Thermodynamic Analysis of Biomass Pyrolysis in an Auger Reactor Coupled with a Fluidized-Bed Reactor for Catalytic Deoxygenation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials Used
2.2. Fe-HZSM-5 Preparation
2.3. Pyrolysis Experimental Setup
2.4. Analytical Analysis for Pyrolytic Products
2.5. Energy and Exergy Evaluation
2.5.1. Gaseous Stream
2.5.2. Solid Stream
2.5.3. Liquid Stream
- Method 1: the first method was based on the energetic contribution of only “model compounds” representative of chemical families of the bio-oil, categorized according to their functional group. Each chemical family’s principal compound was selected to serve as the model molecule. This approach is typically used in the literature [14] to simplify the calculation, knowing that bio-oil comprises a large number of different types of molecules (>150).
- Method 2: In the second method, all compounds identified in the bio-oil were considered for the energy calculation.
2.5.4. Energy and Exergy Efficiency
3. Results and Discussions
3.1. Energy and Exergy Evaluation of Pyrolysis with and Without Catalytic Deoxygenation
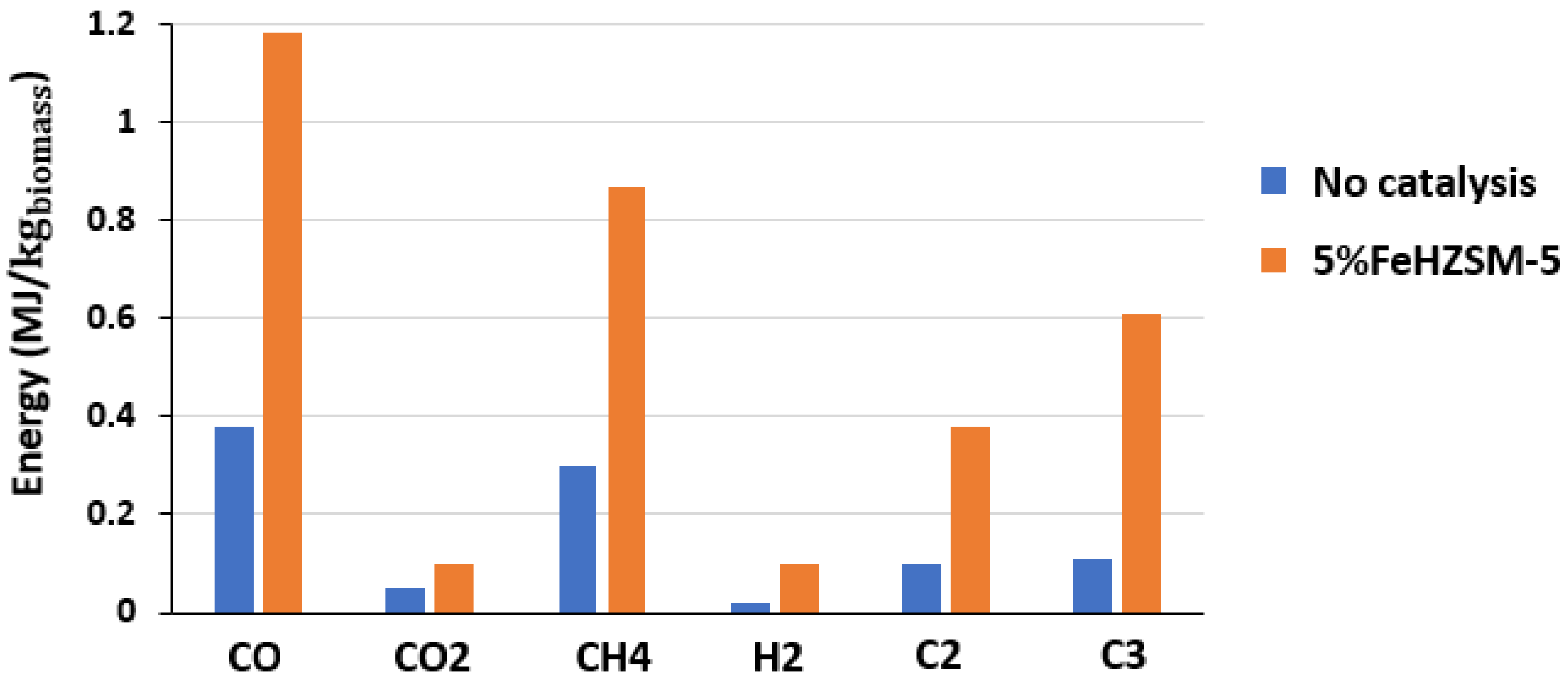
3.1.1. Energy and Exergy Rates of Gas Product
3.1.2. Energy and Exergy Rates of the Bio-Oil
3.1.3. Pyrolysis Heat
3.1.4. Exergetic Efficiency and Exergy Destruction
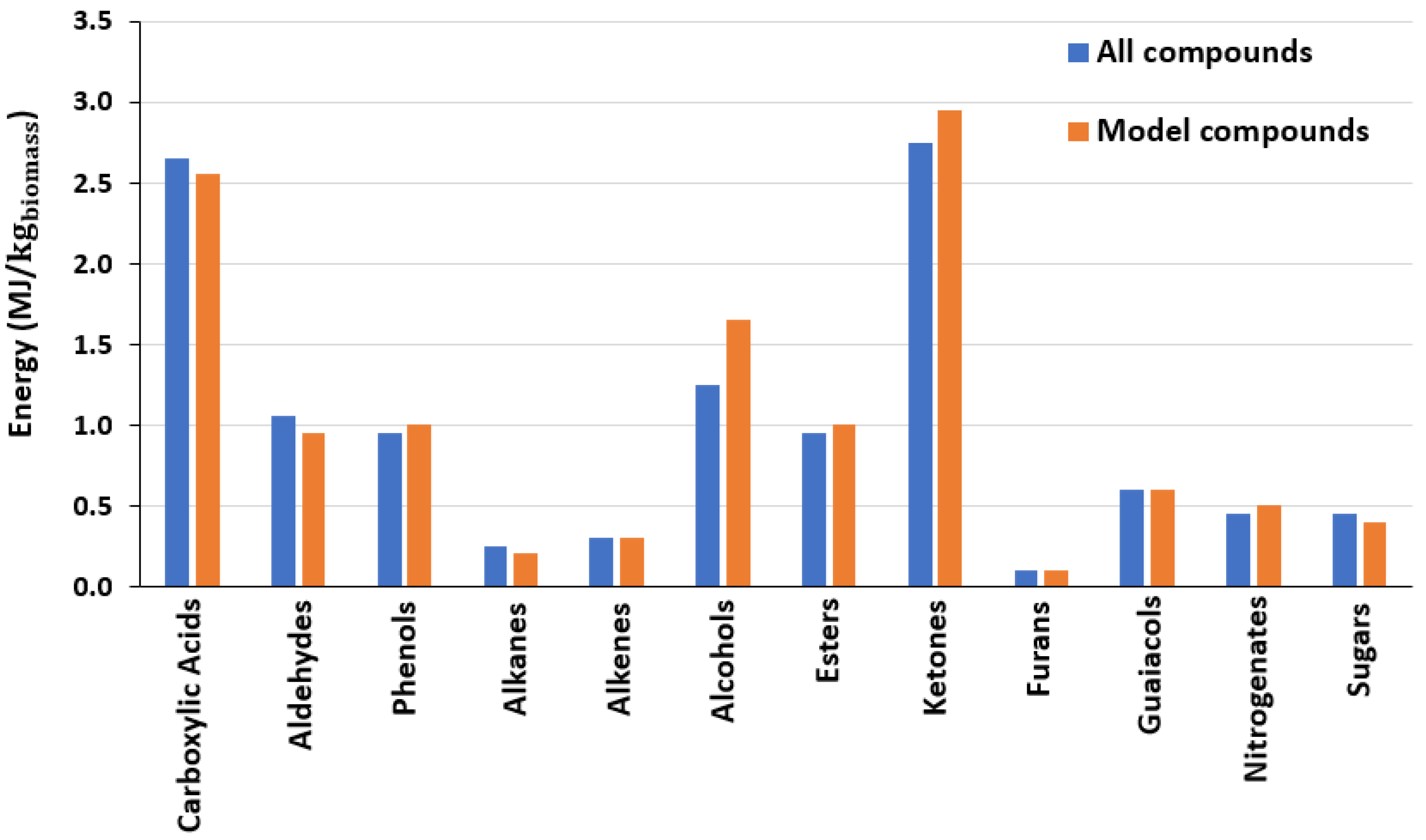
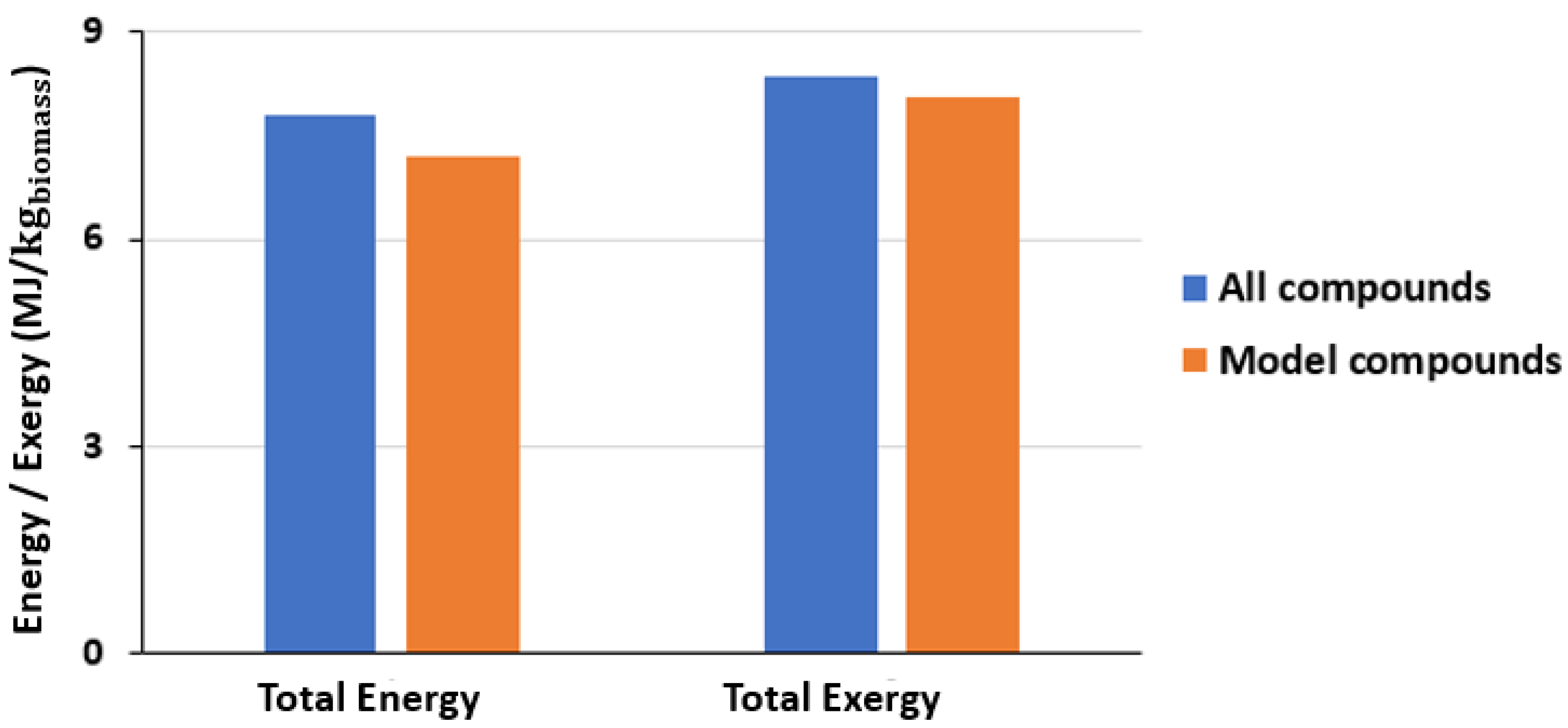
3.2. Comparative Study of Different Methodologies for Thermodynamic Analysis of Bio-Oil
4. Conclusions
- At a temperature of 500 °C, the heat of pyrolysis and the exergy efficiency in the continuous screw reactor were, respectively, 1.20 MJ/kgbiomass and 90.3%.
- The energy yield of the bio-oil improved significantly from 9.3 to 11.3 MJ/kgbiomass in the pyrolysis system with catalytic treatment, and the exergy efficiency rose to 91.6%, indicating less energy degradation.
- The aromatic groups are primarily responsible for the bio-oil increased energy rate. With a value of 6.20 MJ/kgbiomass, they account for 55.1% of the total energy rates, followed by the phenolic group in the second place.
- The two approaches used in the continuous system to estimate the bio-oil total energy and total exergy differed by 6% when compared with respect to the “all compounds” method. In contrast, the two approaches differed significantly in terms of functional categories. The groups with the biggest energy analysis differences in the bio-oil sample from pyrolysis were the nitrogenates, esters, and guaiacols with 20, 16, and 15%, respectively.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Substance | A | b (10−2) | c (10−5) | d (10−9) |
|---|---|---|---|---|
| H2 | 29.11 | −0.1916 | 0.4003 | −0.8704 |
| CO | 28.16 | 0.1675 | 0.5372 | −2.222 |
| CO2 | 22.26 | 5.981 | −3.501 | 7.469 |
| CH4 | 19.89 | 5.024 | 1.269 | −11.01 |
| C2H2 | 21.8 | 9.2143 | −6.527 | 18.21 |
| C2H4 | 3.95 | 15.64 | −8.344 | 17.67 |
| C2H6 | 6.9 | 17.27 | −6.406 | 7.285 |
| C3H6 | 3.15 | 23.83 | −12.18 | 24.62 |
| C3H8 | −4.04 | 30.48 | −15.72 | 31.74 |
| N2 | 28.9 | −0.1571 | 0.8081 | −2.873 |
| H2O(g) | 32.24 | 0.1923 | 1.055 | −3.595 |
| Substance | (kJ/kmol) | (kJ/kmol K) | LHV (kJ/kmol) | (kJ/kmol) |
|---|---|---|---|---|
| H2 | 8469 | 130.57 | 240,420 | 236,100 |
| CO | 8669 | 197.54 | 282,800 | 275,100 |
| CO2 | 9364 | 213.69 | - | 19,870 |
| CH4 | 10,018.7 | 186.16 | 801,280 | 831,650 |
| C2H2 | 10,012 | 200.85 | 1,253,200 | 1,265,000 |
| C2H4 | 10,518 | 219.32 | 1,321,600 | 1,361,100 |
| C2H6 | 10,900 | 229.49 | 1,425,000 | 1,495,000 |
| C3H6 | 14,000 | 266.94 | 1,957,200 | 2,002,700 |
| C3H8 | 14,775.8 | 269.91 | 2,037,200 | 2,152,800 |
| N2 | 8669 | 191.5 | - | 720 |
| H2O(g) | 9904 | 188.84 | - | 9.5 |
| No. | Compounds | Chemical Formula | MM (g/mol) |
|---|---|---|---|
| 1 | 2,5-dimethyl-Furan | C6H8O | 96 |
| 2 | 3-Penten-2-one | C5H8O | 84 |
| 3 | 2-Butenal | C4H6O | 70 |
| 4 | Acetic acid | C2H4O2 | 60 |
| 5 | 2,3-Pentanedione | C5H8O2 | 100 |
| 6 | Propanedioic acid | C3H4O4 | 104 |
| 7 | 1-hydroxy-2-propanone | C3H6O2 | 74 |
| 8 | Benzyl methyl ketone | C9H10O | 134 |
| 9 | 2-Butanone, 3-hydroxy- | C4H8O2 | 88 |
| 10 | 3-Penten-2-one, (E)- | C5H8O | 84 |
| 11 | Isopropyl Alcohol | C3H8O | 60 |
| 12 | 2-Propanol, 2-methyl- | C4H10O | 74 |
| 13 | Propanoic acid | C3H6O2 | 74 |
| 14 | 3-Pentanone, 2,4-dimethyl- | C7H14O | 114 |
| 15 | 1-Methoxy-2-propyl acetate | C6H12O3 | 132 |
| 16 | 2-Hexanone, 3-methyl- | C7H14O | 114 |
| 17 | 3-Hexen-2-one | C6H10O | 98 |
| 18 | Cyclopentanone | C5H8O | 84 |
| 19 | 1-Hydroxy-2-butanone | C4H8O2 | 88 |
| 20 | 1,2-Ethanediol, monoacetate | C4H8O3 | 104 |
| 21 | 3-Furaldehyde | C5H4O2 | 96 |
| 22 | 3-methyl-Cyclohexanol | C7H14O | 114 |
| 23 | Succindialdehyde (butanedial) | C4H6O2 | 86 |
| 24 | 2-Cyclopenten-1-one | C5H6O | 82 |
| 25 | Furfural | C5H4O2 | 96 |
| 26 | 1-Propen-2-ol, acetate | C5H8O2 | 103 |
| 27 | 2-Furanmethanol | C5H6O2 | 98 |
| 28 | 1-(acetyloxy)-2-Propanone | C5H8O3 | 116 |
| 29 | 2-methyl-2-Cyclopenten-1-one | C6H8O | 96 |
| 30 | 2-Butanone | C4H8O | 72 |
| 31 | 1-(2-furanyl)-Ethanone | C6H6O2 | 110 |
| 32 | 1,2-Cyclopentanedione | C5H6O2 | 98 |
| 33 | 2-Furanmethanol, 5-methyl- | C6H8O2 | 112 |
| 34 | 5-methyl-2-Furancarboxaldehyde | C6H6O2 | 110 |
| 35 | Propanoic acid, ethenyl ester | C5H8O2 | 100 |
| 36 | 1-(acetyloxy)-2-Butanone | C6H10O3 | 130 |
| 37 | Spiro [2,4]heptan-4-one | C7H10O | 110 |
| 38 | 3-methyl-2-Cyclopenten-1-one | C6H8O | 96 |
| 39 | Butyrolactone | C4H6O2 | 86 |
| 40 | 2(5H)-Furanone | C4H4O2 | 84 |
| 41 | 5-methyl-2(5H)-Furanone | C5H6O2 | 98 |
| 42 | Cyclohexanone, 2-(hydroxymethyl)- | C7H12O2 | 128 |
| 43 | N-hexyl-1-Hexanamine | C12H27N | 185 |
| 44 | 3-methyl-1,2-Cyclopentanedione | C6H8O2 | 112 |
| 45 | 2-methyl-2-Pentenal | C6H10O | 98 |
| 46 | 2-hydroxy-3-methyl-2-Cyclopenten-1-one | C6H8O2 | 112 |
| 47 | Phenol | C6H6O | 94 |
| 48 | o-Guaiacol | C7H8O2 | 124 |
| 49 | 4-methyl-phenol | C7H8O | 108 |
| 50 | 2-methyl-phenol | C7H8O | 108 |
| 51 | Ethyl Cyclopentenolide | C7H10O2 | 126 |
| 52 | 2,5-dimethyl-phenol | C8H10O | 122 |
| 53 | Heptyl caprylate | C15H30O2 | 242 |
| 54 | p-Cresol | C7H8O | 108 |
| 55 | 2-methoxy-3-methyl-phenol | C8H10O2 | 138 |
| 56 | Creosol | C8H10O2 | 138 |
| 57 | 3-Methyl-2-(2-oxopropyl)furan | C8H10O2 | 138 |
| 58 | 2,6-dimethyl-phenol | C8H10O | 122 |
| 59 | 3,4-Dimethoxytoluene | C9H12O2 | 152 |
| 60 | 2,3,5-trimethyl-1,4-Benzenediol | C9H12O2 | 152 |
| 61 | 4-ethyl-2-methoxy--Phenol | C9H12O2 | 152 |
| 62 | Nonanal | C9H18O | 142 |
| 63 | 4-Piperidinemethanamine | C6H14N2 | 114 |
| 64 | Piperidine, 4-methyl-1-nitroso- | C6H12N2O | 128 |
| 65 | Undecanal | C11H22O | 170 |
| 66 | 1,4:3,6-Dianhydro-α-d-glucopyranose | C6H8O4 | 144 |
| 67 | d-Glycero-d-ido-heptose | C7H14O7 | 210 |
| 68 | p-Vinylguaiacol | C9H10O2 | 150 |
| 69 | 2-methoxy-4-(1-propenyl)-phenol | C10H12O2 | 164 |
| 70 | 2-methoxy-4-propyl-phenol | C10H14O2 | 166 |
| 71 | 2,6-dimethoxy-phenol | C8H10O3 | 154 |
| 72 | Guanosine | C10H13N5O5 | 283 |
| 73 | Isoeugenol | C10H12O2 | 164 |
| 74 | 3-methyl-benzenodiol | C7H8O2 | 124 |
| 75 | Sucrose | C12H22O11 | 342 |
| 76 | trans-Isoeugenol | C10H12O2 | 164 |
| 77 | 3.5-Dimethoxy-4-hydroxytoluene | C9H12O3 | 168 |
| 78 | Vanillin, acetate | C10H10O4 | 194 |
| 79 | Dihydrojasmone | C11H18O | 166 |
| 80 | Hydroquinone | C6H6O2 | 110 |
| 81 | 1,2,3-trimethoxy-5-methyl-Benzene | C10H14O3 | 182 |
| 82 | t-Butylhydroquinone | C10H14O2 | 166 |
| 83 | 4-ethenyl-2,6-dimethoxy-Phenol | C10H12O3 | 180 |
| 84 | 1-(4-hydroxy-3-methoxyphenyl)-2- Propanone | C10H12O3 | 180 |
| 85 | 2,6-dimethoxy-4-(2-propenyl)-Phenol | C11H14O3 | 194 |
| 86 | 4-Hydroxy-2-methylbenzaldehyde | C8H8O2 | 136 |
| 87 | 4-(3-hydroxy-1-propenyl)-2-methoxy-Phenol | C10H12O3 | 180 |
| 88 | 2-Propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)- | C10H10O4 | 194 |
| 89 | Undecanoic acid | C11H22O2 | 186 |
| 90 | 1,6:3,4-Dianhydro-2-O-acetyl-β-dtalopyranose | C8H10O5 | 186 |
| 91 | Levoglucosan | C6H10O5 | 162 |
| 92 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol | C11H14O3 | 194 |
| 93 | Benzaldehyde, 4-hydroxy-3,5-dimethoxy- | C9H10O4 | 182 |
| 94 | D-Mannoheptulose | C7H14O7 | 210 |
| 95 | Homosyringaldehyde | C10H12O4 | 196 |
| 96 | Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | C10H12O4 | 196 |
| 97 | Syringylacetone | C11H14O4 | 210 |
| 98 | 1-Propanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | C11H14O4 | 210 |
| 99 | trans-Sinapyl alcohol | C11H14O4 | 210 |
References
- Elhenawy, Y.; Fouad, K.; Bassyouni, M.; Al-Qabandif, O.A.; Majozi, T. Yield and energy outputs analysis of sawdust biomass pyrolysis. Energy Convers. Manag. X 2024, 22, 100583. [Google Scholar] [CrossRef]
- Sudalaimuthu, P.; Sathyamurthy, R.; Elsheikh, A.H.; Abdul Jameel, A.G. Plastic waste as an alternative sustainable fuel in internal combustion (IC) engines—A comprehensive review. Results Eng. 2025, 26, 104644. [Google Scholar] [CrossRef]
- Buelvas, A.; Quintero-Coronel, D.A.; Vanegas, O.; Ortegon, K.; Bula, A.; Mesa, J.; González-Quiroga, A. Gasification of solid biomass or fast pyrolysis bio-oil: Comparative energy and exergy analyses using AspenPlus®. Eng. Rep. 2024, 6, e12825. [Google Scholar] [CrossRef]
- Yaqoob, H.; Muhammad Ali, H.; Sajjad, U.; Hamid, K. Investigating the potential of plastic pyrolysis oil-diesel blends in diesel engine: Performance, emissions, thermodynamics and sustainability analysis. Results Eng. 2024, 24, 103336. [Google Scholar] [CrossRef]
- Xu, F.; Tian, Y.; Shao, Y.; Wang, J.; Zhang, Y.; Qiao, Y. Parametric investigation of the effects on waste tire pyrolysis oil in a downdraft tube reactor. Energy 2025, 314, 134267. [Google Scholar] [CrossRef]
- Chen, W.H.; Ho, K.Y.; Aniza, R.; Sharma, A.K.; Saravanakumar, A.; Hoang, A.T. A review of noncatalytic and catalytic pyrolysis and co-pyrolysis products from lignocellulosic and algal biomass using Py-GC/MS. J. Ind. Eng. Chem. 2024, 134, 51–64. [Google Scholar] [CrossRef]
- Bieniek, A.; Sieradzka, M.; Wądrzyk, M.; Jerzak, W.; Magdziarz, A. The application of a drop-tube reactor for fast pyrolysis of agricultural biomass: An effective way to valuable products. Chemosphere 2024, 368, 143698. [Google Scholar] [CrossRef]
- Wako, F.M.; Pio, G.; Lofti, A.; Salzano, E.; Farooqui, A.; Mahinpey, N. Performance assessment of drop tube reactor for biomassfast pyrolysis using process simulator. Can. J. Chem. Eng. 2023, 101, 7053–7067. [Google Scholar] [CrossRef]
- Guda, V.K.; Toghiani, H. Catalytic upgrading of pinewood fast pyrolysis vapors using an integrated Auger-packed bed reactor system: Effects of acid catalysts on yields and distribution of pyrolysis products. J. Prod. Ind. 2015, 4, 33–43. [Google Scholar]
- Tang, Y.; Dong, J.; Chi, Y.; Zhou, Z.; Ni, M. Energy and Exergy Analyses of Fluidized-Bed Municipal Solid Waste Air Gasification. Energy Fuels 2016, 30, 7629–7637. [Google Scholar] [CrossRef]
- Hatem, F.A.; Alhumairi, M.K.A.; Al-Obaidi, M.A.; Mohammad, A.T.; Darwish, A.S.K. Upgrading gas turbine efficiency for sustainable power generation: An energy and exergy analyses. Results Eng. 2025, 26, 105489. [Google Scholar] [CrossRef]
- Atsonios, K.; Panopoulos, K.D.; Bridgwater, A.V.; Kakaras, E. Biomass fast pyrolysis energy balance of a 1kg/h test rig. Int. J. Thermodyn. 2015, 18, 267–275. [Google Scholar] [CrossRef][Green Version]
- Thoharudin; Hsiau, S.-S.; Chen, Y.S.; Yang, S. Design optimization of fluidized bed pyrolysis for energy and exergy analysis using a simplified comprehensive multistep kinetic model. Energy 2023, 276, 127615. [Google Scholar] [CrossRef]
- Peters, J.F.; Petrakopoulou, F.; Dufour, J. Exergetic analysis of a fast pyrolysis process for bio-oil production. Fuel Process. Technol. 2014, 119, 245–255. [Google Scholar] [CrossRef]
- Peters, J.F.; Petrakopoulou, F.; Dufour, J. Exergy analysis of synthetic biofuel production via fast pyrolysis and hydro upgrading. Energy 2015, 79, 325–336. [Google Scholar] [CrossRef]
- Keedy, J.; Prymak, E.; Macken, N.; Pourhashem, G.; Spatari, S.; Mullen, C.A.; Boateng, A.A. Exergy Based assessment of the production and conversion of switchgrass, equine waste, and forest residue to bio-oil using fast pyrolysis. Ind. Eng. Chem. Res. 2015, 54, 529–539. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Siyal, A.A.; Gao, Z.; Deng, Z.; Mao, X.; Liu, Y.; Wang, L.; Xing, X.; Chen, Y.; et al. Energy and exergy analyses, and elemental distribution of microwave-assisted auger pyrolysis of textile dyeing sludge and furfural residue. Appl. Therm. Eng. 2024, 248, 123140. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Ma, D.; Chen, C.; Wang, Y.; Wang, W.; Li, A. Exergy and energy analysis of pyrolysis of plastic wastes in rotary kiln with heat carrier. Process Saf. Environ. Prot. 2020, 142, 203–211. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rasul, M.G.; Jahirul, M.I.; Mofijur, M. Fuelling the future: Unleashing energy and exergy efficiency from municipal green waste pyrolysis. Fuel 2024, 357, 129815. [Google Scholar] [CrossRef]
- Boateng, A.A.; Mullen, C.A.; Osgood-Jacobs, L.; Carlson, P.; Macken, N. Mass balance, energy, and exergy analysis of bio-oil production by fast pyrolysis. J. Energy Resour. Technol. 2012, 134, 042001. [Google Scholar] [CrossRef]
- Temireyeva, A.; Sarbassov, Y.; Shah, D. Process simulation of flax straw pyrolysis with kinetic reaction Model: Experimental validation and exergy analysis. Fuel 2024, 367, 131494. [Google Scholar] [CrossRef]
- Mohabeer, C.; Abdelouahed, L.; Marcotte, S.; Taouk, B. Comparative analysis of pyrolytic liquid products of beech wood, flax shives and woody biomass components. J. Anal. Appl. Pyrolysis 2017, 127, 269–277. [Google Scholar] [CrossRef]
- Reyes, L.; Abdelouahed, L.; Mohabeer, C.; Buvat, J.C.; Taouk, B. Energetic and exergetic study of the pyrolysis of lignocellulosic biomasses, cellulose, hemicellulose and lignin. Energy Convers. Manag. 2021, 244, 114459. [Google Scholar] [CrossRef]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Biomass proximate analysis using thermogravimetry. Bioresour. Technol. 2013, 139, 1–4. [Google Scholar] [CrossRef]
- Campusano, B.; Jabbour, M.; Abdelouahed, L.; Mignot, M.; Devouge-Boyer, C.; Taouk, B. Improvement of Properties of Bio-Oil from Biomass Pyrolysis in Auger Reactor Coupled to Fluidized Catalytic Bed Reactor. Processes 2024, 12, 2368. [Google Scholar] [CrossRef]
- Wang, X.; Lv, W.; Guo, L.; Zhai, M.; Dong, P.; Qi, G. Energy and exergy analysis of rice husk high-temperature pyrolysis. Int. J. Hydrogen Energy 2016, 41, 21121–21130. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Boles, M.A. Thermodynamics: An Engineering Approach, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Prestipino, M.; Piccolo, A.; Vilela, C.V.; Galvagno, A. Cogeneration processes based on giant reed gasification combined with ORC and district heating for heat recovery: Comparative energy and exergy analysis. Renew. Energy 2025, 238, 121944. [Google Scholar] [CrossRef]
- Linstrom, P.J. NIST Chemistry WebBook, NIST Standard Reference Database 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Szargut, J. Exergy Method: Technical and Ecological Applications; Environmental Science, Engineering; WIT Press: Southampton, UK, 2005. [Google Scholar]
- Hosokai, S.; Matsuoka, K.; Kuramoto, K.; Suzuki, Y. Modification of Dulong’s formula to estimate the heating value of gas, liquid and solid fuels. Fuel Process. Technol. 2016, 152, 399–405. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Blasiak, W. Energy and Exergy Analysis of High Temperature Agent Gasification of Biomass. Energies 2014, 7, 2107–2122. [Google Scholar] [CrossRef]
- Dupont, C.; Chiriac, R.; Gauthier, G.; Toche, F. Heat capacity measurements of various biomass types and pyrolysis residues. Fuel 2014, 115, 644–651. [Google Scholar] [CrossRef]
- Singh, R.K.; Jena, K.; Chakraborty, J.P.; Sarkar, A. Energy and exergy analysis for torrefaction of pigeon pea stalk (cajanus cajan) and eucalyptus (eucalyptus tereticornis). Int. J. Hydrogen Energy 2020, 45, 18922–18936. [Google Scholar] [CrossRef]
- Baghel, P.; Sakhiya, A.K.; Kaushal, P. Influence of temperature on slow pyrolysis of Prosopis Juliflora: An experimental and thermodynamic approach. Renew. Energy 2022, 185, 538–551. [Google Scholar] [CrossRef]
- Eboh, F.C.; Ahlström, P.; Richards, T. Estimating the specific chemical exergy of municipal solid waste. Energy Sci. Eng. 2016, 4, 217–231. [Google Scholar] [CrossRef]
- Yang, H.; Kudo, S.; Ku, H.P.; Norinaga, K.; Mori, A.; Mašek, O.; Hayashi, J. Estimation of Enthalpy of Bio-Oil Vapor and Heat Required for Pyrolysis of Biomass. Energy Fuels 2013, 27, 2675–2686. [Google Scholar] [CrossRef]
- dos Reis Ferreira, R.A.; da Silva Meireles, C.; Assunção, R.M.N.; Reis Soares, R. Heat required and kinetics of sugarcane straw pyrolysis by TG and DSC analysis in different atmospheres. J. Therm. Anal. Calorim. 2018, 132, 1535–1544. [Google Scholar] [CrossRef]
- Ábrego, J.; Atienza-Martínez, M.; Plou, F.; Arauzo, J. Heat requirement for fixed bed pyrolysis of beechwood chips. Energy 2019, 178, 145–157. [Google Scholar] [CrossRef]
- Torres, E.; Rodriguez-Ortiz, L.A.; Zalazar, D.; Echegaray, M.; Rodriguez, R.; Zhang, H.; Mazza, G. 4-E (environmental, economic, energetic and exergetic) analysis of slow pyrolysis of lignocellulosic waste. Renew. Energy 2020, 162, 296–307. [Google Scholar] [CrossRef]
- Osgood-Jacobs, L.; Boateng, A.A.; Carlson, C.; Mullen, C.A.; Macken, N. Mass Balance and Exergy Analysis of a Fast Pyrolysis System. Energy Syst. Anal. 2011, 4, 21–26. [Google Scholar] [CrossRef]
- Parvez, A.M.; Wu, T.; Afzal, M.T.; Mareta, S.; He, T.; Zhai, M. Conventional and microwave-assisted pyrolysis of gumwood: A comparison study using thermodynamic evaluation and hydrogen production. Fuel Process. Technol. 2019, 184, 1–11. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Baghel, P.; Pathak, S.; Vijay, V.K.; Kaushal, P. Effect of Process Parameters on Slow Pyrolysis of Rice Straw: Product Yield and Energy Analysis. In Proceedings of the International Conference and Utility Exhibition on Energy, Environment and Climate Change (ICUE), Pattaya, Thailand, 20–22 October 2020; pp. 1–9. [Google Scholar] [CrossRef]

