Preparation and Performance Evaluation of Modified Amino-Silicone Supercritical CO2 Viscosity Enhancer for Shale Oil and Gas Reservoir Development
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Materials
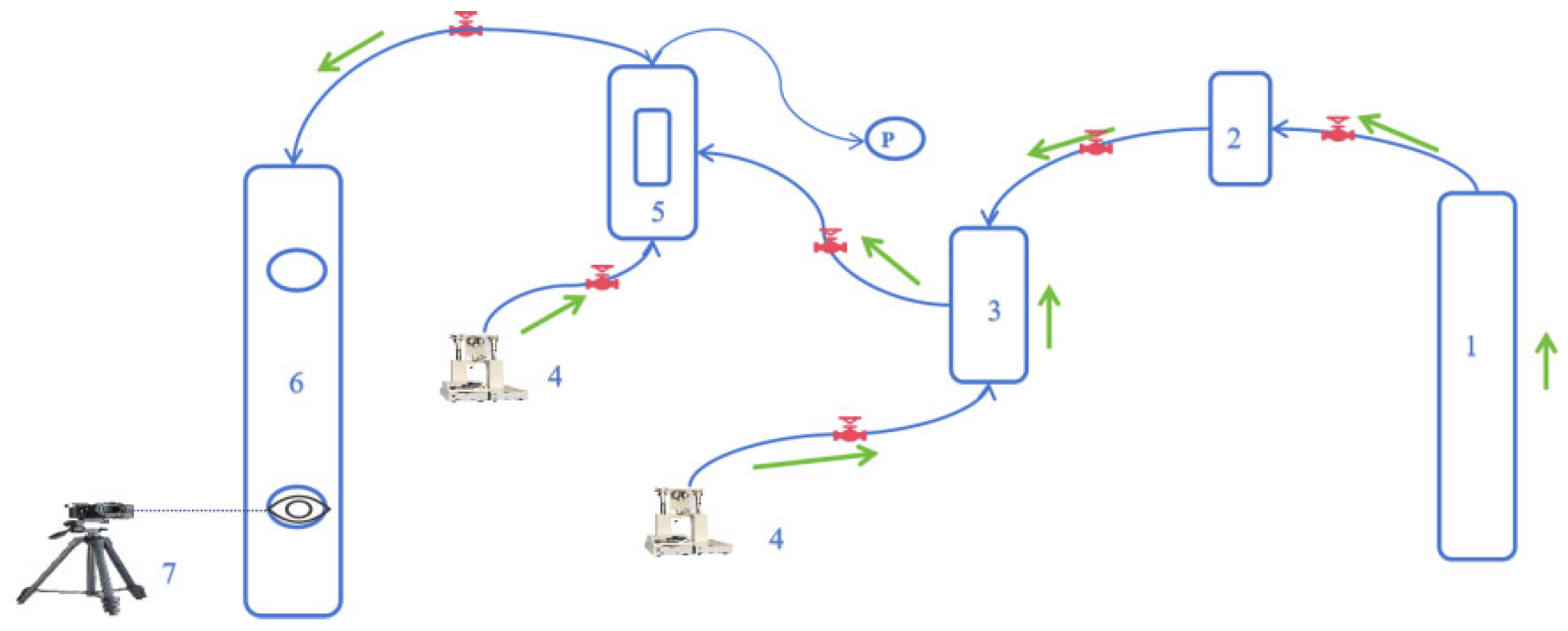
2.2. Measurement Device and Calculation Method
2.2.1. FI-IR Measurements
2.2.2. Cloud Point and Viscosity Measurement
2.3. Preparation Methods
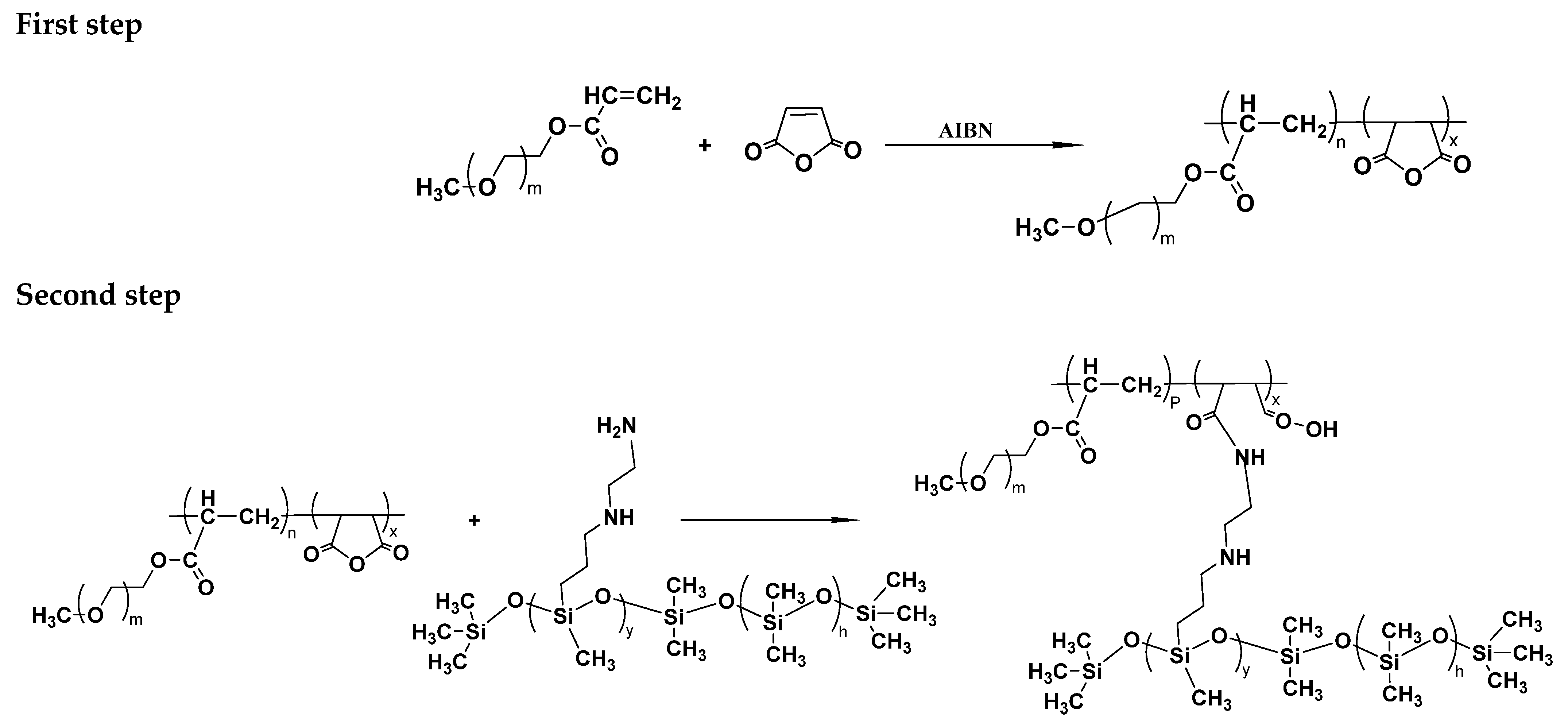
2.3.1. Synthetic Routes
2.3.2. Synthesis of MA-Co-MPEGA-AS
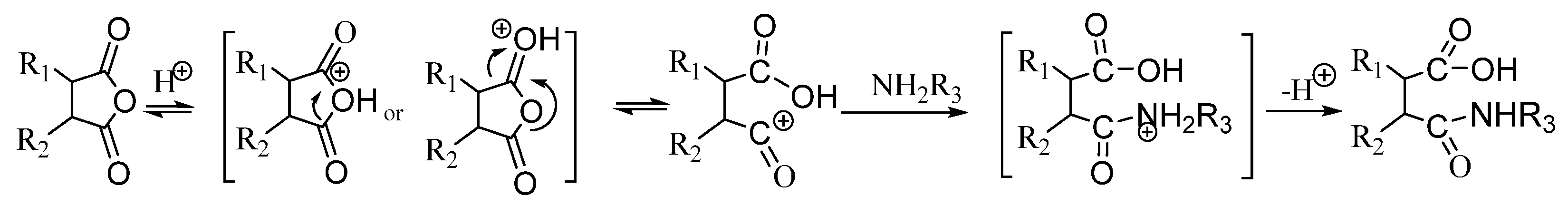
2.3.3. Reaction Mechanism
3. Results and Discussion
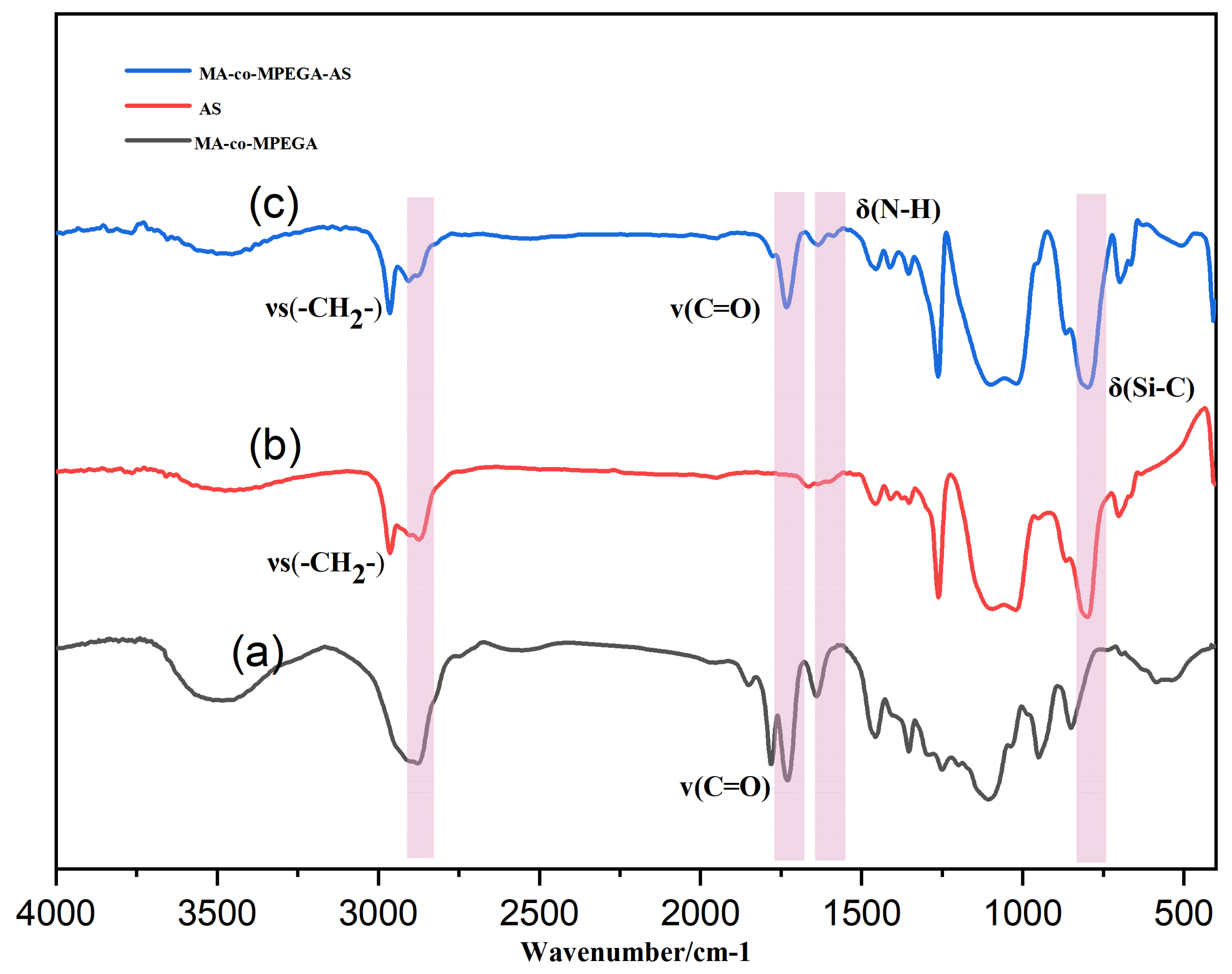
3.1. Fourier Transform Infrared (FTIR) Structural Analysis
3.2. Thickener Performance Evaluation
3.2.1. Effect of Shear Rate on the Viscosity of CO2 Thickening
