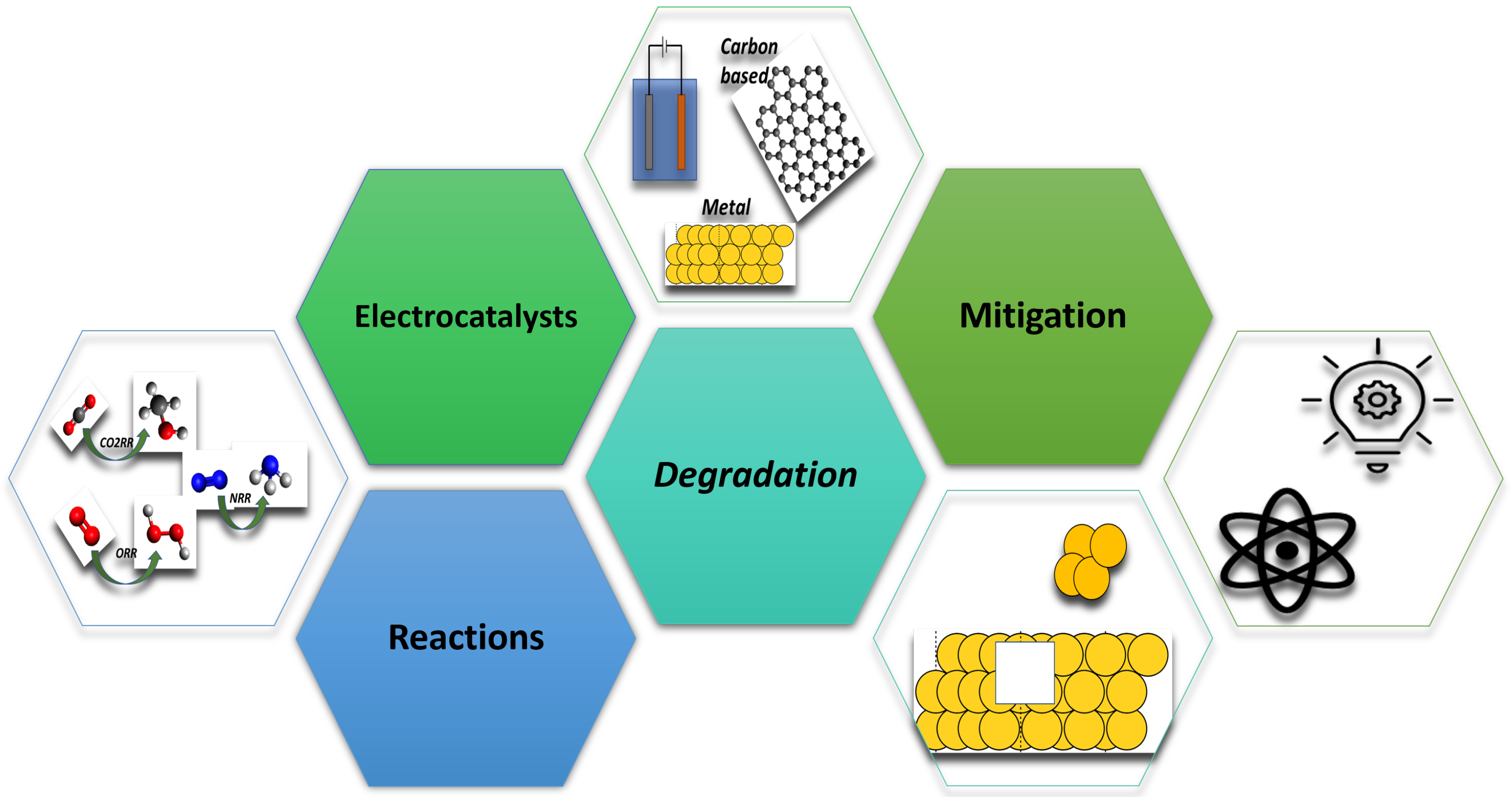
Overview of Topics in Electrocatalysis for Sustainability: Reactions, Electrocatalysts, Degradation, and Mitigation
Abstract
1. Introduction
2. Types of Electrocatalytic Reactions
2.1. Oxygen Reduction Reaction (ORR)
2.2. Hydrogen Evolution Reaction (HER)
2.3. Hydrogen Oxidation Reaction (HOR)
2.4. Oxygen Evolution Reaction (OER)
2.5. Carbon Dioxide Reduction Reaction (CO2RR)
2.6. Nitrogen Reduction Reaction (NRR)
3. Electrocatalysts
3.1. Noble Metal-Based Electrocatalysts
3.1.1. Platinum (Pt)
3.1.2. Other PGMs (Pd, Ir, Ru)
3.2. Non-Noble Metal Electrocatalysts
3.2.1. Metal–Nitrogen–Carbon (M-N-C) Catalysts
3.2.2. Metal Oxides and Hydroxides
3.2.3. Metal Chalcogenides (Sulfides, Selenides)
3.2.4. Metal Phosphides
3.2.5. Metal Carbides and Nitrides
3.3. Metal-Free Carbon-Based Electrocatalysts
3.3.1. Heteroatom-Doped Carbons
3.3.2. Defect-Engineered Carbons
3.3.3. Hybrid Carbon Materials
4. Mechanisms for Degradation
4.1. Corrosion and Oxidation
4.1.1. Carbon Support Corrosion
4.1.2. Metal Oxidation and Dissolution
4.2. Leaching of Active Sites
4.3. Surface Reconstruction
4.4. Poisoning by Reaction Intermediates and Impurities
5. Factors Influencing Degradation
6. Mitigation Strategies
6.1. Composition Engineering
6.2. Structure Modifications
6.3. Interfacial Engineering
6.4. Microenvironment Engineering
6.5. Developing New Materials
7. Techno-Economic and Life-Cycle Considerations
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RHE | Reversible Hydrogen Electrode |
| OER | Oxygen Evolution Reaction |
| ORR | Oxygen Reduction Reaction |
| HER | Hydrogen Evolution Reaction |
| HOR | Hydrogen Oxidation Reaction |
| CO2RR | CO2 Reduction Reaction |
| NRR | Nitrogen Reduction Reaction |
| PGMs | Platinum Group Metals |
| ROS | Reactive Oxygen Species |
| PEMFCs | Proton Exchange Membrane Fuel Cells |
| AEMFCs | Anion Exchange Membrane Fuel Cells |
| M-N-C | Metal–Nitrogen–Carbon |
| TMOs | Transition Metal Oxides |
| TMCs | Transition Metal Chalcogenides |
| MOF | Metal–Organic Framework |
References
- Sherrell, P.C.; Iesalnieks, M.; Ehrnst, Y.; Rezk, A.R.; Šutka, A. Electrocatalysis for Green (er) Chemistry: Limitations and Opportunities with Traditional and Emerging Characterization Methods for Tangible Societal Impact. Adv. Energy Sustain. Res. 2024, 5, 2400008. [Google Scholar] [CrossRef]
- Dey, S.; Mondal, B.; Chatterjee, S.; Rana, A.; Amanullah, S.; Dey, A. Molecular electrocatalysts for the oxygen reduction reaction. Nat. Rev. Chem. 2017, 1, 0098. [Google Scholar] [CrossRef]
- Petersen, H.; Myren, T.; O’Sullivan, S.; Luca, O. Electrochemical methods for materials recycling. Mater. Adv. 2021, 2, 1113–1138. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Overa, S.; Ko, B.H.; Zhao, Y.; Jiao, F. Electrochemical approaches for CO2 conversion to chemicals: A journey toward practical applications. Acc. Chem. Res. 2022, 55, 638–648. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent progresses in oxygen reduction reaction electrocatalysts for electrochemical energy applications. Electrochem. Energy Rev. 2019, 2, 518–538. [Google Scholar] [CrossRef]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Topalov, A.A.; Kostka, A.; Schüth, F.; Mayrhofer, K.J.J. Degradation Mechanisms of Pt/C Fuel Cell Catalysts under Simulated Start–Stop Conditions. ACS Catal. 2012, 2, 832–843. [Google Scholar] [CrossRef]
- Risch, M. Upgrading the detection of electrocatalyst degradation during the oxygen evolution reaction. Curr. Opin. Electrochem. 2023, 38, 101247. [Google Scholar] [CrossRef]
- Pletcher, D. Electrocatalysis: Present and future. J. Appl. Electrochem. 1984, 14, 403–415. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative strategies for electrocatalytic water splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Xiaoding, X.; Moulijn, J. Mitigation of CO2 by chemical conversion: Plausible chemical reactions and promising products. Energ. Fuel. 1996, 10, 305–325. [Google Scholar] [CrossRef]
