Low-Temperature Pretreatment (LT-PT) of Food Waste as a Strategy to Enhance Biomethane Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Food Waste Characteristic
2.2. Setup of Low-Temperature Pretreatment Process
2.3. Physiochemical Analysis of Food Waste
2.4. Molecular Analysis
2.5. Biomethane Potential Test (BMP) and Kinetic Modeling
- V(t)—cumulative methane production at time t (NmL);
- Vm—experimental methane production potential (NmL);
- k—kinetic methanogenesis rate constant (d−1);
- t—cumulative time for the methane production (d);
- e—mathematical constant (2.718282);
- λ—lag phase for methane production (d);
- R—maximum methane production rate (NmL/d),
2.6. Data Analysis
3. Results
3.1. Changes in Physiochemical Parameters of Food Waste
3.2. Microbial Community Structure
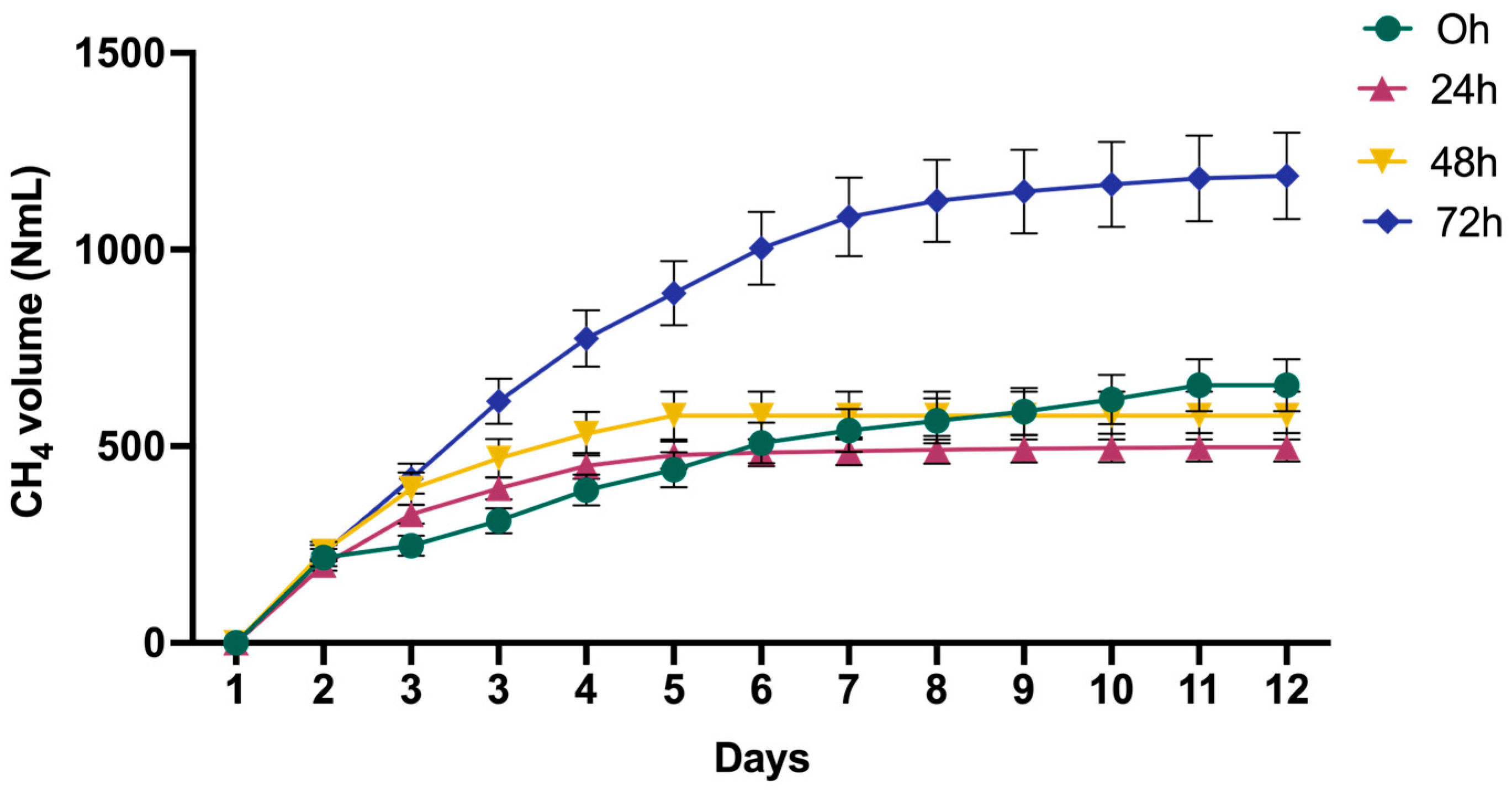
3.3. Biomethane Production and Process Kinetic
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Law, A.W.S.; Rincón, F.R.; van de Vossenberg, J.; Al Saffar, Z.; Welles, L.; Rene, E.R.; Vazquez, C.L. Volatile fatty acid production from food waste: The effect of retention time and lipid content. Bioresour. Technol. 2023, 367, 128298. [Google Scholar] [CrossRef]
- Scholz, K.; Eriksson, M.; Strid, I. Carbon footprint of supermarket food waste. Resour. Conserv. Recycl. 2015, 94, 56–65. [Google Scholar] [CrossRef]
- Eniyan, M.C.; Edwin, M.; Nagarajan, V.A. Mild thermo-mechanical pretreatment method for improving biomethane production: Food waste disintegration and its impact on solubilization. Therm. Sci. Eng. Prog. 2025, 60, 103432. [Google Scholar] [CrossRef]
