Environmental Impact of Biodegradable Packaging Based on Chia Mucilage in Real Water Bodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Collection
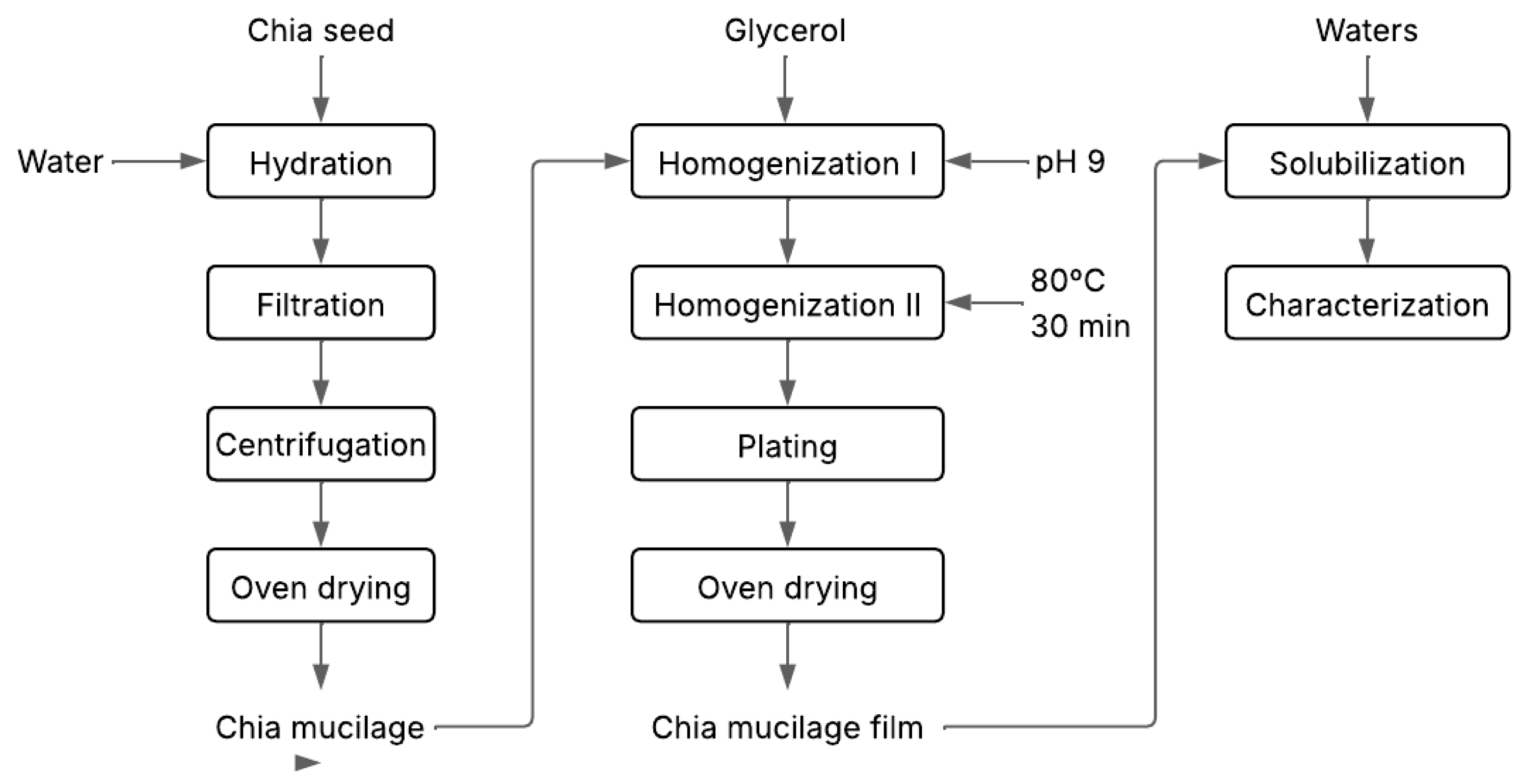
2.2. Development of Biodegradable Film
2.3. Solubilization Test
2.4. Time for Solubilization
2.5. pH
2.6. Salinity
2.7. Turbidity
2.8. Apparent Color
2.9. Total Hardness
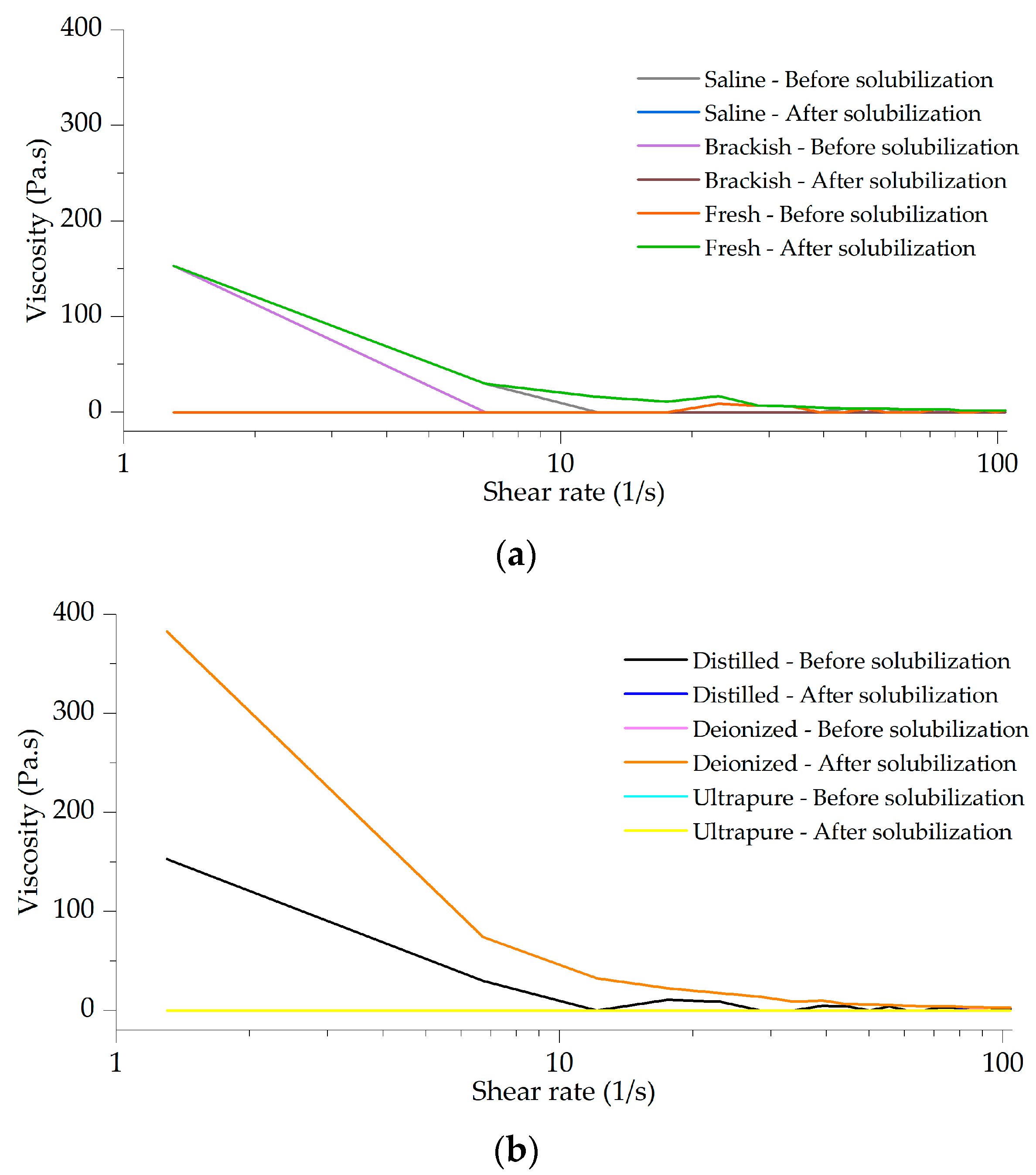
2.10. Viscosity
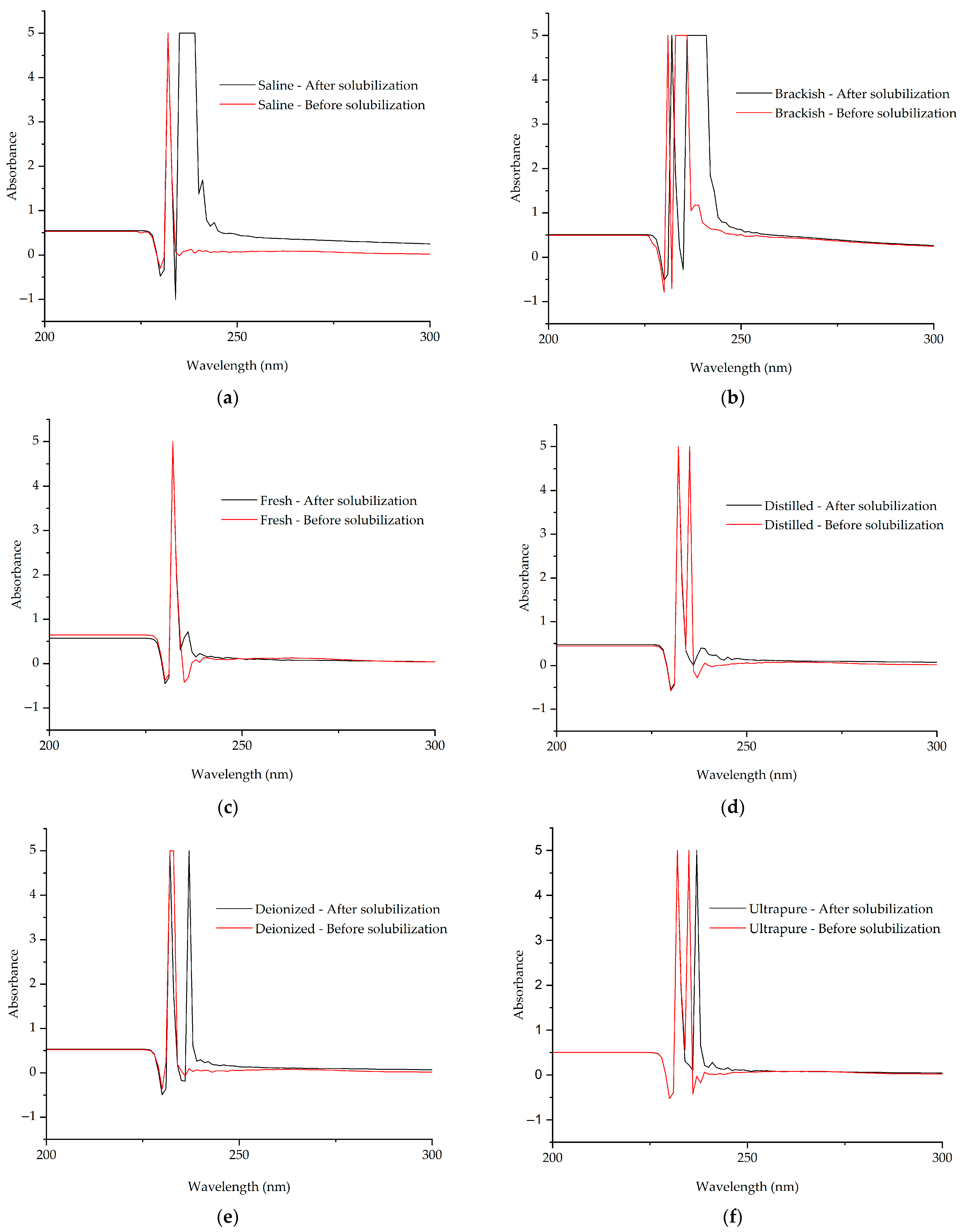
2.11. Ultraviolet and Visible (UV–Vis) Spectra
2.12. Germination Bioassay
2.13. Statistical Analysis
3. Results
3.1. Time for Solubilization and Physical-Chemical Parameters
3.2. Viscosity
3.3. UV-Vis Spectroscopy
3.4. Germination Bioassay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SDGs | Sustainable Development Goals |
| ABNT | Associação Brasileira de Normas Técnicas (Brazilian Association of Technical Standards) |
| NBR | Normativa brasileira (Brazilian regulations) |
| CONAMA | Conselho Nacional do Meio Ambiente (National Environmental Council) |
| EDTA | Ethylenediamine tetraacetic acid |
| UV–Vis | Ultraviolet and visible |
| GR | germination rate |
| RGI | Relative germination index |
| nd | Not detected |
References
- Dang, X.; Cai, Y.; Wang, X. An All-Natural Strategy for Versatile Biomass-Based Active Food Packaging Film with Superior Biodegradability, Antioxidant and Antimicrobial Activity. Food Chem. 2025, 480, 143922. [Google Scholar] [CrossRef]
- ABIPLAST. The Plastic Transformation and Recycling Industries in Brazil—Perfil 2023. Available online: https://www.abiplast.org.br/publicacoes/perfil-2023/ (accessed on 2 July 2025).
