Influence of Ammonium on the Adsorption and Desorption of Heavy Metals in Natural Zeolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Natural Zeolite Properties
2.2. Pre-Processing of Adsorbents
2.3. Analytical Devices and Chemicals
2.4. Competitive Adsorption Batch Tests
Adsorption Kinetics
2.5. Rapid Small-Scale Column Adsorption and Desorption Tests
2.6. Data Evaluation
Hill Adsorption Isotherms
3. Results and Discussion
3.1. Competitive Adsorption of Ammonium and Heavy Metals
3.1.1. Pseudo-Second-Order Adsorption Kinetics
3.1.2. Competitive Heavy Metal Removal in Batch Tests
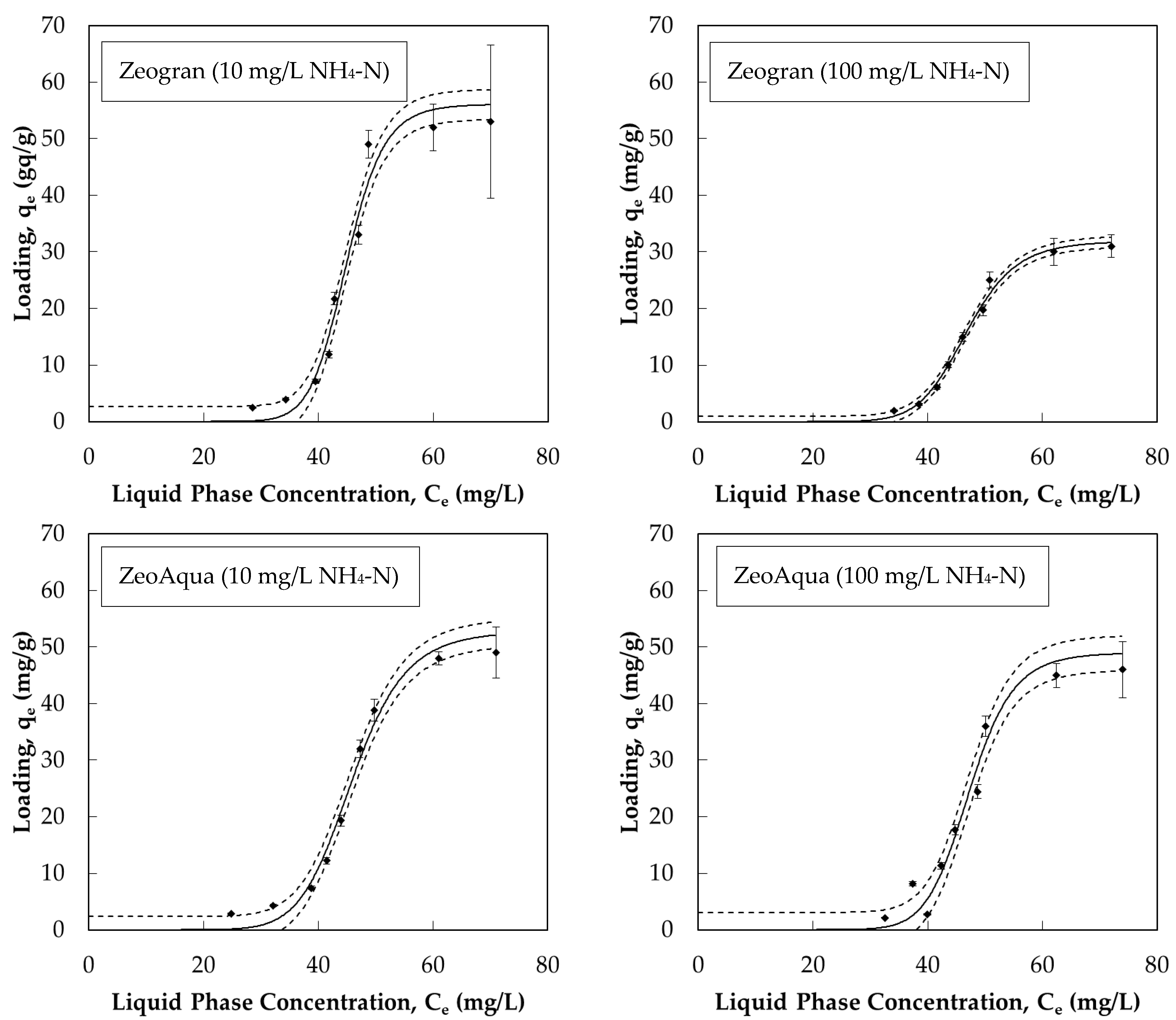
3.1.3. Adsorption Isotherms
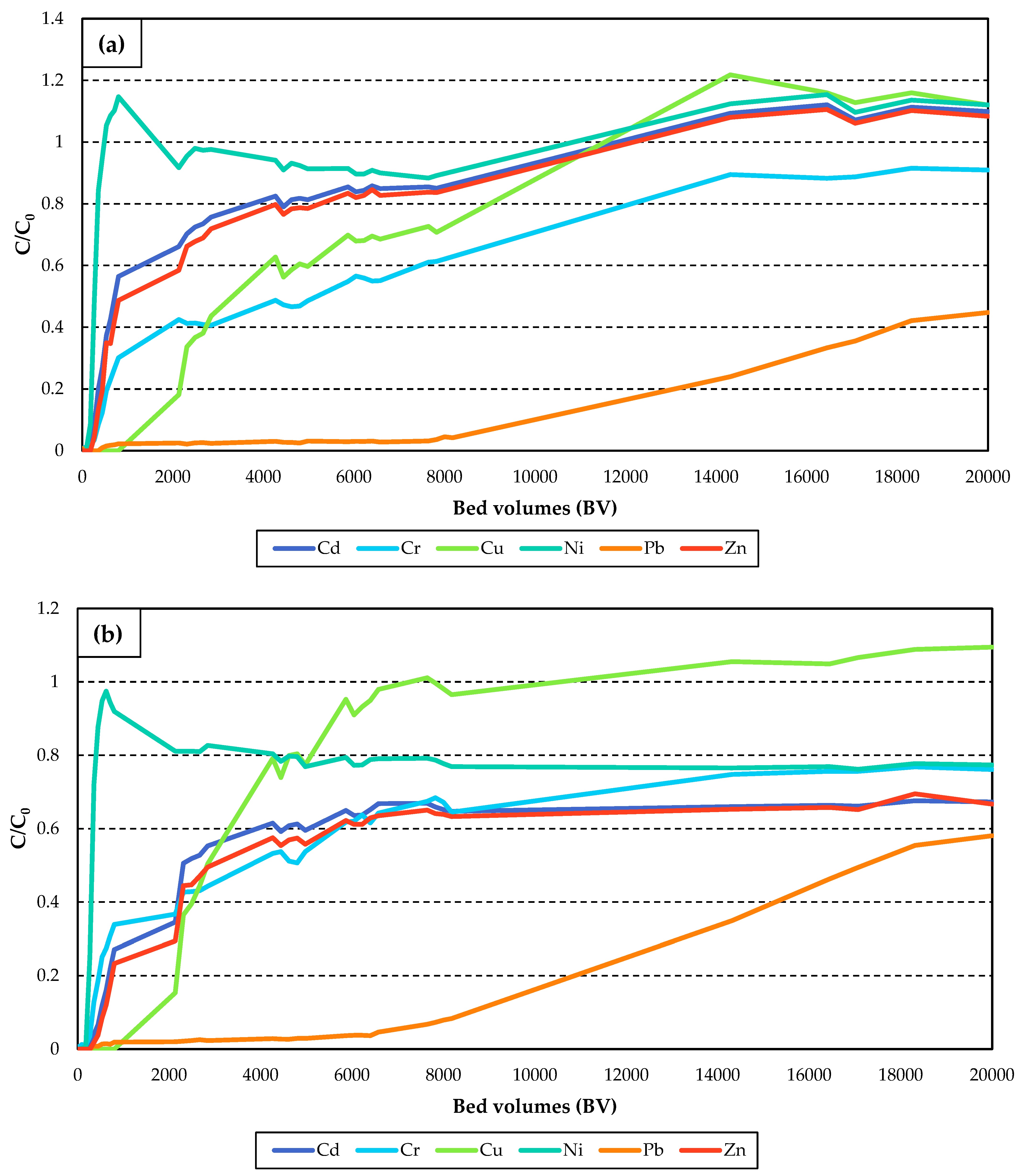
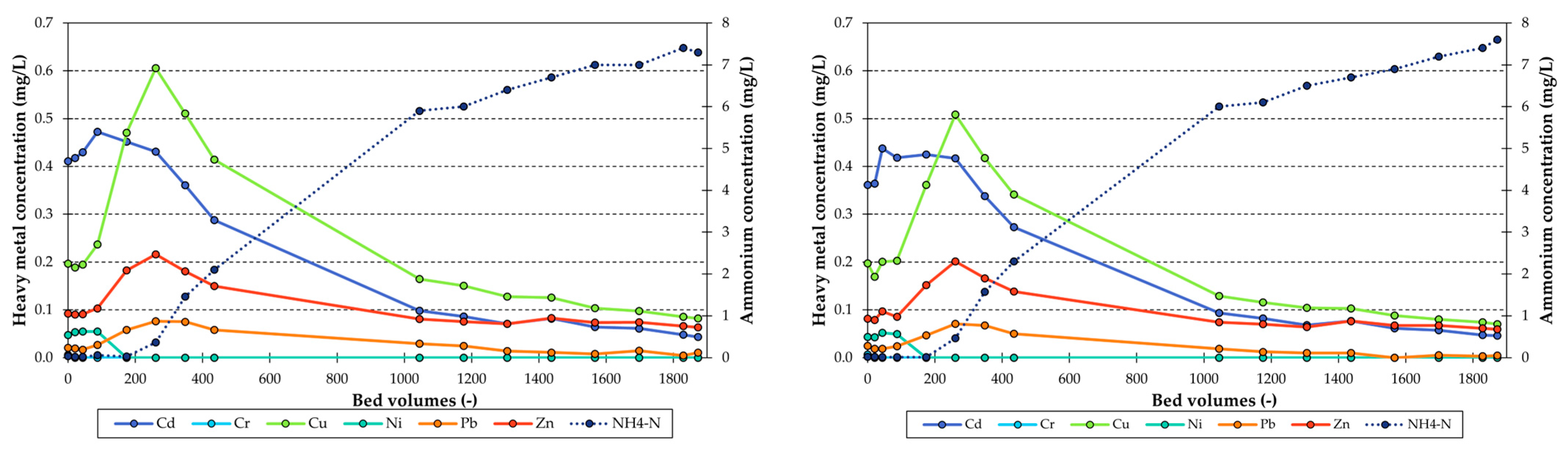
3.2. Heavy Metal Adsorption Column Tests
Competitive Adsorption Column Tests
