Modulation of Enzymatic Activity by Moderate Electric Fields: Perspectives for Prebiotic Epilactose Production via Cellobiose-2-Epimerase
Abstract
1. Introduction
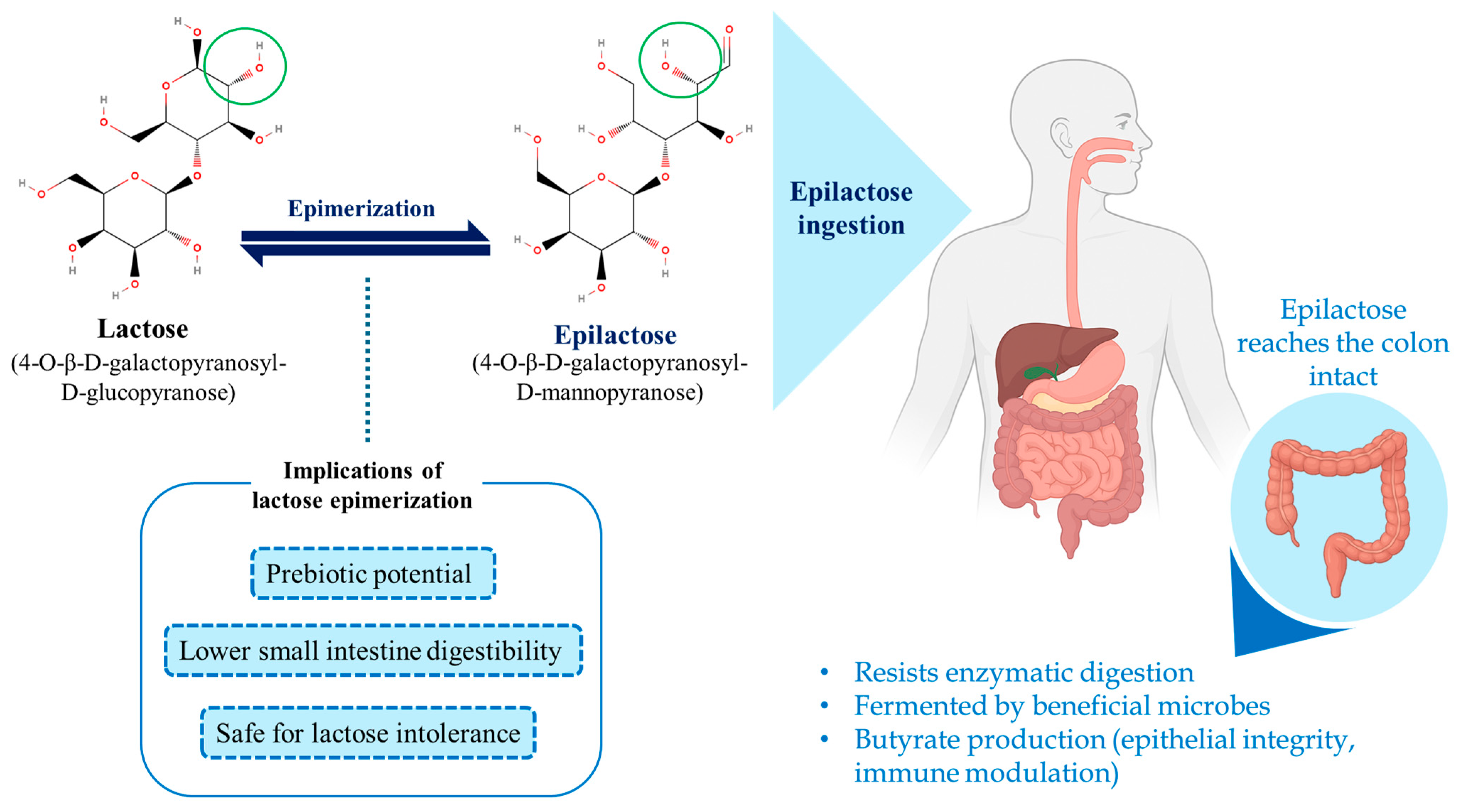
2. Epilactose: Properties and Production Methods
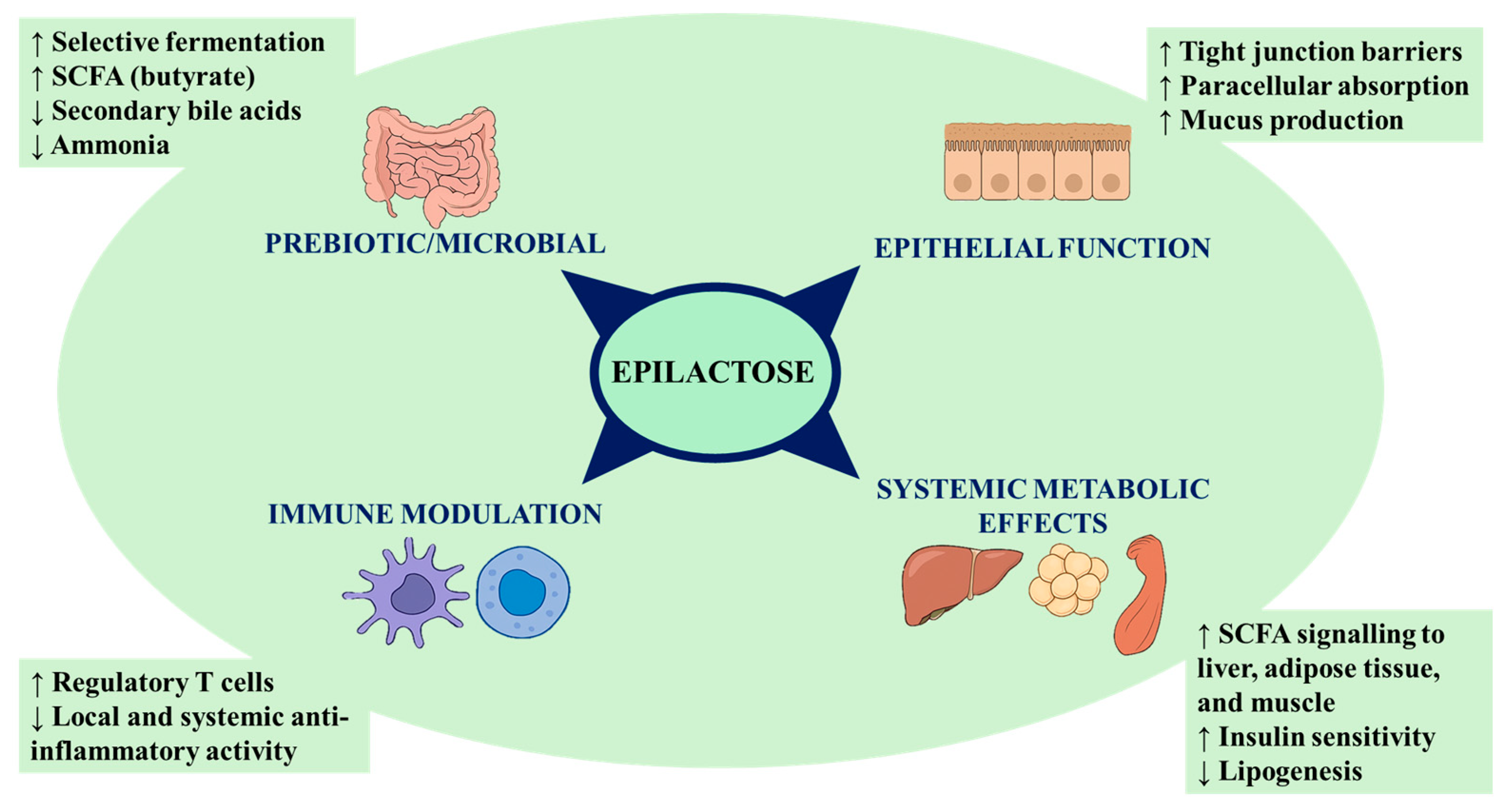
2.1. Structure, Physiological Effects and Prebiotic Action
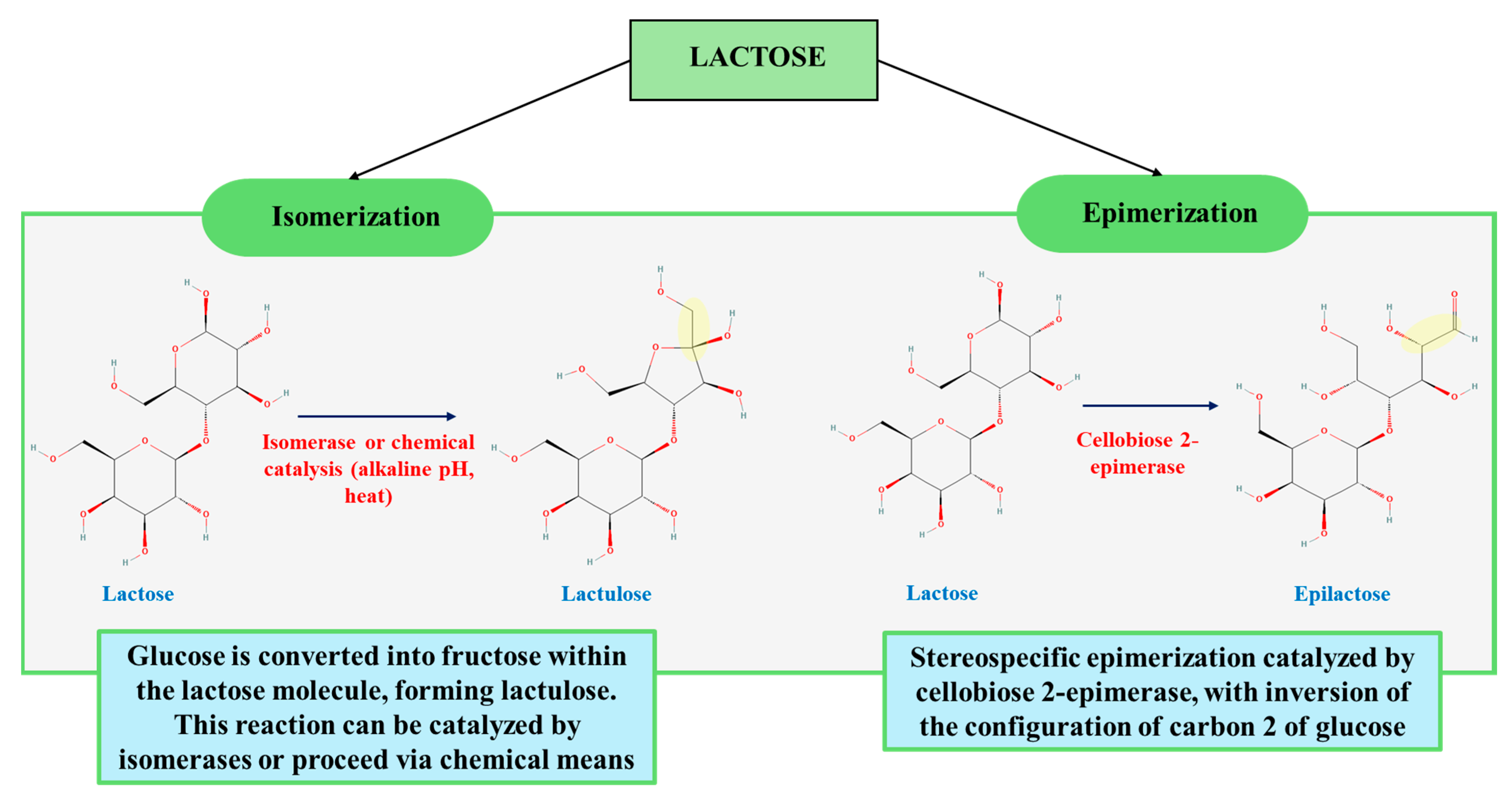
2.2. Epilactose Synthesis
| C2E (Microbial Origin) | Substrate Concentration | Reaction Volume | pH | Assay Temperature (°C) | Reaction Time | Main Results | Ref. |
|---|---|---|---|---|---|---|---|
| Ruminococcus albus NE1 | Lactose, 20 mM (for kinetics); 100 mM (for epilactose synthesis) | 3.2 mL (epilactose synthesis); 100 µL (enzyme assay) | 7.5 (100 mM sodium phosphate buffer) | 30 °C (enzyme assay); 25 °C (epilactose synthesis) | 10 min (enzyme assay); overnight (epilactose synthesis) | Epilactose yield from 100 mM lactose reached 38.8%; Km for lactose = 33 mM, kcat/Km = 1.6 s−1·mM−1; rCE inactive with maltose and other | [49] |
| F. johnsoniae (FjCE) and P. heparinus (PhCE) | Lactose in UHT milk, ~43 g/L (diluted from 48 g/L) | 25 mL (in stirred-tank reactor, STR) | 7.0 (10 mM sodium phosphate buffer used to prepare enzyme solution) | 8 °C (industrial milk processing condition) | 24 h (with maximum production in 9 h) | Epilactose yield: 33.6% (FjCE), 30.5% (PhCE); Final concentration: 14.3 g/L (FjCE), 13.9 g/L (PhCE); No side products detected | [11] |
| Caldicellulosiruptor saccharolyticus | Lactose, 48.5 g/L (from UHT milk) | 25 mL (milk conversion in stirred vessel) | ~6.7 (natural milk pH) | 8 °C and 50 °C | 24 h (at 50 °C); 72 h (at 8 °C) | Epilactose yield: 7.49 g/L (15.5%) at 50 °C; 6.57 g/L (13.6%) at 8 °C | [44] |
| Clostridium sp. TW13 | Lactose, 60 g/L (pure); also tested in whey powder and expired milk | 1.5 L (for purification); 1 mL (standard assay) | 7.5 (50 mM Tris-HCl buffer) | 40 °C (optimum for CsCEase1 activity) | 2 h | Conversion rate: 44.3%; Epilactose yield: 26.58 g/L (pure lactose); 29.65% (whey); 32.69% (expired milk); Purity after purification: >98%; Recovery rate: 66.47% | [19] |
| Caldicellulosiruptor saccharolyticus | Lactose, 100 g/L (ranges of 25–300 g/L tested) | 1 mL (batch reactions for kinetic assays) | 7.0 (100 mM phosphate buffer) | 50 °C (optimal for epilactose production) | 4–72 h depending on E/S ratio | Maximum epilactose yield: 0.256 g/g; highest productivity at 40 °C and pH 8.0; epimerization favored at low E/S ratios (1.417 × 10−7 kat/g); no lactulose formed at early stages; high selectivity achievable | [41] |
| Acidobacteriota bacterium | Lactose, 200 mM (in buffer); 50 g/L (in milk) | 0.4 mL (lactose buffer system); 1 mL (milk system) | 6.0 (PIPES buffer); milk ~6.7 | 50 °C (in buffer); 10 °C (in milk, cold catalysis) | 2–48 h (buffer); 12 h (milk) | 28.5% lactose-to-epilactose conversion at refrigeration temperatures (10 °C) | [48] |
| Rhodothermus marinus | Lactose, 300 g/L | 100 mL (bioconversion system after fed-batch cultivation) | 8.0 (Tris-HCl buffer) | 80 °C | 4 h (max yield at 100 min with 0.5% broth) | Epilactose yield: 29.5%; Productivity: up to 9 g/L/h; Enzyme volumetric activity: 1255 U/mL; Only 0.25% broth needed for 120 kg lactose → 35.4 kg epilactose | [20] |
| Caldicellulosiruptor saccharolyticus | Lactose, 400 g/L | 100 mL | 7.0 (100 mM phosphate buffer) | 50 °C and 70 °C | 48–72 h (epilactose/lactulose synthesis); additional TOS step at 50 °C, pH 4.5 | Up to 81.6 g/L epilactose + 22.4 g/L lactulose from unreacted lactose | [46] |
| Caldicellulosiruptor saccharolyticus | Epilactose 87% purity, final conc. 10 g/L in fermentation medium | 40 mL (batch cultures in 70 mL serum bottles) | 7.0 (growth medium); dropped to ~5.3–5.6 after fermentation | 37 °C (static anaerobic fermentation) | Not applicable (epilactose previously purified) | Epilactose (10 g/L) led to the highest short-chain fatty acid production; butyrate reached 86 mM with the Mediterranean diet | [10] |
