Abstract
With the widespread use of lithium-ion batteries in electric vehicles, energy storage systems, and portable electronic devices, concerns regarding their thermal runaway have escalated, raising significant safety issues. Despite advances in existing thermal management technologies, challenges remain in addressing the complexity and variability of battery thermal runaway. These challenges include the limited heat dissipation capability of passive thermal management, the high energy consumption of active thermal management, and the ongoing optimization of material improvement methods. This paper systematically examines the mechanisms through which three main triggers—mechanical abuse, thermal abuse, and electrical abuse—affect the thermal runaway of lithium-ion batteries. It also reviews the advantages and limitations of passive and active thermal management techniques, battery management systems, and material improvement strategies for enhancing the thermal stability of batteries. Additionally, a comparison of the principles, characteristics, and innovative examples of various thermal management technologies is provided in tabular form. The study aims to offer a theoretical foundation and practical guidance for optimizing lithium-ion battery thermal management technologies, thereby promoting their development for high-safety and high-reliability applications.
1. Introduction
The widespread use of lithium-ion batteries (LIBs) in electric vehicles, renewable energy storage, and portable electronic devices has increasingly drawn attention to their safety. LIBs are favored for their high energy density and long cycle life, but their safety concerns have also become more prominent due to the complex chemical reactions, physical processes, and environmental factors involved in their operation. In particular, thermal runaway is a major cause of catastrophic events such as fires and explosions under extreme conditions. Therefore, effectively preventing thermal runaway has become a critical issue in ensuring battery safety.
The causes of thermal runaway in LIBs are multifaceted, typically including mechanical, thermal, and electrical abuse. These factors can lead to a significant rise in internal battery temperature, triggering uncontrolled chemical reactions that may result in safety hazards. Mechanical abuse refers to external physical forces (e.g., compression, puncture, or collision) that damage the battery structure, thereby initiating thermal runaway. Thermal abuse occurs when the battery is exposed to high temperatures, accelerating internal chemical reactions, generating excess heat, and causing thermal runaway. Electrical abuse arises during charging and discharging processes, where excessive current or improper management leads to temperatures exceeding the safety threshold, which can also trigger thermal runaway. These runaway events can cause severe consequences, including damage to both the internal and external battery structure, electrolyte leakage, and gas release. Therefore, understanding the mechanisms behind thermal runaway under various conditions and developing effective prevention strategies are essential for enhancing the safety of LIBs.
To address these challenges, the design and optimization of thermal management systems for LIBs has become a prominent research area. By regulating the battery’s temperature, the thermal management system ensures safe operating conditions and enhances overall performance. Currently, thermal management methods for LIBs are categorized into two main types: passive and active. Passive thermal management methods regulate temperature by utilizing the thermophysical properties of materials in conjunction with natural heat transfer mechanisms (e.g., phase change materials, heat pipes, heat dissipation fins, etc.). In contrast, active thermal management methods directly cool the battery through external cooling systems, such as liquid or air cooling, to prevent overheating. Additionally, the BMS, as an intelligent monitoring technology, ensures safe operation by continuously monitoring key parameters such as temperature, voltage, and current, employing timely interventions to prevent thermal runaway. Furthermore, advancements in lithium-ion battery materials play a crucial role in improving thermal management performance. Recent research has focused on phase change materials, high thermal conductivity materials, nanoparticles, and porous foams to enhance the thermal stability of the battery by optimizing the thermal conductivity of the internal materials. Additionally, improvements in cathode and anode materials through coating and doping modifications significantly enhance both thermal and chemical stability, thereby reducing the risk of thermal runaway.
This review aims to examine the causes and mechanisms of thermal runaway in LIBs, with a particular focus on analyzing the effects of mechanical, thermal, and electrical abuse. It also reviews recent research on these topics. Section 1 will provide a detailed explanation of the mechanisms of thermal runaway triggered by these three forms of abuse and will assess the current state of research in this area. Section 2 will explore thermal management methods for LIBs, including passive and active thermal management, the application of BMSs, and recent advancements in materials designed to prevent thermal runaway. By analyzing these research developments, this review will offer scientific insights and technical support for improving the safety of LIBs, thus facilitating their broader adoption in various applications.
2. Analysis of the Causes of Thermal Runaway in Lithium Batteries
The root of thermal runaway in LIBs is a self-accelerating, exothermic chain reaction triggered by the disruption of the dynamic balance between heat generation and dissipation within the battery [1]. Its core evolutionary process follows a unified path: abnormal trigger → failure of key components → rapid increase in heat generation rate → critical temperature breach → uncontrollable outburst. Thermal runaway can be initiated by mechanical (e.g., extrusion, puncture), thermal (e.g., high ambient temperature, heat dissipation failure), or electrical (e.g., overcharging, external short-circuiting) abuse, each of which triggers the outburst through three primary mechanisms. The first is the destruction of structural integrity, such as mechanical force causing diaphragm rupture, thermal abuse leading to diaphragm melting, or electrical abuse causing lithium dendrite penetration into the diaphragm, ultimately forming an internal short circuit. The second mechanism is the exacerbation of electrochemical side reactions. When the temperature exceeds a threshold, the SEI membrane decomposes at 80–120 °C, the anode material releases oxygen and reacts violently with the electrolyte at 150 °C, and the electrolyte decomposes at 200 °C. The heat generated in each step can reach hundreds to thousands of joules per gram [2]. The third mechanism involves a positive feedback loop of sudden energy release. The energy released from the short-circuit current or violent chemical reactions further accelerates material degradation, creating a self-reinforcing cycle of increasing temperature → accelerating reaction → increasing heat production. This common mechanism provides a unified theoretical framework for analyzing thermal runaway across different abuse scenarios. The uniqueness of each causative agent is reflected in the variations in their triggering points and evolutionary pathways: mechanical abuse is driven by physical structural damage, thermal abuse by temperature-induced chemical decomposition, and electrical abuse by abnormal electrochemical processes.
2.1. Mechanical Abuse of LIBs
The origin of mechanical abuse lies in the direct damage to the battery’s internal structure caused by external physical stress, such as extrusion, puncture, or collision. This damage initiates a cascading sequence: stress loading → structural failure → electrical short circuit → thermal runaway [3]. When the mechanical load exceeds the material strength threshold, the diaphragm undergoes plastic deformation or fracture, causing direct contact between the positive and negative electrodes. This contact forms a low-impedance short-circuit channel, triggering an instantaneous high-current discharge and the accumulation of joule heat. Meanwhile, the electrode material experiences particle dislodgement or collector deformation under stress, which further exacerbates the rise in local electrical resistance and the formation of hot spots [4]. Through this process, mechanical stress disrupts the battery’s physical isolation barrier and the integrity of its conductive network, disrupting the internal balance between heat generation and dissipation, and ultimately triggering thermal runaway.
Extrusion and puncture are the primary causes of thermal runaway in Li-ion batteries [5], as illustrated in Figure 1. When the battery is subjected to external extrusion forces, its internal structure may undergo significant changes. Specifically, the rupture of the diaphragm due to extrusion directly triggers an internal short circuit, which in turn leads to a rapid release of energy [6], causing a sharp temperature rise that may ultimately result in thermal runaway. Chai et al. [7] developed a mechanical–electrical–thermal coupling model to predict the force, voltage, and temperature responses of batteries under different states of charge (SOC) and loading conditions. Their findings highlighted the critical role of the diaphragm in battery rupture, emphasizing the importance of its integrity and rupture resistance in preventing thermal runaway. This model provides a comprehensive framework for understanding the complex interactions between mechanical stress and thermal runaway, but it relies on accurate calibration of the model parameters, which can be challenging in practical applications. In contrast, Gao et al. [8] conducted experiments to observe internal micro-damage, such as diaphragm rupture and electrode cracks, in batteries subjected to charge/discharge cycling and capacity testing. They quantified the differences in capacity, voltage correlation coefficients, and transient internal resistance between batteries with varying levels of micro-short circuits and normal batteries. This study offers valuable insights into the micro-level damage mechanisms that can lead to thermal runaway, but it primarily focuses on the effects of charge/discharge cycles rather than on external mechanical forces. Wang et al. [9] further analyzed the effects of SOC and loading rate on the mechanical–electrical characteristics of batteries, revealing that diaphragm rupture characteristics significantly impact overall failure behavior. Their work complements the findings of Chai et al. [7] by providing experimental evidence of the relationship between mechanical stress and internal short circuits. However, their study also assumes a specific battery configuration, limiting the generalizability of the results to other battery types and conditions.
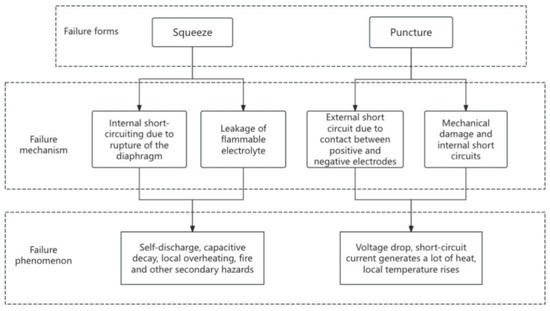
Figure 1.
Mechanical abuse—thermal runaway failure forms, mechanisms, and phenomena.
Penetration, as a more intense form of mechanical abuse [10], primarily leads to failure through external short circuits caused by the contact between the positive and negative electrodes, as well as mechanical damage and internal short circuits [11]. When a foreign object penetrates the battery, it causes direct contact between the positive and negative electrodes, forming an external short circuit. This generates a large amount of joule heat, rapidly increasing the local temperature and triggering a series of thermal runaway phenomena, such as electrolyte decomposition and combustion [12,13]. Chen et al. [14] employed the pinning test to initiate thermal runaway in a high-energy-density 21700-type cylindrical battery, investigating the effects of several parameters, including pinning diameter, pinning speed, penetration location, penetration depth, and SOC. Using high-definition video, thermal imaging, and X-ray CT, they revealed the mechanisms of thermal runaway and sidewall rupture. Additionally, An et al. [15] conducted three-point bending tests on cylindrical LIBs with varying SOCs, electrode thicknesses, and materials. They examined the mechanical response, voltage fluctuations, temperature changes, and microstructural alterations in the electrodes and diaphragm post-testing. Their results showed that when the SOC exceeded 60%, the battery experienced significant thermal runaway events, such as explosions and fire. These studies underscore the critical role of internal structural destruction during penetration in triggering thermal runaway.
To better understand and predict the thermal runaway behavior of LIBs under mechanical abuse, the study of coupled multi-physical field models has become a prominent research area. Li et al. [16] employed a three-dimensional, two-way coupled mechanical-electrochemical–thermal model to clarify the complex process from short circuit to thermal runaway, validating the model’s effectiveness in two common engineering scenarios: mechanical extrusion and pinning. The model predicted the mechanism of internal short circuit initiation and the detailed exothermic reaction process leading to thermal runaway. Li et al. [17] proposed an efficient multi-physics field coupling framework that integrates a 3D mechanical model, a 3D thermal model, a zero-dimensional battery electrical model, and a zero-dimensional short-circuit model, providing visualized simulation results for battery failure analysis.
Significant progress has been made in research focused on optimizing the structural and mechanical properties of batteries to enhance their resistance to mechanical abuse. Chai et al. [18] experimentally investigated the thermal runaway behavior and safety assessment of LiFePO4 batteries under mechanical abuse. The study involved mechanical abuse experiments conducted under various conditions and battery SOC to capture the force, voltage, and temperature responses during failure, as well as to extract the characteristic parameters of thermal runaway behavior. The mechanical abuse conditions were further quantified, and the relationship between experimental conditions and battery parameters was analyzed, culminating in the development of a regression model for defining the battery’s safety boundary and assessing the risk of thermal runaway. Wang et al. [19] reviewed experimental and numerical simulation studies on the mechanical abuse of LIBs, comparing responses under quasi-static and dynamic loading conditions, and discussing the strain-rate dependence of LIBs, along with the impact of mechanical abuse on battery capacity and impedance. Huang et al. [20] conducted a comprehensive investigation into the changes in the electrochemical properties, morphology, and thermal stability of commercial ternary/graphite Li-ion batteries subjected to slight mechanical deformation. The test cells were compressed from 4 mm to 6.8 mm and cycled 620 times. The results indicated that indentation enhanced the cycling performance of the test cells, although the effect became detrimental when the indentation depth exceeded 6.5 mm. Electrochemical impedance spectroscopy measurements revealed that indentation delayed the increase in impedance of the pressed cells during aging, likely due to the increased density of indentation, which attenuates side reactions within the cells.
2.2. Lithium Battery Thermal Abuse
The source of thermal abuse lies in a positive feedback loop of material decomposition and exothermic reactions triggered by the temperature threshold [21]. The process evolves in stages according to specific temperature intervals: When the temperature reaches 80–120 °C, the SEI membrane on the negative electrode begins to decompose, releasing active lithium for an oxidative reaction with the electrolyte [22,23]. As the temperature further increases to 150–200 °C [24,25], the positive electrode material releases oxygen, which reacts violently with the electrolyte, significantly increasing the rate of heat production. Once the temperature exceeds 200 °C, the electrolyte decomposes, and the polyolefin diaphragm melts and shrinks, causing direct contact between the positive and negative electrodes [26,27]. This creates a composite heat source from both “chemical exothermic reactions” and “electrical short-circuit heat generation” [28]. In this process, the temperature rise and exothermic reactions mutually accelerate each other, forming a self-perpetuating thermal runaway chain reaction [29], as shown in Figure 2.