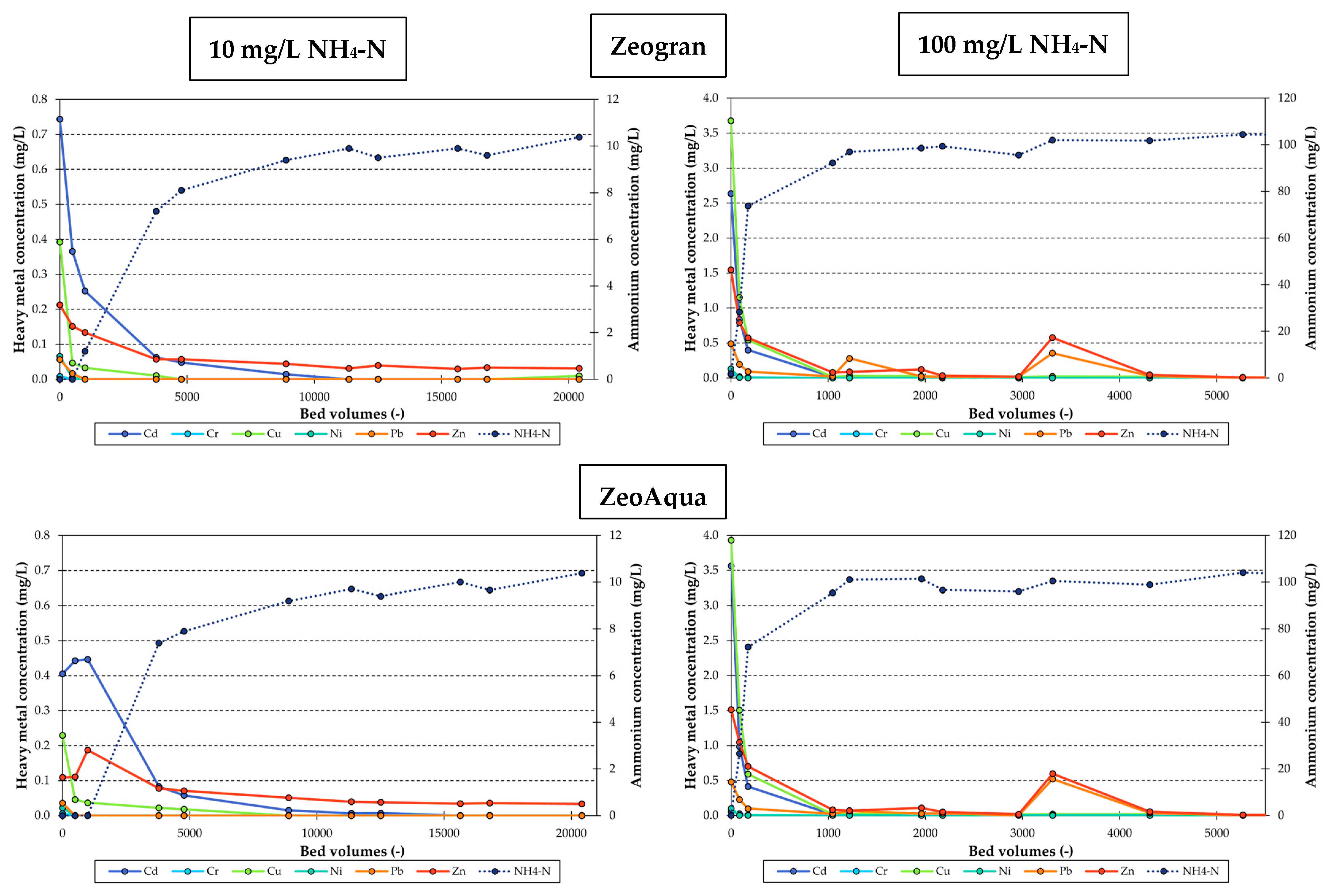
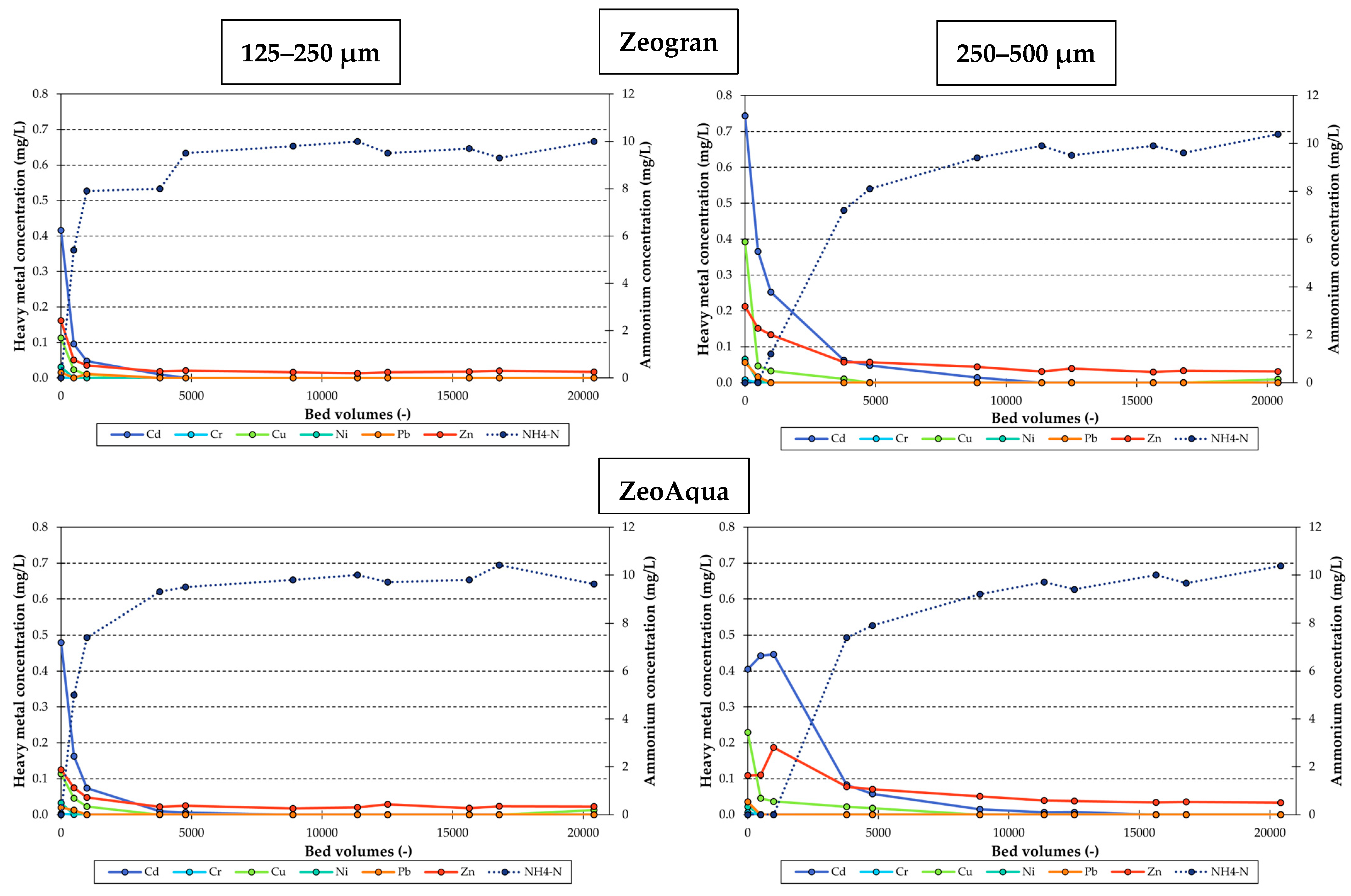
3.3. Heavy Metal Desorption Column Tests
3.3.1. Desorption of Heavy Metals by Elevated Ammonium Concentrations
3.3.2. Influence of the Particle Size on Heavy Metal Desorption
3.3.3. Influence of the pH on Heavy Metal Desorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Finish, N.; Ramos, P.; Borojovich, E.J.C.; Zeiri, O.; Amar, Y.; Gottlieb, M. Zeolite performance in removal of multicomponent heavy metal contamination from wastewater. J. Hazard. Mater. 2023, 457, 131784. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Bradl, H. Heavy Metals in the Environment: Origin, Interaction and Remediation, 1st ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; ISBN 9780080455006. [Google Scholar]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Abedi, T.; Mojiri, A. Constructed