Rapeseed Meal-Derived Three-Dimensional Porous Carbon for High-Performance Lithium–Selenium Batteries
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation
2.3. Characterization
2.4. Electrochemical Measurements
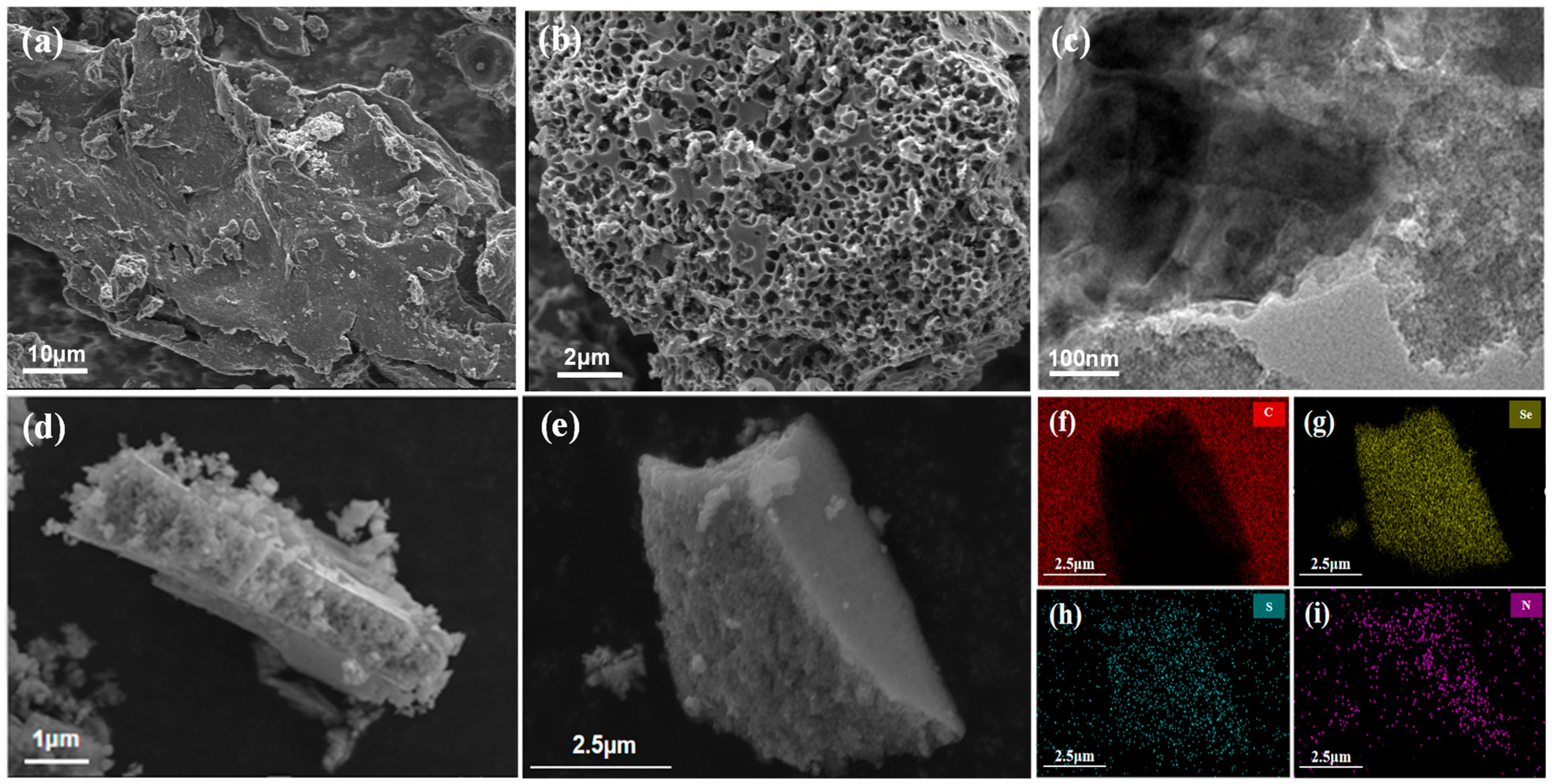
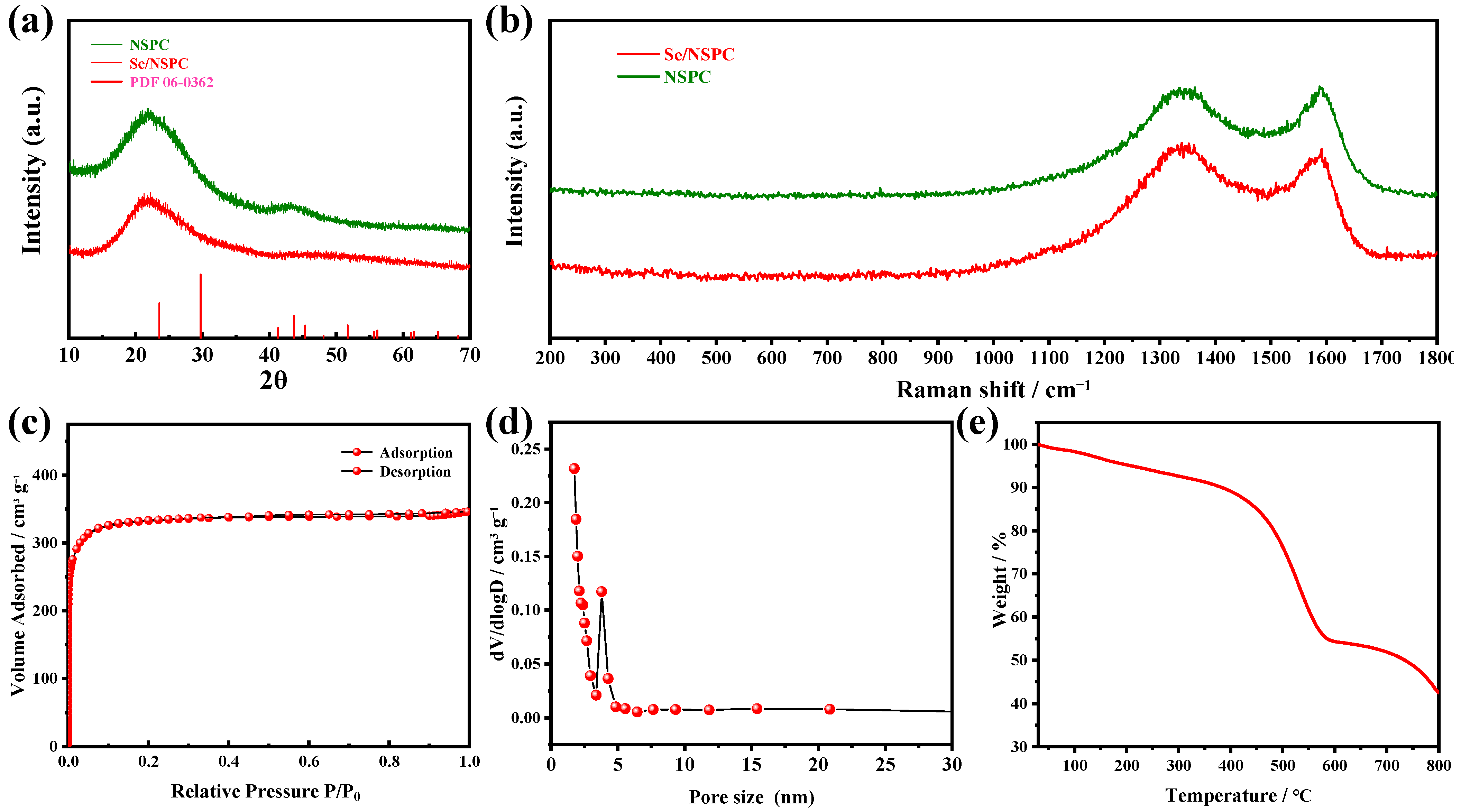
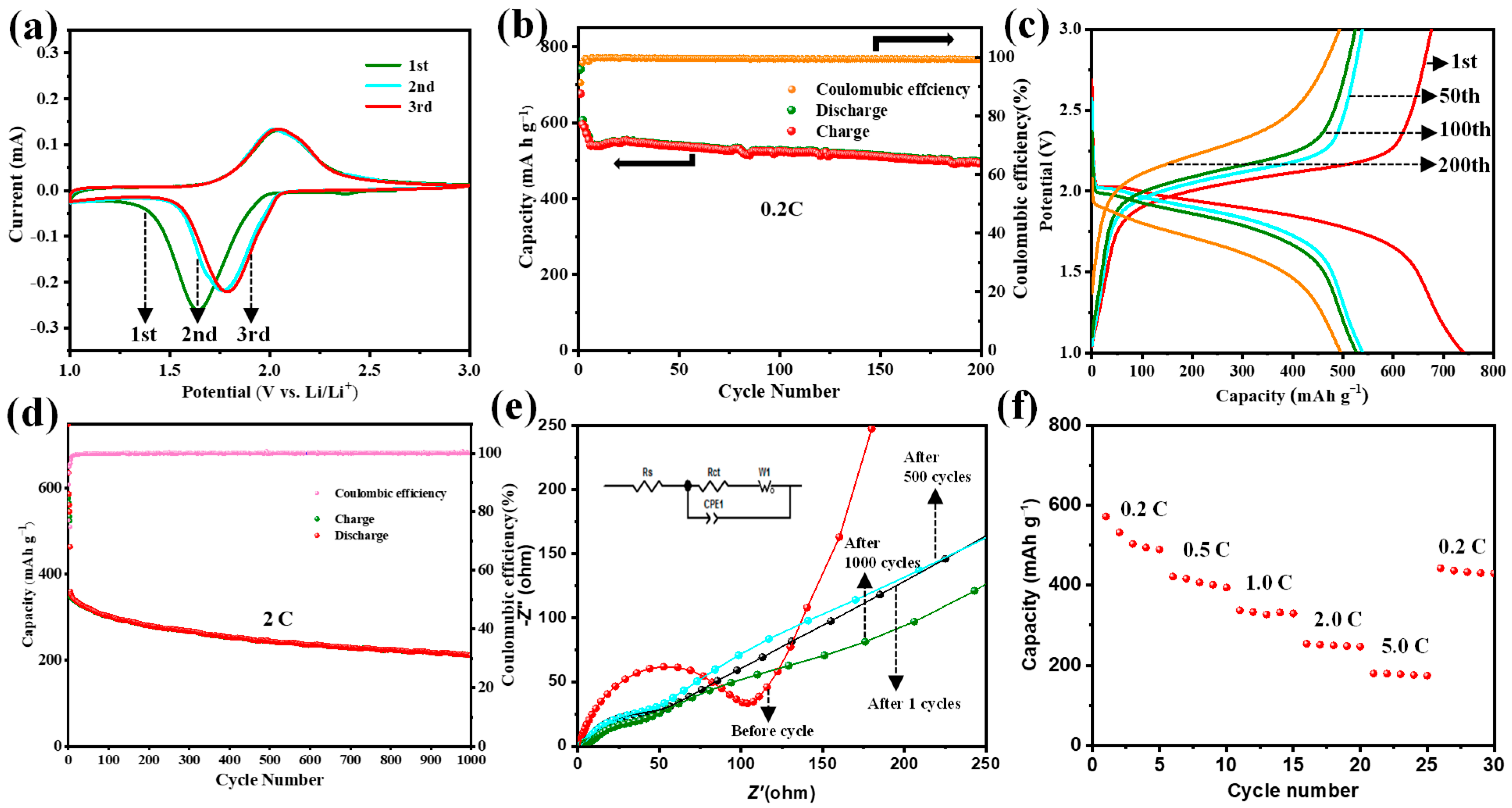
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, N.; Shen, J.; Zhou, X.; Li, S.; Li, J.; Liu, G.; Guo, D.; Deng, W.; Yuan, C.; Liu, X.; et al. Constructing Iron Vacancies in Thiospinel FeIn2S4 to Modulate Fe D-Band Center and Accelerate Sodiation Kinetics Enabling High-Rate and Durable Sodium Storage. Adv. Energy Mater. 2025, 15, 2405729. [Google Scholar] [CrossRef]
- Meng, L.-C.; Zhang, H.; Kang, L.; Zhang, Y.; Yu, N.-F.; Zhang, F.; Du, H.-L. Robust and flexible 3D integrated FeNi@NHCFs air electrode for high-performance rechargeable zinc-air battery. Rare Met. 2024, 43, 5677–5689. [Google Scholar] [CrossRef]
- Li, J.-X.; Lu, Y.-J.; Quan, K.-Y.; Wu, L.-B.; Feng, X.; Wang, W.-Z. One-pot cocrystallization of mononuclear and 1D cobalt(II) complexes based on flexible triclopyr and 2,2′-bipyridine coligands: Structural analyses, conformation comparison, non-covalent interactions and magnetic properties. J. Mol. Struct. 2024, 1297, 136830. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Yu, S.; Johnson, H.M.; Zhao, D.-C.; Tan, S.-C.; Pan, Z.-D.; Wang, Z.-L.; Wu, Y.-T.; Liu, X. Three-dimensional nanostructured Co2VO4-decorated carbon nanotubes for sodium-ion battery anode materials. Rare Met. 2023, 42, 4060–4069. [Google Scholar] [CrossRef]
- Li, J.-X.; Liu, M.-Y.; You, X.; Wang, J.-Q.; Feng, X. One-pot cocrystallization of 1D linear and zigzag cobalt(II) polymers assembled by triclopyr and 4,4′-bipyridine: Structural comparison, conformational analysis, non-covalent interactions as well as the magnetic property of the latter. Polyhedron 2024, 249, 116791. [Google Scholar] [CrossRef]
