In Vitro Degradation of Continuous Iron Wire-Reinforced PLLA Composite Monofilaments for Bioresorbable Vascular Stents Fabricated via a Novel 3D Printer: An Early-Stage Prototype Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
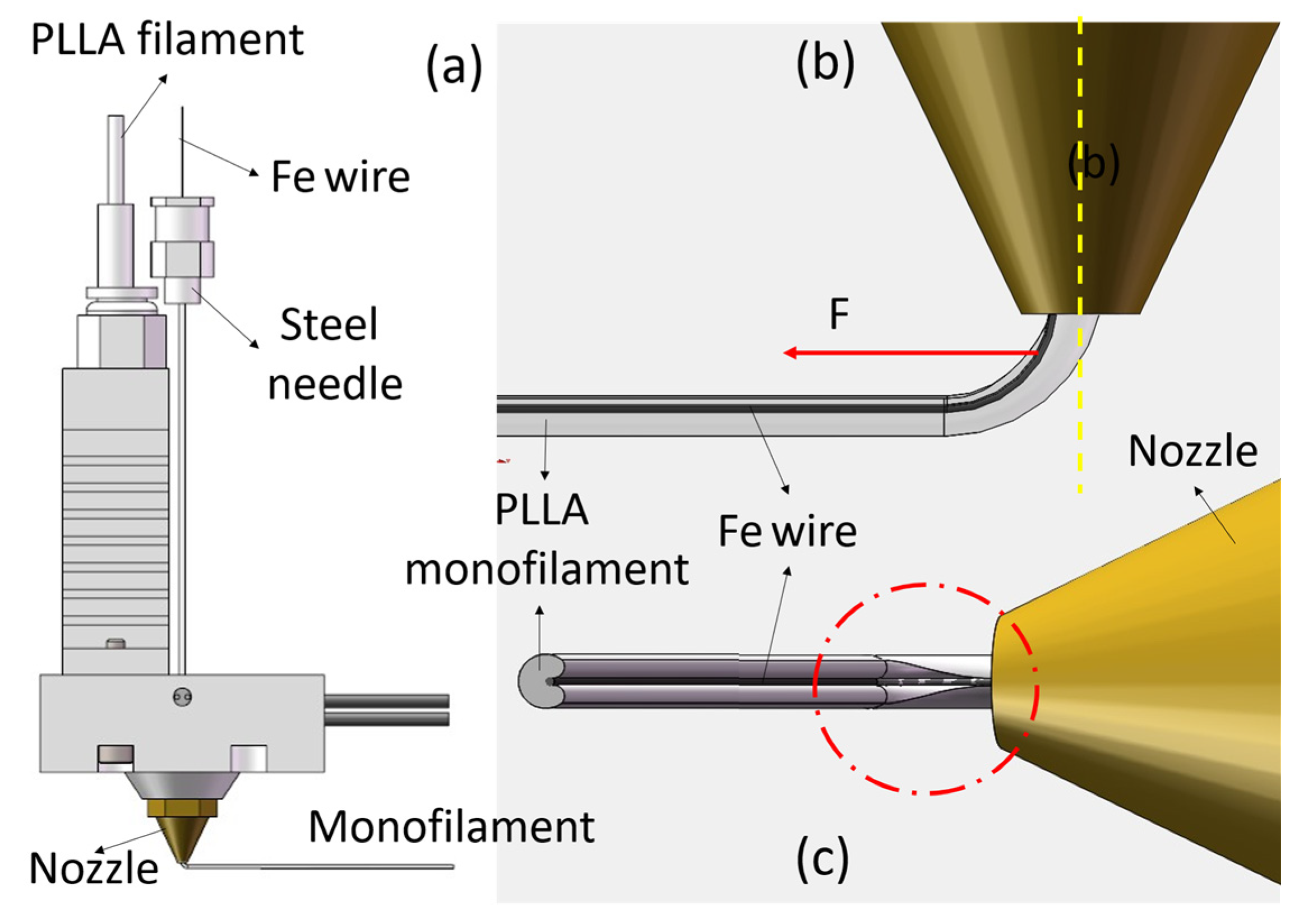
2.2. A Novel Filament–Metal Wire Coextrusion 3D Printer
2.3. Specimen Preparation
2.4. In Vitro-Accelerated Degradation
2.4.1. Simulated Body Fluid (SBF) Preparation
2.4.2. Immersion Test
2.4.3. Iron Ion Concentration Measurement
2.5. Tensile Test
2.6. Mass Loss Percentage
2.7. Gel Permeation Chromatography (GPC) Test
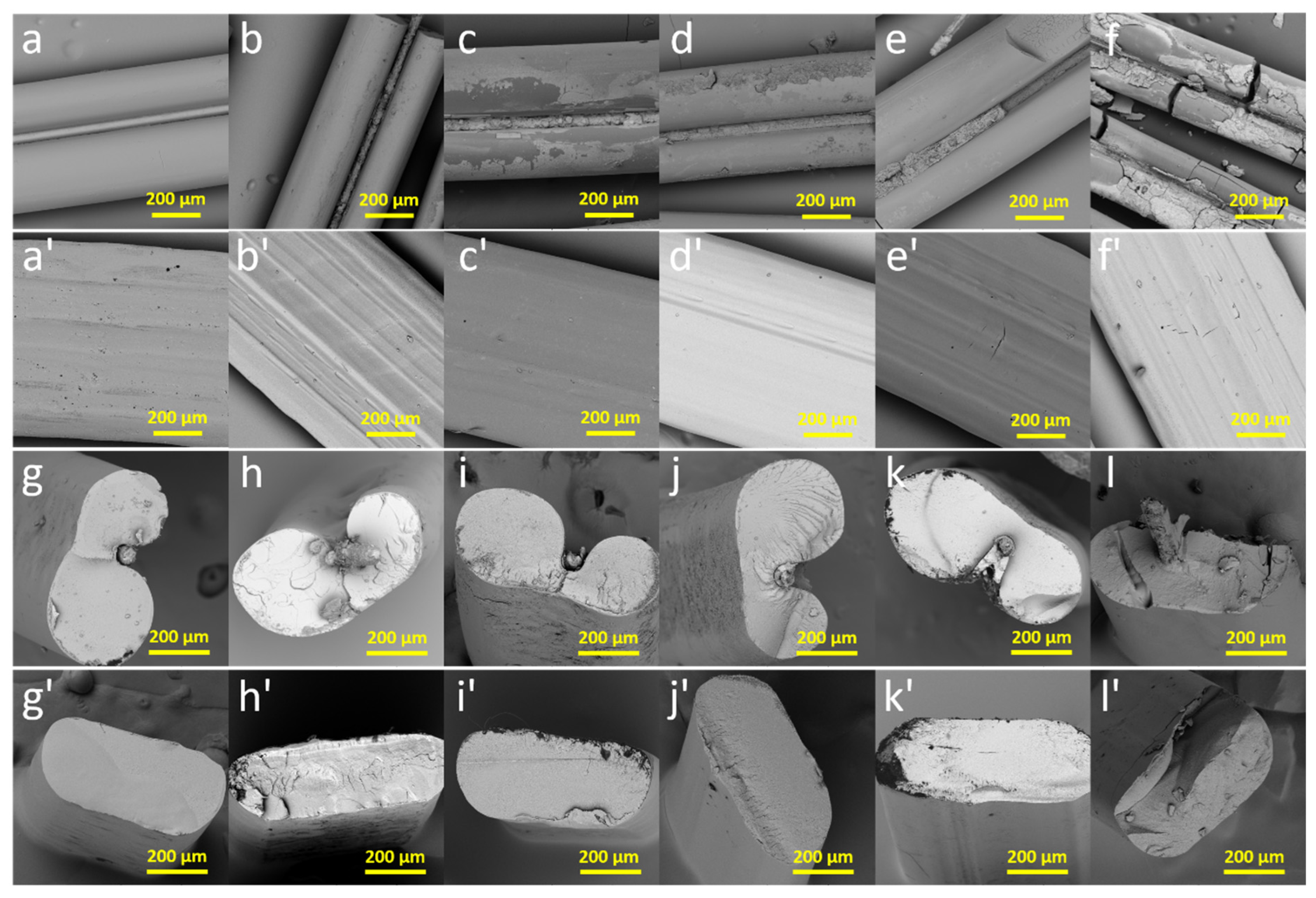
2.8. Surface and Cross-Sectional Surface Morphology
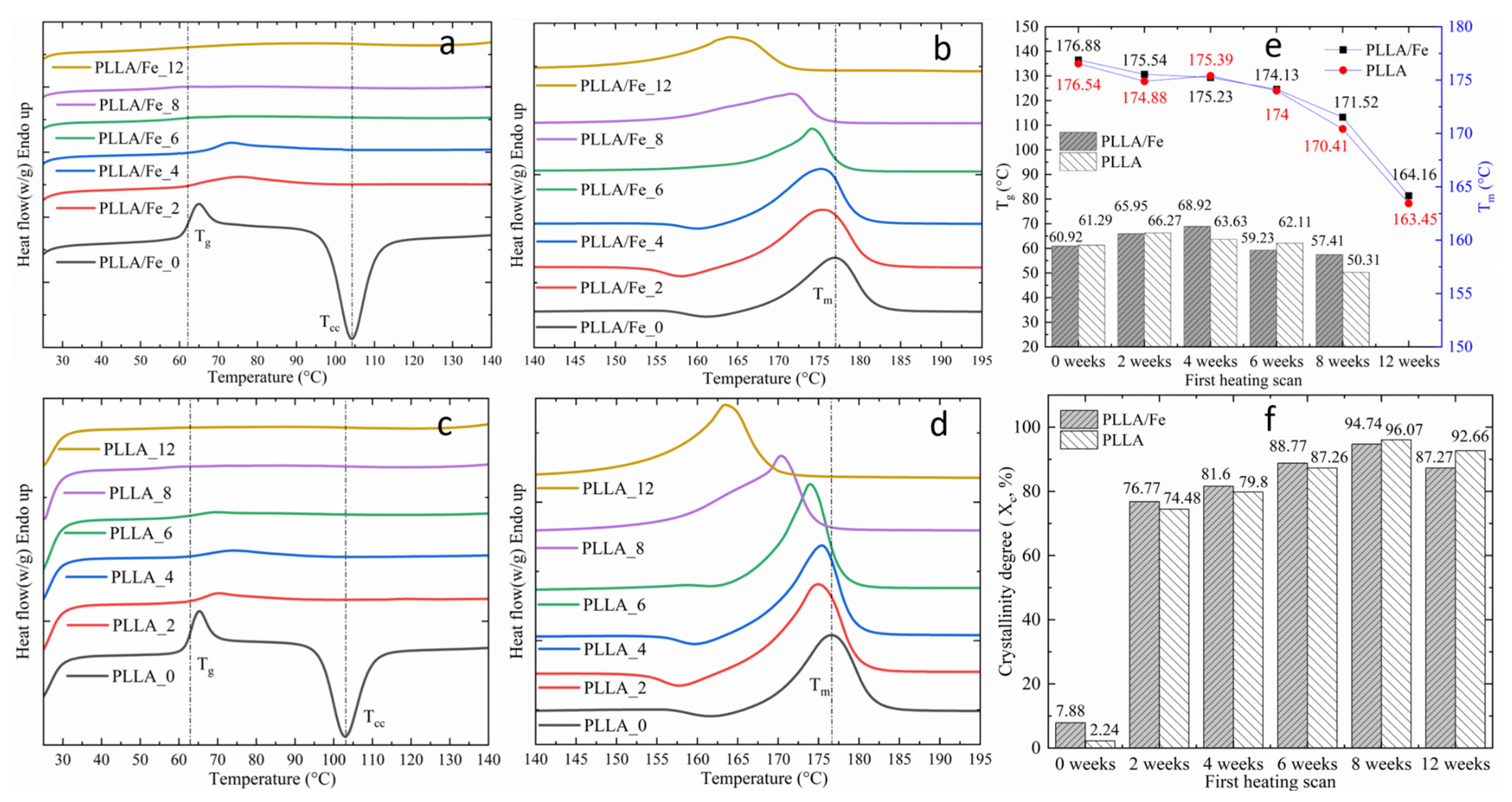
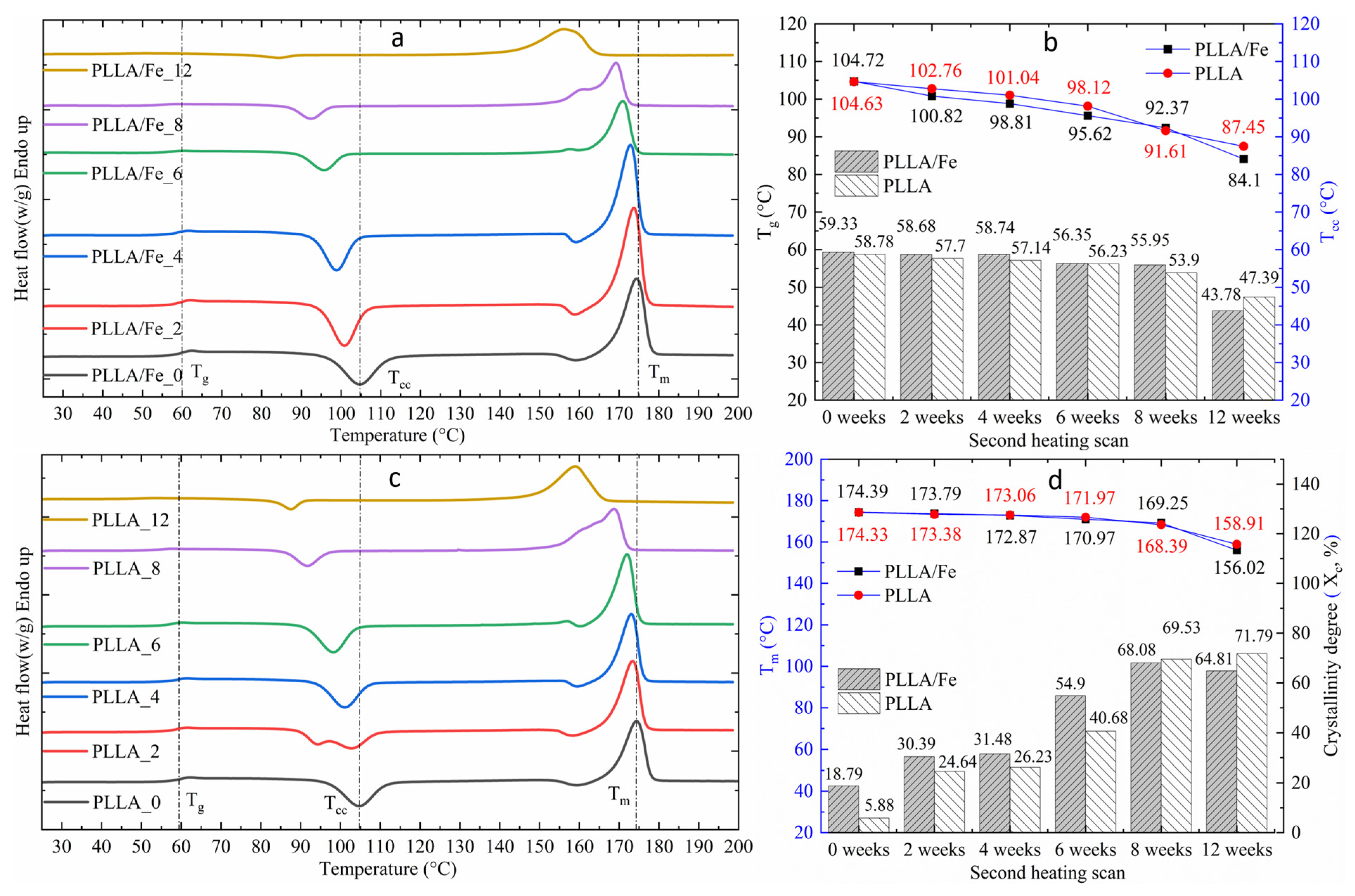
2.9. Differential Scanning Calorimetry
2.10. Statistical Analysis
3. Results and Discussion
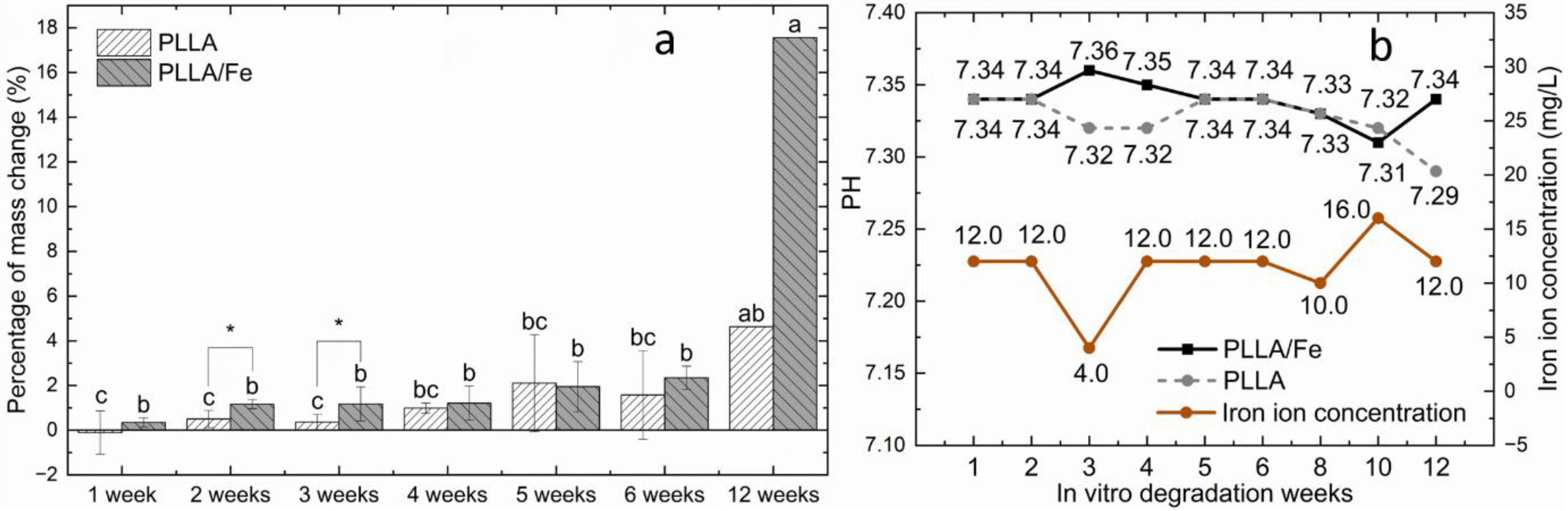
3.1. Mass Change and Acceleration Mechanism of PLLA Matrix
3.1.1. Mass Change
3.1.2. Mechanism of Accelerated Degradation of Iron by PLLA Matrix
3.2. PH and Iron Ion Concentration
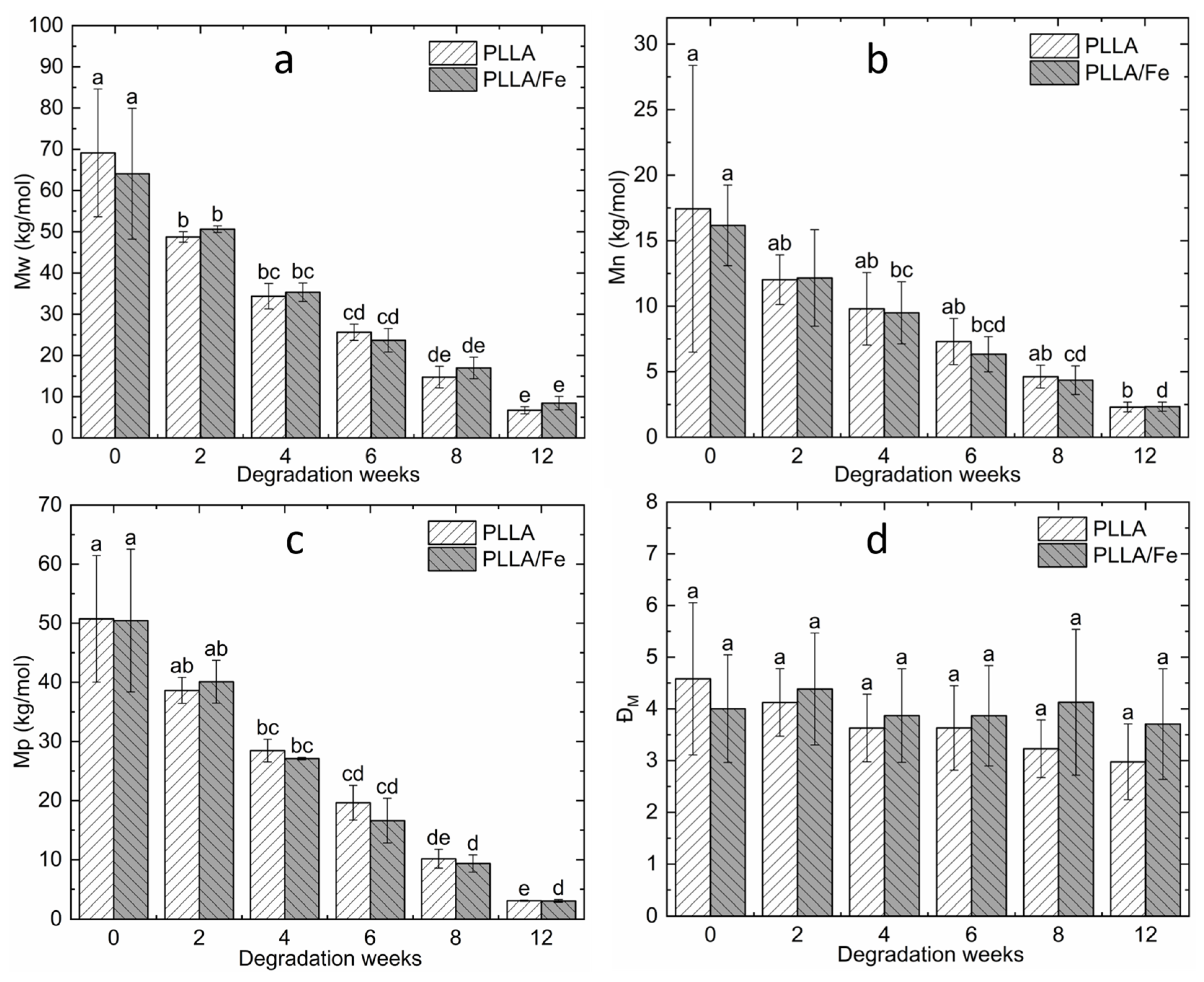
3.3. GPC Analysis
3.4. DSC Analysis
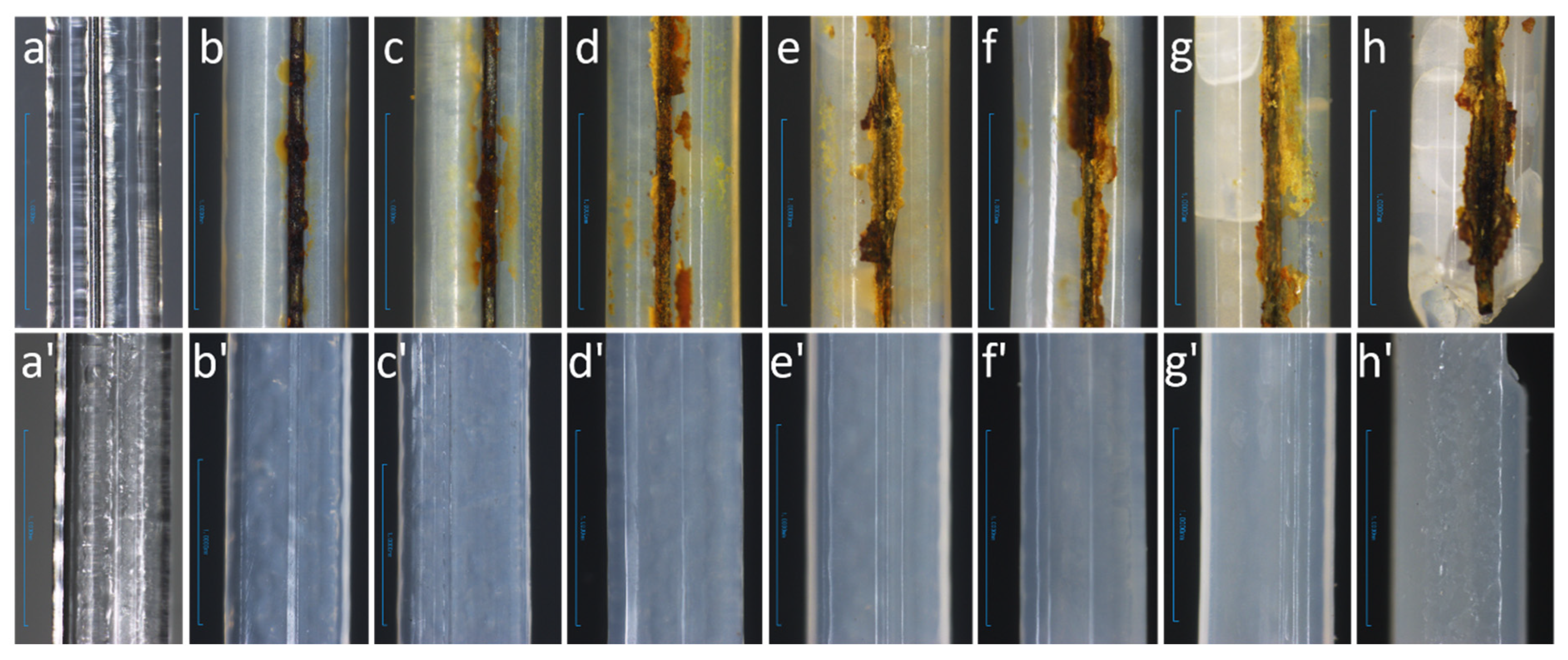
3.5. Sample Appearance and Morphology
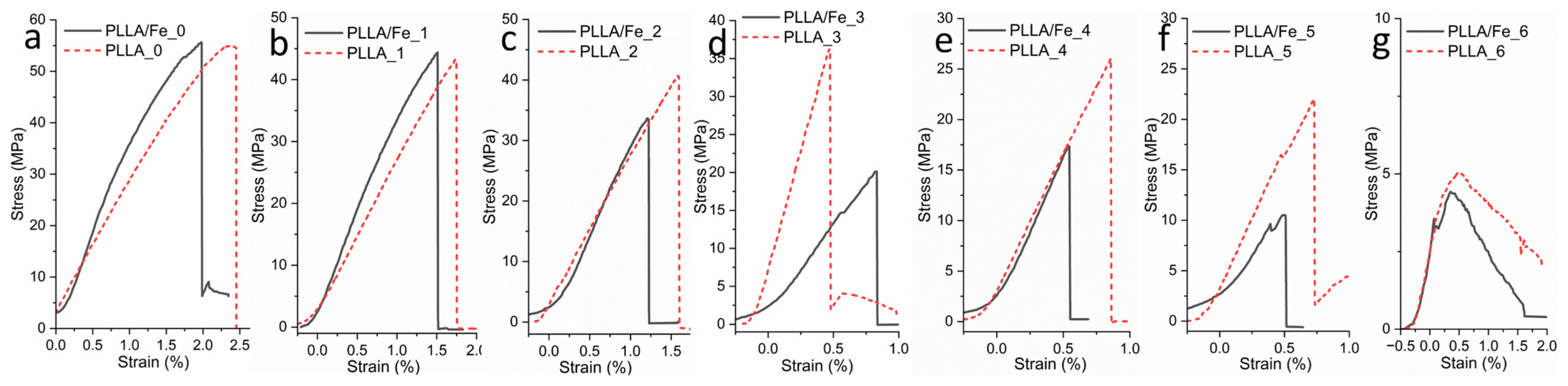
3.6. Tensile Properties
4. Conclusions
5. Limitations and Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, Y.; Jin, X.; Fan, Z.; Li, S.; Lu, Z. In Vivo Degradation of Copolymers Prepared from L-Lactide, 1,3-Trimethylene Carbonate and Glycolide as Coronary Stent Materials. J. Mater. Sci. Mater. Med. 2015, 26, 139. [Google Scholar] [CrossRef]
- Toong, D.W.Y.; Ng, J.C.K.; Cui, F.; Leo, H.L.; Zhong, L.; Lian, S.S.; Venkatraman, S.; Tan, L.P.; Huang, Y.Y.; Ang, H.Y. Nanoparticles-Reinforced Poly-l-Lactic Acid Composite Materials as Bioresorbable Scaffold Candidates for Coronary Stents: Insights from Mechanical and Finite Element Analysis. J. Mech. Behav. Biomed. Mater. 2022, 125, 104977. [Google Scholar] [CrossRef] [PubMed]
- Gąsior, G.; Szczepański, J.; Radtke, A. Biodegradable Iron-Based Materials—What Was Done and What More Can Be Done? Materials 2021, 14, 3381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, H.; Qiu, Z.; Liu, W.; Hu, Y.; Wang, C.; Ding, J. Enhancement of Poly-L-Lactic Acid Stents through Polydopamine Coating: Boosting Endothelialization and Suppressing Inflammation. Alex. Eng. J. 2023, 81, 525–531. [Google Scholar] [CrossRef]
- Im, S.H.; Jung, Y.; Kim, S.H. Current Status and Future Direction of Biodegradable Metallic and Polymeric Vascular Scaffolds for Next-Generation Stents. Acta Biomater. 2017, 60, 3–22. [Google Scholar] [CrossRef]
- Hu, T.; Yang, C.; Lin, S.; Yu, Q.; Wang, G. Biodegradable Stents for Coronary Artery Disease Treatment: Recent Advances and Future Perspectives. Mater. Sci. Eng. C 2018, 91, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mo, Z.; Guo, F.; Shi, D.; Han, Q.Q.; Liu, Q. Drug Loaded Nanoparticle Coating on Totally Bioresorbable PLLA Stents to Prevent In-stent Restenosis. J. Biomed. Mater. Res. 2018, 106, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Johnson, D.M.; Feldman, M.D. Metallic Stents Coated with Bioabsorbable Polymers. Card. Interv. Today 2009, 7, 42–49. [Google Scholar]