| Thermoanaerobacterium saccharolyticum | Lactose, 300 g/L (from whey powder) | 1 L (with whole cell permeabilized B. subtilis) | 7.0 (sodium phosphate buffer) | 60 °C | 1 h | Epilactose yield: 66.9 g/L (22.3%); final purity >98% after purification | [45] |
| Caldicellulosiruptor saccharolyticus | Lactose, 50 g/L (tested range: 10–270 g/L) | 1 mL (standard assay); varied for optimization studies | 7.5 (50 mM Tris-HCl buffer) | 80 °C (optimum); tested: 50–90 °C | 20–120 min | Max epilactose yield: 27% (13.5 g/L); productivity: 9 g/L/h; no lactulose after 20 min; enzyme optimal at 80 °C, pH 7.5; stable up to 60 °C | [10] |
| Caldicellulosiruptor sp. | Lactose, 700 g/L | 1 L (biotransformation using whole cells) | 8.0–9.0 (optimal); 6.5–7.5 still active | 70 °C | 2 h | Epilactose yield: 30.4% (210 g/L); productivity: 105 g/L/h | [2] |
| Caldicellulosiruptor bescii | Lactose, 50 g/L (from cheese whey permeate) | 5 L (stirred fermenter) | 7.0 (100 mM MOPS buffer) | 30 °C (epilactose favored); also tested at 70 °C | 24 h | Epilactose yield: 35% (14.9 g/L) at 30 °C; no epilactose formed chemically; reaction is temperature-dependent | [47] |
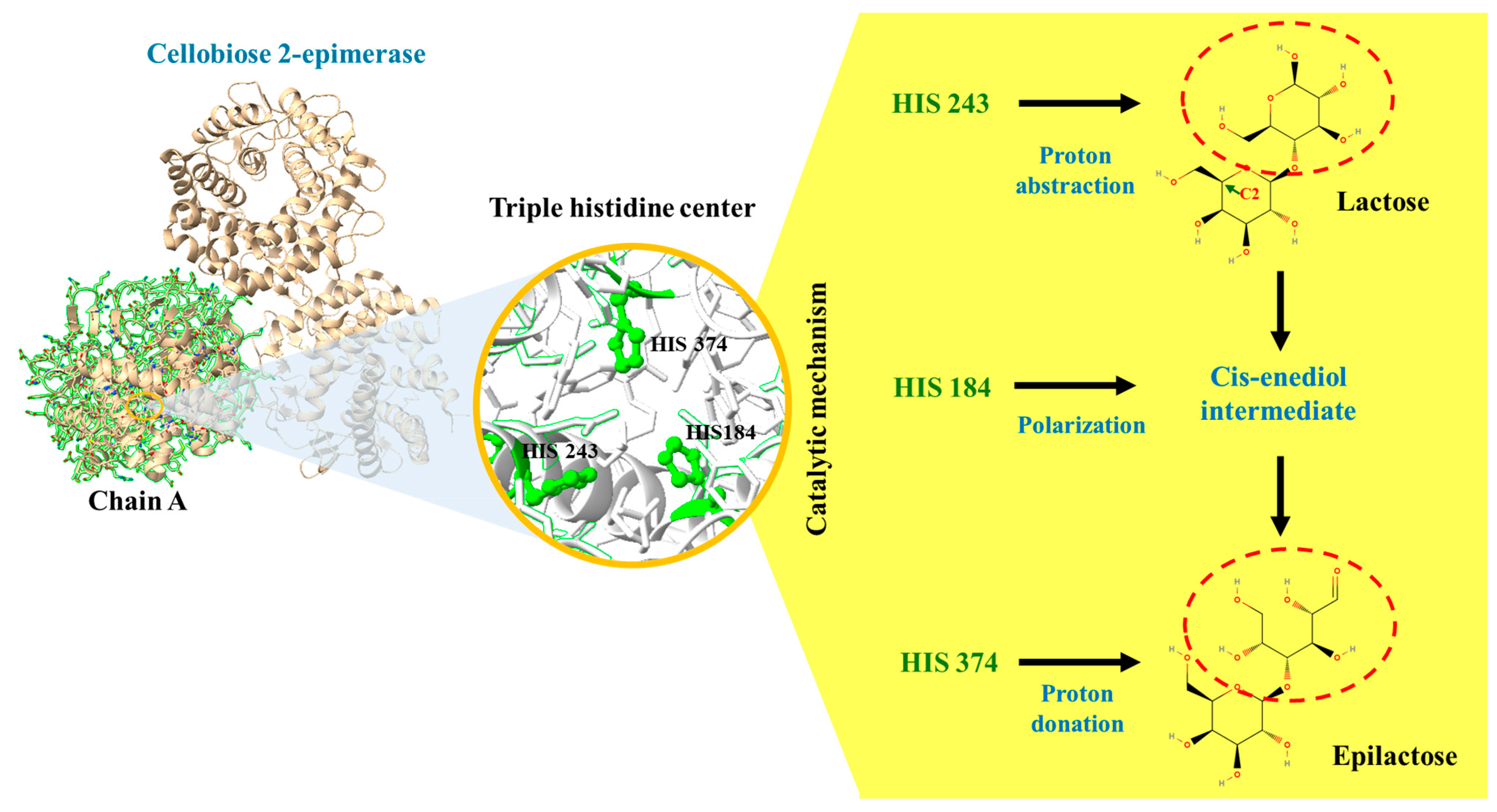
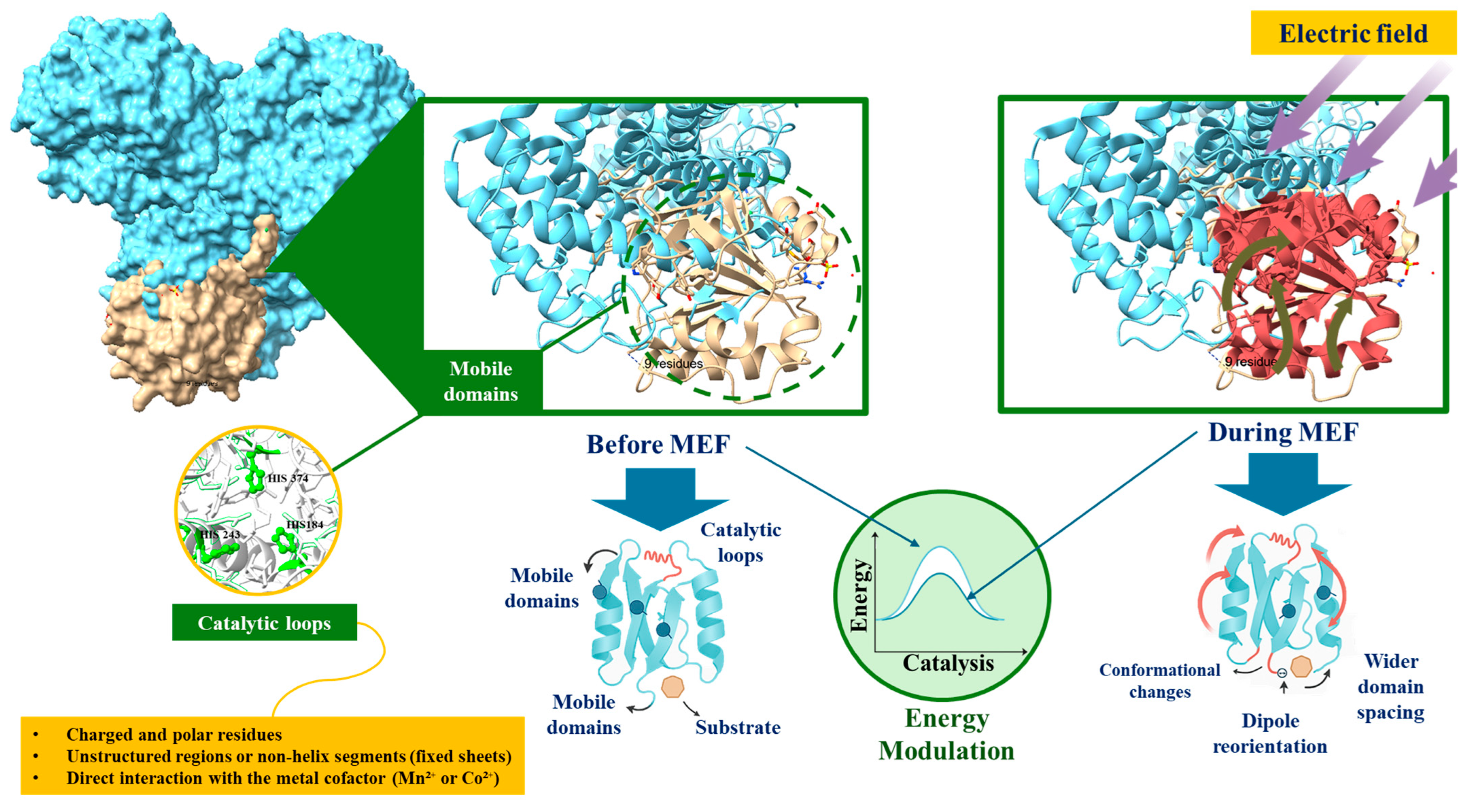
3. Cellobiose 2-Epimerase: Structure, Function, and Applications
4. Moderate Electric Fields (MEFs): Principles and Effects on Enzymes
| Application | Enzyme | Reaction Medium | MEFs Configuration | MEFs Parameters | Assay Temperature (°C) | Effect on Activity | Structural Insights | Ref. |
|---|---|---|---|---|---|---|---|---|
| Non-thermal pasteurization of sugarcane juice | Peroxidase (POD) | Sugarcane juice | MEFs and ohmic heating | 10 to 105 Hz, sinusoidal waveform, 0–16.7 V/cm | 60–80 | Activation (10 Hz, 60 °C); Inactivation (≥50 Hz, 80 °C) | Browning; loss of phenolic compounds | [61] |
| Hydrolysis of corn starch (native and gelatinized); starch modification via MEF | α-amilase | Corn starch suspensions (native and fully gelatinized) | MEFs, alternating current | 2.5 V/cm, 50 Hz, up to 120 min; with or without Ca2+ (0–50 mM) | 40–70 | Activity increased by 23.3% with low-intensity MEF (2.5 V/cm); effects depend on intensity, frequency, Ca2+ ions, temperature, and time | Structural changes (increase in α-helix and β-sheet); changes in fluorescence; greater hydrolysis in gelatinized vs. native starch | [72] |
| Blanching and pasteurization of mushrooms with higher efficiency | Tyrosinase (Agaricus bisporus) | 50 mM phosphate-buffer solution, pH 6.5 | MEFs with ohmic heating (OH), alternating current (AC) | 25, 30, and 35 V/cm; 60 Hz; up to 15 min | 50–58 | OH reduced inactivation time (D-values from 42.14 min to 6.44 min at 58 °C); synergistic effect between electric field and temperature | Suggested denaturation by polarization of the metallic active site; structural alteration facilitated by the electric field | [78] |
| Efficient pasteurization of orange juice | Peroxidase | Orange juice | Ohmic heating (AC), continuous vs. intermittent | 15 V/cm; continuous vs. intermittent | 60 | More efficient inactivation under continuous treatment | Electric field improves heat and mass transfer | [79] |
| Non-thermal preservation of grape juice | Not applicable (enzyme not studied) | Grape juice | MEFs (AC) | 15.6, 18.8, 21.9 V/cm; 50 Hz; 5, 10, 15 min | 25 | Not applicable (emphasis on physicochemical and microbiological parameters) | Reduction in pH; increase in turbidity and conductivity; degradation of phenolic compounds and vitamin C; microbial reduction up to 3.43 log10 | [80] |