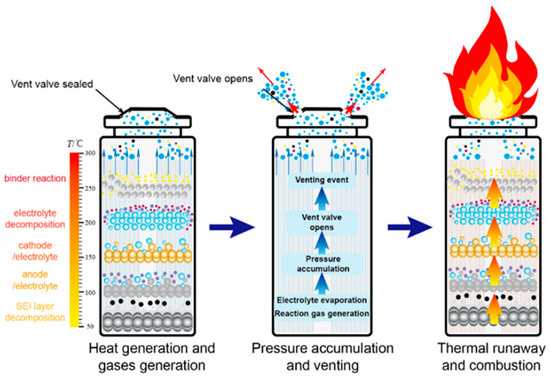
Figure 2.
Schematic diagram of venting, combustion, and thermal runaway mechanisms of lithium batteries under thermal abuse triggers.
The main reaction stages during the occurrence of thermal runaway are as follows:
SEI Film Decomposition: The SEI (solid electrolyte interphase) film is a passivation layer that forms on the surface of the negative electrode during the initial charging and discharging process of the battery. At elevated temperatures, the SEI film decomposes, generating heat, with a typical decomposition temperature range of 80–120 °C. The decomposition reaction of the SEI film can be represented as follows [30]:
Positive Electrode Reaction with Electrolyte: As the battery temperature rises to approximately 150 °C, the positive electrode material decomposes, releasing oxygen and reacting violently with the electrolyte, producing substantial heat [31]. The decomposition reaction of the positive electrode material can be represented as follows:
Electrolyte Decomposition: At temperatures exceeding 200 °C, the electrolyte itself decomposes, releasing additional heat and causing a sharp increase in internal battery pressure. This may result in phenomena such as bulging, valve spouting, and even explosions [32]. The decomposition reactions of the electrolyte can be represented as follows:
Separator Melting and Internal Short Circuit Formation: Due to the continuous reaction between the negative electrode and the electrolyte, the internal temperature of the battery continues to rise. When the temperature reaches the melting point of the separator, the separator melts and forms closed pores, significantly increasing the internal resistance of the battery. The melting points of commonly used PP and PE separators are around 165 °C and 130 °C, respectively, while the melting point of a PP/PE/PP composite separator is around 170 °C, and that of a separator with a ceramic coating is around 200 °C. As the temperature further increases, the separator collapses, and the positive and negative electrodes directly connect, forming an internal short circuit [33]. The internal short circuit generates a large amount of heat, which is a key link in thermal runaway.
Other Reactions: In addition to the above reactions, there are also other reactions occurring in the thermal runaway process, such as the decomposition of binders [34]. When the temperature exceeds 260 °C, fluorinated binders react with the intercalated lithium in the negative electrode to produce H2. As the thermal runaway progresses, the evaporation of the electrolyte inside the battery and the gas produced by side reactions lead to an increase in internal pressure. When the internal pressure reaches the designed threshold, the safety valve ruptures, and flammable gases are expelled. If ignited by an open flame, an explosion may even occur in a confined space.
Yang et al. [35] experimentally investigated the characteristics of SEI film decomposition under thermal abuse conditions and found that it is a key reaction in the early stages of thermal runaway. Negative electrode reaction with electrolyte: Following SEI film decomposition, the negative electrode is directly exposed to the electrolyte, initiating a reaction that further releases heat. Li et al. [36] highlighted that this reaction plays a significant driving role in the thermal runaway process. Positive electrode reaction with electrolyte: As the battery temperature rises to approximately 150 °C, the positive electrode material decomposes, releasing oxygen and reacting violently with the electrolyte, producing substantial heat [37]. Baakes, F. et al. [38] experimentally analyzed the behavior of the positive electrode material at high temperatures and found that its decomposition is a primary source of heat during thermal runaway. Electrolyte decomposition: At temperatures exceeding 200 °C, the electrolyte itself decomposes, releasing additional heat and causing a sharp increase in internal battery pressure. This may result in phenomena such as bulging, valve spouting, and even explosions. Ubaldi et al. [39] emphasized that electrolyte decomposition plays a crucial role in the later stages of thermal runaway.
Many researchers have conducted extensive studies on the thermal runaway characteristics of single-cell batteries under thermal abuse conditions, investigating the effects of various factors—such as battery type, capacity, SOC, and others—on the thermal runaway process through both experimental and simulation methods. Li et al. [40] found that the packing configuration significantly influences the propagation of thermal runaway, as demonstrated by comparing the behaviors of square and soft-packed batteries under thermal abuse conditions. This approach intuitively reveals the differences in thermal stability of different cell structures but does not delve into the intrinsic mechanisms of thermal runaway propagation. Salloum et al. [41] examined the internal short-circuit behavior of 18,650 batteries under thermal abuse conditions and identified a clear relationship between internal short circuits and thermal runaway. However, the experimental conditions are relatively homogeneous, failing to cover a variety of battery types and complex working conditions.
In practical applications, LIBs are typically used in modular form, making the study of thermal runaway propagation within modules highly significant [42]. Related studies have investigated the propagation mode, speed, and influencing factors within the module, such as the contact mode between cells, thermal insulation measures, and module structure [43]. Hu et al. [44] experimentally examined the effect of thermal insulation materials on thermal runaway propagation and found that such materials can significantly delay the propagation speed. In contrast, other studies employed numerical simulations to analyze the impact of heating position and module spacing on thermal runaway propagation, identifying these factors as key determinants of propagation behavior [45].
To mitigate thermal runaway and its propagation caused by thermal abuse, researchers have proposed several strategies [46]. On one hand, thermal safety is primarily enhanced by improving the thermal stability of battery materials, optimizing the structural design of batteries, and enhancing their heat dissipation capability [47]. For example, new cathode materials, diaphragm materials, and electrolyte additives have been developed to reduce the rate of side reactions at high temperatures. On the other hand, external thermal management systems [48], such as liquid cooling, air cooling, and phase change materials, are employed to effectively manage the heat generated by the battery module, ensuring timely heat distribution and preventing high temperatures from triggering thermal runaway. Additionally, some studies focus on incorporating thermal insulation materials and establishing fire barriers within the battery module to slow the propagation of thermal runaway, thereby allowing more time for fire rescue and accident response. Allen et al. [49] experimentally investigated the thermal runaway behavior of ruptured sidewalls and proposed a method to inhibit its propagation through material and structural design. Li et al. [50] developed a thermal runaway warning method based on online electrochemical impedance monitoring, which is able to issue warnings in advance and buy critical time for implementing protective measures, but the accuracy and reliability of the method still need to be verified in additional practical application scenarios.
2.3. Lithium Battery Electrical Abuse
Electrical abuse induces thermal runaway by disrupting the electrochemical equilibrium of the battery, with different types leading to distinct failure modes [51,52]. Under overcharging conditions, lithium dendrite deposition occurs on the negative electrode, penetrating the diaphragm, while excessive lithium delithiation on the positive electrode causes structural collapse and increased oxygen release [53,54]. In the case of overdischarge, lattice destruction in the negative electrode material leads to electrolyte reduction and decomposition [55,56]. An external short circuit generates a transient high current, causing a rapid local temperature increase due to the joule heating effect [57]. Regardless of the form, electrical abuse promotes lithium dendrite growth, electrode material degradation, or sudden energy release through electrochemical anomalies, disrupting the internal thermal balance and ultimately triggering thermal runaway.
The overcharging process in lithium battery abuse significantly influences the growth behavior of lithium dendrites under various SOC [58], as shown in Figure 3. Figure 3a illustrates the growth of lithium dendrites during the overcharging of lithium batteries at different SOC, ranging from 1.00 SOC to 1.85 SOC. As the SOC increases, the growth patterns of the dendrites show significant variations. The formation and extension of these dendrites are critical risk factors for thermal runaway caused by electrical abuse (overcharging) [59]. Dendrites can puncture the diaphragm, leading to an internal short circuit and accelerating thermal runaway [60]. Figure 3b presents SEM images of the anode after overcharging to 1.00 SOC and 1.85 SOC. At 1.00 SOC, the anode’s structure, including materials such as LMO and NCM, is recognizable [61]. However, at 1.85 SOC, the anode material shows noticeable degradation due to overcharging. In the case of electrical abuse (overcharging), the overcharging process not only promotes the abnormal growth of lithium dendrites on the anode but also degrades the anode’s microstructure and composition. These factors collectively compromise the battery’s thermal stability and increase the risk of thermal runaway [62]. Carla et al. [63] experimentally confirmed that these reactions release significant heat, further exacerbating the temperature rise and potentially leading to thermal runaway. Mei et al. [64] found that overcharging induces side reactions, such as the decomposition of the electrolyte and the oxidation of the positive electrode. The overcharging process also generates large amounts of heat, which may contribute to thermal runaway. Oxidation of materials further releases heat, worsening the temperature rise and ultimately resulting in thermal runaway.
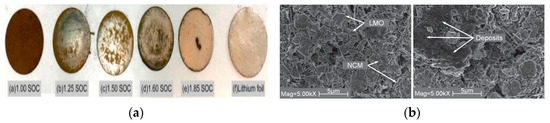
Figure 3.
(a) Lithium dendrite growth phenomenon when overcharging occurs in different SOC; (b) SEM images of the cathodes overcharged to 1.00SOC and 1.85SOC [65].
In recent years, both domestic and international scholars have conducted numerous studies on the thermal runaway behavior of LIBs under electrical abuse, with a primary focus on the development of experimental methods and simulation models. For instance, Ren et al. [65] investigated the overcharging behavior and failure mechanisms of Li-ion batteries under various test conditions. They found that overcharging leads to the growth of lithium dendrites and the decomposition of the electrolyte, which, in turn, triggers thermal runaway. This approach intuitively reveals the effect of overcharging on battery safety but does not delve into the interaction mechanism between lithium dendrite growth and electrolyte decomposition. Mei et al. [64] explored the lithium deposition behavior inside Li-ion batteries induced by overcharging, employing both experimental and numerical simulation methods. They discovered that the direction and morphology of the lithium dendrites significantly influence battery safety. This study provides an important basis for understanding the effect of lithium dendrite growth on thermal runaway, but the simulation results depend on the accuracy of the experimental conditions and do not adequately account for the complexity of the battery structure in practical applications. In experimental research, Owen et al. [66] used ultrasonic monitoring technology to track temperature changes inside LIBs in real time; ultrasonic technology was found to be effective for detecting and preventing thermal runaway. This method provides a new method for real-time monitoring of the internal state of the battery, but the accuracy and reliability of the ultrasonic monitoring technique still need to be verified in more practical application scenarios. Regarding simulation models, Li et al. [67] developed a dynamic compression-based safety performance assessment model for LIBs and verified thermal runaway behavior under different operating conditions through simulations. Jin et al. [68] used the gas capture technique to detect the growth of microscale lithium dendrites and found that early detection can effectively prevent thermal runaway.
3. Prevention of Thermal Runaway in LIBs
In the study of lithium-ion battery thermal runaway prevention, many scholars focus on enhancing the thermal stability and safety of batteries through various technical approaches. This paper systematically summarizes the principles, advantages, and limitations of several rapidly developed thermal management methods, as shown in Table 1. It then provides an in-depth analysis of these methods and reviews recent advancements, with the goal of offering a comprehensive reference for the study, design optimization, and engineering application of lithium-ion battery thermal management technologies.

Table 1.
Summary of battery thermal runaway prevention methods.
3.1. Thermal Management Technology
Thermal management is a key strategy for preventing thermal runaway in LIBs, and it is primarily divided into two categories: passive and active thermal management [69]. Passive thermal management controls battery temperature using the physical properties of materials [70], while active thermal management utilizes external devices and systems for temperature regulation [71].
3.1.1. Passive Thermal Management
Passive thermal management is a technology that efficiently absorbs, stores, and dissipates heat generated by LIBs, utilizing passive heat dissipation elements such as PCM and heat pipes, without requiring external energy input [72,73,74]. The core principle involves phase change materials’ ability to absorb or release substantial amounts of latent heat during phase transitions, effectively controlling battery temperature fluctuations [75]. For example, nano-enhanced phase change materials improve thermal conductivity by incorporating nanoparticles, and experimental studies have shown that these can effectively reduce battery temperature at high discharge rates [76]. Additionally, heat pipes, as efficient heat transfer devices, facilitate rapid heat transfer through the phase change circulation of the internal working fluid, thereby reducing the maximum surface temperature of the battery and improving temperature uniformity [77].
