1. Introduction
Rising anthropogenic greenhouse gas (GHG) emissions, particularly CO
2, are major drivers of global warming, ecosystem degradation, and extreme weather events, underscoring the urgent need for decarbonisation. Hydrogen offers potential for reducing emissions in sectors such as transport, power, and heavy industry [
1]. However, conventional production methods (grey hydrogen) like steam-methane reforming (SMR) emit significant CO
2, approximately 9 kg per kg of hydrogen produced [
1,
2]. Blue hydrogen, defined as hydrogen produced from fossil sources with carbon capture and storage (CCS), is a transitional technology bridging conventional grey hydrogen and green hydrogen from electrolysis. While this approach is commercially mature, it has efficiency penalties and cannot capture 100% of carbon (practical capture rates are typically 90–95%). Over the past decade, significant research has focused on advanced process configurations to improve the energy efficiency and carbon capture rate of blue hydrogen [
3]. Two of the most advanced configurations for blue hydrogen are sorption-enhanced steam methane reforming (SE-SMR) and chemical-looping combustion (CLC), often used in tandem.
SE-SMR integrates a CO
2 sorbent (usually CaO) directly into the reforming reactor, enhancing hydrogen yield by shifting equilibrium reactions and enabling in situ carbon capture [
2]. In effect, SE-SMR can operate auto-thermally, without the large furnace required in standard SMR, as the exothermic CO
2 sorption provides much of the needed heat internally [
4]. SE-SMR has been shown to significantly increase hydrogen yield and purity while simultaneously capturing CO
2 from the reaction mixture. Laboratory and pilot studies over the last decade have validated the SE-SMR concept (current technology readiness level ~4–5) [
5]. For example, the Gas Technology Institute demonstrated a pilot SE-SMR unit producing hydrogen at 71 kW
th scale with >80% H
2 purity [
6].
The challenge in SE-SMR is that the CO
2 sorbent eventually becomes saturated and must be regenerated (releasing the CO
2) in a separate step. A key requirement for blue hydrogen is that the CO
2 released during regeneration be kept separate (concentrated CO
2 suitable for capture, not vented). Conventional regeneration by heating the sorbent with a combustion flue gas (from burning fuel in air) is not viable, because that would dilute the CO
2 with nitrogen [
3]. Thus, various studies have explored regeneration via oxy-fuel combustion and, more recently, CLC [
7,
8].
In the oxy-fuel approach, fuel is combusted with pure O
2 (from an air separation unit (ASU)) to provide heat for regenerating the CO
2 sorbent, producing a concentrated CO
2 stream. Martínez et al. (2019) assessed an oxyfuel SE-SMR process in a hydrogen plant and achieved over 98% carbon capture with an equivalent hydrogen production efficiency of ~75% (lower heating value (LHV) basis) [
9]. This high capture rate is because both sources of CO
2 (the reforming-produced CO
2 and the combustion CO
2 for heat) are captured: the former via the sorbent and the latter via the pure CO
2 from oxy-combustion. However, the need for an ASU imposes an energy and cost penalty. A study by Yan et al. (2020b) [
10] noted that integrating oxy-fuel combustion for sorbent calcination reduced net efficiency by about 2.7 percentage points compared to other regeneration methods, due to the ASU power load.
The second (and increasingly favoured) approach is to integrate CLC with the SE-SMR process. In a CLC system, a solid oxygen carrier (typically a metal oxide like NiO or CuO) is used to transfer oxygen for combustion without direct contact between fuel and air [
3,
11]. The metal oxide is alternately oxidised by air in one reactor and reduced by the fuel (e.g., natural gas or reformer off-gas) in another, releasing heat. Importantly, the fuel in a CLC reduces the metal oxide and produces CO
2 and H
2O without nitrogen dilution, so a pure CO
2 stream can be obtained after condensing water [
3,
12].