- Zong, J.; He, Q.; Liu, Y.; Qiu, M.; Wu, J.; Hu, B. Advances in the Development of Biodegradable Coronary Stents: A Translational Perspective. Mater. Today Bio 2022, 16, 100368. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, G.; Zhao, Y.; Wang, G.; Cai, T. Computational and Experimental Investigation into Mechanical Performances of Poly-L-Lactide Acid (PLLA) Coronary Stents. J. Mech. Behav. Biomed. Mater. 2017, 65, 415–427. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Lu, Y.; Li, X.; Zhou, J.; Zadpoor, A.A.; Wang, L. Additive Manufacturing of Vascular Stents. Acta Biomater. 2023, 167, 16–37. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Yang, J.; Cheng, J.; Zhang, Y.; Lang, J.; Liu, J.; Zhao, G.; Ni, Z. Focus on the Crucial Deformation Region to Adjust the Comprehensive Performance of Poly (L-Lactic Acid) Stent. Int. J. Biol. Macromol. 2023, 230, 123417. [Google Scholar] [CrossRef]
- Zheng, J.-F.; Xi, Z.-W.; Li, Y.; Li, J.-N.; Qiu, H.; Hu, X.-Y.; Luo, T.; Wu, C.; Wang, X.; Song, L.-F.; et al. Long-Term Safety and Absorption Assessment of a Novel Bioresorbable Nitrided Iron Scaffold in Porcine Coronary Artery. Bioact. Mater. 2022, 17, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, W.; Qiu, H.; Zhang, G.; Li, X.; Qi, H.; Guo, J.; Qian, J.; Shi, X.; Gao, X.; et al. A Biodegradable Metal-Polymer Composite Stent Safe and Effective on Physiological and Serum-Containing Biomimetic Conditions. Adv. Healthc. Mater. 2022, 11, 2201740. [Google Scholar] [CrossRef]
- Baek, S.-W.; Kim, D.-S.; Song, D.H.; Kim, H.B.; Lee, S.; Kim, J.H.; Lee, J.-K.; Hong, Y.J.; Park, C.G.; Han, D.K. Reduced Restenosis and Enhanced Re-Endothelialization of Functional Biodegradable Vascular Scaffolds by Everolimus and Magnesium Hydroxide. Biomater. Res. 2022, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, X.; He, Y.; Zhang, D.; Ding, J. Mechanism of Acceleration of Iron Corrosion by a Polylactide Coating. ACS Appl. Mater. Interfaces 2019, 11, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Yingying, H.; Wong, Y.S. Bio-Absorbable Cardiovascular Implants: Status and Prognosis. JOM 2020, 72, 1833–1844. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, H.; Zhang, W.; Qi, H.; Zhang, G.; Qian, J.; Li, X.; Qin, L.; Li, H.; Wang, X.; et al. In Vivo Degradation and Endothelialization of an Iron Bioresorbable Scaffold. Bioact. Mater. 2021, 6, 1028–1039. [Google Scholar] [CrossRef]
- Bakina, O.V.; Ivanova, L.Y.; Toropkov, N.E.; Senkina, E.I.; Lerner, M.I.; Glazkova, E.A.; Krinitsyn, M.G. Core–Shell Fe-Fe3O4 Nanoparticles for Synthesizing PLA Composites with Low Toxicity and High Radiopacity. Phys. Mesomech. 2022, 25, 270–278. [Google Scholar] [CrossRef]
- Wei, H.; Menary, G.; Buchanan, F.; Yan, S.; Nixon, J. Experimental Characterisation on the Behaviour of PLLA for Stretch Blowing Moulding of Bioresorbable Vascular Scaffolds. Int. J. Mater. Form. 2021, 14, 375–389. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, J.; Zhao, G.; Hua, R.; Tian, Y.; Tao, J. Simulation and Experimental Investigation into Mechanical Behaviors of PLLA Stents during Deployment. In Proceedings of the 2020 Ural Symposium on Biomedical Engineering, Radioelectronics and Information Technology (USBEREIT), Yekaterinburg, Russia, 14–15 May 2020; IEEE: Yekaterinburg, Russia, 2020; pp. 203–206. [Google Scholar]
- Chen, D.; Su, Z.; Weng, L.; Cao, L.; Chen, C.; Zeng, S.; Zhang, S.; Wu, T.; Hu, Q.; Xiao, J. Effect of Inflammation on Endothelial Cells Induced by Poly-L-Lactic Acid Degradation In Vitro and In Vivo. J. Biomater. Sci. Polym. Ed. 2018, 29, 1909–1919. [Google Scholar] [CrossRef]
- Yang, F.; Niu, X.; Gu, X.; Xu, C.; Wang, W.; Fan, Y. Biodegradable Magnesium-Incorporated Poly(l-Lactic Acid) Microspheres for Manipulation of Drug Release and Alleviation of Inflammatory Response. ACS Appl. Mater. Interfaces 2019, 11, 23546–23557. [Google Scholar] [CrossRef]
- Lee, H.I.; Heo, Y.; Baek, S.-W.; Kim, D.-S.; Song, D.H.; Han, D.K. Multifunctional Biodegradable Vascular PLLA Scaffold with Improved X-Ray Opacity, Anti-Inflammation, and Re-Endothelization. Polymers 2021, 13, 1979. [Google Scholar] [CrossRef] [PubMed]
- Venezuela, J.; Dargusch, M.S. Addressing the Slow Corrosion Rate of Biodegradable Fe-Mn: Current Approaches and Future Trends. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100822. [Google Scholar] [CrossRef]
- Lin, W.; Qin, L.; Qi, H.; Zhang, D.; Zhang, G.; Gao, R.; Qiu, H.; Xia, Y.; Cao, P.; Wang, X.; et al. Long-Term In Vivo Corrosion Behavior, Biocompatibility and Bioresorption Mechanism of a Bioresorbable Nitrided Iron Scaffold. Acta Biomater. 2017, 54, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Fagali, N.S.; Grillo, C.A.; Puntarulo, S.; Fernández Lorenzo De Mele, M.A. Cytotoxicity of Corrosion Products of Degradable Fe-Based Stents: Relevance of pH and Insoluble Products. Colloids Surf. B Biointerfaces 2015, 128, 480–488. [Google Scholar] [CrossRef]
- Wang, S.; Gu, R.; Wang, F.; Zhao, X.; Yang, F.; Xu, Y.; Yan, F.; Zhu, Y.; Xia, D.; Liu, Y. 3D-Printed PCL/Zn Scaffolds for Bone Regeneration with a Dose-Dependent Effect on Osteogenesis and Osteoclastogenesis. Mater. Today Bio 2022, 13, 100202. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, S.C.; Gavilán, R.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L. In Vitro Degradation of Biodegradable Polylactic Acid/Magnesium Composites: Relevance of Mg Particle Shape. Acta Biomater. 2016, 32, 348–357. [Google Scholar] [CrossRef]
- Shuai, C.; Zan, J.; Qi, F.; Wang, G.; Liu, Z.; Yang, Y.; Peng, S. nMgO-Incorporated PLLA Bone Scaffolds: Enhanced Crystallinity and Neutralized Acidic Products. Mater. Des. 2019, 174, 107801. [Google Scholar] [CrossRef]
- Cai, H.; Meng, J.; Li, X.; Xue, F.; Chu, C.; Guo, C.; Bai, J. In Vitro Degradation Behavior of Mg Wire/Poly(Lactic Acid) Composite Rods Prepared by Hot Pressing and Hot Drawing. Acta Biomater. 2019, 98, 125–141. [Google Scholar] [CrossRef]
- Ko, K.-W.; Choi, B.; Kang, E.Y.; Shin, S.-W.; Baek, S.-W.; Han, D.K. The Antagonistic Effect of Magnesium Hydroxide Particles on Vascular Endothelial Activation Induced by Acidic PLGA Degradation Products. Biomater. Sci. 2021, 9, 892–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, C.; Batalu, D.; Zhang, L.; Hu, J.; Lu, W. In Vitro Study of the PLLA-Mg65Zn30Ca5 Composites as Potential Biodegradable Materials for Bone Implants. J. Magnes. Alloys 2021, 9, 2009–2018. [Google Scholar] [CrossRef]
- Jiang, D.; Ning, F.; Wang, Y. Additive Manufacturing of Biodegradable Iron-Based Particle Reinforced Polylactic Acid Composite Scaffolds for Tissue Engineering. J. Mater. Process. Technol. 2021, 289, 116952. [Google Scholar] [CrossRef]
- Tettey, F.; Saudi, S.; Davies, D.; Shrestha, S.; Johnson, K.; Fialkova, S.; Subedi, K.; Bastakoti, B.P.; Sankar, J.; Desai, S.; et al. Fabrication and Characterization of Zn Particle Incorporated Fibrous Scaffolds for Potential Application in Tissue Healing and Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 48913–48929. [Google Scholar] [CrossRef]
- Qi, Y.; Qi, H.; He, Y.; Lin, W.; Li, P.; Qin, L.; Hu, Y.; Chen, L.; Liu, Q.; Sun, H.; et al. Strategy of Metal–Polymer Composite Stent to Accelerate Biodegradation of Iron-Based Biomaterials. ACS Appl. Mater. Interfaces 2018, 10, 182–192. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Lin, W.; Qiu, H.; Qi, Y.; Ma, X.; Qi, H.; He, Y.; Zhang, H.; Qian, J.; et al. Long-Term Efficacy of Biodegradable Metal–Polymer Composite Stents After the First and the Second Implantations into Porcine Coronary Arteries. ACS Appl. Mater. Interfaces 2020, 12, 15703–15715. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, X.; Zhang, H.; Sun, G.; Zhang, G.; Li, X.; Qi, H.; Guo, J.; Qin, L.; Shi, D.; et al. Maglev-Fabricated Long and Biodegradable Stent for Interventional Treatment of Peripheral Vessels. Nat. Commun. 2024, 15, 7903. [Google Scholar] [CrossRef]