| Selective inactivation of enzymes in tomato pulp | Pectin methyl esterase (PME) and Polygalacturonase (PG) | Tomato pulp | MEFs | Frequência from 0 Hz (DC) to 1 MHz, 0.4 V/cm, 300 s | - | PME: significant inactivation up to 26% at 1–60 Hz; PG | Increased molecular motion at low frequencies | [81] |
| Hydrolysis of pre-gelatinized corn starch via MEF | α-amylase | Corn starch suspension | MEFs (AC) | 2.5 and 5 V/cm, 50 Hz, 30 min | Not controlled | Higher activity at 2.5 V/cm; inactivation at 5 V/cm; increased content of reducing sugars (up to 0.92 mg/mL) | Destruction of semicrystalline structure; formation of new crystals aligned by the field | [77] |
| Inactivation of peroxidase in sugarcane juice | Peroxidase (POD) | Sugarcane juice (liquid, solid, and concentrated fractions) | Ohmic heating and MEFs | 7.8 V/cm, 60 Hz, 75 °C, 25 min | 75 | Inactivation: 78% (liquid), 100% (solid), 96% (concentrated); OH more effective than conventional heating (CH) | Sugar-stabilizing effect; influence on isoenzymes and fraction behavior; thermal effect enhanced by electric current | [82] |
| Inactivation of POD and PPO and preservation of compounds | Peroxidase (POD) and Polyphenol oxidase (PPO) | Sugarcane juice | Ohmic heating and MEFs | 3.57 to 4.39 V/cm, 25 V applied, 60–80 °C, up to 12 min | 60 to 80 | POD: greater inactivation with OH at 80 °C (up to 72%); PPO: almost completely inactivated | Preservation of phenolics in sugarcane juice; possible activation of POD at 60 °C; variations in thermal inactivation phase; improved stability under MEF | [13] |
| Increased enzymatic saccharification of lignocellulose in a bioreactor | Cellulases (endoglucanase, cellobiohydrolase, and β-glucosidase) | Pre-treated rice straw (1% NaOH) | Pulsed electric field | 0.12 V/cm, 48 °C, 96 h, electrode change every 6 h | 48 | Improved conversion efficiency (22.8%); 32.6% increase in saccharification | Greater enzyme mobility and adsorption to cellulose surface; possible denaturation under high field strengths | [83] |
| Microbial and α-amylase inactivation in oat-based plant beverage with pea protein | α-amylase (Bacillus subtilis) | Non-sterilized plant beverage (oat + pea protein) | Pulsed electric field (PEF) | 8.2–10.4 kV/cm, 77–244 kJ/L, 25 s, preheating 30–45 °C | Up to 85 | Inactivation up to 89.2%; effect dependent on energy and preheating | Synergistic effect with preheating; limited thermal stability of the enzyme | [84] |
| Non-thermal inactivation of oxidative enzymes in grape juice | Polyphenol oxidase (PPO) and Peroxidase (POD) | Fresh grape juice (red grape, seedless) | MEFs | 82 to 87 V/cm, 65 to 75 °C, 10 min | 65 to 75 | PPO: complete inactivation in 6–8 min (87 V/cm, 75 °C); POD: complete inactivation in 8–10 min (87 V/cm, 75 °C) | Molecular motion simulation shows increased kinetic energy of enzymes with MEF; field-temperature synergistic effect | [75] |
| Enhance enzymatic cellulose hydrolysis at suboptimal temperatures | Cellulases (Cel7A, Cel6A, Cel7B, etc.) from Trichoderma reesei | Whatman No. 1 filter paper disks (cellulosic substrate model) | MEFs | 8–12 V/cm, 50 Hz, 30–55 °C, up to 24 h | 30 to 55 | Increased activity below the optimum (up to +264% in k1 at 30 °C); activity loss above 50 °C | Molecular motion simulations show increased kinetic energy of enzymes under MEF; synergistic field-temperature effect | [15] |
| In situ control of α-amylase activity by MEF frequency | α-amylase | Diluted potato starch solution in acetate buffer (pH 5.0) | MEFs | 1 V/cm; 1 Hz–1 MHz, 60 °C, 25 s | 60 | Up to 41% increase in in situ activity (1–60 Hz); mild inhibition at high frequencies | Transition from oscillatory to rotational motion below 60 Hz; possible increase in enzyme–substrate collisions | [66] |
| Pasteurization and PPO inactivation in mango pulp | Polyphenol oxidase (PPO) | Mango pulp | Ohmic heating and MEFs | 15–20 V/cm, 72 °C, 15–120 s | 72 | Up to 95.7% inactivation with 15 s (PPO); partial reactivation possible between 45 and 60 s | Increase in soluble fiber and viscosity; electroporation and enzyme structural modification | [85] |