Several studies have examined combining SE-SMR with a Ni-based CLC loop to supply the regeneration heat for the CO
2 sorbent [
8,
10,
11,
12]. In this configuration, the exothermic oxidation of Ni to NiO (in air) produces hot solids that are circulated to provide heat for the endothermic calcination of CaCO
3 (regenerating CaO and releasing CO
2), and the NiO is reduced by fuel in a separate step. Alam (2017) achieved a hydrogen production efficiency of 70.7% with 95.1% of carbon captured using a CLC-integrated SE-SMR process [
12]. Later, Yan et al. (2020b) [
10] evaluated multiple SE-SMR process configurations for blue H
2 at industrial scale, including cases with conventional heating, oxyfuel, and CLC. They showed that integrating SE-SMR with CLC and pressure swing adsorption (PSA) for H
2 purification could achieve nearly 100% CO
2 capture with a net efficiency up to 76.3%, markedly higher than a traditional SMR with CCS. This CLC-integrated design (SE-SMR + CLC + PSA) had the best performance of the six configurations studied by Yan et al., highlighting the efficacy of combining these technologies [
10]. In a subsequent study, Yan et al. (2020a) [
7] demonstrated that SE-SMR integrated with CLC could achieve over 95% CO
2 capture with competitive efficiencies and levelized hydrogen costs (LCOH) ranging between GBP 1.90–2.80 kg H
2.
Overall, the integration of SE-SMR and CLC is a major trend in blue hydrogen research, aiming to maximise carbon capture while minimising efficiency loss. By employing in situ CO
2 capture and using chemical looping to supply regeneration energy (instead of an external furnace), these systems virtually eliminate direct CO
2 emissions. Other process intensification strategies, including membrane-assisted separation and indirect heating methods, are further enhancing the appeal of SE-SMR. These configurations eliminate the need for externally fired heaters and shift the system toward near-zero emissions [
13,
14]. Additionally, applying high-performance CO
2 sorbents and cyclic reactors improves long-term process stability and scalability.
One crucial aspect of hydrogen production is water usage and environmental impact. Large-scale hydrogen production is energy-intensive, and substantial heat must be removed for safe and efficient operation. Traditionally, water-based cooling (via cooling water or evaporative cooling towers) is employed in SMR plants due to water’s high heat capacity and the effectiveness of evaporative cooling. However, this comes at the cost of significant water consumption. Studies estimate that roughly 30% of the total water withdrawals associated with hydrogen production (SMR processes) are consumed by cooling systems [
15]. In fact, adding CCS to SMR further increases cooling water requirements, since CCS (e.g., solvent scrubbing systems) introduce additional cooling and solvent regeneration needs [
16].
Water-cooling systems, typically implemented as once-through, open-loop, or closed-loop evaporative systems, are valued for their compact design and high thermal conductivity. However, their significant water consumption poses challenges, especially in arid or drought-prone areas. For example, an SMR with CCS has been reported to consume about 32.2 L of water per kg H
2 produced (vs. ~30.4 L/kg without CCS), primarily due to extra cooling duties [
16]. Arup (2022) [
17] reported that a blue hydrogen facility employing water cooling could consume between 28 and 35 L of water per kg of hydrogen, depending on the process integration and CO
2 capture efficiency.
Air-cooled heat exchangers or dry cooling systems eliminate most process water consumption by using ambient air to carry away heat, albeit often with larger equipment and fan power requirements. In practice, there are trade-offs between water and air cooling. Evaporative (water) cooling is often more thermodynamically efficient and cost-effective than dry cooling for a given duty [
18]. Ellersdorfer et al. (2025) [
18] found that in large hydrogen electrolysis facilities, evaporative cooling systems could be up to 8× cheaper to implement than equivalent dry cooling systems for the same heat removal capacity. The ability of water cooling to achieve lower temperatures (approaching the water’s wet-bulb temperature) means smaller temperature differentials, and thus smaller required heat exchange area, explaining its lower cost in many cases [
18]. Air cooling, by contrast, typically operates at higher minimum temperatures (limited by ambient air dry-bulb temperature) and often requires larger exchangers and fans, raising capital and operating costs [
18]. Despite this, the advantage of air cooling is the drastic reduction in water use, a critical factor in arid regions or where water resources are constrained [
16]. Recent analyses strongly encourage using “water-efficient cooling technologies such as air cooling” for hydrogen projects in water-scarce areas [
19]. This is echoed by the International Renewable Energy Agency (IRENA), which notes that hydrogen projects in desert climates should minimise freshwater consumption by opting for dry cooling when feasible [
19].