- Taheri Najafabadi, A. CO2 chemical conversion to useful products: An engineering insight to the latest advances toward sustainability. Int. J. Energ. Res. 2013, 37, 485–499. [Google Scholar] [CrossRef]
- Liu, H.; Wei, L.; Liu, F.; Pei, Z.; Shi, J.; Wang, Z.j.; He, D.; Chen, Y. Homogeneous, heterogeneous, and biological catalysts for electrochemical N2 reduction toward NH3 under ambient conditions. ACS Catal. 2019, 9, 5245–5267. [Google Scholar] [CrossRef]
- Geiger, S.; Kasian, O.; Ledendecker, M.; Pizzutilo, E.; Mingers, A.M.; Fu, W.T.; Diaz-Morales, O.; Li, Z.; Oellers, T.; Fruchter, L.; et al. The stability number as a metric for electrocatalyst stability benchmarking. Nat. Catal. 2018, 1, 508–515. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, Y.; Tang, Z. Insight into structural evolution, active sites, and stability of heterogeneous electrocatalysts. Angew. Chem. Int. Ed. 2022, 61, e202110186. [Google Scholar]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2019, 120, 851–918. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Balasubramaniam, R. Uncoupled non-linear equations method for determining kinetic parameters in case of hydrogen evolution reaction following Volmer–Heyrovsky–Tafel mechanism and Volmer–Heyrovsky mechanism. Int. J. Hydrogen Energy 2008, 33, 2178–2188. [Google Scholar] [CrossRef]
- Bullock, R.M.; Helm, M.L. Molecular electrocatalysts for oxidation of hydrogen using earth-abundant metals: Shoving protons around with proton relays. Acc. Chem. Res. 2015, 48, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.C.; Tang, T.; Jiang, Z.; Wang, L.; Hu, J.S.; Wan, L.J. Electrocatalytic hydrogen oxidation in alkaline media: From mechanistic insights to catalyst design. ACS Nano 2022, 16, 5153–5183. [Google Scholar] [CrossRef]
- Cong, Y.; Yi, B.; Song, Y. Hydrogen oxidation reaction in alkaline media: From mechanism to recent electrocatalysts. Nano Energy 2018, 44, 288–303. [Google Scholar] [CrossRef]
- Shao, M. Palladium-based electrocatalysts for hydrogen oxidation and oxygen reduction reactions. J. Power Sources 2011, 196, 2433–2444. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, S.; Deng, J.; Zhang, W.; Feng, Y.; Ma, J. Transition metal carbides in electrocatalytic oxygen evolution reaction. Chin. Chem. Lett. 2021, 32, 291–298. [Google Scholar] [CrossRef]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.J.; Wang, Z.L. Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Lee, D.U.; Xu, P.; Cano, Z.P.; Kashkooli, A.G.; Park, M.G.; Chen, Z. Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal–air batteries. J. Mater. Chem. A 2016, 4, 7107–7134. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Goh, K.; Zhao, L.; Sui, X.L.; Gong, X.F.; Cai, J.J.; Zhou, Q.Y.; Zhang, H.D.; Li, L.; Kong, F.R.; et al. Advanced non-noble materials in bifunctional catalysts for ORR and OER toward aqueous metal–air batteries. Nanoscale 2020, 12, 21534–21559. [Google Scholar] [CrossRef]
- Eftekhari, A. Tuning the electrocatalysts for oxygen evolution reaction. Mater. Today Energy 2017, 5, 37–57. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, R.; Yao, Z.; Wang, T.; Li, Q. Effective approaches for enhancing the stability of ruthenium-based electrocatalysts towards acidic oxygen evolution reaction. Chin. Chem. Lett. 2024, 35, 109416. [Google Scholar] [CrossRef]
- Wan, R.; Yuan, T.; Wang, L.; Li, B.; Liu, M.; Zhao, B. Earth-abundant electrocatalysts for acidic oxygen evolution. Nat. Catal. 2024, 7, 1288–1304. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, J.; He, G.; Chen, H. Current and future trends for spinel-type electrocatalysts in electrocatalytic oxygen evolution reaction. Coord. Chem. Rev. 2023, 475, 214869. [Google Scholar] [CrossRef]