- Gao, R.; Xu, B.; Sun, Z.; Guan, C.; Song, L.; Gao, L.; Li, C.; Cui, J.; Zhang, Y.; Dou, K.; et al. First-in-Human Evaluation of a Novel Ultrathin Sirolimus- Eluting Iron Bioresorbable Scaffold: 3-Year Outcomes of the IBS-FIM Trial. EuroIntervention 2023, 19, 222. [Google Scholar]
- Huang, T.; Cheng, Y.; Zheng, Y. In Vitro Studies on Silver Implanted Pure Iron by Metal Vapor Vacuum Arc Technique. Colloids Surf. B Biointerfaces 2016, 142, 20–29. [Google Scholar] [CrossRef]
- Ahadi, F.; Azadi, M.; Biglari, M.; Bodaghi, M.; Khaleghian, A. Evaluation of Coronary Stents: A Review of Types, Materials, Processing Techniques, Design, and Problems. Heliyon 2023, 9, e13575. [Google Scholar] [CrossRef]
- Feng, P.; Yang, F.; Jia, J.; Zhang, J.; Tan, W.; Shuai, C. Mechanism and Manufacturing of 4D Printing: Derived and beyond the Combination of 3D Printing and Shape Memory Material. Int. J. Extrem. Manuf. 2024, 6, 062011. [Google Scholar] [CrossRef]
- Feng, P.; Liu, L.; Yang, F.; Min, R.; Wu, P.; Shuai, C. Shape/Properties Collaborative Intelligent Manufacturing of Artificial Bone Scaffold: Structural Design and Additive Manufacturing Process. Biofabrication 2025, 17, 012005. [Google Scholar] [CrossRef] [PubMed]
- Flege, C.; Vogt, F.; Höges, S.; Jauer, L.; Borinski, M.; Schulte, V.A.; Hoffmann, R.; Poprawe, R.; Meiners, W.; Jobmann, M.; et al. Development and Characterization of a Coronary Polylactic Acid Stent Prototype Generated by Selective Laser Melting. J. Mater. Sci. Mater. Med. 2013, 24, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.J.; Ciurana, J. 3D-Printed Bioabsordable Polycaprolactone Stent: The Effect of Process Parameters on Its Physical Features. Mater. Des. 2018, 137, 430–437. [Google Scholar] [CrossRef]
- Asyraf Azli, A.; Muhammad, N.; Mustafa Abdullah Albakri, M.; Fathullah Ghazali, M.; Zamree Abd Rahim, S.; Andrei Victor, S. Printing Parameter Optimization of Biodegradable PLA Stent Strut Thickness by Using Response Surface Methodology (RSM). IOP Conf. Ser. Mater. Sci. Eng. 2020, 864, 012154. [Google Scholar] [CrossRef]
- Van Kampen, K.A.; Olaret, E.; Stancu, I.-C.; Moroni, L.; Mota, C. Controllable Four Axis Extrusion-Based Additive Manufacturing System for the Fabrication of Tubular Scaffolds with Tailorable Mechanical Properties. Mater. Sci. Eng. C 2021, 119, 111472. [Google Scholar] [CrossRef]
- Guerra, A.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. 3D-Printed PCL/PLA Composite Stents: Towards a New Solution to Cardiovascular Problems. Materials 2018, 11, 1679. [Google Scholar] [CrossRef]
- Yu, P.; Huang, S.; Yang, Z.; Liu, T.; Qilin, Z.; Feng, J.; Zeng, B. Biomechanical Properties of a Customizable TPU/PCL Blended Esophageal Stent Fabricated by 3D Printing. Mater. Today Commun. 2023, 34, 105196. [Google Scholar] [CrossRef]
- Singh, J.; Singh, G.; Pandey, P.M. Multi-Objective Optimization of Solvent Cast 3D Printing Process Parameters for Fabrication of Biodegradable Composite Stents. Int. J. Adv. Manuf. Technol. 2021, 115, 3945–3964. [Google Scholar] [CrossRef]
- Mahmud, Z.; Nasrin, A.; Hassan, M.; Gomes, V.G. 3D-printed polymer Nanocomposites with Carbon Quantum Dots for Enhanced Properties and in Situ Monitoring of Cardiovascular Stents. Polym. Adv. Technol. 2022, 33, 980–990. [Google Scholar] [CrossRef]
- Feng, P.; Shen, S.; Yang, L.; Kong, Y.; Yang, S.; Shuai, C. Vertical and Uniform Growth of MoS2 Nanosheets on GO Nanosheets for Efficient Mechanical Reinforcement in Polymer Scaffold. Virtual Phys. Prototyp. 2023, 18, e2115384. [Google Scholar] [CrossRef]
- Feng, P.; Tian, H.; Yang, F.; Peng, S.; Pan, H.; Shuai, C. Reduced Graphene Oxide-Mediated Electron-Hole Separation Using Titanium Dioxide Increases the Photocatalytic Antibacterial Activity of Bone Scaffolds. Bio-Des. Manuf. 2025, 8, 100–115. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Jeong, S.; Kim, S.; Kim, H.J.; Jo, J.; Shanmugasundaram, A.; Kim, H.; Choi, E.; Lee, D.-W. 3D-Printed Cardiovascular Polymer Scaffold Reinforced by Functional Nanofiber Additives for Tunable Mechanical Strength and Controlled Drug Release. Chem. Eng. J. 2023, 454, 140118. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Ji, J.; Fang, Y.; Ouyang, L.; Zhang, L.; Sun, W. 3D-Printed Bioresorbable Stent Coated with Dipyridamole-Loaded Nanofiber for Restenosis Prevention and Endothelialization. IJB 2022, 8, 543. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.; Melenka, G.W.; Kempers, R. Additive Manufacturing of Continuous Wire Polymer Composites. Manuf. Lett. 2018, 16, 49–51. [Google Scholar] [CrossRef]
- Güneş, M.; Çayiroğlu, İ. Mechanical Behaviour of 3D Printed Parts with Continuous Steel Wire Reinforcement. El-Cezeri Fen. Ve Mühendislik Derg. 2021, 9, 276–289. [Google Scholar] [CrossRef]
- Li, L.; You, Q.; Liu, W.; Zeng, Q.; Zhou, C.; Wang, J.; Li, Z.; Li, K. Enhanced Flexural Performances of Cementitious Composite Beams with Continuously Graded Steel Fiber Distribution. Mater. Des. 2023, 227, 111728. [Google Scholar] [CrossRef]
- Mishra, V.; Ror, C.K.; Negi, S.; Kar, S. Enhanced Fracture Toughness and Tensile Strength of 3D Printed Recycled ABS Composites Reinforced with Continuous Metallic Fiber for Load-Bearing Application. RPJ 2024, 30, 760–769. [Google Scholar] [CrossRef]
- Mu, Q.; Dunn, C.K.; Wang, L.; Dunn, M.L.; Qi, H.J.; Wang, T. Thermal Cure Effects on Electromechanical Properties of Conductive Wires by Direct Ink Write for 4D Printing and Soft Machines. Smart Mater. Struct. 2017, 26, 045008. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Kempers, R. Effective Thermal Conductivity of 3D-Printed Continuous Wire Polymer Composites. Prog. Addit. Manuf. 2022, 7, 699–712. [Google Scholar] [CrossRef]
- Shemelya, C.; Cedillos, F.; Aguilera, E.; Espalin, D.; Muse, D.; Wicker, R.; MacDonald, E. Encapsulated Copper Wire and Copper Mesh Capacitive Sensing for 3-D Printing Applications. IEEE Sens. J. 2015, 15, 1280–1286. [Google Scholar] [CrossRef]
- Saleh, M.A.; Kempers, R.; Melenka, G.W. Fatigue Behavior and Electromechanical Properties of Additively Manufactured Continuous Wire Polymer Composites for Structural Health Monitoring. Fatigue Fract. Eng. Mat. Struct. 2022, 45, 2630–2645. [Google Scholar] [CrossRef]
- Saleh, M.A.; Kempers, R.; Melenka, G.W. A Comparative Study on the Electromechanical Properties of 3D-Printed Rigid and Flexible Continuous Wire Polymer Composites for Structural Health Monitoring. Sens. Actuators A Phys. 2021, 328, 112764. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Wilson, D.; Gomez-Kervin, E.; Tang, B.; Wang, J. Investigation of Closed-Loop Manufacturing with Acrylonitrile Butadiene Styrene over Multiple Generations Using Additive Manufacturing. ACS Sustain. Chem. Eng. 2019, 7, 13955–13969. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Rojas-Martínez, L.E.; Flores-Hernandez, C.G.; López-Marín, L.M.; Martinez-Hernandez, A.L.; Thorat, S.B.; Reyes Vasquez, C.D.; Del Rio-Castillo, A.E.; Velasco-Santos, C. 3D Printing of PLA Composites Scaffolds Reinforced with Keratin and Chitosan: Effect of Geometry and Structure. Eur. Polym. J. 2020, 141, 110088. [Google Scholar] [CrossRef]
- ISO-13781-2017; Implants for Surgery—Homopolymers, Copolymers and Blends on Poly(Lactide)—In Vitro Degradation Testing. ISO: Geneva, Switzerland, 2017.
- Liu, J.; Wang, B.; Liu, W.; Hu, X.; Zhang, C.; Zhou, Z.; Lang, J.; Wu, G.; Zhang, Y.; Yang, J.; et al. Regulating Mechanical Performance of Poly (l-Lactide Acid) Stent by the Combined Effects of Heat and Aqueous Media. Int. J. Biol. Macromol. 2023, 242, 124987. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, B.; Li, X.; Liu, M.; Tian, Y.; Zhang, J.; Zhang, Y.; Cheng, J.; Yang, J.; Ni, Z. Evaluation of Poly (L-Lactic Acid) Monofilaments with High Mechanical Performance In Vitro Degradation. J. Mater. Sci. 2022, 57, 6361–6371. [Google Scholar] [CrossRef]
- Weir, N.A.; Buchanan, F.J.; Orr, J.F.; Farrar, D.F.; Dickson, G.R. Degradation of Poly-L-Lactide. Part 2: Increased Temperature Accelerated Degradation. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2004, 218, 321–330. [Google Scholar] [CrossRef]
- Srivastava, A.; Ahuja, R.; Bhati, P.; Singh, S.; Chauhan, P.; Vashisth, P.; Kumar, A.; Bhatnagar, N. Fabrication and Characterization of PLLA/Mg Composite Tube as the Potential Bioresorbable/Biodegradable Stent(BRS). Materialia 2020, 10, 100661. [Google Scholar] [CrossRef]
- Srivastava, A.; Bhatnagar, N. Production and Characterisation of New Bioresorbable Radiopaque Mg–Zn–Y Alloy to Improve X-Ray Visibility of Polymeric Scaffolds. J. Magnes. Alloys 2022, 10, 1694–1703. [Google Scholar] [CrossRef]
- Fiuza, C.; Polak-Kraśna, K.; Antonini, L.; Petrini, L.; Carroll, O.; Ronan, W.; Vaughan, T.J. An Experimental Investigation into the Physical, Thermal and Mechanical Degradation of a Polymeric Bioresorbable Scaffold. J. Mech. Behav. Biomed. Mater. 2022, 125, 104955. [Google Scholar] [CrossRef] [PubMed]
- Hayman, D.; Bergerson, C.; Miller, S.; Moreno, M.; Moore, J.E. The Effect of Static and Dynamic Loading on Degradation of PLLA Stent Fibers. J. Biomech. Eng. 2014, 136, 081006. [Google Scholar] [CrossRef] [PubMed]
- Naseem, R.; Zhao, L.; Eswaran, S.K.; Willcock, H. Characterization of Biodegradable Poly(l-lactide) Tube over Accelerated Degradation. Polym. Eng. Sci. 2020, 60, 1430–1436. [Google Scholar] [CrossRef]
- Polak-Kraśna, K.; Abaei, A.R.; Shirazi, R.N.; Parle, E.; Carroll, O.; Ronan, W.; Vaughan, T.J. Physical and Mechanical Degradation Behaviour of Semi-Crystalline PLLA for Bioresorbable Stent Applications. J. Mech. Behav. Biomed. Mater. 2021, 118, 104409. [Google Scholar] [CrossRef]
- ASTM D3822/D3822M-14; Standard Test Method for Tensile Properties of Single Textile Fibers. ASTM: West Conshohocken, PA, USA, 2020. [CrossRef]
- Chen, Y.; Geever, L.M.; Killion, J.A.; Lyons, J.G.; Higginbotham, C.L.; Devine, D.M. Halloysite Nanotube Reinforced Polylactic Acid Composite. Polym. Compos. 2015, 38, 2166–2173. [Google Scholar] [CrossRef]
- Liu, H.; Gong, K.; Portela, A.; Cao, Z.; Dunbar, R.; Chen, Y. Granule-Based Material Extrusion Is Comparable to Filament-Based Material Extrusion in Terms of Mechanical Performances of Printed PLA Parts: A Comprehensive Investigation. Addit. Manuf. 2023, 75, 103744. [Google Scholar] [CrossRef]
- Kang, E.Y.; Lih, E.; Kim, I.H.; Joung, Y.K.; Han, D.K. Effects of Poly (L-Lactide-ε-Caprolactone) and Magnesium Hydroxide Additives on Physico-Mechanical Properties and Degradation of Poly (L-Lactic Acid). Biomater. Res. 2016, 20, 7. [Google Scholar] [CrossRef]
- Jones, R.G. Dispersity in Polymer Science. Polym. Int. 2010, 59, 22. [Google Scholar] [CrossRef]
- Liu, H.; Gong, K.; Portela, A.; Chyzna, V.; Yan, G.; Cao, Z.; Dunbar, R.; Chen, Y. Investigation of Distributed Recycling of Polylactic Acid over Multiple Generations via the Granule-Based Material Extrusion Process. J. Clean. Prod. 2024, 436, 140609. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Portela, A.; Higginbotham, C.; Devine, D. A Closed-Loop Process to Transform Mixed Plant Biomass Waste into Cellulose Acetate Bioplastic as Innovative Growing Substrates in Plant Cultivation. Cellulose 2024, 31, 9565–9581. [Google Scholar] [CrossRef]
- Yan, G.; Cao, Z.; Devine, D.; Penning, M.; Gately, N.M. Physical Properties of Shellac Material Used for Hot Melt Extrusion with Potential Application in the Pharmaceutical Industry. Polymers 2021, 13, 3723. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, S.C.; Frutos, E.; Benavente, R.; Lorenzo, V.; González-Carrasco, J.L. Assessment of Mechanical Behavior of PLA Composites Reinforced with Mg Micro-Particles through Depth-Sensing Indentations Analysis. J. Mech. Behav. Biomed. Mater. 2017, 65, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ye, X.; Zhong, J.; Lin, Z.; Wu, S.; Hu, Y.; Zheng, W.; Zhou, W.; Wei, Y.; Dong, X. Recycling of Waste Crab Shells into Reinforced Poly (Lactic Acid) Biocomposites for 3D Printing. Int. J. Biol. Macromol. 2023, 234, 122974. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Murphy, A.; Scholz, D.; Geever, L.M.; Lyons, J.G.; Devine, D.M. Surface-Modified Halloysite Nanotubes Reinforced Poly(Lactic Acid) for Use in Biodegradable Coronary Stents. J. Appl. Polym. Sci. 2018, 135, 46521. [Google Scholar] [CrossRef]
- Zhou, Z.; Salaoru, I.; Morris, P.; Gibbons, G.J. Additive Manufacturing of Heat-Sensitive Polymer Melt Using a Pellet-Fed Material Extrusion. Addit. Manuf. 2018, 24, 552–559. [Google Scholar] [CrossRef]
- Zhang, Y.; Roux, C.; Rouchaud, A.; Meddahi-Pellé, A.; Gueguen, V.; Mangeney, C.; Sun, F.; Pavon-Djavid, G.; Luo, Y. Recent Advances in Fe-Based Bioresorbable Stents: Materials Design and Biosafety. Bioact. Mater. 2024, 31, 333–354. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, L.; Liu, Y.; Wang, J.; Zhang, G.; Qi, H.; Zhang, W.; Zhang, D.; Wang, J. Study on the Chronic Toxicity and Carcinogenicity of Iron-Based Bioabsorbable Stents. Nanotechnol. Rev. 2023, 12, 20220507. [Google Scholar] [CrossRef]