In summary, air versus water cooling presents a trade-off between water conservation and cost-effectiveness. Water cooling remains the predominant choice for most current hydrogen plants (including blue hydrogen from SMR) due to its lower cost and excellent heat rejection capability. Indeed, most literature studies on hydrogen production assume conventional water-cooling utilities (cooling towers or once-through water) as part of the plant design. For instance, an advanced blue hydrogen process study by Eluwah et al. (2023) assumed an external cooling water supply at 20 °C for condensing and heat recovery duties [
20]. However, as sustainability concerns grow, researchers are increasingly recognising the need to evaluate dry cooling. The techno-economic impact of switching to air cooling can be significant: while it virtually eliminates process water consumption, it may slightly reduce overall plant efficiency (due to higher condensing temperatures) and increase costs. Some hydrogen production scenarios find that employing dry/air cooling would avoid approximately 6000–20,000 GL of water per year for a large future hydrogen facility (versus wet cooling), albeit with a moderate energy penalty [
18]. Ultimately, the choice of cooling method in blue hydrogen production must balance water availability, environmental impact, energy efficiency, and economics. Emerging literature underscores that in regions with abundant water, traditional water cooling offers cost and efficiency benefits, whereas in water-limited areas, air cooling can be justified to ensure the water sustainability of hydrogen production [
16].
Lin et al. (2025) assessed different hydrogen production technologies for water footprint of the process. They found that blue hydrogen production using amine scrubbing as the CCS method, consumed more water (~1.8 L/kg H
2) than a conventional SMR process [
16]. Within the literature, there is a tendency to focus on technologies with a high readiness level such as amine scrubbing, especially when assessing further environmental metrics such as the water footprint. Lower technology readiness level (TRL) processes such as SE-SMR-CLC are often overlooked as they tend to focus on improved production efficiency [
20]. Eluwah et al. (2023) developed an industrial SE-SMR process coupled with CLC, finding a high thermal efficiency of ~97.5% at a LCOH of USD 1.6/kg H
2 [
20]. Whilst showing high efficiency and low cost, further exploration of the system is required.
Despite extensive research into integrating SE-SMR and CLC technologies for blue hydrogen production, the techno-economic implications of cooling strategies within these configurations remain underexplored. Most existing studies assume conventional water-based cooling without considering regional variations in water availability or the potential performance trade-offs associated with alternative methods such as dry (air) cooling. This oversight is particularly critical given that cooling systems not only significantly contribute to the overall water footprint of hydrogen production but also impact equipment sizing, land use, and operating efficiency (factors that can vary dramatically between temperate and arid climates). The current study addresses this gap by conducting a comprehensive comparative assessment of air versus water cooling in an SE-SMR-CLC process for blue hydrogen production, by further focusing on two climatically distinct regions (i.e., UK and Saudi Arabia). This research evaluates how location-dependent parameters influence the selection and performance of the cooling methods. These scenarios are systematically analysed (water and air cooling in each location), concentrating on energy demand, heat exchanger sizing, land requirements, and cost implications. Additionally, a detailed sensitivity analysis of operational expenditure is undertaken to better understand the economic trade-offs and robustness of each configuration. Through this approach, the study aims to provide critical insights into how cooling infrastructure decisions affect the scalability and sustainability of next-generation blue hydrogen systems, ultimately supporting more location-sensitive and resource-aware deployment strategies, for early-TRL blue hydrogen production processes.
