Recovery of High-Alkali-Grade Feldspar Substitute from Phonolite Tailings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
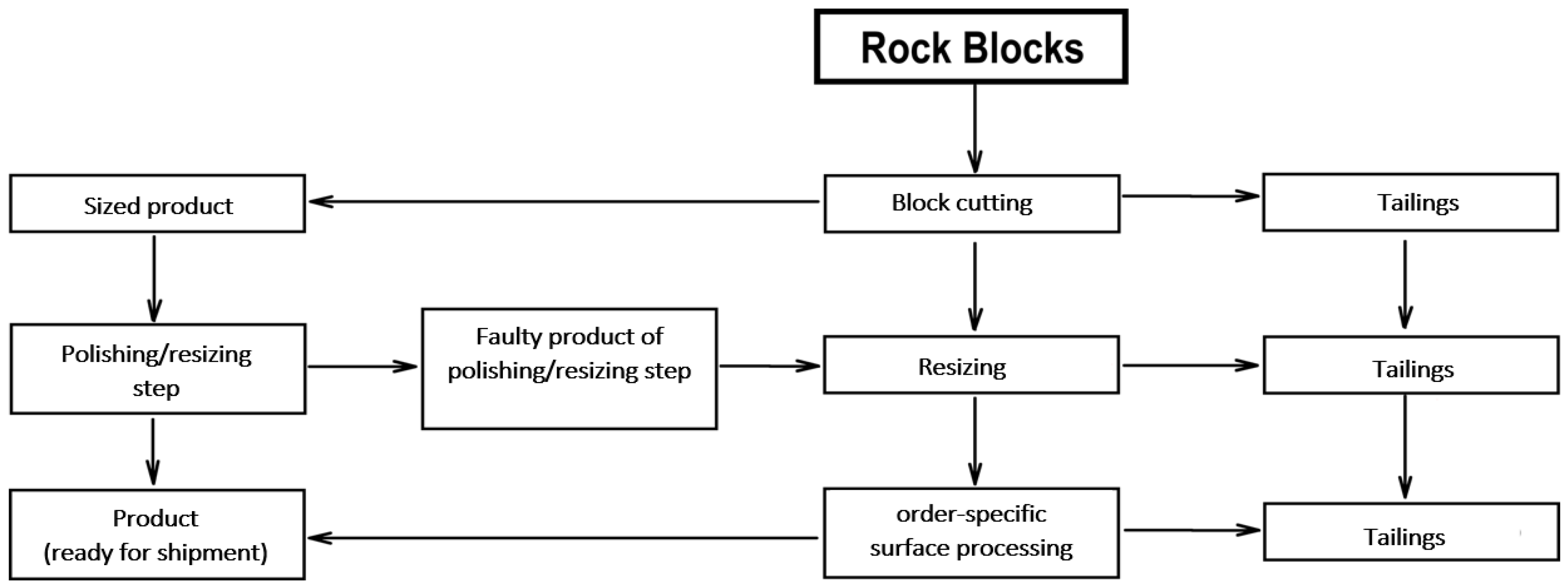
2.2. Method
3. Results and Discussion
3.1. Magnetic Separation Tests
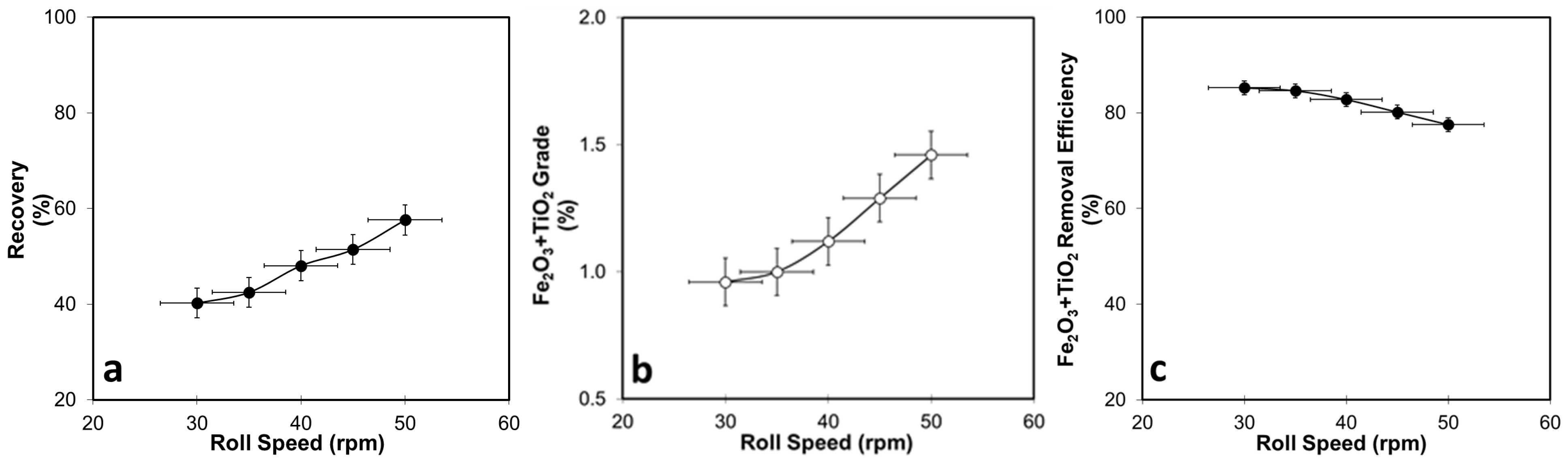
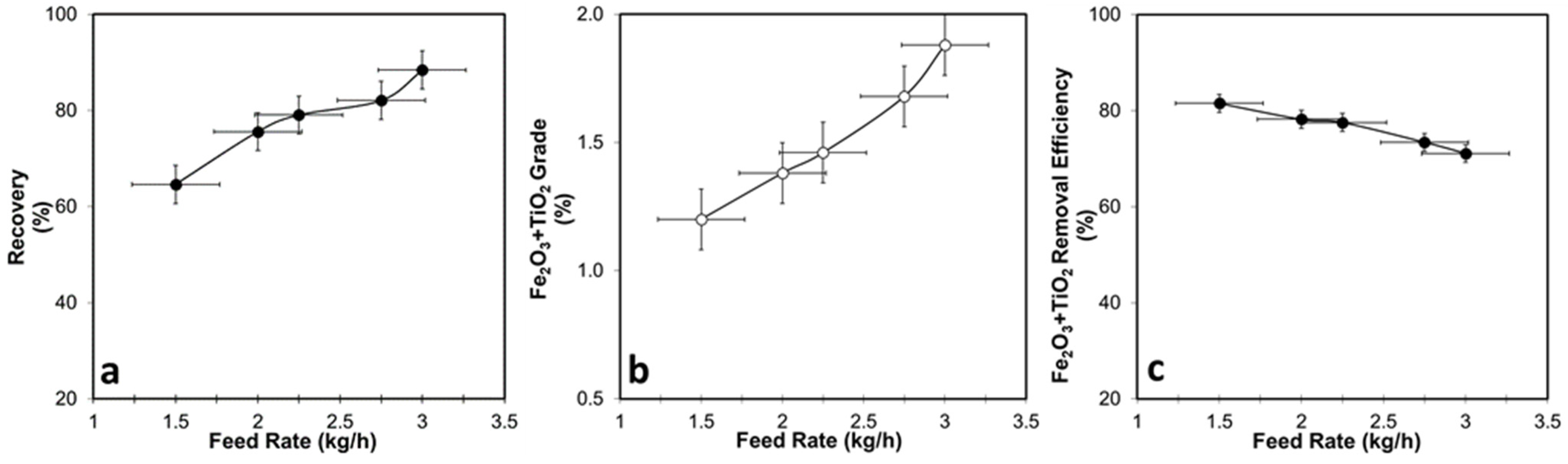
3.1.1. DHIMS Tests
3.1.2. WHIMS Tests
3.2. Flotation Tests
3.3. Magnetic Separation Tests for Flotation Concentrate
3.3.1. DHIMS Tests for Flotation Concentrate
3.3.2. WHIMS Tests for Flotation Concentrate
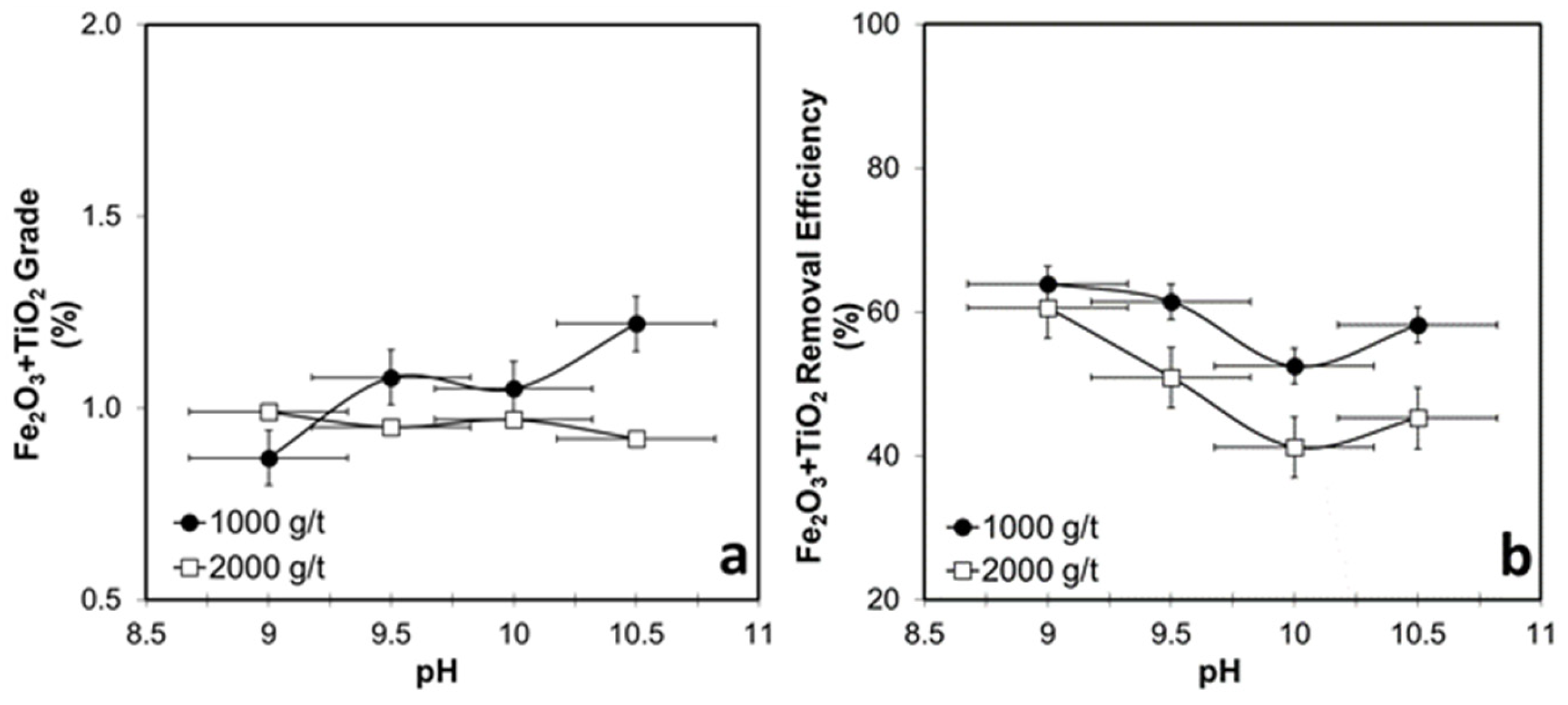
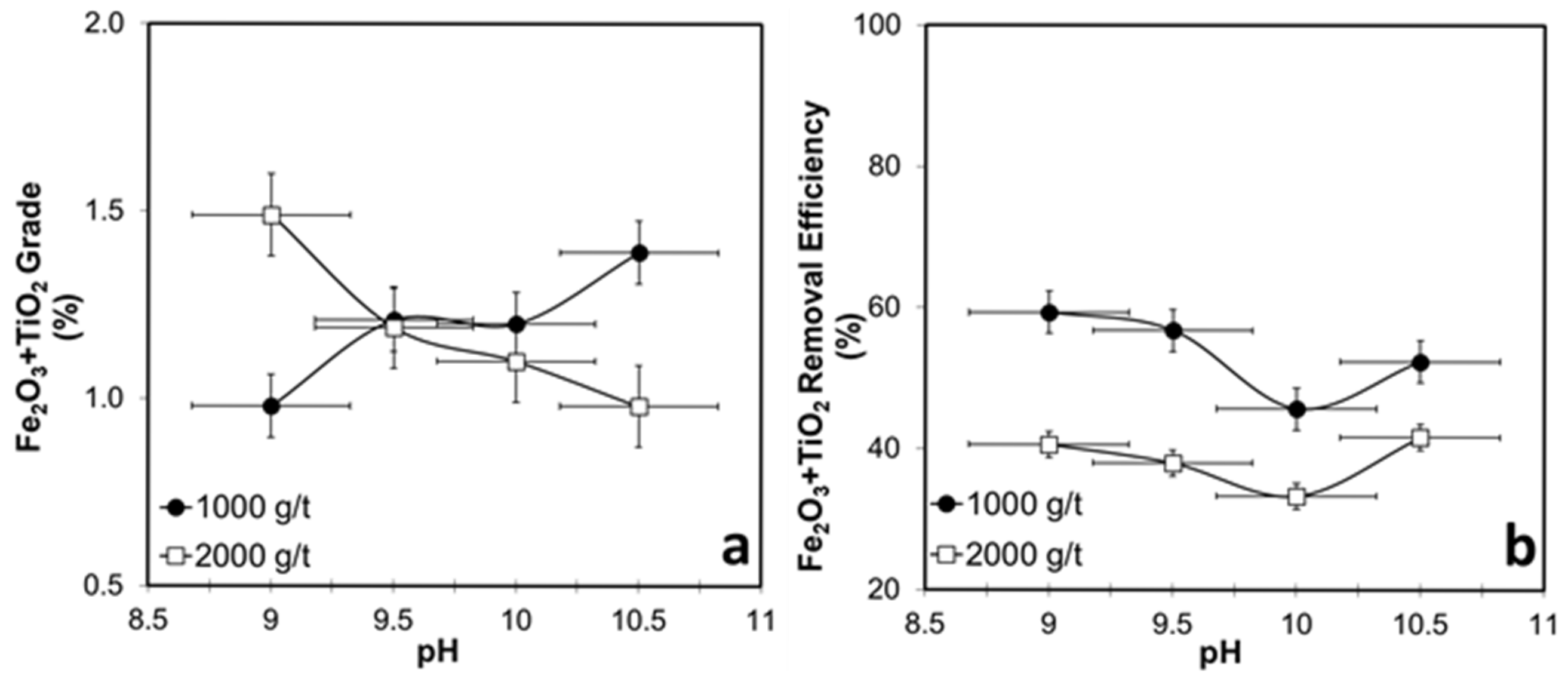
3.4. Flotation Tests for WHIMS Concentrate
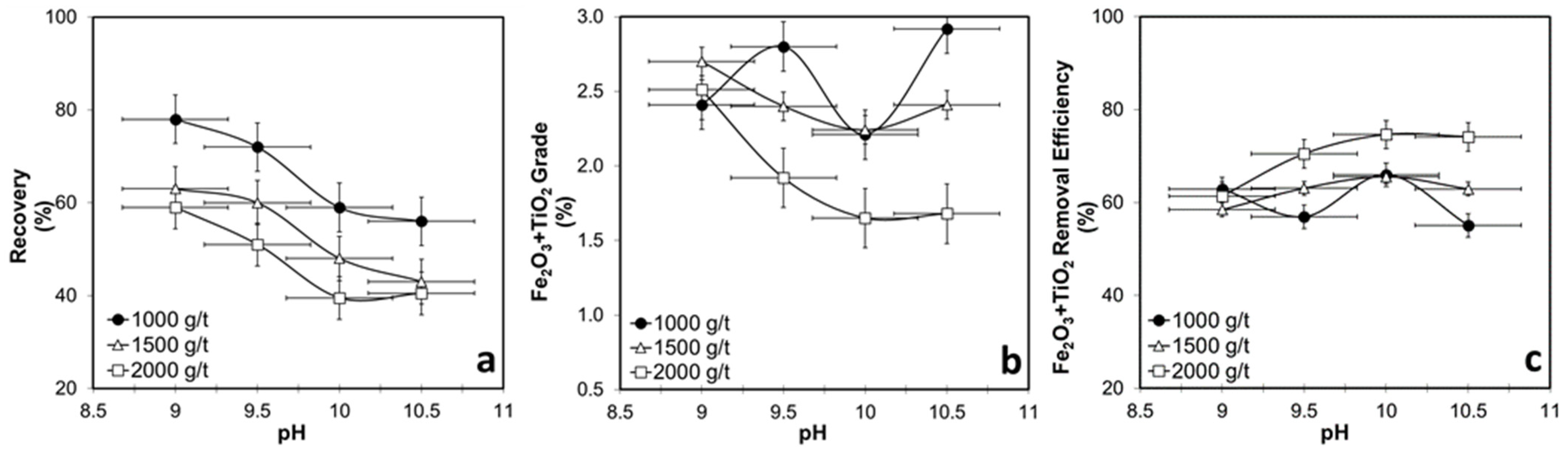
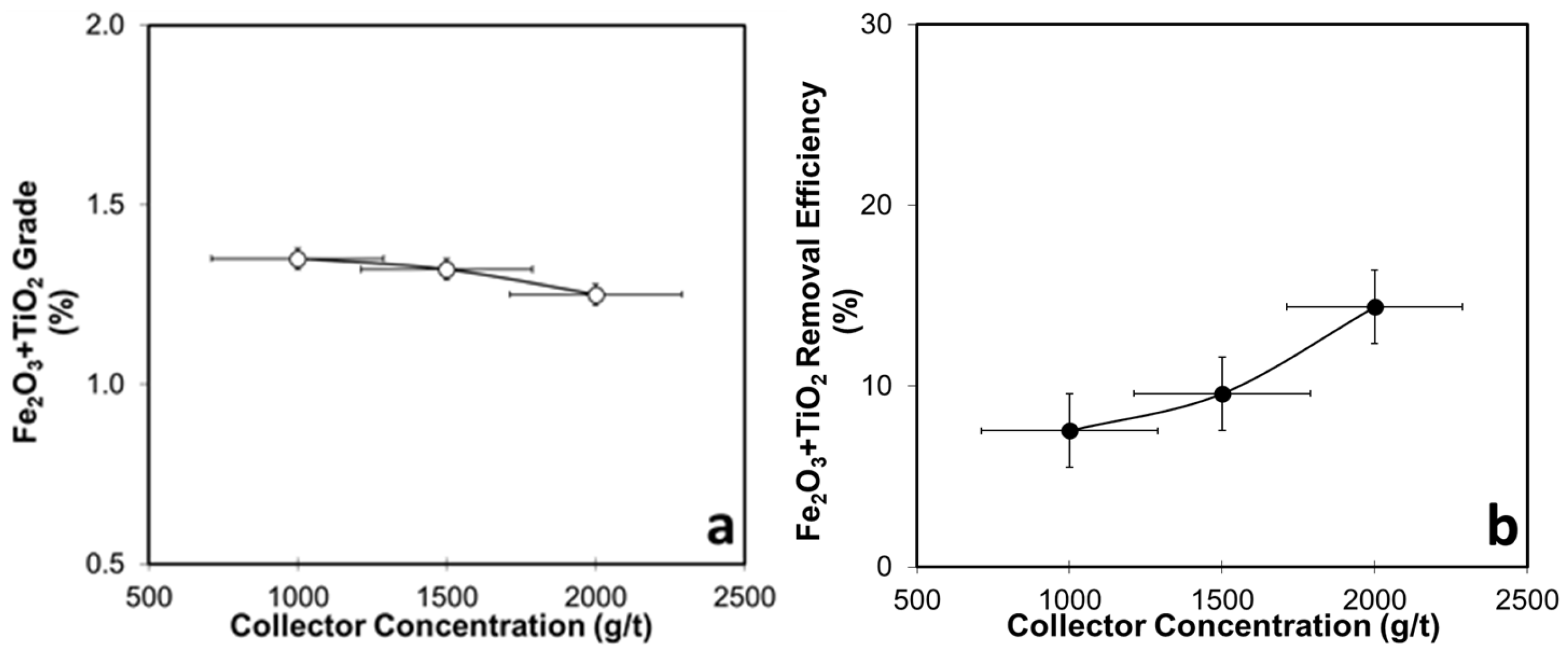
3.4.1. Collector-Concentration-Dependent Flotation Tests for WHIMS Concentrate
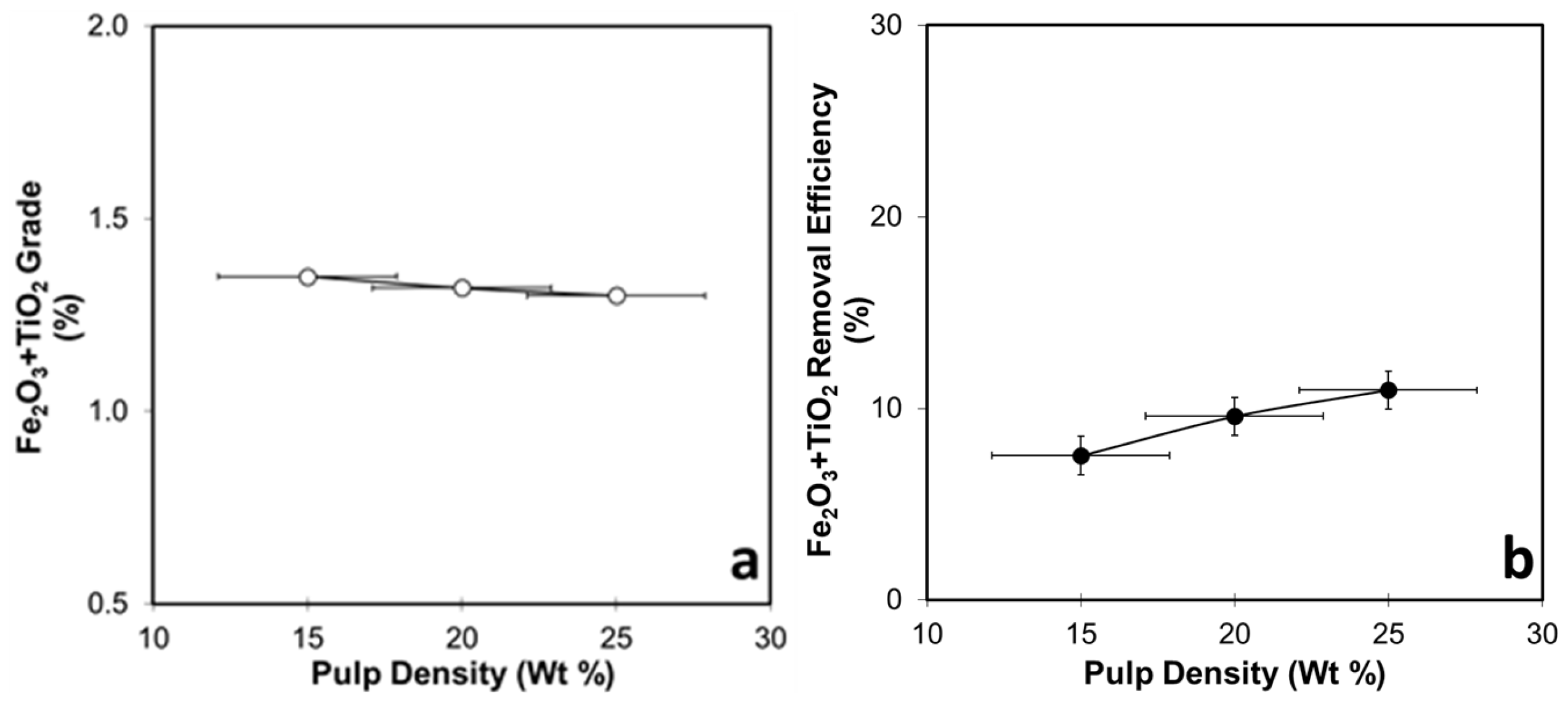
3.4.2. Pulp-Density-Dependent Flotation Tests for WHIMS Concentrate
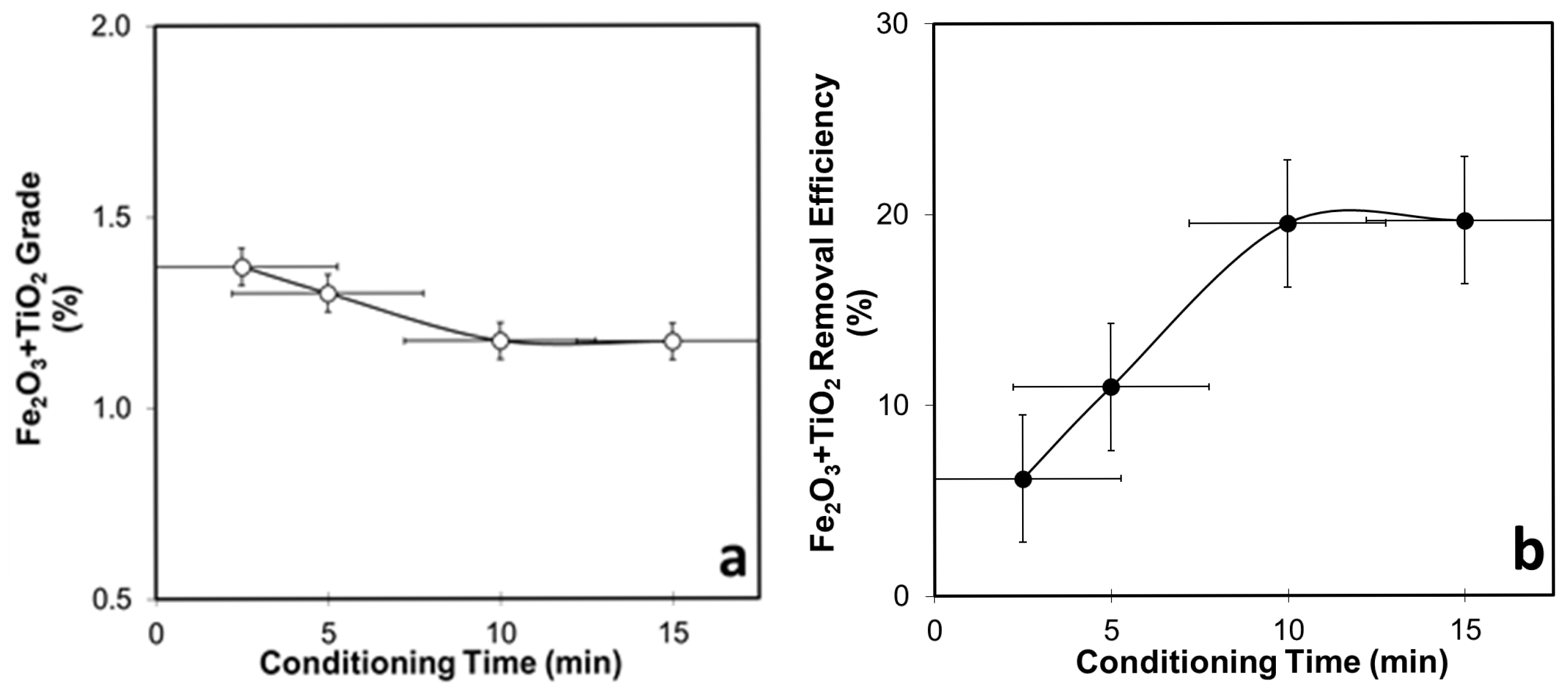
3.4.3. Conditioning-Time-Dependent Flotation Tests for WHIMS Concentrate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heimann, R.B. Classic and Advanced Ceramics: From Fundamentals to Applications; Wiley VCH Verlag GMBH & Co: Hoboken, NJ, USA, 2010. [Google Scholar]