Numerous scholars have conducted in-depth studies on passive thermal management techniques, achieving significant progress in the field. Bahrami et al. [78] systematically investigated the enhancement of heat dissipation by adding a phase change material layer to the external surface of the battery in a microgravity environment, combined with the use of heat dissipation fins, nanoparticles, and porous foams. They found that optimizing the combination of these passive strategies, as shown in Figure 4, achieved the most effective thermal management under various working conditions. The fin spacing influences the temperature field distribution by regulating both the heat transfer path and convective heat transfer efficiency. The underlying mechanism of this phenomenon is that smaller fin spacing increases the heat dissipation area but can impede airflow, leading to the thickening of the thermal boundary layer. In contrast, larger fin spacing enhances heat dissipation uniformity by optimizing the convection path. This insight is crucial for optimizing the fin parameters in passive thermal management systems. For instance, in high-power discharge scenarios, a fin spacing of 2–3 mm effectively balances compactness with heat dissipation efficiency and can be combined with phase change materials for improved performance. Additionally, Song et al. [79] demonstrated that optimizing the flow rate and channel structure can significantly improve cooling efficiency in an immersion cooling system. This approach excels in improving heat transfer efficiency, but the complexity and high cost of immersion cooling systems may limit their use in practical applications. Feng Yi et al. [80] developed an L-shaped ultra-thin steam chamber that exhibited excellent thermal management performance under different tilt angles and vibration conditions.
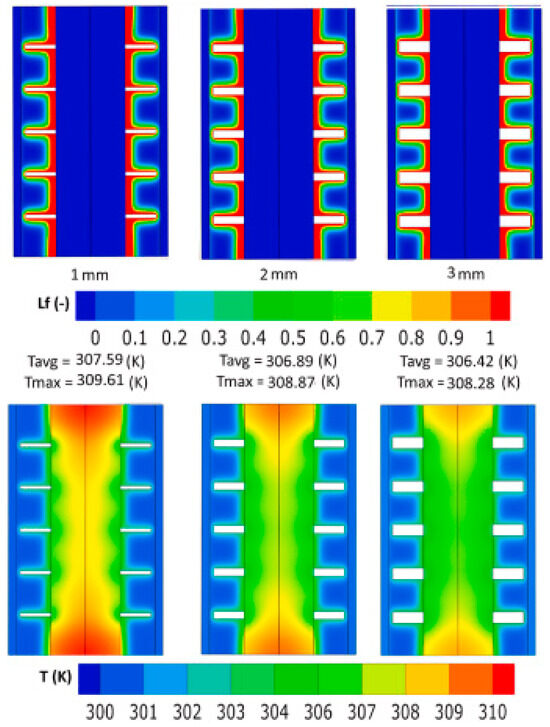
Figure 4.
Comparison of thermal management effect of batteries with different fin structure sizes [78].
The application of composite phase change materials has attracted significant attention. Li et al. [81] designed dual-network, aerogel-based composite phase change materials, as shown in Figure 5, which exhibit high energy density and excellent cycle durability, enabling all-weather thermal management of LIBs. In passive thermal management technology, the design of composite aerogels emphasizes the innovative use of porous materials for heat dissipation. The core mechanism involves the high thermal conductivity network of GNPs, which rapidly transfers heat away from localized hotspots. Meanwhile, the phase change thermal storage properties of hydrogels buffer temperature fluctuations, and the nanofiber skeleton prevents structural collapse of the aerogel at high temperatures. This combination provides a three-in-one solution—thermal conductivity, thermal storage, and structural support—for passive thermal management. The composite aerogel serves as an effective thermal conductor, particularly under extreme conditions where its intrinsic properties help prevent the spread of thermal runaway. MoraliU [82] demonstrated that the thickness of the phase change material significantly affects the thermal management system’s performance, with a 1 mm thick material effectively reducing battery temperature under high discharge conditions. However, the study did not fully consider the effect of material thickness on the overall weight and volume of the system. Additionally, Bian and Gao [83,84,85] developed a passive thermal management system combining heat pipes and phase change materials which demonstrated excellent thermal performance across varying discharge rates, significantly reducing the maximum temperature of the battery pack and improving temperature uniformity.
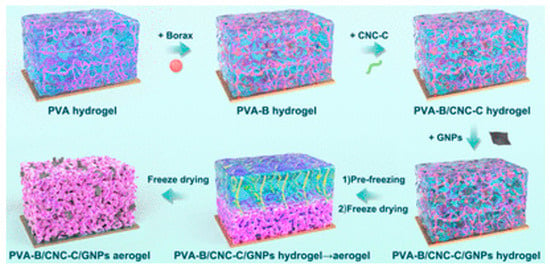
Figure 5.
Logic diagram for the design of PVA-B/CNC-C/GNPs composite aerogel [81].
Other emerging passive thermal management approaches are rapidly advancing. Zhao et al. [86] analyzed and optimized distributed liquid cooling panels to enhance the thermal management performance of cylindrical power batteries. Xiao et al. [87] numerically simulated and optimized a composite cooling strategy that combines phase change materials and cooling air to improve the heat dissipation performance of batteries in hot climates. Wang et al. [88] employed a multi-objective optimization method based on an optimal support vector machine to balance thermal performance and lightweight design in batteries.
However, passive thermal management systems display certain limitations. Lin et al. [89] led a study on the performance degradation of phase change materials under extreme operating conditions. Furthermore, improving the heat transfer performance of phase change materials and the overall thermal management efficiency of the system remains a significant challenge in current research. With ongoing advancements in materials science and thermal management technology, passive thermal management solutions are expected to offer more reliable and efficient thermal management for the large-scale application of LIBs.
3.1.2. Active Thermal Management
Active thermal management is a commonly used technology in the thermal management systems of LIBs. It regulates the battery’s temperature by consuming external energy, effectively maintaining the operating temperature within a predefined safe range [90]. The active thermal management system primarily relies on several key heat dissipation mechanisms: liquid cooling, which absorbs and radiates heat through the circulation of coolant in the battery pack [91], with the coolant flow driven by a water pump for rapid heat removal; air cooling, which uses a fan to circulate air and dissipate the heat generated by the battery [92]; and thermoelectric cooling, which leverages the semiconductor Peltier effect for directional heat transfer through the application of direct current [93]. A typical structure of the system is shown in Figure 6; the lithium battery is placed in a temperature control box, where a thermoelectric cooler directs heat transfer. A heater simulates various thermal load conditions, while a resistance, voltage, and current detection module monitors the system’s working state in real time. A thermocouple array collects temperature data, which is then analyzed by the system. The core mechanism of this structure involves actively consuming electricity to achieve precise temperature control, allowing for dynamic simulation of critical thermal runaway conditions. This setup provides an experimental platform for verifying the synergistic effect of the coupled thermoelectric system, assisted by liquid/air cooling strategies. These methods enhance heat transfer by actively consuming external energy, enabling precise control of the battery temperature.
Regarding the current research on active thermal management, numerous scholars and research institutes have conducted extensive studies. Ouyang et al. [94] proposed a battery thermal management system (BTMS) that combines pyrolytic graphite flakes, copper foam phase change material, and liquid cooling, experimentally verifying the system’s excellent cooling performance across various scenarios. They also explored the effects of copper foam phase change material, along with coolant flow rate and direction, on BTMS performance. This approach achieves efficient thermal management through a combination of cooling technologies, but the complexity and cost of the system are high. Maiorano, L.P. et al. [95] designed a novel low-porosity metal foam material (Guefoams), significantly enhancing heat transfer efficiency and offering a new approach for heat dissipation in high-performance electronic devices. However, the complexity of the preparation process of this material may limit its large-scale application. Hao et al. [96] conducted simulations to investigate the performance of micro-/minitubes in phase change cooling, focusing on pressure drop and integrated performance. They proposed an integrated performance factor that considers both pressure drop and heat transfer characteristics, optimizing tube structure parameters. Additionally, Venkatesh, R.J. et al. [97] introduced a hybrid active–passive thermal management strategy for prismatic LIBs, combining phase change materials and porous-filled microchannels. This strategy optimizes thermal management through large kernel attention graph convolutional networks (LKAGCN) and leaf in wind optimization algorithms (LWOA). He et al. [98] presented a method to improve thermal management and enhance the current capability of power devices under stochastic convection, maximizing short-term current capability by considering thermal inertia while preventing overheating. Zhang et al. [99] investigated air-cooled thermal management for LIBs used in energy storage, analyzing temperature variations in both single cells and battery packs at different currents, with numerical simulations conducted using ANSYS2022R1 Fluent software. Lucas et al. [100] explored the active thermal management of electric motors and generators using thermoelectric (Peltier effect) technology, demonstrating the potential of these solid-state devices to regulate motor temperature, reduce moisture intrusion, and assist with heat removal. This approach offers promising applications in thermal management of electric motors and generators, but the performance and cost of thermoelectric materials still need to be further optimized. Gao, Y. et al. [101] achieved active thermal management, controllable energy storage, and mechanical flexibility for supercapacitors by using microgel-enhanced, heat-sensitive hydrogel electrolytes. Waqas et al. [102] experimentally analyzed the impact of various thermal management methods on lithium-ion battery pack performance, finding that active cooling (air cooling) significantly reduces temperature differences within the packs. Xin et al. [103] studied the effect of active thermal management on grooved flat heat pipes under electrostatic fluid effects, discovering that electrostatic forces can enhance the heat and mass transfer process inside the heat pipe, improving its heat transfer capability and thermal conductivity. Other studies have explored technologies such as microchannel heat exchangers, thermal barrier coatings, and thermoelectric coolers, all of which exhibit significantly advanced thermal management efficiency, reduced energy consumption, and optimized system design.
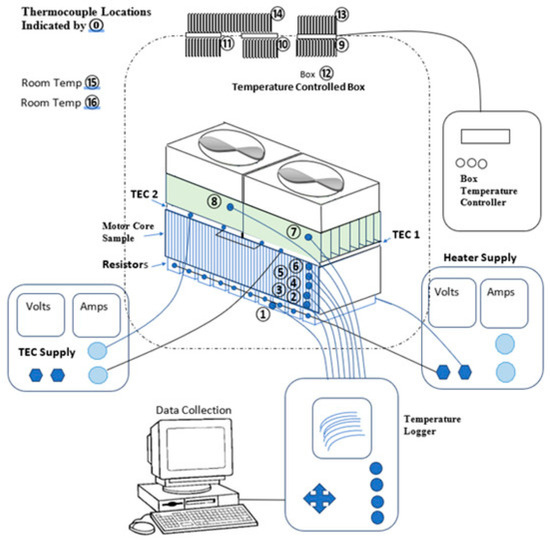
Figure 6.
Typical thermoelectric cooling structure of a lithium battery [100].
In summary, active thermal management technology plays a vital role and holds substantial potential in the thermal management of LIBs. By continuously optimizing active heat dissipation methods, such as liquid cooling, air cooling, and thermoelectric cooling, and integrating advanced thermal management system designs with intelligent control strategies, the safety, stability, and service life of LIBs can be significantly enhanced. This approach provides more reliable thermal management solutions for applications such as electric vehicles and energy storage systems.
3.2. Battery Management System
The BMS is a crucial component in a lithium-ion battery pack, ensuring that the battery operates safely and efficiently by monitoring and controlling its charging and discharging processes [104]. The primary functions of the BMS include monitoring the voltage, current, and temperature of the batteries, balancing the charge across each cell in the pack, and providing a protection mechanism in the event of abnormalities [105]. Its operation relies on precise battery state monitoring and control strategies powered by intelligent algorithms, with the overall system architecture depicted in Figure 7. By continuously collecting key battery parameters in real time, the BMS can accurately estimate the SOC and state of health (SOH) of the battery, adjusting the charging and discharging processes accordingly to optimize performance and extend battery life [106]. For instance, the BMS can employ incremental capacity analysis (ICA) and adaptive neuro-fuzzy inference systems (ANFIS) to improve SOH estimation accuracy [107]. Although both References [106,107] employ intelligent algorithms to improve the performance of the BMS, there are differences in the focus of their respective attention. Reference [106] focuses on improving the accuracy of SOH estimation through ICA and ANFIS, while Reference [107] further introduces GA to optimize the global search capability of the algorithm and prevent it from falling into local optimal solutions. This suggests that in the study of BMSs, different algorithms can be optimized for different performance metrics, but the complementarity between the algorithms must also be taken into account to achieve a more comprehensive performance improvement. Jiang et al. [108] further incorporated a genetic algorithm (GA) to address local minimum problems. Additionally, deep reinforcement learning-based strategies have been applied to optimize the energy and thermal management systems of hydrogen fuel cell trains, allow them to adapt to dynamic environmental changes and enhancing their energy conversion efficiency [109]. Moreover, Richard et al. [110] employed a complex hesitant fuzzy multi-criteria decision-making technique combined with the Einstein operator to manage uncertainty in battery energy storage systems, improving decision-making accuracy.