- Frank, S.; Havlík, P.; Soussana, J.-F.; Levesque, A.; Valin, H.; Wollenberg, E.; Kleinwechter, U.; Fricko, O.; Gusti, M.; Herrero, M. Reducing greenhouse gas emissions in agriculture without compromising food security? Environ. Res. Lett. 2017, 12, 105004. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste–Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food waste to energy: An overview of sustainable approaches for food waste management and nutrient recycling. BioMed Res. Int. 2017, 2017, 2370927. [Google Scholar] [CrossRef]
- Grimberg, S.J.; Hilderbrandt, D.; Kinnunen, M.; Rogers, S. Anaerobic digestion of food waste through the operation of a mesophilic two phase pilot scale digester–assessment of variable loadings on system performance. Bioresour. Technol. 2015, 178, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Shukla, K.A.; Sofian, A.D.A.B.A.; Singh, A.; Chen, W.H.; Show, P.L.; Chan, Y.J. Food waste management and sustainable waste to energy: Current efforts, anaerobic digestion, incinerator and hydrothermal carbonization with a focus in Malaysia. J. Clean. Prod. 2024, 448, 141457. [Google Scholar] [CrossRef]
- Askarniya, Z.; Sun, X.; Wang, Z.; Boczkaj, G. Cavitation-based technologies for pretreatment and processing of food wastes: Major applications and mechanisms–A review. Chem. Eng. J. 2023, 454, 140388. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef]
- Climent, M.; Ferrer, I.; Baeza, M.d.M.; Artola, A.; Vazquez, F.; Font, X. Effects of thermal and mechanical pretreatments of secondary sludge on biogas production under thermophilic conditions. Chem. Eng. J. 2007, 133, 335–342. [Google Scholar] [CrossRef]