- Ibrahim, S.S.; Mohamed, H.A.; Boulos, T.R. Dry magnetic separation of nepheline syenite ores. Physicochem. Probl. Miner. Process. 2002, 36, 173–183. [Google Scholar]
- Deniz, K.; Kadioglu, Y.K.; Koralay, T.; Gullu, B. The distribution of elements in the alteration of feldspatic minerals. Bull. Miner. Res. Explor. 2021, 166, 167–188. [Google Scholar] [CrossRef]
- Kangal, M.O.; Bulut, G.; Yesilyurt, Z.; Guven, O.; Burat, F. An alternative source for ceramics and glass raw materials: Augen-gneiss. Minerals 2017, 7, 70. [Google Scholar] [CrossRef]
- Vasić, M.V.; Mijatović, N.; Radojević, Z. Aplitic granite waste as raw material for the production of outdoor ceramic floor tiles. Materials 2022, 15, 3145. [Google Scholar] [CrossRef]
- Ozun, S.; Atalay, M.U.; Demirci, S. Study of adsorption characteristics of long chain alkyl amine and petroleum sulfonate on silicates by electrokinetic potential, microflotation, FTIR, and AFM analyses. Part. Sci. Technol. 2019, 37, 492–503. [Google Scholar] [CrossRef]
- Gaied, M.E.; Gallala, W. Beneficiation of feldspar ore for application in the ceramic industry: Influence of composition on the physical characteristics. Arab. J. Chem. 2015, 8, 186–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Sun, N.; Liu, R.; Wang, Z.; Wang, L.; Sun, W. Systematic review of feldspar beneficiation and its comprehensive application. Miner. Eng. 2018, 128, 141–152. [Google Scholar] [CrossRef]