- United Nations. The 17 Sustainable Development Goals (SDGs). Available online: https://sdgs.un.org/goals (accessed on 2 July 2025).
- Grisales-Mejía, J.F.; Martínez-Correa, H.A.; Andrade-Mahecha, M.M. Biodegradable and Antioxidant Films with Barrier Properties to Visible and Ultraviolet Light Using Hass Avocado (Persea americana Mill.) by-Products. Food Bioprod. Process. 2024, 148, 154–164. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Salas-Mellado, M.D.L.M. Addition of Chia Seed Mucilage for Reduction of Fat Content in Bread and Cakes. Food Chem. 2017, 227, 237–244. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Cardoso, P.D.; Egea, M.B.; Martínez, J.P.Q.; Campos, M.R.S.; Otero, D.M. Chia Mucilage Carrier Systems: A Review of Emulsion, Encapsulation, and Coating and Film Strategies. Food Res. Int. 2023, 172, 113125. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Romani, V.P.; da Silva Filipini, G.; Martins, V.G. Chia Seeds to Develop New Biodegradable Polymers for Food Packaging: Properties and Biodegradability. Polym. Eng. Sci. 2020, 60, 2214–2223. [Google Scholar] [CrossRef]
- Eskandari, Z.; Osanloo, M.; Mazloomi, S.M.; Zarenezhad, E.; Mollakhalili-Meybodi, N.; Nematollahi, A. Bioactive Edible Films for Chicken Breast Packaging: Integration of Chia Seed Mucilage with Nanoemulsions of Cinnamomum Zeylanicum and Satureja Khuzestanica. Appl. Food Res. 2025, 5, 100959. [Google Scholar] [CrossRef]
- Forouzan, S.; Pirsa, S.; Alirezalu, A. Biodegradable Photocatalytic Film Based on Chia Seed Mucilage (Xylose, Glucose, and Methyl Glucuronic Acid Polysaccharides) Containing Barberry Extract and SnO2 Nanoparticles. Carbohydr. Polym. Technol. Appl. 2024, 8, 100592. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, K. Xanthoceras Sorbifolium Bunge Leaf Extract Activated Chia Seeds Mucilage/Chitosan Composite Film: Structure, Performance, Bioactivity, and Molecular Dynamics Perspectives. Food Hydrocoll. 2023, 144, 109050. [Google Scholar] [CrossRef]
- Çelik, I.; Sariçoban, C. The Interaction of Chia Mucilage-Based Edible Film and Transglutaminase Enzyme on Spent Hen Meat Nuggets. Br. Poult. Sci. 2023, 64, 710–717. [Google Scholar] [CrossRef]
- Ribba, L.; Lopretti, M.; de Oca-Vásquez, G.M.; Batista, D.; Goyanes, S.; Vega-Baudrit, J.R. Biodegradable Plastics in Aquatic Ecosystems: Latest Findings, Research Gaps, and Recommendations. Environ. Res. Lett. 2022, 17, 033003. [Google Scholar] [CrossRef]
- Lavagnolo, M.C.; Poli, V.; Zampini, A.M.; Grossule, V. Biodegradability of Bioplastics in Different Aquatic Environments: A Systematic Review. J. Environ. Sci. 2024, 142, 169–181. [Google Scholar] [CrossRef]
- ABNT—Associação Brasileira de Normas Técnicas NBR 9898—Preservação e Técnicas de Amostragem de Efluentes Líquidos e Corpos Receptores. 1987. Available online: https://sistema.ceteclins.com.br/Uploads/PDF/B8E15D63-B64B-4106-81AB-0E807999C6DD_29012020115558.pdf (accessed on 5 June 2025).
- Brasil Resolução CONAMA N° 357, de 17 de Março de 2005 (Retificada). 2005. Available online: https://conama.mma.gov.br/?option=com_sisconama&task=arquivo.download&id=450 (accessed on 5 June 2025).