- Hu, X.; Wang, R.; Feng, W.; Xu, C.; Wei, Z. Electrocatalytic oxygen evolution activities of metal chalcogenides and phosphides: Fundamentals, origins, and future strategies. J. Energy Chem. 2023, 81, 167–191. [Google Scholar] [CrossRef]
- Li, L.; Cao, X.; Huo, J.; Qu, J.; Chen, W.; Liu, C.; Zhao, Y.; Liu, H.; Wang, G. High valence metals engineering strategies of Fe/Co/Ni-based catalysts for boosted OER electrocatalysis. J. Energy Chem. 2023, 76, 195–213. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liu, J.; Wu, H.; Wu, K.; Lyu, C.; Wu, J.; Lau, W.M.; Wu, Q.; Zheng, J. The role of manganese-based catalyst in electrocatalytic water splitting: Recent research and progress. Mater. Today Phys. 2023, 36, 101169. [Google Scholar] [CrossRef]
- Kumar, L.; Antil, B.; Kumar, A.; Das, M.R.; López-Estrada, O.; Siahrostami, S.; Deka, S. Experimental and computational insights into the overall water splitting reaction by the Fe–Co–Ni–P electrocatalyst. ACS Appl. Mater. Interfaces 2023, 15, 54446–54457. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wang, Z.; Bao, A.; Tang, X.; Huang, X.; Yi, H.; Zhao, S.; Sun, T.; Wang, J.; Gao, F. Recent advances on electrocatalytic CO2 reduction to resources: Target products, reaction pathways and typical catalysts. Chem. Eng. J. 2023, 453, 139663. [Google Scholar] [CrossRef]
- Perry, S.C.; Leung, P.k.; Wang, L.; de León, C.P. Developments on carbon dioxide reduction: Their promise, achievements, and challenges. Curr. Opin. Electrochem. 2020, 20, 88–98. [Google Scholar] [CrossRef]
- Jeyachandran, N.; Yuan, W.; Giordano, C. Cutting-Edge Electrocatalysts for CO2RR. Molecules 2023, 28, 3504. [Google Scholar] [CrossRef]
- Saha, P.; Amanullah, S.; Dey, A. Selectivity in electrochemical CO2 reduction. Acc. Chem. Res. 2022, 55, 134–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Zhang, X.; Bond, A.M.; Zhang, J. Mechanistic understanding of the electrocatalytic CO2 reduction reaction – New developments based on advanced instrumental techniques. Nano Today 2020, 31, 100835. [Google Scholar] [CrossRef]
- Wu, H.; Singh-Morgan, A.; Qi, K.; Zeng, Z.; Mougel, V.; Voiry, D. Electrocatalyst microenvironment engineering for enhanced product selectivity in carbon dioxide and nitrogen reduction reactions. ACS Catal. 2023, 13, 5375–5396. [Google Scholar] [CrossRef]
- Raciti, D.; Wang, C. Recent advances in CO2 reduction electrocatalysis on copper. ACS Energy Lett. 2018, 3, 1545–1556. [Google Scholar] [CrossRef]
- Chen, T.W.; Chen, S.M.; Anushya, G.; Kannan, R.; G. Al-Sehemi, A.; Alargarsamy, S.; Gajendran, P.; Ramachandran, R. Development of different kinds of electrocatalyst for the electrochemical reduction of carbon dioxide reactions: An overview. Molecules 2023, 28, 7016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 reduction: From homogeneous to heterogeneous electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264. [Google Scholar] [CrossRef]
- Tavella, F.; Giusi, D.; Ampelli, C. Nitrogen reduction reaction to ammonia at ambient conditions: A short review analysis of the critical factors limiting electrocatalytic performance. Curr. Opin. Green Sustain. Chem. 2022, 35, 100604. [Google Scholar] [CrossRef]
- Wan, Y.; Xu, J.; Lv, R. Heterogeneous electrocatalysts design for nitrogen reduction reaction under ambient conditions. Mater. Today 2019, 27, 69–90. [Google Scholar] [CrossRef]
- Majumder, M.; Saini, H.; Dedek, I.; Schneemann, A.; Chodankar, N.R.; Ramarao, V.; Santosh, M.S.; Nanjundan, A.K.; Kment, S.; Dubal, D.; et al. Rational design of graphene derivatives for electrochemical reduction of nitrogen to ammonia. ACS Nano 2021, 15, 17275–17298. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.W.; Chang, N.; Tsai, M.L.; Chen, C.L. Decarbonizing the energy supply chain: Ammonia as an energy carrier for renewable power systems. Fuel 2024, 360, 130627. [Google Scholar] [CrossRef]
- Shetty, A.U.; Sankannavar, R. Exploring nitrogen reduction reaction mechanisms in electrocatalytic ammonia synthesis: A comprehensive review. J. Energy Chem. 2024, 92, 681–697. [Google Scholar] [CrossRef]