- Ulutas, S. Investigation on Recovery of Ceramic Raw Material from Foid-Bearing Rocks. Master’s Thesis, Graduate School of Natural and Applied Sciences, Suleyman Demirel University, Isparta, Turkey, 2017. [Google Scholar]
- Tian, J.; Xu, L.; Wu, H.; Fang, S.; Deng, W.; Peng, T.; Sun, W.; Hu, Y. A novel approach for flotation recovery of spodumene, mica and feldspar from a lithium pegmatite ore. J. Clean. Prod. 2018, 174, 625–633. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Guedes, D.G.; Da Costa, F.P.; Rodrigues, A.M.; Neves, G.D.A.; Menezes, R.R.; De Santana, L.N.L. Sustainable ceramic materials manufactured from ceramic formulations containing quartzite and scheelite tailings. Sustainability 2020, 12, 9417. [Google Scholar] [CrossRef]
- Johansson, G.; Pugh, R.J. The influence of particle size and hydrophobicity on the stability of mineralized froths. Int. J. Min. Process 1992, 34, 1–21. [Google Scholar] [CrossRef]
- Orhan, E.C.; Bayraktar, I. Amine-oleate interactions in feldspar flotation. Miner. Eng. 2005, 19, 48–55. [Google Scholar] [CrossRef]
