A Review on Sustainable Upcycling of Plastic Waste Through Depolymerization into High-Value Monomer
Abstract
1. Introduction
2. Overview of Plastic Waste and Its Environmental Impact
2.1. Types of Plastics and Their Applications (PET, PE, PP, PS, PVC, etc.)
Recycling Codes of Common Plastics
2.2. Global Statistics on Plastic Waste Generation
2.3. Environmental Consequences of Plastic Accumulation
2.4. Need for Circular Economy and Sustainable Waste Management
3. Concept of Plastic Upcycling
3.1. Definition of and Distinction Between Recycling, Downcycling, and Upcycling
3.2. Advantages of Upcycling over Traditional Recycling Methods
3.3. Role of Upcycling in Achieving a Circular Economy
4. Depolymerization Techniques for Plastic Waste
4.1. Overview of Depolymerization Processes
4.1.1. Thermal Depolymerization
4.1.2. Chemical Depolymerization
4.1.3. Catalytic Depolymerization
4.1.4. Biological Depolymerization
4.2. Comparative Analysis of Methods: Efficiency, Scalability, Environmental Impact
4.3. Emerging Hybrid and Green Technologies
5. Conversion of Depolymerized Polymers to Useful Monomers
5.1. Target Monomers and Their Industrial Significance
5.2. Pathways from Depolymerized Products to Monomer Recovery
5.3. Case Studies: PET to BHET/TPA, PU to Polyols, etc.
5.4. Quality and Purity Concerns in Monomer Production
6. Life Cycle Assessment and Sustainability Considerations
6.1. Energy Consumption and Carbon Footprint
6.2. Environmental Benefits of Monomer Recovery
6.3. Economic Feasibility and Scalability of Processes
6.4. Comparison with Conventional Plastic Management Strategies
6.5. Limitations and Comparative Assessment of Plastic Waste Valorization Approaches
6.6. Comparative Analysis: Upcycling vs. Downcycling vs. Recycling
7. Recent Advances and Future Perspectives
7.1. Recent Breakthroughs in Upcycling and Depolymerization
7.2. Integration with Renewable Energy and Green Chemistry
7.3. Policy Support, Regulations, and Public–Private Partnerships
7.4. Research Gaps and Future Research Directions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Single-Use Plastics: A Roadmap for Sustainability; UNEP: Nairobi, Kenya, 2018. [Google Scholar]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD Publishing: Paris, France, 2022. [Google Scholar]
- PlasticsEurope. Plastics—The Facts 2021: An Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope: Brussels, Belgium, 2021. [Google Scholar]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Policy: Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. B 2009, 364, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Davidson, M.G.; Furlong, R.A.; McManus, M.C. Developments in the life cycle assessment of chemical recycling of plastic waste—A review. J. Clean. Prod. 2021, 293, 126163. [Google Scholar] [CrossRef]
- Eschenbacher, A.; vom Stein, T.; Schutyser, W. From plastics to monomers and back: Recent advances in chemical recycling of plastics. Sustain. Chem. 2021, 2, 56–79. [Google Scholar]
- Laldinpuii, Z.; Lalhmangaihzuala, S.; Pachuau, Z.; Vanlaldinpuia, K. Depolymerization of poly(ethylene terephthalate) waste with biomass-waste derived recyclable heterogeneous catalyst. Waste Manag. 2021, 126, 1–10. [Google Scholar] [CrossRef]
- Shen, L.; Worrell, E.; Patel, M.K. Open-loop recycling: A LCA case study of PET bottle-to-fibre recycling. Resour. Conserv. Recycl. 2010, 55, 34–52. [Google Scholar] [CrossRef]
- Shukla, S.R.; Harad, A.M. Glycolysis of polyethylene terephthalate waste. Polym. Degrad. Stab. 2006, 91, 1850–1854. [Google Scholar] [CrossRef]
- Rahimi, A.; Ulbrich, J.; Coon, J.J.; Stahl, S.S. Formic-acid-induced depolymerization of waste polycarbonate to recover bisphenol A and dimethyl carbonate. Nature 2014, 515, 249–252. [Google Scholar] [CrossRef]
- Gu, J.D. Microbiological deterioration and degradation of synthetic polymeric materials: Recent research advances. Int. Biodeterior. Biodegrad. 2003, 52, 69–91. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Wang, P.; Yu, B.; Zhang, T.; Zhao, Z.; Li, Y.; Zhan, S. From Plastic Waste to Treasure: Selective Upcycling through Catalytic Technologies. Adv. Energy Mater. 2023, 13, 2302008. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; Maniar, D.; Zhu, H.; Maniar, D. A perspective on PEF synthesis, properties, and end-life. Front. Chem. 2020, 8, 585190. [Google Scholar] [CrossRef]
- Sinha, V.; Patel, M.R.; Patel, J.V. PET waste management by chemical recycling: A review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Ignatyev, I.A.; Thielemans, W.; Vander Beke, B. Recycling of polymers: A review. ChemSusChem 2014, 7, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Paci, M.; La Mantia, F.P. Influence of small amounts of polyvinylchloride on the recycling of polyethyleneterephthalate. Polym. Degrad. Stab. 1999, 63, 11–14. [Google Scholar] [CrossRef]
- Demirel, B.; Yaraş, A.; Kalkan, D.; Tufan, M. Chemical recycling of polyurethane foam wastes. J. Polym. Environ. 2021, 29, 3090–3104. [Google Scholar]