- Gontard, N.; Duchez, C.; Cuq, J.-L.; Guilbert, S. Composite Films of Wheat Gluten and Lipids: Water Vapour Permeability and Other Physical Properties. Int. J. Food Sci. Technol. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- Cuervo Lumbaque, E.; Gomes, M.F.; Da Silva Carvalho, V.; de Freitas, A.M.; Tiburtius, E.R.L. Degradation and Ecotoxicity of Dye Reactive Black 5 after Reductive-Oxidative Process: Environmental Science and Pollution Research. Environ. Sci. Pollut. Res. 2017, 24, 6126–6134. [Google Scholar] [CrossRef]
- Bezerra, J.J.L.; Leonardo, A.B.F.; Barbosa, I.C.; de Oliveira, A.F.M. Phytochemical Analysis and Allelopathic Effects of Aqueous Extracts of Solanum americanum Mill. and Solanum stramoniifolium Jacq. on Lactuca sativa L. Ecol. Front. 2025, 45, 829–835. [Google Scholar] [CrossRef]
- Priac, A.; Badot, P.M.; Crini, G. Évaluation de La Phytotoxicité d’eaux de Rejets via Lactuca Sativa: Paramètres Des Tests de Germination et d’élongation. Comptes Rendus Biol. 2017, 340, 188–194. [Google Scholar] [CrossRef]
- Deroiné, M.; Le Duigou, A.; Corre, Y.M.; Le Gac, P.Y.; Davies, P.; César, G.; Bruzaud, S. Accelerated Ageing of Polylactide in Aqueous Environments: Comparative Study between Distilled Water and Seawater. Polym. Degrad. Stab. 2014, 108, 319–329. [Google Scholar] [CrossRef]
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia Seeds: Microstructure, Mucilage Extraction and Hydration. J. Food Eng. 2012, 108, 216–224. [Google Scholar] [CrossRef]
- da Silveira Ramos, I.F.; Fernandes, V.L.; de Alencar Lucena, M.; Geronço, M.S.; da Costa, M.P.; dos Santos Rizzo, M.; Ribeiro, A.B. Chia Seed Mucilage (Salvia hispanica L.): An Emerging Biopolymer for Industrial Application. Braz. J. Dev. 2023, 9, 2237–2258. [Google Scholar] [CrossRef]
- Silva, J.M.; Caridade, S.G.; Costa, R.R.; Alves, N.M.; Groth, T.; Picart, C.; Reis, R.L.; Mano, J.F. PH Responsiveness of Multilayered Films and Membranes Made of Polysaccharides. Langmuir 2015, 31, 11318–11328. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Soetaert, K.; Hagens, M. Ocean Alkalinity, Buffering and Biogeochemical Processes. Rev. Geophys. 2020, 58, e2019RG000681. [Google Scholar] [CrossRef]
- Boyd, C.E. PH, Carbon Dioxide, and Alkalinity. In Water Quality; Springer International Publishing: Cham, Germany, 2015. [Google Scholar]
- Molot, L.A. Color of Aquatic Ecosystems. In Encyclopedia of Inland Waters; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Ikumi, P.W.; Mburu, M.; Njoroge, D.; Gikonyo, N.; Benjamin, M. Evaluation of the Mineral Composition of Chia (Salvia hispanica L.) Seeds from Selected Areas in Kenya. J. Food Res. 2023, 12. [Google Scholar] [CrossRef]
- Singh, H. Advancements in Biodegradable Materials: Impacts on Soil and Water Quality. Stallion J. Multidiscip. Assoc. Res. Stud. 2024, 3, 2635–3490. [Google Scholar] [CrossRef]
- Hussain, N.; Ishak, I.; Sulaiman, R.; Fauzi, N.M.; Coorey, R. Influence of Processing Conditions on Rheological Properties of Aqueous Extract Chia (Salvia hispanica L.) Mucilage. Food Res. 2020, 4, 227–236. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Ye, R.; Duan, Q. Advances on Water Quality Detection by Uv-Vis Spectroscopy. Appl. Sci. 2020, 10, 6874. [Google Scholar]
| Water Type | Time for Solubilization (min) | pH | Salinity (%) | ||