- Kasinath, A.; Byliński, H.; Artichowicz, W.; Remiszewska–Skwarek, A.; Szopińska, M.; Zaborowska, E.; Łuczkiewicz, A.; Fudala–Ksiazek, S. Biochemical assays of intensified methane content in biogas from low-temperature processing of waste activated sludge. Energy 2023, 282, 128855. [Google Scholar] [CrossRef]
- Remiszewska-Skwarek, A.; Wierzchnicki, R.; Roubinek, O.K.; Kasinath, A.; Jeżewska, A.; Jasinska, M.; Byliński, H.; Chmielewski, A.G.; Czerwionka, K. The Influence of Low-Temperature Disintegration on the Co-Fermentation Process of Distillation Residue and Waste-Activated Sludge. Energies 2022, 15, 482. [Google Scholar] [CrossRef]
- Kondusamy, D.; Kalamdhad, A.S. Pre-treatment and anaerobic digestion of food waste for high rate methane production–A review. J. Environ. Chem. Eng. 2014, 2, 1821–1830. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Water Works Association (AWWA): Denver, CO, USA; Water Environment Federation (WEF): Washington, DC, USA, 2005. [Google Scholar]
- Azizi, S.M.M.; Dastyar, W.; Meshref, M.N.; Maal-Bared, R.; Dhar, B.R. Low-temperature thermal hydrolysis for anaerobic digestion facility in wastewater treatment plant with primary sludge fermentation. Chem. Eng. J. 2021, 426, 130485. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Dahiya, S.; Mohan, S.V. Selective control of volatile fatty acids production from food waste by regulating biosystem buffering: A comprehensive study. Chem. Eng. J. 2019, 357, 787–801. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Mei, M.; Wang, T.; Chen, S.; Li, J. A Ca-rich biochar derived from food waste digestate with exceptional adsorption capacity for arsenic (III) removal via a cooperative mechanism. Sep. Purif. Technol. 