- Papong, S.; Malakul, P. Life-cycle energy and environmental analysis of bio-based and petrochemical-based plastic: Comparative study. Int. J. Life Cycle Assess. 2010, 15, 284–296. [Google Scholar]
- Meys, R.; Frick, F.; Westhues, S.; Sternberg, A.; Klankermayer, J.; Bardow, A. Towards a circular economy for plastic packaging wastes—The environmental potential of chemical recycling. Resour. Conserv. Recycl. 2020, 162, 105010. [Google Scholar] [CrossRef]
- Uihlein, A.; Schebek, L. Environmental impacts of a lignin-derived bioaromatic monomer compared to fossil-derived alternatives. Int. J. Life Cycle Assess. 2009, 14, 226–234. [Google Scholar]
- Larrain, M.; Van Passel, S.; Thomassen, G. Techno-economic assessment of chemical recycling of plastics: State-of-the-art and future prospects. Resour. Conserv. Recycl. 2022, 180, 106183. [Google Scholar]
- Saygin, D.; Patel, M.K. Life cycle assessment of PET bottle recycling. Int. J. Life Cycle Assess. 2010, 15, 100–113. [Google Scholar]
- Seitz, M.; Schröter, S. Catalytic Depolymerization of Polyolefinic Plastic Waste. Chem. Ing. Tech. 2022, 94, 720–726. [Google Scholar] [CrossRef]
- Jehanno, C.; Flores, I.; Dove, A.P.; Müller, R.J.; Wierckx, N.; Samorì, C.; von Harpe, A.; Esposito, F.; Delogu, F.; Brondi, A.; et al. Organocatalysis for depolymerization of PET: Towards closed-loop recycling. Angew. Chem. Int. Ed. 2022, 61, e202117508. [Google Scholar]
- Rorrer, N.A.; Nicholson, S.; Carpenter, A.C.; Biddy, M.J.; Grundl, N.J.; Beckham, G.T. Combining reclaiming of PET with valorization of lignin-derived monomers. Green Chem. 2020, 22, 6323–6330. [Google Scholar]
- Saha, N.; Banivaheb, S.; Reza, M.T. Towards solvothermal upcycling of mixed plastic wastes: Depolymerization pathways of waste plastics in sub- and supercritical toluene. Energy Convers. Manag. X 2022, 13, 100158. [Google Scholar] [CrossRef]
- Moon, H.; Jeon, J.; Song, Y.; Kim, K.; Kim, H.; Kim, S.; Lee, J.W. Green chemical pathways for plastic upcycling. Chem. Eng. J. 2023, 451, 138384. [Google Scholar]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Rahimi, A.; García, J.M. Emerging technologies to tackle plastic waste: Current understanding and future research needs. Science 2022, 376, 1070–1076. [Google Scholar]
- Singh, A.; Ruj, B.; Sadhukhan, A.K. Pyrolysis of plastic waste: A review. Waste Manag. 2022, 141, 37–58. [Google Scholar]
- Ragaert, P.; Hubo, S.; Veelaert, L.; De Meulenaer, B. Upcycling of food-grade recycled plastics into food-contact packaging materials. TrAC Trends Anal. Chem. 2020, 124, 115772. [Google Scholar]
- Bahl, S.; Choudhary, A.; Khullar, S.; Bhatia, R.K.; Arya, S.K. Recent advances in biodegradable polymers and plastics for sustainable packaging. ACS Appl. Polym. Mater. 2021, 3, 2204–2225. [Google Scholar]
- Deloitte. New Circular Economy Business Models in the EU Plastics Industry; Deloitte Sustainability Report; Deloitte: New York, NY, USA, 2020. [Google Scholar]
- PlasticsEurope. The Circular Economy for Plastics: A European Overview; PlasticsEurope: Brussels, Belgium, 2020. [Google Scholar]
- Lomwongsopon, P.; Varrone, C. Critical Review on the Progress of Plastic Bioupcycling Technology as a Potential Solution for Sustainable Plastic Waste Management. Polymers 2022, 14, 4996. [Google Scholar] [CrossRef] [PubMed]
- Brems, A.; Baeyens, J.; Dewil, R.; Jong, D. Polymeric waste to fuels via pyrolysis: Review of commercial status. J. Hazard. Mater. 2012, 200–201, 82–88. [Google Scholar]
- La Rosa, A.D.; Blanco, I.; Banatao, D.R. Recycling of end-of-life composite materials and sustainability: A review. J. Compos. Sci. 2020, 4, 42. [Google Scholar]
- Korley, L.T.J.; Epps, T.H.; Helms, B.A.; Ryan, A.J. Toward polymer upcycling adding value and tackling circularity. Science 2021, 373, 66–69. [Google Scholar] [CrossRef]
- Wei, R.; Zimmermann, W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: How far are we? Microb. Biotechnol. 2017, 10, 1308–1322. [Google Scholar] [CrossRef]
- Bejgarn, S.; MacLeod, M.; Bogdal, C.; Breitholtz, M. Toxicity of leachate from weathering plastics: An overlooked issue for risk assessment of marine plastics? Mar. Pollut. Bull. 2015, 102, 227–234. [Google Scholar]
- Zhen, X.; Yu, Z.; Zhang, X.; Jin, Y.; Wang, X. Pyrolysis and catalytic pyrolysis of plastic waste: Fundamentals, mechanisms, and reactor design. Renew. Sustain. Energy Rev. 2022, 153, 111776. [Google Scholar]
- Kohli, K.; Chandrasekaran, S.R.; Prajapati, R.; Kunwar, B.; Al-Salem, S.; Moser, B.R.; Sharma, B.K. Pyrolytic Depolymerization Mechanisms for Post-Consumer Plastic Wastes. Energies 2022, 15, 8821. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.; Kwon, E.E. Biohydrogen production by catalytic hydrothermal gasification of plastic wastes: A review. Chem. Eng. J. 2021, 418, 129426. [Google Scholar]
- Wang, J.; Yuan, M.; Sun, Y.; Li, Y.; Lu, J. Integrated processes for plastic waste recycling: State-of-the-art and future prospects. Chem. Eng. J. 2022, 443, 137406. [Google Scholar]