3.2.2. Effect of Temperature on the Viscosity of CO2 Thickening Systems
3.2.3. Effect of Pressure on the Viscosity of CO2 Thickening Systems
3.2.4. Effect of Thickener Concentration on the Viscosity of CO2 Thickening Systems
3.3. Thickening Mechanism of Modified Amino Silicone Oil Polymer in CO2 Thickening Systems
4. Conclusions
- Mechanistic Elucidation of Anhydride–Amino Reactions: The nucleophilic acyl substitution mechanism is validated, where the reaction between maleic anhydride and amino silicone oil proceeds via a tetrahedral intermediate, eliminating the need for noble metal catalysts (e.g., chloroplatinic acid), as shown in Scheme 1 of the reference. This confirms the feasibility of Lewis acid–base catalysis for efficient amide bond formation.
- Green Synthesis and Low cost Conditions: The process operates at room temperature (25–110 °C) and ambient pressure, reducing energy consumption compared to traditional high-temperature processes. Noble-Metal-Free Catalysis: By leveraging the inherent reactivity of acid anhydrides, the synthesis avoids toxic catalysts, aligning with the “atom economy” principle of green chemistry.
- Rheological Performance and Solubility enhancement mechanism of molecular Design: The polyether segments in MA-co-MPEGA-AS interact with CO2 through Lewis base coordination (ether oxygen as electron donor), improving solubility in supercritical fluid. Viscosity Modulation: Polydimethylsiloxane side chains form a three-dimensional network, increasing viscosity via intermolecular entanglement, as observed in NCA polymerization.
