Mg/Fe Layered Double Hydroxide Modified Biochar for Synergistic Removal of Phosphate and Ammonia Nitrogen from Chicken Farm Wastewater: Adsorption Performance and Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Raw Materials
2.2. Preparation of Modified Biochar
2.3. Characterization and Analysis
2.4. Batch Adsorption Experiments
2.5. Adsorption Regeneration Experiment
2.6. Data Analysis
3. Results and Discussion
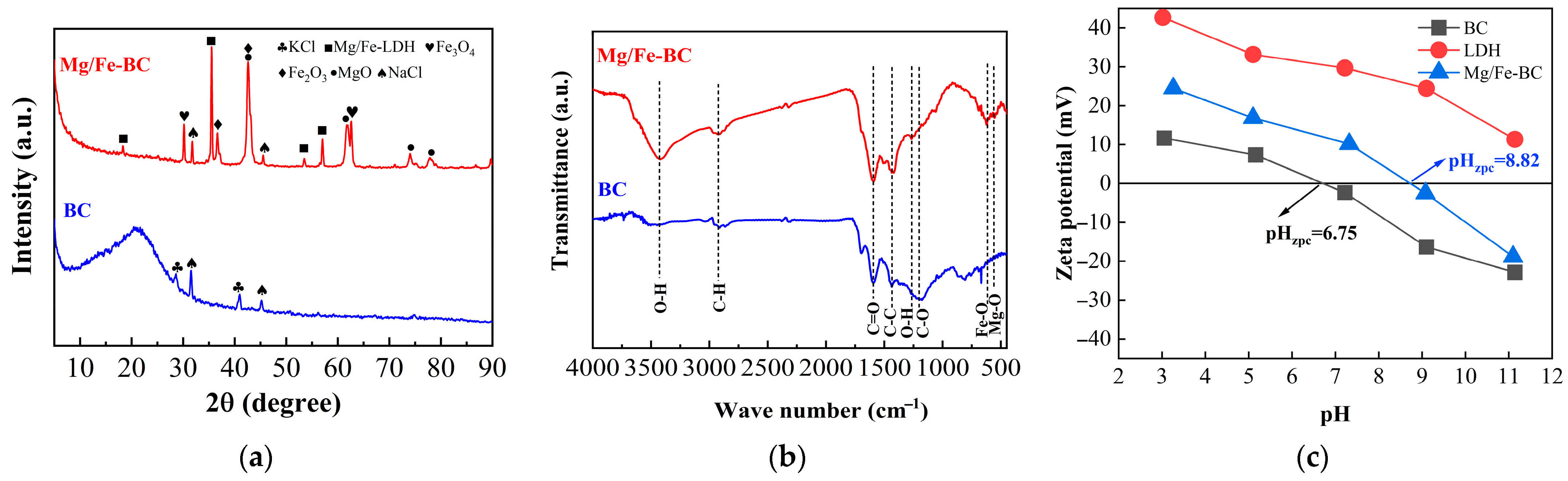
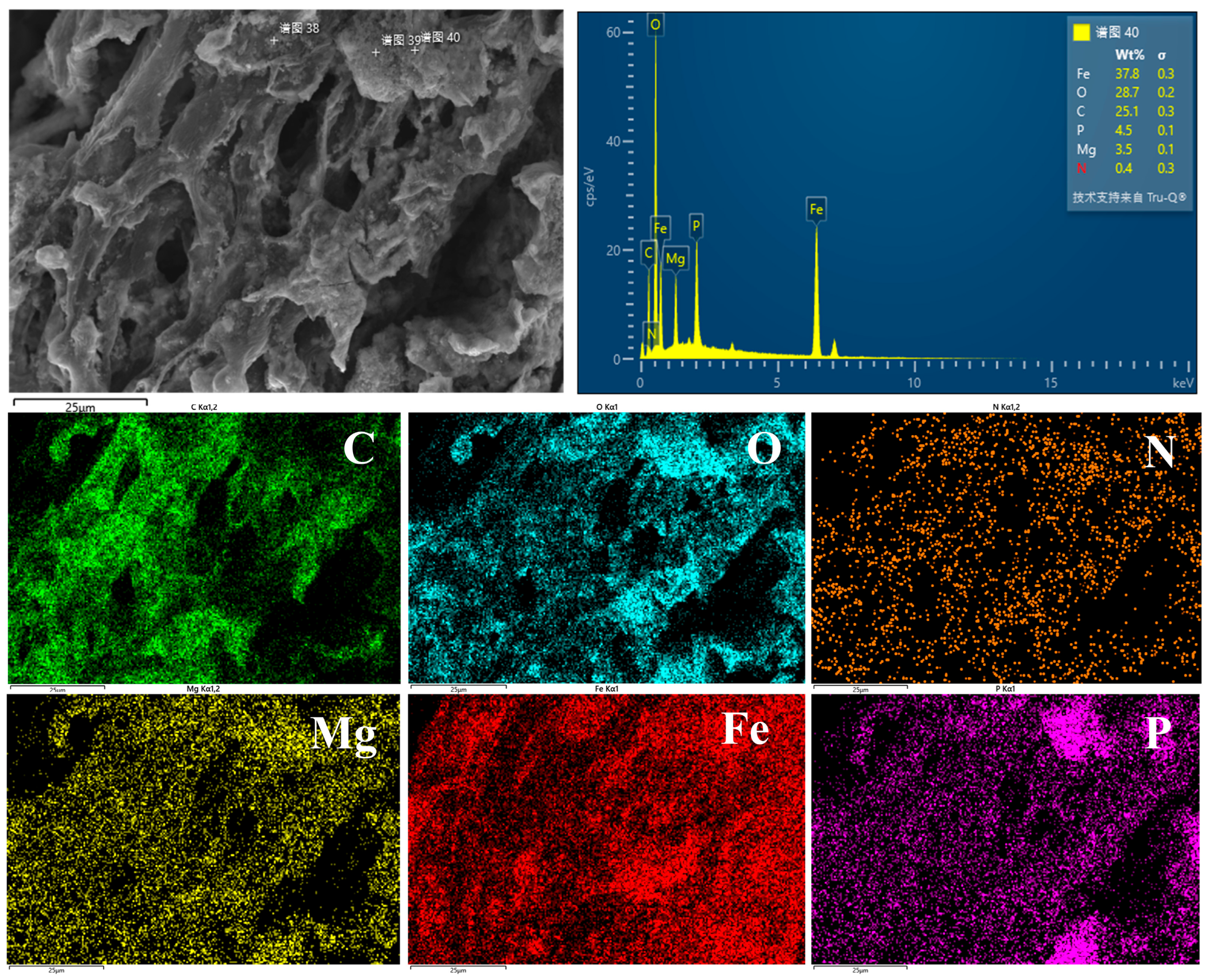
3.1. Characterization of Composite Materials
3.2. The Adsorption Performance of Mg/Fe-BC for PO43−-P and NH4+-N in a Single Aqueous Solution
3.3. Adsorption of PO43−-P and NH4+-N in Mixed Solutions by Mg/Fe-BC
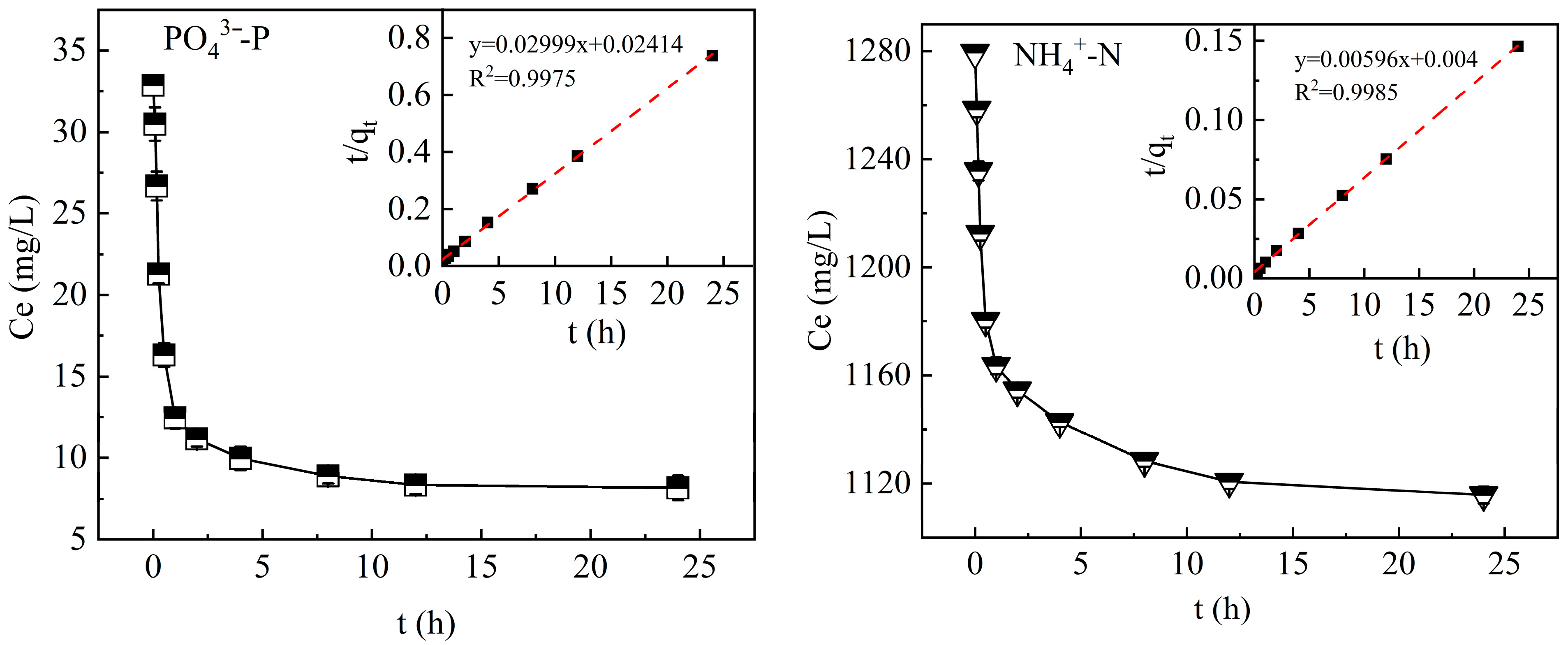
3.3.1. Adsorption Kinetics
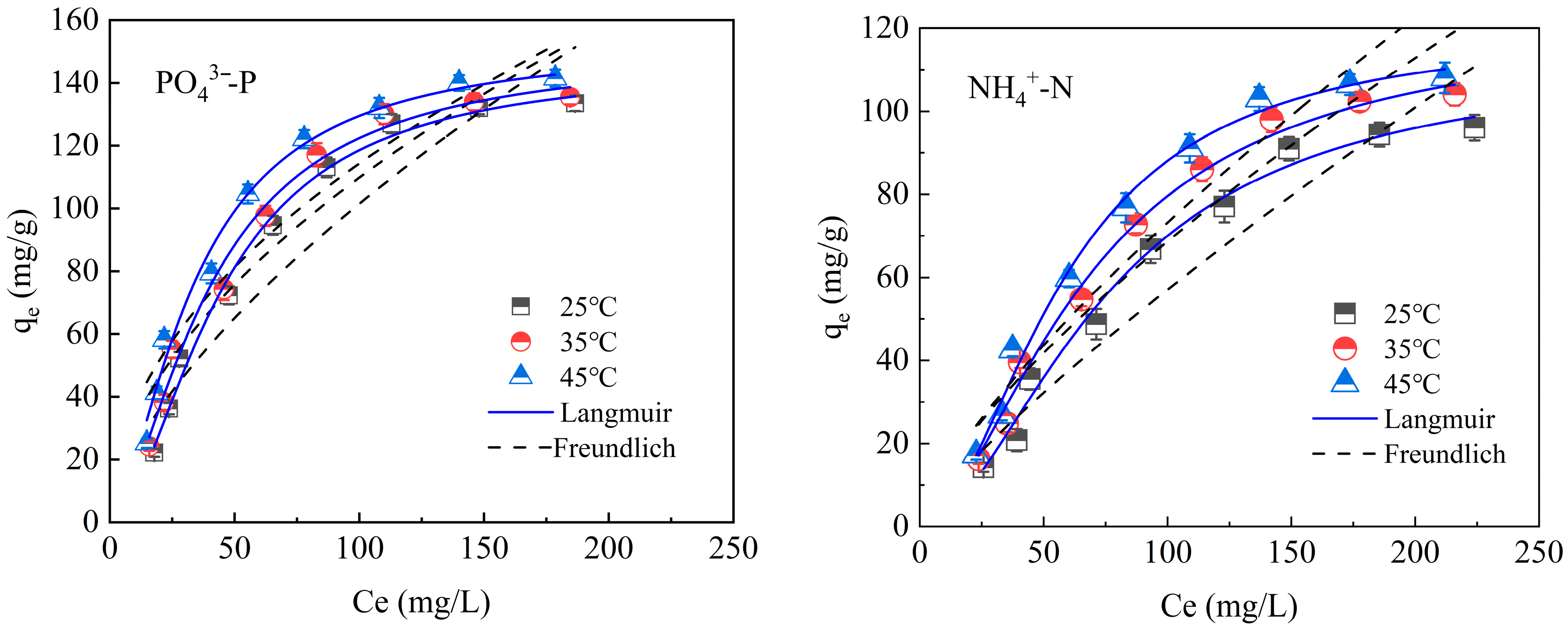
3.3.2. Adsorption Isotherm
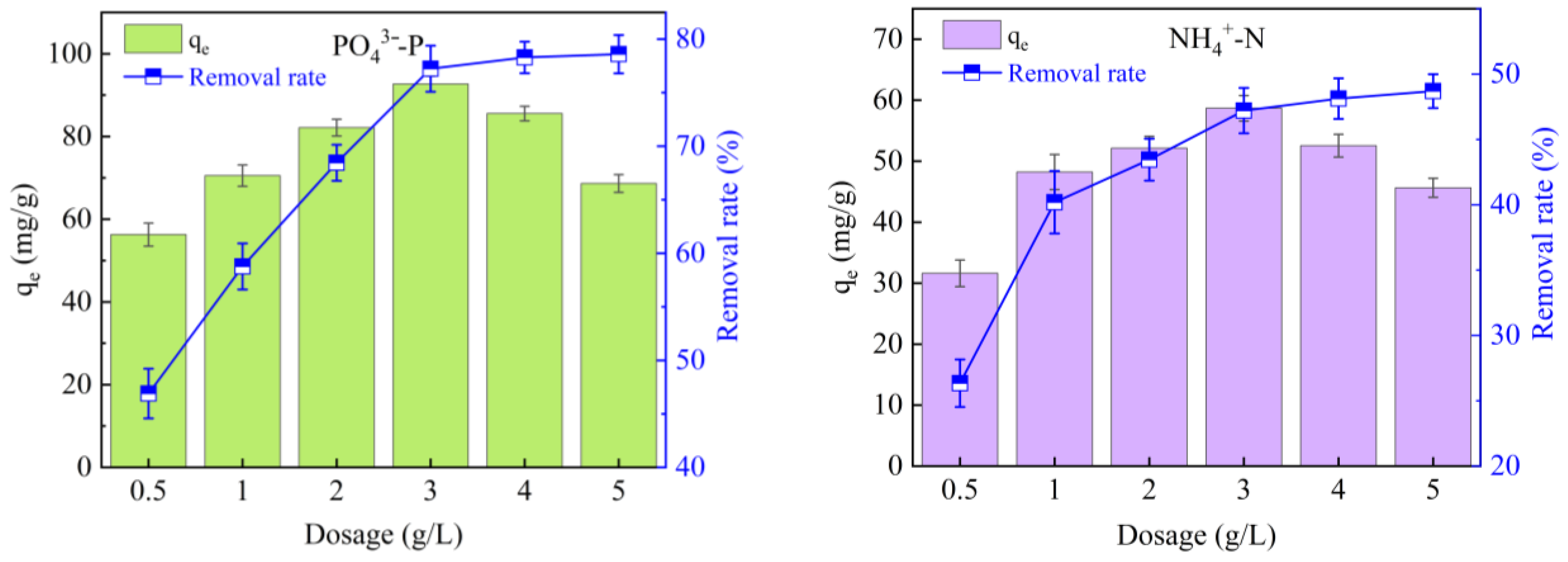
3.4. Factors Influencing Simultaneous PO43−-P and NH4+-N Adsorption by Mg/Fe-BC
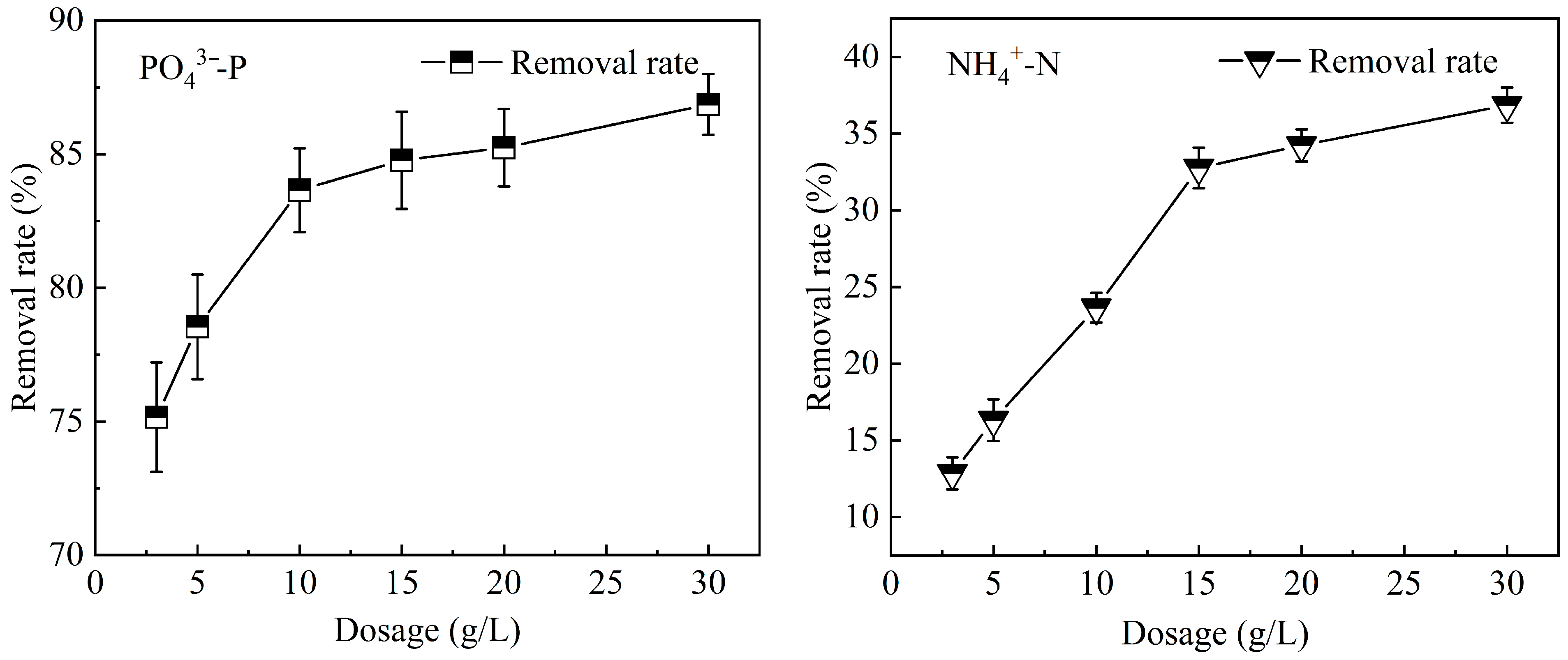
3.4.1. Dosage
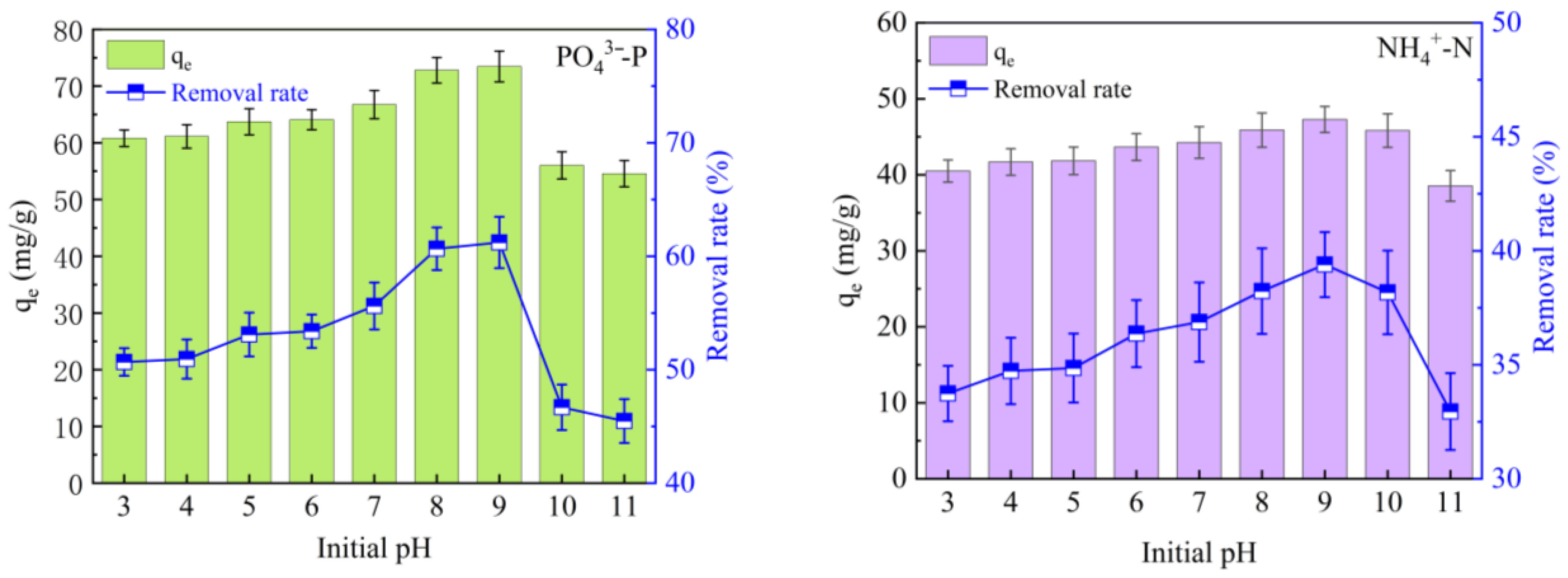
3.4.2. Initial pH
3.4.3. Coexisting Ions
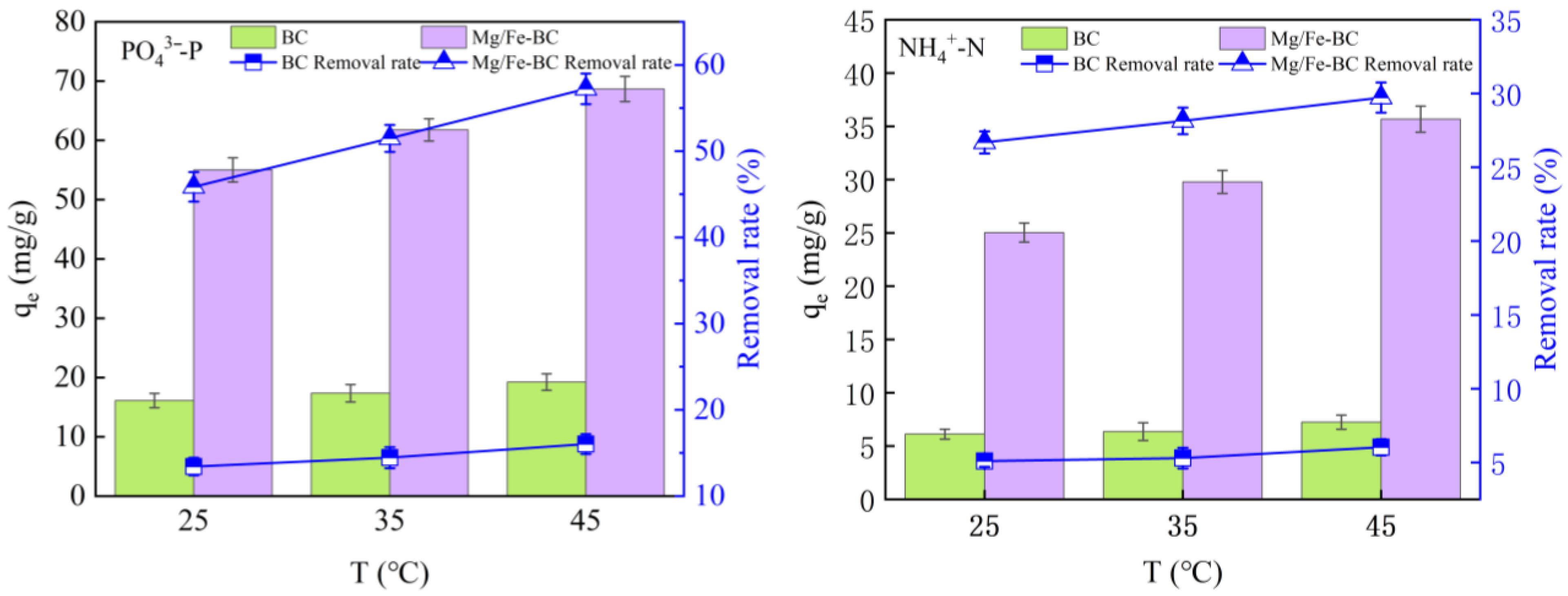
3.5. Adsorption Performance of Mg/Fe-BC in Chicken Manure Biogas Slurry
3.5.1. The Influence of Dosage on the Adsorption of PO43−-P and NH4+-N
3.5.2. Effect of Contact Time on PO43−-P and NH4+-N Adsorption
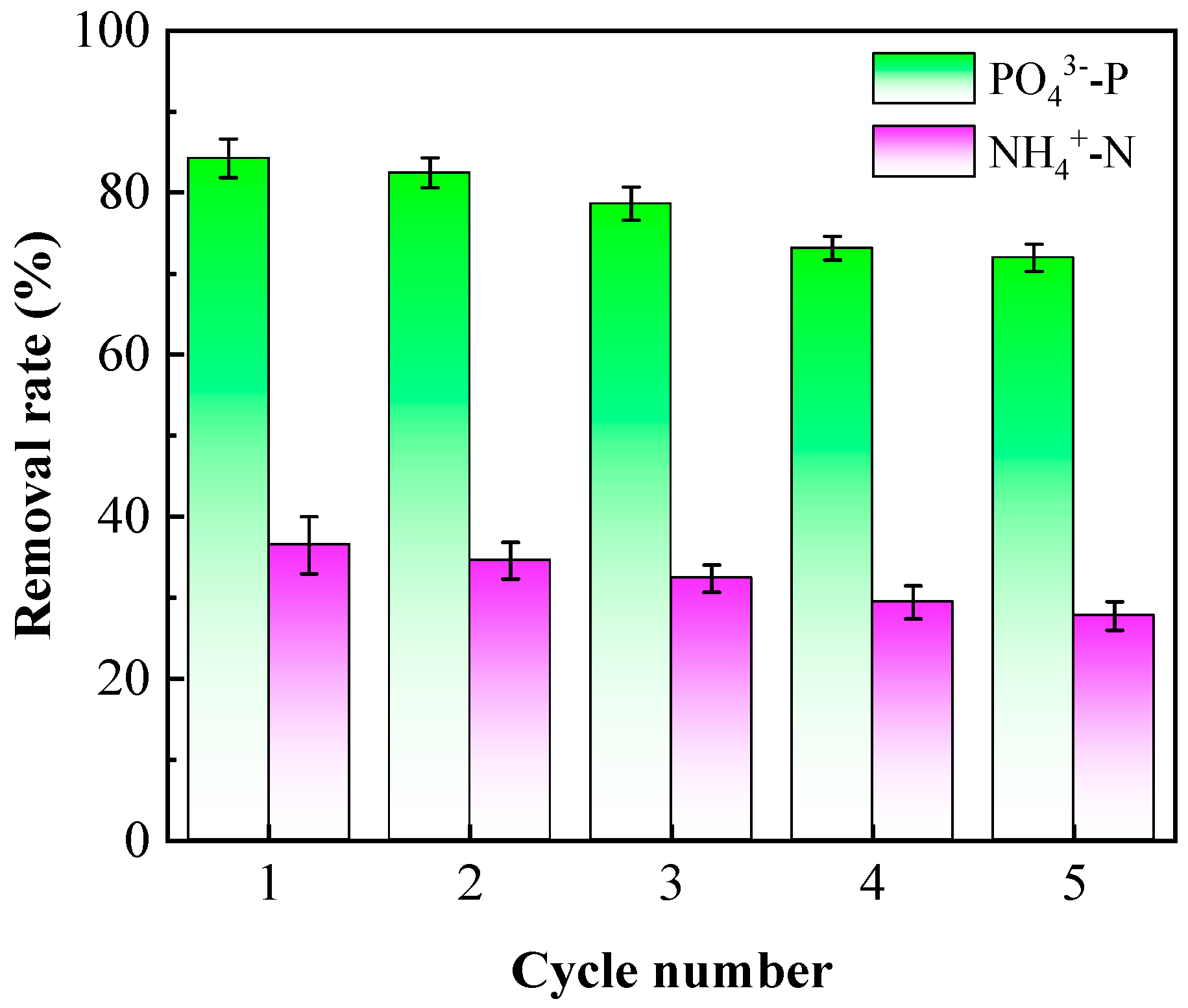
3.6. Regeneration Experiment
3.7. Adsorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, Z.; Ji, J.; Sheng, H.; Jiang, S.; Chen, T.; Liu, X.; Liu, X.; Zhuang, Y.; Zhang, L. Animal Based Diets and Environment: Perspective from Phosphorus Flow Quantifications of Livestock and Poultry Raising in China. J. Environ. Manag. 2019, 244, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Sui, L.; Tang, C.; Li, J.; Cheng, K.; Xue, Q. Sustainable Advances on Phosphorus Utilization in Soil via Addition of Biochar and Humic Substances. Sci. Total Environ. 2021, 768, 145106. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Huang, Q.; Khan, S.; Nawab, J.; Khan, M.A.; Ali, A.; Ullah, R.; Kubar, A.A.; Guo, G.; Yaseen, M.; et al. Methods for the Removal and Recovery of Nitrogen and Phosphorus Nutrients from Animal Waste: A Critical Review. Ecol. Front. 2024, 44, 2–14. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Chen, X.; Cui, S.; Hofstra, N.; Kroeze, C.; Ma, L.; Xu, W.; Zhang, Q.; Zhang, F.; et al. Multi-Pollutant Assessment of River Pollution from Livestock Production Worldwide. Water Res. 2022, 209, 117906. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A.M. A Comprehensive Review of Green Policy, Anaerobic Digestion of Animal Manure and Chicken Litter Feedstock Potential–Global and Irish Perspective. Renew. Sust. Energ. Rev. 2022, 154, 111884. [Google Scholar] [CrossRef]
- Huang, X.; Wang, S.; Shi, Z.; Fang, L.; Yin, C. Challenges and Strategies for Biogas Production in the Circular Agricultural Waste Utilization Model: A Case Study in Rural China. Energy 2022, 241, 122889. [Google Scholar] [CrossRef]
- Duan, N.; Zhang, D.; Lin, C.; Zhang, Y.; Zhao, L.; Liu, H.; Liu, Z. Effect of Organic Loading Rate on Anaerobic Digestion of Pig Manure: Methane Production, Mass Flow, Reactor Scale and Heating Scenarios. J. Environ. Manag. 2019, 231, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Liu, X.; Du, B.; Wang, Y.; Zheng, Y.; Li, Q. Component Analysis and Risk Assessment of Biogas Slurry from Biogas Plants. Chin. J. Chem. Eng. 2022, 44, 182–191. [Google Scholar] [CrossRef]
- Wicker, R.; Bhatnagar, A. Application of Nordic Microalgal-Bacterial Consortia for Nutrient Removal from Wastewater. Chem. Eng. J. 2020, 398, 125567. [Google Scholar] [CrossRef]
- Karri, R.R.; Sahu, J.N.; Chimmiri, V. Critical Review of Abatement of Ammonia from Wastewater. J. Mol. Liq. 2018, 261, 21–31. [Google Scholar] [CrossRef]
- Ma, L.L.; Ma, C.; Zhang, H.F.; Wang, B.H. Identifying Influential Spreaders in Complex Networks Based on Gravity Formula. Phys. A 2016, 451, 205–212. [Google Scholar] [CrossRef]
- Qiu, J.; Hou, H.; Liang, S.; Yang, L.; Gan, Q.; Tao, S.; Yu, W.; Lv, R.; Ding, L.; Xiao, K.; et al. Hierarchically Porous Biochar Preparation and Simultaneous Nutrient Recovery from Sewage Sludge via Three Steps of Alkali-Activated Pyrolysis, Water Leaching and Acid Leaching. Resour. Conserv. Recycl. 2022, 176, 105953. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.; Chen, H.; Yu, P.; Chen, C. Evaluating Biochar for Adsorption of Ammonium Nitrogen in Wastewater: Insights into Modifications and Mechanisms. Environ. Res. 2025, 277, 121615. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ali, A.; Gao, Y.; Zhao, P.; Li, R.; Li, X.; Liu, J.; Luo, Y.; Peng, Y.; Wang, H.; et al. Preparation and Characterization of MgO Hybrid Biochar and Its Mechanism for High Efficient Recovery of Phosphorus from Aqueous Media. Biochar 2022, 4, 171. [Google Scholar] [CrossRef]
- Cao, J.; Wang, R.; Zhu, H.; Cao, S.; Duan, Z. Effect of Fenton Pre-Oxidation on the Physicochemical Properties of Sludge-Based Biochar and Its Adsorption Mechanisms for Ammonia Nitrogen Removal. J. Environ. Chem. Eng. 2023, 11, 110689. [Google Scholar] [CrossRef]
- Rybka, K.; Matusik, J.; Marzec, M. Mg/Al and Mg/Fe Layered Double Hydroxides Derived from Magnesite and Chemicals: The Effect of Adsorbent Features and Anions Chemistry on Their Removal Efficiency. J. Clean. Prod. 2022, 332, 130084. [Google Scholar] [CrossRef]
- Fang, Q.; Ye, S.; Yang, H.; Yang, K.; Zhou, J.; Gao, Y.; Lin, Q.; Tan, X.; Yang, Z. Application of Layered Double Hydroxide-Biochar Composites in Wastewater Treatment: Recent Trends, Modification Strategies, and Outlook. J. Hazard. Mater. 2021, 420, 126569. [Google Scholar] [CrossRef]
- Yang, H.; Ye, S.; Zeng, Z.; Zeng, G.; Tan, X.; Xiao, R.; Wang, J.; Song, B.; Du, L.; Qin, M.; et al. Utilization of Biochar for Resource Recovery from Water: A Review. Chem. Eng. J. 2020, 397, 125502. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhao, Y.; Wang, L.; Feng, J.; Sun, F. Efficient Simultaneous Phosphate and Ammonia Adsorption Using Magnesium-Modified Biochar Beads and Their Recovery Performance. J. Environ. Chem. Eng. 2023, 11, 110875. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Li, T.; Cheng, X. Characterization of Magnesium-Iron Modified Biochar to Alleviate Ammonia Inhibition and Enhance Anaerobic Digestion of Chicken Manure. Waste Biomass Valorization 2025, 25, 2894. [Google Scholar] [CrossRef]
- Bian, H.; Wang, M.; Han, J.; Hu, X.; Xia, H.; Wang, L.; Fang, C.; Shen, C.; Man, Y.B.; Wong, M.H.; et al. MgFe-LDH@Biochars for Removing Ammonia Nitrogen and Phosphorus from Biogas Slurry: Synthesis Routes, Composite Performance, and Adsorption Mechanisms. Chemosphere 2023, 324, 138333. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qi, G.; Wang, B.; Yue, D.; Wang, Y.; Sato, T. Fulvic Acid Anchored Layered Double Hydroxides: A Multifunctional Composite Adsorbent for the Removal of Anionic Dye and Toxic Metal. J. Hazard. Mater. 2018, 343, 19–28. [Google Scholar] [CrossRef]