- Bayat, O.; Arslan, V.; Cebeci, Y. Combined application of different collectors in the flotation concentration of Turkish feldspars. Miner. Eng. 2006, 19, 98–101. [Google Scholar] [CrossRef]
- Aslan, N.; Kaya, H. Beneficiation of chromite concentration waste by multi-gravity separator and high intensity Induced-roll magnetic separator. Arab. J. Sci. Eng. 2009, 34, 285–297. [Google Scholar]
- Pourghahramani, P. Effects of ore characteristics on product shape properties and breakage mechanisms in industrial SAG mills. Miner. Eng. 2012, 32, 30–37. [Google Scholar] [CrossRef]
- Seifelnassr, A.A.S.; Moslim, E.M.; Abouzeid, A.Z.M. Effective processing of low-grade iron ore through gravity and magnetic separation techniques. Physicochem. Probl. Miner. Process. 2012, 48, 567–578. [Google Scholar] [CrossRef]
- Quast, K. Literature review on the interaction of oleate with non-sulphide minerals using zeta potential. Miner. Eng. 2016, 94, 10–20. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Suresh, N. Influence of particle size on dry high-intensity magnetic separation of paramagnetic mineral. Adv. Powder Technol. 2017, 28, 1092–1102. [Google Scholar] [CrossRef]
- Aumond, J.J.; Scheibe, L.F. O fonolito de Lages–SC, um novo fundente cerâmico brasileiro. Cerã¢mica Ind. 1996, 1, 17–21. [Google Scholar]