- Müh, U.; Weigand, L. Catalytic depolymerization of polyolefins and polyesters: From fundamentals to future technologies. Ind. Eng. Chem. Res. 2021, 60, 10273–10291. [Google Scholar]
- Zhang, Y.; Huang, K.; Wang, Y.; Yu, H.; Zhou, Y. Recent advances in machine learning-assisted design for plastic recycling. Chem. Eng. J. 2023, 455, 140843. [Google Scholar]
- Khopade, K.V.; Chikkali, S.H.; Barsu, N. Metal-catalyzed plastic depolymerization. Cell Rep. Phys. Sci. 2023, 4, 101341. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Wei, R. Protein engineering for plastic recycling. Angew. Chem. Int. Ed. 2021, 60, 21048–21058. [Google Scholar]
- Ma, Y.; Yao, M.; Li, B.; Ding, M.; He, B.; Chen, S. Enhanced enzymatic degradation of PET through fusion of a hydrophobin. Biotechnol. Bioeng. 2018, 115, 2790–2796. [Google Scholar]
- Kawai, F.; Kawabata, T.; Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 2020, 104, 8053–8064. [Google Scholar] [CrossRef]
- Taniguchi, I.; Yoshida, S.; Hiraga, K.; Miyamoto, K.; Kimura, Y.; Oda, K. Biodegradation of PET: Current status and application aspects. ACS Catal. 2019, 9, 4089–4105. [Google Scholar] [CrossRef]
- Milios, L.; Christensen, L.H.; McKinnon, D.; Christensen, C.; Rasch, M.K.; Hallstrøm, E.R. Plastic recycling in the Nordics: A value chain market analysis. Waste Manag. 2018, 76, 180–189. [Google Scholar] [CrossRef]
- Ögmundarson, Ó.; Herrgård, M.J.; Fantke, P. Towards sustainable plastics: Tackling the environmental and health impacts of plastics throughout the life cycle. Sustain. Mater. Technol. 2020, 25, e00136. [Google Scholar]
- Morão, A.; de Bie, F. Life cycle impact assessment of polylactic acid (PLA) produced from sugarcane in Thailand. J. Polym. Environ. 2020, 28, 466–481. [Google Scholar] [CrossRef]
- Spierling, S.; Knüpffer, E.; Behnsen, H.; Mudersbach, M.; Krieg, H.; Springer, S.; Albrecht, S.; Herrmann, C.; Endres, H.-J. Bio-based plastics-A review of environmental, social and economic impact assessments. J. Clean. Prod. 2018, 185, 476–491. [Google Scholar] [CrossRef]
- Zeng, W.; Qiu, Y.; Chen, Y.; Yang, W.; Chen, J.; Wu, H. Recent advances in catalytic depolymerization of polyolefins. Chem. Soc. Rev. 2023, 52, 2451–2484. [Google Scholar]
- Singh, J.; Lee, J. Depolymerization of waste plastics into fuels and chemicals. ACS Sustain. Chem. Eng. 2021, 9, 12487–12506. [Google Scholar]
- Ügdüler, S.; Van Geem, K.M.; Roosen, M.; Delbeke, E.I.; De Meester, S. Challenges and opportunities of solvent-based additive extraction methods for plastic recycling. Waste Manag. 2020, 104, 148–182. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Breite, D.; Song, C.; Gräsing, D.; Ploss, T.; Hille, P.; Schwerdtfeger, R.; Matysik, J.; Schulze, A.; Zimmermann, W. Biocatalytic degradation efficiency of postconsumer polyethylene terephthalate packaging determined by their polymer microstructures. Adv. Sci. 2019, 6, 1900491. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Krüger, C.; Russ, M.; Horlacher, M.; Antony, F.; Hann, S.; Azapagic, A. Life cycle environmental impacts of chemical recycling via pyrolysis of mixed plastic waste in comparison with mechanical recycling and energy recovery. Sci. Total Environ. 2021, 769, 144483. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.-X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Sardon, H.; Dove, A.P. Plastics recycling with a difference. Nat. Catal. 2018, 1, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Jehanno, C.; Pérez-Madrigal, M.M.; Demarteau, J.; Ruipérez, F.; Sardon, H. Organocatalysis for depolymerization. Polym. Chem. 2019, 10, 172–186. [Google Scholar] [CrossRef]
- Stache, E.E.; Ertelt, M.J.; Loy, B.A.; Dove, A.P. Closed-loop recycling of poly(methyl methacrylate) via a photo-mediated process. J. Am. Chem. Soc. 2022, 144, 13445–13455. [Google Scholar]
- Kaminsky, W. Chemical recycling of plastics by fluidized bed pyrolysis. Fuel Commun. 2021, 6, 100036. [Google Scholar] [CrossRef]
- Ügdüler, S.; Van Geem, K.M.; Roosen, M.; Delbeke, E.I.; De Meester, S. Towards closed-loop recycling of multilayer and colored PET plastic waste by alkaline hydrolysis. Resour. Conserv. Recycl. 2021, 165, 105219. [Google Scholar]
- Lu, F.; Xu, F.; Wang, J.; Liu, Y.; Sun, X.; Song, J. Upcycling plastic waste into carbon-based materials for energy applications. Green Chem. 2022, 24, 715–735. [Google Scholar]
- Hernandez, H.L.; Lee, O.P.; Possanza, C.C.M.; Kaitz, J.A.; Park, C.W.; Plantz, C.L.; Moore, J.S.; White, S.R. Accelerated Thermal Depolymerization of Cyclic Polyphthalaldehyde with a Polymeric Thermoacid Generator. Macromol. Rapid Commun. 2018, 39, 1800046. [Google Scholar] [CrossRef]
- Häfliger, F.; Truong, N.P.; Wang, H.S.; Anastasaki, A. Fate of the RAFT End-Group in the Thermal Depolymerization of Polymethacrylates. ACS Macro Lett. 2023, 12, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Geng, X.; Wang, H.; Liu, J. Enzymatic depolymerization of plastic wastes: A green and sustainable solution. Trends Biotechnol. 2021, 39, 672–675. [Google Scholar]