|---|---|---|---|---|---|
| Before Solubilization | After Solubilization | Before Solubilization | After Solubilization | ||
| Saline | 230.00 ± 14.14 a | 7.79 ± 0.17 a,A | 7.76 ± 0.04 a,A | 30.10 ± 0.26 a,A | 30.67 ± 0.72 a,A |
| Brackish | 155.00 ± 10.00 b | 6.74 ± 0.13 b,A | 6.90 ± 0.13 b,A | 1.27 ± 0.01 b,A | 1.27 ± 0.03 b,A |
| Fresh | 156.67 ± 11.55 b | 6.35 ± 0.04 c,A | 6.62 ± 0.18 c,A | 0.05 ± 0.01 c,A | 0.06 ± 0.01 c,A |
| Distilled | 40.00 ± 0.00 c | 7.05 ± 0.04 d,A | 6.11 ± 0.08 d,B | nd | nd |
| Deionized | 40.00 ± 0.00 c | 6.02 ± 0.05 e,B | 6.17 ± 0.03 d,A | nd | nd |
| Ultrapure | 40.00 ± 0.00 c | 5.78 ± 0.08 e,B | 6.00 ± 0.04 d,A | nd | nd |
| Water Type | Turbidity (NTU) | Apparent Color (PCU) | Total Hardness (mg CaCO3/L) | |||
|---|---|---|---|---|---|---|
| Before Solubilization | After Solubilization | Before Solubilization | After Solubilization | Before Solubilization | After Solubilization | |
| Saline | 82.23 ± 1.29 a,B | 88.23 ± 1.91 a,A | 430.50 ± 2.12 a,B | 507.00 ± 12.73 a,A | 2167.56 ± 54.31 a,B | 2535.41 ± 7.41 a,A |
| Brackish | 25.85 ± 1.30 b,B | 34.70 ± 0.56 b,A | 193.33 ± 7.64 b,B | 274.00 ± 5.66 b,A | 278.97 ± 17.28 b,A | 36.13 ± 14.81 b,A |
| Fresh | 7.86 ± 0.11 c,B | 20.20 ± 0.17 c,A | 21.00 ± 1.00 c,B | 77.33 ± 2.08 c,A | 35.80 ±1.23 c,B | 43.20 ± 3.70 b,A |
| Distilled | 1.58 ± 0.13 d,B | 9.60 ± 0.42 e,A | nd | 49.50 ± 4.95 d | nd | nd |
| Deionized | 1.04 ± 0.07 d,B | 15.73 ± 0.61 d,A | nd | 44.00 ± 4.24 d,e | nd | nd |
| Ultrapure | 0.22 ± 0.03 d,B | 9.66 ± 0.48 e,A | nd | 33.50 ± 3.54 e | nd | nd |
| Treatment | Solubilization | GR (%) | Radicle Length (cm) | RGI (%) |
|---|---|---|---|---|
| Saline | Before | 0.00 ± 0.00 a | 0.00 ± 0.00 a | - |
| After | 0.00 ± 0.00 a | 0.00 ± 0.00 a | ||
| Brackish | Before | 90.00 ± 7.07 a | 4.24 ± 0.30 b | 148.46 ± 17.20 A |
| After | 97.50 ± 3.54 a | 6.26 ± 0.28 a | ||
| Fresh | Before | 87.50 ± 17.68 a | 4.39 ± 0.47 b | 128.75 ± 12.00 A |
| After | 82.50 ± 3.54 a | 5.61 ± 0.09 a | ||
| Distilled | Before | 87.50 ± 3.54a | 3.69 ± 0.11 b | 142.37 ± 0.66 A |
| After | 85.00 ± 0.00 a | 5.26 ± 0.18 a | ||
| Deionized | Before | 92.50 ± 3.54 a | 3.54 ± 0.48 b | 149.93 ± 26.56 A |
| After | 92.50 ± 10.61 a | 5.22 ± 0.21 a | ||
| Ultrapure | Before | 95.00 ± 7.07 a | 3.67 ± 0.15 b | 142.55 ± 27.89 A |
| After | 85.00 ± 14.14 a | 5.20 ± 0.80 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.M.P.d.; Atanes, S.P.; Fernandes, S.S. Environmental Impact of Biodegradable Packaging Based on Chia Mucilage in Real Water Bodies. Processes 2025, 13, 2381. https://doi.org/10.3390/pr13082381
Silva RMPd, Atanes SP, Fernandes SS. Environmental Impact of Biodegradable Packaging Based on Chia Mucilage in Real Water Bodies. Processes. 2025; 13(8):2381. https://doi.org/10.3390/pr13082381
Chicago/Turabian StyleSilva, Renata Machado Pereira da, Stefanny Pereira Atanes, and Sibele Santos Fernandes. 2025. "Environmental Impact of Biodegradable Packaging Based on Chia Mucilage in Real Water Bodies" Processes 13, no. 8: 2381. https://doi.org/10.3390/pr13082381
APA StyleSilva, R. M. P. d., Atanes, S. P., & Fernandes, S. S. (2025). Environmental Impact of Biodegradable Packaging Based on Chia Mucilage in Real Water Bodies. Processes, 13(8), 2381. https://doi.org/10.3390/pr13082381