4. Policy Implications: How to Incentivise Blue Hydrogen Production Effectively
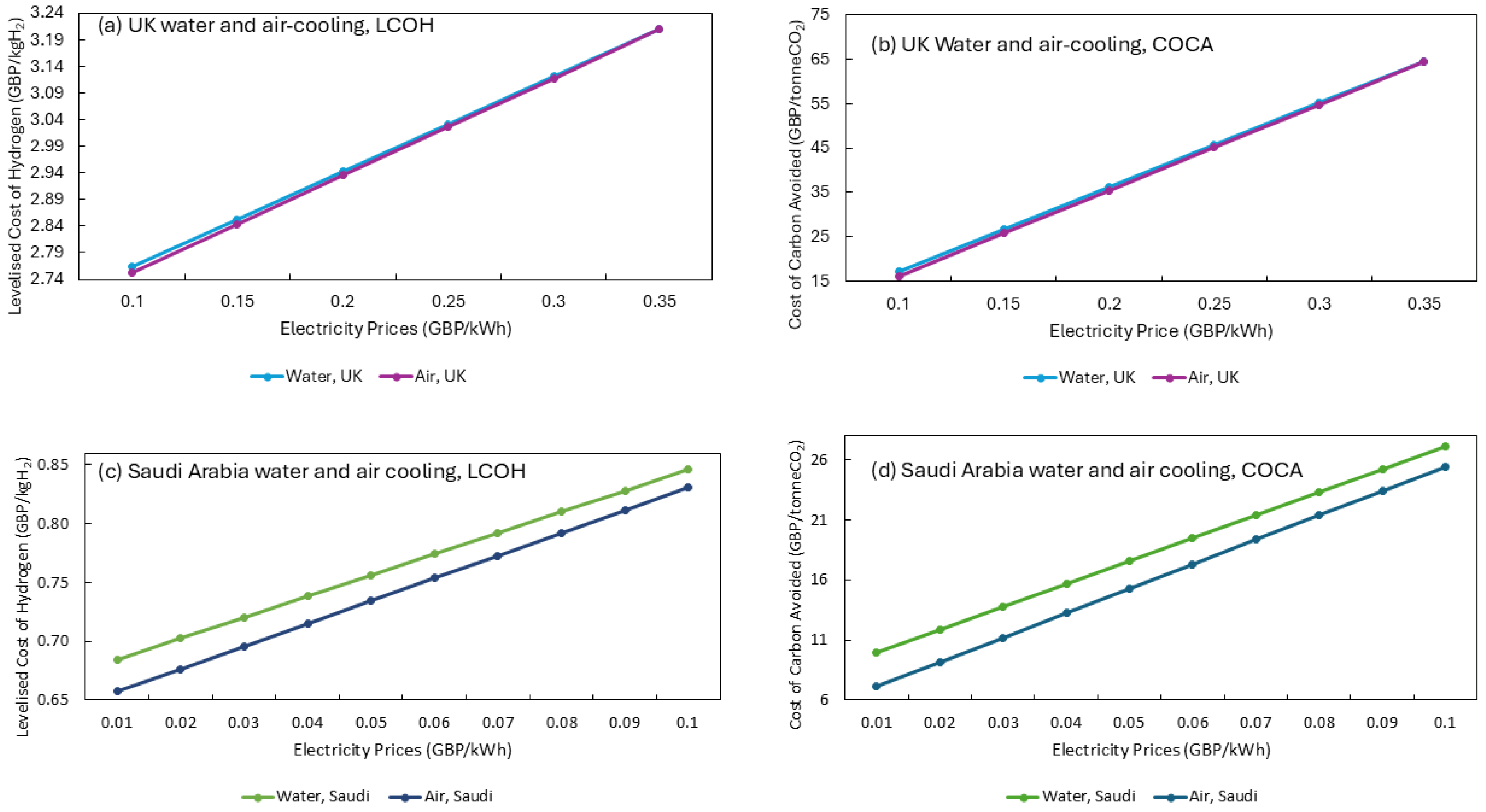
Blue hydrogen has emerged as a critical transitional fuel in the global shift towards low-carbon energy systems. While technological advancements have significantly improved efficiency and reduced costs, effective policy mechanisms are required to accelerate large-scale deployment. Governments must focus on targeted incentives that address economic viability, infrastructure development, and sustainable cooling requirements to ensure widespread adoption. One of the most effective ways to promote blue hydrogen production is through direct financial incentives. Governments can implement subsidies that reduce the LCOH, making it competitive with conventional fuels [
46]. For example, in regions such as the United Kingdom, where the LCOH is relatively high at GBP 2.94/kg H
2, subsidies can be structured to offset production costs, ensuring blue hydrogen remains a viable alternative. Tax credits and grants for industries investing in blue hydrogen infrastructure can further drive adoption. In Saudi Arabia, where the cost is significantly lower (around GBP 0.70–0.72/kg H
2), incentives could focus on supporting infrastructure and scaling up production to meet growing energy demands [
47].