- Tennakoon, A.; Wu, X.; Paterson, A.L.; Patnaik, S.; Pei, Y.; LaPointe, A.M.; Ammal, S.C.; Hackler, R.A.; Heyden, A.; Sadow, A.D.; et al. Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat. Catal. 2020, 3, 893–901. [Google Scholar] [CrossRef]
- McNeill, I.C.; Memetea, L.; Cole, W.J. A study of the products of PVC pyrolysis. Polym. Degrad. Stab. 1995, 49, 181–191. [Google Scholar] [CrossRef]
- Celik, G.; Kennedy, R.M.; Hackler, R.A.; Ferrandon, M.; Tennakoon, A.; Patnaik, S.; LaPointe, A.M.; Ammal, S.C.; Heyden, A.; Perras, F.A.; et al. Upcycling single-use polyethylene into high-quality liquid products. ACS Cent. Sci. 2019, 5, 1795–1803. [Google Scholar] [CrossRef]
- Rose, C.; Woodward, R.T.; Song, Y.; King, M.E.; Matson, J.B. Reversible, acid-catalyzed depolymerization of bottlebrush polymers for on-demand release. J. Am. Chem. Soc. 2022, 144, 11326–11330. [Google Scholar]
- Zhu, H.; Liu, W.; Yang, F.; Song, J.; Jiang, Z.; Zhou, M.; Huang, W. Toward sustainable polyester upcycling via catalytic hydrogenolysis. ChemSusChem 2022, 15, e202200016. [Google Scholar]
- Kumagai, S.; Shimaoka, T. Influence of polymer types on the behavior of heavy metals during the pyrolysis of plastic waste. Waste Manag. 2013, 33, 479–485. [Google Scholar]
- Garcia, J.M.; Clark, K.E.; Schwarz, J.N.; Yao, N.; Sisco, S.W. Chemical recycling of end-of-life thermosets into virigin-quality epoxies. Science 2023, 379, 78–84. [Google Scholar]
- Kim, H.E.; Park, I.; Kim, S.; Kim, J.F.; Choi, Y.H.; Lee, H. Chemical recycling of polyethylene terephthalate using a micro-sized MgO-incorporated SiO2 catalyst to produce highly pure bis(2-hydroxyethyl) terephthalate in high yield. Chem. Eng. J. 2024, 499, 155865. [Google Scholar]
- Cai, S.; Li, Y.; Wang, Y.; Guo, Z.; Liu, B.; Huang, L.; Beiyuan, J.; Liu, D.; Cha, R.; Yuan, W. Highly efficient kilogram-scale mechanochemical catalytic depolymerization of PET polyester waste into reusable monomers. Chem. Eng. J. 2024, 500, 157131. [Google Scholar] [CrossRef]
- East, A.J.; Rorrer, J.E.; Beckham, G.T. Waste polyester depolymerization catalyzed by earth-abundant metal salts. ACS Catal. 2022, 12, 14968–14980. [Google Scholar]
- Xiong, M.; Lu, Y.; Zhang, Y.; Li, X.; Li, H. Room-temperature chemical recycling of PET to DMT using deep eutectic solvents. Green Chem. 2023, 25, 1581–1591. [Google Scholar]
- Biswas, B.; Kumar, A.; Krishna, B.B.; Baltrusaitis, J.; Adhikari, S.; Bhaskar, T. Catalytic Depolymerization of Lignin for the Selective Production of Phenolic Monomers over Cobalt-Supported Calcium Catalysts. Energy Fuels 2023, 37, 3813–3824. [Google Scholar] [CrossRef]
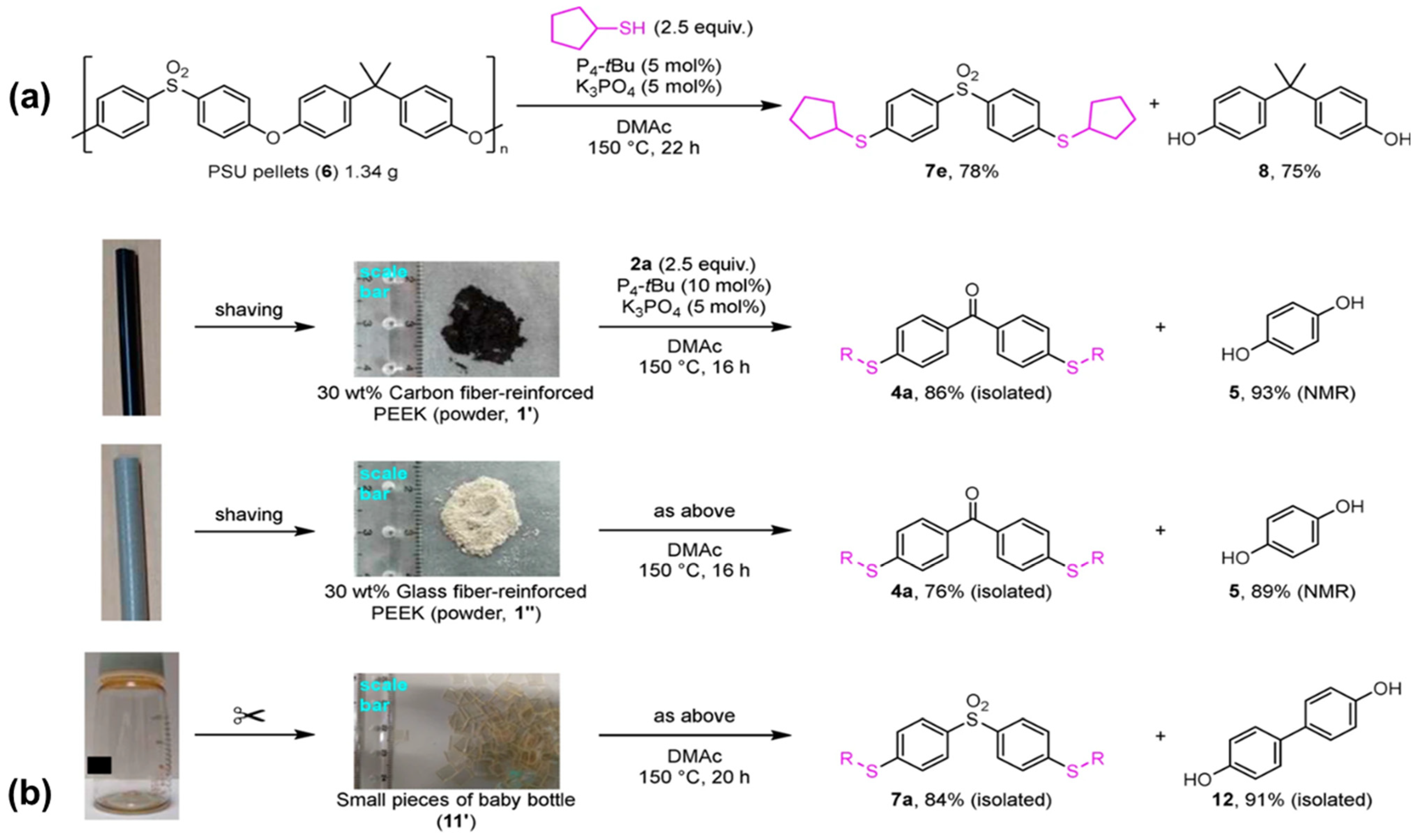
- Minami, Y.; Imamura, S.; Matsuyama, N.; Nakajima, Y.; Yoshida, M. Catalytic thiolation-depolymerization-like decomposition of oxyphenylene-type super engineering plastics via selective carbon–oxygen main chain cleavages. Commun. Chem. 2024, 7, 37. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Zhang, C.; Huang, H.; Yuan, T. Catalytic degradation of waste plastics in supercritical fluids: A review. J. Supercrit. Fluids 2020, 160, 104800. [Google Scholar]
- Wei, R.; Oeser, T.; Then, J.; Kühnel, S.; Zimmermann, W. Engineered cutinases for enhanced PET hydrolysis. Biotechnol. Bioeng. 2016, 113, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.-S.; Imam, S.H.; Orts, W.J.; Chiellini, E. Thermal, mechanical and morphological characterization of PLA plasticized with hydrolyzable esters. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.P.; Sridhar, M.; Rao, R.G. Biological depolymerization of lignin using laccase harvested from the autochthonous fungus Schizophyllum commune employing various production methods and its efficacy in augmenting in vitro digestibility in ruminants. Sci. Rep. 2022, 12, 11170. [Google Scholar] [CrossRef]
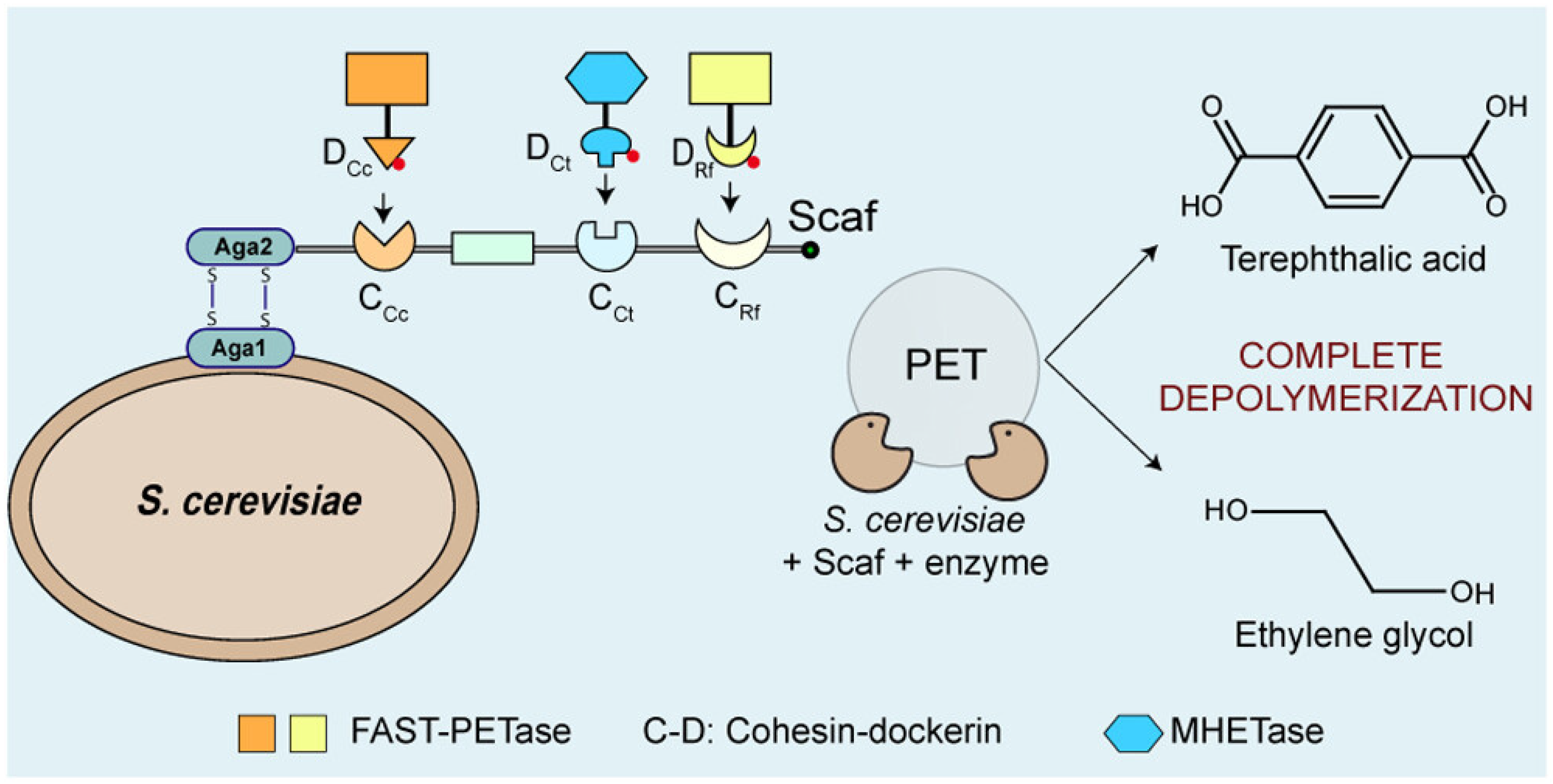
- Gulati, S.; Sun, Q. Complete Enzymatic Depolymerization of Polyethylene Terephthalate (PET) Plastic Using a Saccharomyces cerevisiae-Based Whole-Cell Biocatalyst. Environ. Sci. Technol. Lett. 2025, 12, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.-P. Managing plastic waste Sorting, recycling, disposal, and product redesign. ACS Sustain. Chem. Eng. 2021, 9, 15722–15738. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Liu, L.; Zhang, M. Advances in ionic liquids for polymer recycling. Prog. Polym. Sci. 2022, 126, 101496. [Google Scholar]
- Zhang, H.; Tian, M.; Zhu, L.; Ma, J.; Cui, Y. Bio-upcycling of PET plastics via microbial metabolic pathways. Biotechnol. Adv. 2022, 60, 108015. [Google Scholar]
- Go, J.; Kim, H.; Kim, S.; Lee, J. Catalytic cracking of plastic waste: A review on catalysts, mechanism, and reactor design. Catalysts 2021, 11, 248. [Google Scholar]