| Non-thermal inactivation of PPO in apple juice | Polyphenol oxidase (PPO) | Fresh apple juice (Red Delicious) | MEFs | 85–110 V/cm, 50 °C (on-off); 92.5–98 V/cm, 50–65 °C (constant) | 50 to 65 | Inactivation up to 89% (65 °C); inactivation rate doubled compared to thermal control (50 °C) | Synergistic field-temperature effect; lower specific energy required with on-off mode | [74] |
| Green wine clarification with PEC under MEF | Pectinase (PEC) | Green wine (simulated and real) | MEFs | 7 V/cm, 20 kHz, 15–35 °C | 15–35 | Activity increased up to 29% (15 °C) and 21% (20 °C); lower activity above 25 °C | 42% reduction in pectin content in must at 20 °C with MEF; lower thermal stability | [60] |
| Internal heating and PPO and POD inactivation in kiwi juice | Polyphenol oxidase (PPO) and Peroxidase (POD) | Fresh kiwi juice (Actinidia chinensis) | Induced electric field (IEF) by alternating magnetic field | 1800 V *, 6 mL/min, 60 kHz, 7.7 s | 65.4 | PPO: 94.8% inactivation; POD: 92.7% inactivation | Lower loss of phenolics (7.1%) and flavonoids (2.7%); better color preservation; lower browning index | [86] |
| Enzymatic hydrolysis of soy isoflavone glycosides assisted by PEF | β-glucosidase (β-GLU) | Soy isoflavone glycosides in acetate buffer (pH 5.0) | Pulsed electric field (PEF) | 5–25 kV/cm; 6–10 pulses; 1000–3000 Hz; 2 μs pulse width | 20–23 | Up to 122.1% activity increase at 15 kV/cm; α-helix increase | Ea, ΔH, ΔS reduction, and ΔS; increase in α-helix content; conformational changes (CD and fluorescence) | [73] |
| Preservation of polyphenols and increased bioavailability in fruits and vegetables | PPO/POD | Various fruit juices and pulps (e.g., jujube juice, kiwi, apple, etc.) | Pulsed electric fields (PEF), cold plasma (CP), and high-pressure processing (HPP) (as comparative reference) | 20–65 kHz (CP), 25–35 kV/cm (PEF), 300–600 MPa (HPP); duration ranging from 1 to 30 min | 60 | Reduction or inactivation of PPO/POD by structural disruption or oxidative stress | Increased total phenolic content (TPC); cell membrane rupture; induction of plant stress | [87] |
5. Perspectives on the Use of Moderate Electric Fields (MEFs) for Enzymatic Modulation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Qiao, Y.; Xu, X.; Peng, Q.; Ren, J.; Ma, L.; Tian, D.; Gong, Y.; Feng, D.; Shi, B. In Vitro Fermentation of Epilactose and Epilactitol by Human Faecal Microbiota. Int. Dairy J. 2023, 144, 105697. [Google Scholar] [CrossRef]
- Liangfei, L.; Yafeng, Z.; Kai, X.; Zheng, X. Identification of a Thermostable Cellobiose 2-Epimerase from Caldicellulosiruptor sp. Rt8.B8 and Production of Epilactose Using Bacillus subtilis. J. Sci. Food Agric. 2022, 102, 85–94. [Google Scholar] [CrossRef]
- Fujiwara, T.; Saburi, W.; Inoue, S.; Mori, H.; Matsui, H.; Tanaka, I.; Yao, M. Crystal Structure of Ruminococcus Albus Cellobiose 2-epimerase: Structural Insights into Epimerization of Unmodified Sugar. FEBS Lett. 2013, 587, 840–846. [Google Scholar] [CrossRef]
- Cardoso, B.B.; Amorim, C.; Franco-Duarte, R.; Alves, J.I.; Barbosa, S.G.; Silvério, S.C.; Rodrigues, L.R. Epilactose as a Promising Butyrate-Promoter Prebiotic via Microbiota Modulation. Life 2024, 14, 643. [Google Scholar] [CrossRef]
- Grand View Research. Prebiotics Market Size, Share & Trends Analysis Report By Ingredients (FOS, Inulin, GOS, MOS), By Application (Food & Beverages, Dietary Supplements, Animal Feed), By Region, And Segment Forecasts, 2022–2030. Grand View Research: San Francisco, CA, USA, 2022. [Google Scholar]
- Precedence Research Precedence Research. Available online: https://www.precedenceresearch.com/prebiotic-ingredients-market (accessed on 14 August 2025).
- Pang, B.; Yang, J.; Song, M.; Zhang, W.; Qian, S.; Xu, M.; Chen, X.; Huang, Y.; Gu, R.; Wang, K. Advances and Prospects on Production of Lactulose and Epilactose by Cellobiose 2-Epimerases: A Review. Int. J. Biol. Macromol. 2025, 305, 141283. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Saburi, W.; Ojima, T.; Taguchi, H.; Mori, H.; Matsui, H. Immobilization of a Thermostable Cellobiose 2-Epimerase from Rhodothermus marinus JCM9785 and Continuous Production of Epilactose. Biosci. Biotechnol. Biochem. 2012, 76, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Tian, Y.; Zhao, S.; Lu, F.; Lin, X.; Lu, S.; Hu, Y.; Wang, T.; Xu, Z. High-Efficiency Secretion Expression of Cellobiose 2-Epimerase in Escherichia Coli and Its Applications. Int. J. Biol. Macromol. 2025, 307, 142205. [Google Scholar] [CrossRef]
- Cardoso, B.B.; Silvério, S.C.; Rodrigues, J.L.; Rodrigues, L.R. Epilactose Biosynthesis Using Recombinant Cellobiose 2-Epimerase Produced by Saccharomyces cerevisiae. ACS Food Sci. Technol. 2021, 1, 1578–1584. [Google Scholar] [CrossRef]
- Krewinkel, M.; Gosch, M.; Rentschler, E.; Fischer, L. Epilactose Production by 2 Cellobiose 2-Epimerases in Natural Milk. J. Dairy Sci. 2014, 97, 155–161. [Google Scholar] [CrossRef]
- Kraljić, K.; Balbino, S.; Filipan, K.; Herceg, Z.; Stuparević, I.; Ivanov, M.; Vukušić Pavičić, T.; Jakoliš, N.; Škevin, D. Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part II—Non-Thermal Technique. Processes 2023, 11, 3283. [Google Scholar] [CrossRef]
- Brochier, B.; Mercali, G.D.; Marczak, L.D.F. Influence of Moderate Electric Field on Inactivation Kinetics of Peroxidase and Polyphenol Oxidase and on Phenolic Compounds of Sugarcane Juice Treated by Ohmic Heating. LWT 2016, 74, 396–403. [Google Scholar] [CrossRef]
- Ali, M.; Liao, L.; Zeng, X.-A.; Manzoor, M.F.; Mazahir, M. Impact of Sustainable Emerging Pulsed Electric Field Processing on Textural Properties of Food Products and Their Mechanisms: An Updated Review. J. Agric. Food Res. 2024, 15, 101076. [Google Scholar] [CrossRef]
- Durham, E.K.; Sastry, S.K. Moderate Electric Field Treatment Enhances Enzymatic Hydrolysis of Cellulose at Below-Optimal Temperatures. Enzyme Microb. Technol. 2020, 142, 109678. [Google Scholar] [CrossRef]
- Giteru, S.G.; Oey, I.; Ali, M.A. Feasibility of Using Pulsed Electric Fields to Modify Biomacromolecules: A Review. Trends Food Sci. Technol. 2018, 72, 91–113. [Google Scholar] [CrossRef]
- Chaturvedi, S.S.; Bím, D.; Christov, C.Z.; Alexandrova, A.N. From Random to Rational: Improving Enzyme Design through Electric Fields, Second Coordination Sphere Interactions, and Conformational Dynamics. Chem. Sci. 2023, 14, 10997–11011. [Google Scholar] [CrossRef]
- Eat, S.; Wulansari, S.; Ketbot, P.; Waeonukul, R.; Pason, P.; Uke, A.; Kosugi, A.; Ratanakhanokchai, K.; Tachaapaikoon, C. A Novel Cellobiose 2-Epimerase from Anaerobic Halophilic Iocasia Fonsfrigidae and Its Ability to Convert Lactose in Fresh Goat Milk into Epilactose. J. Sci. Food Agric. 2024, 104, 8529–8540. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, Y.; Yan, Y.; Chen, W.; Ren, T.; Ma, A.; Li, S.; Jia, Y. Characterization of an Epilactose-Producing Cellobiose 2-Epimerase from Clostridium sp. TW13 and Reutilization of Waste Milk. Food Chem. 2025, 480, 143948. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Huang, Z.; Ding, J.; Ni, D.; Mu, W. Improvement of Cellobiose 2-Epimerase Expression in Bacillus subtilis for Efficient Bioconversion of Lactose to Epilactose. Int. J. Biol. Macromol. 2024, 280, 136063. [Google Scholar] [CrossRef]
- Canani, R.B. Potential Beneficial Effects of Butyrate in Intestinal and Extraintestinal Diseases. World J. Gastroenterol. 2011, 17, 1519. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Nishimukai, M.; Taguchi, H.; Senoura, T.; Hamada, S.; Matsui, H.; Yamamoto, T.; Wasaki, J.; Hara, H.; Ito, S. Prebiotic Properties of Epilactose. J. Dairy Sci. 2008, 91, 4518–4526. [Google Scholar] [CrossRef]
- Nishimukai, M.; Watanabe, J.; Taguchi, H.; Senoura, T.; Hamada, S.; Matsui, H.; Yamamoto, T.; Wasaki, J.; Hara, H.; Ito, S. Effects of Epilactose on Calcium Absorption and Serum Lipid Metabolism in Rats. J. Agric. Food Chem. 2008, 56, 10340–10345. [Google Scholar] [CrossRef]
- Suzuki, T.; Nishimukai, M.; Takechi, M.; Taguchi, H.; Hamada, S.; Yokota, A.; Ito, S.; Hara, H.; Matsui, H. The Nondigestible Disaccharide Epilactose Increases Paracellular Ca Absorption via Rho-Associated Kinase-and Myosin Light Chain Kinase-Dependent Mechanisms in Rat Small Intestines. J. Agric. Food Chem. 2010, 58, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Nishimukai, M.; Shinoki, A.; Taguchi, H.; Fukiya, S.; Yokota, A.; Saburi, W.; Yamamoto, T.; Hara, H.; Matsui, H. Ingestion of Epilactose, a Non-Digestible Disaccharide, Improves Postgastrectomy Osteopenia and Anemia in Rats through the Promotion of Intestinal Calcium and Iron Absorption. J. Agric. Food Chem. 2010, 58, 10787–10792. [Google Scholar] [CrossRef]
- Murakami, Y.; Ojima-Kato, T.; Saburi, W.; Mori, H.; Matsui, H.; Tanabe, S.; Suzuki, T. Supplemental Epilactose Prevents Metabolic Disorders through Uncoupling Protein-1 Induction in the Skeletal Muscle of Mice Fed High-Fat Diets. Br. J. Nutr. 2015, 114, 1774–1783. [Google Scholar] [CrossRef]
- van Trijp, M.P.H.; Rios-Morales, M.; Witteman, B.; Abegaz, F.; Gerding, A.; An, R.; Koehorst, M.; Evers, B.; van Dongen, K.C.V.; Zoetendal, E.G.; et al. Intraintestinal Fermentation of Fructo- and Galacto-Oligosaccharides and the Fate of Short-Chain Fatty Acids in Humans. iScience 2024, 27, 109208. [Google Scholar] [CrossRef] [PubMed]
- Moens, F.; Verce, M.; De Vuyst, L. Lactate- and Acetate-Based Cross-Feeding Interactions between Selected Strains of Lactobacilli, Bifidobacteria and Colon Bacteria in the Presence of Inulin-Type Fructans. Int. J. Food Microbiol. 2017, 241, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Karakan, T.; Tuohy, K.M.; Janssen-van Solingen, G. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Front. Nutr. 2021, 8, 672925. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Lin, H.; Lehmann, C.; Dylla, N.P.; Cole, C.G.; Mostad, J.D.; Pappas, T.E.; Ramaswamy, R.; Moran, A.; Hutchison, A.L.; et al. Bifidobacteria Metabolize Lactulose to Optimize Gut Metabolites and Prevent Systemic Infection in Patients with Liver Disease. Nat. Microbiol. 2023, 8, 2033–2049. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Stahl, B.; Vinderola, G.; Szajewska, H. Infant Formula Supplemented with Biotics: Current Knowledge and Future Perspectives. Nutrients 2020, 12, 1952. [Google Scholar] [CrossRef]