- Prospects of Industrial Application in Energy Extraction.The polymer enables a 114-fold viscosity increase in CO2-based fluids, enhancing proppant transport efficiency in hydraulic fracturing without cosolvents. Cost Reduction: Eliminating precious metal catalysts reduces production costs, while mild conditions lower equipment investment and operational risks.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| scCO2 | supercritical carbon dioxide |
| AS | amino silicone oil |
| PDMS | polydimethylsiloxane |
| MA | maleic anhydride |
| MPEGA | methoxy polyethylene glycol acrylate |
| MA-co-MPEGA-AS | modified amino silicone oil polymer |
| γ | shear rate |
References
- Xie, W.; Chen, S.; Wang, M.; Yu, Z.; Wang, H. Progress and Prospects of Supercritical CO2 Application in the Exploitation of Shale Gas Reservoirs. Energy Fuels 2021, 35, 18370–18384. [Google Scholar] [CrossRef]
- Wanniarachchi, W.A.M.; Ranjith, P.G.; Perera, M.S.A. Perera 1Shale gas fracturing using foam-based fracturing fluid a review. Environ. Earth Sci. 2017, 76–91. [Google Scholar]
- Li, Y.; Lei, Z.; Shen, Y.; Dai, M.; Song, X.; Zhang, J.; Cheng, P.; Yang, Y.; Huang, W. Understanding the Interaction Mechanism of CO2 and Position Isomeric Organic Absorbents by Density Functional Theory. Comput. Theor. Chem. 2023, 1226, 114191. [Google Scholar] [CrossRef]
- Polikhronidi, N.; Batyrova, R.; Aliev, A.; Abdulagatov, I. Supercritical CO2: Properties and Technological Applications—A Review. J. Therm. Sci. 2019, 28, 394–430. [Google Scholar]
- Middleton, R.S.; Carey, J.W.; Currier, R.P.; Hyman, J.D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.; Porter, M.L.; Viswanathan, H.S. Shale Gas and Non-Aqueous Fracturing Fluids: Opportunities and Challenges for Supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef]
- Goicochea, A.G.; Firoozabadi, A. CO2 Viscosification by Functional Molecules from Mesoscale Simulations. J. Phys. Chem. C 2019, 123, 29461–29467. [Google Scholar] [CrossRef]
- Fu, H.; Song, K.; Pan, Y.; Song, H.; Meng, S.; Liu, M.; Bao, R.; Hao, H.; Wang, L.; Fu, X. Application of polymeric CO2 thickener Polymer-Viscosity-Enhance in extraction of low-permeability tight sandstone. Polymers 2024, 16, 299. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Rakesh, M.; Medhi, S.; Trivedi, J.; Sangwai, J.S. Pore-Scale Flow Simulation of Supercritical CO2 and Oil Flow for Simultaneous CO2 Geo-Sequestration and Enhanced Oil Recovery. Environ. Sci. Pollut. Res. 2022, 29, 76003–76025. [Google Scholar] [CrossRef]
- Ricky, E.X.; Mwakipunda, G.C.; Nyakilla, E.E.; Kasimu, N.A.; Wang, C.; Xu, X. A Comprehensive Review on CO2 Thickeners for CO2 Mobility Control in Enhanced Oil Recovery: Recent Advances and Future Outlook. J. Ind. Eng. Chem. 2023, 126, 69–91. [Google Scholar] [CrossRef]
- Tapriyal, D.; Wang, Y.; Enick, R.M.; Johnson, J.K.; Crosthwaite, J.; Thies, M.C.; Paik, I.H.; Hamilton, A.D. Poly(Vinyl Acetate), Poly((1-O-(Vinyloxy)Ethyl-2, 3, 4, 6-Tetra-O-Acetyl-D-Glucopyranoside) and Amorphous Poly(Lactic Acid) Are the Most CO2-Soluble Oxygenated Hydrocarbon-Based Polymers. J. Supercrit. Fluids 2008, 46, 252. [Google Scholar] [CrossRef]
- Card, R.J. Direct Thickeners for Mobility Control of CO2 Floods. Soc. Pet. Eng. J. 1985, 25, 679–686. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, W.; Yang, Q.; Niu, H.; Liu, Q.; Liu, Y.; Gao, M.; Xu, M.; Xu, A.; Liu, S.; et al. Preliminary investigation on cytotoxicity of fluorinated polymer nanoparticles. J. Environ. Sci. 2017, 69, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, W.N.; Li, Y.; Ren, P.F. Progress in research on pollution status and hazards of perfluorinated organic compounds (PFCs) in surface water. Environ. Eng. 2015, 33, 34–39. [Google Scholar]
- Zang, X.; Chang, M.; Zheng, L.; Zhou, Y.; Wang, Y.; Ren, J.; Wu, L.; Han, X.; Wang, Q.; Wu, J. Synthesis and characterization of novel poly(ionic liquid)s and their viscosity-increasing effect. J. Mol. Liq. 2020, 298, 112044. [Google Scholar] [CrossRef]