- Esposito, L.; Salem, A.; Tucci, A.; Gualtieri, A.; Jazayeri, S.H. The use of nepheline-syenite in a body mix for porcelain stoneware tiles. Ceram. Int. 2005, 31, 233–240. [Google Scholar] [CrossRef]
- Tavares Brasileiro, C.; Conte, S.; Contartesi, F.; Melchiades, F.G.; Zanelli, C.; Dondi, M.; Boschi, A.O. Effect of strong mineral fluxes on sintering of porcelain stoneware tiles. J. Eur. Ceram. Soc. 2021, 41, 5755–5767. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Le Maitre, R.W.; Woolley, A.R. The construction of the total alkali-silica chemical classification of volcanic rocks. Min. Pet. 1992, 46, 1–22. [Google Scholar] [CrossRef]
- Christ, R.; Bourscheid, I.; Pacheco, F.; Da Silva, M.G.; Ehrenbring, H.Z.; Da Silva, A.B.; Tutikian, B.F. Effect of firing temperature and mineral composition on the mechanical properties of silty clays. Matéria 2023, 28, 20230181. [Google Scholar] [CrossRef]
- Dondi, M. Feldspathic fluxes for ceramics: Sources, production trends and technological value. Resour. Conserv. Recycl. 2018, 133, 191–205. [Google Scholar] [CrossRef]
- Silva, A.C.; Carolina, S.D.; Sousa, D.N.; Silva, E.M.S. Feldspar production from dimension stone tailings for application in the ceramic industry. J. Mater. Res. Technol. 2019, 8, 1–7. [Google Scholar] [CrossRef]