- De Preter, V.; Falony, G.; Windey, K.; Hamer, H.M.; De Vuyst, L.; Verbeke, K. The Prebiotic, Oligofructose-enriched Inulin Modulates the Faecal Metabolite Profile: An In Vitro Analysis. Mol. Nutr. Food Res. 2010, 54, 1791–1801. [Google Scholar] [CrossRef]
- Gonçalves, D.A.; González, A.; Roupar, D.; Teixeira, J.A.; Nobre, C. How Prebiotics Have Been Produced from Agro-Industrial Waste: An Overview of the Enzymatic Technologies Applied and the Models Used to Validate Their Health Claims. Trends Food Sci. Technol. 2023, 135, 74–92. [Google Scholar] [CrossRef]
- Braga, J.D.; Yang, Y.; Nagao, T.; Kato, N.; Yanaka, N.; Nishio, K.; Okada, M.; Kuroda, M.; Yamaguchi, S.; Kumrungsee, T. Fructooligosaccharides and Aspergillus Enzymes Increase Brain GABA and Homocarnosine by Modulating Microbiota in Adolescent Mice. NPJ Sci. Food 2025, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, Y.; Yang, Z.; Ying, J.; Guan, F.; Liu, B.; Miao, M.; Mohamed, A.; Wei, X.; Yang, Y.; et al. Enhancing the Synthesis Efficiency of Galacto-Oligosaccharides of a β-Galactosidase from Paenibacillus Barengoltzii by Engineering the Active and Distal Sites. Food Chem. 2025, 483, 144208. [Google Scholar] [CrossRef]
- Leangnim, N.; Shank, L.; Chanawanno, K.; Khanongnuch, C.; Kanpiengjai, A. Biotechnological Production and Current Feasible Applications of Neokestose: A Review. Carbohydr. Polym. Technol. Appl. 2025, 10, 100798. [Google Scholar] [CrossRef]
- Ryabtseva, S.; Khramtsov, A.; Shpak, M.; Lodygin, A.; Anisimov, G.; Sazanova, S.; Tabakova, Y. Biotechnology of Lactulose Production: Progress, Challenges, and Prospects. Food Process. Tech. Technol. 2023, 53, 97–122. [Google Scholar] [CrossRef]
- Panagopoulos, V.; Karabagias, I.K.; Dima, A.; Boura, K.; Kanellaki, M.; Bosnea, L.; Nigam, P.S.N.; Koutinas, A. Promotion of Lactose Isomerization to Fructose and Lactulose in One Batch by Immobilized Enzymes on Bacterial Cellulose Membranes. Food Chem. 2024, 457, 140127. [Google Scholar] [CrossRef]
- Ojima, T.; Saburi, W.; Yamamoto, T.; Mori, H.; Matsui, H. Identification and Characterization of Cellobiose 2-Epimerases from Various Aerobes. Biosci. Biotechnol. Biochem. 2013, 77, 189–193. [Google Scholar] [CrossRef]
- Huerta, M.; Cornejo, F.; Hofflinger, H.; Illanes, A.; Vera, C.; Guerrero, C. Reaction Conditions for the Isomerization and Epimerization of Lactose with a Mutant Cellobiose 2-Epimerase. LWT 2025, 217, 117380. [Google Scholar] [CrossRef]
- Chen, Q.; Levin, R.; Zhang, W.; Zhang, T.; Jiang, B.; Stressler, T.; Fischer, L.; Mu, W. Characterisation of a Novel Cellobiose 2-epimerase from Thermophilic Caldicellulosiruptor obsidiansis for Lactulose Production. J. Sci. Food Agric. 2017, 97, 3095–3105. [Google Scholar] [CrossRef]
- Wang, M.; Hua, X.; Yang, R.; Shen, Q. Immobilization of Cellobiose 2-Epimerase from Caldicellulosiruptor Saccharolyticus on Commercial Resin Duolite A568. Food Biosci. 2016, 14, 47–53. [Google Scholar] [CrossRef]
- Krewinkel, M.; Kaiser, J.; Merz, M.; Rentschler, E.; Kuschel, B.; Hinrichs, J.; Fischer, L. Novel Cellobiose 2-Epimerases for the Production of Epilactose from Milk Ultrafiltrate Containing Lactose. J. Dairy Sci. 2015, 98, 3665–3678. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, E.; Schuh, K.; Krewinkel, M.; Baur, C.; Claaßen, W.; Meyer, S.; Kuschel, B.; Stressler, T.; Fischer, L. Enzymatic Production of Lactulose and Epilactose in Milk. J. Dairy Sci. 2015, 98, 6767–6775. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; He, W.; Yan, X.; Zhang, T.; Jiang, B.; Stressler, T.; Fischer, L.; Mu, W. Construction of an Enzymatic Route Using a Food-Grade Recombinant Bacillus subtilis for the Production and Purification of Epilactose from Lactose. J. Dairy Sci. 2018, 101, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.K.; Mathiesen, G.; Pope, P.B.; Westereng, B.; La Rosa, S.L. Biochemical Characterization of Two Cellobiose 2-Epimerases and Application for Efficient Production of Lactulose and Epilactose. Curr. Res. Biotechnol. 2021, 3, 57–64. [Google Scholar] [CrossRef]
- Huerta, M.; San Martín, A.; Arancibia, B.; Cornejo, F.A.; Arenas, F.; Illanes, A.; Guerrero, C.; Vera, C. Integrating the Enzymatic Syntheses of Lactulose, Epilactose and Galacto-Oligosaccharides. Food Bioprod. Process. 2024, 147, 474–482. [Google Scholar] [CrossRef]
- Zeng, Q.; Lyu, X. Identification of a Novel Cellobiose 2-Epimerase from Acidobacteriota Bacterium and Its Application for in-Situ Milk Catalysis. Front. Microbiol. 2025, 16, 1575725. [Google Scholar] [CrossRef]
- Ito, S.; Taguchi, H.; Hamada, S.; Kawauchi, S.; Ito, H.; Senoura, T.; Watanabe, J.; Nishimukai, M.; Ito, S.; Matsui, H. Enzymatic Properties of Cellobiose 2-Epimerase from Ruminococcus Albus and the Synthesis of Rare Oligosaccharides by the Enzyme. Appl. Microbiol. Biotechnol. 2008, 79, 433–441. [Google Scholar] [CrossRef]
- Fujiwara, T.; Saburi, W.; Matsui, H.; Mori, H.; Yao, M. Structural Insights into the Epimerization of β-1,4-Linked Oligosaccharides Catalyzed by Cellobiose 2-Epimerase, the Sole Enzyme Epimerizing Non-Anomeric Hydroxyl Groups of Unmodified Sugars. J. Biol. Chem. 2014, 289, 3405–3415. [Google Scholar] [CrossRef]
- Saburi, W. Functions, Structures, and Applications of Cellobiose 2-Epimerase and Glycoside Hydrolase Family 130 Mannoside Phosphorylases. Biosci. Biotechnol. Biochem. 2016, 80, 1294–1305. [Google Scholar] [CrossRef]
- Kim, J.-E.; Kim, Y.-S.; Kang, L.-W.; Oh, D.-K. Characterization of a Recombinant Cellobiose 2-Epimerase from Dictyoglomus Turgidum That Epimerizes and Isomerizes β-1,4- and α-1,4-Gluco-Oligosaccharides. Biotechnol. Lett. 2012, 34, 2061–2068. [Google Scholar] [CrossRef]
- de Freitas, M.d.F.M.; Hortêncio, L.C.; de Albuquerque, T.L.; Rocha, M.V.P.; Gonçalves, L.R.B. Simultaneous Hydrolysis of Cheese Whey and Lactulose Production Catalyzed by β-Galactosidase from Kluyveromyces Lactis NRRL Y1564. Bioprocess Biosyst. Eng. 2020, 43, 711–722. [Google Scholar] [CrossRef]
- Ramstadab, M.V.; Markussen, S.; Ellingsen, T.E.; Skjåk-Bræk, G.; Levine, D.W. Influence of Environmental Conditions on the Activity of the Recombinant Mannuronan C-5-Epimerase AlgE2. Enzyme Microb. Technol. 2001, 28, 57–69. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Oh, D.-K. Lactulose Production from Lactose as a Single Substrate by a Thermostable Cellobiose 2-Epimerase from Caldicellulosiruptor Saccharolyticus. Bioresour. Technol. 2012, 104, 668–672. [Google Scholar] [CrossRef]
