 AA (HC15). Acrylic acid addition significantly promoted deoxygenation, reducing the O/C ratio from 0.67 in raw biomass to 0.21, thereby improving fuel quality. FT-IR and XRD analyses indicated enhanced aromatization and partial graphitization with increasing acid concentration, while SEM images revealed carbon microspheres and porous morphologies. Thermogravimetric analysis showed that HC15 exhibited the lowest mass loss and highest residual carbon, indicating superior thermal stability. GC-MS analysis demonstrated that acrylic acid markedly increased phenolic derivatives, with phenol content rising from 19.47% (without catalyst) to 40.92% (1.00 mol L−1 AA). The aqueous phase contained TOC values of 14,280–28,728 mg/L and COD values of 43,227–113,920 mg/L. Overall, acrylic acid-assisted HTC enhances both the energy-related properties of hydrochars and the chemical diversity of liquid products, providing a sustainable route for valorizing Stevia rebaudiana waste into value-added fuels and chemicals.
1. Introduction
As a key driver of economic growth and human development, energy is essential; yet its production—primarily from fossil fuels—has serious environmental consequences. Shifting to alternative energy sources is crucial for sustainability [1]. Biomass refers to organic matter derived from organisms that contain energy (primarily carbon) and has been extensively researched and used as a raw material for producing solid, liquid, or gaseous fuels as alternatives to fossil fuels [2]. The increasing demand for sustainable energy and the urgent need to reduce the environmental impacts of fossil fuels have accelerated the advancement of renewable biomass conversion technologies. Hydrothermal carbonization (HTC) has emerged as an efficient and environmentally friendly thermochemical process that converts wet biomass into carbon-rich solids (hydrochars) without requiring energy-intensive drying [3,4]. Operating under subcritical water conditions (180–250 °C), HTC facilitates reactions such as hydrolysis, dehydration, decarboxylation, and polymerization, producing hydrochar with enhanced fuel properties [5]. In this context, Stevia rebaudiana is considered a highly promising lignocellulosic feedstock for hydrothermal carbonization (HTC) due to its high carbohydrate content, richness in phenolic compounds, and significant lignin composition. Stevia rebaudiana, a perennial herb of the Asteraceae family, is widely cultivated for its natural zero-calorie sweeteners, primarily stevioside and rebaudioside A [6,7,8]. Stevia rebaudiana leaves generate a significant amount of lignocellulosic residue after glycoside extraction, which is often underutilized and disposed of as waste. The industrial-scale extraction of stevioside and rebaudioside A involves hot water extraction followed by ultrafiltration steps during which a substantial amount of leaf-derived residues rich in lignin and cellulose is generated, and these residues possess a complex composition including cellulose, hemicellulose, lignin, soluble sugars, proteins, and secondary metabolites [9,10]. Stevia rebaudiana biomass is considered a renewable resource with carbon-neutral characteristics, as it absorbs carbon dioxide during its growth phase, thereby helping to offset CO2 emissions [11,12]. Recent studies have shown that Stevia rebaudiana is noteworthy not only as a natural sweetener but also for its suitability in biomass conversion applications. Considering the widespread cultivation of Stevia rebaudiana and its relatively short life cycle, the substantial amount of residual biomass left after harvest poses significant environmental concerns. A large portion of this waste is often carelessly discarded or openly burned, leading to serious environmental pollution and resource wastage. To address this issue, it is essential to prioritize proper disposal methods instead of resorting to open burning or random field dumping. Research has shown that Stevia rebaudiana biomass contains a high amount of volatile matter and a moderate level of fixed carbon, making it a suitable feedstock for thermochemical conversion processes [13]. Despite its rich composition and abundance, research on the thermochemical valorization of Stevia rebaudiana biomass remains limited [14]. Few studies have investigated its potential for bioenergy applications such as pyrolysis or gasification. However, HTC presents a more suitable approach for processing Stevia rebaudiana residues due to their inherent moisture content and structural characteristics. Moreover, catalytic HTC—especially in the presence of organic acids—has shown promise in enhancing the carbonization efficiency, fixed carbon yield, and aromaticity of the resulting hydrochars [15]. Organic acids like acrylic acid can facilitate dehydration and aromatization reactions, thereby improving the quality and stability of the produced hydrochars [16]. Catalysts, especially organic acids, play a crucial role in hydrothermal carbonization by accelerating dehydration, decarboxylation, and aromatization reactions. In particular, acrylic acid was chosen over other acids due to its unique dual functionality: the carboxylic group effectively promotes dehydration and decarboxylation reactions, while the unsaturated double bond enables cross-linking and polymerization during hydrothermal treatment. Compared to strong mineral acids, acrylic acid offers milder catalytic conditions, reduces corrosion risks, and directs product formation toward aromatic and phenolic compounds, which are valuable for fuel and chemical applications [15,16]. Various studies in the literature have thoroughly examined the suitability of both plant-based and organic waste feedstocks for the HTC process, as well as the fuel-related and functional potential of the resulting products. Hoekman et al. conducted a systematic investigation into the hydrothermal carbonization (HTC) of six distinct biomass feedstocks, comprising three woody and three herbaceous types, under reaction conditions of 175–295 °C for 30 min. The study involved comprehensive characterization of the resulting hydrochar, aqueous, and gaseous products. Findings demonstrated that increasing the reaction temperature led to a notable decline in hydrochar yield, while simultaneously enhancing its energy density—highlighting the thermal upgrading potential of HTC at elevated temperatures [17]. Iryani et al. investigated the hydrothermal carbonization (HTC) of sugarcane bagasse in a batch-type reactor at temperatures ranging from 200 to 300 °C and reaction times between 3 and 30 min. After solid–liquid separation, 34–88% of the initial feedstock was recovered as solid products. The higher heating value (HHV) of these solid products increased by a factor between 1.1 and 1.9 compared to the raw material. These findings underscore the potential of the HTC process as a promising method to reduce the environmental impact of wet sugarcane bagasse waste generated by the sugar industry while simultaneously improving energy efficiency [18]. Martinez et al. investigated the hydrothermal carbonization (HTC) processes of lignocellulosic biomass types such as giant bamboo, coffee tree, eucalyptus, and coffee parchment at temperatures ranging from 180 to 240 °C for 3 h. As the temperature increased, fixed carbon content, energy density, and higher heating value (HHV) rose, while volatile matter content and mass yield decreased. Hydrochars produced at temperatures above 220 °C exhibited HHVs ranging from 24.6 to 29.2 MJ kg−1, indicating high potential as solid fuels. Coffee parchment showed the lowest mass yield among the samples. HTC liquors had low pH values due to the presence of organic acids. The results demonstrated that all tested biomass types are suitable for energy and value-added product generation via HTC and that integration with bioenergy plants could enhance process efficiency [19]. Mohammed et al. investigated the conversion of corn stalks into a high-quality energy source through hydrothermal carbonization (HTC). The HTC process was carried out at temperatures ranging from 250 to 350 °C, with residence times of 30 to 60 min, and biomass-to-water ratios between 0.09 and 0.14. The effects of process parameters were optimized using response surface methodology. The maximum hydrochar yield, energy yield, and higher heating value (HHV) were found to be 29.91% (dry weight), 42.38% (dry weight), and 26.03 MJ kg−1, respectively. The optimal conditions were determined as 305 °C, 60 min of residence time, and a biomass-to-water ratio of 0.114, under which a hydrochar with an HHV of 25.42 MJ kg−1 was produced. The results confirm that corn stalks are a viable and efficient feedstock for sustainable energy production via HTC [20]. Wu et al. applied hydrothermal carbonization (HTC) at various temperatures to agricultural waste collected from corn fields and examined the physicochemical properties, combustion behavior, kinetic characteristics, as well as slagging and fouling tendencies of the resulting hydrochar. The highest energy yield of 68.54% was achieved at 220 °C. With increasing HTC temperature, the H/C and O/C atomic ratios decreased, while porosity initially increased and then declined. It was reported that higher temperatures promoted the formation of aromatic structures but reduced the abundance of oxygen-containing functional groups [21]. Qi et al. synthesized functional carbon materials through one-step hydrothermal carbonization of glucose in the presence of sulfosalicylic acid or acrylic acid as co-monomers. Notably, the most remarkable aspect of this study is that the carbon microspheres prepared via hydrothermal carbonization of glucose can be used directly, without any modification or in situ functionalization, and were found to be efficient for cellulose hydrolysis [22]. Gan et al. synthesized a highly acidic, lignin-derived carbon-based catalyst through hydrothermal carbonization in the presence of acrylic acid, followed by sulfonation. This catalyst was proven to be highly effective for the catalytic hydrolysis of cellulose into reducing sugars in a [BMIM] Cl–H2O solvent system. Prepared with the addition of 40 wt% acrylic acid, the catalyst demonstrated excellent performance for cellulose hydrolysis (with a TRS yield of 75.4%) at a 100:1 mass ratio, outperforming conventional solid acid catalyst [23]. Although several studies have explored the use of organic acids in hydrothermal carbonization, the present work provides a novel contribution by systematically examining the catalytic role of acrylic acid in the HTC of Stevia rebaudiana biomass. Unlike commonly studied agricultural residues, Stevia rebaudiana is rich in glycosides and phenolic precursors, offering a distinctive chemical composition that influences HTC pathways. This study highlights both the improvement of hydrochar fuel properties through enhanced deoxygenation and aromatization, and the selective formation of phenolic derivatives in the aqueous phase. By integrating comprehensive analyses (FT-IR, XRD, SEM, TGA/DTG, GC-MS), this work establishes a dual valorization approach that differentiates it from previously reported organic acid-assisted HTC processes. The main objectives are: (i) to investigate the effects of temperature, residence time, and catalyst concentration on hydrochar properties; (ii) to analyze the structural, thermal, and surface characteristics of the hydrochars; and (iii) to assess the composition of the aqueous phase for its valorization potential. The findings contribute to optimizing catalytic HTC processes for the production of high-performance solid biofuels and value-added chemical intermediates, thereby advancing biomass valorization strategies for energy and environmental applications.
2. Materials and Methods
2.1. Metarials
Stevia rebaudiana leaves were obtained from a producer located in Balıkesir, Turkey. The leaves were ground using a blender (average size was 500 μm), packaged in plastic bags, and stored at +4 °C. Acrylic acid used were of analytical grade purchased from Sigma–Aldrich Company.
2.2. HTC Experiments
Hydrothermal carbonization experiments were performed in a 500 mL stainless-steel reactor (Parr 4848 High Pressure Reactor, Parr Instrument Co., Moline, IL, USA) capable of reaching temperatures up to 500 °C and pressures up to 35 MPa. Following a standard protocol, the reactor was charged with 15 g of lignocellulosic biomass sample (dry basis) and 150 mL of deionized water. After loading, the reactor was placed in a furnace, sealed tightly, and exposed to temperatures of 185, 215, 245, and 275 °C for durations of 30, 60, and 90 min, respectively. In catalytic runs, in addition to using the same amount of feedstock, 0.25, 0.50, and 1.00 mol L−1 acrylic acid solutions were used. After the HTC experiments were completed, the reactor was allowed to cool to room temperature. Subsequently, the reactor contents were transferred to a beaker. Solid products were collected using vacuum filtration and washed with 150 mL of deionized water. After filtration, the solids were dried at 105 °C for 24 h to obtain the final product. Each of the catalytic and non-catalytic HTC processes was repeated three times, and standard deviation values as error bars in hydrochar yield were shown in the yield graph. Standard deviations in hydrochar yields were determined to range from 0% to 2.5%, strengthening the reliability of the results. Hydrochar yields were determined using the following formula:
2.3. Analytical Methods
Fourier-transform infrared spectroscopy (FTIR) analysis was performed to determine the functional groups of the raw material and hydrochars. FTIR-ATR analyses were carried out using a Perkin Elmer-Frontier Spectrometer 100 instrument in the wavenumber range of 550–4000 cm−1. Morphological examinations of the surfaces of Stevia rebaudiana powder and hydrothermally carbonized Stevia rebaudiana powders were performed using a scanning electron microscope (FEI Quanta FEG 450, Hillsboro, OR, USA). X-ray diffraction (XRD) patterns of hydrochars were obtained using a Panalytical Empyrean Powder X-ray diffractometer (Almelo, The Netherlands) with Cu Kα radiation (λ = 1.5406 Å), operating at 45 kV and 40 mA, in the 10–90° scanning range. Thermogravimetric (TG) analyses were performed under a nitrogen atmosphere using a Seiko EXSTAR 7200 TG/DTA instrument (Chiba, Japan). TG and DTG curves were obtained from approximately 5 mg samples at a heating rate of 15 °C min−1 over a temperature range from room temperature to 850 °C.
Organic compounds in the aqueous phase were extracted using dichloromethane, and their volatile fractions were determined using a gas chromatography-mass spectrometry (GC-MS) instrument (Agilent 6890, CA, USA). A phenylmethyl siloxane capillary column (30 m × 0.25 mm i.d. × 0.25 mm film thickness, Agilent 19091S-433, CA, USA) was used for separation. The GC oven temperature was programmed as follows: initial temperature of 40 °C, held for 5 min, ramped to 170 °C at a rate of 2 °C/min, held for 5 min, ramped to 270 °C at a rate of 5 °C/min, held for 10 min, ramped to 280 °C at a rate of 5 °C/min, and held at this final temperature for 5 min. The injector temperature was 280 °C, and the injector was operated in split mode with a split ratio of 10:1 and a split flow rate of 9.9 mL/min. The total flow rate was 13.9 mL/min. Helium was used as the carrier gas. The end of the column was directly inserted into the ion source of an Agilent 5973 series mass selective detector, operating in electron impact ionization mode. Data were analyzed using G1035A software in conjunction with the National Institute of Standards and Technology (NIST) library. The Total Organic Carbon measurement of the aqueous phase was performed using a Shimadzu TOCL series TOC analyzer, which operates with a 680 °C combustion catalytic oxidation method. The Chemical Oxygen Demand (COD) of the aqueous phase obtained with HTC was measured using the closed reflux standard method: 5220 C. The pH of the aqueous phase was measured using a Mettler Toledo SE S470-K pH meter (Switzerland).
The elemental composition of the hydrochars was analyzed using a LECO CHNS-932 analyzer (MI, USA), with the oxygen content determined by difference. The proximate analysis (moisture, volatile matter, and ash content) of the hydrochars was performed according to ASTM D3174-12 and D3175-20 standards. The fixed carbon content (FC) was calculated using the following equation:
The higher heating value (HHV) of the hydrochars obtained from the Stevia rebaudiana was determined according to the Dulong formula (where C, H, O, N, and S represent the mass percentages) specified below:
The energy densification ratio (EDR) of hydrochars derived from the Stevia rebaudiana was determined using the following equation:
The energy yield (EY, %) of hydrochars obtained from the Stevia rebaudiana was determined using the following equation:
The following equation was used to determine reaction severity [24].
R0 is the reaction severity, and its unit is time (minutes); T is the HTC temperature (Celsius), and t is the residence time (minutes). This equation assumes that the reaction is first-order and that the temperature dependence follows the Arrhenius constant [25]. The severity factor (SF) is the logarithm of the reaction ordinate (log R0).
The fuel ratio (FR) value, which indicates the combustion quality of hydrochars obtained from the Stevia rebaudiana, was calculated using the following Equation:
3. Results and Discussion
3.1. Solid Phase Characteristics
3.1.1. Hydrochar Yields and Characteristics
The effects of temperature and duration on the yield of hydrochar derived from the Stevia rebaudiana are shown in Figure 1a. It was observed that the hydrochar yield increased slightly with increasing temperature and then decreased. The hydrochar yield was 45.0% (wt) at 185 °C for 60 min, while it decreased to 35.0% (wt) when the temperature was increased to 275 °C. The highest hydrochar yield was determined to be 45.7% (wt) at a temperature of 200 °C and a reaction time of 60 min. It was observed that increasing the temperature to 275 °C reduced hydrochar yields at all reaction times. The lowest hydrochar yield (35.0%) was obtained at a reaction time of 60 min at 275 °C. The decrease in hydrochar yields with increasing temperature and residence time can be explained by the enhanced degradation, solubility, and decomposition of organic compounds [26,27]. Figure 1b shows the hydrochar yields obtained from HTC with and without catalyst at a temperature of 215 °C and a reaction time of 60 min. Use of acrylic acid at a concentration of 0.25 mol L−1 resulted in an increase in hydrochar yield compared to the process without catalyst. However, as the concentration increased, the hydrochar yield decreased. The highest hydrochar yield was found to be 48.5% (by weight) at a concentration of 0.25 mol L−1, while the lowest yield was determined to be 44.4% (by weight) at a concentration of 1.00 mol L−1. The concentrations of acrylic acid (0.25, 0.50, and 1.00 mol L−1) were selected to represent low, medium, and high catalytic loadings within the typical range reported for organic acid-assisted HTC. Concentrations below 0.25 mol L−1 generally show negligible catalytic influence, whereas values above 1.0 mol L−1 may cause excessive acidity, equipment corrosion, and reduced operational safety. Thus, the chosen range provided a systematic basis to evaluate the effect of acrylic acid on hydrochar yield, structure, and liquid-phase composition under feasible and safe experimental conditions.
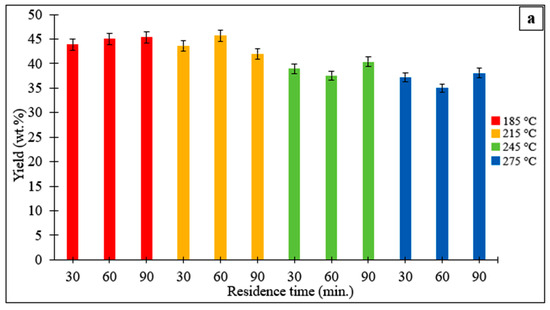
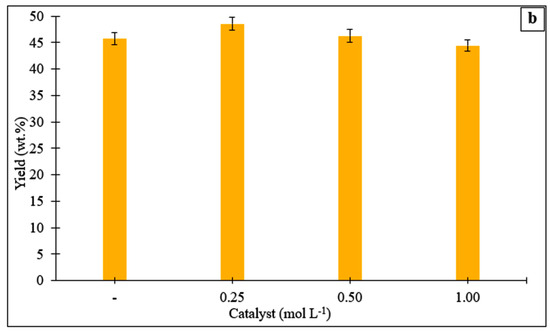
Figure 1.
(a) Yields of hydrochars obtained from the hydrothermal processing of the Stevia rebaudiana at different temperatures and reaction times. (b) Yields of hydrochars obtained from the hydrothermal conversion of the Stevia rebaudiana in the presence of a catalyst (acrylic acid) at a temperature of 215 °C and a reaction time of 60 min.
In addition to hydrochar yields, the properties of the obtained hydrochars for potential biofuel applications are also becoming increasingly important. The proximate and elemental analysis results of hydrochars obtained from the HTC of the Stevia rebaudiana are shown in Table 1. As expected, with increasing temperature, the percentage of volatile matter decreased while the percentage of fixed carbon increased as a result of carbonization. At higher temperatures or with longer residence times, the decomposition of organic matter leads to increased release of volatile compounds, which results in a rise in the percentage by weight of fixed carbon. Although Stevia rebaudiana waste is a plant-based agricultural lignocellulosic residue with a distinct chemical composition, it has demonstrated results comparable to those reported in previous studies on hydrothermal carbonization (HTC) [18,19,28]. Low volatile matter and high fixed carbon indicate improved combustion efficiency of hydrochar [29]. The highest fixed carbon content (34.27 wt%) was obtained at the highest temperature (275 °C) and the longest reaction time (90 min). The O/C atomic ratio of raw Stevia rebaudiana was 0.67; it decreased to 0.22 after the HTC process (215 °C and 60 min), indicating that the process provided deoxygenation. The O/C and H/C atomic ratios decreased significantly with increasing temperatures at longer reaction times. The H/C atomic ratios in hydrochar decreased with increasing holding time at all temperatures except 185 °C. It can be considered that long reaction times increase the aromatic content in hydrochar. Figure 2 presents a van Krevelen diagram in which the atomic H/C ratios are plotted against the atomic O/C ratios for the Stevia rebaudiana and the hydrochars derived from it. The resulting hydrochars are located near the coal region on the diagram, indicating their potential suitability as solid fuels. The higher heating values of hydrochar were higher than that of raw Stevia rebaudiana under all tested conditions. The higher heating value increased with increasing re-action time at all temperatures except 275 °C. All tested conditions exhibited a high degree of deoxygenation and dehydration. The higher heating value (HHV) of the raw Stevia rebaudiana biomass was determined to be 19.8 MJ kg−1, while the HHVs of the resulting hydrochars ranged from 26.95 to 36.61 MJ kg−1. The highest HHV was obtained at 275 °C with a reaction time of 60 min. The HHV values of the hydrochars produced in this study were consistent with those reported in the literature for hydrochars derived from agricultural lignocellulosic waste. For example, the higher heating value (HHV) of hazelnut shell obtained under HTC-250 conditions was found to be 32.03 MJ kg−1 [30]. Using the volatile matter (VM) to fixed carbon (FC) ratio (VM/FC) is an effective method for estimating H/C and O/C ratios in assessing biochar stability. A low VM/FC ratio (e.g., <0.88) indicates long-term stability, with a predicted biochar half-life of over 1000 years [31]. In this study, the FR values of hydrochars derived from Stevia rebaudiana biomass ranged from 0.28 to 0.58, indicating that most of the samples exhibited moderately stable biofuel characteristics. The highest FR value was obtained under the experimental conditions of 275 °C and 90 min of reaction time. The ash content of all the produced hydrochars is lower than that of the raw material. The ash content of the raw material was determined to be 7.46 wt%, while the ash contents of the hydrochars obtained from catalytic and thermal processes ranged between 1.68 wt% and 6.72 wt% (Table 1). The ash contents of the hydrochars examined in this study were found to be lower than those of various fossil-based coals [32,33]. Hydrochars are among the most promising solid biofuels within the scope of renewable energy sources, offering various environmental and technical advantages, particularly as alternatives to fossil coal. Their low ash content, high fixed carbon ratio, and low emission potential during combustion make them attractive candidates for use in energy production systems. Owing to their scalability, the application of hydrochars in boiler systems and thermal power plants not only provides integrated solutions for waste management but also contributes to reducing dependence on fossil fuels, thereby supporting sustainable energy policies. To fully evaluate the energy efficiency and environmental impacts of these fuels, advanced combustion analyses are essential. In this context, establishing a solid foundation for the integration of hydrothermal carbonization products into modern energy technologies becomes increasingly feasible.

Table 1.
HTC processing and composition of Stevia rebaudiana-based hydrochars with and without catalysts.
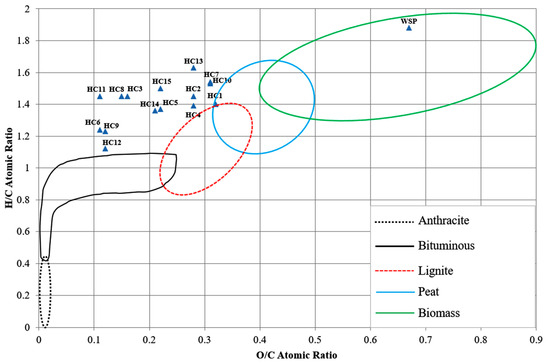
Figure 2.
Van Krevelen diagram of the waste Stevia rebaudiana (WS) and hydrochars (HC1–HC15) produced via hydrothermal carbonization using acrylic acid as a catalyst at 185, 215, 245, and 275 °C, under varying reaction times (30, 60, and 90 min) and catalyst.
3.1.2. Structural Characteristics of Hydrochars
The FT-IR spectra of the hydrochars presented in Figure 3 reveal the presence of functional groups observed in carbonized materials. Characteristic functional groups have been assigned and indicated within the spectra. Although no significant structural changes are observed with increasing catalyst concentration, the intensity and clarity of the peaks become more pronounced. Several characteristic vibration bands observed during hydrothermal carbonization indicate the occurrence of aromatization processes. In all spectra, the broad band around 3400 cm−1 corresponds to the O–H stretching of phenolic and aliphatic groups, while the band around 2900 cm−1 is attributed to the C–H stretching in methyl groups [34]. However, it was observed that the intensity of the absorption band at 1690 cm−1, corresponding to the carbonyl group, increased with the amount of acrylic acid in the sample; this confirms a higher degree of carboxylic groups in the hydrochars used as catalysts [16]. The peak at 1613 cm−1 is attributed to the stretching vibrations of aromatic C=C bonds, confirming the presence of polyphenols, which are known constituents of Stevia rebaudiana, while the peak at 1425 cm−1 corresponds to the CH bending vibrations of alkyl groups, and the peaks at 1077 cm−1 and 1033 cm−1 are assigned to the C–O stretching vibrations of carbohydrates and glycosides [35]. Overall, the FT-IR spectra indicate that, despite increasing catalyst concentrations, only minor intensity variations occur, while the chemical functionality of the hydrochars remains largely preserved. This suggests that the main transformations occurring during HTC are predominantly structural rather than involving significant changes in functional groups. These findings highlight the chemical stability of hydrochars under varying HTC conditions.
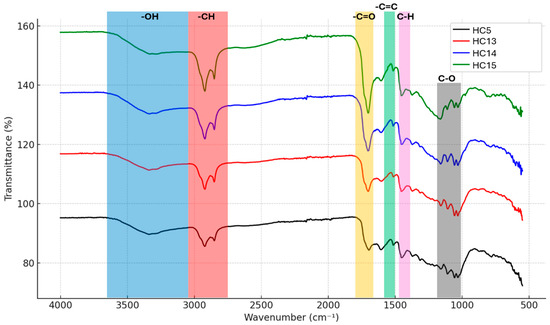
Figure 3.
FT-IR Spectra of hydrochars obtained from the hydrothermal carbonization of Stevia rebaudiana under catalytic (acrylic acid) and non-catalytic conditions (215 °C, 60 min).
XRD, a non-destructive technique for determining the crystalline structure of carbon-based materials, has been widely accepted. To evaluate the structural transformation of biomass derived from the Stevia rebaudiana after hydrothermal carbonization (HTC), XRD patterns of hydrochars synthesized at 215 °C for 60 min with varying concentrations of acrylic acid (AA) were analyzed and are presented in Figure 4. The samples coded as HC5 (without catalyst), HC13 (0.25 mol L−1 AA), HC14 (0.50 mol L−1 AA), and HC15 (1.00 mol L−1 AA) exhibit broad and low-intensity diffraction peaks, particularly around 2θ ≈ 22°. These patterns are characteristic of carbonaceous materials with amorphous structures, rather than well-ordered graphitic crystals [36]. Baysal et al. [37] and Sonilbare et al. [38] have stated that aromatization and carbonization reactions may be the main reasons for the transformation from the crystalline phase to the amorphous phase. In the catalyst-free sample (HC5), the peaks are notably broad and of low intensity, indicating that the carbon structures formed during HTC are largely disordered and lack crystalline phases. As the concentration of acrylic acid increases, the diffraction peaks in the XRD patterns become narrower and more distinct. This is particularly evident in samples HC14 and HC15, where increased peak intensity suggests that acrylic acid promotes partial graphitization and a more organized carbon framework [16,39]. Among the samples, HC15 displays the most developed diffraction pattern with semi-crystalline characteristics and enhanced aromatic structures. This outcome highlights the catalytic role of AA during the HT process and its contribution to improving the structural organization within the carbon matrix. It is well known that organic acids, particularly carboxylic groups, support dehydration and cross-linking mechanisms during carbonization [40]. Nonetheless, the absence of sharp peaks corresponding to crystalline phases in all patterns indicates that the resulting hydrochars are predominantly amorphous or semi-crystalline. Such structures are advantageous for environmental applications (e.g., adsorbent catalyst supports) due to their high surface area, abundance of functional groups, and porosity [41].
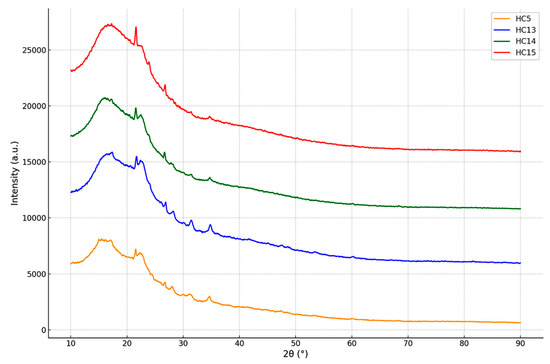
Figure 4.
XRD patterns of hydrochars (sample codes HC5, HC13, HC14, and HC15 as referenced in Table 1).
Scanning electron microscopy (SEM) images of raw Stevia rebaudiana biomass and hydrochars produced via hydrothermal carbonization (HTC) at 215 °C for 60 min with different concentrations of acrylic acid (AA) are shown in Figure 5. These images offer valuable insights into the surface morphology and structural changes of the biomass under various catalytic conditions. The untreated biomass (WS) displays a well-preserved fibrous structure, characterized by elongated, compact vascular bundles and a porous cellular matrix typical of lignocellulosic materials. After HTC treatment without a catalyst (HC5), significant morphological degradation occurs. The original fibrous architecture collapses, resulting in irregular, fused aggregates with little structural order, indicative of thermal decomposition and carbon matrix shrinkage caused by dehydration and devolatilization reactions [42]. With the addition of acrylic acid as a catalytic agent, significant morphological transformations are observed. In HC13 (0.25 mol L−1 AA), uniformly distributed microspheres appear across the surface, likely formed through localized nucleation during carbonization. These spherical structures become more prominent in HC14 (0.50 mol L−1 AA), where a hierarchically porous network embedded with well-defined carbon spheres is evident. This progression indicates that moderate concentrations of AA promote dehydration, crosslinking, and aromatization reactions, resulting in a more organized and porous carbon framework [43,44]. At the highest concentration (HC15, 1.00 mol L−1 AA), the surface morphology is densely populated with aggregated spherical particles, forming a continuous carbon matrix with reduced macroporosity. The increase in particle size and compaction corresponds to enhanced carbon ordering, which has previously been linked to the catalytic effects of carboxylic acids in promoting condensation and cyclization reactions during HTC. Additionally, the observed surface roughness and particle cohesion suggest the formation of a denser carbonaceous phase, which may affect the hydrochar’s physicochemical properties, such as the surface area, conductivity, and adsorption performance. The progressive morphological refinement observed with increasing AA concentration confirms the catalytic role of organic acids, not only in tuning chemical structure but also in directing particle morphology during HTC. These spherical carbon architectures have been reported to enhance performance in environmental applications, such as adsorption, capacitive energy storage, and catalyst support systems, owing to their tunable surface functionality and structural stability [45,46].
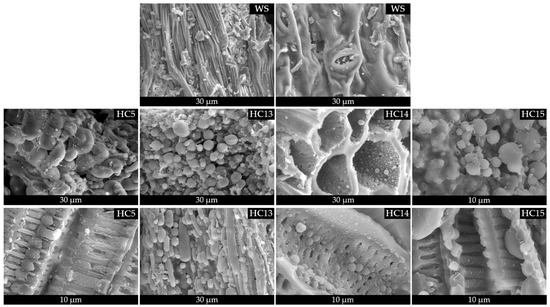
Figure 5.
SEM images showing the morphological evolution of Stevia rebaudiana derived hydrochars obtained without catalyst (HC5) and with increasing acrylic acid concentrations (HC13–HC15), compared to raw biomass (WS).
Similar to our findings with acrylic acid-assisted HTC, recent studies have also confirmed that acid treatment can substantially improve the surface chemistry and morphology of carbonaceous materials. For instance, Zhang et al. demonstrated that hydrochloric acid activation of sycamore bark-derived biochar significantly increased surface area, porosity, and oxygen-containing functional groups, thereby enhancing adsorption capacity toward microplastics [47]. These results highlight the common role of acidic environments in promoting structural reorganization and functionalization of biochars, which is consistent with the morphological and chemical improvements observed in acrylic acid-catalyzed hydrochars in this study.
3.1.3. Thermal Stability of Hydrochars
The thermal behavior of hydrochars derived from Stevia rebaudiana was examined using thermogravimetric analysis (TGA) and derivative thermogravimetric analysis (DTG) to assess the impact of varying acrylic acid (AA) concentrations after hydrothermal carbonization (HTC) at 215 °C for 60 min. The TGA and DTG curves for the samples labeled HC5 (no catalyst), HC13 (0.25 mol L−1 AA), HC14 (0.50 mol L−1 AA), and HC15 (1.00 mol L−1 AA) are shown in Figure 6. The active decomposition region lies between 200 and 500 °C, during which the weak bonds within the polymeric structures of lignocellulosic biomass components—namely hemicellulose, cellulose, and lignin—undergo thermal cleavage [48]. As expected, moisture loss occurred between 25 and 100 °C. The second thermal degradation stage took place around 275 °C, with a shoulder appearing in all samples—more pronounced in the DT sample [49]. The second stage (~200–400 °C) is attributed to the degradation of hemicellulose and volatile components, and the third stage (~400–600 °C) reflects the decomposition of condensed aromatic structures [50,51,52,53]. The primary decomposition of cellulose occurs at approximately 350 °C, where a distinct peak indicating the maximum degradation rate is observed [49]. HC13 and HC14 exhibited greater mass losses, while HC15 showed the least degradation and the highest residual carbon content. DTG curves indicated that HC15 experienced a delayed and less intense decomposition rate, suggesting enhanced thermal stability. These results confirm that higher concentrations of acrylic acid promote the formation of more condensed and thermally robust carbon structures, supporting its catalytic role during HTC.
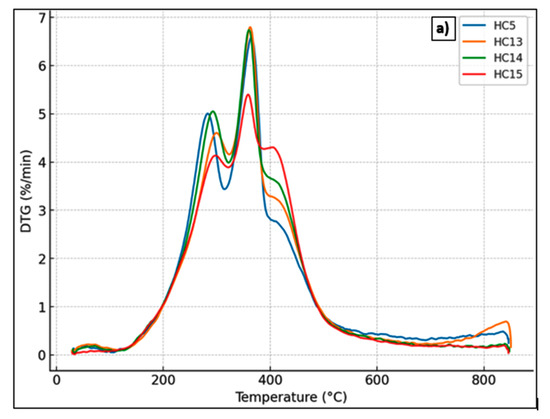
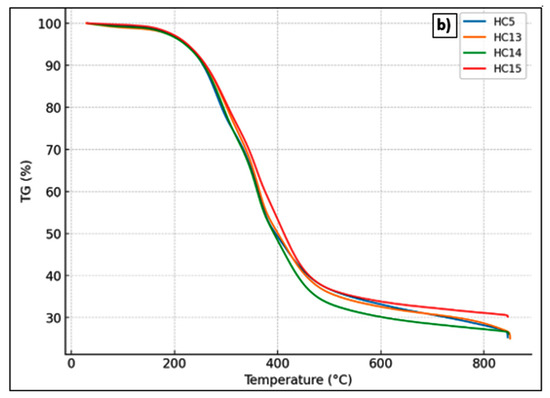
Figure 6.
(a) DTG curves and (b) TG curves of hydrochars produced from Stevia rebaudiana by hydrothermal carbonization at 215 °C for 60 min: HC5 (without catalyst), HC13 (0.25 mol L−1 AA), HC14 (0.50 mol L−1 AA), and HC15 (1.00 mol L−1 AA).
3.2. Aqueous Phase Characteristics
As shown in Table 1, the pH values of the aqueous phase resulting from the hydrothermal carbonization (HTC) of Stevia rebaudiana biomass ranged from 3.30 to 5.32, depending on process parameters such as temperature, reaction time, and acrylic acid concentration. No clear or consistent trend was observed in pH variation across the different experimental conditions. However, in samples treated with acrylic acid, the pH decreased proportionally with increasing acid concentration.
Table 2 presents the compounds identified by GC-MS in the organic fraction extracted with dichloromethane from the aqueous phase obtained after hydrothermal carbonization, along with their corresponding retention times. GC-MS results indicate that the presence and concentration of acrylic acid catalyst in the aqueous phases obtained by hydrothermal carbonization of Stevia rebaudiana significantly influence the type and distribution of the resulting organic compounds. Under catalyst-free conditions (HC5), the product profile is largely limited to phenolic derivatives (e.g., 19.47% phenol) and simple cyclic ketones, while the addition of the catalyst not only preserves these structures but also leads to the formation of new functional groups. The percentage of phenolic compounds increases significantly with acrylic acid. The phenol compound, which is 19.47% under catalyst-free conditions, nearly doubles to 40.92% in the presence of 1.00 mol L−1 acrylic acid. During the hydrothermal process, acidic conditions lead to both the depolymerization and repolymerization of lignin [54]. As a result, the formation of phenolic compounds is considered an indicator of lignin degradation. In particular, conjugated carbonyl compounds such as Ethanone, 1-(2-furanyl)-; 2-Methyl-2-cyclopenten-1-one, and 2-cyclohexen-1-one are formed in HC13, while 1-Proline derivatives, nitrogenous heterocyclic compounds, ketones, and esters are formed in the HC14 sample; in HC15, the percentage of phenolic compounds reaches its maximum values. This indicates that acrylic acid promotes the formation and stabilization of aromatic rings during the HTC process and also enhances acid-catalyzed dehydration, condensation, and esterification reactions, thereby expanding structural diversity [16,23]. Additionally, the identification of phenolic structures and furan derivatives confirms the occurrence of dehydration and decarboxylation reactions during the carbonization process, which is also consistent with the results presented in the van Krevelen diagram [55]. However, high concentrations of acrylic acid may suppress some compounds or cause such structures to become unstable due to the acidity of the reaction medium. Furthermore, the diversity of compounds such as carboxylic esters, furan derivatives, and cyclic ketones increases, especially when comparing HC13-HC15, demonstrating that acrylic acid promotes complex rearrangement reactions in the HTC environment. Moreover, phenolic compounds derived from plant-based biomass are considered valuable and renewable raw materials for the production of phenolic precursors, polymer derivatives, carbon fibers, natural antioxidants, and green aromatic-based chemicals [56]. Consequently, acrylic acid effectively guides the product range by lowering the pH of the medium and altering reaction pathways, thus clearly demonstrating its effectiveness as a catalyst that significantly contributes to both the product profile and chemical diversity.

Table 2.
GC-MS identified compounds in the aqueous phase obtained from the hydrothermal carbonization of Stevia rebaudiana at 215 °C for 60 min: HC5 (no catalyst), HC13 (0.25 mol L−1 AA), HC14 (0.50 mol L−1 AA), and HC15 (1.00 mol L−1 AA).
Table 3 presents the Total Organic Carbon (TOC) and Chemical Oxygen Demand (COD) values of the aqueous phase obtained from the hydrothermal carbonization (HTC) of Stevia rebaudiana biomass at different temperatures and acrylic acid concentrations. These results indicate that, during the HTC process carried out in the presence of acrylic acid, a significant portion of dissolved organic compounds is transferred into the liquid phase as a result of biomass hydrolysis and thermal degradation. It should be noted that although the aqueous phase contained high-value phenolic compounds, the COD levels were extremely high (up to 113,920 mg/L), indicating a heavy organic load. This highlights a potential environmental drawback, as such effluents cannot be directly discharged and would require further treatment. Therefore, any valorization strategy for the aqueous fraction must consider both the recovery of valuable compounds and the necessity of appropriate wastewater management. Although this organic-rich aqueous phase may pose challenges for conventional wastewater treatment systems, the soluble compounds it contains also represent valuable resources that can potentially be recovered through sustainable approaches such as anaerobic digestion, biogas production, or nutrient recovery processes.

Table 3.
Total Organic Carbon (TOC) and Chemical Oxygen Demand (COD) values of the aqueous phase obtained after hydrothermal carbonization of Stevia rebaudiana biomass at different temperatures and acrylic acid concentrations.
4. Conclusions
This study demonstrated the catalytic role of acrylic acid in the hydrothermal carbonization (HTC) of Stevia rebaudiana biomass under moderate conditions (215 °C, 60 min). The use of acrylic acid at different concentrations significantly influenced both the yield and properties of the hydrochars. The highest hydrochar yield (48.5%) was obtained at 0.25 mol L−1 AA, while the highest HHV (32.20 MJ kg−1) and thermal stability were achieved at 1.00 mol L−1 AA (HC15). Although the non-catalytic sample showed relatively higher fixed carbon content, the addition of acrylic acid promoted deoxygenation, aromatization, and partial graphitization, resulting in improved energy values and enhanced structural features. FT-IR analysis confirmed the increased aromaticity of the hydrochars, and XRD analysis indicated partial graphitization, while SEM revealed the formation of more uniform microspheres and porous structures at higher acid concentrations. Thermogravimetric analysis further supported the enhanced stability of the catalytically produced hydrochars.
In the liquid phase, acrylic acid catalysis led to higher organic carbon concentrations and a notable increase in phenolic derivatives. GC-MS analysis revealed that phenol content rose from 19.47% in the absence of catalyst to 40.92% in the presence of 1.00 mol L−1 AA. This effect can be attributed to the carboxyl group of acrylic acid facilitating dehydration and decarboxylation reactions, while its unsaturated double bond favors condensation and aromatic stabilization.
Overall, these findings indicate that acrylic acid-assisted HTC provides a promising approach for the dual valorization of Stevia reb biomass by producing hydrochars with enhanced fuel properties and aqueous phases enriched in phenolic compounds. While the improvements are clear compared to the non-catalytic condition, further studies are required to benchmark these results against other biomass feedstocks and catalytic systems.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Mignogna, D.; Szabó, M.; Ceci, P.; Avino, P. Biomass energy and biofuels: Perspective, potentials, and challenges in the energy transition. Sustainability 2024, 16, 7036. [Google Scholar] [CrossRef]
- Alper, K.; Tekin, K.; Karagöz, S.; Ragauskas, A.J. Sustainable energy and fuels from biomass: A review focusing on hydrothermal biomass processing. Sustain. Energy Fuels 2024, 4, 4390–4414. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuel Bioprod. Bior. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Wang, R.; Jia, J.; Jin, Q.; Chen, H.; Liu, H.; Yin, Q.; Zhao, Z. Forming mechanism of coke microparticles from polymerization of aqueous organics during hydrothermal carbonization process of biomass. Carbon 2022, 192, 50–60. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Emmerich, K.H. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, S.; Dhyani, D.; Ahuja, P.S. A review on the improvement of stevia [Stevia rebaudiana(Bertoni)]. Can. J. Plant Sci. 2011, 91, 1–27. [Google Scholar] [CrossRef]
- Tavarini, S.; Angelini, L.G. Stevia rebaudiana Bertoni as a source of bioactive compounds: The effect of harvest time, experimental site and crop age on steviol glycoside content and antioxidant properties. J. Sci. Food Agric. 2013, 93, 2121–2129. [Google Scholar] [CrossRef]
- Yildiz, M.; Karhan, M. Characteristics of some beverages adjusted with stevia extract, and persistence of steviol glycosides in the mouth after consumption. Int. J. Gastron. Food Sci. 2021, 24, 100326. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Zura-Bravo, L.; Ah-Hen, K. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef]
- Yokoyama, J.T.; Cazetta, A.L.; Bedin, K.C.; Spessato, L.; Fonseca, J.M.; Carraro, P.S.; Almeida, V.C. Stevia residue as new precursor of CO2-activated carbon: Optimization of preparation condition and adsorption study of triclosan. Ecotoxicol. Environ. Saf. 2019, 172, 403–410. [Google Scholar] [CrossRef]
- Zhang, B.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal carbonization of fruit wastes: A promising technique for generating hydrochar. Energies 2018, 11, 2022. [Google Scholar] [CrossRef]
- Chen, W.H.; Cheng, C.L.; Lee, K.T.; Lam, S.S.; Ong, H.C.; Ok, Y.S.; Hsieh, T.H. Catalytic level identification of ZSM-5 on biomass pyrolysis and aromatic hydrocarbon formation. Chemosphere 2021, 271, 129510. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, X.; Zhang, L.; Wang, H.; Chen, J.; Hong, L.; Wu, K. Co-pyrolysis of Stevia rebaudiana straw and polystyrene: A study on biochars production and characterization. Biomass Convers. Biorefin. 2024, 14, 31501–31512. [Google Scholar] [CrossRef]
- Thakur, B.K.; Sharma, S.; Sharma, A.; Singh, K.K.; Pal, P.K. Integration of biochar with nitrogen in acidic soil: A strategy to sequester carbon and improve the yield of stevia via altering soil properties and nutrient recycling. J. Environ. Manag. 2023, 345, 118872. [Google Scholar] [CrossRef]
- Rasaq, W.A.; Okpala, C.O.R.; Igwegbe, C.A.; Białowiec, A. Catalyst-enhancing hydrothermal carbonization of biomass for hydrochar and liquid fuel production—A review. Materials 2024, 17, 2579. [Google Scholar] [CrossRef] [PubMed]
- Demir-Cakan, R.; Baccile, N.; Antonietti, M.; Titirici, M.M. Carboxylate-rich carbonaceous materials via one-step hydrothermal carbonization of glucose in the presence of acrylic acid. Chem. Mater. 2009, 21, 484–490. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Zielinska, B.; Felix, L. Hydrothermal carbonization (HTC) of selected woody and herbaceous biomass feedstocks. Biomass Convers. Biorefin 2013, 3, 113–126. [Google Scholar] [CrossRef]
- Iryani, D.A.; Kumagai, S.; Nonaka, M.; Sasaki, K.; Hirajima, T. Characterization and production of solid biofuel from sugarcane bagasse by hydrothermal carbonization. Waste Biomass Valori. 2017, 8, 1941–1951. [Google Scholar] [CrossRef]
- Martinez, C.L.M.; Sermyagina, E.; Saari, J.; de Jesus, M.S.; Cardoso, M.; de Almeida, G.M.; Vakkilainen, E. Hydrothermal carbonization of lignocellulosic agro-forest based biomass residues. Biomass Bioenergy 2021, 147, 106004. [Google Scholar] [CrossRef]
- Mohammed, I.S.; Na, R.; Kushima, K.; Shimizu, N. Investigating the effect of processing parameters on the products of hydrothermal carbonization of corn stover. Sustainability 2020, 12, 5100. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Cui, D.; Sun, H.; Yin, H.; Xu, F.; Wang, Z. Evaluation of fuel properties and combustion behaviour of hydrochar derived from hydrothermal carbonisation of agricultural wastes. J. Energy Inst. 2023, 108, 101209. [Google Scholar] [CrossRef]
- Qi, X.; Lian, Y.; Yan, L.; Smith Jr, R.L. One-step preparation of carbonaceous solid acid catalysts by hydrothermal carbonization of glucose for cellulose hydrolysis. Catal. Commun. 2014, 57, 50–54. [Google Scholar] [CrossRef]