- Bayliss, P.; Bernstein, L.R.; McDonald, A.M.; Roberts, A.C.; Sabina, A.P.; Smith, D.K. Mineral Powder Diffraction File Databook, Sets 1–50; International Center for Diffraction Data (ICDD): Newtown Square, PA, USA, 2001. [Google Scholar]
- Gol, F.; Saritas, Z.G.; Cibuk, S.; Ture, C.; Kacar, E.; Yilmaz, A.; Arslan, M.; Sen, F. Coloring effect of iron oxide content on ceramic glazes and their comparison with the similar waste containing materials. Ceram. Int. 2022, 48, 2241–2249. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, F.; Shen, Y.; Liu, S.; Liu, S. A glass-ceramic approach to prepare porous TiO2 with self-supported nano-flakes: The phase evolution and the thermal stability of the product. Ceram. Int. 2022, 48, 18745–18752. [Google Scholar] [CrossRef]
- Mercier, G.; Duchesne, J.; Blackburn, D. Removal of metals from contaminated soils by mineral processing techniques followed by chemical leaching. Water Air Soil Pollut. 2002, 135, 105–130. [Google Scholar] [CrossRef]
- Svoboda, J. The effect of magnetic field strenght on the efficiency of magnetic separation. Miner. Eng. 1994, 7, 747–757. [Google Scholar] [CrossRef]