- Li, J.-X.; Xie, Q.; Zhao, Y.; Zhao, P.; Zhang, S.; Huang, W. Unveiling morphology evolution and performance enhancement of tin-doped Co3O4 porous nanoarrays anchored on stainless-steel mesh for advanced lithium-ion battery anodes. J. Energy Storage 2024, 88, 111605. [Google Scholar] [CrossRef]
- Li, J.-X.; Zhang, J.; Zhao, Y.; Zhao, P.; Xie, Q.; Zhang, S. Heterostructured δ-MnO2/Fe2O3 nanoarrays layer-by-layer assembled on stainless-steel mesh as free-standing anodes for lithium ion batteries towards enhanced performance. Mater. Today Commun. 2022, 32, 104034. [Google Scholar] [CrossRef]
- Li, J.-X.; Xie, Q.; Zhao, P.; Li, C. EDTA-Co(II) sodium complex derived Co(OH)2/Co3O4/Co nanoparticles embedded in nitrogen-enriched graphitic porous carbon as lithium-ion battery anode with superior cycling stability. Appl. Surf. Sci. 2020, 504, 144515. [Google Scholar] [CrossRef]
- Deng, W.; Xu, Z.; Deng, Z.; Wang, X. Enhanced polysulfide regulation via honeycomblike carbon with catalytic MoC for lithium-sulfur batteries. J. Mater. Chem. A 2021, 9, 21760. [Google Scholar] [CrossRef]
- An, C.-H.; Li, Y.-Q.; Wu, S.; Gao, L.-X.; Lin, L.-Y.; Deng, Q.-B.; Hu, N. Matched MnO@C anode and porous carbon cathode for Li-ion hybrid supercapacitors. Rare Met. 2023, 42, 1959–1968. [Google Scholar] [CrossRef]
- Shao, Q.; Zhu, S.; Chen, J. A review on lithium-sulfur batteries: Challenge, development, and perspective. Nano Res. 2023, 16, 8097–8138. [Google Scholar] [CrossRef]
- Rao, X.-Y.; Xiang, S.-F.; Zhou, J.; Zhang, Z.; Xu, X.-Y.; Xu, Y.-Y.; Zhou, X.-C.; Pan, Z.-D.; Tan, S.-C.; Dong, S.-X.; et al. Recent progress and strategies of cathodes toward polysulfides shuttle restriction for lithium-sulfur batteries. Rare Met. 2024, 43, 4132–4161. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, S.; Zhang, N.; Deng, W.; Seo, M.H.; Wang, X. Efficient Zn metal anode enabled by O, N-codoped carbon microflowers. Nano Lett. 2022, 22, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yue, X.-A.; Xu, X.-Y.; Xu, P.; Zhang, F.; Fan, H.-S.; Wang, Z.-L.; Wu, Y.-T.; Liu, X.; Zhang, Y. AN/Co co-doped three-dimensional porous carbon as cathode host for advanced lithium–selenium batteries. Rare Met. 2023, 42, 2670–2678. [Google Scholar] [CrossRef]
- Kan, X.; Yuan, J.; Zhu, Q.; Qiu, Y.; Zhong, S.; Liu, Z.; Zheng, A.; Liu, F.; Jiang, L. Edge-nitrogen rich porous carbons for acid gases capture. Chem. Eng. J. 2025, 512, 162353. [Google Scholar] [CrossRef]
- Huo, T.; Wang, Y.; Wang, J.; Chen, C.; Zhao, Y.; Guo, L.; Wu, X. O Co-Doped Porous Carbon Decorated on Porous Graphene for Zinc Ion Hybrid Capacitor. Adv. Sustain. Syst. 2025, 9, 2400807. [Google Scholar] [CrossRef]
- Xu, F.; Neshumayev, D.; Konist, A. Synthesis strategies and hydrogen storage performance of porous carbon materials derived from bio-oil. Chem. Eng. J. 2025, 505, 159381. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.; Su, Z.; Hu, S.; Li, H.; Yan, Y. Electrospun polyporous VN nanofibers for symmetric all-solid-state supercapacitors. J. Adv. Ceram. 2018, 7, 246–255. [Google Scholar] [CrossRef]
- Ren, M.; Kong, F.; Zhou, C.; Fakayode, O.A.; Liang, J.; Li, H.; Zhou, M.; Fan, X. Green, one-pot biomass hierarchical utilization strategy for lignin-containing cellulose nanofibrils and fractionated lignin preparation. Ind. Crops Prod. 2023, 203, 117193. [Google Scholar] [CrossRef]
- Liu, G.; Qin, S.; Zhang, X.; You, D.; Zhang, Y.; Zeng, X.; Zhang, Y.; Zhu, Z.; Zhang, Y.; Li, X. A new opportunity for biomass-derived carbon in highly stable Li-O2 battery: A review. Nano Res. Energy 2025, 4, e9120142. [Google Scholar] [CrossRef]
- Wei, L.; Yang, H.; Niu, Y.; Zhang, Y.; Xu, L.; Chai, X. Wheat biomass, yield, and straw-grain ratio estimation from multi-temporal UAV-based RGB and multispectral images. Biosyst. Eng. 2023, 234, 187–205. [Google Scholar] [CrossRef]