- Bhatia, A.; Khatri, A.; Yadav, M.; Kumari, A.; Mona, S.; Bhateria, R. Potential of Iron Oxide Nanoparticles in Enhancing Growth and Development of Plants: A Review. Physiol. Mol. Plant Pathol. 2025, 139, 102746. [Google Scholar] [CrossRef]
- Roy Choudhury, M.; Christopher, J.; Das, S.; Apan, A.; Menzies, N.W.; Chapman, S.; Mellor, V.; Dang, Y.P. Detection of Calcium, Magnesium, and Chlorophyll Variations of Wheat Genotypes on Sodic Soils Using Hyperspectral Red Edge Parameters. Environ. Technol. Innov. 2022, 27, 102469. [Google Scholar] [CrossRef]
- Tan, W.T.; Zhou, H.; Tang, S.F.; Zeng, P.; Gu, J.F.; Liao, B.H. Enhancing Cd(II) Adsorption on Rice Straw Biochar by Modification of Iron and Manganese Oxides. Environ. Pollut. 2022, 300, 118899. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Navarathna, C.M.; Krishna Das, N.; Alchouron, J.; Reneau, P.; Stokes, S.; Thirumalai, R.V.K.G.; Perez, F.; Barbary Hassan, E.; Mohan, D. High Capacity Aqueous Phosphate Reclamation Using Fe/Mg-Layered Double Hydroxide (LDH) Dispersed on Biochar. J. Colloid Interface Sci. 2021, 597, 182–195. [Google Scholar] [CrossRef]
- Liu, H.; Shan, J.; Chen, Z.; Lichtfouse, E. Efficient Recovery of Phosphate from Simulated Urine by Mg/Fe Bimetallic Oxide Modified Biochar as a Potential Resource. Sci. Total Environ. 2021, 784, 147546. [Google Scholar] [CrossRef]
- Fang, L.; Li, J.S.; Donatello, S.; Cheeseman, C.R.; Poon, C.S.; Tsang, D.C.W. Use of Mg/Ca Modified Biochars to Take Up Phosphorus from Acid-Extract of Incinerated Sewage Sludge Ash (ISSA) for Fertilizer Application. J. Clean. Prod. 2020, 244, 118853. [Google Scholar] [CrossRef]
- Li, X.; Shi, J. Simultaneous Adsorption of Tetracycline, Ammonium and Phosphate from Wastewater by Iron and Nitrogen Modified Biochar: Kinetics, Isotherm, Thermodynamic and Mechanism. Chemosphere 2022, 293, 133574. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, Q.; Xing, Z. Adsorption of Ammonia Nitrogen in Low Temperature Domestic Wastewater by Modification Bentonite. J. Clean. Prod. 2019, 233, 720–730. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, W.; Xu, H.; Li, S.; Peng, Y. Adsorption Removal and Reuse of Phosphate from Wastewater Using a Novel Adsorbent of Lanthanum-Modified Platanus Biochar. Process Saf. Environ. 2020, 140, 221–232. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, M.; Wu, P.; Zhang, X.; Wang, S.; Yu, Z.; Wang, B. Simultaneous Reclaiming Phosphate and Ammonium from Aqueous Solutions by Calcium Alginate-Biochar Composite: Sorption Performance and Governing Mechanisms. Chem. Eng. J. 2022, 429, 132166. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.; Yu, F.; Zhu, X.; Dong, M.; Li, Q. Recovery of Phosphate from Aqueous Solution by Modified Biochar with Concentrated Seawater and Its Potential Application as Fertilizer. J. Environ. Chem. Eng. 2024, 12, 112646. [Google Scholar] [CrossRef]
- Yang, X.; Yang, F.; Liu, C.; Sun, H.; Hou, D.; Zheng, Y.; Gao, H.; Shi, R.; He, X.; Lin, X. Adsorption Characteristics and Mechanism Insights of K2FeO4 Coupling with ZnCl2-Assisted Modified Functionalized Biochar for Pb (II) in Wastewater. J. Environ. Chem. Eng. 2025, 13, 115279. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Xie, X.; Wang, Z. Bifunctional MnFe2O4/Chitosan Modified Biochar Composite for Enhanced Methyl Orange Removal Based on Adsorption and Photo-Fenton Process. Colloids Surf. A 2021, 613, 126104. [Google Scholar] [CrossRef]
- Cui, X.; Dai, X.; Khan, K.Y.; Li, T.; Yang, X.; He, Z. Removal of Phosphate from Aqueous Solution Using Magnesium-Alginate/Chitosan Modified Biochar Microspheres Derived from Thalia Dealbata. Bioresour. Technol. 2016, 218, 1123–1132. [Google Scholar] [CrossRef]
- Tan, M.; Li, Y.; Chi, D.; Wu, Q. Efficient Removal of Ammonium in Aqueous Solution by Ultrasonic Magnesium-Modified Biochar. Chem. Eng. J. 2023, 461, 142072. [Google Scholar] [CrossRef]
- Gong, Y.P.; Ni, Z.Y.; Xiong, Z.Z.; Cheng, L.H.; Xu, X.H. Phosphate and Ammonium Adsorption of the Modified Biochar Based on Phragmites Australis after Phytoremediation. Environ. Sci. Pollut. R. 2017, 24, 8326–8335. [Google Scholar] [CrossRef]
- Li, B.; Jing, F.; Hu, Z.; Liu, Y.; Xiao, B.; Guo, D. Simultaneous Recovery of Nitrogen and Phosphorus from Biogas Slurry by Fe-Modified Biochar. J. Saudi Chem. Soc. 2021, 25, 101213. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Li, A.Y.; Deng, H.; Ye, C.H.; Wu, Y.Q.; Linmu, Y.D.; Hang, H.L. Characteristics of Nitrogen and Phosphorus Adsorption by Mg-Loaded Biochar from Different Feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef]
- Luo, L.; Wang, G.; Shi, G.; Zhang, M.; Zhang, J.; He, J.; Xiao, Y.; Tian, D.; Zhang, Y.; Deng, S.; et al. The Characterization of Biochars Derived from Rice Straw and Swine Manure, and Their Potential and Risk in N and P Removal from Water. J. Environ. Manag. 2019, 245, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moulessehoul, A.; Gallart-Mateu, D.; Harrache, D.; Djaroud, S.; de la Guardia, M.; Kameche, M. Conductimetric Study of Struvite Crystallization in Water as a Function of pH. J. Cryst. Growth 2017, 471, 42–52. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Zhang, Z.; Liu, S.; Lei, S.; Xiao, R. Simultaneous Capture Removal of Phosphate, Ammonium and Organic Substances by MgO Impregnated Biochar and Its Potential Use in Swine Wastewater Treatment. J. Clean. Prod. 2017, 147, 96–107. [Google Scholar] [CrossRef]
- Shin, J.; Rho, H.; Cho, Y.; Son, C.; Kwak, J.; Kim, S.; Ki, S.; Kim, H.J.; Lee, Y.-G.; Park, Y.; et al. Functionalization of Coffee Waste Biochars with Ca/Fe Layered Double Hydroxides for Enhanced Removal and Selectivity of Phosphate Ions: Mechanisms and Reusability. J. Water Process. Eng. 2025, 76, 108123. [Google Scholar] [CrossRef]
- Yan, H.; Shih, K. Effects of Calcium and Ferric Ions on Struvite Precipitation: A New Assessment Based on Quantitative X-Ray Diffraction Analysis. Water Res. 2016, 95, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Ye, Z.L. Concentration-Dependent Adsorption Behaviors and Mechanisms for Ammonium and Phosphate Removal by Optimized Mg-Impregnated Biochar. J. Clean. Prod. 2022, 349, 131453. [Google Scholar] [CrossRef]
- Chung, H.K.; Kim, W.H.; Park, J.; Cho, J.; Jeong, T.Y.; Park, P.K. Application of Langmuir and Freundlich Isotherms to Predict Adsorbate Removal Efficiency or Required Amount of Adsorbent. J. Ind. Eng. Chem. 2015, 28, 241–246. [Google Scholar] [CrossRef]
- Pan, F.; Wei, H.; Huang, Y.; Song, J.; Gao, M.; Zhang, Z.; Teng, R.; Jing, S. Phosphorus Adsorption by Calcium Chloride-Modified Buckwheat Hulls Biochar and the Potential Application as a Fertilizer. J. Clean. Prod. 2024, 444, 141233. [Google Scholar] [CrossRef]
- Lou, L.; Li, W.; Yao, H.; Luo, H.; Liu, G.; Fang, J. Corn Stover Waste Preparation Cerium-Modified Biochar for Phosphate Removal from Pig Farm Wastewater: Adsorption Performance and Mechanism. Biochem. Eng. J. 2024, 212, 109530. [Google Scholar] [CrossRef]
- Wang, B.; Hu, X.; Li, L.; Wang, H.; Huang, H.; Wang, R.; Zhou, D.; Yuan, J.; Chen, L. Application and Functionalization of Toxic Waste Sludge-Derived Biochar for Efficient Phosphate Separation from Aqueous Media: Toxicity Diminution, Robust Adsorption, and Inner Mechanism. Chem. Eng. J. 2023, 468, 143745. [Google Scholar] [CrossRef]
- Tang, Y.; Alam, M.S.; Konhauser, K.O.; Alessi, D.S.; Xu, S.; Tian, W.; Liu, Y. Influence of Pyrolysis Temperature on Production of Digested Sludge Biochar and Its Application for Ammonium Removal from Municipal Wastewater. J. Clean. Prod. 2019, 209, 927–936. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, K.; Feng, Y.; He, Q.; Zhang, K.; Shen, S.; Wang, F. Synthesis of a La(OH)3 Nanorod/Walnut Shell Biochar Composite for Reclaiming Phosphate from Aqueous Solutions. Colloid Surf. A 2021, 610, 125736. [Google Scholar] [CrossRef]
- Cichy, B.; Kuzdzal, E.; Krzton, H. Phosphorus Recovery from Acidic Wastewater by Hydroxyapatite Precipitation. J. Environ. Manag. 2019, 232, 421–427. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Yang, R.; Fu, Y.; Zhu, B. Superior Properties of Biochar Contribute to Soil Carbon Sequestration and Climate Change Mitigation. J. Environ. Chem. Eng. 2025, 13, 116936. [Google Scholar] [CrossRef]





| Parameter | pH | SCOD (mg/L) | TN (mg/L) | NH4+-N (mg/L) | PO43−-P (mg/L) |
|---|---|---|---|---|---|
| Biogas slurry | 8.16 | 3067.84 | 2466.57 | 1279.45 | 32.86 |
| Sample | BET (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | Productivity (%) |
|---|---|---|---|---|
| BC | 3.43 | 0.0134 | 13.92 | 35.17 |
| Mg/Fe-BC | 71.69 | 0.1574 | 9.35 | 58.12 |
| Adsorption Kinetic Model | PO43−-P | NH4+-N | |
|---|---|---|---|
| Pseudo-first-order | qe (mg/g) | 67.88 | 45.16 |
| K1(min−1) | 1.48 | 1.09 | |
| R2 | 0.97 | 0.95 | |
| Pseudo-second-order | qe (mg/g) | 74.27 | 50.22 |
| K2 (g/(mg·min)) | 0.02 | 0.02 | |