- Svoboda, J.; Fujita, T. Recent developments in magnetic methods of material separation. Miner. Eng. 2003, 16, 785–792. [Google Scholar] [CrossRef]
- Xie, S.; Hu, Z.; Lu, D.; Zhao, Y. Dry permanent magnetic separator: Present status and future prospects. Minerals 2022, 12, 1251. [Google Scholar] [CrossRef]
- Nakai, Y.; Mishima, F.; Akiyama, Y.; Nishijima, S. Development of high gradient magnetic separation system under dry condition. Phys. C Supercond. 2010, 470, 1812–1817. [Google Scholar] [CrossRef]
- Jobin, P.; Mercier, G.; Blais, J.-F. Magnetic and density characteristics of a heavily polluted soil with municipal solid waste incinerator residues: Significance for remediation strategies. Int. J. Miner. Process. 2016, 149, 119–126. [Google Scholar] [CrossRef]
- Veetil, S.P.; Mercier, G.; Blais, J.F.; Cecchi, E.; Kentish, S. Magnetic separation of serpentinite mining residue as a precursor to mineral carbonation. Int. J. Miner. Process. 2015, 140, 19–25. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J.A. Magnetic and Electrical Separation. In Wills’ Mineral Processing Technology (8th Edition) An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 8th ed.; Butterworth-Heinemann: Oxford, UK; Elsevier: Amsterdam, The Netherlands, 2016; pp. 381–407. [Google Scholar] [CrossRef]