Another method to incentivise low-carbon technologies is through carbon pricing mechanisms, including carbon taxes and cap-and-trade systems, which can create market conditions that favour blue hydrogen production [
48]. By increasing the cost of carbon-intensive alternatives, industries will be incentivised to transition towards cleaner hydrogen solutions. Setting aggressive emission reduction targets for heavy industries can further push investments in blue hydrogen technologies such as SE-SMR-CLC. Ethical considerations in carbon pricing frameworks are essential in guiding responsible corporate behaviour, particularly in emissions reduction [
49]. In the UK, the COCA determined within
Section 3.3.1 shows that a carbon tax of ~GBP 30/tonne CO
2 is needed to ensure it is cost competitive with grey hydrogen production routes. Both processes are impacted by the NG costs, meaning that policy should incentivise reducing emissions within the process. The carbon tax can provide an approach to incentive capturing CO
2.
Scaling blue hydrogen production requires robust infrastructure, including storage, transport, and distribution networks. Governments can play a pivotal role by investing in hydrogen transport corridors, supporting pipeline development, and integrating hydrogen refuelling stations into existing energy grids. In geographically diverse regions, policies should address local energy requirements by optimising cooling methods. For instance, in arid environments such as Saudi Arabia, air cooling has proven viable method within minimal impact on the LCOH (GBP 0.70/kg H
2), despite its higher electricity consumption [
50]. Policies should support energy-efficient cooling methods through research grants and technology development incentives. Based on the analysis, H
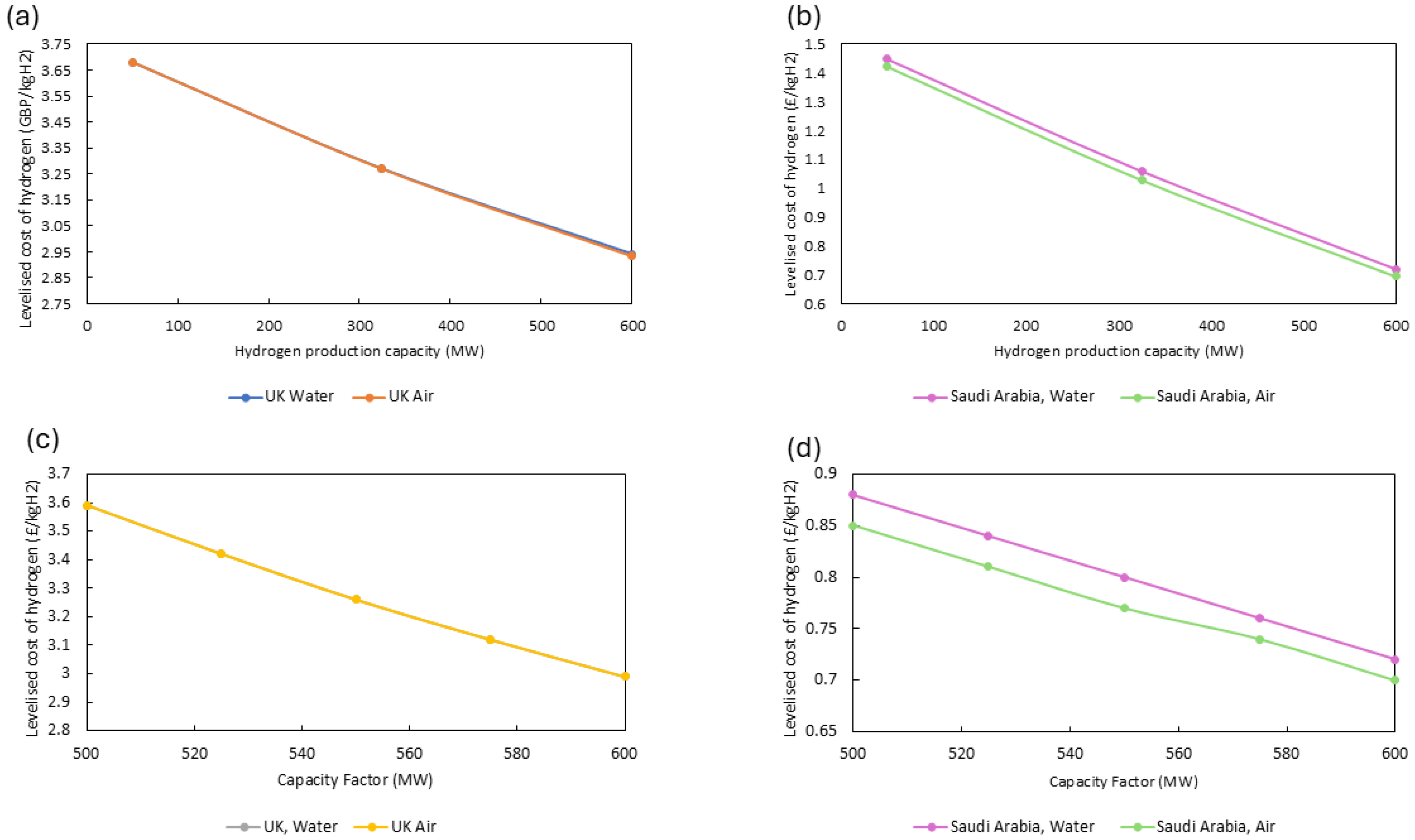
2 production capacity significantly impacts the LCOH for both the UK and Saudi Arabia. Developing large-scale production facilities ensures a lower LCOH: GBP 2.94/kg H
2 for the UK and GBP 0.70–0.72/kg H
2 for Saudi Arabia. Ensuring a market demand for hydrogen is vital. This demand must coincide with developing hydrogen infrastructure. In the UK, this development is particularly slow. For example, only 16 refuelling stations exist within the UK, which is significantly fewer than the 92 refuelling stations in Germany [
51,
52]. This adoption of hydrogen infrastructure is vital as the hydrogen produced should be linked to a sustainable end use, and without it, the large-scale production capacities are mostly redundant, leading to increased LCOH.
Furthermore, robust infrastructure is required for the storage and transportation of CO