- Li, G.; Kim, S.; Han, S.H.; Chang, H.; Du, D.; Son, Y. Precipitation affects soil microbial and extracellular enzymatic responses to warming. Soil Biol. Biochem. 2018, 120, 212–221. [Google Scholar] [CrossRef]
- Jia, R.; Tan, Y.; Li, A.; Wang, Y.; Cheng, C. 3D ordered RuO2/WO3 heterostructure inverse opal arrays for highly-active and stable acidic oxygen evolution reaction. Nano Res. Energy 2025, 4, e9120141. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, D.; Guo, Q.; Qiu, F.; Yang, D.; Ou, Z. Preparation of a renewable biomass carbon aerogel reinforced with sisal for oil spillage clean-up: Inspired by green leaves to green Tofu. Food Bioprod. Process. 2019, 114, 154–162. [Google Scholar] [CrossRef]
- Jing, Z.; Ding, J.; Zhang, T.; Yang, D.; Qiu, F.; Chen, Q.; Xu, J. Flexible, versatility and superhydrophobic biomass carbon aerogels derived from corn bracts for efficient oil/water separation. Food Bioprod. Process. 2019, 115, 134–142. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, B.; Chen, Q.; Peng, X.; Yang, D.; Qiu, F. Layered double hydroxide functionalized biomass carbon fiber for highly efficient and recyclable fluoride adsorption. Appl. Biol. Chem. 2019, 62, 12. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Fang, Z.; Shi, G.; Lou, L.; Ren, K.; Cai, Q. Italian ryegrass–rice rotation system for biomass production and cadmium removal from contaminated paddy fields. J. Soils Sediments 2020, 20, 874–882. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Ali, S.S.; Gao, L.; Ni, X.; Li, X.; Wu, Y.; Jiang, J. Identification and expression analysis of Sorghum bicolor gibberellin oxidase genes with varied gibberellin levels involved in regulation of stem biomass. Ind. Crops Prod. 2020, 145, 111951. [Google Scholar] [CrossRef]