- Zhai, X.; Reinhardt, C.J.; Malabanan, M.M.; Amyes, T.L.; Richard, J.P. Enzyme Architecture: Amino Acid Side-Chains That Function To Optimize the Basicity of the Active Site Glutamate of Triosephosphate Isomerase. J. Am. Chem. Soc. 2018, 140, 8277–8286. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Stuyver, T.; Shaik, S.; Dubey, K.D. Designed Local Electric Fields—Promising Tools for Enzyme Engineering. JACS Au 2023, 3, 3259–3269. [Google Scholar] [CrossRef] [PubMed]
- Szőri-Dorogházi, E.; Maróti, G.; Szőri, M.; Nyilasi, A.; Rákhely, G.; Kovács, K.L. Analyses of the Large Subunit Histidine-Rich Motif Expose an Alternative Proton Transfer Pathway in [NiFe] Hydrogenases. PLoS ONE 2012, 7, e34666. [Google Scholar] [CrossRef]
- Zheng, C.; Ji, Z.; Mathews, I.I.; Boxer, S.G. Enhanced Active-Site Electric Field Accelerates Enzyme Catalysis. Nat. Chem. 2023, 15, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Queirós, M.; Pereira, G.; Leite, A.C.; Leal, R.; Rodrigues, R.; Teixeira, J.A.; Pereira, R.N. Tunning Pectinase Activity under the Effects of Electric Fields in the Enhanced Clarification of Wine Must. Front. Sustain. Food Syst. 2023, 7, 1053013. [Google Scholar] [CrossRef]
- Brochier, B.; Mercali, G.D.; Marczak, L.D.F. Effect of Moderate Electric Field on Peroxidase Activity, Phenolic Compounds and Color during Ohmic Heating of Sugarcane Juice. J. Food Process. Preserv. 2019, 43, e14254. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Li, C.; Huang, W.; Guo, C.; Jin, W.; Shen, W. Structural Transitions of Alpha-Amylase Treated with Pulsed Electric Fields: Effect of Coexisting Carrageenan. Foods 2022, 11, 4112. [Google Scholar] [CrossRef]
- Wang, L.H.; Pyatkovskyy, T.; Yousef, A.; Zeng, X.A.; Sastry, S.K. Mechanism of Bacillus subtilis Spore Inactivation Induced by Moderate Electric Fields. Innov. Food Sci. Emerg. Technol. 2020, 62, 102349. [Google Scholar] [CrossRef]
- Wang, H.; Wang, N.; Chen, X.; Wu, Z.; Zhong, W.; Yu, D.; Zhang, H. Effects of Moderate Electric Field on the Structural Properties and Aggregation Characteristics of Soybean Protein Isolate. Food Hydrocoll. 2022, 133, 107911. [Google Scholar] [CrossRef]
- Poojary, M.M.; Roohinejad, S.; Koubaa, M.; Barba, F.J.; Passamonti, P.; Režek Jambrak, A.; Oey, I.; Greiner, R. Impact of Pulsed Electric Fields on Enzymes. In Handbook of Electroporation; Springer International Publishing: Cham, Germany, 2017; pp. 2369–2389. [Google Scholar]
- Samaranayake, C.P.; Sastry, S.K. In-Situ Activity of α-Amylase in the Presence of Controlled-Frequency Moderate Electric Fields. LWT 2018, 90, 448–454. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Avelar, Z.; Machado, L.; Pereira, R.N.; Vicente, A.A. Electric Field Effects on Proteins—Novel Perspectives on Food and Potential Health Implications. Food Res. Int. 2020, 137, 109709. [Google Scholar] [CrossRef] [PubMed]
- Bekard, I.; Dunstan, D.E. Electric Field Induced Changes in Protein Conformation. Soft Matter 2014, 10, 431–437. [Google Scholar] [CrossRef]
- Fried, S.D.; Boxer, S.G. Electric Fields and Enzyme Catalysis. Annu. Rev. Biochem. 2017, 86, 387–415. [Google Scholar] [CrossRef]
- Hekstra, D.R.; White, K.I.; Socolich, M.A.; Henning, R.W.; Šrajer, V.; Ranganathan, R. Electric-Field-Stimulated Protein Mechanics. Nature 2016, 540, 400–405. [Google Scholar] [CrossRef]
- Zhang, Q.; Shao, D.; Xu, P.; Jiang, Z. Effects of an Electric Field on the Conformational Transition of the Protein: Pulsed and Oscillating Electric Fields with Different Frequencies. Polymers 2021, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, C.; Tao, Y.; Huang, Y.; Wang, P.; Han, Y. Changes in the Structural and Catalytic Characteristics of α-Amylase under Moderate Electric Field. Food Hydrocoll. 2022, 130, 107717. [Google Scholar] [CrossRef]
- Lu, C.; Li, F.; Yan, X.; Mao, S.; Zhang, T. Effect of Pulsed Electric Field on Soybean Isoflavone Glycosides Hydrolysis by β-Glucosidase: Investigation on Enzyme Characteristics and Assisted Reaction. Food Chem. 2022, 378, 132032. [Google Scholar] [CrossRef]
- Samaranayake, C.P.; Mok, J.H.; Heskitt, B.F.; Sastry, S.K. Nonthermal Inactivation of Polyphenol Oxidase in Apple Juice Influenced by Moderate Electric Fields: Effects of Periodic on-off and Constant Exposure Electrical Treatments. Innov. Food Sci. Emerg. Technol. 2022, 77, 102955. [Google Scholar] [CrossRef]
- Samaranayake, C.P.; Mok, J.H.; Heskitt, B.F.; Sastry, S.K. Nonthermal Inactivation Effects on Oxidative Enzymes in Grape Juice Influenced by Moderate Electric Fields: Effect of Constant Exposure Electrical Treatments Combined with Temperature. J. Food Eng. 2023, 340, 111288. [Google Scholar] [CrossRef]
- Roobab, U.; Abida, A.; Chacha, J.S.; Athar, A.; Madni, G.M.; Ranjha, M.M.A.N.; Rusu, A.V.; Zeng, X.A.; Aadil, R.M.; Trif, M. Applications of Innovative Non-Thermal Pulsed Electric Field Technology in Developing Safer and Healthier Fruit Juices. Molecules 2022, 27, 4031. [Google Scholar] [CrossRef]
- Li, D.; Yu, X.; Wang, P.; Cui, B.; Xu, E.; Tao, Y.; Han, Y. Effect of Pre-Gelatinization on α-Amylase-Catalyzed Hydrolysis of Corn Starch under Moderate Electric Field. Int. J. Biol. Macromol. 2022, 221, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Barrón-García, O.Y.; Morales-Sánchez, E.; Gaytán-Martínez, M. Inactivation Kinetics of Agaricus Bisporus Tyrosinase Treated by Ohmic Heating: Influence of Moderate Electric Field. Innov. Food Sci. Emerg. Technol. 2019, 56, 102179. [Google Scholar] [CrossRef]
- Samaranayake, C.P.; Mok, J.H.; Heskitt, B.F.; Sastry, S.K. Impact of Intermittent and Continuous Electric Fields on Peroxidase Inactivation in Orange Juice: An Experimental and Molecular Dynamics Analysis. J. Food Eng. 2024, 367, 111890. [Google Scholar] [CrossRef]
- Rajeswari; Vidyalakshmi, R.; Radhakrishnan, M.; Tito Anand, M. Exploring the Impact of Moderate Electric Field Treatment on Grape Juice: Physicochemical Characteristics and Microbial Reduction. J. Food Process Eng. 2025, 48, e70067. [Google Scholar] [CrossRef]
- Samaranayake, C.P.; Sastry, S.K. Effects of Controlled-Frequency Moderate Electric Fields on Pectin Methylesterase and Polygalacturonase Activities in Tomato Homogenate. Food Chem. 2016, 199, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Brochier, B.; Hertz, P.F.; Marczak, L.D.F.; Mercali, G.D. Influence of Ohmic Heating on Commercial Peroxidase and Sugarcane Juice Peroxidase Inactivation. J. Food Eng. 2020, 284, 110066. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhang, L.; Xu, T.; Ding, K. Improving Lignocellulose Enzymatic Saccharification in a Bioreactor with an Applied Electric Field. Ind. Crops Prod. 2017, 109, 404–409. [Google Scholar] [CrossRef]