- Cui, Y.; Sun, Y.; Jiang, Z.; Liu, Y.; Tan, T. Depolymerization of polycarbonate plastic waste using bio-based solvents and heterogeneous catalysis. Green Chem. 2021, 23, 8690–8702. [Google Scholar]
- Meng, X.; Chen, Y.; Feng, S.; Wang, J.; Ding, S.-Y.; Zhang, H. Chemical recycling of plastics to monomers and fuels using solar energy. Chem. Soc. Rev. 2022, 51, 5039–5057. [Google Scholar]
- Zhang, X.; MacArthur, M.R.; Rorrer, N.A.; Nicholson, S.R.; Carpenter, A.C.; Walker, T.W.; Beckham, G.T. Biocatalytic upcycling of PET to monomers and bioproducts. Green Chem. 2020, 22, 3721–3731. [Google Scholar]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.; Inuwa, I.M. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Barlow, C.Y.; Morgan, D.C. Polymer film packaging for food: An environmental assessment. Resour. Conserv. Recycl. 2013, 78, 74–80. [Google Scholar] [CrossRef]
- Dufaud, O.; Basset, J.M. Catalytic hydrogenolysis of polyethylene and polystyrene into lubricants and waxes. Angew. Chem. Int. Ed. 1998, 37, 806–810. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Change Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Yoo, M.; Jeong, Y.; Kim, M.; Lee, S. Life cycle assessment of chemical recycling technologies for PET. Sustainability 2020, 12, 10401. [Google Scholar]
- Ma, Y.; Xin, X.; Wang, C.; Guo, X.; Zhang, W. Advances in acid/base catalyzed depolymerization of polyesters. Catal. Sci. Technol. 2021, 11, 1348–1364. [Google Scholar]
- Olarte, M.V.; Ferrell, J.R.; Christensen, E.D.; Padmaperuma, A.B.; Zacher, A.H.; Harmon, M.E. Catalytic hydrothermal liquefaction of plastics: A review. ACS Sustain. Chem. Eng. 2020, 8, 9245–9264. [Google Scholar]
- Chen, Y.; Jiang, X.; Liu, Y.; Ding, S. Upcycling of plastic waste to functional materials: A review. Chem. Eng. J. 2022, 445, 136759. [Google Scholar]
- Walker, T.W.; Frelka, N.; Shen, Z.; Chew, A.K.; Banick, J.; Grey, S.; Kim, M.S.; Dumesic, J.A.; Van Lehn, R.C.; Huber, G.W. Recycling of multilayer plastic packaging materials by solvent-targeted recovery and precipitation. Sci. Adv. 2020, 6, eaba7599. [Google Scholar] [CrossRef]
- Trinh, T.K.; Kwon, E.E.; Kim, J. Solvent-free catalytic upcycling of plastic waste into aromatic chemicals. J. Hazard. Mater. 2021, 402, 123771. [Google Scholar]
- Rorrer, J.E.; Nicholson, S.R.; Carpenter, A.C.; Zhang, X.; Beckham, G.T. Combining bio- and chemocatalysis for plastics upcycling. Trends Chem. 2021, 3, 761–774. [Google Scholar]
- Upadhyayula, V.K.K. Enzymatic and microbial depolymerization of synthetic plastics. Crit. Rev. Biotechnol. 2021, 41, 594–611. [Google Scholar]
- Dey, K.; Mondal, S.; Das, A.K.; Pal, T. Smart catalytic systems for green depolymerization of plastic waste: A review. J. Environ. Chem. Eng. 2022, 10, 107152. [Google Scholar]
- Ji, L.; Meng, J.; Li, C.; Wang, M.; Jiang, X. From Polyester Plastics to Diverse Monomers via Low-Energy Upcycling. Adv. Sci. 2024, 11, 2403002. [Google Scholar] [CrossRef]
- Amalia, L.; Chang, C.; Wang, S.S.S.; Yeh, Y.; Tsai, S. Recent advances in the biological depolymerization and upcycling of polyethylene terephthalate. Curr. Opin. Biotechnol. 2024, 85, 103053. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sajwan, D.; Sharma, D.; Krishnan, V. Reductive Upcycling of Polyolefins, Polyesters and Mixed Plastic Wastes to Valuable Chemicals: Bridging Chemical Catalysis With Plastic Waste Management. Adv. Sustain. Syst. 2025, 9, 2500003. [Google Scholar] [CrossRef]
- Nyam, T.T.; Ayeleru, O.O.; Ramatsa, I.M.; Olubambi, P.A. Conversion of Waste Plastics into Value-added Materials: A Global Perspective. Plast. Waste Manag. Methods Appl. 2024, 2024, 227–258. [Google Scholar]
- Li, C.; Yan, G.; Dong, Z.; Zhang, G.; Zhang, F. Upcycling waste commodity polymers into high-performance polyarylate materials with direct utilization of capping agent impurities. Nat. Commun. 2025, 16, 2482. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, W.; Zhao, X.; Liu, Y.; Yan, L.; Liu, X.; Xu, S.; Wang, Z. Adjustable Upcycling of Polyethylene Terephthalate to Biodegradable Polymer Monomers by Mn-Catalyzed Solvent Switching Strategy. Angew. Chem. Int. Ed. 2025; in press. [Google Scholar] [CrossRef]
- Deng, T.; Chen, Y.; Hou, D. Practically simple, catalyst-free, and scalable approach for all-component upcycling of mixed PVC/PA plastics. Waste Manag. 2025, 205, 115002. [Google Scholar] [CrossRef]
- Wu, Y.; Nguyen, P.T.; Wong, S.S.; Feng, M.; Han, P.; Yao, B.; He, Q.; Sum, T.C.; Zhang, T.; Yan, N. Photocatalytic upcycling of polylactic acid to alanine by sulfur vacancy-rich cadmium sulfide. Nat. Commun. 2025, 16, 846. [Google Scholar] [CrossRef]
- Hourtoule, M.; Trienes, S.; Ackermann, L. Anodic Commodity Polymer Recycling: The Merger of Iron-Electrocatalysis with Scalable Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2024, 63, e202412689. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, S.; Tang, J.; Fu, L. Sustainably Recycling and Upcycling of Single-Use Plastic Wastes through Heterogeneous Catalysis. Catalysts 2022, 12, 818. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Nie, M.; Liu, X.; Wang, Q.; Chen, N.; Zhang, C. Lifecycle Management for Sustainable Plastics: Recent Progress from Synthesis, Processing to Upcycling. Adv. Mater. 2024, 36, 2404115. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Tu, W.; Yang, S.; Zhang, C.; Li, Q.; Zhang, Q.; Chen, J. Sustainable chemical upcycling of waste polyolefins by heterogeneous catalysis. SusMat 2022, 2, 161–185. [Google Scholar] [CrossRef]
- Wei, X.; Zheng, W.; Chen, X.; Qiu, J.; Sun, W.; Xi, Z.; Zhao, L. Chemical upcycling of poly(ethylene terephthalate) with binary mixed alcohols toward value-added copolyester by depolymerization and repolymerization strategy. Chem. Eng. Sci. 2024, 294, 120103. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, Q.; Zhang, Y.; Guo, H.; Wu, Y.; Sun, M.; Zhu, X.; Zhang, J.; Gong, S.; Liu, P.; et al. Depolymerization of polyesters by a binuclear catalyst for plastic recycling. Nat. Sustain. 2023, 6, 965–973. [Google Scholar] [CrossRef]
- Shi, Y.; Diao, X.; Ji, N.; Ding, H.; Ya, Z.; Xu, D.; Wei, R.; Cao, K.; Zhang, S. Advances and Challenges for Catalytic Recycling and Upgrading of Real-World Mixed Plastic Waste. ACS Catal. 2025, 15, 841–868. [Google Scholar] [CrossRef]
- Zeng, M.; Lee, Y.-H.; Strong, G.; LaPointe, A.M.; Kocen, A.L.; Qu, Z.; Coates, G.W.; Scott, S.L.; Abu-Omar, M.M. Chemical Upcycling of Polyethylene to Value-Added α,ω-Divinyl-Functionalized Oligomers. ACS Sustain. Chem. Eng. 2021, 9, 13926–13936. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Z.; Wei, W.; Ni, B. Perspectives on sustainable plastic treatment: A shift from linear to circular economy. TrAC Trends Anal. Chem. 2024, 173, 117631. [Google Scholar] [CrossRef]
- Kumari, A.; Debbarma, S.; Maurya, P.; Anand, V. Depolymerization of waste plastics and chemicals. Green Chem. Approaches Environ. Sustain. 2023, 2023, 337–356. [Google Scholar]
- Xu, S.; Han, Z.; Yuan, K.; Qin, P.; Zhao, W.; Lin, T.; Zhou, T.; Huang, F. Upcycling chlorinated waste plastics. Nat. Rev. Methods Primers 2023, 3, 44. [Google Scholar] [CrossRef]
- Saito, K.; Eisenreich, F.; Türel, T.; Tomović, Ž. Closed-Loop Recycling of Poly(Imine-Carbonate) Derived from Plastic Waste and Bio-based Resources. Angew. Chem. 2022, 134, e202211806. [Google Scholar] [CrossRef]
- Musa, A.; Jaseer, E.A.; Barman, S.; Garcia, N. Review on Catalytic Depolymerization of Polyolefin Waste by Hydrogenolysis: State-of-the-Art and Outlook. Energy Fuels 2024, 38, 1676–1691. [Google Scholar] [CrossRef]
- Ng, K.W.J.; Yu, E.; Hu, C.-P.; Liang, Y.N.; Periasamy, K.; Chen, H.; Hu, X. Continuous Rapid Depolymerization Process to Upcycle Polyethylene Terephthalate into Polyols. ACS Sustain. Chem. Eng. 2025, 13, 4170–4181. [Google Scholar] [CrossRef]
- Spicer, A.J.; Brandolese, A.; Dove, A.P. Selective and Sequential Catalytic Chemical Depolymerization and Upcycling of Mixed Plastics. ACS Macro Lett. 2024, 13, 189–194. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, M.; Jiao, Y.; Li, Y.; Sun, B.; Xiao, D.; Wang, M.; Ma, D. Co-upcycling of polyvinyl chloride and polyesters. Nat. Sustain. 2023, 6, 1685–1692. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, Y.; Yuan, Y.; Han, Y.; Su, T.; Qi, Q. Upcycling of PET oligomers from chemical recycling processes to PHA by microbial co-cultivation. Waste Manag. 2023, 172, 51–59. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Z.; Wei, W.; Ni, J. Electrocatalysis-driven sustainable plastic waste upcycling. Electron 2024, 2, e34. [Google Scholar] [CrossRef]
- Wang, C.; El-Sepelgy, O. Reductive depolymerization of plastics catalyzed with transition metal complexes. Curr. Opin. Green Sustain. Chem. 2021, 32, 100547. [Google Scholar] [CrossRef]
- Kim, J.; Masoumilari, S.; Park, Y.; Lee, S.; Kyung, D.; Masoumi, Z. Advancements in the Electrochemical Upcycling of Waste Plastics into High-Value Products. Crystals 2025, 15, 293. [Google Scholar] [CrossRef]
- Pantazidis, C.; Saito, K.; Chen, R.; Tomović, Ž. Closed-Loop Recyclable Polyhexahydrotriazine Aerogels Derived from PET Waste. Small 2025, 21, 2502885. [Google Scholar] [CrossRef]
- Yu, Y.; Qi, Y.; Tang, J.; Yan, B.; Lou, L.; Wu, W.; Mei, Q. Selective oxidative upcycling of PET plastic waste into aniline and terephthalic acid using nitrobenzene. Chem. Eng. J. 2025, 508, 160970. [Google Scholar] [CrossRef]
- Tuli, V.; Luo, C.; Robinson, B.; Hu, J.; Wang, Y. Microwave-assisted catalytic technology for sustainable production of valuable chemicals from plastic waste with enhanced catalyst reusability. Chem. Eng. J. 2024, 489, 151551. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, X.; Zhang, J.; Anandita, S.; Liu, W.; Koh, S.W.; Yu, S.; Li, C.; Chen, Z.; Xu, R.; et al. Solar-Driven Photoelectrochemical Upcycling of Polyimide Plastic Waste with Safe Green Hydrogen Generation. Adv. Energy Mater. 2024, 14, 2400037. [Google Scholar] [CrossRef]