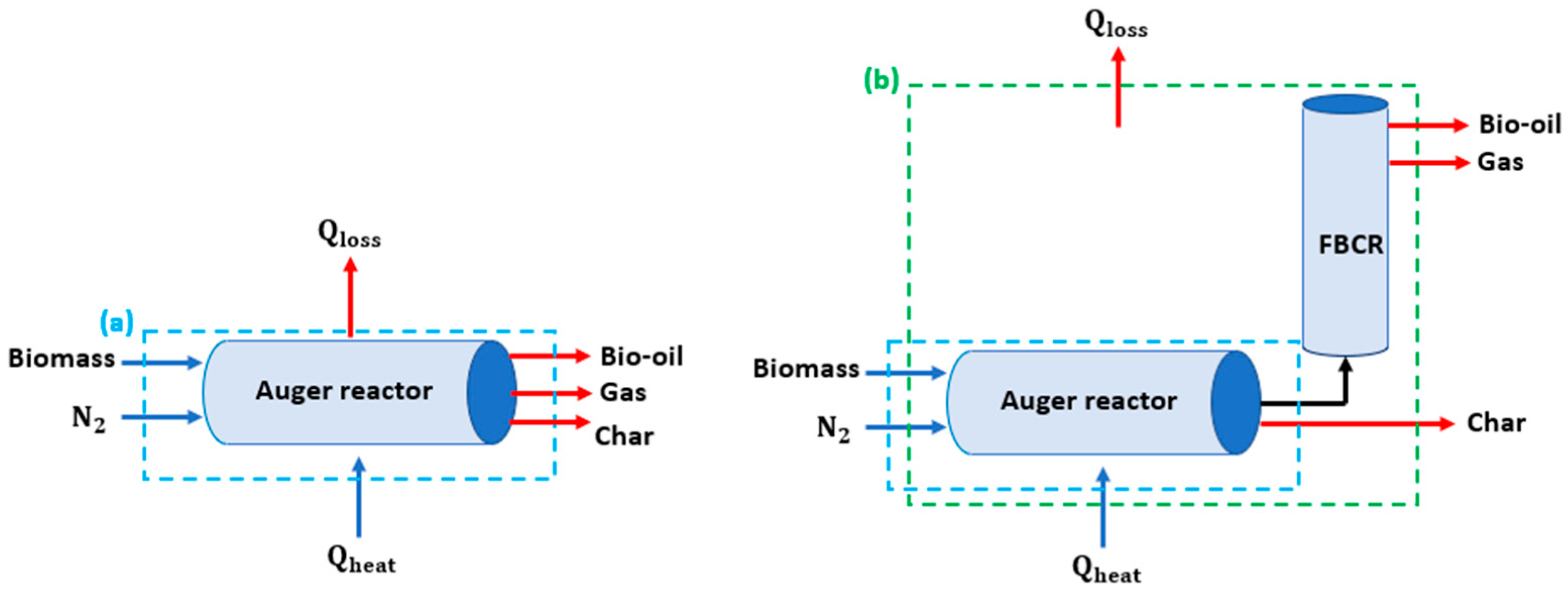
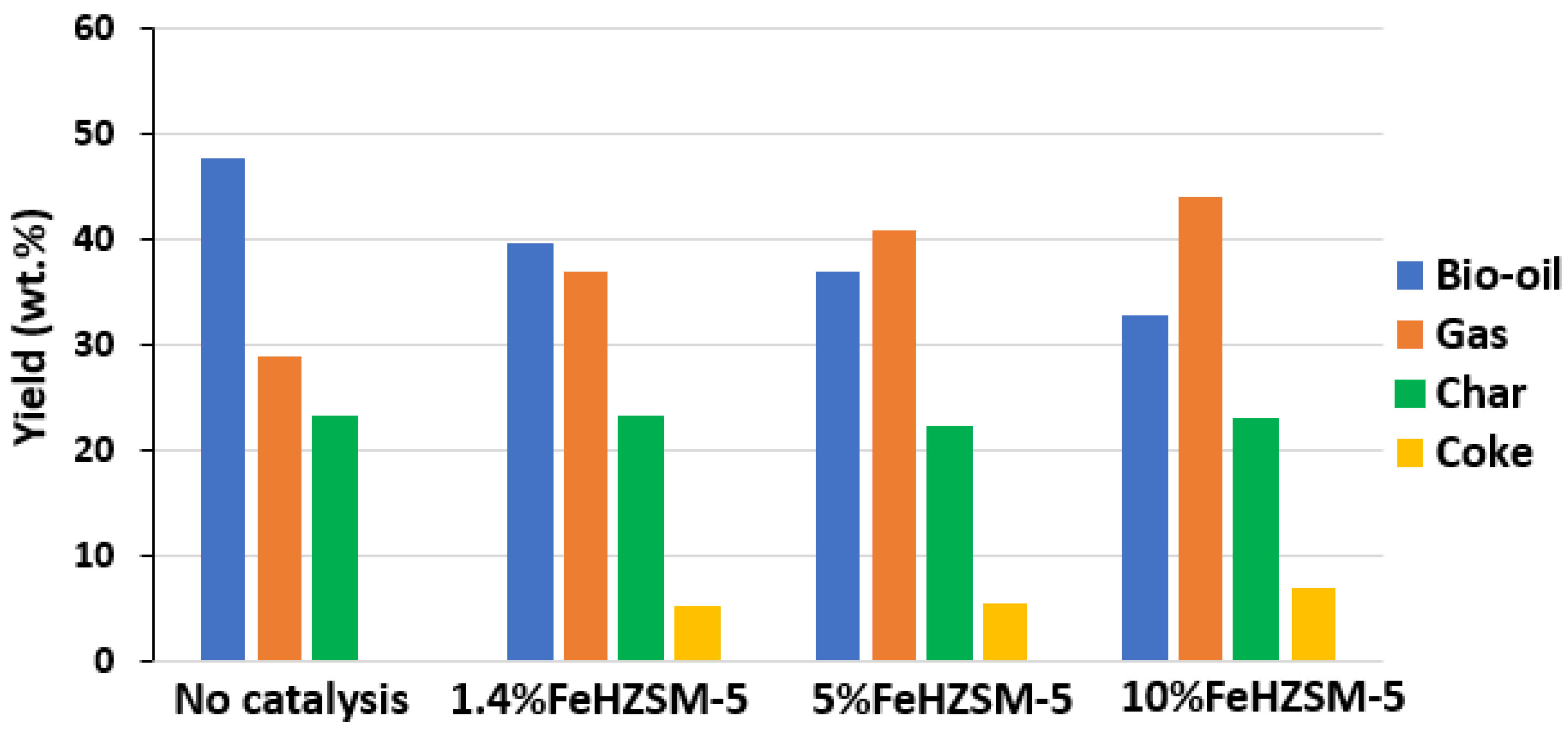
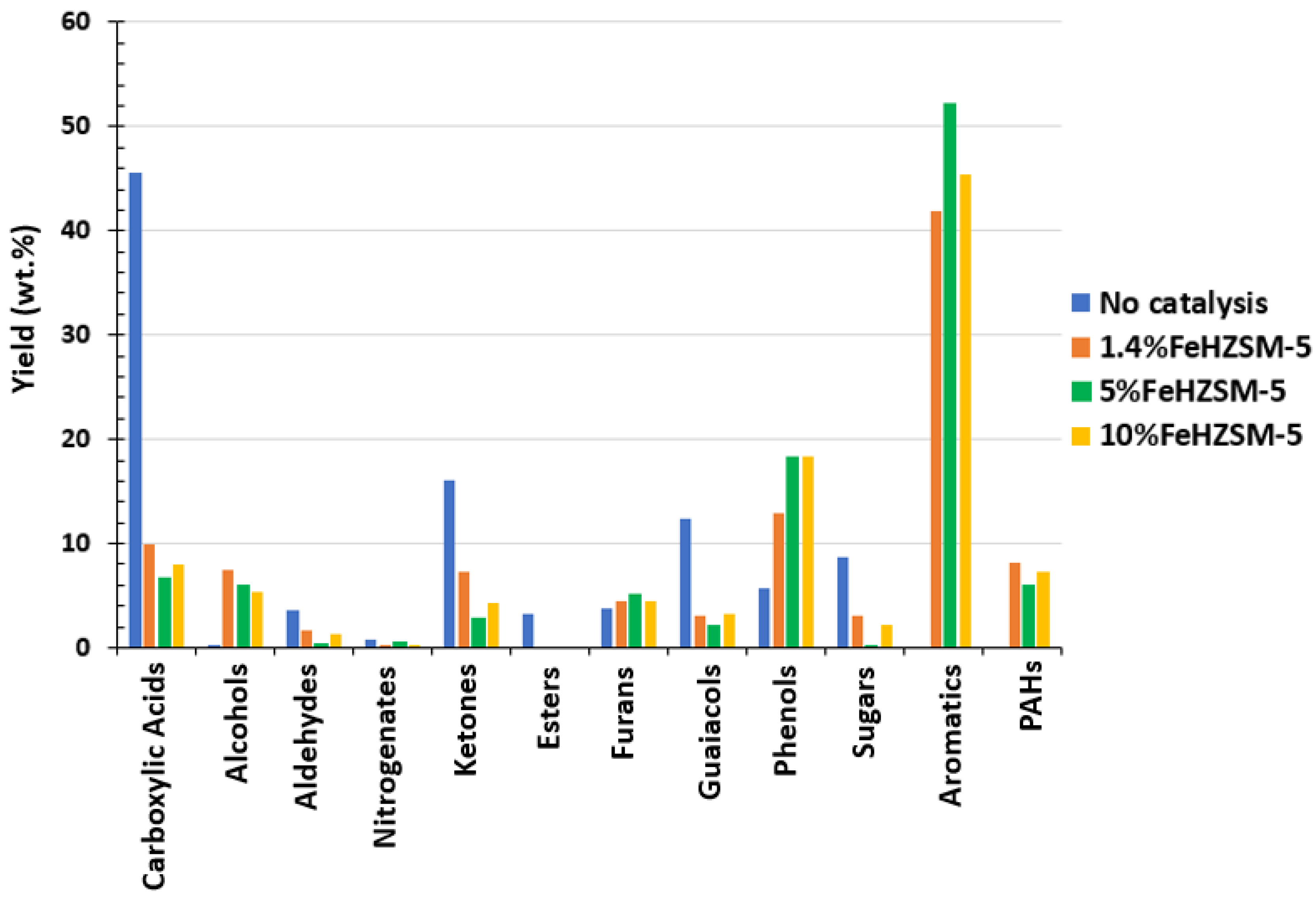
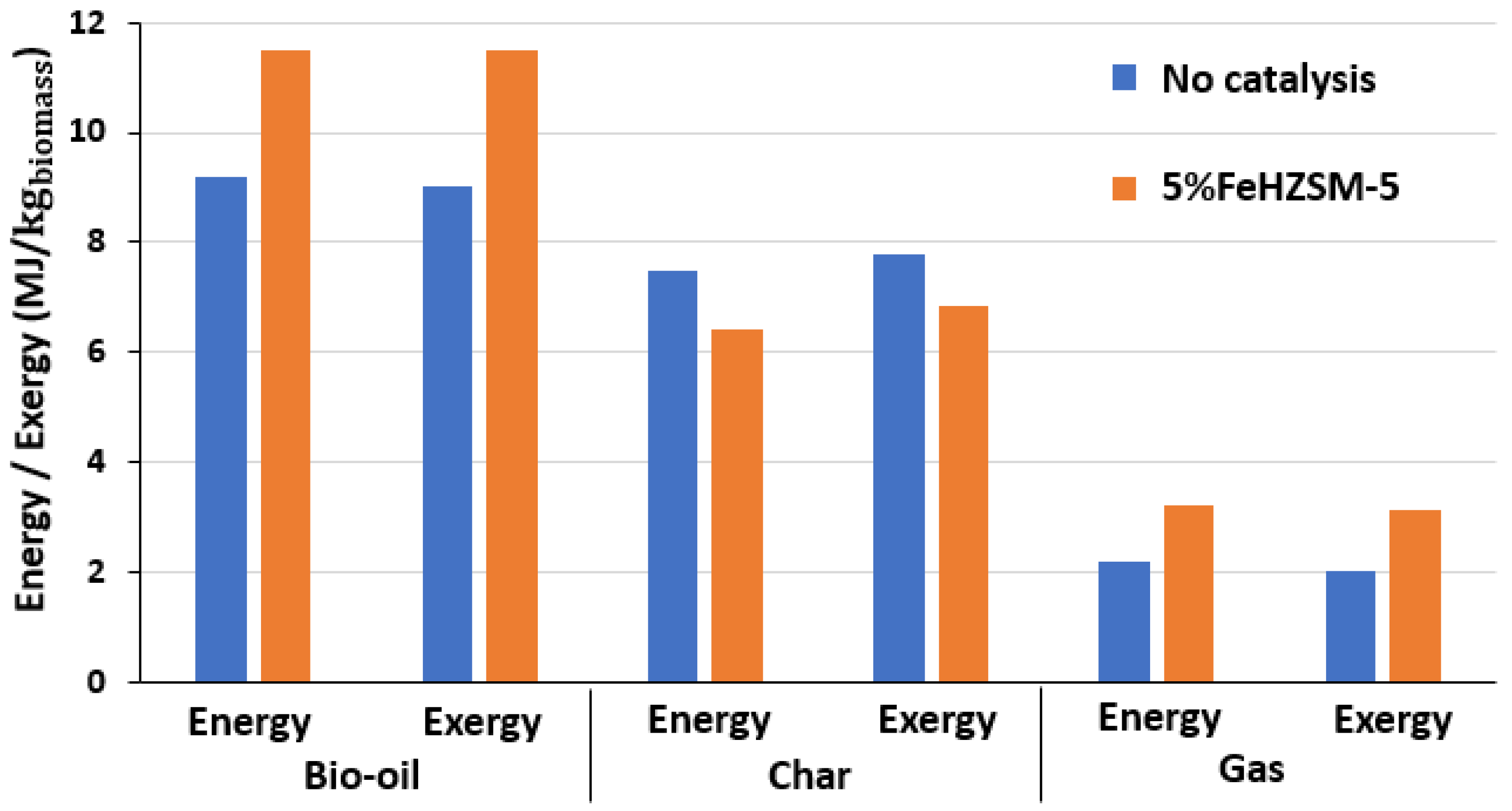



| Biomass | Energy Efficiency (%) | Exergy Efficiency (%) | References |
|---|---|---|---|
| Textile dyeing sludge | 38.93 | 36.65 | [14] |
| Furfural residue | 82.24 | 81.82 | [14] |
| Plastic mixture | 60.9–67.3 | 59.4–66.0 | [15] |
| Waste | 72.89 | 68.4 | [16] |
| Elemental analysis (wt.%) | Carbon 44.77 | Hydrogen 5.72 | Nitrogen 0.23 | Oxygen a 49.28 |
| Proximate analysis (wt.%) | Humidity 6.23 | Volatile matter 75.4 | Fixed Carbon 17.54 | Ash 0.83 |
| No Catalysis | 1.4%FeHZSM-5 | 5%FeHZSM-5 | 10%FeHZSM-5 | |
|---|---|---|---|---|
| CO | 42 | 44 | 41.5 | 42.5 |
| CO2 | 47 | 35 | 35 | 32 |
| CH4 | 7 | 10.5 | 11.5 | 10 |
| H2 | 1 | 2.5 | 4 | 7.5 |
| C2 (C2H2, C2H4, C2H6) | 2 | 3.75 | 4 | 4 |
| C3 (C3H4, C3H6, C3H8) | 1 | 4.25 | 4 | 4 |
| Chemical Family | Model Molecule | Formula |
|---|---|---|
| Acids | Acetic acid | C2H4O2 |
| Phenols | Phenol, 2,6-dimethyl- | C8H10O |
| Aldehydes | Furfural | C5H4O2 |
| Alcohols | Cyclohexanol, 3-methyl | C7H14O |
| Amides | 4-piperidinmethanamine | C6H14N2 |
| Ketones | 2-propanone, 1-hydroxy- | C3H6O2 |
| Esters | 1-propen-2-ol, acetate | C5H8O2 |
| Furans | Furan, 2,5-dimethyl- | C6H8O2 |
| Guaiacol | Phenol, 2,6-dimethoxy- | C8H10O3 |
| Sugars | Levoglucosan | C6H10O5 |
| En (MJ/kgbiomass) | Ex (MJ/kgbiomass) | |||
|---|---|---|---|---|
| No Catalysis | 5%FeHZSM-5 | No Catalysis | 5%FeHZSM-5 | |
| Acids | 2.88 | 0.57 | 2.77 | 0.55 |
| Phenols | 0.76 | 1.78 | 0.75 | 1.74 |
| Aldehydes | 0.35 | 0.07 | 0.35 | 0.07 |
| Alcohols | 0.04 | 0.40 | 0.03 | 0.39 |
| Nitrogenates | 0.07 | 0.04 | 0.06 | 0.04 |
| Ketones | 2.22 | 0.76 | 2.19 | 0.75 |
| Esters | 0.34 | 0.00 | 0.34 | 0.00 |
| Furans | 0.47 | 0.48 | 0.47 | 0.48 |
| Guaiacols | 1.45 | 0.32 | 1.44 | 0.31 |
| Sugar | 0.67 | 0.13 | 0.64 | 0.12 |
| Aromatics | 0.00 | 6.20 | 0.00 | 6.23 |
| PAH | 0.00 | 0.50 | 0.00 | 0.50 |
| Total | 9.26 | 11.25 | 9.05 | 11.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campusano, B.; Jabbour, M.; Abdelouahed, L.; Taouk, B. Thermodynamic Analysis of Biomass Pyrolysis in an Auger Reactor Coupled with a Fluidized-Bed Reactor for Catalytic Deoxygenation. Processes 2025, 13, 2496. https://doi.org/10.3390/pr13082496
Campusano B, Jabbour M, Abdelouahed L, Taouk B. Thermodynamic Analysis of Biomass Pyrolysis in an Auger Reactor Coupled with a Fluidized-Bed Reactor for Catalytic Deoxygenation. Processes. 2025; 13(8):2496. https://doi.org/10.3390/pr13082496
Chicago/Turabian StyleCampusano, Balkydia, Michael Jabbour, Lokmane Abdelouahed, and Bechara Taouk. 2025. "Thermodynamic Analysis of Biomass Pyrolysis in an Auger Reactor Coupled with a Fluidized-Bed Reactor for Catalytic Deoxygenation" Processes 13, no. 8: 2496. https://doi.org/10.3390/pr13082496
APA StyleCampusano, B., Jabbour, M., Abdelouahed, L., & Taouk, B. (2025). Thermodynamic Analysis of Biomass Pyrolysis in an Auger Reactor Coupled with a Fluidized-Bed Reactor for Catalytic Deoxygenation. Processes, 13(8), 2496. https://doi.org/10.3390/pr13082496