Recent research has focused extensively on optimizing and expanding the functionality of BMSs. The application of machine learning and deep learning algorithms in BMSs has emerged as a significant research area, enabling real-time analysis of battery data and fault diagnosis to enhance system intelligence [111]. Ofoegbu et al. [112] demonstrated that neural networks are highly effective in predicting the SOC with high accuracy and low time complexity. Additionally, Pinter [113] explored reconfigurable battery systems based on rule-based and model predictive control (MPC) algorithms to balance the SOC and SOH of the battery pack, ensuring system robustness and performance. In the realm of thermal management, BMS integration with other systems has been explored. For instance, Luo et al. [114] utilized a thermal management system incorporating thermoelectric cooling technology to optimize thermal performance by analyzing the thermal stress between the thermoelectric leg and the ceramic plate. Qin et al. [115] examined the impact of battery thermal characteristics on energy management strategies, employing deep reinforcement learning in a plug-in hybrid electric vehicle to control battery and motor temperatures within a safe range and enhance overall vehicle performance. Furthermore, Kumaresan et al. [116] applied an adaptive neuro-fuzzy inference system to optimize the power integration of batteries and supercapacitors in electric vehicles, addressing the limitations of conventional LIBs. Jiang et al. [117] proposed a deep reinforcement learning-based co-optimized energy and thermal management strategy for hydrogen fuel cell trains, enabling real-time balanced control of energy and thermal management.
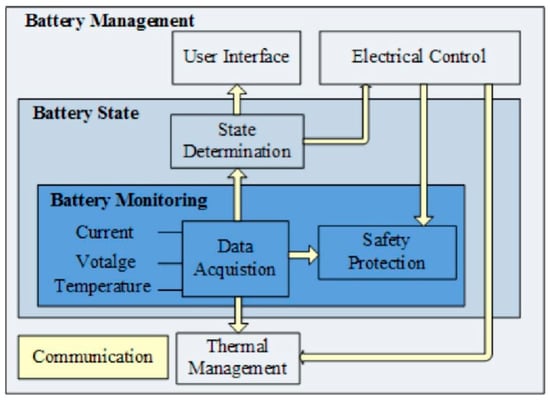
Figure 7.
Diagrammatic representation of the battery management system [112].
In conclusion, BMSs are essential for the thermal management and safe operation of LIBs. As the performance requirements for electric vehicles and energy storage systems continue to evolve, further advancements and innovations in BMS technology will be pivotal in ensuring the large-scale adoption of LIBs.
3.3. Material Improvements
Material improvement plays a crucial role in the thermal management and prevention of thermal runaway in LIBs [118]. The relationship between material improvement and prevention of thermal runaway of Li-ion batteries is shown in Figure 8. Enhancing the thermal conductivity and thermal capacity of materials can effectively reduce heat accumulation, thereby minimizing the risk of thermal runaway. Additionally, the use of highly efficient thermal isolation and regulation materials improves heat dissipation and equalizes internal temperatures, preventing localized overheating. Furthermore, functionalized materials, such as phase change materials and thermally conductive polymers, can buffer temperature fluctuations, further improving thermal management. Therefore, material enhancement is essential for ensuring the safety and performance of LIBs.
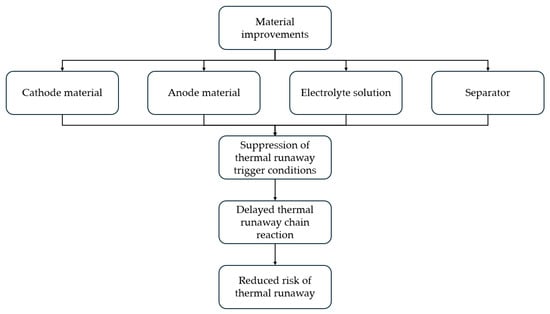
Figure 8.
Plot of material improvement versus prevention of thermal runaway in lithium batteries.
3.3.1. Cathode Material
The cathode material is a critical component of LIBs, with its performance directly influencing the energy density, cycle life, and safety of the battery. In the context of thermal management, optimizing cathode materials is essential for preventing thermal runaway [119]. Anode materials also undergo complex chemical reactions during charging and discharging; if uncontrolled, these reactions can lead to thermal runaway [120].
The thermal runaway triggered by positive electrode materials is primarily linked to their chemical stability at high temperatures [121]. For instance, during overcharging, overdischarging, or exposure to external short circuits and other abnormal conditions, the cathode material may decompose, releasing oxygen and other gases that react violently with other battery components, such as the electrolyte, generating significant heat. This heat causes a rapid increase in battery temperature, ultimately leading to thermal runaway. Furthermore, the interfacial reaction between the cathode material and electrolyte can also influence the thermal stability of the battery. Unstable interfacial reactions may result in issues such as increased interfacial resistance and localized overheating, thereby amplifying the risk of thermal runaway [122].
Surface cladding modification not only optimizes electrochemical cycling stability, but also enhances thermal safety by suppressing high-temperature interfacial side reactions. In recent years, significant advancements have been made in the research of cathode materials. Researchers have employed various strategies to enhance the performance of cathode materials and reduce the likelihood of thermal runaway. On one hand, surface coating and doping modifications have effectively improved the chemical and thermal stability of cathode materials. For example, the application of a cladding layer can prevent direct contact between the cathode material and electrolyte, thereby reducing interfacial side reactions and lowering the risk of thermal runaway under high-temperature conditions. On the other hand, the development of novel cathode materials offers new opportunities to enhance the thermal safety of batteries. However, these modification methods may affect the energy density and cycle life of the battery, and a balance needs to be found between performance and safety.
Wang et al. [123] demonstrated the use of highly crystalline covalent organic frameworks (COFs) as cathode materials, which significantly enhanced the stability and safety of batteries during charging and discharging. This approach focuses on structural modifications to improve thermal and chemical stability. In contrast, Kim et al. [124] developed an aqueous treatment solution for anode fabrication which mitigates interfacial side reactions and structural degradation in high-nickel anode materials. This method highlights the importance of surface treatments in enhancing thermal stability. Liu et al. [125] designed an optimized cathode–electrolyte interface (CEI) for high-voltage LIBs. This interface effectively regulates the electrode–electrolyte interfacial reaction, reduces irreversible phase transitions in cathode materials, and enhances the oxidative stability of the electrolyte, thereby improving the cycling stability and thermal safety of the battery.
In summary, optimizing cathode materials plays a crucial role in the thermal management of LIBs. Through strategies such as material modification, the development of new materials, and the enhancement of interface stability, the risk of thermal runaway caused by cathode materials can be significantly reduced, thereby improving the overall safety and reliability of the battery.
3.3.2. Anode Material
Anode materials are critical to the performance of LIBs, directly influencing energy density, cycle life, and safety [126]. In the context of thermal management, optimizing anode materials is essential to prevent thermal runaway [127]. During battery charging and discharging, anode materials undergo complex physical and chemical changes, which, if uncontrolled, may lead to thermal runaway [128].
The thermal runaway triggered by anode materials is primarily associated with their volume changes and interfacial reactions during charging and discharging [129]. For example, silicon (Si), a highly promising anode material, has garnered significant attention due to its high specific capacity. However, silicon undergoes substantial volume expansion during charging and discharging, which not only damages the electrode structure but also increases internal stresses, heightening the risk of localized overheating and thermal runaway. Furthermore, the interfacial reaction between the anode material and the electrolyte can influence the battery’s thermal stability. Unstable interfacial reactions may result in increased interfacial resistance and localized overheating, further exacerbating the risk of thermal runaway [130].
In recent years, significant progress has been made in the research of anode materials. Researchers have employed various strategies to optimize their performance and reduce the likelihood of thermal runaway. One approach involves enhancing the chemical and thermal stability of anode materials through surface coating and doping modifications. For example, Li et al. [131] investigated the effects of different sintering temperatures on the performance and structure of anode materials. By coating a layer of stabilized solid electrolyte on the anode material surface, they effectively isolated the anode from the electrolyte, reducing interfacial reactions and consequently, lowering the risk of thermal runaway under high-temperature conditions. Another strategy focuses on developing new anode materials to improve battery thermal safety. Li et al. [132] utilized waste photovoltaic silicon encapsulated in nanocages through electrospinning technology and introduced titanium dioxide and silver particles to create silicon–carbon nanofiber composites with excellent electrochemical properties. This design not only enhances the structural stability of silicon but also mitigates the volume expansion during charging and discharging, thus reducing the risk of thermal runaway.
Additionally, researchers have focused on developing anode materials with specialized structures to enhance their stability and safety during charging and discharging. Jiang et al. [133] synthesized C@Si composites by incorporating biomass carbon derived from the high-temperature carbonization of Chinese rose petals and embedding silicon nanoparticles into it. This composite material demonstrates excellent cycling stability and performance during charging and discharging, while effectively mitigating silicon volume changes and reducing the risk of thermal runaway. Xu et al. [134] studied a lithium–boron alloy with a three-dimensional skeleton structure as the anode material for solid-state lithium batteries. The unique structure of this material prevents the collapse of the structure during lithium ion de-intercalation, thus improving both cycling stability and thermal safety.
In conclusion, optimizing anode materials is a key focus of the research on thermal management of LIBs. By employing strategies such as material modification, development of new materials, and design of specialized structures, the risk of thermal runaway caused by anode materials can be significantly reduced, thereby enhancing the overall safety and reliability of the battery.
3.3.3. Electrolyte Solution
The performance of the electrolyte directly impacts the energy density, cycle life, and safety of batteries [135]. In the context of thermal management in LIBs, optimizing the electrolyte is crucial for preventing thermal runaway. The electrolyte not only serves as a medium for lithium-ion transport [136], but also participates in chemical reactions at the electrode interface. Its composition, stability, and compatibility are critical factors in preventing thermal runaway under abnormal conditions [137].
The principle of electrolyte-induced thermal runaway is primarily associated with its chemical stability and interfacial reactions [138]. In conventional liquid electrolytes, organic solvents such as carbonates possess volatile and flammable characteristics, which can easily trigger combustion and thermal runaway under abnormal conditions, such as high temperatures or short circuits within the battery. Furthermore, interfacial reactions between the electrolyte and electrode materials can lead to the formation of unstable SEI films. These films may rupture and repair during battery charging and discharging, resulting in irreversible capacity loss [139] and heat generation, further increasing the risk of thermal runaway.
In recent years, significant progress has been made in the optimization of electrolytes. Researchers have employed various strategies to enhance electrolyte performance and reduce the likelihood of triggering thermal runaway. On one hand, the development of solid-state electrolytes has offered a novel approach to improving battery safety. Zhu et al. [140] investigated the mechano–electrochemical coupling modes of sulfide- and halide-based solid-state electrolytes in all-solid-state Li-ion batteries through dynamic pressure modulation. Their findings revealed that dynamic pressure control can regulate the electrolyte’s creep behavior and maintain the stability of interfacial contact, thereby improving battery cycling performance and thermal stability. On the other hand, the preparation of composite polymer electrolytes has also provided new avenues for improving electrolyte performance. Ho et al. [141] developed ultra-stable composite polymer electrolytes by dispersing silane-modified TiO2 nanoparticles in a PEO matrix, which not only enhanced ionic conductivity but also exhibited a high oxidation potential and low overpotential, effectively reducing heat generation during the charging and discharging processes.
Additionally, researchers have focused on developing novel electrolyte systems to enhance their thermal and interfacial stability. Chen et al. [142] proposed a method for preparing fluorine-doped chloride electrolytes through a mixed liquid-phase synthesis, which significantly improved their oxidative and cycling stability. Wang et al. [143] designed a quasi-solid-state electrolyte by chemically grafting amphoteric sulfobetaine onto the surface of PVDF-HFP and LLZTO, thereby constructing a three-dimensional, continuous Li+ transport channel. This approach effectively reduced solid–solid interfacial resistance and decreased dependence on liquid electrolytes, ultimately improving the thermal safety of the battery. Meanwhile, gel polymer electrolytes (GPEs) have gained attention for their combination of the high ionic conductivity of liquid electrolytes and the structural integrity of solid-state polymer electrolytes. Xue et al. [144] conducted a comprehensive analysis of GPE technology, highlighting advancements in fabrication methods, advantages, and challenges, thereby offering valuable insights for future developments in GPE technology.
Researchers have made significant advancements in improving the thermal stability and safety of electrolytes. Liu et al. [145] developed self-healing quasi-solid electrolytes through in situ polymerization, using hydroxyethyl acrylate as a molecular bridge to link acrylate and polyurethane. This approach facilitated rapid Li+ transport and enhanced its self-healing ability, while also ensuring excellent solid-state electrolyte/electrode interfacial contact, effectively repairing interfacial defects and improving the thermal stability and cycle life of the battery. Additionally, ionic liquid electrolytes have gained attention due to their non-volatile and non-flammable properties. Santis et al. [146] proposed a novel ionic liquid electrolyte based on tetrabutylphosphonium and 1-ethyl-3-methylimidazole cations, combined with per-(fluoroalkylsulfonyl)imide anions, for high-temperature lithium-ion battery systems. This technology significantly enhances the safety and performance of batteries under high-temperature conditions.
In summary, the optimization of electrolytes is a critical area of research for the thermal management of LIBs. By developing new electrolyte materials, composite polymer electrolytes, and gel polymer electrolytes, as well as enhancing their thermal and interfacial stability, the risk of thermal runaway can be significantly reduced, thereby improving the battery’s overall safety and reliability.
3.3.4. Separator
The primary function of the diaphragm is to separate the positive and negative electrodes, preventing short circuits while allowing lithium ions to move freely during charging and discharging [147]. The diaphragm’s performance directly impacts the ion transfer efficiency, thermal stability, and safety of the battery [148]. In the context of thermal management in LIBs, optimizing the diaphragm is crucial for preventing thermal runaway [149].
The principle behind diaphragm-induced thermal runaway is primarily linked to its shrinkage behavior at high temperatures and its low lithium-ion transport number [150]. Conventional polyolefin diaphragms (e.g., polypropylene (PP) and polyethylene (PE)) are susceptible to shrinkage under high temperatures, which can result in short-circuiting within the battery, triggering thermal runaway. Moreover, the low lithium-ion transport number of polyolefin diaphragms can lead to uneven deposition of lithium ions on the electrode surface, promoting the formation of lithium dendrites. These dendrites can puncture the diaphragm, causing a short circuit and initiating thermal runaway [151].
In recent years, significant advancements have been made in diaphragm optimization research. Researchers have employed various strategies to enhance diaphragm performance, thereby reducing the likelihood of triggering thermal runaway. One approach involves improving the diaphragm’s thermal stability and lithium-ion transport performance through surface modification and functionalization. For example, Shao et al. [152] proposed a polyolefin diaphragm integrated with a Li2S@C sacrificial layer, which replenishes the lithium-ion stock via pre-lithiation treatment. Additionally, the lithium–nickel–cobalt–manganese oxide (LiNi0.8Co0.1Mn0.1O2) anode interface forms a lithium polysulfide-rich, high-voltage-tolerant interface, significantly enhancing the battery’s thermal stability and cycling performance. On the other hand, the development of new diaphragm materials provides additional avenues for improving battery safety. Chaykina et al. [153] reviewed various types of solid electrolytes (including oxides, sulfides, halides, polymers, and composites), with a particular focus on sulfide-based solid electrolytes. Their study highlights that sulfide-based materials offer the optimal balance between performance and processability.
Furthermore, researchers have focused on developing composite diaphragms with multifunctional properties to enhance the thermal stability and safety of batteries. He et al. [154] introduced a bilayer composite diaphragm by coating commercial polypropylene diaphragms with methoxyl-modified graphyne (OMe-GDY). This diaphragm not only exhibits excellent electronic and ionic conductivity but also promotes uniform lithium deposition, inhibits lithium dendrite growth, and significantly extends battery life. Vinci et al. [155] proposed a method for functionalizing a mono-ionic polymer electrolyte on the surface of a porous polyolefin diaphragm using wet-coating and ultraviolet cross-linking processes. This method significantly improves the diaphragm’s wettability, electrolyte uptake capacity, and ionic conductivity, thereby enhancing the interfacial resistance and cycling performance of the battery.
Researchers have achieved significant advancements in enhancing the thermal stability and mechanical strength of diaphragms. Yu et al. [156] proposed a dual in situ curing strategy that combines external UV curing with internal thermal curing to prepare a diaphragm-free gel polymer electrolyte. This approach not only improved the interfacial properties of the diaphragm on both the positive and negative sides, but also eliminated the need for a nonionic conductive diaphragm, effectively suppressed lithium dendrite growth, and enhanced both thermal stability and cycle life of the batteries. Liang et al. [157] developed a functionalized diaphragm by coating polyolefin diaphragms with nonconductive attapulgite (ATP) nanorods and conductive tungsten trioxide (WO3) spherical nanoparticles. This composite diaphragm not only improves the wettability and thermal stability of the electrolyte, but also regulates ion transport and promotes uniform lithium ion deposition.
In summary, diaphragm optimization plays a crucial role in the thermal management of LIBs. Through strategies such as surface modification, functionalization, the development of new diaphragm materials, and the preparation of multifunctional composite diaphragms, both thermal stability and lithium-ion transport performance can be significantly enhanced. These improvements reduce the risk of thermal runaway and enhance the overall safety and reliability of the battery.
4. Results
This paper systematically discusses the causes of thermal runaway of lithium batteries and its prevention methods, focusing on analyzing the influence mechanisms of three main factors, namely mechanical abuse, thermal abuse, and electrical abuse, on the thermal runaway of batteries, as well as the related research progress. In addition, this paper discusses the current status and development of thermal management methods for Li-ion batteries, including the application of passive thermal management, active thermal management, BMSs, and the role of improved materials in enhancing thermal management performance.
This paper first analyzes the thermal runaway mechanisms in lithium batteries triggered by mechanical, thermal, and electrical abuse. Mechanical abuse results in structural damage to the battery due to external physical forces, while thermal abuse involves runaway chemical reactions caused by excessive temperature. Electrical abuse primarily arises from temperature increases due to excessive current during charging and discharging processes. These factors significantly impact the thermal stability of LIBs, leading to abnormal internal reactions and the potential for thermal runaway, which presents safety risks. Analyzing these triggers provides theoretical support for designing more effective protective measures and thermal management systems. Secondly, this paper reviews the advancements in thermal management research for lithium batteries and highlights the characteristics and advantages of passive and active thermal management methods. Passive methods leverage the physical properties of materials, such as phase change materials and heat pipes, to dissipate heat. These methods are simple and cost-effective but exhibit limited heat dissipation capacity for high-power batteries. Active methods, on the other hand, employ external cooling systems, such as liquid and air cooling, to regulate temperature and maintain battery stability during high-power operations. Furthermore, the BMS, an intelligent monitoring technology, can effectively prevent thermal runaway by continuously monitoring battery parameters—such as temperature, voltage, and current—and adjusting the battery’s operating conditions in real time. Finally, this paper emphasizes the critical role of material improvements in thermal management. By optimizing the thermal conductivity and stability of materials within the battery, the overall thermal management performance can be substantially enhanced. Recent advancements in phase change materials, thermally conductive materials, and composite materials have demonstrated significant improvements in heat dissipation and thermal stability for batteries.
Despite advances in lithium battery thermal management technology, several challenges remain. As the energy density of batteries continues to increase and application requirements diversify, the complexity of thermal management escalates. Future research should focus on the following areas: first, further optimizing the thermal management system to enhance heat dissipation efficiency and reduce energy loss; second, exploring new high-performance materials to improve thermal stability and extend the cycle life of batteries; and third, strengthening research on the mechanisms of thermal runaway under various operating conditions to ensure a more comprehensive guarantee of safety for lithium batteries.
5. Discussion
The thermal management Li-ion batteries remains a challenging research area that urgently requires further theoretical investigation and technological innovation. Through multi-disciplinary collaboration, integrating advanced materials science, thermodynamics, and intelligent control technologies, a more robust foundation can be established for ensuring the safety, performance, and reliability of lithium batteries. This study offers valuable insights for the future optimization and innovation of lithium battery thermal management systems.
Funding
This research was funded by the National Natural Science Foundation of China (52107225), the Natural Science Foundation of Jiangsu Province (BK20210765), the Science and Technology Plan Project of Zhenjiang (CQ2022004), and the 2023 Suzhou Innovation Consortium Project “Suzhou Advanced Packaging Substrate Technology Innovation Consortium” (LHT202329).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
Author Wanlin Wang was employed by Vonergy Technology Limited (Zhenjiang). Author Weiran Jiang was employed by Farasis Energy USA, Inc. Authors Simeng Zhu and Qingliang Sheng were employed by AKM Electronic Technology (Suzhou) Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Duah, I.B.; Ming, X.Y.; Yuan, Y.L. Optimization of infrared-drying parameters for Ginkgo biloba L. seed and evaluation of product quality and bioactivity. Ind. Crops Prod. 2020, 160, 113108. [Google Scholar]
- Huang, C.; Zhu, H.; Ma, Y. Evaluation of lithium battery immersion thermal management using a novel pentaerythritol ester coolant. Energy 2023, 284, 129250. [Google Scholar] [CrossRef]
- Lin, S.; Zhou, L. Enhanced cooling design of serpentine mini-channel for optimizing energy consumption in battery thermal management. Therm. Sci. Eng. Prog. 2025, 62, 103625. [Google Scholar] [CrossRef]
- Xi, W.; Zhang, Q.; Cao, G.; Zheng, L.; Zhang, X. An experimental investigation into the thermal management of prismatic lithium battery utilizing liquid-vapor phase change cooling. Therm. Sci. 2025, 29, 427–439. [Google Scholar] [CrossRef]
- Zhu, X.; Liao, X.; Kang, S.; Sun, L.; Zhao, Y. Optimization design of lithium battery management system based on Z-F composite air cooling structure. J. Energy Storage 2024, 102, 114068. [Google Scholar] [CrossRef]
- Liu, H.; Han, X.; Fadiji, T.; Li, Z.; Ni, J. Prediction of the cracking susceptibility of tomato pericarp: Three-point bending simulation using an extended finite element method. Postharvest Biol. Technol. 2022, 187, 111876. [Google Scholar] [CrossRef]
- Chai, Z.; Liu, Z.; Xue, Q.; Xiao, Y.; Tan, P.; Qiu, M. Efficient coupled mechanical-electrical-thermal modeling and safety assessment of lithium-ion battery under mechanical abuse. J. Energy Storage 2025, 114, 115917. [Google Scholar] [CrossRef]
- Gao, R.; Liang, H.; Zhang, Y.; Zhao, H.; Chen, Z. Characterization of lithium-ion batteries after suffering micro short circuit induced by mechanical stress abuse. Appl. Energy 2024, 374, 123931. [Google Scholar] [CrossRef]
- Wang, C.; Wang, R.; Zhang, C.; Yu, Q. Coupling effect of state of charge and loading rate on internal short circuit of lithium-ion batteries induced by mechanical abuse. Appl. Energy 2024, 375, 124138. [Google Scholar] [CrossRef]
- Du, Z.; Hu, Y.; Buttar, N.A. Analysis of mechanical properties for tea stem using grey relational analysis coupled with multiple linear regression. Sci. Hortic. 2020, 260, 108886. [Google Scholar] [CrossRef]
- Faheem, M.; Liu, J.; Chang, G.; Abbas, I.; Xie, B.; Shan, Z.; Yang, K. Experimental Research on Grape Cluster Vibration Signals during Transportation and Placing for Harvest and Post-Harvest Handling. Agriculture 2021, 11, 902. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Hu, J.; Xu, G.; Liu, W.; Ma, G.; Ding, Q.; He, R. Quantitative Evaluation of Post-Tillage Soil Structure Based on Close-Range Photogrammetry. Agriculture 2024, 14, 2124. [Google Scholar] [CrossRef]
- Tang, Z.; Li, Y.; Zhang, B.; Wang, M.; Li, Y. Controlling Rice Leaf Breaking Force by Temperature and Moisture Content to Reduce Breakage. Agronomy 2020, 10, 628. [Google Scholar] [CrossRef]
- Chen, H.; Kalamaras, E.; Abaza, A.; Tripathy, Y.; Page, J.; Barai, A. Comprehensive analysis of thermal runaway and rupture of lithium-ion batteries under mechanical abuse conditions. Appl. Energy 2023, 349, 121610. [Google Scholar] [CrossRef]