- Wang, C.; He, G.; Meng, J.; Wang, S.; Kong, Y.; Jiang, J.; Hu, R.; Zhou, G. Improved lignocellulose saccharification of a Miscanthus reddish stem mutant induced by heavy-ion irradiation. GCB Bioenergy 2020, 12, 1066–1077. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, H.; Zhang, W.; Hanan, J.S.; Ge, D.; Cao, J.; Xia, J.A.; Xuan, S.; Liang, W.; Zhang, L.; et al. An aboveground biomass partitioning coefficient model for rapeseed (Brassica napus L.). Field Crops Res. 2020, 259, 107966. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crops Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
- Sun, L.; Liu, L.; Yang, L.; Wang, Y.; Dabbour, M.; Mintah, B.K.; He, R.; Ma, H. Effects of low-intensity ultrasound on the biomass and metabolite of Ganoderma lucidum in liquid fermentation. J. Food Process Eng. 2021, 44, e13601. [Google Scholar] [CrossRef]
- Rehman, W.; Ma, Y.; Xu, J. Biomass-derived carbon materials for batteries: Navigating challenges, structural diversities, and future perspective. Next Mater. 2025, 7, 100450. [Google Scholar] [CrossRef]
- Li, J.; Ji, K.; Li, B.; Xu, M. Rechargeable Biomass Battery for Electricity Storage/generation and Concurrent Valuable Chemicals Production. Angew. Chem. Int. Ed. 2023, 62, e202304852. [Google Scholar] [CrossRef]
- Wu, P.; Shi, B.; Tu, H.; Guo, C.; Liu, A.; Yan, G.; Yu, Z. Pomegranate-type Si/C anode with SiC taped, well-dispersed tiny Si particles for lithium-ion batteries. J. Adv. Ceram. 2021, 10, 1129–1139. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Fu, J.; Duan, H. Highly Stable Halide-Electrolyte-Based All-Solid-State Li–Se Batteries. Adv. Mater. 2022, 34, 2200856. [Google Scholar] [CrossRef] [PubMed]
- Miao, B.; Wang, J.; Li, J.; Gu, S.; Wang, L.; Jiang, W. Sintering and mechanical properties of carbon bulks from ordered mesoporous carbon and nano diamond. J. Adv. Ceram. 2022, 11, 1815–1823. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Q.; Zeng, K.; Liang, S.; Mao, W. Mechanical properties and thermal stability of carbon fiber cloth reinforced sol-derived mullite composites. J. Adv. Ceram. 2019, 8, 218–227. [Google Scholar] [CrossRef]