- Qing, G.; Ghazfar, R.; Jackowski, S.T.; Habibzadeh, F.; Ashtiani, M.M.; Chen, C.P.; Smith, M.R., III; Hamann, T.W. Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem. Rev. 2020, 120, 5437–5516. [Google Scholar] [CrossRef]
- Tang, T.; Wang, Z.; Guan, J. A review of defect engineering in two-dimensional materials for electrocatalytic hydrogen evolution reaction. Chin. J. Catal. 2022, 43, 636–678. [Google Scholar] [CrossRef]
- Zhao, X.; Sasaki, K. Advanced Pt-based core–shell electrocatalysts for fuel cell cathodes. Acc. Chem. Res. 2022, 55, 1226–1236. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Ke, C.; Wei, G.; Priest, C.; Qiao, Z.; Wu, G.; Zhang, J. Platinum-group-metal catalysts for proton exchange membrane fuel cells: From catalyst design to electrode structure optimization. EnergyChem 2020, 2, 100023. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H. Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 2013, 46, 1848–1857. [Google Scholar] [CrossRef]
- Huang, L.; Zaman, S.; Tian, X.; Wang, Z.; Fang, W.; Xia, B.Y. Advanced platinum-based oxygen reduction electrocatalysts for fuel cells. Acc. Chem. Res. 2021, 54, 311–322. [Google Scholar] [CrossRef]
- Mukoyoshi, M.; Kitagawa, H. Nanoalloys Composed of Platinum Group Metals and p-Block Elements for Innovative Catalysis. Adv. Energy Sustain. Res. 2025, 6, 2400270. [Google Scholar] [CrossRef]
- Kumar, K.; Dubau, L.; Jaouen, F.; Maillard, F. Review on the degradation mechanisms of metal-NC catalysts for the oxygen reduction reaction in acid electrolyte: Current understanding and mitigation approaches. Chem. Rev. 2023, 123, 9265–9326. [Google Scholar] [CrossRef]
- Kumar, L.; Antil, B.; Kumar, A.; Das, M.R.; Deka, S. A superior and stable electrocatalytic oxygen evolution reaction by one-dimensional FeCoP colloidal nanostructures. ACS Appl. Mater. Interfaces 2022, 14, 5468–5477. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zelenay, P. Activity versus stability of atomically dispersed transition-metal electrocatalysts. Nat. Rev. Mater. 2024, 9, 643–656. [Google Scholar] [CrossRef]
- Manjunatha, R.; Karajić, A.; Liu, M.; Zhai, Z.; Dong, L.; Yan, W.; Wilkinson, D.P.; Zhang, J. A review of composite/hybrid electrocatalysts and photocatalysts for nitrogen reduction reactions: Advanced materials, mechanisms, challenges and perspectives. Electrochem. Energy Rev. 2020, 3, 506–540. [Google Scholar] [CrossRef]
- Alom, M.S.; Kananke-Gamage, C.C.; Ramezanipour, F. Perovskite oxides as electrocatalysts for hydrogen evolution reaction. ACS Omega 2022, 7, 7444–7451. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Liu, H.; Chu, W.; Wu, C.; Xie, Y. Structural transformation of heterogeneous materials for electrocatalytic oxygen evolution reaction. Chem. Rev. 2021, 121, 13174–13212. [Google Scholar] [CrossRef]
- Zeb, Z.; Huang, Y.; Chen, L.; Zhou, W.; Liao, M.; Jiang, Y.; Li, H.; Wang, L.; Lin, W.; Wang, H.; et al. Comprehensive overview of polyoxometalates for electrocatalytic hydrogen evolution reaction. Coordin. Chem. Rev. 2023, 482, 215058. [Google Scholar] [CrossRef]
- Jin, W.; Maduraiveeran, G. Recent advances of porous transition metal-based nanomaterials for electrochemical energy conversion and storage applications. Mater. Today Energy 2019, 13, 64–84. [Google Scholar] [CrossRef]
- Su, H.; Pan, X.; Li, S.; Zhang, H.; Zou, R. Defect-engineered two-dimensional transition metal dichalcogenides towards electrocatalytic hydrogen evolution reaction. Carbon Energy 2023, 5, e296. [Google Scholar] [CrossRef]
- Tan, Z.H.; Kong, X.Y.; Ng, B.J.; Soo, H.S.; Mohamed, A.R.; Chai, S.P. Recent advances in defect-engineered transition metal dichalcogenides for enhanced electrocatalytic hydrogen evolution: Perfecting imperfections. ACS Omega 2023, 8, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, L.; Wang, L.; Cheng, F. The electrochemical tuning of transition metal-based materials for electrocatalysis. Electrochem. Energy Rev. 2021, 4, 146–168. [Google Scholar] [CrossRef]