2022, 295, 121359. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, L.; Li, H.; Deng, Z.; Liu, J. Lactic acid production from mesophilic and thermophilic fermentation of food waste at different pH. J. Environ. Manag. 2022, 304, 114312. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Zhao, S.; Wang, D.; Zheng, X.; Luo, J. Lactic acid accumulation from sludge and food waste to improve the yield of propionic acid-enriched VFA. Biochem. Eng. J. 2014, 84, 28–35. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, J.; Zhang, J.; Yu, D.; Wei, Y. The performance evaluation and kinetics response of advanced anaerobic digestion for sewage sludge under different SRT during semi-continuous operation. Bioresour. Technol. 2020, 308, 123239. [Google Scholar] [CrossRef]
- Mishra, P.; Panda, B. Polyhydroxybutyrate (PHB) accumulation by a mangrove isolated cyanobacteria Limnothrix planktonica using fruit waste. Int. J. Biol. Macromol. 2023, 252, 126503. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of Current Potentials and Applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Zamanzadeh, M.; Hagen, L.H.; Svensson, K.; Linjordet, R.; Horn, S.J. Anaerobic digestion of food waste–effect of recirculation and temperature on performance and microbiology. Water Res. 2016, 96, 246–254. [Google Scholar] [CrossRef]
- Shin, S.G.; Han, G.; Lim, J.; Lee, C.; Hwang, S. A comprehensive microbial insight into two-stage anaerobic digestion of food waste-recycling wastewater. Water Res. 2010, 44, 4838–4849. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Yao, T.; Su, M.; Li, J.; Liu, Z.; Xin, Y.; Wang, L.; Chen, J.; Gun, S. Effects of microbial inoculation on enzyme activity, available nitrogen content, and bacterial succession during pig manure composting. Bioresour. Technol. 2020, 306, 123167. [Google Scholar] [CrossRef] [PubMed]
- Cardinali-Rezende, J.; Colturato, L.F.; Colturato, T.D.; Chartone-Souza, E.; Nascimento, A.M.; Sanz, J.L. Prokaryotic diversity and dynamics in a full- scale municipal solid waste anaerobic reactor from start-up to steady-state conditions. Bioresour. Technol. 2012, 119, 373–383. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, W.; Li, Y.; Zheng, J.; Ma, X.; Wang, L.; Niu, L. How nutrient loading leads to alternative stable states in microbially mediated N-cycle pathways: A new insight into bioavailable nitrogen removal in urban rivers. Water Res. 2023, 236, 119938. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Khurana, S.M.P. Importance of Actinobacteria for Bioremediation. In Plant Biotechnology: Progress in Genomic Era; Khurana, S., Gaur, R., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Lu, T.; Yang, Y.; Feng, W.J.; Jin, Q.C.; Wu, Z.G.; Jin, Z.H. Effect of the compound bacterial agent on microbial community of the aerobic compost of food waste. Lett. Appl. Microbiol. 2022, 74, 32–43. [Google Scholar] [CrossRef]
- Cogan, T.M.; Jordan, K.N. Metabolism of Leuconostoc bacteria. J. Dairy Sci. 1994, 77, 2704–2717. [Google Scholar] [CrossRef]