- Stevanovic, S.; Milovanovic, J.; Padamati, R.B.; Cosovic, V.R.; Milosevic, D.; Argirusis, C.; Sourkouni, G.; Nikodinovic-Runic, J.; Ponjavic, M. Upcycling PET plastic waste into bacterial nanocellulose based electro catalyst efficient in direct methanol fuel cells. Carbon Resour. Convers. 2025; in press. [Google Scholar] [CrossRef]
- Waribam, P.; Rajeendre Katugampalage, T.; Opaprakasit, P.; Ratanatawanate, C.; Chooaksorn, W.; Pang Wang, L.; Liu, C.; Sreearunothai, P. Upcycling plastic waste: Rapid aqueous depolymerization of PET and simultaneous growth of highly defective UiO-66 metal-organic framework with enhanced CO2 capture via one-pot synthesis. Chem. Eng. J. 2023, 473, 145349. [Google Scholar] [CrossRef]
- Feng, Y.; Quan, X.; Wang, Q.; Zhang, Y.; Liu, C.; Yuan, X.; Zhao, S.; Yang, J.; He, W.; Guo, K. Recent Advances in the Chemical Recycling of Polyamide for a Sustainable Circular Economy. Ind. Eng. Chem. Res. 2025, 64, 2516–2530. [Google Scholar] [CrossRef]
- Garratt, A.; Nguyen, K.; Brooke, A.; Taylor, M.J.; Francesconi, M.G. Photocatalytic Hydrolysis—A Sustainable Option for the Chemical Upcycling of Polylactic Acid. ACS Environ. Au 2023, 3, 342–347. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, D.; Huang, B.; Shen, Y.; Li, Z. Chemical Upcycling of Poly(3-hydroxybutyrate) (P3HB) toward Functional Poly(amine-alt-ester) via Tandem Degradation and Ring-Opening Polymerization. Macromolecules 2022, 55, 9697–9704. [Google Scholar] [CrossRef]
- Ruan, J.; Cao, Q.; Li, X.; Ren, Q.; Li, M.; Dong, S.; Li, N.; Xu, Q.; Li, H.; Lu, J.; et al. Morphology Optimization of Spinel Catalysts for High-Efficiency Photothermal Catalytic Upcycling of Polyethylene Terephthalate. Adv. Mater. 2025, 37, 2500090. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, B.; Xiong, L.; Liu, W.; Wang, D.; Ma, W.; Jiang, L.; Yang, J.; Wang, P.; Xiao, T.; et al. Highly selective upcycling of plastic mixture waste by microwave-assisted catalysis over Zn/b-ZnO. Nat. Commun. 2025, 16, 1726. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, Y.T.; Tsang, Y.F.; Lee, J. Sustainable ethylene production: Recovery from plastic waste via thermochemical processes. Sci. Total Environ. 2023, 903, 166789. [Google Scholar] [CrossRef]
- Razote, B.J.; Saabome, S.M.; Hong, J.S.; Ahn, K.H. Integrating thermodynamic and kinetic approaches for the design of effective and sustainable PET chemical upcycling systems. Chem. Eng. J. 2024, 499, 156438. [Google Scholar] [CrossRef]
- Yue, S.; Zhao, Z.; Zhang, T.; Li, F.; Wang, P.; Zhan, S. Photoreforming of Plastic Waste to Sustainable Fuels and Chemicals: Waste to Energy. Environ. Sci. Technol. 2024, 58, 22865–22879. [Google Scholar] [CrossRef] [PubMed]
- Hernández, B.; Kots, P.; Selvam, E.; Vlachos, D.G.; Ierapetritou, M.G. Techno-Economic and Life Cycle Analyses of Thermochemical Upcycling Technologies of Low-Density Polyethylene Waste. ACS Sustain. Chem. Eng. 2023, 11, 7170–7181. [Google Scholar]
- Liu, Y.; Duan, H. Recent progress in upcycling of plastic wastes into value-added chemicals via photo-, electro- and photoelectro-catalytic strategies. Fundam. Res. 2025, 5, 913–918. [Google Scholar] [CrossRef]
- Lyu, J.; Lee, S.; Jung, H.; Park, Y.I.; Ahn, J.; Jin, Y.; Jeong, J.; Kim, J.C. Low-temperature chemical upcycling of poly(ethylene terephthalate) waste to recyclable polyurethane thermosets using biomass-derived materials. Chem. Eng. J. 2024, 501, 157535. [Google Scholar] [CrossRef]
- Gupta, V.; Majumdar, S.; Das, D.; Ashish, P.K.; Sarkar, R. Upcycling of waste PET via reprocessable thermoset-like covalent adaptable networks. Polymer 2025, 328, 128438. [Google Scholar] [CrossRef]
- Wang, H.; Smith, R.L.; Qi, X. Upcycling of monomers derived from waste polyester plastics via electrocatalysis. J. Energy Chem. 2025, 101, 535–561. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Wu, F.; Mincheva, R.; Hakkarainen, M.; Raquez, J.; Mielewski, D.F.; Narayan, R.; Netravali, A.N.; Misra, M. Sustainable polymers. Nat. Rev. Methods Primers 2022, 2, 46. [Google Scholar] [CrossRef]
- Qi, J.; Xia, Y.; Meng, X.; Li, J.; Yang, S.; Zou, H.; Ma, Y.; Zhang, Y.; Du, Y.; Zhang, L.; et al. Cation-Vacancy Engineering in Cobalt Selenide Boosts Electrocatalytic Upcycling of Polyester Thermoplastics at Industrial-Level Current Density. Adv. Mater. 2025, 37, 2419058. [Google Scholar] [CrossRef]
- Xing, C.; Cai, H.; Kang, D.; Sun, W. Photothermal Catalysis: An Emerging Green Approach to Upcycling Plastic Waste. Adv. Energy Sustain. Res. 2023, 4, 2300015. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Tsang, S.C.E. Beyond Hydroconversion: A Paradigm Shift for Sustainable Plastic Waste Upcycling. ACS Sustain. Chem. Eng. 2025, 13, 9367–9369. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, S.; Zhang, M.; Yu, Y.; Qin, T.; Tang, L.; Liu, Y.; Wu, W.; Mei, Q. Green recycling of waste PET plastic monomers by banana peel extract. Chem. Eng. J. 2023, 474, 145697. [Google Scholar] [CrossRef]