- Carlson, J.J.; Kawatra, S.K. Factors affecting zeta potential of iron oxides. Miner. Process. Extr. Metall. Rev. 2011, 34, 269–303. [Google Scholar] [CrossRef]
- Kose, M.; Hosten, C.; Topkaya, Y.; Akser, M. Selective Flotation of Ilmenite from Ilmenite-Rutile Mixtures. In Mineral Processing on the Verge of the 21st Century; Hicyilmaz, C., Ed.; Routledge: London, UK, 2017. [Google Scholar] [CrossRef]
- Pugh, R.J. Selective coagulation in quartz-hematite and quartz-rutile suspensions. Colloid Polym. Sci. 1974, 252, 400–406. [Google Scholar] [CrossRef]
- Shibata, J.; Fuerstenau, D.W. Flocculation and flotation characteristics of fine hematite with sodium oleate. Int. J. Miner. Process. 2003, 72, 25–32. [Google Scholar] [CrossRef]
- Somasundaran, P.; Ananthapadmanabhan, K.P.; Ivanov, I.B. Dimerization of oleate in aqueous solutions. J. Colloid Interface Sci. 1984, 99, 128–135. [Google Scholar] [CrossRef]
- Somasundaran, P.; Wang, D. Solution Chemistry: Minerals and Reagents, Developments in Mineral Processing; Elsevier: Amsterdam, The Netherlands, 2006; Volume 17, pp. 5–72. [Google Scholar]
- Ozun, S.; Atalay, M.U. Flotation and adsorption characteristics of albite and quartz with oleic acid-based collector. Colloids Surf. A Physicochem. Eng. