Effect of Acrylic Acid Concentration on the Hydrothermal Carbonization of Stevia rebaudiana Biomass and Resulting Hydrochar Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Metarials
2.2. HTC Experiments
2.3. Analytical Methods
3. Results and Discussion
3.1. Solid Phase Characteristics
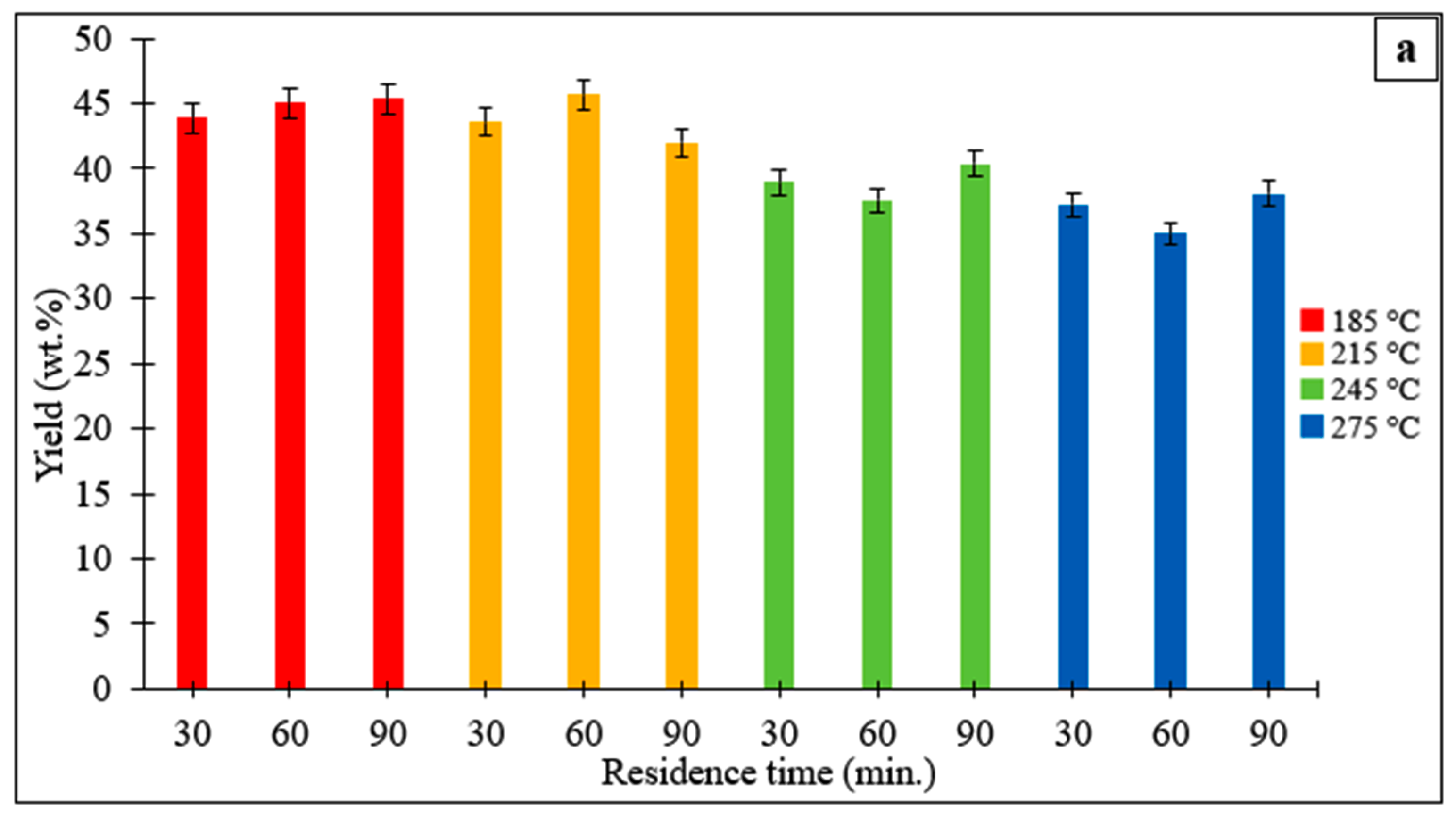
3.1.1. Hydrochar Yields and Characteristics
3.1.2. Structural Characteristics of Hydrochars
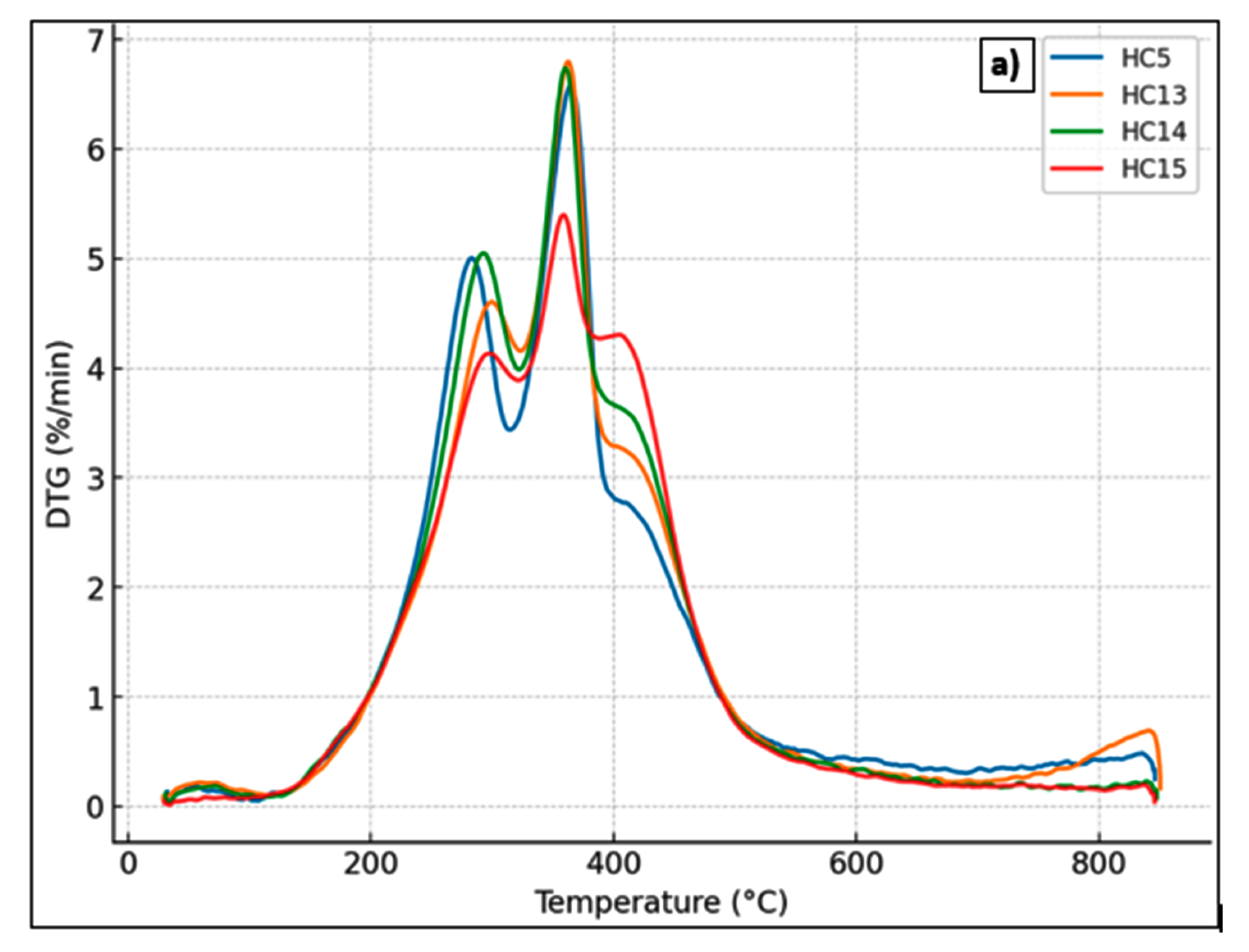
3.1.3. Thermal Stability of Hydrochars
3.2. Aqueous Phase Characteristics
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mignogna, D.; Szabó, M.; Ceci, P.; Avino, P. Biomass energy and biofuels: Perspective, potentials, and challenges in the energy transition. Sustainability 2024, 16, 7036. [Google Scholar] [CrossRef]
- Alper, K.; Tekin, K.; Karagöz, S.; Ragauskas, A.J. Sustainable energy and fuels from biomass: A review focusing on hydrothermal biomass processing. Sustain. Energy Fuels 2024, 4, 4390–4414. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuel Bioprod. Bior. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Wang, R.; Jia, J.; Jin, Q.; Chen, H.; Liu, H.; Yin, Q.; Zhao, Z. Forming mechanism of coke microparticles from polymerization of aqueous organics during hydrothermal carbonization process of biomass. Carbon 2022, 192, 50–60. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Emmerich, K.H. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, S.; Dhyani, D.; Ahuja, P.S. A review on the improvement of stevia [Stevia rebaudiana(Bertoni)]. Can. J. Plant Sci. 2011, 91, 1–27. [Google Scholar] [CrossRef]
- Tavarini, S.; Angelini, L.G. Stevia rebaudiana Bertoni as a source of bioactive compounds: The effect of harvest time, experimental site and crop age on steviol glycoside content and antioxidant properties. J. Sci. Food Agric. 2013, 93, 2121–2129. [Google Scholar] [CrossRef]
- Yildiz, M.; Karhan, M. Characteristics of some beverages adjusted with stevia extract, and persistence of steviol glycosides in the mouth after consumption. Int. J. Gastron. Food Sci. 2021, 24, 100326. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Zura-Bravo, L.; Ah-Hen, K. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef]