- Nath, M.; Singh, H.; Saxena, A. Progress of transition metal chalcogenides as efficient electrocatalysts for energy conversion. Curr. Opin. Electrochem. 2022, 34, 100993. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, S.; Zheng, Y.; Rong, X.; Bai, M.; Tang, Y.; Ma, T.; Cheng, C.; Zhao, C. Emerging electrocatalytic activities in transition metal selenides: Synthesis, electronic modulation, and structure-performance correlations. Chem. Eng. J. 2023, 451, 138514. [Google Scholar] [CrossRef]
- Yang, W.; Chen, S. Recent progress in electrode fabrication for electrocatalytic hydrogen evolution reaction: A mini review. Chem. Eng. J. 2020, 393, 124726. [Google Scholar] [CrossRef]
- Singh, S.; Patidar, R.; Srivastava, V.C.; Lo, S.L.; Nidheesh, P. A critical review on the degradation mechanism of textile effluent during electrocatalytic oxidation: Removal optimization and degradation pathways. J. Environ. Chem. Eng. 2023, 11, 111277. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, H.; Qian, T.; Yan, C. Interfacial engineering of carbon-based materials for efficient electrocatalysis: Recent advances and future. EnergyChem 2022, 4, 100074. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Z.; Dai, L. Carbon-based electrocatalysts for advanced energy conversion and storage. Sci. Adv. 2015, 1, e1500564. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. NPJ Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Jia, Y.; Yao, X. Defects in carbon-based materials for electrocatalysis: Synthesis, recognition, and advances. Acc. Chem. Res. 2023, 56, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Graphene-based electrocatalysts: Hydrogen evolution reactions and overall water splitting. Int. J. Hydrogen Energy 2021, 46, 21401–21418. [Google Scholar] [CrossRef]
- Liu, T.; You, J.; Zhao, Y.; Zhang, J.; Wang, J. Progress of 3D Graphene-Based Electrocatalytic Oxygen Evolution Reaction Catalysts. Langmuir 2025, 41, 7965–7979. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Peng, Y.; Hu, X.; Miao, L.; Ishizaki, T. Recent progress of carbon-based electrocatalytic materials in Lithium-based batteries. Sustain. Mater. Technol. 2022, 32, e00384. [Google Scholar] [CrossRef]
- Qiu, R.; Ma, D.; Zheng, H.; Liu, M.; Cai, J.; Yan, W.; Zhang, J. Performance degradation mechanisms and mitigation strategies of hard carbon anode and solid electrolyte interface for sodium-ion battery. Nano Energy 2024, 128, 109920. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Y. Degradation of carbon materials in electrocatalysis. Curr. Opin. Electrochem. 2022, 36, 101159. [Google Scholar] [CrossRef]
- Singh, H.; Zhuang, S.; Ingis, B.; Nunna, B.B.; Lee, E.S. Carbon-based catalysts for oxygen reduction reaction: A review on degradation mechanisms. Carbon 2019, 151, 160–174. [Google Scholar] [CrossRef]
- Bodner, M.; Senn, J.; Hacker, V. Degradation mechanisms and their lifetime. In Fuel Cells and Hydrogen; Elsevier: Amsterdam, The Netherlands, 2018; pp. 139–154. [Google Scholar]
- Wei, X.; Wang, R.Z.; Zhao, W.; Chen, G.; Chai, M.R.; Zhang, L.; Zhang, J. Recent research progress in PEM fuel cell electrocatalyst degradation and mitigation strategies. EnergyChem 2021, 3, 100061. [Google Scholar] [CrossRef]
- Chowdury, M.S.K.; Park, Y.; Park, S.B.; Park, Y.i. Degradation Mechanisms, Long-Term durability Challenges, and mitigation methods for proton exchange membranes and membrane electrode assemblies with Pt/C electrocatalysts in Low-Temperature and High-Temperature fuel Cells: A comprehensive review. J. Electroanal. Chem. 2024, 975, 118712. [Google Scholar] [CrossRef]
- Li, L.; Hu, L.; Li, J.; Wei, Z. Enhanced stability of Pt nanoparticle electrocatalysts for fuel cells. Nano Res. 2015, 8, 418–440. [Google Scholar] [CrossRef]