- Kilic, S.; Michalik, S.; Wang, Y.; Johnson, J.K.; Enick, R.M.; Beckman, E.J. Effect of Grafted Lewis Base Groups on the Phase Behavior of Model Poly(Dimethyl Siloxanes) in CO2. Ind. Eng. Chem. Res. 2003, 42, 6415–6424. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Perry, R.J.; Doherty, M.D.; Lee, J.J.; Dhuwe, A.; Beckman, E.J.; Enick, R.M. Anthraquinone Siloxanes as Thickening Agents for Supercritical CO2. Energy Fuels 2016, 30, 5990–5998. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Liang, L.; Zeng, Y. Achieving Solubility Alteration with Functionalized Polydimethylsiloxane for Improving the Viscosity of Supercritical CO2 Fracturing Fluids. RSC Adv. 2021, 11, 17197–17205. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Z.; Tang, J. Research on Polyether-Based Hydrocarbon Thickener for CO2. Fluid Phase Equilibria 2021, 532, 11293219. [Google Scholar] [CrossRef]
- Zhao, M. Formulation and Performance Evaluation of Polymer-Thickened Supercritical CO2 Fracturing Fluid. J. Pet. Sci. Eng. 2021, 35, 88–93. [Google Scholar] [CrossRef]
- Luo, X.; Wang, S.; Wang, Z.; Jing, Z.; Lv, M.; Zhai, Z.; Han, T. Experimental Investigation on Rheological Properties and Friction Performance of Thickened CO2 Fracturing Fluid. J. Pet. Sci. Eng. 2015, 133, 410–420. [Google Scholar] [CrossRef]
- Byun, H.-S. Phase behavior for the poly(dimethylsiloxane) in supercritical fluid solvents. J. Ind. Eng. Chem. 2013, 19, 665–669. [Google Scholar] [CrossRef]
- Szwarc, M. The kinetics and mechanism of N-carboxy-α-amino-acid anhydride (NCA) polymerisation to poly-amino acids. Adv. Polym. Sci. 1965, 4, 1–65. [Google Scholar]
- Shi, C.; Huang, Z.; J. Beckman, E.; M. Enick, R. Semi-Fluorinated Trialkyltin Fluorides and Fluorinated Telechelic Ionomers as Viscosity-Enhancing Agents for Carbon Dioxide. Ind. Eng. Chem. Res. 2001, 40, 908–913. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, Y.; Yao, L.; Liu, H.; Xu, P.; Li, H. Progress in research of closed supercritical carbon dioxide Brayton cycle system. J. Beijing Univ. Aeronaut. Astronaut. 2022, 48, 1643–1677. [Google Scholar]
- Cabral, B.J.C.; Rivelino, R.; Coutinho, K.; Canuto, S. A First Principles Approach to the Electronic Properties of Liquid and Supercritical CO2. J. Chem. Phys. 2015, 142, 024501. [Google Scholar] [CrossRef]
- White, R.P.; Lipson, J.E.G. Polymer free volume and its connection to the glass transition. Macromolecules 2016, 49, 3987–4007. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Kohlmeyer, A.; Klein, M.L. Ab initio molecular dynamics study of supercritical carbon dioxide including dispersion corrections. J. Chem. Phys. 2009, 131, 144506. [Google Scholar] [CrossRef]
- Heller, J.P. Polar Attributes of Supercritical Carbon Dioxide. Acc. Chem. Res. 2005, 38, 478–485. [Google Scholar]
- Kannangara, P.B.; West, C.T.; Peebles, S.A.; Peebles, R.A. Towards microsolvation of fluorocarbons by CO2: Two isomers of fluoroethylene-(CO2)2 observed using chirped-pulse Fourier-transform microwave spectroscopy. Chem. Phys. Lett. 2018, 706, 538–542. [Google Scholar] [CrossRef]
- Xue, P.; Shi, J.; Cao, X.; Yuan, S. Molecular dynamics simulation of thickening mechanism of supercritical CO2 thickener. Chem. Phys. Lett. 2018, 706, 658–664. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Zou, Y.; Wang, F.; Chen, B.; Guan, L.; Wu, Y. Models for the solubility calculation of a CO2/polymer system: A review. Mater. Today Commun. 2020, 24, 101277. [Google Scholar] [CrossRef]

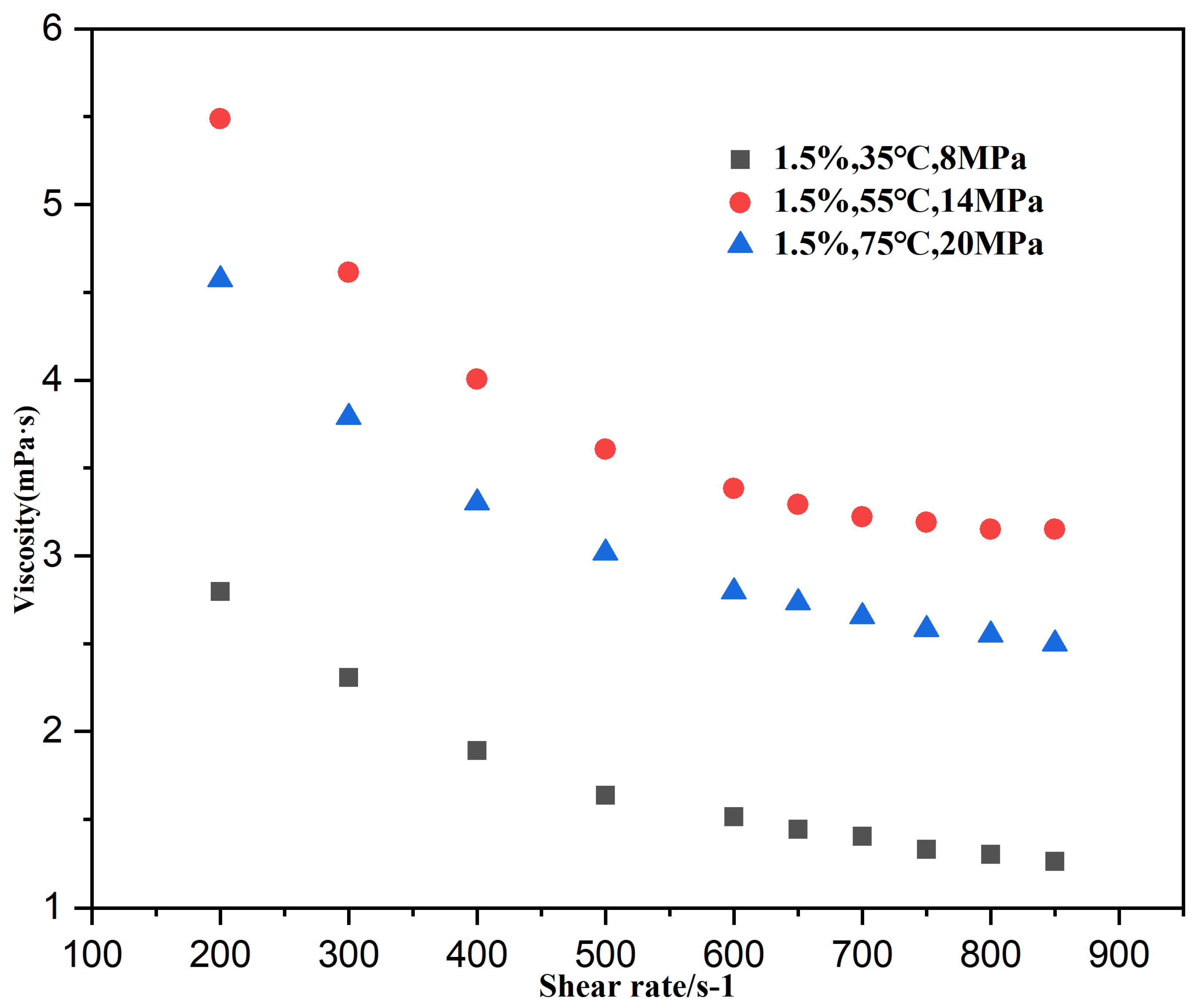


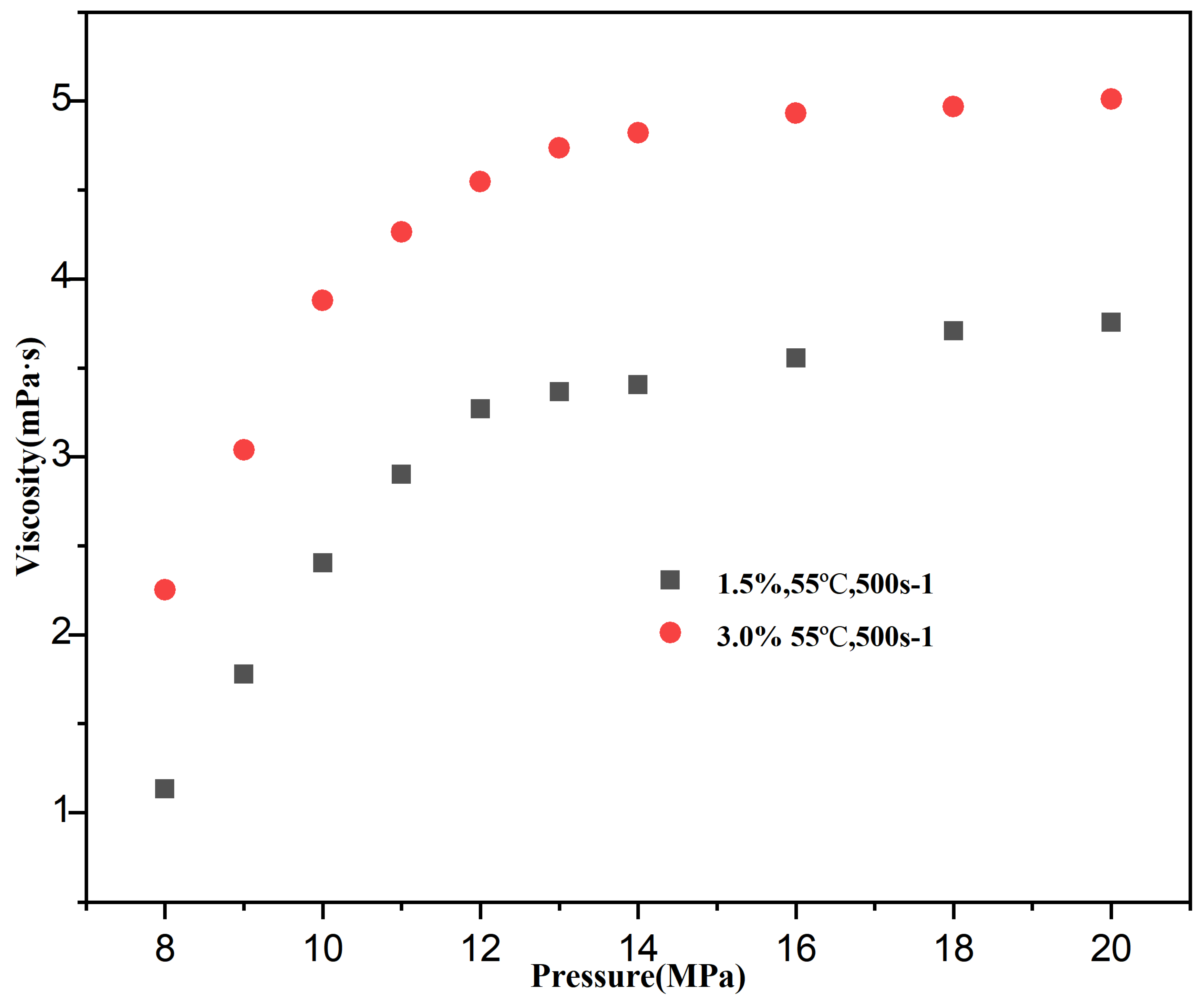
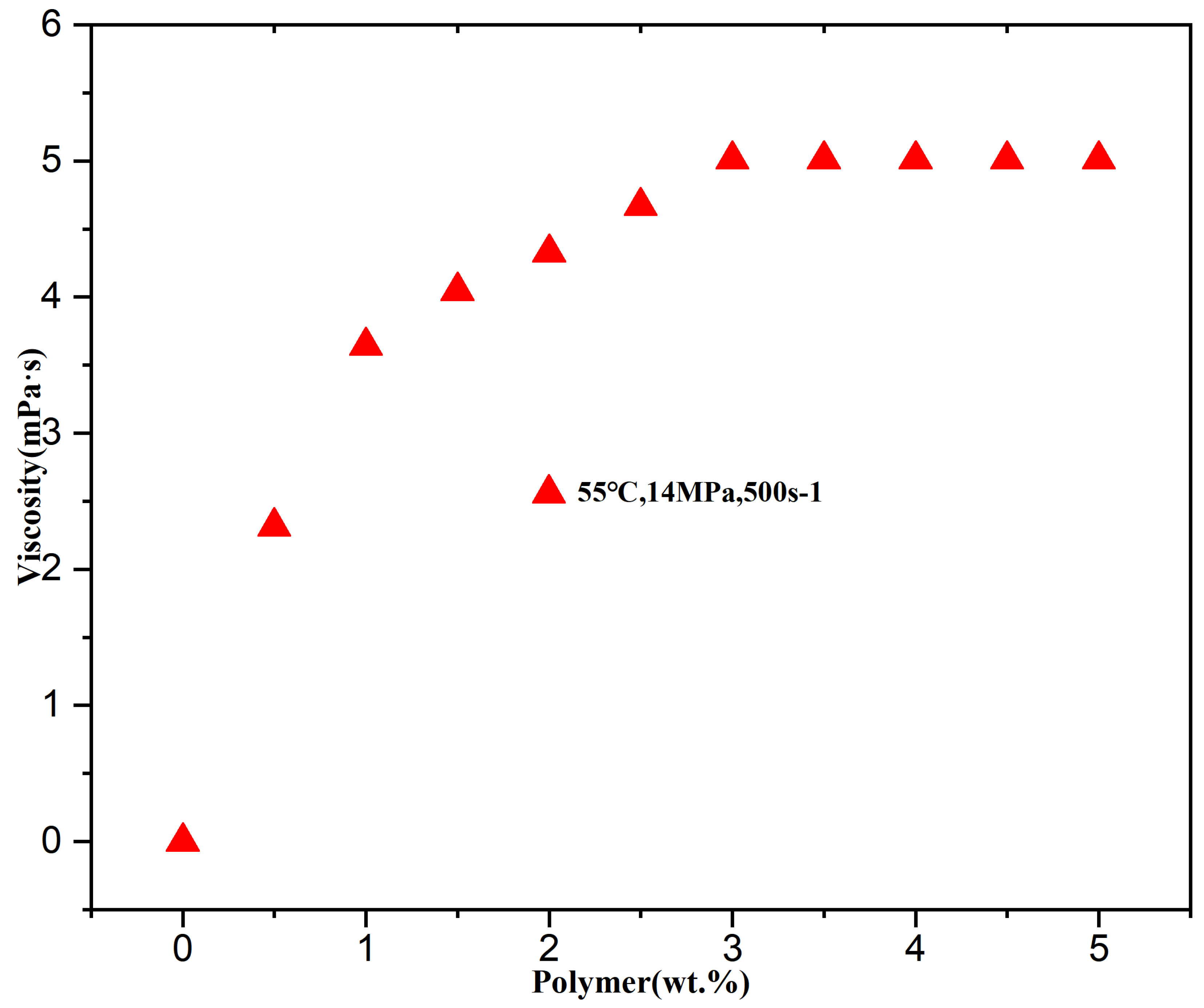
| Temperature (°C) | Pressure (MPa) | Polymer (wt%) | CO2 (mPa·s) | System Viscosity (mPa·s) | Viscosity Increase Factor |
|---|---|---|---|---|---|
| 35 | 8 | 1.5 | 0.026 | 1.637 | 63 |
| 35 | 8 | 3.0 | 0.026 | 2.791 | 107 |
| 35 | 14 | 1.5 | 0.051 | 4.249 | 83 |
| 35 | 14 | 3.0 | 0.051 | 5.546 | 109 |
| 35 | 20 | 1.5 | 0.067 | 4.765 | 71 |
| 35 | 20 | 3.0 | 0.067 | 5.811 | 87 |
| 55 | 8 | 1.5 | 0.023 | 1.485 | 65 |
| 55 | 8 | 3.0 | 0.023 | 2.361 | 103 |
| 55 | 14 | 1.5 | 0.045 | 3.608 | 80 |
| 55 | 14 | 3.0 | 0.045 | 5.150 | 114 |
| 55 | 20 | 1.5 | 0.062 | 3.933 | 63 |
| 55 | 20 | 3.0 | 0.062 | 5.093 | 82 |
| 75 | 8 | 1.5 | 0.021 | 1.137 | 54 |
| 75 | 8 | 3.0 | 0.021 | 2.143 | 102 |