- Yokoyama, J.T.; Cazetta, A.L.; Bedin, K.C.; Spessato, L.; Fonseca, J.M.; Carraro, P.S.; Almeida, V.C. Stevia residue as new precursor of CO2-activated carbon: Optimization of preparation condition and adsorption study of triclosan. Ecotoxicol. Environ. Saf. 2019, 172, 403–410. [Google Scholar] [CrossRef]
- Zhang, B.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal carbonization of fruit wastes: A promising technique for generating hydrochar. Energies 2018, 11, 2022. [Google Scholar] [CrossRef]
- Chen, W.H.; Cheng, C.L.; Lee, K.T.; Lam, S.S.; Ong, H.C.; Ok, Y.S.; Hsieh, T.H. Catalytic level identification of ZSM-5 on biomass pyrolysis and aromatic hydrocarbon formation. Chemosphere 2021, 271, 129510. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, X.; Zhang, L.; Wang, H.; Chen, J.; Hong, L.; Wu, K. Co-pyrolysis of Stevia rebaudiana straw and polystyrene: A study on biochars production and characterization. Biomass Convers. Biorefin. 2024, 14, 31501–31512. [Google Scholar] [CrossRef]
- Thakur, B.K.; Sharma, S.; Sharma, A.; Singh, K.K.; Pal, P.K. Integration of biochar with nitrogen in acidic soil: A strategy to sequester carbon and improve the yield of stevia via altering soil properties and nutrient recycling. J. Environ. Manag. 2023, 345, 118872. [Google Scholar] [CrossRef]
- Rasaq, W.A.; Okpala, C.O.R.; Igwegbe, C.A.; Białowiec, A. Catalyst-enhancing hydrothermal carbonization of biomass for hydrochar and liquid fuel production—A review. Materials 2024, 17, 2579. [Google Scholar] [CrossRef] [PubMed]
- Demir-Cakan, R.; Baccile, N.; Antonietti, M.; Titirici, M.M. Carboxylate-rich carbonaceous materials via one-step hydrothermal carbonization of glucose in the presence of acrylic acid. Chem. Mater. 2009, 21, 484–490. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Zielinska, B.; Felix, L. Hydrothermal carbonization (HTC) of selected woody and herbaceous biomass feedstocks. Biomass Convers. Biorefin 2013, 3, 113–126. [Google Scholar] [CrossRef]
- Iryani, D.A.; Kumagai, S.; Nonaka, M.; Sasaki, K.; Hirajima, T. Characterization and production of solid biofuel from sugarcane bagasse by hydrothermal carbonization. Waste Biomass Valori. 2017, 8, 1941–1951. [Google Scholar] [CrossRef]
- Martinez, C.L.M.; Sermyagina, E.; Saari, J.; de Jesus, M.S.; Cardoso, M.; de Almeida, G.M.; Vakkilainen, E. Hydrothermal carbonization of lignocellulosic agro-forest based biomass residues. Biomass Bioenergy 2021, 147, 106004. [Google Scholar] [CrossRef]
- Mohammed, I.S.; Na, R.; Kushima, K.; Shimizu, N. Investigating the effect of processing parameters on the products of hydrothermal carbonization of corn stover. Sustainability 2020, 12, 5100. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Cui, D.; Sun, H.; Yin, H.; Xu, F.; Wang, Z. Evaluation of fuel properties and combustion behaviour of hydrochar derived from hydrothermal carbonisation of agricultural wastes. J. Energy Inst. 2023, 108, 101209. [Google Scholar] [CrossRef]
- Qi, X.; Lian, Y.; Yan, L.; Smith Jr, R.L. One-step preparation of carbonaceous solid acid catalysts by hydrothermal carbonization of glucose for cellulose hydrolysis. Catal. Commun. 2014, 57, 50–54. [Google Scholar] [CrossRef]
- Gan, L.; Zhu, J.; Lv, L. Cellulose hydrolysis catalyzed by highly acidic lignin-derived carbonaceous catalyst synthesized via hydrothermal carbonization. Cellulose 2017, 24, 5327–5339. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. London Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Leland, A.; Felix, L. Hydrothermal carbonization (HTC) of biomass for energy applications. In Biomass Preprocessing and Pretreatments for Production of Biofuels; CRC Press: Boca Raton, FL, USA, 2018; pp. 196–254. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Pandey, D.S.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonisation of poultry litter: Effects of treatment temperature and residence time on yields and chemical properties of hydrochars. Bioresour. Technol. 2016, 216, 373–380. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Xie, W.; Wang, Y.; Kang, J. Effects of temperature, time and acidity of hydrothermal carbonization on the hydrochar properties and nitrogen recovery from corn stover. Biomass Bioenergy 2019, 122, 175–182. [Google Scholar] [CrossRef]