wetland modified by biochar/zeolite addition for enhanced wastewater treatment. Environ. Technol. Innov. 2019, 16, 100472. [Google Scholar] [CrossRef]
- He, H.; Duan, Z.; Wang, Z.; Yue, B. The removal efficiency of constructed wetlands filled with the zeolite-slag hybrid substrate for the rural landfill leachate treatment. Environ. Sci. Pollut. Res. Int. 2017, 24, 17547–17555. [Google Scholar] [CrossRef]
- Guo, X.; Cui, X.; Li, H. Effects of fillers combined with biosorbents on nutrient and heavy metal removal from biogas slurry in constructed wetlands. Sci. Total Environ. 2020, 703, 134788. [Google Scholar] [CrossRef]
- Ofiera, L.M.; Wintgens, T.; Kazner, C. Adsorbent modified constructed wetlands for advanced removal of bulk organics and heavy metals from municipal wastewater effluent. Environ. Sci. Water Res. Technol. 2025. [Google Scholar] [CrossRef]
- Jiménez-Reyes, M.; Almazán-Sánchez, P.T.; Solache-Ríos, M. Radioactive waste treatments by using zeolites. A short review. J. Environ. Radioact. 2021, 233, 106610. [Google Scholar] [CrossRef]
- Ren, H.; Jiang, J.; Wu, D.; Gao, Z.; Sun, Y.; Luo, C. Selective Adsorption of Pb(II) and Cr(VI) by Surfactant-Modified and Unmodified Natural Zeolites: A Comparative Study on Kinetics, Equilibrium, and Mechanism. Water Air Soil Pollut. 2016, 227, 101. [Google Scholar] [CrossRef]
- Wasielewski, S.; Rott, E.; Minke, R.; Steinmetz, H. Evaluation of Different Clinoptilolite Zeolites as Adsorbent for Ammonium Removal from Highly Concentrated Synthetic Wastewater. Water 2018, 10, 584. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Armijos, C.; Cortina, J.L. Simultaneous phosphate and ammonium removal from aqueous solution by a hydrated aluminum oxide modified natural zeolite. Chem. Eng. J. 2015, 271, 204–213. [Google Scholar] [CrossRef]
- Zhan, L.; You, Y.; Zhao, L.; Hao, N.; Bate, B. A study on the competitive adsorption process of NH4+ and Zn2+ on activated carbon and zeolite. Int. J. Environ. Sci. Technol. 2022, 20, 6039–6052. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, T.; Yi, W.; Zhang, T.; Zhang, Y. The release mechanism of heavy metals from lab-scale vertical flow constructed wetlands treating road runoff. Environ. Sci. Pollut. Res. Int. 2019, 26, 16588–16595. [Google Scholar] [CrossRef] [PubMed]
- DIN EN ISO 11885:2009-09; Water Quality-Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). Beuth Verlag GmbH: Berlin, Germany, 2009.
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 9781118103777. [Google Scholar]
- Ofiera, L.M.; Bose, P.; Kazner, C. Removal of Heavy Metals and Bulk Organics towards Application in Modified Constructed Wetlands Using Activated Carbon and Zeolites. Water 2024, 16, 511. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Hill, A.V. The possible effects of the aggregation of the molecules of hemoglobin on its dissociation curves. J. Physiol. 1910, 40, iv–vii. [Google Scholar]
- Stefan, M.I.; Le Novère, N. Cooperative binding. PLoS Comput. Biol. 2013, 9, e1003106. [Google Scholar] [CrossRef]
- Yuna, Z. Review of the Natural, Modified, and Synthetic Zeolites for Heavy Metals Removal from Wastewater. Environ. Eng. Sci. 2016, 33, 443–454. [Google Scholar] [CrossRef]
- Panayotova, M.; Velikov, B. Influence of zeolite transformation in a homoionic form on the removal of some heavy metal ions from wastewater. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2003, 38, 545–554. [Google Scholar] [CrossRef]
- Richens, D.T. The Chemistry of Aqua Ions: Synthesis, Structure and Reactivity: A Tour Through the Periodic Table of the Elements; Wiley: Chichester, UK, 1997; ISBN 0471970581. [Google Scholar]
- Biblioteca, I.; Sambucci, M.; Valente, M. Zeolite-Clinoptilolite conditioning for improved heavy metals ions removal: A preliminary assessment. Ceram. Int. 2023, 49, 39649–39656. [Google Scholar] [CrossRef]
- Alvarez-Ayuso, E.; García-Sánchez, A.; Querol, X. Purification of metal electroplating waste waters using zeolites. Water Res. 2003, 37, 4855–4862. [Google Scholar] [CrossRef] [PubMed]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Res. 2002, 36, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. Adsorption of Heavy Metals from Aqueous Solutions on Synthetic Zeolite. Res. J. Environ. Sci. 2008, 2, 13–22. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Buszewski, B.; Terzyk, A.P.; Namieśnik, J. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef]
- Kuldeyev, E.; Seitzhanova, M.; Tanirbergenova, S.; Tazhu, K.; Doszhanov, E.; Mansurov, Z.; Azat, S.; Nurlybaev, R.; Berndtsson, R. Modifying Natural Zeolites to Improve Heavy Metal Adsorption. Water 2023, 15, 2215. [Google Scholar] [CrossRef]
- Lashchenko, D.I.; Kukushkin, R.G.; Danilova, I.G.; Yakovlev, I.V.; Bulavchenko, O.A.; Lyulyukin, A.P.; Rodina, V.O.; Yakovlev, V.A. Effect of the alumina binder content on the mechanical strength and the Si/Al ratio in the zeolite framework in the shaped γ-Al2O3-ZSM-5 composites. Mater. Today Chem. 2025, 46, 102735. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Medykowska, M.; Wiśniewska, M.; Szewczuk-Karpisz, K.; Panek, R. Interaction mechanism of heavy metal ions with the nanostructured zeolites surface–Adsorption, electrokinetic and XPS studies. J. Mol. Liq. 2022, 357, 119144. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Vollprecht, D.; Frühauf, S.; Stocker, K.; Ellersdorfer, M. Ammonium Sorption from Landfill Leachates Using Natural and Modified Zeolites: Pre-Tests for a Novel Application of the Ion Exchanger Loop Stripping Process. Minerals 2019, 9, 471. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, W.; Chen, Q.; Peng, C.; He, D.; Zhou, K. Removal of ammonia-nitrogen in wastewater using a novel poly ligand exchanger-Zn(II)-loaded chelating resin. Water Sci. Technol. 2019, 79, 126–136. [Google Scholar] [CrossRef]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Sheikh Abdul Kadir, S.H.; Mohd Sokri, M.N.; Tai, Z.S.; Iwamoto, Y.; Tanemura, M.; Honda, S.; Puteh, M.H.; A Rahman, M.; et al. Influence of the Natural Zeolite Particle Size Toward the Ammonia Adsorption Activity in Ceramic Hollow Fiber Membrane. Membranes 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.S.; Chao, C.Y.H.; Kot, S.C. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Emerson, K.; Russo, R.C.; Lund, R.E.; Thurston, R.V. Aqueous Ammonia Equilibrium Calculations: Effect of pH and Temperature. J. Fish. Res. Board Can. 1975, 32, 2379–2383. [Google Scholar] [CrossRef]
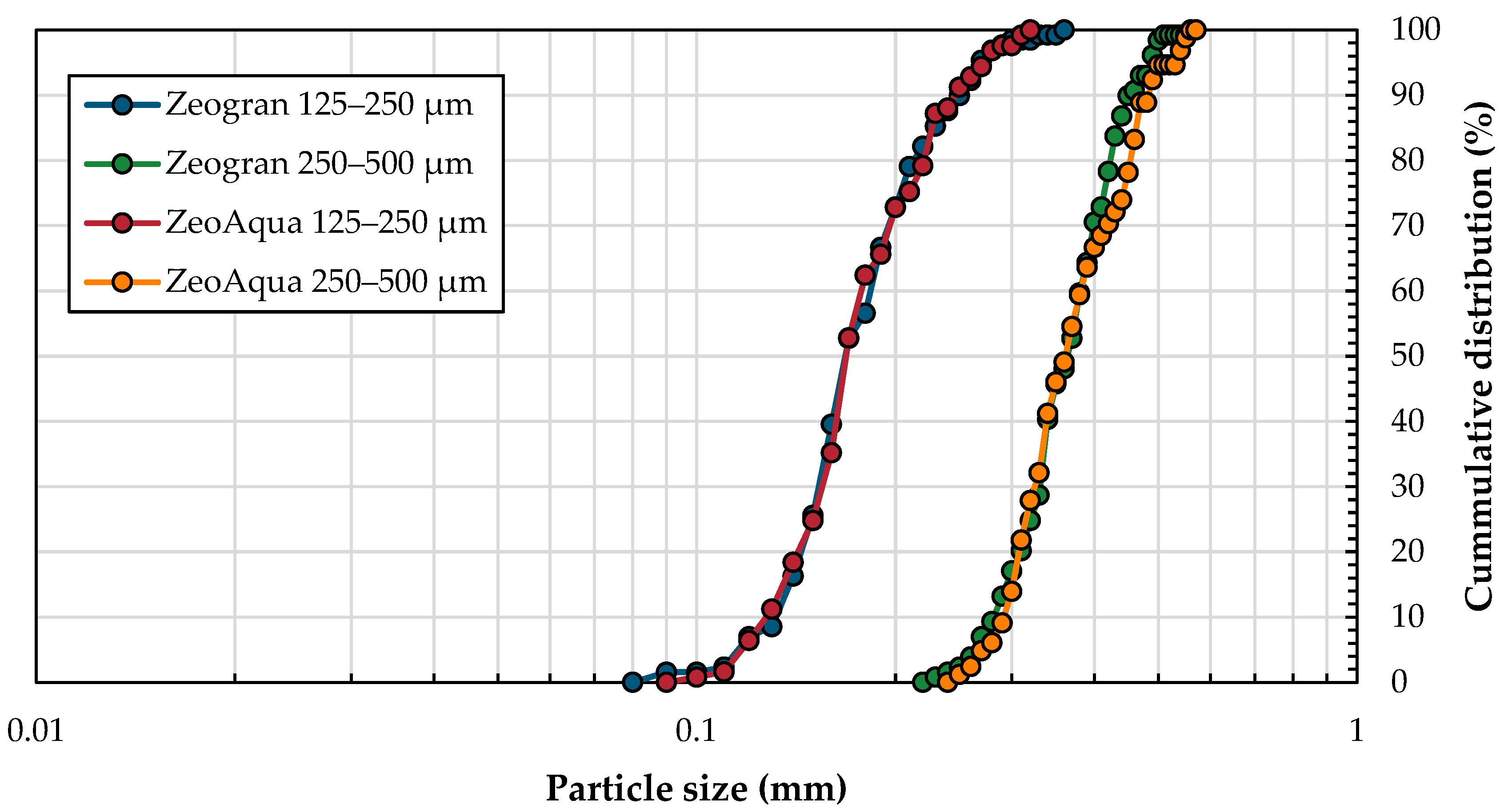



| Supplier | Zeolite | BET (m2/g) | SiO3 (%) | Al2O3 (%) | Fe2O3 (%) | CaO (%) | K2O (%) |
|---|---|---|---|---|---|---|---|
| Zeobon GmbH, Dattenberg, Germany | Zeogran K 80 | 27.41 | 65.47 | 11.63 | 1.43 | 2.91 | 3.81 |
| Zeocem Inc., Bystré, Slovakia | ZeoAqua | 33.03 | 66.99 | 11.81 | 1.51 | 2.80 | 3.81 |
| Ion | Charge | Hydrated Radius (nm) | Ionic Radius (nm) | Polarizability | Hydration Enthalpy (kJ/mol) |
|---|---|---|---|---|---|
| Cd | +2 | 0.426 | 0.097 | Moderate | −1800 |
| Cr | +3 | 0.401 | 0.062 | High | −4600 |
| Cu | +2 | 0.419 | 0.073 | High | −2100 |
| Ni | +2 | 0.404 | 0.069 | Low | −2105 |
| Pb | +2 | 0.401 | 0.119 | Very high | −1480 |
| Zn | +2 | 0.430 | 0.074 | Moderate | −2045 |
| NH4 | +1 | 0.331 | 0.148 | Low | −300 |
| Zeolite | Parameters | Cd | Cr | Cu | Ni | Pb | Zn | All HMs |
|---|---|---|---|---|---|---|---|---|