| 75 | 14 | 1.5 | 0.042 | 2.581 | 61 |
| 75 | 14 | 3.0 | 0.042 | 3.938 | 94 |
| 75 | 20 | 1.5 | 0.060 | 3.018 | 50 |
| 75 | 20 | 3.0 | 0.060 | 4.385 | 73 |
| Temperature (°C) | Pressure (MPa) | Viscosity Enhancement Factor Trend | Key Data Examples |
|---|---|---|---|
| 35 | 8 → 20 | 63 → 71 (Δ + 13%) | 3.0 wt.%, 8 → 20 MPa |
| 55 | 8 → 14 | 103 → 114 (Δ + 11%) | 3.0 wt.%, 8 → 14 MPa |
| 75 | 8 → 20 | 102 → 73 (Δ − 28%) | 3.0 wt.%, 8 → 20 MPa |
| Concentration (wt.%) | Average Viscosity Enhancement Factor (Fold) | Enhancement Slope (Fold/wt.%) |
|---|---|---|
| 1.5 | 66 ± 11 | - |
| 3.0 | 96 ± 13 | 20.5 (R2 = 0.23) |
| Pressure (MPa) | 1.5 wt.% Viscosity (mPa·s) | 3.0 wt.% Viscosity (mPa·s) | Viscosity Increase (%) |
|---|---|---|---|
| 8 | 1.485 | 2.361 | - |
| 14 | 3.608 | 5.150 | 1.5%: 128.4% |
| 20 | 3.393 | 5.093 | 3.0%: 118.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Tang, L.; Zheng, X.; Zhu, Y.; Zheng, C.; Liu, G.; Lai, N. Preparation and Performance Evaluation of Modified Amino-Silicone Supercritical CO2 Viscosity Enhancer for Shale Oil and Gas Reservoir Development. Processes 2025, 13, 2337. https://doi.org/10.3390/pr13082337
Yang R, Tang L, Zheng X, Zhu Y, Zheng C, Liu G, Lai N. Preparation and Performance Evaluation of Modified Amino-Silicone Supercritical CO2 Viscosity Enhancer for Shale Oil and Gas Reservoir Development. Processes. 2025; 13(8):2337. https://doi.org/10.3390/pr13082337
Chicago/Turabian StyleYang, Rongguo, Lei Tang, Xuecheng Zheng, Yuanqian Zhu, Chuanjiang Zheng, Guoyu Liu, and Nanjun Lai. 2025. "Preparation and Performance Evaluation of Modified Amino-Silicone Supercritical CO2 Viscosity Enhancer for Shale Oil and Gas Reservoir Development" Processes 13, no. 8: 2337. https://doi.org/10.3390/pr13082337
APA StyleYang, R., Tang, L., Zheng, X., Zhu, Y., Zheng, C., Liu, G., & Lai, N. (2025). Preparation and Performance Evaluation of Modified Amino-Silicone Supercritical CO2 Viscosity Enhancer for Shale Oil and Gas Reservoir Development. Processes, 13(8), 2337. https://doi.org/10.3390/pr13082337