- Smith, A.M.; Ross, A.B. The influence of residence time during hydrothermal carbonisation of miscanthus on bio-coal combustion chemistry. Energies 2019, 12, 523. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Pavkov, I.; Radojčin, M.; Stamenković, Z.; Bikić, S.; Tomić, M.; Bukurov, M.; Despotović, B. Hydrothermal carbonization of agricultural biomass: Characterization of hydrochar for energy production. Solid Fuel Chem. 2022, 56, 225–235. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H.; Li, H.; Li, J.; Zhou, W. Biochar stability assessment methods: A review. Sci. Total Environ. 2019, 647, 210–222. [Google Scholar] [CrossRef]
- Das, B.; Suresh, A.; Dash, P.S.; Chandra, S.; Díaz, M.C.; Stevens, L.A.; Snape, C.E. Understanding the unusual fluidity characteristics of high ash Indian bituminous coals. Fuel Process. Technol. 2018, 176, 258–266. [Google Scholar] [CrossRef]
- Song, B.; Zhai, X.; Ma, T.; Wang, B.; Hao, L.; Zhou, Y. Effect of water immersion on pore structure of bituminous coal with different metamorphic degrees. Energy 2023, 274, 127449. [Google Scholar] [CrossRef]
- Pourbaba, R.; Abdulkhani, A.; Rashidi, A.; Ashori, A.; Braving, A. Sustainable production of hierarchically porous carbon from lignin-acrylic acid copolymers. J. Polym. Environ. 2024, 32, 2660–2678. [Google Scholar] [CrossRef]
- Elabbasy, M.T.; El Bayomi, R.M.; Abdelkarim, E.A.; Hafez, A.E.S.E.; Othman, M.S.; Ghoniem, M.E.; Hussein, M.A. Harnessing stevia rebaudiana for zinc oxide nanoparticle green synthesis: A sustainable solution to combat multidrug-resistant bacterial pathogens. Nanomater 2025, 15, 369. [Google Scholar] [CrossRef]
- Wang, L.; Lü, K.; Chang, Y.; Cao, X.; Huo, Q. Mesoporous carbon material prepared from sewage sludge hydrochar using Pluronic F127 as template for efficient removal of phenolic compounds: Experimental study and mechanism interpretation via advanced statistical physics model. J. Environ. Manag. 2023, 326, 116841. [Google Scholar] [CrossRef] [PubMed]
- Baysal, M.; Yürüm, A.; Yıldız, B.; Yürüm, Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. [Google Scholar] [CrossRef]
- Sonibare, O.O.; Haeger, T.; Foley, S.F. Structural characterization of Nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy. Energy 2010, 35, 5347–5353. [Google Scholar] [CrossRef]