- Horlacher, N.; Oey, I.; Leong, S.Y. Effect of Pulsed Electric Field Processing on Microbial and Enzyme Inactivation in Blended Plant-Based Milk Alternatives: A Case Study on a Microbial Challenge Test for a Non-Presterilized Oat-Based Beverage Enriched with Pea Protein. Innov. Food Sci. Emerg. Technol. 2024, 94, 103699. [Google Scholar] [CrossRef]
- Barrón-García, O.Y.; Gaytán-Martínez, M.; Ramírez-Jiménez, A.K.; Luzardo-Ocampo, I.; Velazquez, G.; Morales-Sánchez, E. Physicochemical Characterization and Polyphenol Oxidase Inactivation of Ataulfo Mango Pulp Pasteurized by Conventional and Ohmic Heating Processes. LWT 2021, 143, 111113. [Google Scholar] [CrossRef]
- He, C.; Yang, N.; Jin, Y.; Wu, S.; Pan, Y.; Xu, X.; Jin, Z. Application of Induced Electric Field for Inner Heating of Kiwifruit Juice and Its Analysis. J. Food Eng. 2021, 306, 110609. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Zhao, T.; Yang, X.; Zhang, J.; Yang, H. Bioavailability and Mechanisms of Dietary Polyphenols Affected by Non-Thermal Processing Technology in Fruits and Vegetables. Curr. Res. Food Sci. 2024, 8, 100715. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Hamada, S.; Ito, H.; Matsui, H.; Ozawa, T.; Taguchi, H.; Ito, S. Site-Directed Mutagenesis of Possible Catalytic Residues of Cellobiose 2-Epimerase from Ruminococcus Albus. Biotechnol. Lett. 2009, 31, 1065–1071. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Polyphenol Extraction from Food (by) Products by Pulsed Electric Field: A Review. Int. J. Mol. Sci. 2023, 24, 15914. [Google Scholar] [CrossRef] [PubMed]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed Electric Field (PEF): Avant-Garde Extraction Escalation Technology in Food Industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Ghoshal, G. Comprehensive Review on Pulsed Electric Field in Food Preservation: Gaps in Current Studies for Potential Future Research. Heliyon 2023, 9, e17532. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.; Avelar, Z.; Leite, A.C.; Leal, R.; Pereira, R.S.; Vicente, A. Electrical Fields in the Processing of Protein-Based Foods. Foods 2024, 13, 577. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, R.; Hu, J.; Luan, Y.; Liu, R.; Ge, Q.; Yu, H.; Wu, M. Moderate Pulsed Electric Field-Induced Structural Unfolding Ameliorated the Gelling Properties of Porcine Muscle Myofibrillar Protein. Innov. Food Sci. Emerg. Technol. 2022, 81, 103145. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Vicente, A.A.; Petersen, S.B.; Pereira, R.N. Electric Field Effects on β-Lactoglobulin Thermal Unfolding as a Function of PH—Impact on Protein Functionality. Innov. Food Sci. Emerg. Technol. 2019, 52, 1–7. [Google Scholar] [CrossRef]
- Sjöblom, J.; Mhatre, S.; Simon, S.; Skartlien, R.; Sørland, G. Emulsions in External Electric Fields. Adv. Colloid Interface Sci. 2021, 294, 102455. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xiao, S.; Wang, L.; Niu, D.; Gao, W.; Zeng, X.-A.; Woo, M.; Han, Z.; Wang, R. Pulsed Electric Field Enhances Glucose Glycation and Emulsifying Properties of Bovine Serum Albumin: Focus on Polarization and Ionization Effects at a High Reaction Temperature. Int. J. Biol. Macromol. 2024, 257, 128509. [Google Scholar] [CrossRef] [PubMed]
- Rozynek, Z.; Bielas, R.; Józefczak, A. Efficient Formation of Oil-in-Oil Pickering Emulsions with Narrow Size Distributions by Using Electric Fields. Soft Matter 2018, 14, 5140–5149. [Google Scholar] [CrossRef]
- Samaranayake, C.P.; Sastry, S.K. Effect of Moderate Electric Fields on Inactivation Kinetics of Pectin Methylesterase in Tomatoes: The Roles of Electric Field Strength and Temperature. J. Food Eng. 2016, 186, 17–26. [Google Scholar] [CrossRef]
- Chen, Q.; Xiao, Y.; Zhang, W.; Stressler, T.; Fischer, L.; Jiang, B.; Mu, W. Computer-Aided Search for a Cold-Active Cellobiose 2-Epimerase. J. Dairy Sci. 2020, 103, 7730–7741. [Google Scholar] [CrossRef]
- Dubey, K.D.; Stuyver, T.; Shaik, S. Local Electric Fields: From Enzyme Catalysis to Synthetic Catalyst Design. J. Phys. Chem. B 2022, 126, 10285–10294. [Google Scholar] [CrossRef]
- Singh, A.K.; Sathaye, S.B.; Rai, A.K.; Singh, S.P. Novel Cellobiose 2-Epimerase from Thermal Aquatic Metagenome for the Production of Epilactose. J. Agric. Food Chem. 2025, 73, 9690–9700. [Google Scholar] [CrossRef]
- Wang, M.; Yang, R.; Hua, X.; Shen, Q.; Zhang, W.; Zhao, W. Lactulose Production from Lactose by Recombinant Cellobiose 2-epimerase in Permeabilised Escherichia coli Cells. Int. J. Food Sci. Technol. 2015, 50, 1625–1631. [Google Scholar] [CrossRef]
- Yu, S.; Vermeeren, P.; Hamlin, T.A.; Bickelhaupt, F.M. How Oriented External Electric Fields Modulate Reactivity. Chem. Eur. J. 2021, 27, 5683–5693. [Google Scholar] [CrossRef]
- Subaşı, B.G.; Jahromi, M.; Casanova, F.; Capanoglu, E.; Ajalloueian, F.; Mohammadifar, M.A. Effect of Moderate Electric Field on Structural and Thermo-Physical Properties of Sunflower Protein and Sodium Caseinate. Innov. Food Sci. Emerg. Technol. 2021, 67, 102593. [Google Scholar] [CrossRef]
- Welborn, V.V.; Head-Gordon, T. Fluctuations of Electric Fields in the Active Site of the Enzyme Ketosteroid Isomerase. J. Am. Chem. Soc. 2019, 141, 12487–12492. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albuquerque, T.L.d.; Pereira, R.N.; Silvério, S.C.; Rodrigues, L.R. Modulation of Enzymatic Activity by Moderate Electric Fields: Perspectives for Prebiotic Epilactose Production via Cellobiose-2-Epimerase. Processes 2025, 13, 2671. https://doi.org/10.3390/pr13092671
Albuquerque TLd, Pereira RN, Silvério SC, Rodrigues LR. Modulation of Enzymatic Activity by Moderate Electric Fields: Perspectives for Prebiotic Epilactose Production via Cellobiose-2-Epimerase. Processes. 2025; 13(9):2671. https://doi.org/10.3390/pr13092671
Chicago/Turabian StyleAlbuquerque, Tiago Lima de, Ricardo N. Pereira, Sara C. Silvério, and Lígia R. Rodrigues. 2025. "Modulation of Enzymatic Activity by Moderate Electric Fields: Perspectives for Prebiotic Epilactose Production via Cellobiose-2-Epimerase" Processes 13, no. 9: 2671. https://doi.org/10.3390/pr13092671
APA StyleAlbuquerque, T. L. d., Pereira, R. N., Silvério, S. C., & Rodrigues, L. R. (2025). Modulation of Enzymatic Activity by Moderate Electric Fields: Perspectives for Prebiotic Epilactose Production via Cellobiose-2-Epimerase. Processes, 13(9), 2671. https://doi.org/10.3390/pr13092671