- Mo, Z.; Yang, W.; Gao, S.; Shang, J.K.; Ding, Y.; Sun, W.; Li, Q. Efficient oxygen reduction reaction by a highly porous, nitrogen-doped carbon sphere electrocatalyst through space confinement effect in nanopores. J. Adv. Ceram. 2021, 10, 714–728. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Baek, J. Design of Topology-Controlled Polyethers toward Robust Cooperative Hydrogen Bonding. Adv. Funct. Mater. 2023, 33, 2302086. [Google Scholar] [CrossRef]
- Wu, N.; He, W.; Shi, S.; Yuan, X.; Li, J.; Cao, J.; Yuan, C.; Liu, X. Bamboo fiber-derived carbon support for the immobilization of Pt nanoparticles to enhance hydrogen evolution reaction. J. Colloid Interface Sci. 2025, 684, 658–667. [Google Scholar] [CrossRef]
- Wu, N.; Zhao, Z.; Zhang, Y.; Hua, R.; Li, J.; Liu, G.; Guo, D.; Zhao, J.; Cao, A.; Sun, G.; et al. Revealing the fast reaction kinetics and interfacial behaviors of CuFeS2 hollow nanorods for durable and high-rate sodium storage. J. Colloid Interface Sci. 2025, 679, 990–1000. [Google Scholar] [CrossRef]
- Pan, Z.; Ren, J.; Guan, G.; Fang, X.; Wang, B.; Doo, S.-G.; Son, I.H.; Huang, X.; Peng, H. Synthesizing Nitrogen-Doped Core–Sheath Carbon Nanotube Films for Flexible Lithium Ion Batteries. Adv. Energy Mater. 2016, 6, 1600271. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, Y.; Tong, Y.; Liu, X.; Sun, Y.; Li, H.; Niu, L. High Capacity and Fast Kinetics of Potassium-Ion Batteries Boosted by Nitrogen-Doped Mesoporous Carbon Spheres. Nano-Micro Lett. 2021, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Liu, Z.; Wang, F.; Xue, L.; Li, L.; Ye, C.; Zhang, C.; Liu, Q.; Tan, J. The C-S/C=S Bonds Synergistically Modify Porous Hollow-Carbon-Nanocages Anode for Durable and Fast Sodium-Ion Storage. Adv. Funct. Mater. 