2. As shown in this work, transportation and storage costs significantly impact the LCOH and COCA; in the UK, high CO
2 transportation and storage costs can increase the LCOH to GBP 3.05/tonneCO
2. Developing the infrastructure to ensure low transportation and storage costs is vital for the adoption of blue hydrogen. Similarly to hydrogen infrastructure, the surrounding infrastructure for CO
2 transportation and storage is key to ensure lower costs. As outlined by Brownsort et al. (2016), an approach to reducing the CAPEX costs is via sharing of pipelines within industrial clusters [
53]. This development of industrial clusters is a key UK policy for industrial decarbonisation with two currently under development (HyNet and Humber) [
54]. This policy allows for shared costs of CO
2 transportation and storage.
Governments should prioritise funding for research and development of advanced hydrogen technologies. This includes improving process efficiency, reducing reliance on energy-intensive cooling methods, and integrating CCS solutions. Academic institutions and private-sector partnerships should be encouraged through targeted grants, fostering innovation that enhances hydrogen production viability [
55]. Studies indicate that investments in sustainability-focused technological advancements have a positive impact on financial markets and industry adoption [
56]. In Saudi Arabia, the use of water-cooling approaches shows an increased LCOH at GBP 0.72/kg H
2, due to the increased costs of water in the region. The development of hybrid cooling approaches should focus on improving the energy efficiency of air-cooling processes and how this could further reduce LCOH [
57].
Given the disparity in hydrogen production costs between regions, international collaboration is essential. Trade agreements can facilitate cross-border hydrogen exports, ensuring regions with lower production costs, such as Saudi Arabia, can supply countries with higher costs. Cooperative efforts in regulatory standards, shared infrastructure development, and policy harmonisation can further streamline the global hydrogen supply chain [
50,
58]. To effectively incentivise blue hydrogen production, policymakers must adopt a multifaceted approach. By implementing financial incentives, carbon pricing strategies, infrastructure support, research funding, and international collaboration, governments can create favourable conditions for large-scale adoption. Tailoring policies to regional economic and environmental realities will ensure a balanced and sustainable transition towards clean hydrogen energy.