- Lu, X.; Jordan, B.; Berge, N.D. Thermal conversion of municipal solid waste via hydrothermal carbonization: Comparison of carbonization products to products from current waste management techniques. Waste Manag. 2012, 32, 1353–1365. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal carbonization of biomass for energy and crop production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Zhao, P.; Shen, Y.; Ge, S.; Yoshikawa, K. Energy recycling from sewage sludge by producing solid biofuel with hydrothermal carbonization. Energy Convers. Manag. 2014, 78, 815–821. [Google Scholar] [CrossRef]
- Pala, M.; Kantarli, I.C.; Buyukisik, H.B.; Yanik, J. Hydrothermal carbonization and torrefaction of grape pomace: A comparative evaluation. Bioresour. Technol. 2014, 161, 255–262. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhang, H.; Shao, L.M.; Lü, F.; He, P.J. Preparation and application of hierarchical porous carbon materials from waste and biomass: A review. Waste Biomass Valori. 2021, 12, 1699–1724. [Google Scholar] [CrossRef]
- Waribam, P.; Ngo, S.D.; Tran, T.T.V.; Kongparakul, S.; Reubroycharoen, P.; Chanlek, N.; Samart, C. Waste biomass valorization through production of xylose-based porous carbon microspheres for supercapacitor applications. Waste Manag. 2020, 105, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, D.; Liu, Z.; Xu, D.; Yang, F.; Tang, Q.; Jia, Q. From waste to Resource: Engineering biochar through optimized HCl activation for microplastic mitigation. Chem. Eng. Sci. 2025, 304, 121091. [Google Scholar] [CrossRef]
- Mahdi, T.S.; Elhafiz, D.R.A.; Helal, N.M.; Akkad, S.S.E. Hydrothermal conversion of mango wood wastes and sugarcane bagasse for biofuel production. Biomass Convers. Biorefin. 2024, 1–15. [Google Scholar] [CrossRef]
- Jusic, J.; Giannoni, T.; Zikeli, F.; Tamantini, S.; Pierpaoli, V.; Barbanera, M.; Romagnoli, M. Chemical and thermogravimetric characterization of chestnut tree biomass waste: Towards sustainable resource utilization in the tannin industry. Eur. J. Wood Wood Prod. 2025, 83, 113. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.; Kim, H.J.; Yang, H.S. Thermal properties of bio-flour-filled polyolefin composites with different compatibilizing agent type and content. Thermochim. Acta 2006, 451, 181–188. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M. Structural differences between wood species: Evidence from chemical composition, FTIR spectroscopy, and thermogravimetric analysis. J. Appl. Polym. Sci. 2012, 126, E337–E344. [Google Scholar] [CrossRef]