2024, 34, 2400020. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Wang, Z.; Wang, Z.; Kang, L.; Qi, M.; Chen, M.; Liu, W.; Gong, W.; Lu, W.; et al. Rational Construction of Self-Standing Sulfur-Doped Fe2O3 Anodes with Promoted Energy Storage Capability for Wearable Aqueous Rechargeable NiCo-Fe Batteries. Adv. Energy Mater. 2020, 10, 2001064. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Pan, S.; Wang, Z.-J.; Wang, S.; Chen, Y.; Chen, L. Crafting of Photothermal Cobalt/Sulfur Doped Manganese Selenide for Extreme-Temperature-Tolerant Flexible Zinc-Air Batteries. Adv. Funct. Mater. NA 2025, 35, 2425430. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Liu, Y.; Huang, Y. Confined selenium within porous carbon nanospheres as cathode for advanced Li-Se batteries. Nano Energy 2014, 9, 229–236. [Google Scholar] [CrossRef]
- Fan, J.; Sun, W.; Fu, Y.; Guo, W. Activation of Li2S Cathode by an Organoselenide Salt Mediator for All-Solid-State Lithium–Sulfur Batteries. Adv. Funct. Mater. 2024, 34, 2407166. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z.; Guan, M.; Wu, F.; Chen, R. Sodium-Ion Batteries: Toward Rapid-Charging Sodium-Ion Batteries using Hybrid-Phase Molybdenum Sulfide Selenide-Based Anodes. Adv. Mater. 2020, 32, 2070302. [Google Scholar] [CrossRef]
- Chu, L.; Shen, H.; Wei, H.; Chen, H.; Ma, G.; Yan, W. Morphology engineering of ZnO micro/nanostructures under mild conditions for optoelectronic application. Int. J. Miner. Metall. Mater. 2025, 32, 498. [Google Scholar] [CrossRef]
- Xiao, Z.; Xiao, X.; Kong, L.B.; Dong, H.; Li, X.; He, B.; Ruan, S.; Zhai, J.; Zhou, K.; Huang, Q.; et al. Preparation of MXene-based hybrids and their application in neuromorphic devices. Int. J. Extrem. Manuf. 2024, 6, 022006. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, P.; Yang, F.; Zhu, Y.; Pan, Y.; Xue, H.; Mao, W.; Chu, L. Ferroelectric polarization in Bi0.9Dy0.1FeO3/g-C3N4 Z-scheme heterojunction boosts photocatalytic hydrogen evolution. Sci. China Mater. 2024, 67, 3160–3167. [Google Scholar] [CrossRef]