- Yu, S.; Yu, X.; Yang, H.; Li, F.; Li, S.; Kang, Y.S.; Zheng, J.Y. Mechanism, modification and stability of tungsten oxide-based electrocatalysts for water splitting: A review. J. Energy Chem. 2024, 99, 23–49. [Google Scholar] [CrossRef]
- Liu, W.; Su, X.; Wu, Y.; Yi, G.; Guo, X.; Shi, S.; Zhang, C.; Zhang, Y. A comprehensive review of PbO2 electrodes in electrocatalytic degradation of organic pollutants. Environ. Res. 2025, 279, 121885. [Google Scholar] [CrossRef]
- Jones, T.E.; Teschner, D.; Piccinin, S. Toward realistic models of the electrocatalytic oxygen evolution reaction. Chem. Rev. 2024, 124, 9136–9223. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.H.; Girod, R.; Tileli, V. Insights into electrocatalyst transformations studied in real time with electrochemical liquid-phase transmission electron microscopy. Acc. Chem. Res. 2023, 56, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yang, R.; Zeng, Z. Advances in Electrochemical Liquid-Phase Transmission Electron Microscopy for Visualizing Rechargeable Battery Reactions. ACS Nano 2024, 18, 12598–12609. [Google Scholar] [CrossRef]
- Fratarcangeli, M.; Vigil, S.A.; Moreno-Hernandez, I.A. Understanding Electrochemical Degradation via Liquid Phase Transmission Electron Microscopy. J. Phys. Chem. C 2025, 129, 7612–7624. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, Y.; Zeng, R.; Lu, X.; Krumov, M.; Huang, X.; Xu, W.; Wang, H.; DiSalvo, F.J.; Brock, J.D.; et al. Operando methods in electrocatalysis. ACS Catal. 2021, 11, 1136–1178. [Google Scholar] [CrossRef]
- Liu, S.; D’Amario, L.; Jiang, S.; Dau, H. Selected applications of operando Raman spectroscopy in electrocatalysis research. Curr. Opin. Electrochem. 2022, 35, 101042. [Google Scholar] [CrossRef]
- Claudel, F.; Dubau, L.; Berthomé, G.; Sola-Hernandez, L.; Beauger, C.; Piccolo, L.; Maillard, F. Degradation mechanisms of oxygen evolution reaction electrocatalysts: A combined identical-location transmission electron microscopy and X-ray photoelectron spectroscopy study. ACS Catal. 2019, 9, 4688–4698. [Google Scholar] [CrossRef]
- Pittkowski, R.K. Shedding Light on Electrocatalysts: Practical Considerations for Operando Studies with High-Energy X-Rays. ChemElectroChem 2024, 11, e202400171. [Google Scholar] [CrossRef]
- Simondson, D.; Tesch, M.F.; Spanos, I.; Jones, T.E.; Guo, J.; Kerr, B.V.; Chatti, M.; Bonke, S.A.; Golnak, R.; Johannessen, B.; et al. Decoupling the catalytic and degradation mechanisms of cobalt active sites during acidic water oxidation. Nat. Energy 2025, 10, 1013–1024. [Google Scholar] [CrossRef]
- Prajapati, A.; Hahn, C.; Weidinger, I.M.; Shi, Y.; Lee, Y.; Alexandrova, A.N.; Thompson, D.; Bare, S.R.; Chen, S.; Yan, S.; et al. Best practices for in situ and operando techniques within electrocatalytic systems. Nat. Commun. 2025, 16, 2593. [Google Scholar] [CrossRef]
- Perry, M.L.; Patterson, T.; Reiser, C. Systems strategies to mitigate carbon corrosion in fuel cells. ECS Trans. 2006, 3, 783. [Google Scholar] [CrossRef]
- Patil, V.; Reshmi, P.; Prajna, S.; Yashaswi; Yashaswini; Haleshappa, D.; Jayarama, A.; Pinto, R. Degradation mechanisms in PEM fuel cells: A brief review. Mater. Today Proc. 2023; in press. [Google Scholar]
- Mohtadi, R.; Lee, W.K.; Van Zee, J. The effect of temperature on the adsorption rate of hydrogen sulfide on Pt anodes in a PEMFC. Appl. Catal. B 2005, 56, 37–42. [Google Scholar] [CrossRef]
- Uribe, F.A.; Gottesfeld, S.; Zawodzinski, T.A. Effect of ammonia as potential fuel impurity on proton exchange membrane fuel cell performance. J. Electrochem. Soc. 2002, 149, A293. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Z.J. Mechanistic Insights into Cation Effects in Electrolytes for Electrocatalysis. Angew. Chem. Int. Ed. 2025, 64, e202505022. [Google Scholar]