- Wu, J.; Lu, X.; Tan, L.; Zhang, B.; Yang, K. Effect of Hydrion Evolution by Polylactic-Co-Glycolic Acid Coating on Degradation Rate of Pure Iron: Effect of Hydrion Evolution. J. Biomed. Mater. Res. 2013, 101, 1222–1232. [Google Scholar] [CrossRef]
- Shi, D.; Kang, Y.; Zhang, G.; Gao, C.; Lu, W.; Yang, C.; Zou, H.; Jiang, H. A Comparative Study on In Vitro Degradation Behavior of PLLA-Based Copolymer Monofilaments. Polym. Degrad. Stab. 2018, 158, 148–156. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, J.; Cheng, J.; Wu, G.; Zhang, Y.; Ni, Z.; Zhao, G. A Poly(L-lactic Acid) Monofilament with High Mechanical Properties for Application in Biodegradable Biliary Stents. J. Appl. Polym. Sci. 2021, 138, 49656. [Google Scholar] [CrossRef]
- Khalaj Amnieh, S.; Mosaddegh, P.; Mashayekhi, M.; Kharaziha, M. Biodegradation Evaluation of Poly (Lactic Acid) for Stent Application: Role of Mechanical Tension and Temperature. J. Appl. Polym. Sci. 2021, 138, 50389. [Google Scholar] [CrossRef]
- Zamiri, P.; Kuang, Y.; Sharma, U.; Ng, T.F.; Busold, R.H.; Rago, A.P.; Core, L.A.; Palasis, M. The Biocompatibility of Rapidly Degrading Polymeric Stents in Porcine Carotid Arteries. Biomaterials 2010, 31, 7847–7855. [Google Scholar] [CrossRef] [PubMed]
- Abaei, A.R.; Vaughan, T.J.; Ronan, W. Micromechanical Modelling of Biodegradable Semi-Crystalline Polymers: The Evolution of Anisotropic Mechanical Properties during Degradation. Int. J. Solids Struct. 2023, 279, 112366. [Google Scholar] [CrossRef]
- Wang, A.; Venezuela, J.; Dargusch, M.S. Enhancing the Corrodibility of Biodegradable Iron and Zinc Using Poly(Lactic) Acid (PLA) Coating for Temporary Medical Implant Applications. Prog. Org. Coat. 2023, 174, 107301. [Google Scholar] [CrossRef]
- Chausse, V.; Iglesias, C.; Bou-Petit, E.; Ginebra, M.-P.; Pegueroles, M. Chemical vs Thermal Accelerated Hydrolytic Degradation of 3D-Printed PLLA/PLCL Bioresorbable Stents: Characterization and Influence of Sterilization. Polym. Test. 2023, 117, 107817. [Google Scholar] [CrossRef]
- Shuai, C.; Wang, Z.; Zhang, H.; Jia, J.; Huang, L.; Wang, D.; Chen, S.; Feng, P. Biosoluble Ceramic Fiber Reinforced Poly(L-Lactic Acid) Bone Scaffold: Degradation and Bioactivity. npj Mater. Degrad. 2022, 6, 87. [Google Scholar] [CrossRef]
- Oosterbeek, R.N.; Kwon, K.-A.; Duffy, P.; McMahon, S.; Zhang, X.C.; Best, S.M.; Cameron, R.E. Tuning Structural Relaxations, Mechanical Properties, and Degradation Timescale of PLLA during Hydrolytic Degradation by Blending with PLCL-PEG. Polym. Degrad. Stab. 2019, 170, 109015. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, G.; Cao, P.; Zhang, D.; Zheng, Y.; Wu, R.; Qin, L.; Wang, G.; Wen, T. Cytotoxicity and Its Test Methodology for a Bioabsorbable Nitrided Iron Stent: Cytocompatibility of Bioabsorbable Iron-Based Materials. J. Biomed. Mater. Res. 2015, 103, 764–776. [Google Scholar] [CrossRef]
- Ekinci, A.; Gleadall, A.; Johnson, A.A.; Li, L.; Han, X. Mechanical and Hydrolytic Properties of Thin Polylactic Acid Films by Fused Filament Fabrication. J. Mech. Behav. Biomed. Mater. 2021, 114, 104217. [Google Scholar] [CrossRef]
- Moetazedian, A.; Gleadall, A.; Han, X.; Ekinci, A.; Mele, E.; Silberschmidt, V.V. Mechanical Performance of 3D Printed Polylactide during Degradation. Addit. Manuf. 2021, 38, 101764. [Google Scholar] [CrossRef]
- Zhao, G.; Li, X.; Tian, Y.; Wu, G.; Zhang, Y.; Jiang, W.; Yang, J.; Ni, Z. Poly(l-Lactic Acid) Monofilaments for Biodegradable Braided Self-Expanding Stent. J. Mater. Sci. 2021, 56, 12383–12393. [Google Scholar] [CrossRef]
- Van de Voorde, B.; Katalagarianakis, A.; Huysman, S.; Toncheva, A.; Raquez, J.M.; Duretek, I.; Holzer, C.; Cardon, L.; Bernaerts, K.V.; Van Hemelrijck, D.; et al. Effect of Extrusion and Fused Filament Fabrication Processing Parameters of Recycled Poly(Ethylene Terephthalate) on the Crystallinity and Mechanical Properties. Addit. Manuf. 2022, 50, 102518. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Melenka, G.W.; Kempers, R. Fabrication and Tensile Testing of 3D Printed Continuous Wire Polymer Composites. RPJ 2018, 24, 1131–1141. [Google Scholar] [CrossRef]
- Li, X.; Hu, J.; Yu, Z.; Liu, M.; Xiao, X.; Qin, G.; Yang, L.; Zhang, E. A Study on the In Vitro and In Vivo Degradation Behaviour and Biocompatibility of a Mg-Mn-Zn Alloy with PLLA and Micro Arc Oxidation Composite Coating. Surf. Coat. Technol. 2023, 471, 129894. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, C.; Sun, B.; Zhang, Y.; Sun, X.; EL-Newehy, M.; EL-Hamshary, H.; Morsi, Y.; Gu, H.; Wang, W.; et al. 3D Printed Personalized, Heparinized and Biodegradable Coronary Artery Stents for Rabbit Abdominal Aorta Implantation. Chem. Eng. J. 2022, 450, 138202. [Google Scholar] [CrossRef]
- Wang, B.; Liu, M.; Liu, J.; Tian, Y.; Liu, W.; Wu, G.; Cheng, J.; Zhang, Y.; Zhao, G.; Ni, Z. Key Factors of Mechanical Strength and Toughness in Oriented Poly(l-Lactic Acid) Monofilaments for a Bioresorbable Self-Expanding Stent. Langmuir 2022, 38, 13477–13487. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.A.; Sikora-Jasinska, M.; Demir, A.G.; Guillory, R.J. Recent Advances and Directions in the Development of Bioresorbable Metallic Cardiovascular Stents: Insights from Recent Human and In Vivo Studies. Acta Biomater. 2021, 127, 1–23. [Google Scholar] [CrossRef]
- Lan, Z.; Lyu, Y.; Xiao, J.; Zheng, X.; He, S.; Feng, G.; Zhang, Y.; Wang, S.; Kislauskis, E.; Chen, J.; et al. Novel Biodegradable Drug-Eluting Stent Composed of Poly-L-Lactic Acid and Amorphous Calcium Phosphate Nanoparticles Demonstrates Improved Structural and Functional Performance for Coronary Artery Disease. J. Biomed. Nanotechnol. 2014, 10, 1194–1204. [Google Scholar] [CrossRef]
- Duek, E.A.R.; Zavaglia, C.A.C.; Belangero, W.D. In Vitro Study of Poly(Lactic Acid) Pin Degradation. Polymer 1999, 40, 6465–6473. [Google Scholar] [CrossRef]