- Gong, J.; Ji, S.; Li, J.; Wei, H.; Mao, W.; Hu, J.; Huang, W.; He, X.; Li, X.; Chu, L. Three-dimensional/one-dimensional perovskite heterostructures for stable tri-state synaptic memristors. Sci. China Mater. 2024, 67, 2848–2855. [Google Scholar] [CrossRef]
- Chu, L.; Rong, Z.; Ma, M.; Guo, F.; Tian, S.; Yang, J. Dodecylamine-mediated synthesis of caron-supported platinum-ruthenium alloy nanoparticles with ultrafine sizes towards high-efficiency methanol oxidation electrocatalysis. Prog. Nat. Sci. Mater. Int. 2025, 35, 215. [Google Scholar] [CrossRef]
- Chu, L.; Cao, J.; Wu, C. Methylammonium-Free Perovskite Photovoltaic Modules. ACS Nano 2025, 19, 13527–13548. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Shu, X.; Zhang, Y.; Meng, L.; Yu, N.; Fang, B. Rapeseed Meal-Derived Three-Dimensional Porous Carbon for High-Performance Lithium–Selenium Batteries. Processes 2025, 13, 2596. https://doi.org/10.3390/pr13082596
Yang Y, Shu X, Zhang Y, Meng L, Yu N, Fang B. Rapeseed Meal-Derived Three-Dimensional Porous Carbon for High-Performance Lithium–Selenium Batteries. Processes. 2025; 13(8):2596. https://doi.org/10.3390/pr13082596
Chicago/Turabian StyleYang, Yuanjiang, Xiaoyan Shu, Yi Zhang, Leichao Meng, Nengfei Yu, and Baizeng Fang. 2025. "Rapeseed Meal-Derived Three-Dimensional Porous Carbon for High-Performance Lithium–Selenium Batteries" Processes 13, no. 8: 2596. https://doi.org/10.3390/pr13082596
APA StyleYang, Y., Shu, X., Zhang, Y., Meng, L., Yu, N., & Fang, B. (2025). Rapeseed Meal-Derived Three-Dimensional Porous Carbon for High-Performance Lithium–Selenium Batteries. Processes, 13(8), 2596. https://doi.org/10.3390/pr13082596