- Vos, J.G.; Venugopal, A.; Smith, W.A.; Koper, M.T. Competition and interhalogen formation during parallel electrocatalytic oxidation of bromide and chloride on Pt. J. Electrochem. Soc. 2020, 167, 046505. [Google Scholar] [CrossRef]
- Xiao, Y.; Xia, C.; Qian, Q.; Chen, J.; Wang, X.; Park, H.S.; Xia, B.Y. Halogen Cocatalysis in Electrocatalytic Systems. Small 2025, e05412. [Google Scholar] [CrossRef]
- Navodye, S.K.; Gunasooriya, G.K.K. Acid electrolyte anions adsorption effects on IrO2 electrocatalysts for oxygen evolution reaction. J. Phys. Chem. C 2024, 128, 6041–6052. [Google Scholar] [CrossRef]
- Li, L.; Yang, H.; Yuan, Z.; Tan, Y.; Zhang, Y.; Miao, C.; Chen, D.; Li, G.; Han, W. The organic ligand etching method for constructing in situ terraced protective layer toward stable aqueous Zn anode. Small 2023, 19, 2305554. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jiang, J.; Li, G.; Song, T.; Pei, Y.; Wang, X.; Wu, X.; Chen, L.; Deng, Q.; Long, B. Co-solvent electrolyte induces hybrid solid electrolyte interphase for ultra-stable zinc-ion batteries. J. Energy Storage 2024, 99, 113354. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, C.; Zeng, Y.; Spendelow, J.S.; Wu, G. Advanced nanocarbons for enhanced performance and durability of platinum catalysts in proton exchange membrane fuel cells. Small 2021, 17, 2006805. [Google Scholar] [CrossRef] [PubMed]
- Nemiwal, M.; Gosu, V.; Zhang, T.C.; Kumar, D. Metal organic frameworks as electrocatalysts: Hydrogen evolution reactions and overall water splitting. Int. J. Hydrogen Energy 2021, 46, 10216–10238. [Google Scholar] [CrossRef]
- Cherevko, S. Stability and dissolution of electrocatalysts: Building the bridge between model and “real world” systems. Curr. Opin. Electrochem. 2018, 8, 118–125. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, D.; Yao, H.; Chu, T.; Li, B. Investigation of Performance Degradation and Control Strategies of PEMFC under Three Typical Operating Conditions. J. Electrochem. Soc. 2024, 171, 054510. [Google Scholar] [CrossRef]
- Ding, J.; Guo, D.; Wang, N.; Wang, H.F.; Yang, X.; Shen, K.; Chen, L.; Li, Y. Defect engineered metal–organic framework with accelerated structural transformation for efficient oxygen evolution reaction. Angew. Chem. Int. Ed. 2023, 62, e202311909. [Google Scholar] [CrossRef]
- Park, M.G.; Hwang, J.; Deng, Y.P.; Lee, D.U.; Fu, J.; Hu, Y.; Jang, M.J.; Choi, S.M.; Feng, R.; Jiang, G.; et al. Longevous Cycling of Rechargeable Zn-Air Battery Enabled by “Raisin-Bread” Cobalt Oxynitride/Porous Carbon Hybrid Electrocatalysts. Adv. Mater. 2024, 36, 2311105. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wu, M.; Zhou, L.; Chen, W.; Han, L.; Gao, G.; Cui, Y.; Sun, Z.; Cabot, A. Enhancing Electrocatalytic Activity Through Targeted Local Electrolyte Micro-Environment. Adv. Funct. Mater. 2025, 35, 2419328. [Google Scholar] [CrossRef]
- Patel, P.; Schwartz, D.; Wang, X.; Lin, R.; Ajao, O.; Seifitokaldani, A. Technoeconomic and life-cycle assessment for electrocatalytic production of furandicarboxylic acid. ACS Sustain. Chem. Eng. 2022, 10, 4206–4217. [Google Scholar] [CrossRef]
- Paladin, G.; Manzardo, A.; Nale, A.; Negro, E.; Di Noto, V. A comparative life cycle assessment of Pt nanoalloy/carbon nitride/graphene electrocatalysts for PEMFC stacks. Chem. Eng. J. 2025, 505, 159251. [Google Scholar] [CrossRef]



| Electrocatalyst | Reaction Type | Overpotential @10 mA/cm2 (mV) | Tafel Slope (mV/dec) | Stability (h) | Ref. |
|---|---|---|---|---|---|
| Pt/C | HER/ORR | ∼30 (HER) | ∼30 | >100 | [17,52] |
| IrO2 | OER | ∼300 | ∼40 | >100 | [24] |
| RuO2 | OER | ∼280 | ∼55 | ∼50 | [24,29] |
| Pd/C | HOR, ORR | ∼50 (HOR) | ∼40 | ∼60 | [22] |
| NiFe LDH | OER | ∼270 | ∼35 | >200 | [30] |
| MoS2 (exfoliated) | HER | ∼150 | ∼60 | ∼20 | [64] |
| CoP | HER | ∼120 | ∼50 | ∼80 | [69] |
| Fe–N–C | ORR | ∼400 | ∼70 | 30–50 | [56] |
| MnOx | OER | ∼350 | 60–80 | ∼50 | [33] |
| Mo2C | HER/CO2RR | ∼180 (HER)/∼600 (CO2RR) | ∼50–70 | ∼40 | [23,42] |