| R2 | 0.99 | 0.99 | |
| Elovich | α | 336.16 | 103.77 |
| β | 0.08 | 0.10 | |
| R2 | 0.93 | 0.92 | |
| Index | T | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| KL (L/mg) | qm (mg/g) | R2 | KF | 1/n | R2 | ||
| PO43−-P | 25 °C | 0.01 | 145.97 | 0.99 | 5.28 | 0.64 | 0.92 |
| 35 °C | 0.03 | 150.39 | 0.98 | 9.28 | 0.54 | 0.91 | |
| 45 °C | 0.04 | 153.05 | 0.98 | 11.85 | 0.49 | 0.91 | |
| NH4+-N | 25 °C | 0.04 | 112.63 | 0.98 | 2.37 | 0.75 | 0.92 |
| 35 °C | 0.04 | 119.60 | 0.99 | 1.29 | 0.82 | 0.94 | |
| 45 °C | 0.07 | 123.51 | 0.99 | 2.53 | 0.72 | 0.93 | |
| Adsorbent | Target Pollutant Ions | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|
| Biochar derived from iron-rich sludge | PO43−-P | 1.83 | [35] |
| Mg-alginate modified biochar | PO43−-P | 46.56 | [36] |
| NBC, NMBC | NH4+-N | 13.59, 23.78 | [37] |
| MPB | NH4+-N | 30.00 | [38] |
| CA-MB | PO43−-P, NH4+-N | 31.80, 10.15 | [32] |
| Fe-modified biochar | PO43−-P, NH4+-N | 26.14, 11.68 | [39] |
| Mg-loaded biochar | PO43−-P, NH4+-N | 31.15, 24.04 | [40] |
| Mg/Fe-BC | PO43−-P, NH4+-N | 145.97, 112.63 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Li, J.; Li, Z.; Cheng, X. Mg/Fe Layered Double Hydroxide Modified Biochar for Synergistic Removal of Phosphate and Ammonia Nitrogen from Chicken Farm Wastewater: Adsorption Performance and Mechanisms. Processes 2025, 13, 2504. https://doi.org/10.3390/pr13082504
Li T, Li J, Li Z, Cheng X. Mg/Fe Layered Double Hydroxide Modified Biochar for Synergistic Removal of Phosphate and Ammonia Nitrogen from Chicken Farm Wastewater: Adsorption Performance and Mechanisms. Processes. 2025; 13(8):2504. https://doi.org/10.3390/pr13082504
Chicago/Turabian StyleLi, Tao, Jinping Li, Zengpeng Li, and Xiuwen Cheng. 2025. "Mg/Fe Layered Double Hydroxide Modified Biochar for Synergistic Removal of Phosphate and Ammonia Nitrogen from Chicken Farm Wastewater: Adsorption Performance and Mechanisms" Processes 13, no. 8: 2504. https://doi.org/10.3390/pr13082504
APA StyleLi, T., Li, J., Li, Z., & Cheng, X. (2025). Mg/Fe Layered Double Hydroxide Modified Biochar for Synergistic Removal of Phosphate and Ammonia Nitrogen from Chicken Farm Wastewater: Adsorption Performance and Mechanisms. Processes, 13(8), 2504. https://doi.org/10.3390/pr13082504