- Wätjen, A.P.; De Vero, L.; Carmona, E.N.; Sberveglieri, V.; Huang, W.; Turner, M.S.; Bang-Berthelsen, C.H. Leuconostoc performance in soy-based fermentations–Survival, acidification, sugar metabolism, and flavor comparisons. Food Microbiol. 2023, 115, 104337. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Phosphate-solubilizing bacteria: Their agroecological function and optimistic application for enhancing agro-productivity. Sci. Total Environ. 2023, 901, 166468. [Google Scholar] [CrossRef]
- Sung, T.Y.; Patel, A.K.; Lin, S.R.; Huang, C.T.; Huang, Y.T. Strategic carbon source supplementation enhances nitrite degradation by Pantoea sp. A5 in variable temperature conditions. Bioresour. Technol. 2025, 425, 132299. [Google Scholar] [CrossRef]
- Browne, J.D.; Murphy, J.D. Assessment of the resource associated with biomethane from food waste. Appl. Energy 2013, 104, 170–177. [Google Scholar] [CrossRef]
- Ahire, P.D.; Upadhyay, A.; Talwar, P.; Khatri, H.; Singh, R.; Lindenberger, C.; Vivekanand, V. Tool development for estimation of biomethane potential of different food waste for a sustainable bioeconomy. Biomass Bioenergy 2024, 182, 107107. [Google Scholar] [CrossRef]
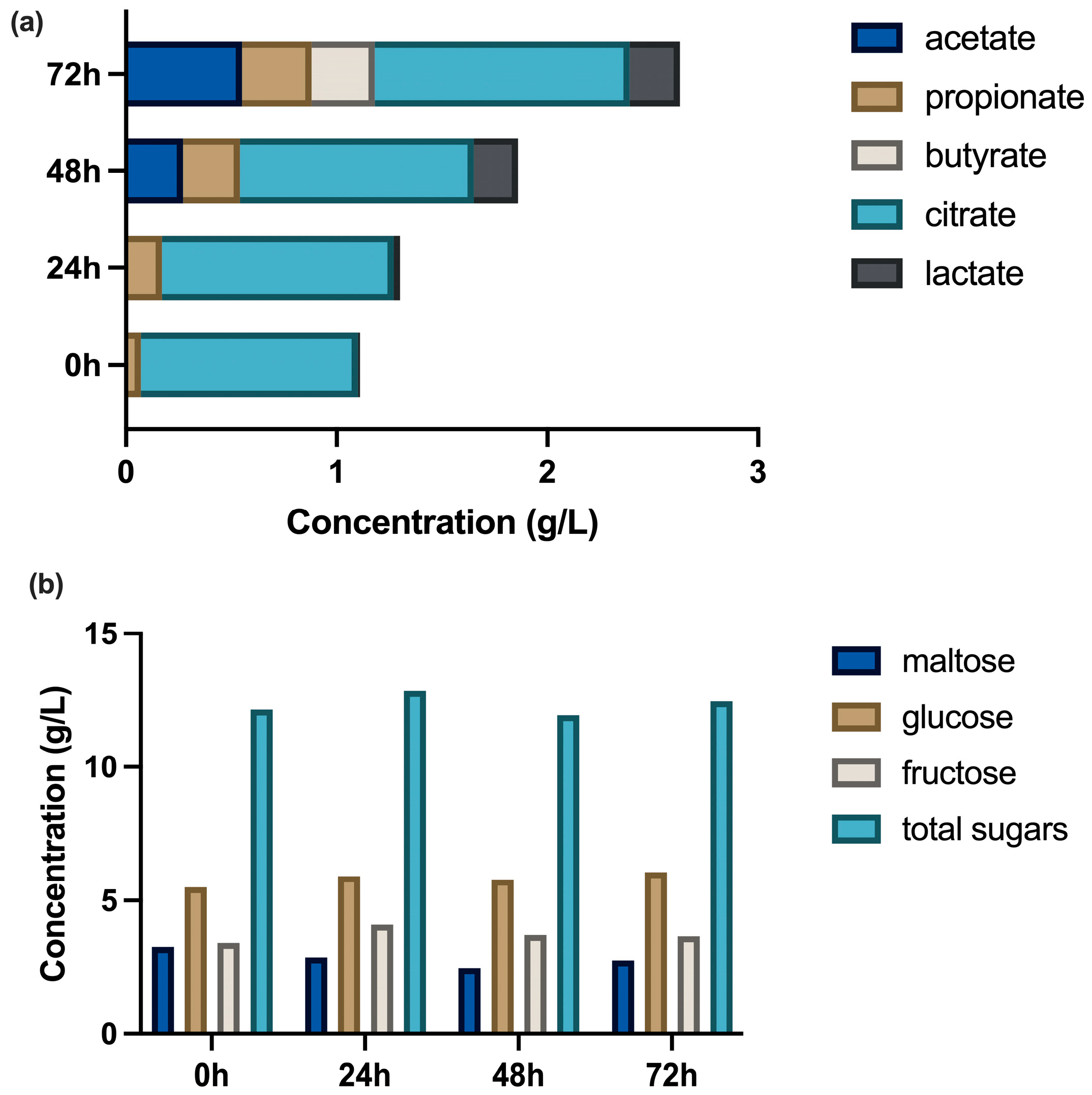
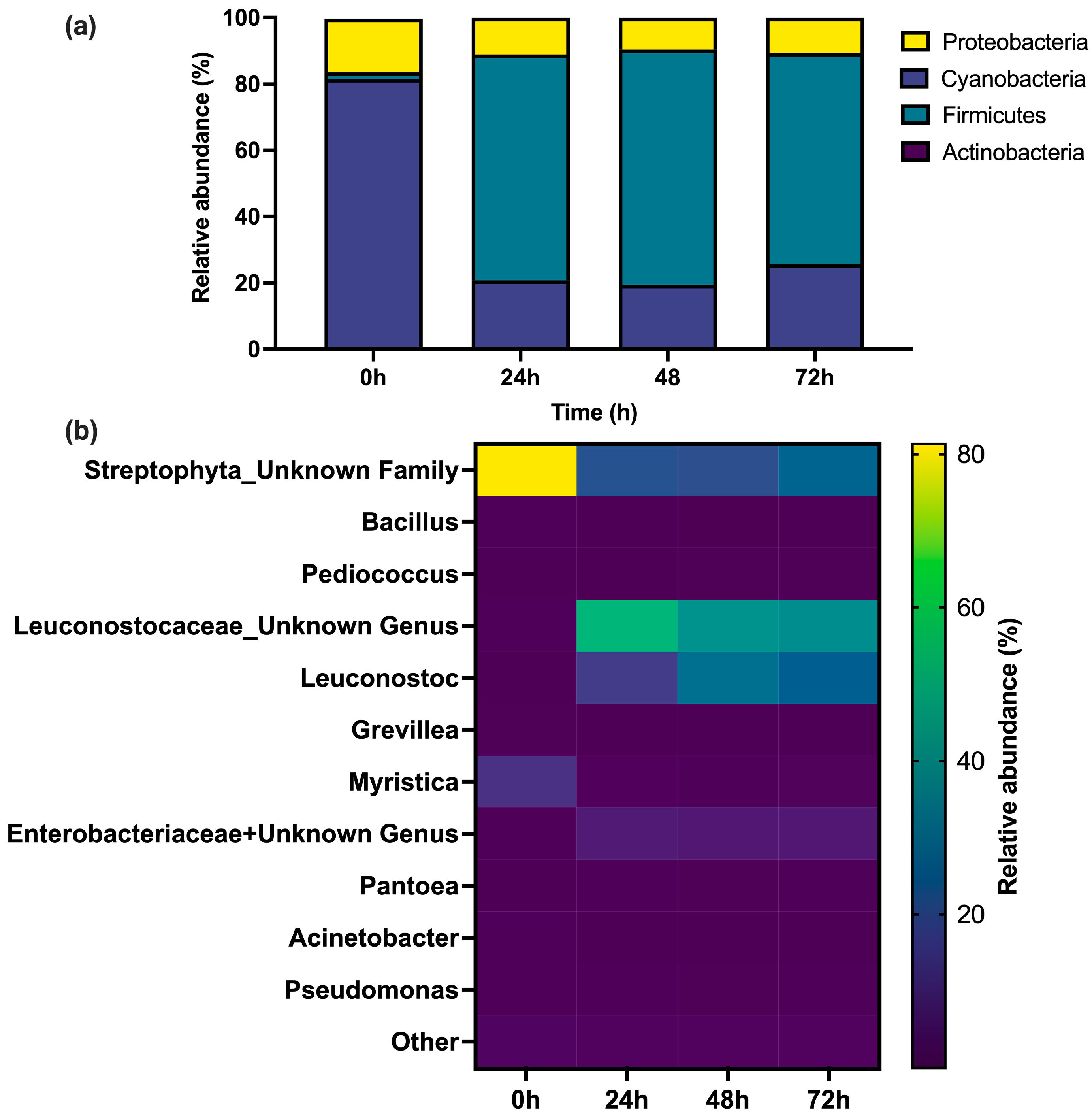
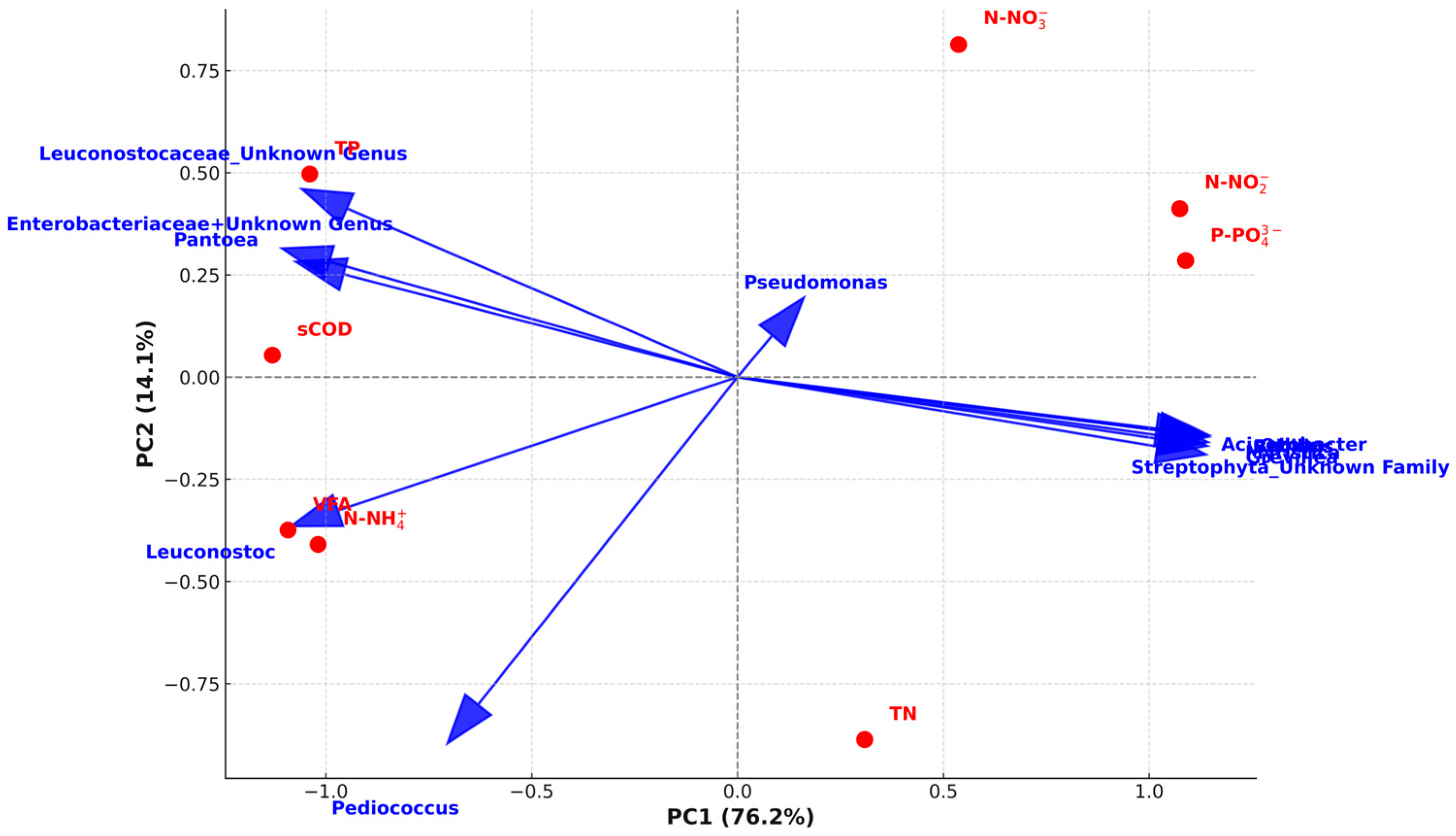
| Time of LT-PT | sCOD (g/L) | VFA (g/L) | TN (mg/L) | N-NH4+ (mg/L) | N-NO2− (mg/L) | N-NO3− (mg/L) | TP (mg/L) | P-PO43− (mg/L) |
|---|---|---|---|---|---|---|---|---|
| 0 h | 18 | 0.77 | 240 | 10.2 | 0.027 | 26.4 | 49.4 | 1.355 |
| 24 h | 23 | 0.95 | 225 | 19.6 | 0.017 | 27.0 | 62.4 | 0.892 |
| 48 h | 23 | 1.11 | 232 | 25.8 | 0.008 | 23.6 | 59.6 | 0.475 |
| 72 h | 24 | 1.09 | 245 | 31.9 | 0.007 | 25.6 | 58 | 0.719 |
| LT-PT Time | First-Order Model | Modified Gompertz Model | Experimental | ||||
|---|---|---|---|---|---|---|---|
| k (d−1) | R2 | Vm (NmL) | Rm (NmL/d) | λ (d) | R2 | Vm (NmL) | |
| 0 h | 147.0087 | 0.4425 | 653.9598 | 80.5392 | −0.8084 | 0.9729 | 641 |
| 24 h | 193.9367 | 0.6562 | 531.8467 | 178.9863 | −0.0445 | 0.9903 | 539 |
| 48 h | 193.2452 | 0.6602 | 507.9874 | 179.8553 | −0.0033 | 0.9912 | 509 |
| 72 h | 143.5602 | 0.3906 | 1175.8891 | 205.2382 | 0.0944 | 0.9964 | 1170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamoń, F.; Nowakowska, M.; Ronowicz, K.; Rosicki, K.; Szopińska, M.; Byliński, H.; Łuczkiewicz, A.; Fudala-Książek, S. Low-Temperature Pretreatment (LT-PT) of Food Waste as a Strategy to Enhance Biomethane Production. Processes 2025, 13, 2682. https://doi.org/10.3390/pr13092682
Gamoń F, Nowakowska M, Ronowicz K, Rosicki K, Szopińska M, Byliński H, Łuczkiewicz A, Fudala-Książek S. Low-Temperature Pretreatment (LT-PT) of Food Waste as a Strategy to Enhance Biomethane Production. Processes. 2025; 13(9):2682. https://doi.org/10.3390/pr13092682
Chicago/Turabian StyleGamoń, Filip, Martyna Nowakowska, Kacper Ronowicz, Kacper Rosicki, Małgorzata Szopińska, Hubert Byliński, Aneta Łuczkiewicz, and Sylwia Fudala-Książek. 2025. "Low-Temperature Pretreatment (LT-PT) of Food Waste as a Strategy to Enhance Biomethane Production" Processes 13, no. 9: 2682. https://doi.org/10.3390/pr13092682
APA StyleGamoń, F., Nowakowska, M., Ronowicz, K., Rosicki, K., Szopińska, M., Byliński, H., Łuczkiewicz, A., & Fudala-Książek, S. (2025). Low-Temperature Pretreatment (LT-PT) of Food Waste as a Strategy to Enhance Biomethane Production. Processes, 13(9), 2682. https://doi.org/10.3390/pr13092682