| MoSe2 | CO2RR | ∼500–700 | - | ∼10–20 | [68] |
| N-doped graphene | ORR/NRR | ∼350 | ∼80 | ∼40 | [71,74] |
| Electrolyte Factor | Activity or Selectivity | Effect on Stability | Example Reactions |
|---|---|---|---|
| Li+ | N2 polarization, HER suppression | Reduces local proton activity | NRR, CO2RR |
| K+ or Cs+ | Stabilizes •N2−, •CO2− intermediates | Minimizes parasitic reactions | NRR, CO2RR |
| Halides (Cl−, Br−) | Modulate binding energies | Possible surface adsorption | HER, CO2RR |
| SO42− (anion) | Inert behavior, supports intrinsic activity | Minimal interaction | ORR, OER |
| Water-in-salt electrolytes | Suppress HER, improve selectivity | Reduce catalyst leaching | NRR, CO2RR |
| Organic co-solvents | Stabilize intermediates | Lower solvent- driven degradation | CO2RR, NRR |
| Degradation Mechanism | Reaction Types Affected | Typical Catalyst Types | Example Catalysts (Refs) | Mitigation Strategies (Refs) |
|---|---|---|---|---|
| Carbon Support Corrosion | ORR, OER (fuel cells, metal–air) | Pt/C, PGM/C (Pt, Pd, Ir, Ru) | Pt/C, PtCo/C [7,80,81] | Use graphitized carbon supports [80], alloy catalysts [54], protective coatings [110], control start-stop cycles [111] |
| Metal Oxidation & Dissolution | ORR, OER, HER | Pt, Ir, Ru, Pd | Pt [7], RuO2 [28,29], IrO2 [24] | Alloying with stable metals (PtCo, PtNi) [54], core–shell structures [51], potential cycling control [82] |
| Leaching of Active Sites | ORR, OER, NRR | M–N–C (Fe, Co), Ru-based | Fe–N–C [56,58], Ru-based OER [28,29] | Stronger metal–support interaction [29], interfacial engineering [112], anchoring atomically dispersed sites [56] |
| Ostwald Ripening & Aggregation | ORR, HER, CO2RR | Pt, Pd nanoparticles, Cu for CO2RR | Pt/C [84,85], Cu nanoparticles [41] | Optimize particle size [85], porous supports [113], stabilizers to suppress migration [54] |
| Surface Reconstruction | OER, HER, CO2RR | Transition metal oxides, chalcogenides | NiFeOx [30], MoS2 [64] | Operando monitoring [92], design self-reconstructing catalysts [61], interface stabilization [61] |
| Poisoning by Impurities | ORR, CO2RR, NRR | Pt (CO poisoning), Cu (CO2RR), Fe–N–C (NRR) | Pt (CO) [98,100], Cu (CO2RR) [38], Fe–N–C (NRR) [46] | Microenvironment engineering [40], hydrophobic layers, selective binding sites [40], impurity filtration [99] |
| Electrolyte Effects (pH/Ions) | HOR, HER, ORR, CO2RR, NRR | Pt, M–N–C, Cu | Pt (HOR) [20,21], Cu (CO2RR) [38], Fe–N–C (NRR) [46] | Electrolyte optimization [114], buffer layers [40], ion-selective membranes [99] |
| Catalyst Class | Material Cost | Element Abundance | Synthetic Scalability | Recyclability | Life-Cycle Impact |
|---|---|---|---|---|---|
| Pt/C, IrO2, RuO2 | High | Low | Moderate | Moderate | High |
| Fe–N–C, Co–N–C | Low | High | Moderate–High | High | Moderate |
| Metal Oxides | Low | High | High | High | Low |
| Metal Phosphides | Medium | Moderate | Moderate | Unknown | Moderate |
| MOF-derived Catalysts | Medium–High | Variable | Often Complex | Unknown | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purohit, V.; Datar, A. Overview of Topics in Electrocatalysis for Sustainability: Reactions, Electrocatalysts, Degradation, and Mitigation. Processes 2025, 13, 2659. https://doi.org/10.3390/pr13082659
Purohit V, Datar A. Overview of Topics in Electrocatalysis for Sustainability: Reactions, Electrocatalysts, Degradation, and Mitigation. Processes. 2025; 13(8):2659. https://doi.org/10.3390/pr13082659
Chicago/Turabian StylePurohit, Varada, and Avdhoot Datar. 2025. "Overview of Topics in Electrocatalysis for Sustainability: Reactions, Electrocatalysts, Degradation, and Mitigation" Processes 13, no. 8: 2659. https://doi.org/10.3390/pr13082659
APA StylePurohit, V., & Datar, A. (2025). Overview of Topics in Electrocatalysis for Sustainability: Reactions, Electrocatalysts, Degradation, and Mitigation. Processes, 13(8), 2659. https://doi.org/10.3390/pr13082659