- An, Z.; Shi, T.; Du, X.; An, X.; Zhang, D.; Bai, J. Experimental study on the internal short circuit and failure mechanism of lithium-ion batteries under mechanical abuse conditions. J. Energy Storage 2024, 89, 111819. [Google Scholar] [CrossRef]
- Li, H.; Zhou, D.; Zhang, M.; Liu, B.; Zhang, C. Multi-field interpretation of internal short circuit and thermal runaway behavior for lithium-ion batteries under mechanical abuse. Energy 2023, 263, 126027. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Lin, C.; Zuo, F. High-efficiency multiphysics coupling framework for cylindrical lithium-ion battery under mechanical abuse. J. Clean. Prod. 2021, 286, 125451. [Google Scholar]
- Chai, Z.; Li, J.; Liu, Z.; Liu, Z.; Jin, X. Experimental analysis and safety assessment of thermal runaway behavior in lithium iron phosphate batteries under mechanical abuse. Sci. Rep. 2024, 14, 8673. [Google Scholar] [CrossRef]
- Wang, G.; Guo, X.; Chen, J.; Han, P.; Su, Q.; Guo, M.; Wang, B.; Song, H. Safety Performance and Failure Criteria of Lithium-Ion Batteries under Mechanical Abuse. Energies 2023, 16, 6346. [Google Scholar] [CrossRef]
- Huang, P.; Liu, S.; Ma, J.; Zheng, G.; Li, E.; Wei, M.; Wang, Q.; Bai, Z. Comprehensive investigation on the durability and safety performances of lithium-ion batteries under slight mechanical deformation. J. Energy Storage 2023, 66, 107450. [Google Scholar] [CrossRef]
- Irina, V.-G.; Adrian, P.; Dayana, C.; Alexis, D.; Karla, V.; Isabel, B.; Genoveva, G.-A.; Hugo, R.-M.; Maurizio, B.; Francesca, G. Effect of thermal liquefaction on quality, chemical composition and antibiofilm activity against multiresistant human pathogens of crystallized eucalyptus honey. Food Chem. 2021, 365, 130519. [Google Scholar] [CrossRef]
- Zhang, J.; Yagoub, A.E.A.; Sun, Y.; Arun, M.S.; Ma, H.; Zhou, C. Role of thermal and non-thermal drying techniques on drying kinetics and the physicochemical properties of shiitake mushroom. J. Sci. Food Agric. 2021, 102, 214–222. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X. Thermal and non-thermal processing affect Maillard reaction products, flavor, and phytochemical profiles of Ginkgo biloba seed. Food Biosci. 2021, 41, 101044. [Google Scholar] [CrossRef]
- Gong, C.; Li, Y.; Gao, R. Preservation of sturgeon using a photodynamic non-thermal disinfection technology mediated by curcumin. Food Biosci. 2020, 36, 100594. [Google Scholar] [CrossRef]
- Khan, R.A.; Salam, A.; Li, G. Nanoparticles and their crosstalk with stress mitigators: A novel approach towards abiotic stress tolerance in agricultural systems. Crop J. 2024, 12, 1280–1298. [Google Scholar] [CrossRef]
- Ji, W.; Qian, Z.; Xu, B. Grasping damage analysis of apple by end-effector in harvesting robot. J. Food Process Eng. 2017, 40, e12589.1. [Google Scholar] [CrossRef]
- Su, D.; Sun, W.; Li, B.; Yang, Y.; Wang, Y.; Lv, W.; Li, D.; Wang, L. Influence of Ultrasonic Pretreatments on Microwave Hot-air Flow Rolling Drying Mechanism, Thermal Characteristics and Rehydration Dynamics of Pleurotus eryngii. J. Sci. Food Agric. 2021, 102, 2100–2109. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yan, M.; Naeem, M.; Chen, M.; Chen, Y.; Ni, Z.; Chen, H. Enhancing Manganese Peroxidase: Innovations in Genetic Modification, Screening Processes, and Sustainable Agricultural Applications. J. Agric. Food Chem. 2024, 72, 26040–26056. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, Y.; Okoye, C.O.; Gao, L.; Chen, X.; Wang, Y.; Jiang, J. Fermentation profile and bioactive component retention in honeysuckle residue silages inoculated with lactic acid bacteria: A promising feed additive for sustainable agriculture. Ind. Crops Prod. 2025, 224, 120315. [Google Scholar] [CrossRef]
- Zhou, Z.; Fu, Z.; Zhang, L.; Zhao, D.; Yu, S.; Chen, Q. Energy management strategy for low hydrogen consumption in hybrid power systems consisting of dual fuel cells and a single lithium battery. Int. J. Green Energy 2024, 21, 2441–2456. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, H.; Xie, M.; Li, B. Design of energy management for composite energy storage system consisting of lithium battery and flywheel based on adaptive wavelet–fuzzy control strategy. J. Braz. Soc. Mech. Sci. Eng. 2024, 46, 434. [Google Scholar] [CrossRef]
- An, Z.; Gao, W.; Zhang, J.; Liu, H.; Gao, Z. Enhancing heat dissipation of thermal management system utilizing modular dual bionic cold plates for prismatic lithium batteries. J. Energy Storage 2024, 87, 111541. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Cheng, D.; Mao, J.; Zhang, K. Numerical investigation and parameter optimization on a rib-grooved liquid-cooled plate for lithium battery thermal management system. J. Energy Storage 2024, 85, 111085. [Google Scholar] [CrossRef]
- Yu, X.; Tao, Y.; Deng, Q. Experimental study on thermal management of batteries based on the coupling of metal foam-paraffin composite phase change materials and air cooling. J. Energy Storage 2024, 84, 110891. [Google Scholar] [CrossRef]
- Yang, T.; Xu, H.; Xie, C.; Xu, L.; Liu, M.; Chen, L.; Xin, Q.; Zeng, J.; Zhang, H.; Xiao, J. A Thermal Runaway Protection Strategy for Prismatic Lithium-Ion Battery Modules Based on Phase Change and Thermal Decomposition of Sodium Acetate Trihydrate. Batteries 2025, 11, 198. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Li, X.; Sun, T.; Li, Q. Investigation of Thermal Runaway in Prismatic Batteries with Dual-Parallel Jelly-Roll Architecture Under Thermal Abuse Conditions. Batteries 2025, 11, 196. [Google Scholar] [CrossRef]
- Osae, R.; Essilfie, G.; Alolga, R.N.; Akaba, S.; Song, X.; Owusu-Ansah, P.; Zhou, C. Application of non-thermal pretreatment techniques on agricultural products prior to drying: A review. J. Sci. Food Agric. 2020, 100, 2585–2599. [Google Scholar] [CrossRef]
- Baakes, F.; Song, R.; Bernet, T.; de Leon, J.V.G.; Jackson, G.; Adjiman, C.S.; Galindo, A.; Krewer, U. Pressure evolution and gas solubility of Li-ion battery electrolytes during thermal abuse conditions. J. Power Sources 2025, 640, 236619. [Google Scholar] [CrossRef]
- Ubaldi, S.; Russo, P. Toxicity Assessment of Gas, Solid and Liquid Emissions from Li-Ion Cells of Different Chemistry Subjected to Thermal Abuse. Energies 2024, 17, 4402. [Google Scholar] [CrossRef]
- Li, K.; Gao, X.; Wang, S.; Peng, S.; Zhang, W.; Wu, W.; Wang, H.; Liu, P.; Han, X.; Cao, Y.C.; et al. Comparative analysis of multidimensional signals evolution in prismatic and pouch LiFePO4 batteries under thermal abuse. Appl. Energy 2024, 372, 123818. [Google Scholar] [CrossRef]
- Salloum, R.; Rabuel, F.; Abada, S.; Morcrette, M. Investigating the internal short-circuit in 18650 cells under thermal abuse conditions. J. Power Sources 2025, 628, 235905. [Google Scholar] [CrossRef]
- Xie, F.; Guo, Z.; Li, T.; Feng, Q.; Zhao, C. Dynamic Task Planning for Multi-Arm Harvesting Robots Under Multiple Constraints Using Deep Reinforcement Learning. Horticulturae 2025, 11, 88. [Google Scholar] [CrossRef]
- Wei, W.; Xiaolan, L.; Zhongyi, Y. Parameter Optimization of Reciprocating Cutter for Chinese Little Greens Based on Finite Element Simulation and Experiment. Agriculture 2022, 12, 2131. [Google Scholar] [CrossRef]
- Hu, X.; Gao, F.; Xiao, Y.; Yang, Y. Passive Thermal Control Strategies for Lithium-Ion Batteries under Thermal Abuse Conditions: An Experimental Study on Thermal Insulation Efficacy. J. Energy Eng. 2025, 151, 04025028. [Google Scholar] [CrossRef]
- Abhilash, K.; Jadhav, A.; Kalamkar, V.R.; Jilte, R.D. Numerical study on thermal runaway in a cell and battery pack at critical heating conditions with variation in heating powers. J. Energy Storage 2024, 90, 111813. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, P.; Li, Z.; Li, J.; Tchuenvou-Magaia, F.; Ni, J. Thermo-biomechanical coupling analysis for preventing tomato fruit cracking during ripening. J. Food Eng. 2023, 341, 111336. [Google Scholar] [CrossRef]
- Chen, K.; Li, T.; Yan, T.; Xie, F.; Feng, Q.; Zhu, Q.; Zhao, C. A Soft Gripper Design for Apple Harvesting with Force Feedback and Fruit Slip Detection. Agriculture 2022, 12, 1802. [Google Scholar] [CrossRef]
- Qiu, S.; Yu, Y.; Feng, Y.; Tang, Z.; Cui, Q.; Yuan, X. Crushing Characteristics of Sorghum Grains Subjected to Compression and Impact Loading at Different Moisture Contents. Agriculture 2022, 12, 1422. [Google Scholar] [CrossRef]
- Allen, J.P.C.; Jones, S.; Marco, J. A deterministic approach to achieving repeatable sidewall rupture failure modes within lithium-ion thermal abuse studies. J. Energy Storage 2025, 105, 114706. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, L.; Zhang, N.; Wei, Z.; Mei, W.; Duan, Q.; Sun, J.; Wang, Q. Early warning method for thermal runaway of lithium-ion batteries under thermal abuse condition based on online electrochemical impedance monitoring. J. Energy Chem. 2024, 92, 74–86. [Google Scholar] [CrossRef]
- Xu, J.; Wang, B.; Wang, Y.; Zhang, M.; Chitrakar, B. Effect of Applied Voltage on the Aggregation and Conformational Changes in Peroxidase Under Electrospray. Food Bioprocess Technol. 2020, 13, 245–255. [Google Scholar] [CrossRef]
- Tang, L.; Syed, A.u.A.; Otho, A.R.; Junejo, A.R.; Tunio, M.H.; Hao, L.; Ali, M.N.H.A.; Brohi, S.A.; Otho, S.A.; Channa, J.A. Intelligent Rapid Asexual Propagation Technology—A Novel Aeroponics Propagation Approach. Agronomy 2024, 14, 2289. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Liu, F.; Zou, X.; Xu, Y.; Xu, X. A smart-phone-based electrochemical platform with programmable solid-state-microwave flow digestion for determination of heavy metals in liquid food. Food Chem. 2020, 303, 125378. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, H.T.; Jianmin, G.; Mashooque, A.T.; Imran, A.L.; Farman, A.C.; Sher, A.S.; Kashif, A.S. Effects of different irrigation frequencies and incorporation of rice straw on yield and water productivity of wheat crop. Int. J. Agric. Biol. Eng. 2020, 13, 138–145. [Google Scholar] [CrossRef]
- Zhou, M.; Shan, Y.; Xue, X.; Yin, D. Theoretical analysis and development of a mechanism with punching device for transplanting potted vegetable seedlings. Int. J. Agric. Biol. Eng. 2020, 13, 85–92. [Google Scholar] [CrossRef]
- Xia, J.; Liu, F.; Yan, L.; Suo, H.; Qian, J.; Zou, B. Simultaneous determination of tert-butylhydroquinone, butylated hydroxyanisole and phenol in plant oil by metalloporphyrin-based covalent organic framework electrochemical sensor. J. Food Compos. Anal. 2023, 122, 105486. [Google Scholar]
- Zhu, C.; Liu, D.; Li, Y.; Chen, T.; You, T. Label-free ratiometric homogeneous electrochemical aptasensor based on hybridization chain reaction for facile and rapid detection of aflatoxin B1 in cereal crops. Food Chem. 2022, 373, 131443. [Google Scholar] [CrossRef]
- Cui, B.; Cui, X.; Wei, X.; Zhu, Y.; Ma, Z.; Zhao, Y.; Liu, Y. Design and Testing of a Tractor Automatic Navigation System Based on Dynamic Path Search and a Fuzzy Stanley Model. Agriculture 2024, 14, 2136. [Google Scholar] [CrossRef]
- Yu, R.; Wu, Y.; Xing, D. The Differential Response of Intracellular Water Metabolism Derived from Intrinsic Electrophysiological Information in Morus alba L. and Broussonetia papyrifera (L.) Vent. Subjected to Water Shortage. Horticulturae 2022, 8, 182. [Google Scholar] [CrossRef]
- Zheng, S.; Zhao, X.; Fu, H.; Tan, H.; Zhai, C.; Chen, L. Design and Experimental Evaluation of a Smart Intra-Row Weed Control System for Open-Field Cabbage. Agronomy 2025, 15, 112. [Google Scholar] [CrossRef]
- Srinivasan, R.; Demirev, P.A.; Carkhuff, B.G. Rapid monitoring of impedance phase shifts in lithium-ion batteries for hazard prevention. J. Power Sources 2018, 405, 30–36. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, X.; Zhang, C.; Shi, J.; Huang, X.; Li, Z.; Zou, X.; Gong, Y.; Holmes, M.; Povey, M.; et al. Agar/TiO2/radish anthocyanin/neem essential oil bionanocomposite bilayer films with improved bioactive capability and electrochemical writing property for banana preservation. Food Hydrocoll. 2022, 123, 107187. [Google Scholar] [CrossRef]
- Carla, M.; Stefano, C.; Vincenzo, S.; Livia, D.S.; Roberto, B. Experimental Investigation of Overdischarge Effects on Commercial Li-Ion Cells. Energies 2022, 15, 8440. [Google Scholar] [CrossRef]
- Mei, W.; Zhang, L.; Sun, J.; Wang, Q. Experimental and numerical methods to investigate the overcharge caused lithium plating for lithium ion battery. Energy Storage Mater. 2020, 32, 91–104. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Lu, L.; He, X.; Ouyang, M. Overcharge behaviors and failure mechanism of lithium-ion batteries under different test conditions. Appl. Energy 2019, 250, 323–332. [Google Scholar] [CrossRef]
- Owen, R.E.; Wiśniewska, E.; Braglia, M.; Stocker, R.; Shearing, P.R.; Brett, D.J.L.; Robinson, J.B. Operando Ultrasonic Monitoring of the Internal Temperature of Lithium-ion Batteries for the Detection and Prevention of Thermal Runaway. J. Electrochem. Soc. 2024, 171, 040525. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Lin, C.; Yang, X.; Zuo, F. A Safety Performance Estimation Model of Lithium-ion Batteries for Electric Vehicles under Dynamic Compression. Energy 2020, 215, 119050. [Google Scholar]
- Jin, Y.; Zheng, Z.; Wei, D.; Jiang, X.; Lu, H.; Sun, L.; Tao, F.; Guo, D.; Liu, Y.; Gao, J.; et al. Detection of Micro-Scale Li Dendrite via H2 Gas Capture for Early Safety Warning. Joule 2020, 4, 1714–1729. [Google Scholar] [CrossRef]
- Owusu-Ansah, P.; Yu, X.; Osae, R.; Mustapha, A.T.; Zhang, R.; Zhou, C. Inactivation of Bacillus cereus from pork by thermal, non-thermal and single-frequency/multi-frequency thermosonication: Modelling and effects on physicochemical properties. LWT-Food Sci. Technol. 2020, 133, 109939. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.; Wang, D.; Sun, Z.; Du, L.; Wang, D. The synergistic effects of phenyllactic acid and slightly acid electrolyzed water to effectively inactivate Klebsiella oxytoca planktonic and biofilm cells. Food Control 2021, 125, 107804. [Google Scholar] [CrossRef]
- Wang, L.; Tian, H.; Liu, W.; Zheng, H.; Wu, H.; Guan, Y.; Lu, Q.; Lv, Z. Effects of EPS-producing Leuconostoc mesenteroides XR1 on texture, rheological properties, microstructure and volatile flavor of fermented milk. Food Biosci. 2023, 56, 103371. [Google Scholar] [CrossRef]
- Wu, X.; Wu, C.; Bian, Z.; Ye, Z.; Meng, L.; Xia, L.; Bao, E.; Cao, K. Abscisic acid and reactive oxygen species were involved in slightly acidic electrolyzed water-promoted seed germination in watermelon. Sci. Hortic. 2022, 291, 110581. [Google Scholar] [CrossRef]
- Taha, M.F.; Mao, H.; Mousa, S.; Zhou, L.; Wang, Y.; Elmasry, G.; Rejaie, S.A.; Elwakeel, A.E.; Wei, Y.; Qiu, Z. Deep Learning-Enabled Dynamic Model for Nutrient Status Detection of Aquaponically Grown Plants. Agronomy 2024, 14, 2290. [Google Scholar] [CrossRef]
- Li, Z.; Guo, D.; Li, X.; Tang, Z.; Ling, X.; Zhou, T.; Zhang, B. Heat-Moisture Treatment Further Reduces In Vitro Digestibility and Enhances Resistant Starch Content of a High-Resistant Starch and Low-Glutelin Rice. Foods 2021, 10, 2562. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Q.; Belwal, T.; Lin, X.; Luo, Z. Insights into chemometric algorithms for quality attributes and hazards detection in foodstuffs using Raman/surface enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2476–2507. [Google Scholar] [CrossRef]
- Li, A.; Wang, C.; Ji, T.; Wang, Q.; Zhang, T. D3-YOLOv10: Improved YOLOv10-Based Lightweight Tomato Detection Algorithm Under Facility Scenario. Agriculture 2024, 14, 2268. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, X.; Zou, X.; Shi, J.; Huang, X.; Li, Z.; Gong, Y.; Holmes, M.; Povey, M.; Xiao, J. Bilayer pH-sensitive colorimetric films with light-blocking ability and electrochemical writing property: Application in monitoring crucian spoilage in smart packaging. Food Chem. 2021, 336, 127634. [Google Scholar] [CrossRef]
- Bahrami, H.R.; Ghaedi, M. Strategies for passive thermal management of lithium-ion batteries in microgravity: Combining PCMs, metal foams, fins, and nanoparticles. Case Stud. Therm. Eng. 2025, 71, 106247. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Jia, D.; Yuan, D.; Chang, Y.; Zheng, X.; Zhang, S. Effect of immersion cooling design optimization on thermal management for lithium battery module. Appl. Therm. Eng. 2025, 272, 126401. [Google Scholar] [CrossRef]
- Yi, F.; Gan, Y.; Li, R. Thermal performance analysis of L-shaped ultra-thin vapor chamber for lithium battery thermal management considering tilt angle and vibration. Energy 2025, 320, 135477. [Google Scholar] [CrossRef]
- Li, Z.; Hao, Y.; Cao, F.; Zhang, Y.; Wang, Y.; Zhang, S.; Tang, B. Double-Network Aerogel-Based Composite Phase Change Material Inspired by Beaver Damming for All-Weather Thermal Management of Lithium Batteries. ACS Appl. Mater. Interfaces 2025, 17, 16072–16084. [Google Scholar] [CrossRef]
- Morali, U. Effect of Thickness on Performance of Thermal Management System for a Prismatic Lithium-Ion Battery Using Phase Change Material. Energy Storage 2025, 7, e70135. [Google Scholar] [CrossRef]
- Bian, X.; Tao, H.; Li, Y.; Chu, Z.; Bai, X.; Xian, Y.; Yang, L.; Zhang, Z. Heat pipe/phase change material passive thermal management of power battery packs under different driving modes. Appl. Therm. Eng. 2024, 248, 123172. [Google Scholar] [CrossRef]
- Gao, C.; Sun, K.; Song, K.; Zhang, K.; Hou, Q. Performance improvement of a thermal management system for Lithium-ion power battery pack by the combination of phase change material and heat pipe. J. Energy Storage 2024, 82, 110512. [Google Scholar] [CrossRef]
- Sutheesh, P.M.; Nichit, R.B.; Rohinikumar, B. Numerical investigation of thermal management of lithium ion battery pack with nano-enhanced phase change material and heat pipe. J. Energy Storage 2024, 77, 109972. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, T.; Wang, C.; Tang, Y.; Xi, H. Analysis and optimization of thermal management system for cylindrical power battery based on distributed liquid cooling plates. Sci. China Technol. Sci. 2024, 67, 3695–3706. [Google Scholar] [CrossRef]
- Xiao, H.; E, J.; Tian, S.; Huang, Y.; Song, X. Effect of composite cooling strategy including phase change material and liquid cooling on the thermal safety performance of a lithium-ion battery pack under thermal runaway propagation. Energy 2024, 295, 131093. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Guo, P.; Hu, X.; Zhu, J.; Yu, T. Multi-objective optimization of phase change cooling battery module based on optimal support vector machineoptimal support Vector Machine. Appl. Therm. Eng. 2024, 236, 121386. [Google Scholar] [CrossRef]
- Lin, S.; Zhou, L. Research on the performance failure of phase change materials thermal management for lithium battery. Appl. Therm. Eng. 2024, 244, 122743. [Google Scholar] [CrossRef]
- Muhammad, F.M.; Zahoor, A.; Nazir, A.; Emad, K.; Abdur, R.; Rana, M.A.; Ammar, A.; Muhammad, W.I.; Abdul, R.; Zeng, X. Probing the combined impact of pulsed electric field and ultra-sonication on the quality of spinach juice. J. Food Process. Preserv. 2021, 45, e15475. [Google Scholar]
- Xu, M.; Sun, J.; Yao, K.; Wu, X.; Shen, J.; Cao, Y.; Zhou, X. Nondestructive detection of total soluble solids in grapes using VMD-RC and hyperspectral imaging. J. Food Sci. 2021, 87, 326–338. [Google Scholar] [CrossRef]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J.; et al. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. Nutr. 2021, 63, 21–29. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, X.; Gao, C.; Gai, Z.; He, Y.; Qian, Z.; Liu, Y.; Lei, H.; Sun, Y.; Xu, Z. Development of a highly sensitive and selective electrochemical immunosensor for controlling of rhodamine B abuse in food samples. Food Control 2022, 133, 108662. [Google Scholar] [CrossRef]
- Ouyang, T.; Zuo, K.; Wang, Z.; Hao, J.; Chen, Y. Enhancing thermal performance for electric vehicle lithium-ion battery pack based on active thermal management. Int. J. Heat Mass Transf. 2025, 245, 127026. [Google Scholar] [CrossRef]
- Maiorano, L.P.; Guidoum, M.; Molina, J.M. Low-porosity aluminum foams with steel guest phases as disruptive materials for active thermal management. Appl. Mater. Today 2025, 42, 102530. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, J.; Hu, F.; Zhang, J.; Jin, H.; Huang, Y. Enhancement in Active Thermal Management Efficiency of Micro/Mini-Pipes Based on Phase Change to Consider Pressure Drop. Int. J. Thermophys. 2024, 45, 171. [Google Scholar] [CrossRef]
- Venkatesh, R.J.; Prasad Bhemuni, V.; Prakash Chinnam, D.S.; Gift, M.D.M. Optimizing Hybrid Active–Passive Thermal Management of Prismatic Li-Ion Batteries Using Phase Change Materials and Porous-Filled Mini-Channels. Energy Storage 2024, 6, e70060. [Google Scholar] [CrossRef]
- He, W.; Zhu, Y.; Liu, Z.; Ying, Z.; Zu, W. Active Thermal Management Method for Improving Current Capability of Power Devices under Influence of Random Convection. Energies 2024, 17, 3249. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, X.; Zhang, M.; Yang, H.; Li, S.; Zhou, T. Research on air-cooled thermal management of energy storage lithium battery. Asia-Pac. J. Chem. Eng. 2023, 18, e2924. [Google Scholar] [CrossRef]
- Lucas, S.; Marian, R.; Lucas, M.; Ogunwa, T.; Chahl, J. Active Thermal Management of Electric Motors and Generators Using Thermoelectric (Peltier Effect) Technology. Energies 2023, 16, 3844. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Zheng, B.; Wang, Z.; Zhao, G.; Zhang, H.; Liu, H.; Jin, X.; Chen, W.; Ma, A.; et al. Microgel-enhanced thermal-sensitive hydrogel electrolyte enables active heat management, controllable energy storage and mechanical flexibility of supercapacitors. Chem. Eng. J. 2023, 465, 142923. [Google Scholar] [CrossRef]
- Waqas, N.M.; Naseem, I.; Majid, A.; Hassan, N.; Bin, A.M.Z. Thermal management of Li-ion battery by using active and passive cooling method. J. Energy Storage 2023, 61, 106800. [Google Scholar] [CrossRef]
- Xin, F.; Wang, Q.; Yan, Y.; Tian, W. Experimental investigation on the active thermal management of grooved flat heat pipe under the electrohydrodynamic effect. Int. Commun. Heat Mass Transf. 2023, 141, 106604. [Google Scholar] [CrossRef]
- Chen, P.; Ding, X.; Chen, M.; Song, H.; Imran, M. The Impact of Resource Spatial Mismatch on the Configuration Analysis of Agricultural Green Total Factor Productivity. Agriculture 2024, 15, 23. [Google Scholar] [CrossRef]
- Azam, K.; Akhtar, S.; Gong, Y.Y.; Routledge, M.N.; Ismail, A.; Oliveira, C.A.; Iqbal, S.Z.; Ali, H. Evaluation of the impact of activated carbon-based filtration system on the concentration of aflatoxins and selected heavy metals in roasted coffee. Food Control 2021, 121, 107583. [Google Scholar] [CrossRef]
- Rong, Y.; Ali, S.; Ouyang, Q.; Wang, L.; Li, H.; Chen, Q. Development of a Bimodal Sensor based on Upconversion Nanoparticles and Surface Enhance Raman for the Sensitive Determination of Dibutyl Phthalate in Food. J. Food Compos. Anal. 2021, 121, 103929. [Google Scholar] [CrossRef]
- Li, Z.; Mao, H.; Li, L.; Wei, Y.; Yu, Y.; Zhao, M.; Liu, Z. A Flexible Wearable Sensor for In Situ Non-Destructive Detection of Plant Leaf Transpiration Information. Agriculture 2024, 14, 2174. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, M.; Zhong, M.; Tao, K.; Wu, Y. State of health estimation for lithium-ion batteries through feature enhanced and genetic algorithm optimized adaptive neuro-fuzzy inference system. Ionics 2025, 31, 6889–6906. [Google Scholar] [CrossRef]
- Hafida, W.; Abiola, F.O.; Taiye, M.A.; Cunshan, Z.; Mokhtar, D. Application and potential of multifrequency ultrasound in juice industry: Comprehensive analysis of inactivation and germination of Alicyclobacillus acidoterrestris spores. Crit. Rev. Food Sci. Nutr. 2022, 64, 21–26. [Google Scholar] [CrossRef]
- Rehman, U.u.; Mahmood, T.; Waqas, H.M. Managing uncertainty in battery energy storage system evaluation using complex hesitant fuzzy multi-criteria decision-making technique with Einstein operators. J. Energy Storage 2025, 125, 116968. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Ali, S.; Haruna, S.A.; He, P.; Li, H.; Ouyang, Q.; Chen, Q. Development of a fluorescence aptasensor for rapid and sensitive detection of Listeria monocytogenes in food. Food Control 2021, 122, 107808. [Google Scholar] [CrossRef]
- Ofoegbu, E.O. State of charge (SOC) estimation in electric vehicle (EV) battery management systems using ensemble methods and neural networks. J. Energy Storage 2025, 114, 115833. [Google Scholar] [CrossRef]
- Pinter, Z.M.; Rohde, G.; Marinelli, M. Comparative analysis of rule-based and model-predictive control algorithms in reconfigurable battery systems for EV fast-charging stations. J. Energy Storage 2025, 116, 116008. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, A.; Pang, D.; Wang, B. Dynamic performance analysis of a thermoelectric cooling-based thermal management system for lithium-ion power batteries. Case Stud. Therm. Eng. 2025, 72, 106315. [Google Scholar] [CrossRef]
- Qin, J.; Huang, H.; Lu, H.; Li, Z. Energy management strategy for hybrid electric vehicles based on deep reinforcement learning with consideration of electric drive system thermal characteristics. Energy Convers. Manag. 2025, 332, 119697. [Google Scholar] [CrossRef]
- Kumaresan, N.; Rammohan, A. Adaptive neuro fuzzy inference system based optimized energy management strategy for the power integration of battery and supercapacitor in electric vehicle. J. Energy Storage 2025, 126, 117073. [Google Scholar] [CrossRef]
- Jiang, K.; Tian, Z.; Wen, T.; Song, K.; Hillmansen, S.; Ochieng, W.Y. Collaborative optimization strategy of hydrogen fuel cell train energy and thermal management system based on deep reinforcement learning. Appl. Energy 2025, 393, 126057. [Google Scholar] [CrossRef]
- Xu, C.; Lu, C.; Piao, J.; Wang, Y.; Zhou, T.; Zhou, Y.; Li, S. Rice virus release from the planthopper salivary gland is independent of plant tissue recognition by the stylet. Pest. Manag. Sci. 2020, 76, 3208–3216. [Google Scholar] [CrossRef]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Xia, J.; Zou, B.; Liu, F.; Wang, P.; Yan, Y. Sensitive glucose biosensor based on cyclodextrin modified carbon nanotubes for detecting glucose in honey. J. Food Compos. Anal. 2022, 105, 104221. [Google Scholar] [CrossRef]
- Shi, N.; Li, S.; He, L.; Feng, Y.; Saeed, M.; Ma, Y.; Ni, Z.; Zhu, D.; Chen, H. High-throughput screening and identification of lignin peroxidase based on spore surface display of Bacillus subtilis. J. Sci. Food Agric. 2024, 105, 2179–2189. [Google Scholar] [CrossRef]
- Lin, H.; Duan, Y.; Man, Z.; Zareef, M.; Wang, Z.; Chen, Q. Quantitation of volatile aldehydes using chemoselective response dyes combined with multivariable data analysis. Food Chem. 2021, 353, 129485. [Google Scholar] [CrossRef]
- Wang, C.; Ni, Y.; Zhu, C.; Li, Y.; Yan, Z.; Lu, Y.; Chen, J. A Highly Crystalline Covalent Organic Framework with Rich Redox Active Sites as Cathode Material for Lithium Batteries. Angew. Chem. 2025, 137, e202423992. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, W.Y.; Kim, S.; Kim, J.; Lee, S.J.; Park, N.; Han, S.P.; Ryu, K.; Kim, J.; Lee, W.B. Kosmotropic aqueous processing solution for green lithium battery cathode manufacturing. Nat. Commun. 2025, 16, 1686. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Kamenskii, M.; Volkov, F.; Eliseeva, S.; Ma, J. Research progress on cathode electrolyte interphase in high-voltage lithium batteries. Acta Phys. Chim. Sin. 2025, 41, 100011. [Google Scholar] [CrossRef]
- Su, Z.; Li, Y.; Dong, Y.; Tang, Z.; Liang, Z. Simulation of rice threshing performance with concentric and non-concentric threshing gaps. Biosyst. Eng. 2020, 197, 270–284. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Li, C.; Naa, Y.O.P.; Cunshan, Z. Facile preparation of sugarcane bagasse-derived carbon supported MoS2 nanosheets for hydrogen evolution reaction. Ind. Crops Prod. 2021, 172, 114064. [Google Scholar] [CrossRef]
- Xu, H.; Dai, C.; Tang, Y.; Xu, X.; Umego, E.C.; He, R.; Ma, H. The selective breeding and mutagenesis mechanism of high-yielding surfactin Bacillus subtilis strains with atmospheric and room temperature plasma. J. Sci. Food Agric. 2021, 102, 1851–1861. [Google Scholar] [CrossRef]
- Selva, S.A.; Shujat, A.; Devaraj, S.; Marimuthu, M.; Huanhuan, L.; Quansheng, C. Recent progress on graphene quantum dots-based fluorescence sensors for food safety and quality assessment applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5765–5801. [Google Scholar]
- Dai, C.; Huang, X.; Sun, J.; Tian, X.; Aheto, J.H.; Niu, S. Development of a portable electronic nose for in-situ detection of submerged fermentation of Tremella aurantialba. J. Food Saf. 2021, 41, e12902. [Google Scholar] [CrossRef]
- Li, J.; Ma, M.; Mao, Y.; Zhang, F.; Feng, J.; Lyu, Y.; Lan, T.; Li, Y.; Liu, Z. Chemical Compatibility of Li1.3Al0.3Ti1.7(PO4)3 Solid-State Electrolyte Co-Sintered with Li4Ti5O12 Anode for Multilayer Ceramic Lithium Batteries. Materials 2025, 18, 851. [Google Scholar] [CrossRef]
- Li, Y.; Guan, J.; Zhang, X.; Yang, J.; Chen, S.; Guo, Y.; Lin, D.; Xu, Q.; Wu, Y.; Yuan, H.; et al. Conversion of waste photovoltaic silicon into silicon-carbon nanocages for lithium batteries anodes preparation. Mater. Today Sustain. 2024, 28, 101050. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, B.; Liu, X.; Cao, J.; Ge, J.; Men, K.; Jiang, Y.; Wang, X. Synthesis of C@Si composite materials for lithium battery anode using Chinese rose as carbon source. J. Alloys Compd. 2024, 1008, 176742. [Google Scholar] [CrossRef]
- Xu, S.; Xu, J. Three-Dimensional Skeleton Structure Li-B Alloys as Anode for Solid-State Lithium Batteries. High Energy Chem. 2024, 58, 347–356. [Google Scholar] [CrossRef]
- Lu, B.; Han, F.; Aheto, J.H.; Rashed, M.M.; Pan, Z. Artificial bionic taste sensors coupled with chemometrics for rapid detection of beef adulteration. Food Sci. Nutr. 2021, 9, 5220–5228. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yue, S.; Wu, Y.; Li, H.; Ying, Z.; Xing, D. Comparison on the Nutrient Plunder Capacity of Orychophragmus violaceus and Brassica napus L. Based on Electrophysiological Information. Horticulturae 2021, 7, 206. [Google Scholar] [CrossRef]
- Alkassoumi, H.H.; Zhang, J.; Li, H.; Chen, C.; Xu, B. Modulating the glycemic response of starch-based foods using organic nanomaterials: Strategies and opportunities. Crit. Rev. Food Sci. Nutr. 2022, 63, 21–25. [Google Scholar] [CrossRef]
- Chauhdary, J.N.; Li, H.; Pan, X.; Zaman, M.; Anjum, S.A.; Yang, F.; Akbar, N.; Azamat, U. Modeling effects of climate change on crop phenology and yield of wheat-maize cropping system and exploring sustainable solutions. J. Sci. Food Agric. 2025, 105, 3679–3700. [Google Scholar] [CrossRef]
- Li, J.; Shi, J.; Wang, T.; Huang, X.E.; Zou, X.; Li, Z.; Zhang, D.; Zhang, W.; Xu, Y. Effects of pulsed electric field pretreatment on mass transfer kinetics of pickled lotus root (Nelumbo nucifera Gaertn.). LWT-Food Sci. Technol. 2021, 151, 112205. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, H.; Ben, L.; Hao, J.; Sun, Q.; Zhang, X.; Qiao, R.; Cen, G.; Yao, X.; Zhang, H.; et al. Mechanical-electrochemical coupling patterns in all-solid-state lithium batteries employing sulfide- and halide-based solid electrolyte. Solid State Ion. 2025, 427, 116887. [Google Scholar] [CrossRef]
- Ho, N.X.; Dien, P.T.; Viet, C.D.; Tran, N.A.; Do, L.T.; Dao, V.D.; Vu, N.H. Silane-assisted dispersion of TiO2 nanoparticles into PEO matrix as ultra-stable composite polymer electrolytes for solid-state lithium batteries. J. Power Sources 2025, 647, 237205. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, P.; Li, J.; Liu, T.; Wu, J. Mixed liquid-phase synthesis of high voltage halide electrolytes for high-performance all-solid-state lithium batteries. Chem. Eng. J. 2025, 515, 163713. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Hou, H.; Lv, A.; Yu, X.; Rong, J.; Xiong, S. The meticulous equilibrium between the electrochemical performances and liquid electrolyte in the solid-state lithium batteries. J. Energy Storage 2025, 122, 116758. [Google Scholar] [CrossRef]
- Xue, W.; Ahangaran, F.; Wang, H.; Theato, P.; Cheng, Y.J. Gel Polymer Electrolytes for Lithium Batteries: Advantages, Challenges, and Perspectives. Macromol. Rapid Commun. 2025, e2500207. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Luo, S.; Yang, Y.; Zhao, X.; Huang, G.; Jin, X.; Zhong, T.; Guan, M.; Liu, J.; Li, Y. In-situ polymerization formed self-healing quasi-solid electrolyte for high-loading lithium batteries. Energy Storage Mater. 2025, 78, 104250. [Google Scholar] [CrossRef]
- Santis, E.D.; Aurora, A.; Bergamasco, S.; Rinaldi, A.; Araneo, R.; Appetecchi, G.B. Ionic Liquid Electrolyte Technologies for High-Temperature Lithium Battery Systems. Int. J. Mol. Sci. 2025, 26, 3430. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Hu, X.; Zhang, X.; Huang, X.; Li, Z.; Li, M.; Zou, X.; Shi, J. Efficient preparation of dual-emission ratiometric fluorescence sensor system based on aptamer-composite and detection of bis(2-ethylhexyl) phthalate in pork. Food Chem. 2021, 352, 129352. [Google Scholar] [CrossRef]
- Junyi, M.; Faisal, I.; Ahsan, A.; Rouyi, F.; Fakhir, H.; Ahsan, F.M.; Basharat, A.; Huang, Q.; Sun, R.; Zhou, W. Wood vinegar induces salinity tolerance by alleviating oxidative damages and protecting photosystem II in rapeseed cultivars. Ind. Crops Prod. 2022, 189, 115763. [Google Scholar]
- El-Mesery, H.S.; Sarpong, F.; Atress, A.S. Statistical interpretation of shelf-life indicators of tomato (Lycopersicon esculentum) in correlation to storage packaging materials and temperature. J. Food Meas. Charact. 2021, 16, 366–376. [Google Scholar] [CrossRef]
- Zhu, Z.; Chai, X.; Xu, L.; Li, Q.; Yuan, C.; Tian, S. Design and performance of a distributed electric drive system for a series hybrid electric combine harvester. Biosyst. Eng. 2023, 236, 160–174. [Google Scholar] [CrossRef]
- Muhammad, N.M.; Boateng, F.E.; Umair, M.; Jing, Q.; Taiye, M.A.; Yan, W.; Zhuang, H.; Zhang, J. Dielectric barrier discharge cold atmospheric plasma: Influence of processing parameters on microbial inactivation in meat and meat products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2626–2659. [Google Scholar] [CrossRef]
- Shao, A.; Wang, H.; Zhang, M.; Liu, J.; Cheng, L.; Li, Y.; Guo, Y.; Wang, Z.; Jia, Q.; Wang, X.; et al. Multiscale interfacial stabilization via prelithiation separator engineering for Ah-level anode-free lithium batteries. Nat. Commun. 2025, 16, 4145. [Google Scholar] [CrossRef]
- Chaykina, D.; Ghosh, M.; Kudu, Ö.U. Critical outlook on separator layers for solid-state lithium batteries: Solid electrolyte materials, anode interface engineering,; scalable separator production. J. Power Sources 2025, 643, 237014. [Google Scholar] [CrossRef]
- He, J.; Hu, G.; Chen, J.; Wu, X.; Li, Y. Ultrahigh stability of lithium batteries improved by methoxy-modified graphdiyne composite separators. Chem. Eng. J. 2025, 511, 161941. [Google Scholar] [CrossRef]
- Vinci, V.; Flachard, D.; Henke, H.; Bouchet, R.; Drockenmuller, E. Enhancing the Performances of Lithium Batteries through Functionalization of Porous Polyolefin Separators with Cross-Linked Single-Ion Polymer Electrolytes. ACS Appl. Mater. Interfaces 2025, 17, 25742–25753. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, S.; Jiang, Z.; Tang, X.; Tian, T.; Hu, Z.; Du, P.; Wang, Y.; Tang, H. Dual in-situ curing gel polymer electrolyte for solid-state lithium battery. Mater. Today Commun. 2025, 451, 12414. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, J.; Cai, S.; Ren, H.; Wu, B.; Hua, Y.; Wei, Z.; Zhao, Y. Ionic transport regulation separator co-functionalized by conductive nanoparticles and nonconductive nanorods for high performance lithium battery. J. Power Sources 2025, 640, 236725. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).