Mechanisms and Potential Assessment of CO2 Sequestration in the Baijiahai Uplift, Junggar Basin
Abstract
1. Introduction
2. Research Area
3. Research Method
3.1. Technical Roadmap
3.2. Sample Preparation
3.3. CO2 Immersion and Displacement Experiments
3.4. MICP, NMR, and Mechanical Testing
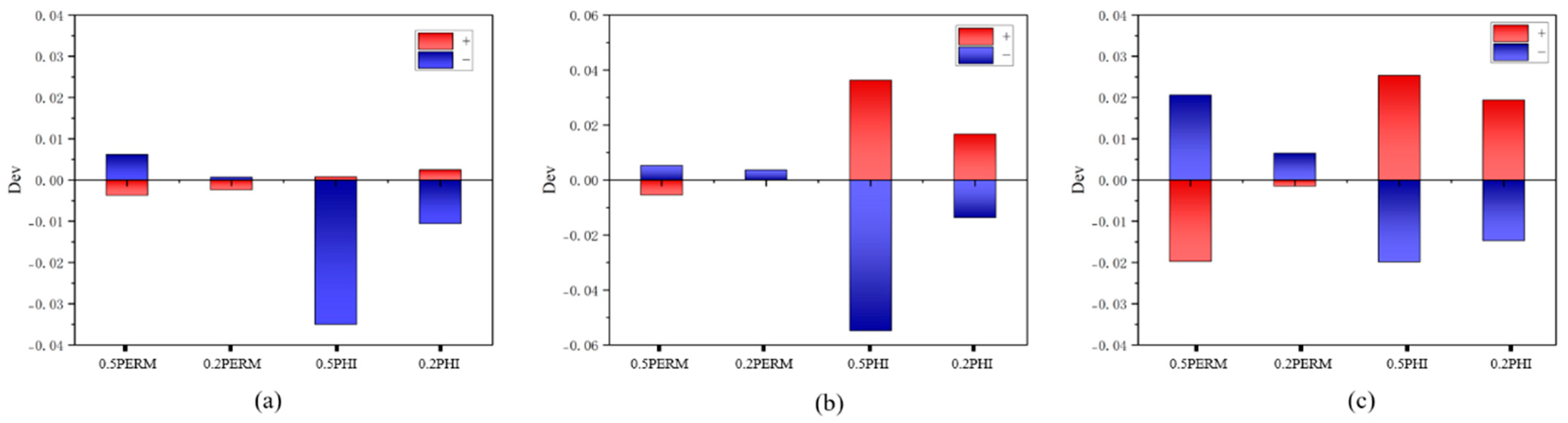
4. Experimental Results
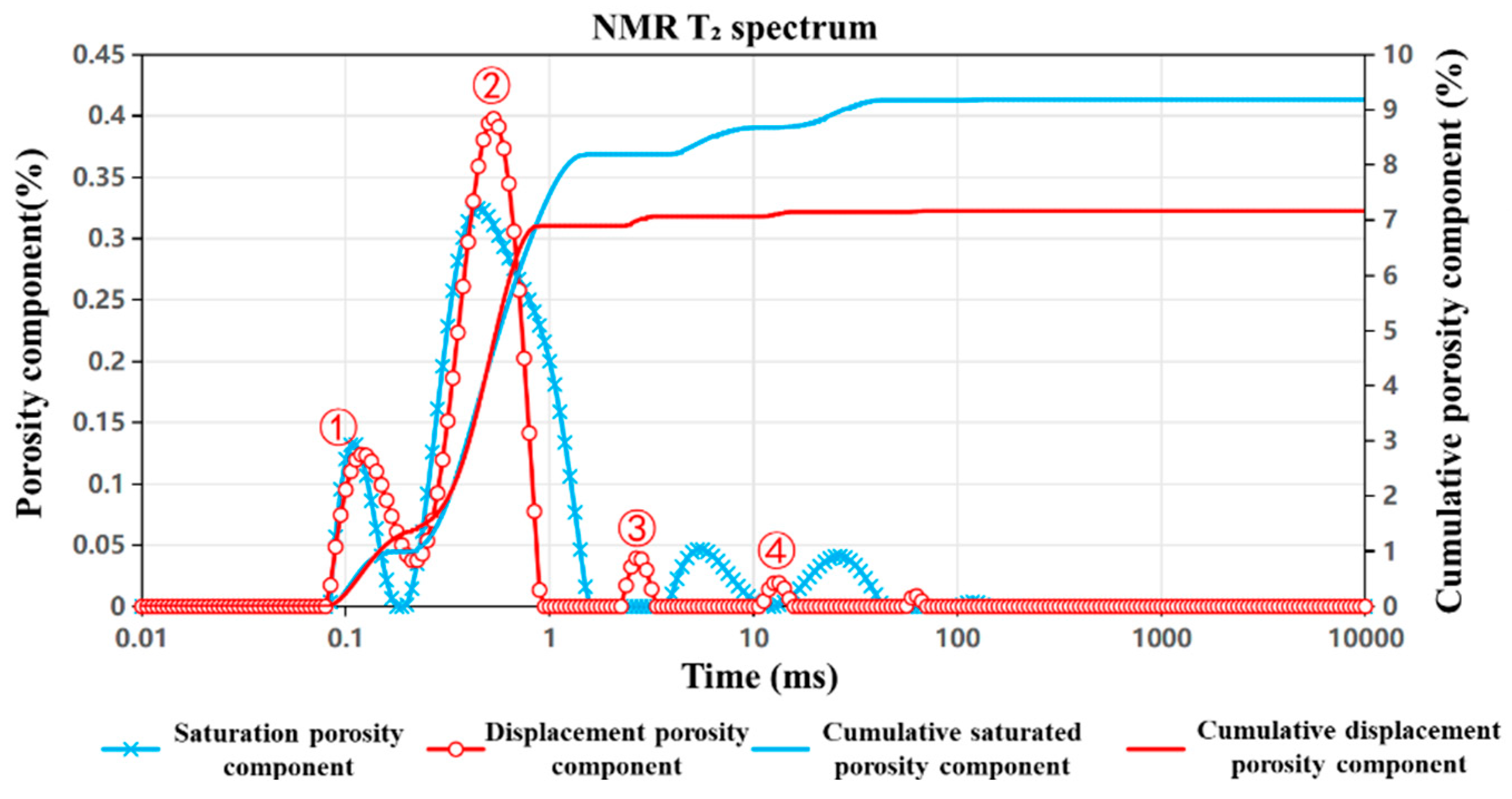
4.1. NMR Tests Before and After Displacement
4.1.1. Nuclear Magnetic Resonance Results
- (1)
- Within the 0.1–1 ms interval, both the number and size of Peak ① (small pores) decrease.
- (2)
- Peak ② (small-to-medium pores) shows a reduction in number but an increase in size.
- (3)
- In the 1–10 ms interval, Peaks ③ and ④ (medium pores) display a significant increase in number.
- (4)
- In the 10–100 ms range, Peak ⑤ (medium-to-large pores) decreases in number while its size remains largely unchanged, and Peak ⑥ (large pores) exhibits an increase in pore size.
- (1)
- In the 0.01–1 ms range, the intensity of Peak ① (small pores) increases, though the corresponding pore sizes diminish.
- (2)
- Peak ② (small-to-medium pores) retains a stable amplitude while shifting toward smaller sizes.
- (3)
- Within the 1–10 ms range, Peak ③ (small-to-medium pores) exhibits increased amplitude, while Peak ④ (medium pores) remains largely unchanged in both size and number.
- (4)
- In the 10–100 ms range, Peak ⑤ (medium-to-large pores)—representing the principal pore-throat domain for CO2 storage—undergoes a significant decline in amplitude with stable size, whereas Peak ⑥ (large pores) shows a slight increase in pore size.
- (1)
- In the 0.01–1 ms range, there is no significant change in Peak ① (small pores).
- (2)
- The number of Peak ② (small-to-medium pores) increases, although their size remains constant.
- (3)
- In the 1–10 ms range, the amplitude of Peak ③ (medium pores) decreases.
- (4)
- In the 10–100 ms range, both the amplitude and size of Peak ④ (large pores) decrease.
4.1.2. Displacement Results
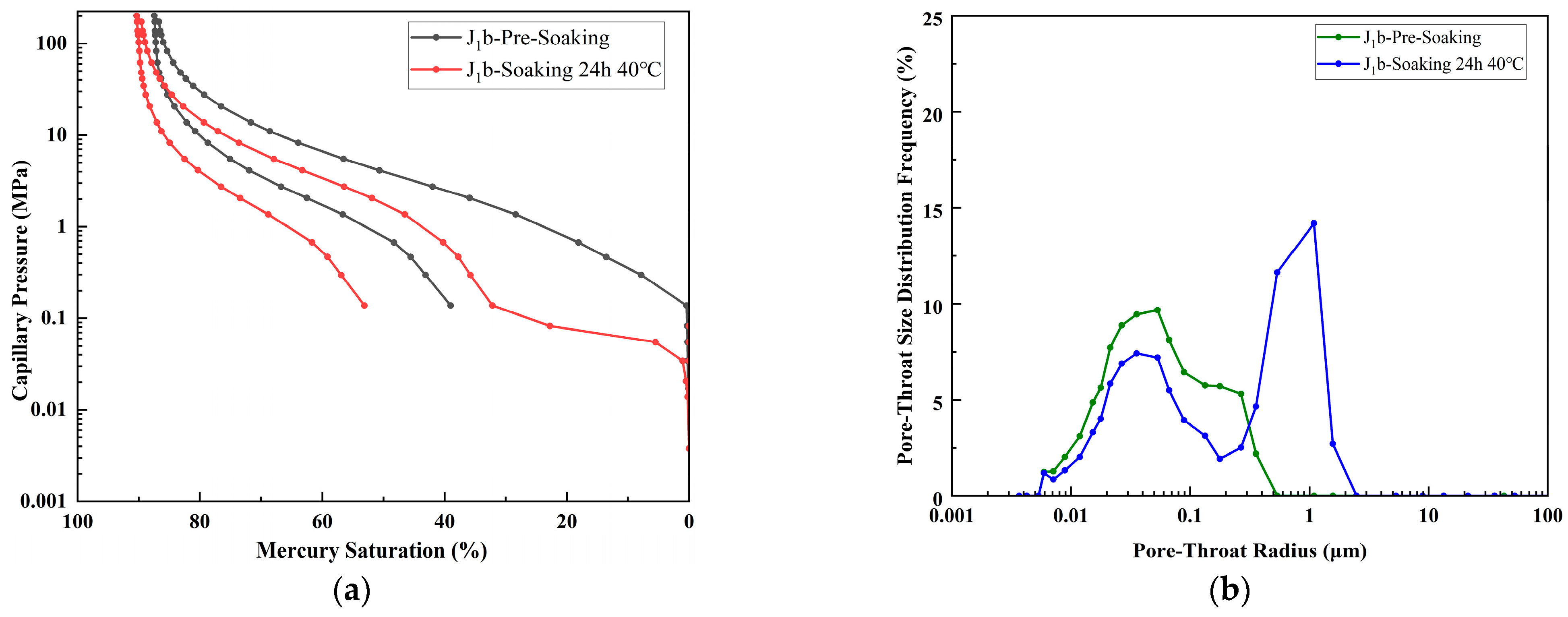
4.2. Mercury Injection Test
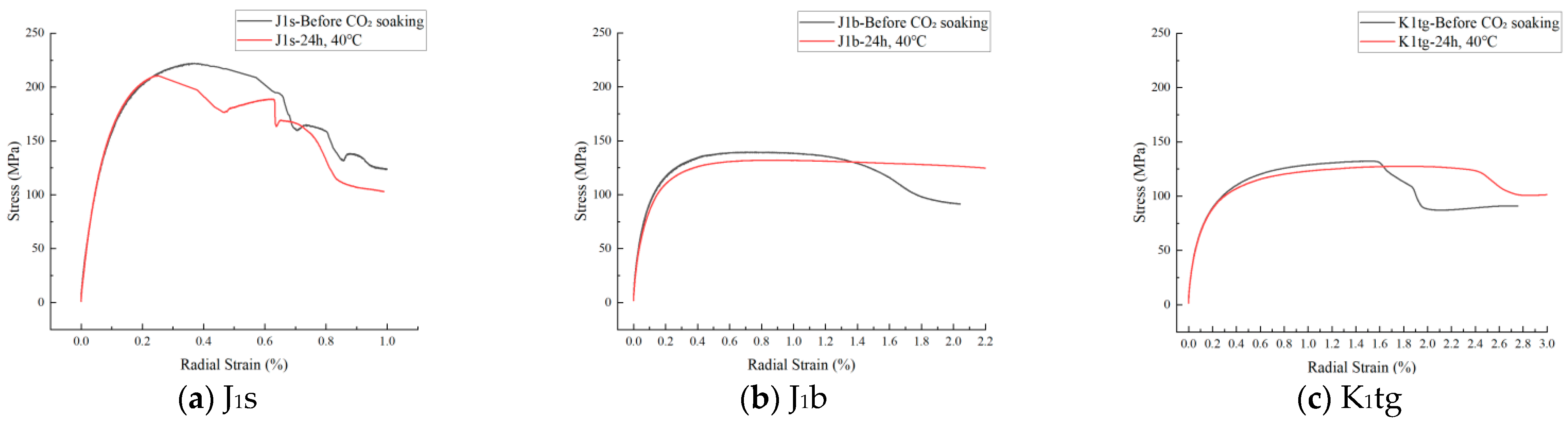
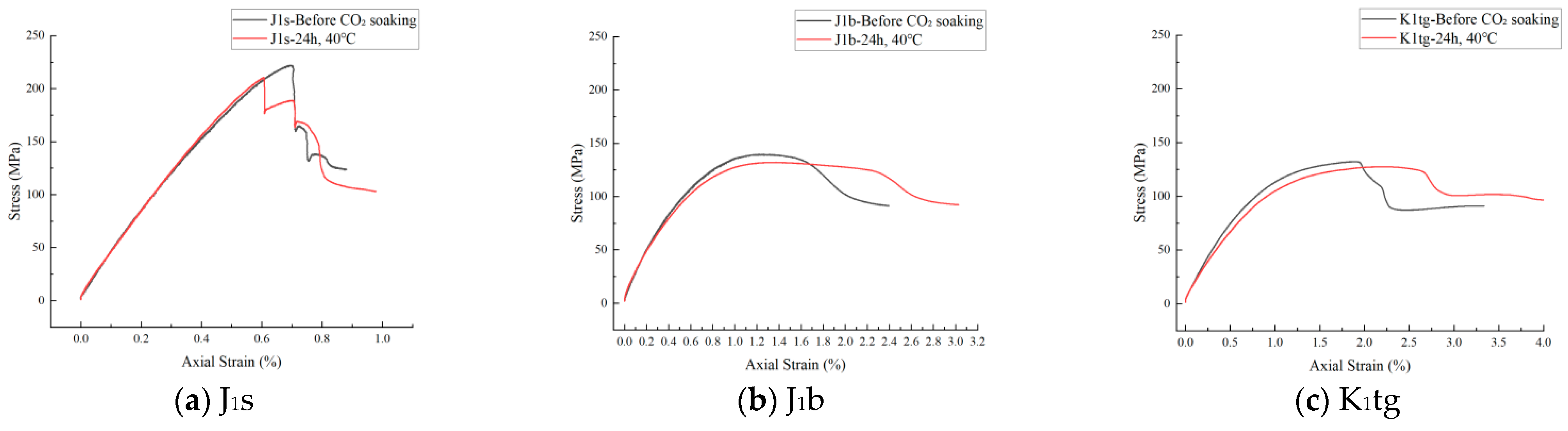
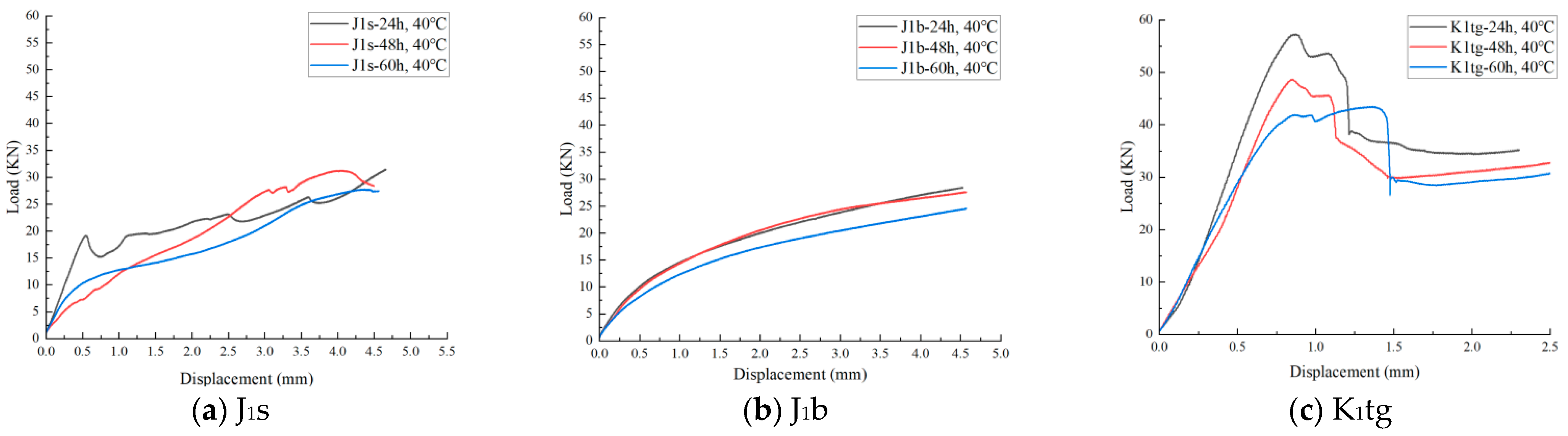
4.3. The Evolution Law of Mechanical Characteristics
4.3.1. Uniaxial Compressive Strength Test
4.3.2. Uniaxial Shear Resistance Test
4.3.3. Geochemical Alterations Coupling with Geomechanical Properties
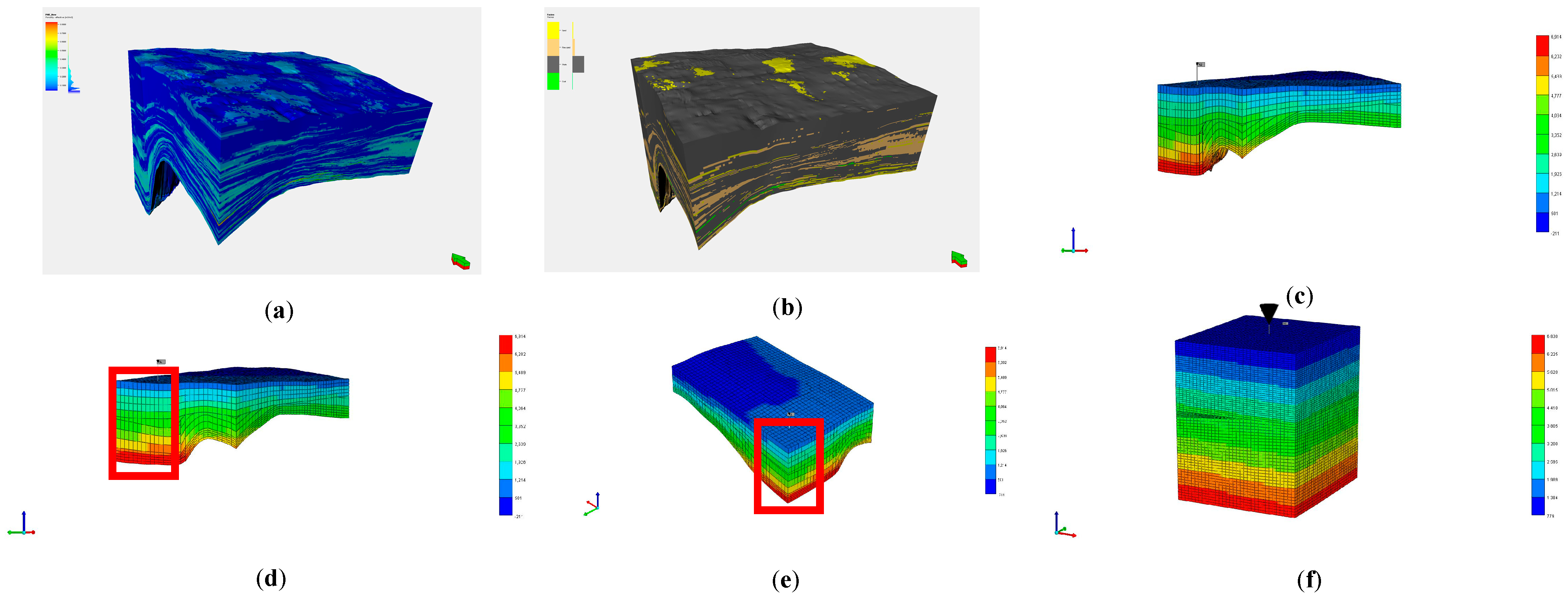
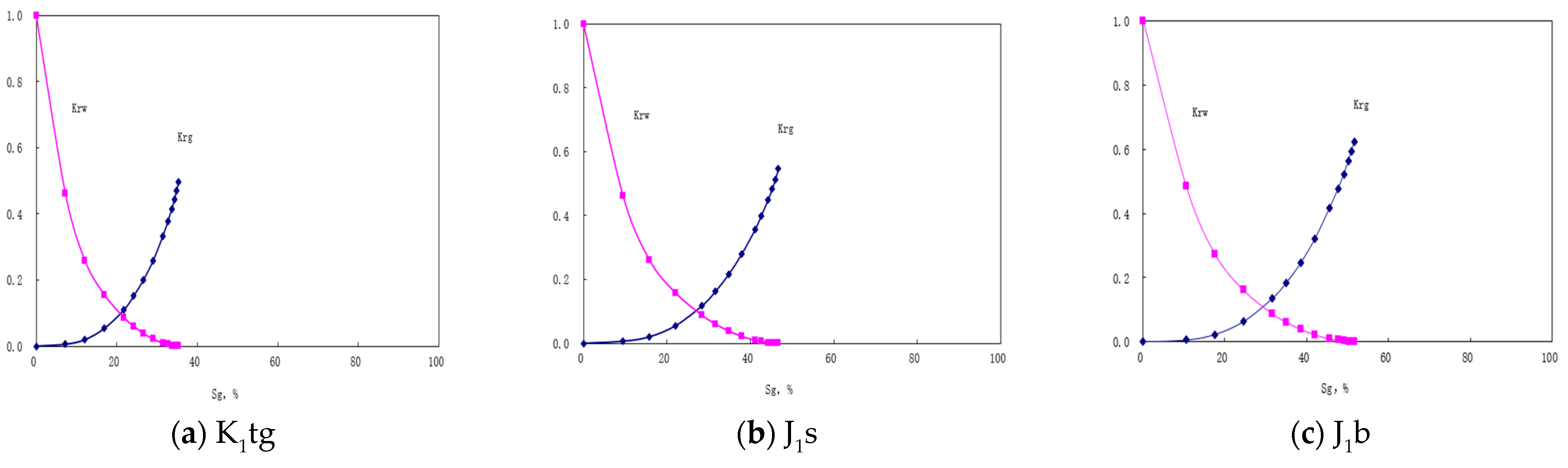
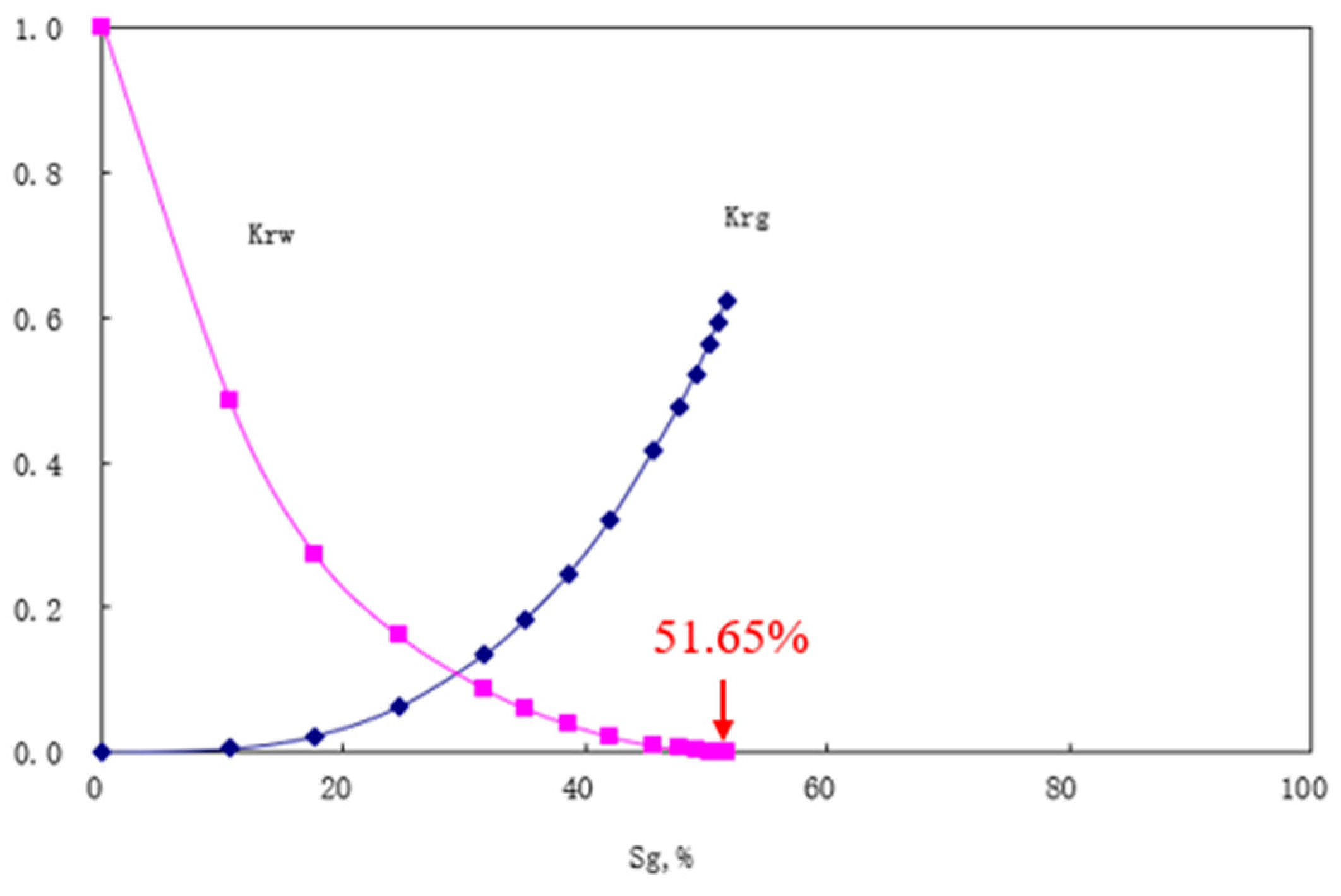
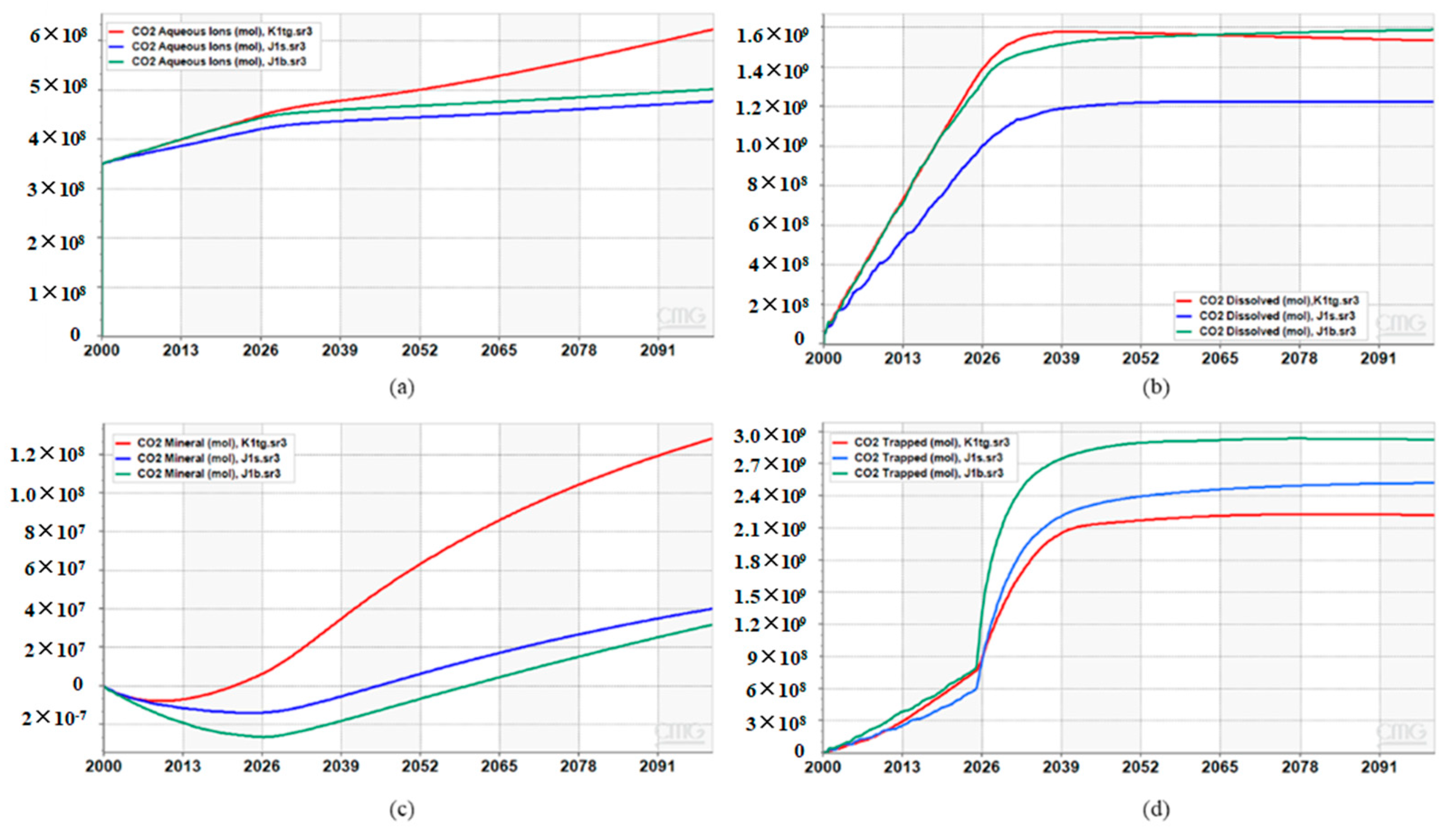
5. Simulation Results
6. Discussions and Conclusions
- The Jurassic Badaowan formation (J1b) is characterized by high quartz content and kaolinite-dominated clay minerals, while the Jurassic Sangonghe formation (J1s) consists mainly of quartz and plagioclase with lower and more diverse clay mineral content. The Cretaceous Tugulu Group (K1tg) is rich in calcite and illite–smectite mixed-layer clays.
- Displacement experiments reveal sequestration efficiency rankings: J1b(8.4%) > K1tg(2.0%) > J1s(1.2%). J1b exhibits increased small pores and dominant large-pore sequestration; J1s shows pore homogenization; K1tg experiences preferential displacement in large pores with stable bound water in small pores. The superior capacity of J1b is attributed to clay mineral (kaolinite) swelling and large-pore plugging effects.
- XRD and NMR analyses indicate that while J1b has the smallest original pore-throat radius (median, 0.183 μm), it demonstrates the most comprehensive improvement in porosity and permeability after CO2 immersion. J1s achieves significant breakthroughs in permeability and a maximum pore-throat radius, whereas K1tg forms a medium-pore preponderance due to calcite dissolution but with limited permeability enhancement.
- Mechanical tests show that J1b has the highest sequestration potential but the poorest mechanical stability, requiring measures to prevent formation collapse. J1s experiences a rapid 20% decline in shear strength within 24 h, necessitating injection pressure control during short-term operations.
- Numerical simulations confirm that J1b achieves the highest total storage capacity through strong capillary trapping in clay minerals, despite weak mineralization. K1tg ranks second due to calcite-driven mineralization and early aqueous storage, while J1s performs mediocrely across all mechanisms.
- Optimal reservoir for CCUS: J1b requires wellbore reinforcement technologies to address mechanical degradation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le Quéré, C.; Andrew, R.M.; Friedlingstein, P.; Sitch, S.; Hauck, J.; Pongratz, J.; Pickers, P.A.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G. Global carbon budget 2018. Earth Syst. Sci. Data 2018, 10, 2141–2194. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–28. [Google Scholar] [CrossRef]
- Soeder, D.J. Greenhouse gas sources and mitigation strategies from a geosciences perspective. Adv. Geo-Energy Res. 2021, 5, 274–285. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Global CO2 Emissions in 2023; IEA: Paris, France, 2024; Available online: https://www.iea.org/reports/co2-emissions-in-2023 (accessed on 2 June 2025).
- Dunn, R.J.H.; Aldred, F.; Gobron, N.; Miller, J.B.; Willett, K.M.; Ades, M.; Adler, R.; Allan, R.P.; Allan, R.; Anderson, J.; et al. Global climate. Bull. Am. Meteorol. Soc. 2021, 102, S11–S142. [Google Scholar] [CrossRef]
- Khanal, A.; Shahriar, M.F. Optimization of CO2 Huff-n-Puff in Unconventional Reservoirs with a Focus on Pore Confinement Effects, Fluid Types, and Completion Parameters. Energies 2023, 16, 2311. [Google Scholar] [CrossRef]
- Zhan, J.; Niu, Z.; Li, M.; Zhang, Y.; Ma, X.; Fan, C.; Wang, R. Numerical simulation and modeling on CO2 sequestration coupled with enhanced gas recovery in shale gas reservoirs. Geofluids 2021, 2021, 9975296. [Google Scholar] [CrossRef]
- Shahriar, M.F.; Khanal, A. Fundamental investigation of reactive-convective transport: Implications for long-term carbon dioxide(CO2) sequestration. Int. J. Greenh. Gas Control. 2023, 127, 103916. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Ma, X.; Atrens, A.; Ni, H. Modeling study of enhanced thermal brine extraction using supercritical CO2. Bull. Geol. Sci. Technol. 2014, 33, 233–240. [Google Scholar]
- Li, Q.; Cai, B.; Chen, F.; Liu, G.; Liu, L. Review of environmental risk assessment methods for carbon dioxide geological storage. Environ. Eng. 2019, 37, 13–21. [Google Scholar]
- Goodman, A.; Sanguinito, S.; Tkach, M.; Natesakhawat, S.; Kutchko, B.; Fazio, J.; Cvetic, P. Investigating the role of water on CO2-utica shale interactions for carbon storage and shale gas extraction activities: Evidence for pore scale alterations. Fuel 2019, 242, 744–755. [Google Scholar] [CrossRef]
- Cook, P.J. CCS research development and deployment in a clean energy future: Lessons from Australia over the past two decades. Engineering 2017, 3, 477–484. [Google Scholar] [CrossRef]
- Jin, J.; Yang, G.; Liu, S.; Ma, X.; Zhang, Y.; Han, S. Effects of CO2-saltwater-rock interaction on reservoir porosity: A case study of the Donggou Formation sandstone layer in the Junggar Basin. Nonferrous Met. (Extr. Metall.) 2025, 204–216. Available online: https://link.oversea.cnki.net/doi/10.20237/j.issn.1007-7545.2025.02.023 (accessed on 2 June 2025).
- Cai, B.; Li, Q.; Zhang, X.; Ouyang, T. Annual Report of Carbon Dioxide Capture, Utilization and Storage (CCUS) in China (2021)—China CCUS Path Research; Environmental Planning Institute of the Ministry of Ecology and Environment: Beijing, China, 2021. [Google Scholar]
- Jafari, M.; Cao, S.C.; Jung, J. Geological CO2 sequestration in saline aquifers: Implication on potential solutions of China’s power sector. Resour. Conserv. Recycl. 2017, 121, 137–155. [Google Scholar] [CrossRef]
- Vishal, V.; Singh, T. (Eds.) Geologic Carbon Sequestration: Understanding Reservoir Behavior; Springer: Cham, Switzerland, 2016; Volume 16, pp. 47–133. [Google Scholar]
- Machado, M.V.B.; Delshad, M.; Sepehrnoori, K. Injectivity assessment for CCS field-scale projects with considerations of salt deposition, mineral dissolution, fines migration, hydrate formation, and non-Darcy flow. Fuel 2023, 353, 129148. [Google Scholar] [CrossRef]
- Meguerdijian, S.; Pawar, R.J.; Harp, D.R.; Jha, B. Thermal and solubility effects on fault leakage during geologic carbon storage. Int. J. Greenh. Gas Control. 2022, 116, 103633. [Google Scholar] [CrossRef]
- Li, Y. Progress in Carbon Neutralization and Carbon Capture, Utilization and Storage Technology; China Petrochemical Press Ltd.: Beijing, China, 2021; Available online: https://ricn.sjtu.edu.cn/Web/Show/77 (accessed on 2 June 2025).
- Ye, H.; Hao, N.; Liu, Q. Review on Key Parameters and Characterization Technology of CO2 Sequestration Mechanism in saline aquifers. Power Gener. Technol. 2022, 43, 562–573. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, B.; Cao, C.; Zhang, L.; Zhou, X.; Huang, C.; Rui, Y.; Li, J. Research progress of evaluation of CO2 storage potential and suitability assessment indexes in saline aquifers. Pet. Reserv. Eval. Dev. 2023, 13, 484–494. [Google Scholar]
- Li, Y.; Pang, Z. Capacity and suitability assessment of deep saline aquifers for CO2 sequestration in the Bohai Bay Basin, East China. Environ. Earth Sci. 2016, 75, 402. [Google Scholar] [CrossRef]
- Gholami, R.; Raza, A. CO2 sequestration in sandstone reservoirs: How does reactive flow alter trapping mechanisms. Fuel 2022, 324, 124781. [Google Scholar] [CrossRef]
- Edem, D.; Abba, M.; Nourian, A.; Babaie, M.; Naeem, Z. Experimental investigation of the extent of the impact of Halite precipitation on CO2 injection in deep saline aquifers. In Proceedings of the SPE Europec featured at EAGE Conference and Exhibition, SPE, Virtual, 1–3 December 2020. [Google Scholar] [CrossRef]
- Haizheng, J.; Baiyang, L.; Zhao, L. Experimental study on the interaction between CO2 and Jimsar reservoir rocks. Chem. Eng. Oil Gas 2021, 50, 76–80. [Google Scholar]
- Amin, S.M.; Weiss, D.J.; Blunt, M.J. Reactive transport modelling of geologic CO2 sequestration in saline aquifers: The influence of pure CO2 and of mixtures of CO2 with CH4 on the sealing capacity of cap rock at 37 °C and 100 bar. Chem. Geol. 2014, 367, 39–50. [Google Scholar] [CrossRef]
- Alzayer, H.; Zahrani, T.; Shubbar, A. Modeling CO2 Sequestration in Deep Saline Aquifers–Best Practices. In Proceedings of the International Petroleum Technology Conference, IPTC, Riyadh, Saudi Arabia, 21–23 February 2022. [Google Scholar] [CrossRef]
- Youjun, J.; Xiaoyang, W.; Tiyao, Z.; Zegen, W.; Guobin, J. Effect of CO2 geological sequestration on reservoir mechanical properties and gas migration and Diffusion Under Multi field coupling. Environ. Eng. 2023, 41 (Suppl. S2), 442–446. [Google Scholar]
- Zhou, Y.; Tang, L.; Song, Z.; Pan, B.; Yue, M.; Liu, J.; Song, H. Research on CO2 sequestration in saline aquifers with different relative permeability considering CO2 phase conditions. Energy 2024, 313, 133739. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Y.; Li, S.; Wang, X.; Cui, G.; He, Y. Migration characteristics and storage forms of liquid and supercritical CO2 in saline aquifers. Coal Geol. Explor. 2025, 53, 99–106. [Google Scholar]
- Fatima, S.; Khan, H.M.M.; Tariq, Z.; Abdalla, M.; Mahmoud, M. An experimental and simulation study of CO2 sequestration in an underground formations; impact on geomechanical and petrophysical properties. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, SPE, Event Canceled, 28 November–1 December 2021. [Google Scholar] [CrossRef]
- Jin, Y.J.; Wang, J.L.; Pan, Z.Y. Determination of CO2 Solubility in Saline Solutions Under Geological Storage Conditions Using Raman Spectroscopy Combined with a Quartz Capillary Equilibrium Still; Chinese Society for Mineralogy, Petrology and Geochemistry; Abstracts of the 17th Annual Conference of the Chinese Society for Mineralogy, Petrology and Geochemistry; College of Environmental Science and Engineering, Zhejiang University of Technology: Hangzhou, China, 2019; p. 1037. [Google Scholar]
- Temitope, A.; Gomes, J.S.; Al Kobaisi, M.; Hu, J. Characterization and quantification of the CO2 sequestration potential of a carbonate aquifer in Falaha Syncline, onshore Abu Dhabi. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, One Petro, Abu Dhabi, United Arab Emirates, 7–10 November 2016. [Google Scholar] [CrossRef]
- Rezk, M.G.; Ibrahim, A.F. Impact of rock mineralogy on reactive transport of CO2 during carbon sequestration in a saline aquifer. J. Pet. Explor. Prod. Technol. 2025, 15, 10. [Google Scholar] [CrossRef]
- GB/T 29172-2012; Practices for Core Analysis. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China: Beijing, China, 2012.

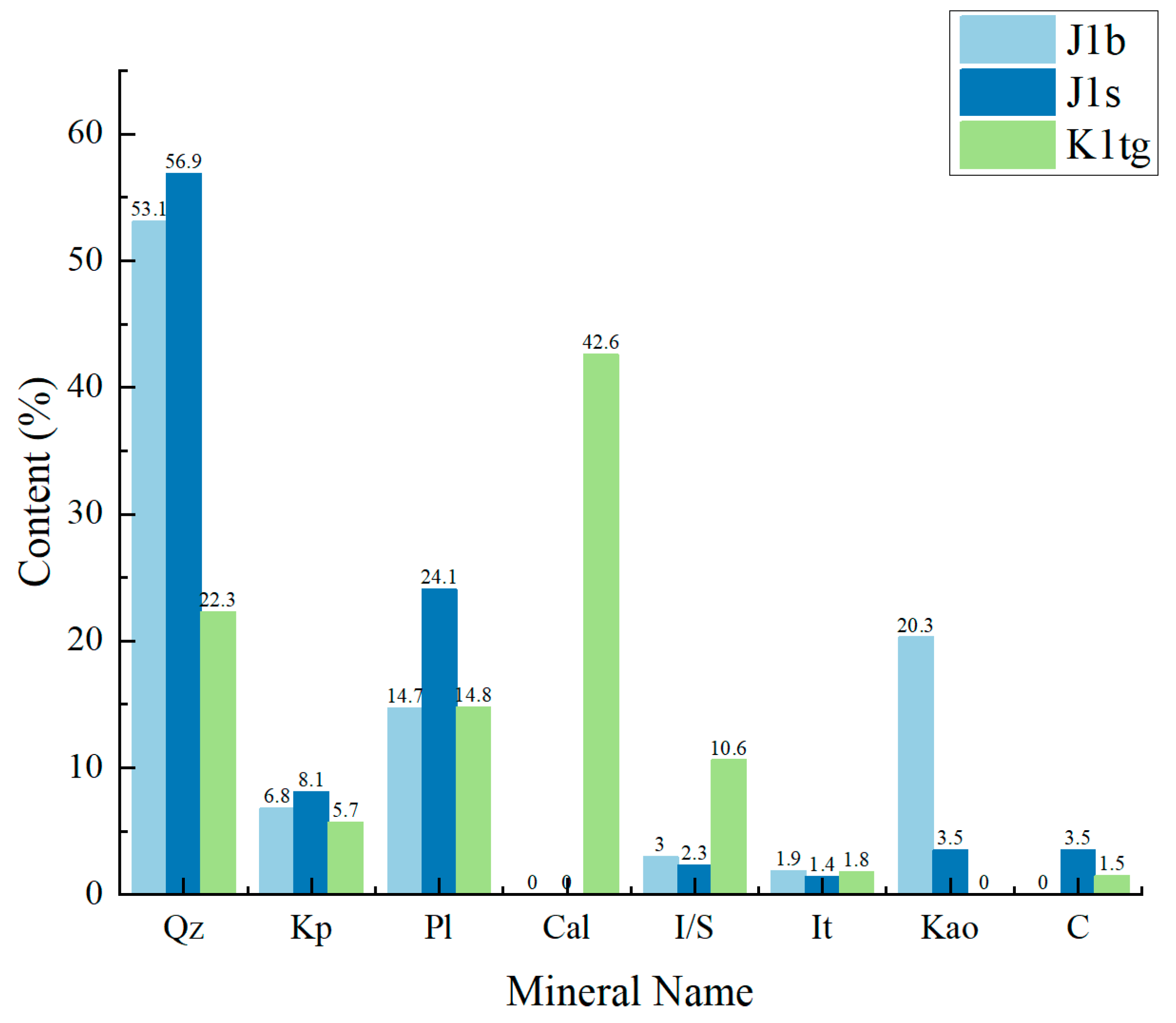








| Test | Formation | CO2 Immersion Time | CO2 Immersion Temperature |
|---|---|---|---|
| Mercury Injection Test | J1s | 24 h | 40 °C |
| J1b | |||
| K1tg | |||
| SEM Test | J1s | 24 h | 40 °C |
| J1b | |||
| K1tg | |||
| Uniaxial Compression Test | J1s | 24 h | 40 °C |
| J1b | |||
| K1tg | |||
| Uniaxial Shear Test | J1s | 24 h, 48 h, 60 h | 40 °C |
| J1b | 24 h, 48 h, 60 h | 40 °C | |
| K1tg | 24 h, 48 h, 60 h | 40 °C |
| Length cm | Diameter cm | Porosity % | Permeability mD | Injection Pressure MPa | Outlet Pressure MPa | Temperature °C |
|---|---|---|---|---|---|---|
| 5.208 | 2.514 | 26.559 | 13.140 | 11.6 | 11.5 | 40 |
| Stratum | CO2 Aqueous (mol) | CO2 Dissolved (mol) | CO2 Mineral (mol) | CO2 Super Critical (mol) | CO2 Trapped (mol) | Total Storage Capacity (mol) |
|---|---|---|---|---|---|---|
| K1tg | 4.92821 × 108 | 1.49032 × 109 | 3.00589 × 107 | 2.65337 × 109 | 2.64899 × 109 | 4.666569 × 109 |
| J1b | 4.97961 × 108 | 1.41702 × 109 | 4.50067 × 107 | 3.09315 × 109 | 3.06720 × 109 | 5.053138 × 109 |
| J1s | 6.05990 × 108 | 1.41933 × 109 | 1.20637 × 108 | 2.12958 × 109 | 1.99623 × 109 | 4.275537 × 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, W.; Wang, Q.; Wang, K.; Qin, S.; Wang, T. Mechanisms and Potential Assessment of CO2 Sequestration in the Baijiahai Uplift, Junggar Basin. Processes 2025, 13, 2648. https://doi.org/10.3390/pr13082648
Wang X, Zhang W, Wang Q, Wang K, Qin S, Wang T. Mechanisms and Potential Assessment of CO2 Sequestration in the Baijiahai Uplift, Junggar Basin. Processes. 2025; 13(8):2648. https://doi.org/10.3390/pr13082648
Chicago/Turabian StyleWang, Xiaohui, Wen Zhang, Qun Wang, Kepeng Wang, Saisai Qin, and Tianyu Wang. 2025. "Mechanisms and Potential Assessment of CO2 Sequestration in the Baijiahai Uplift, Junggar Basin" Processes 13, no. 8: 2648. https://doi.org/10.3390/pr13082648
APA StyleWang, X., Zhang, W., Wang, Q., Wang, K., Qin, S., & Wang, T. (2025). Mechanisms and Potential Assessment of CO2 Sequestration in the Baijiahai Uplift, Junggar Basin. Processes, 13(8), 2648. https://doi.org/10.3390/pr13082648