- Han, M.; Zhu, S.; Xia, C.; Yang, B. Photocatalytic upcycling of poly(ethylene terephthalate) plastic to high-value chemicals. Appl. Catal. B Environ. 2022, 316, 121662. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Zhang, Y.; Xu, H.; Wang, L.; Xu, S.; Wang, Z. Dual Photo-Responsive Diphenylacetylene Enables PET In-Situ Upcycling with Reverse Enhanced UV-Resistance and Strength. Angew. Chem. 2023, 135, e202314448. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Zhang, L.; Chen, Y.; Ma, W. Tandem pyrolysis catalytic reforming of plastic waste to high-yield monocyclic aromatics by one-step microwave catalysis. Energy Convers. Manag. 2024, 312, 118571. [Google Scholar] [CrossRef]
- Chen, X.; Bai, X. A single-step upcycling of PVC-containing municipal solid waste compositions for greener chemicals and clean solids as fuel or oil absorbent. J. Energy Inst. 2023, 111, 101405. [Google Scholar] [CrossRef]
- Ranganathan, P.; Chen, Y.; Rwei, S.; Lee, Y. Biomass upcycling of waste rPET to higher-value new-easy-recyclable microcellular thermoplastic (co)polyamide foams and hot-melt adhesives. Mater. Today Chem. 2022, 26, 101101. [Google Scholar] [CrossRef]
- Su, H.; Li, T.; Wang, S.; Zhu, L.; Hu, Y. Low-temperature upcycling of PET waste into high-purity H2 fuel in a one-pot hydrothermal system with in situ CO2 capture. J. Hazard. Mater. 2023, 443, 130120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, J.; Guo, X.; Chen, Y.; Sun, W.; Peng, C. Electrocatalytic reforming of polyethylene terephthalate waste plastics into high-value-added chemicals with green hydrogen generation. J. Colloid Interface Sci. 2025, 685, 29–37. [Google Scholar] [CrossRef] [PubMed]


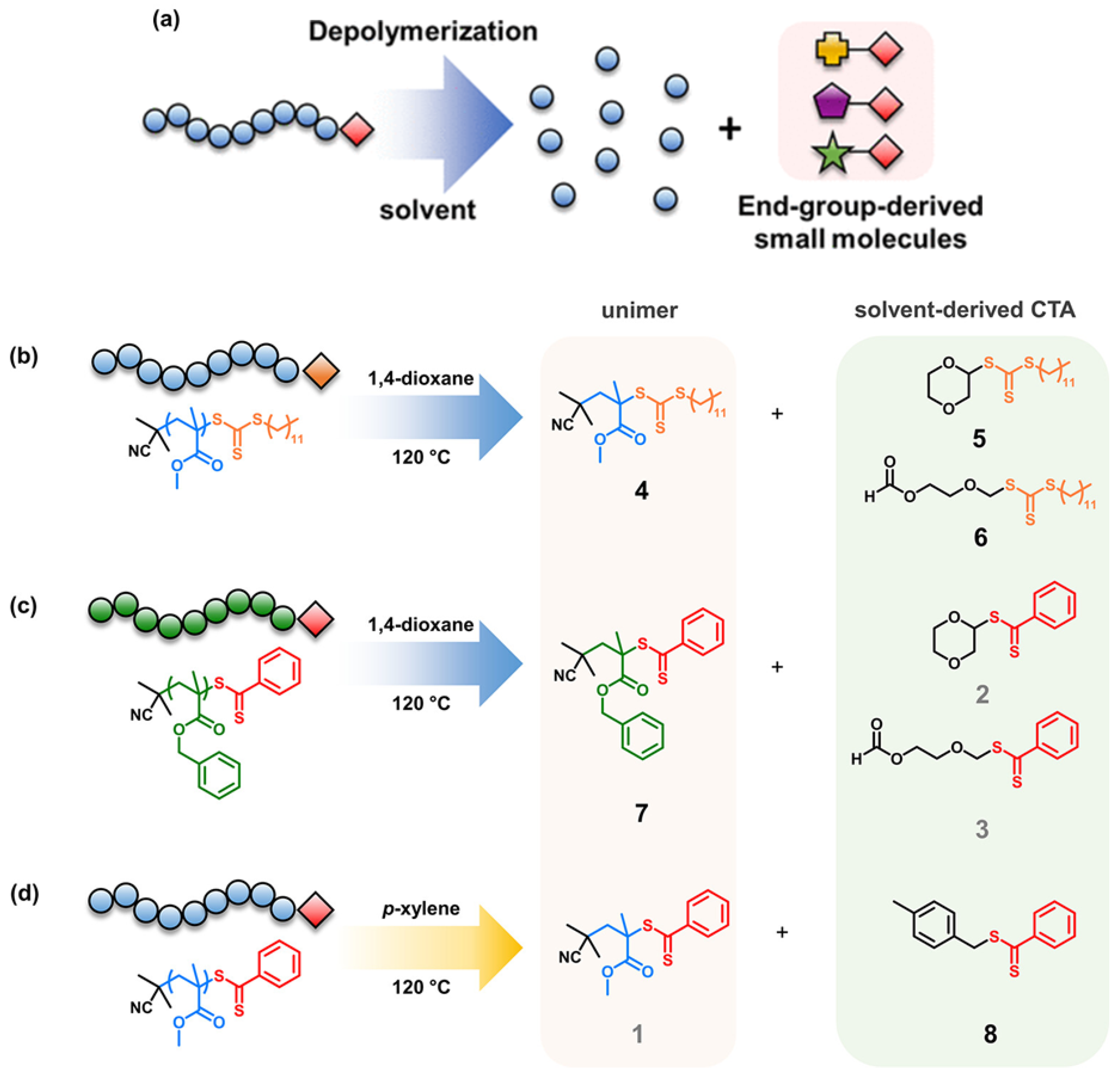
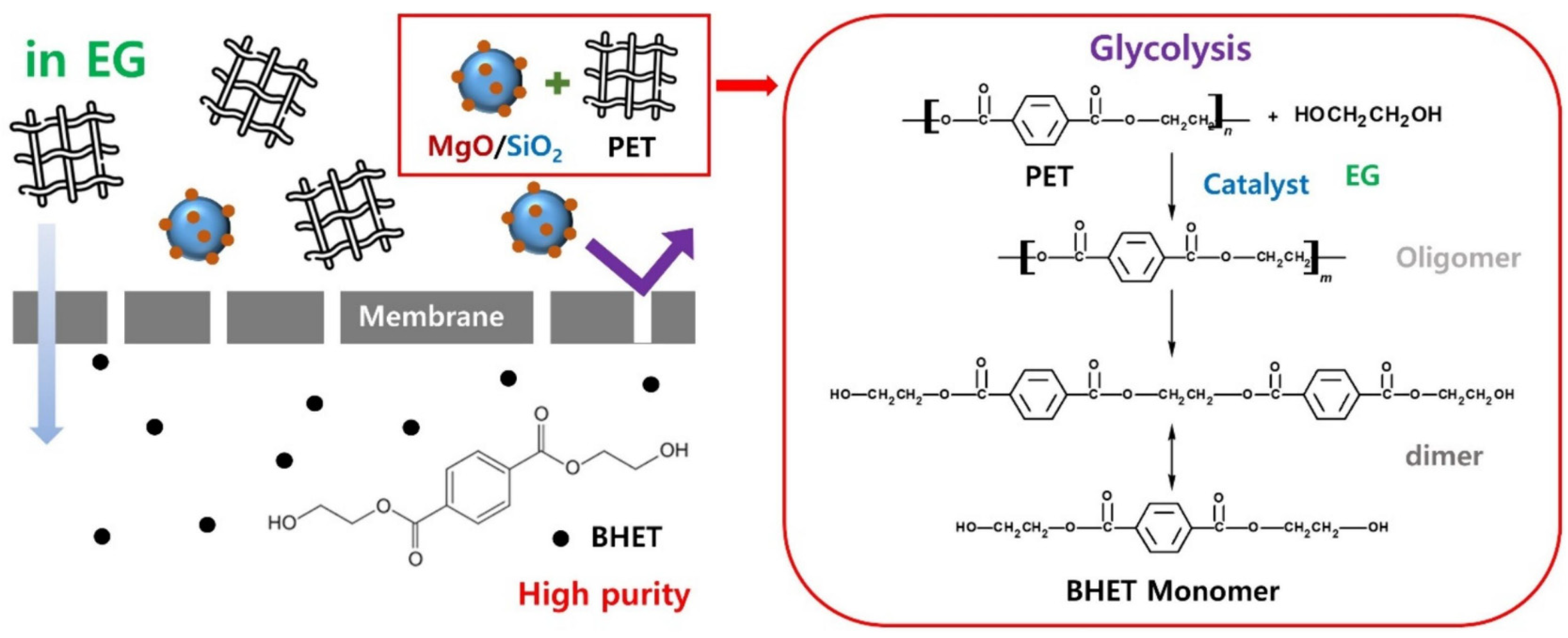
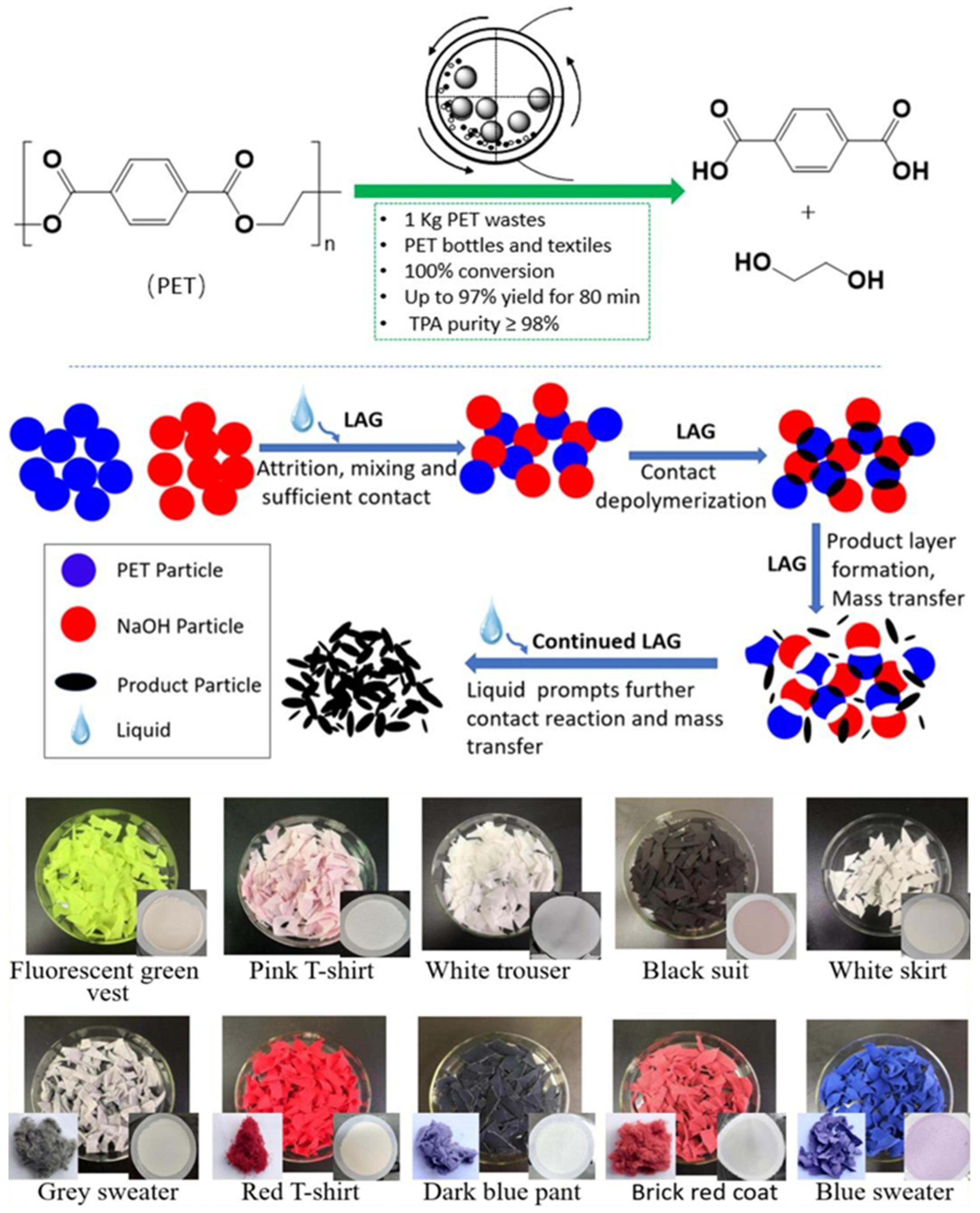
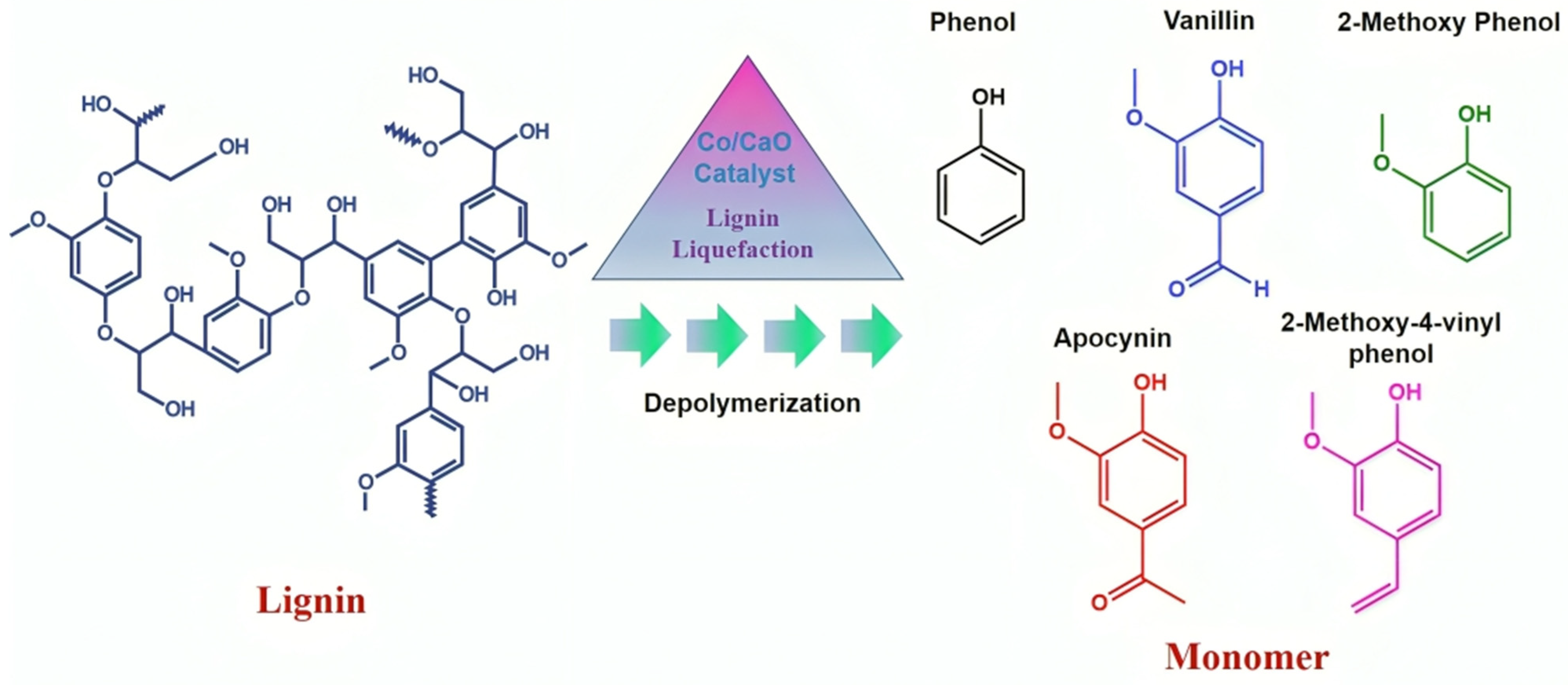

| Plastic Type | Upcycling Method | Process Conditions | Products Obtained | TRL Level |
|---|---|---|---|---|
| PET | Chemical Glycolysis | 180–220 °C, EG, catalyst (e.g., ZnAc2, MgO/SiO2) | BHET, TPA | 7–8 |
| PET | Enzymatic Depolymerization | 30–70 °C, pH 7–9, engineered PETase/MHETase | TPA, EG | 5–6 |
| PU | Chemical Hydrolysis/Glycolysis | 150–220 °C, acid/base/glycol | Polyols, amines | 6–7 |
| PS | Thermal Pyrolysis | 450–550 °C, inert atmosphere | Styrene monomer, gases, oils | 7–8 |
| PE/PP | Catalytic Hydrogenolysis | 200–300 °C, H2, Ru/CeO2 catalyst | Alkanes, fuels, waxes | 5–6 |
| Mixed Plastics | Supercritical Solvolysis | >350 °C, 250 bar, water/ethanol | Monomers, oils | 4–5 |
| PLA | Alcoholysis | 130–160 °C, ethanol or methanol | Lactate esters | 6–7 |
| Nylon-6 | Acid Hydrolysis | 200–250 °C, HCl, water | Caprolactam | 6–8 |
| Criteria | Thermal Depolymerization | Chemical Depolymerization | Catalytic Depolymerization | Biological Depolymerization |
|---|---|---|---|---|
| Target Polymers | PE, PP, PS, Mixed plastics | PET, PU, Nylon, PC | PE, PP, PET, PS | PET, PLA, PCL |
| Temperature Range | 300–800 °C | 150–250 °C | 180–350 °C | 30–70 °C |
| Reaction Time | Minutes–hours | Hours | Minutes–hours | Hours–days |
| Catalyst Requirement | Optional | Often not required (acid/base catalysts used) | Required (e.g., metal, zeolite, MOF, ILs) | Enzymes (e.g., PETase, cutinase) |
| Selectivity | Low (broad product range) | High (monomer-targeted) | High to moderate | Very high (bond cleavage) |
| Feedstock Flexibility | High (mixed, contaminated waste) | Moderate–low (requires clean feedstock) | Moderate | Low (pure polymers preferred) |
| Energy Consumption | Very high | Moderate | Moderate to high | Low |
| Environmental Impact | High (CO2, VOCs emissions) | Moderate (chemical use, waste generation) | Moderate (depends on catalyst and conditions) | Low (greenest method) |
| Scalability | High (already commercialized) | Medium (semi-commercial) | Medium (emerging tech) | Low (lab/pilot scale) |
| Main Products | Hydrocarbons, oils, gases | Monomers (e.g., BHET, TPA, EG) | Monomers, fuels, lubricants | Monomers (e.g., TPA, EG) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanaraj, R.; Suresh Kumar, S.M.; Kim, S.C.; Santhamoorthy, M. A Review on Sustainable Upcycling of Plastic Waste Through Depolymerization into High-Value Monomer. Processes 2025, 13, 2431. https://doi.org/10.3390/pr13082431
Vanaraj R, Suresh Kumar SM, Kim SC, Santhamoorthy M. A Review on Sustainable Upcycling of Plastic Waste Through Depolymerization into High-Value Monomer. Processes. 2025; 13(8):2431. https://doi.org/10.3390/pr13082431
Chicago/Turabian StyleVanaraj, Ramkumar, Subburayan Manickavasagam Suresh Kumar, Seong Cheol Kim, and Madhappan Santhamoorthy. 2025. "A Review on Sustainable Upcycling of Plastic Waste Through Depolymerization into High-Value Monomer" Processes 13, no. 8: 2431. https://doi.org/10.3390/pr13082431
APA StyleVanaraj, R., Suresh Kumar, S. M., Kim, S. C., & Santhamoorthy, M. (2025). A Review on Sustainable Upcycling of Plastic Waste Through Depolymerization into High-Value Monomer. Processes, 13(8), 2431. https://doi.org/10.3390/pr13082431