- Wikberg, H.; Ohra-aho, T.; Pileidis, F.; Titirici, M.M. Structural and morphological changes in kraft lignin during hydrothermal carbonization. ACS Sustain. Chem. Eng. 2015, 3, 2737–2745. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Nowicki, P.; Nosal-Wiercińska, A.; Pietrzak, R.; Szewczuk-Karpisz, K.; Ostolska, I.; Sternik, D. Adsorption of poly (acrylic acid) on the surface of microporous activated carbon obtained from cherry stones. Colloids Surf. A Physicochem. Eng. 2017, 514, 137–145. [Google Scholar] [CrossRef]
- Garrote, G.D.H.P.; Dominguez, H.; Parajó, J.C. Hydrothermal processing of lignocellulosic materials. Holz Als Roh-Und Werkst. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.M.; Baré, W.; Bilodeau, M. Depolymerization of steam-treated lignin for the production of green chemicals. Bioresour. Technol. 2011, 102, 4917–4920. [Google Scholar] [CrossRef]






| Process Conditions | Proximate Analysis (wt%) | Elemental Analysis (daf) a (wt%) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Temperature (°C) | Time (min) | Catalyst (mol L−1) | VM b | FC c | Ash a | C | H | N | O d | H/C e | O/C e | HHV f | EDR g | EY h | SF i | FR j | pH k |
| WS | - | - | - | 77.87 | 14.67 | 7.46 | 47.53 | 7.43 | 2.51 | 42.53 | 1.88 | 0.67 | 19.08 | - | - | - | 0.19 | - |
| HC1 | 185 | 30 | - | 74.69 | 20.79 | 4.53 | 62.87 | 7.32 | 3.20 | 26.61 | 1.40 | 0.32 | 26.95 | 1.41 | 31.05 | 3.98 | 0.28 | 4.06 |
| HC2 | 185 | 60 | - | 72.65 | 22.84 | 4.51 | 64.54 | 7.80 | 3.54 | 24.12 | 1.45 | 0.28 | 28.65 | 1.50 | 30.00 | 4.28 | 0.31 | 4.08 |
| HC3 | 185 | 90 | - | 70.06 | 25.24 | 4.70 | 72.56 | 8.74 | 2.91 | 15.79 | 1.45 | 0.16 | 34.19 | 1.79 | 25.34 | 4.46 | 0.36 | 4.11 |
| HC4 | 215 | 30 | - | 67.90 | 26.89 | 5.20 | 64.86 | 7.51 | 3.50 | 24.13 | 1.39 | 0.28 | 28.34 | 1.49 | 29.34 | 4.86 | 0.40 | 4.34 |
| HC5 | 215 | 60 | - | 64.64 | 29.96 | 5.39 | 68.48 | 7.83 | 3.34 | 20.35 | 1.37 | 0.22 | 30.70 | 1.61 | 28.41 | 5.16 | 0.46 | 4.37 |
| HC6 | 215 | 90 | - | 63.48 | 30.90 | 5.62 | 77.36 | 7.97 | 3.05 | 11.62 | 1.24 | 0.11 | 35.45 | 1.86 | 22.60 | 5.34 | 0.49 | 4.64 |
| HC7 | 245 | 30 | - | 65.17 | 28.87 | 5.96 | 62.11 | 7.95 | 3.95 | 25.99 | 1.54 | 0.31 | 27.71 | 1.45 | 26.85 | 5.75 | 0.44 | 4.78 |