| Zeogran (0 mg/L NH4-N) | qe (mg/g) | 0.41 | 1.33 | 0.79 | 0.27 | 5.84 | 1.21 | 9.36 |
| min) | 0.714 | 2.573 | 0.124 | 0.012 | 2.162 | 0.628 | 0.315 | |
| R2 | 0.82 | 0.90 | 0.93 | 0.59 | 1.00 | 0.86 | 0.94 | |
| ZeoAqua (0 mg/L NH4-N) | qe (mg/g) | 0.53 | 0.97 | 1.20 | 0.70 | 5.86 | 1.49 | 9.74 |
| min) | 3.252 | 7.214 | 0.107 | 0.001 | 12.407 | 1.199 | 0.608 | |
| R2 | 0.50 | 0.67 | 0.90 | 0.86 | 1.00 | 0.78 | 0.86 | |
| Zeogran (10 mg/L NH4-N) | qe (mg/g) | 0.44 | 0.84 | 0.75 | 0.35 | 3.97 | 0.25 | 5.82 |
| min) | 0.029 | 1.679 | 0.022 | 0.048 | 0.271 | 0.058 | 0.084 | |
| R2 | 0.59 | 0.44 | 0.89 | 0.59 | 0.98 | 0.52 | 0.78 | |
| ZeoAqua (10 mg/L NH4-N) | qe (mg/g) | 0.42 | 0.89 | 0.58 | 0.28 | 4.18 | 0.46 | 6.34 |
| min) | 0.084 | 1.256 | 0.160 | 0.162 | 0.485 | 0.058 | 0.127 | |
| R2 | 0.69 | 0.70 | 0.94 | 0.57 | 1.00 | 0.72 | 0.86 | |
| Zeogran (100 mg/L NH4-N) | qe (mg/g) | 0.37 | 0.45 | 0.45 | 0.37 | 3.25 | 0.32 | 5.11 |
| min) | 0.055 | 0.107 | 0.107 | 0.060 | 0.227 | 0.045 | 0.102 | |
| R2 | 0.69 | 0.74 | 0.74 | 0.82 | 0.94 | 0.72 | 0.77 | |
| ZeoAqua (100 mg/L NH4-N) | qe (mg/g) | 0.25 | 0.84 | 0.44 | 0.27 | 3.61 | 0.26 | 5.43 |
| min) | 0.221 | 2.721 | 0.129 | 0.272 | 0.327 | 0.188 | 0.132 | |
| R2 | 0.41 | 0.56 | 0.71 | 0.49 | 0.99 | 0.49 | 0.85 |
| Isotherm Model | Constants | Zeogran 10 mg/L NH4-N | ZeoAqua 10 mg/L NH4-N | Zeogran 100 mg/L NH4-N | ZeoAqua 100 mg/L NH4-N |
|---|---|---|---|---|---|
| Hill | qmax (mg/g) | 56.14 | 52.85 | 31.93 | 49.03 |
| nH (-) | 13.87 | 9.81 | 10.92 | 12.38 | |
| KD (mg/L) | 44.91 | 45.88 | 46.84 | 47.27 | |
| R2 (-) | 0.9999 | 0.9999 | 0.9998 | 0.9997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ofiera, L.M.; Kazner, C. Influence of Ammonium on the Adsorption and Desorption of Heavy Metals in Natural Zeolites. Processes 2025, 13, 2647. https://doi.org/10.3390/pr13082647
Ofiera LM, Kazner C. Influence of Ammonium on the Adsorption and Desorption of Heavy Metals in Natural Zeolites. Processes. 2025; 13(8):2647. https://doi.org/10.3390/pr13082647
Chicago/Turabian StyleOfiera, Luca Marco, and Christian Kazner. 2025. "Influence of Ammonium on the Adsorption and Desorption of Heavy Metals in Natural Zeolites" Processes 13, no. 8: 2647. https://doi.org/10.3390/pr13082647
APA StyleOfiera, L. M., & Kazner, C. (2025). Influence of Ammonium on the Adsorption and Desorption of Heavy Metals in Natural Zeolites. Processes, 13(8), 2647. https://doi.org/10.3390/pr13082647