- Tsuji, H. In Vitro Hydrolysis of Blends from Enantiomeric Poly(Lactide)s Part 1. Well-Stereo-Complexed Blend and Non-Blended Films. Polymer 2000, 41, 3621–3630. [Google Scholar] [CrossRef]
- Tsuji, H. In Vitro Hydrolysis of Blends from Enantiomeric Poly(Lactide)s. Part 4: Well-Homo-Crystallized Blend and Nonblended Films. Biomaterials 2003, 24, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Tsuji, H. Effects of Molecular Weight and Small Amounts of D-Lactide Units on Hydrolytic Degradation of Poly(l-Lactic Acid)s. Polym. Degrad. Stab. 2006, 91, 1665–1673. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-Based Alloys for Degradable Vascular Stent Applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J.; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, C.; Sun, B.; Wu, Y.; Yu, X.; Cui, J.; Zhang, M.; EL-Newehy, M.; EL-Hamshary, H.; Barlis, P.; et al. Development of 3D Printed Biodegradable, Entirely X-ray Visible Stents for Rabbit Carotid Artery Implantation. Adv. Healthc. Mater. 2024, 13, 2304293. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; Von Schnakenburg, C. Long-Term Biocompatibility of a Corrodible Peripheral Iron Stent in the Porcine Descending Aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef]
- Wang, H.-T.; Chiang, P.-C.; Tzeng, J.-J.; Wu, T.-L.; Pan, Y.-H.; Chang, W.-J.; Huang, H.-M. In Vitro Biocompatibility, Radiopacity, and Physical Property Tests of Nano-Fe3O4 Incorporated Poly-l-Lactide Bone Screws. Polymers 2017, 9, 191. [Google Scholar] [CrossRef]
- Li, M.; Deng, D.; Chen, Z.; Liu, W.; Zhao, G.; Zhang, Y.; Yang, F.; Ni, Z. Magnetic Nanoparticle Loaded Biodegradable Vascular Stents for Magnetic Resonance Imaging and Long-Term Visualization. J. Mater. Chem. B 2023, 11, 3669–3678. [Google Scholar] [CrossRef]
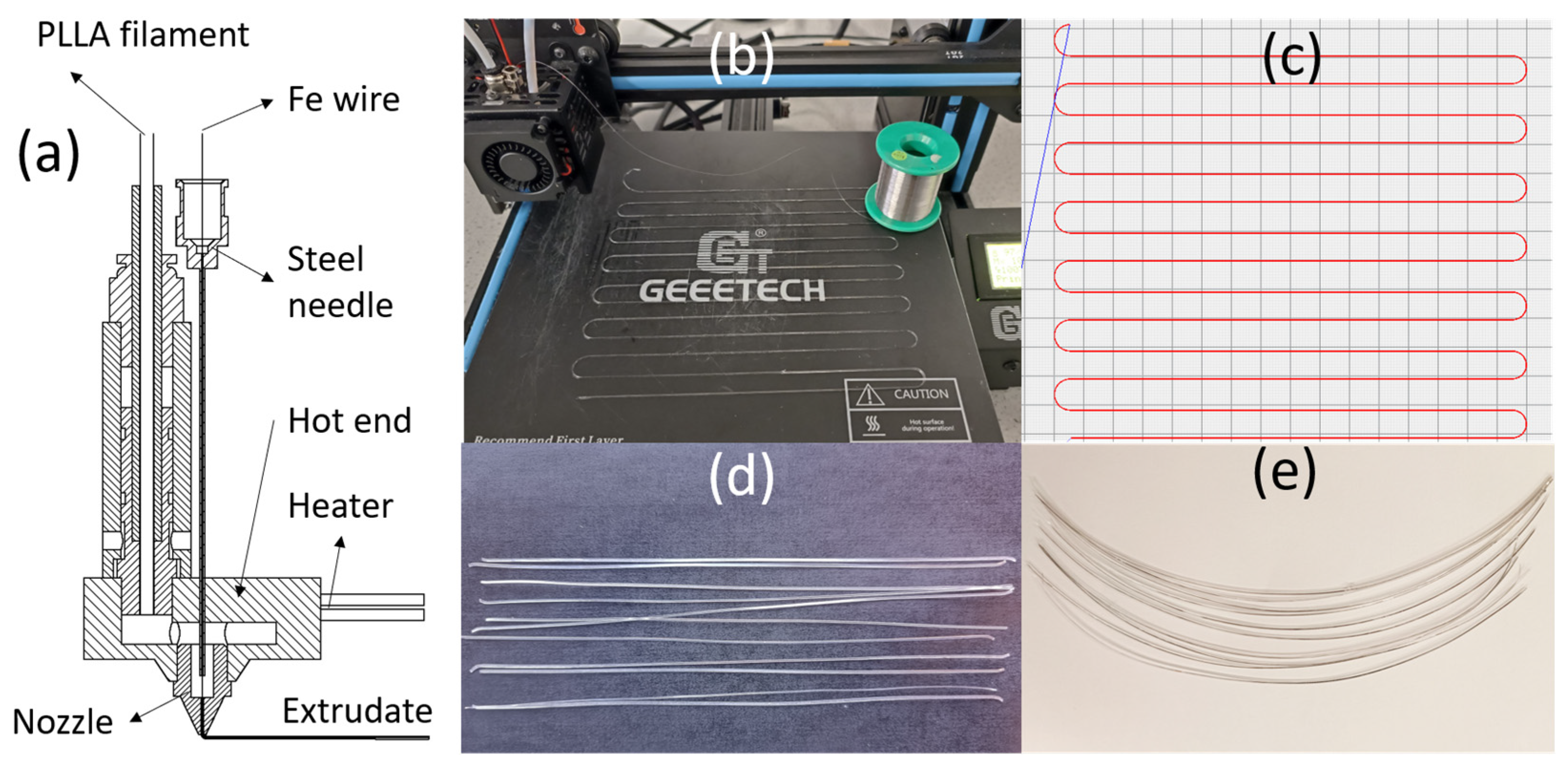


| Property | PLLA | Iron Wire | Unit |
|---|---|---|---|
| Diameter | 1.75 | 0.065 | mm |
| Density | 1.24 | 7.87 | g/cm3 |
| Melt temperature | 175 | 1535 | °C |
| Nozzle temperature (recommended) | 180–220 | - | °C |
| Tensile strength at break | 50 | 180–210 | MPa |
| Tensile elongation at break | ≤5% | - | |
| Young’s modulus | 3.5 | 211.4 | GPa |
| Flexural modulus | 3.35 | - | GPa |
| Charpy impact strength | 21 | - | kJ/m2 |
| Order | Reagent | Amount | Supplier |
|---|---|---|---|
| 1 | NaCl (≥99.5%) | 7.996 g | Sigma-Aldrich (Taufkirchen, Germany) |
| 2 | NaHCO3 (≥99.5%) | 0.350 g | Sigma-Aldrich (Taufkirchen, Germany) |
| 3 | KCl (≥99.0%) | 0.224 gt | Honeywell (Seelze, Germany) |
| 4 | K2HPO4·3H2O (≥99.0%) | 0.228 g | Sigma-Aldrich (Taufkirchen, Germany) |
| 5 | MgCl2·6H2O (≥99.0%) | 0.305 g | Sigma-Aldrich (Taufkirchen, Germany) |
| 6 | 1.0 M-HCl | 39 mL | TCI (Zwijndrecht, BE) |
| 7 | CaCl2 | 0.278 g | Sigma-Aldrich (Taufkirchen, Germany) |
| 8 | Na2SO4 (≥99.0%) | 0.071 g | Sigma-Aldrich (Taufkirchen, Germany) |
| 9 | (CH2OH)3CNH2 (>99.0%) | 6.057 g | TCI (Zwijndrecht, Belgium) |
| 10 | 1.0 M-HCl: adjusting pH to 7.4 | 0–5 mL | TCI (Zwijndrecht, Belgium) |
| PLLA/Fe Wire Monofilament | Pure PLLA Monofilament | ||
|---|---|---|---|
| Composition | PLLA | Iron wire | PLLA |
| Dimensions (μm) | 310.30 ± 36.77 × 650.96 ± 10.80 | Ø 65.28 ± 0.41 | 292.40 ± 13.06 × 617.38 ± 23.53 |
| Parameters | Value | Unit |
|---|---|---|
| Nozzle diameter | 0.6 | mm |
| Layer height | 0.3 | mm |
| Extrusion head temperature | 200 | °C |
| Bed temperature | 60 | °C |
| Cooling fan speed | 100 | % |
| Filling pattern | Line | |
| Fill angle (X axis) | ±0 | ° |
| Filling density | 100 | % |
| Print speed on slicer | 1.2 | mm/s |
| Initial Mass Sum of the Monofilament Group in Each Degradation Tube (mg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Week_1 | Week_2 | Week_3 | Week_4 | Week_5 | Week_6 | Week_8 | Week_10 | Week_12 | Mean ± SD |
| PLLA/Fe | 327.88 | 378.86 | 363.7 | 349.13 | 364.74 | 343.11 | 355.79 | 357.59 | 368.62 | 356.60 ± 15.09 |
| PLLA | 333.18 | 341.44 | 339.33 | 340.86 | 345.52 | 336.56 | 336.98 | 342.36 | 329.39 | 338.40 ± 4.95 |
| Week 0 | Week 2 | Week 4 | Week 6 | Week 8 | Week 12 | |
|---|---|---|---|---|---|---|
| Mw | 0.71 | 0.10 | 0.68 | 0.39 | 0.36 | 0.18 |
| Mn | 0.86 | 0.96 | 0.89 | 0.49 | 0.75 | 0.93 |
| Mp | 0.98 | 0.58 | 0.29 | 0.33 | 0.55 | 0.84 |
| ĐM | 0.61 | 0.74 | 0.73 | 0.76 | 0.36 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Portela, A.; Xu, H.; Chyzna, V.; Lu, Y.; Gong, K.; Fitzpatrick, D.P.; Yan, G.; Dunbar, R.; Chen, Y. In Vitro Degradation of Continuous Iron Wire-Reinforced PLLA Composite Monofilaments for Bioresorbable Vascular Stents Fabricated via a Novel 3D Printer: An Early-Stage Prototype Study. Processes 2025, 13, 2621. https://doi.org/10.3390/pr13082621
Liu H, Portela A, Xu H, Chyzna V, Lu Y, Gong K, Fitzpatrick DP, Yan G, Dunbar R, Chen Y. In Vitro Degradation of Continuous Iron Wire-Reinforced PLLA Composite Monofilaments for Bioresorbable Vascular Stents Fabricated via a Novel 3D Printer: An Early-Stage Prototype Study. Processes. 2025; 13(8):2621. https://doi.org/10.3390/pr13082621
Chicago/Turabian StyleLiu, Handai, Alexandre Portela, Han Xu, Vlasta Chyzna, Yinshi Lu, Ke Gong, Daniel P. Fitzpatrick, Guangming Yan, Ronan Dunbar, and Yuanyuan Chen. 2025. "In Vitro Degradation of Continuous Iron Wire-Reinforced PLLA Composite Monofilaments for Bioresorbable Vascular Stents Fabricated via a Novel 3D Printer: An Early-Stage Prototype Study" Processes 13, no. 8: 2621. https://doi.org/10.3390/pr13082621
APA StyleLiu, H., Portela, A., Xu, H., Chyzna, V., Lu, Y., Gong, K., Fitzpatrick, D. P., Yan, G., Dunbar, R., & Chen, Y. (2025). In Vitro Degradation of Continuous Iron Wire-Reinforced PLLA Composite Monofilaments for Bioresorbable Vascular Stents Fabricated via a Novel 3D Printer: An Early-Stage Prototype Study. Processes, 13(8), 2621. https://doi.org/10.3390/pr13082621