| HC8 | 245 | 60 | - | 63.05 | 30.98 | 5.97 | 72.69 | 8.81 | 4.03 | 14.47 | 1.45 | 0.15 | 34.57 | 1.81 | 20.72 | 6.05 | 0.49 | 4.88 |
| HC9 | 245 | 90 | - | 60.97 | 32.71 | 6.31 | 77.11 | 7.91 | 3.06 | 11.92 | 1.23 | 0.12 | 35.23 | 1.85 | 21.86 | 6.22 | 0.54 | 4.98 |
| HC10 | 275 | 30 | - | 62.33 | 31.08 | 6.59 | 62.36 | 7.97 | 3.94 | 25.73 | 1.53 | 0.31 | 27.87 | 1.46 | 25.51 | 6.63 | 0.50 | 5.15 |
| HC11 | 275 | 60 | - | 61.40 | 32.05 | 6.55 | 75.59 | 9.14 | 4.13 | 11.14 | 1.45 | 0.11 | 36.61 | 1.92 | 18.24 | 6.93 | 0.52 | 5.24 |
| HC12 | 275 | 90 | - | 59.01 | 34.27 | 6.72 | 77.25 | 7.23 | 3.09 | 12.43 | 1.12 | 0.12 | 34.22 | 1.79 | 21.25 | 7.11 | 0.58 | 5.32 |
| HC13 | 215 | 60 | 0.25 | 69.69 | 26.69 | 3.62 | 63.98 | 8.70 | 3.36 | 23.96 | 1.63 | 0.28 | 29.77 | 1.56 | 31.08 | 5.16 | 0.38 | 3.89 |
| HC14 | 215 | 60 | 0.50 | 72.97 | 25.17 | 1.86 | 69.32 | 7.83 | 3.25 | 19.60 | 1.36 | 0.21 | 31.11 | 1.63 | 28.37 | 5.16 | 0.35 | 3.48 |
| HC15 | 215 | 60 | 1.00 | 74.03 | 24.29 | 1.68 | 69.36 | 8.66 | 1.73 | 20.25 | 1.50 | 0.22 | 32.20 | 1.69 | 26.33 | 5.16 | 0.33 | 3.30 |
| Retention Time (min) | Compounds | Area (%) | |||
|---|---|---|---|---|---|
| HC5 | HC13 | HC14 | HC15 | ||
| 5.593 | 4-Hydroxy-3-hexanone | - | 1.96 | 2.33 | 2.60 |
| 5.934 | 2-methyl-2-Cyclopenten-1-one | 1.90 | 3.50 | 2.71 | 2.22 |
| 6.220 | 1-(2-furanyl)-Ethanone | - | 2.29 | - | - |
| 6.864 | 2-Cyclohexen-1-one | - | 2.72 | 4.20 | 3.52 |
| 7.507 | 2-methyl-Propanoic acid-2-ethylhexyl ester | - | 0.44 | - | - |
| 9.605 | Phenol | 19.47 | 34.82 | 33.84 | 40.92 |
| 11.237 | 3-methyl-1,2-Cyclopentanedione | 6.59 | 3.64 | 5.51 | 4.36 |
| 14.265 | 2-methoxy-Phenol | - | 3.66 | 0.66 | - |
| 14.276 | Mequinol | - | - | - | 1.08 |
| 16.195 | 5-butyldihydro-2(3H)-Furanone | - | - | 2.17 | 1.33 |
| 16.887 | 2-ethyl-Cyclohexanone | - | 0.76 | - | 2.85 |
| 17.168 | 3-Furanmethanol | - | - | - | 1.47 |
| 26.451 | 2,6-dimethoxy-Phenol | - | 1.28 | - | - |
| 33.208 | 3,5-bis(1,1-dimethylethyl)-Phenol | - | 0.48 | - | - |
| 33.211 | 2,6-bis(1,1-dimethylethyl)-Phenol | - | - | 0.77 | - |
| 33.214 | 2,4-bis(1,1-dimethylethyl)-Phenol | 1.73 | - | - | - |
| 34.025 | 2-methyl-Cyclohexanone | - | - | 2.35 | 3.81 |
| 40.853 | 3,6-Diisopropylpiperazin-2,5-dione | - | 0.66 | 1.96 | 1.00 |