- Gan, L.; Zhu, J.; Lv, L. Cellulose hydrolysis catalyzed by highly acidic lignin-derived carbonaceous catalyst synthesized via hydrothermal carbonization. Cellulose 2017, 24, 5327–5339. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. London Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Leland, A.; Felix, L. Hydrothermal carbonization (HTC) of biomass for energy applications. In Biomass Preprocessing and Pretreatments for Production of Biofuels; CRC Press: Boca Raton, FL, USA, 2018; pp. 196–254. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Pandey, D.S.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonisation of poultry litter: Effects of treatment temperature and residence time on yields and chemical properties of hydrochars. Bioresour. Technol. 2016, 216, 373–380. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Xie, W.; Wang, Y.; Kang, J. Effects of temperature, time and acidity of hydrothermal carbonization on the hydrochar properties and nitrogen recovery from corn stover. Biomass Bioenergy 2019, 122, 175–182. [Google Scholar] [CrossRef]
- Smith, A.M.; Ross, A.B. The influence of residence time during hydrothermal carbonisation of miscanthus on bio-coal combustion chemistry. Energies 2019, 12, 523. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Pavkov, I.; Radojčin, M.; Stamenković, Z.; Bikić, S.; Tomić, M.; Bukurov, M.; Despotović, B. Hydrothermal carbonization of agricultural biomass: Characterization of hydrochar for energy production. Solid Fuel Chem. 2022, 56, 225–235. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H.; Li, H.; Li, J.; Zhou, W. Biochar stability assessment methods: A review. Sci. Total Environ. 2019, 647, 210–222. [Google Scholar] [CrossRef]
- Das, B.; Suresh, A.; Dash, P.S.; Chandra, S.; Díaz, M.C.; Stevens, L.A.; Snape, C.E. Understanding the unusual fluidity characteristics of high ash Indian bituminous coals. Fuel Process. Technol. 2018, 176, 258–266. [Google Scholar] [CrossRef]
- Song, B.; Zhai, X.; Ma, T.; Wang, B.; Hao, L.; Zhou, Y. Effect of water immersion on pore structure of bituminous coal with different metamorphic degrees. Energy 2023, 274, 127449. [Google Scholar] [CrossRef]
- Pourbaba, R.; Abdulkhani, A.; Rashidi, A.; Ashori, A.; Braving, A. Sustainable production of hierarchically porous carbon from lignin-acrylic acid copolymers. J. Polym. Environ. 2024, 32, 2660–2678. [Google Scholar] [CrossRef]
- Elabbasy, M.T.; El Bayomi, R.M.; Abdelkarim, E.A.; Hafez, A.E.S.E.; Othman, M.S.; Ghoniem, M.E.; Hussein, M.A. Harnessing stevia rebaudiana for zinc oxide nanoparticle green synthesis: A sustainable solution to combat multidrug-resistant bacterial pathogens. Nanomater 2025, 15, 369. [Google Scholar] [CrossRef]
- Wang, L.; Lü, K.; Chang, Y.; Cao, X.; Huo, Q. Mesoporous carbon material prepared from sewage sludge hydrochar using Pluronic F127 as template for efficient removal of phenolic compounds: Experimental study and mechanism interpretation via advanced statistical physics model. J. Environ. Manag. 2023, 326, 116841. [Google Scholar] [CrossRef] [PubMed]
- Baysal, M.; Yürüm, A.; Yıldız, B.; Yürüm, Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. [Google Scholar] [CrossRef]
- Sonibare, O.O.; Haeger, T.; Foley, S.F. Structural characterization of Nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy. Energy 2010, 35, 5347–5353. [Google Scholar] [CrossRef]
- Lu, X.; Jordan, B.; Berge, N.D. Thermal conversion of municipal solid waste via hydrothermal carbonization: Comparison of carbonization products to products from current waste management techniques. Waste Manag. 2012, 32, 1353–1365. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal carbonization of biomass for energy and crop production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Zhao, P.; Shen, Y.; Ge, S.; Yoshikawa, K. Energy recycling from sewage sludge by producing solid biofuel with hydrothermal carbonization. Energy Convers. Manag. 2014, 78, 815–821. [Google Scholar] [CrossRef]
- Pala, M.; Kantarli, I.C.; Buyukisik, H.B.; Yanik, J. Hydrothermal carbonization and torrefaction of grape pomace: A comparative evaluation. Bioresour. Technol. 2014, 161, 255–262. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhang, H.; Shao, L.M.; Lü, F.; He, P.J. Preparation and application of hierarchical porous carbon materials from waste and biomass: A review. Waste Biomass Valori. 2021, 12, 1699–1724. [Google Scholar] [CrossRef]
- Waribam, P.; Ngo, S.D.; Tran, T.T.V.; Kongparakul, S.; Reubroycharoen, P.; Chanlek, N.; Samart, C. Waste biomass valorization through production of xylose-based porous carbon microspheres for supercapacitor applications. Waste Manag. 2020, 105, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, D.; Liu, Z.; Xu, D.; Yang, F.; Tang, Q.; Jia, Q. From waste to Resource: Engineering biochar through optimized HCl activation for microplastic mitigation. Chem. Eng. Sci. 2025, 304, 121091. [Google Scholar] [CrossRef]
- Mahdi, T.S.; Elhafiz, D.R.A.; Helal, N.M.; Akkad, S.S.E. Hydrothermal conversion of mango wood wastes and sugarcane bagasse for biofuel production. Biomass Convers. Biorefin. 2024, 1–15. [Google Scholar] [CrossRef]
- Jusic, J.; Giannoni, T.; Zikeli, F.; Tamantini, S.; Pierpaoli, V.; Barbanera, M.; Romagnoli, M. Chemical and thermogravimetric characterization of chestnut tree biomass waste: Towards sustainable resource utilization in the tannin industry. Eur. J. Wood Wood Prod. 2025, 83, 113. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.; Kim, H.J.; Yang, H.S. Thermal properties of bio-flour-filled polyolefin composites with different compatibilizing agent type and content. Thermochim. Acta 2006, 451, 181–188. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M. Structural differences between wood species: Evidence from chemical composition, FTIR spectroscopy, and thermogravimetric analysis. J. Appl. Polym. Sci. 2012, 126, E337–E344. [Google Scholar] [CrossRef]
- Wikberg, H.; Ohra-aho, T.; Pileidis, F.; Titirici, M.M. Structural and morphological changes in kraft lignin during hydrothermal carbonization. ACS Sustain. Chem. Eng. 2015, 3, 2737–2745. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Nowicki, P.; Nosal-Wiercińska, A.; Pietrzak, R.; Szewczuk-Karpisz, K.; Ostolska, I.; Sternik, D. Adsorption of poly (acrylic acid) on the surface of microporous activated carbon obtained from cherry stones. Colloids Surf. A Physicochem. Eng. 2017, 514, 137–145. [Google Scholar] [CrossRef]
- Garrote, G.D.H.P.; Dominguez, H.; Parajó, J.C. Hydrothermal processing of lignocellulosic materials. Holz Als Roh-Und Werkst. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.M.; Baré, W.; Bilodeau, M. Depolymerization of steam-treated lignin for the production of green chemicals. Bioresour. Technol. 2011, 102, 4917–4920. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).