| 43.745 | (Z)-3-methyl-2-Decene | - | - | - | 1.37 |
| 43.750 | 2,2,4,4-tetramethyl-6-Oxabicyclo [3.1.0] hexan-3-one | - | 1.61 | - | - |
| 44.302 | trans-octahydro-2H-1-Benzothiopyran | 8.78 | - | - | - |
| 44.318 | l-Proline, N-neopentyloxycarbonyl-butyl ester | - | - | 5.01 | - |
| 44.334 | Hexahydro-2-Benzimidazolinethione | - | - | - | 3.98 |
| 44.340 | 3,6-Diisopropylpiperazin-2,5-dione | - | 4.41 | - | - |
| 44.556 | 3,5-dimethoxy-Phenol | 4.53 | 4.12 | - | 3.14 |
| 47.551 | l-Proline, N-allyloxycarbonyl-isohexyl ester | 8.52 | - | - | - |
| 47.616 | l-Proline, N-butoxycarbonyl-hexyl ester | - | 10.93 | - | - |
| 47.627 | 5,10-Diethoxy-2,3,7,8-tetrahydro-1H,6H-dipyrrolo [1,2-a:1′,2′-d] pyrazine | 12.51 | - | 11.78 | 8.59 |
| 47.886 | 3,5-dimethoxy-Phenol | 23.97 | - | 12.95 | 9.47 |
| 47.924 | 3-Isobutylhexahydropyrrolo [1,2-a] pyrazine-1,4-dione | - | 11.75 | - | - |
| 59.180 | 3-Benzyl-6-isopropyl-2,5-piperazinedione | - | 1.68 | - | - |
| 63.251 | Cyclo(D-phenylalanyl-L-prolyl) | 4.69 | 4.37 | 2.26 | 2.02 |
| 74.961 | Bis(2-ethylhexyl)phthalate | 4.20 | - | - | - |
| Temperature (°C) | Time (min) | Catalyst (mol L−1) | TOC (mg/L) | COD (mg/L) |
|---|---|---|---|---|
| 215 | 60 | - | 14,280 | 43,227 |
| 215 | 60 | 0.25 | 19,403 | 71,200 |
| 215 | 60 | 0.50 | 23,940 | 78,320 |
| 215 | 60 | 1.00 | 28,728 | 113,920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alper, K. Effect of Acrylic Acid Concentration on the Hydrothermal Carbonization of Stevia rebaudiana Biomass and Resulting Hydrochar Properties. Processes 2025, 13, 2731. https://doi.org/10.3390/pr13092731
Alper K. Effect of Acrylic Acid Concentration on the Hydrothermal Carbonization of Stevia rebaudiana Biomass and Resulting Hydrochar Properties. Processes. 2025; 13(9):2731. https://doi.org/10.3390/pr13092731
Chicago/Turabian StyleAlper, Koray. 2025. "Effect of Acrylic Acid Concentration on the Hydrothermal Carbonization of Stevia rebaudiana Biomass and Resulting Hydrochar Properties" Processes 13, no. 9: 2731. https://doi.org/10.3390/pr13092731
APA StyleAlper, K. (2025). Effect of Acrylic Acid Concentration on the Hydrothermal Carbonization of Stevia rebaudiana Biomass and Resulting Hydrochar Properties. Processes, 13(9), 2731. https://doi.org/10.3390/pr13092731








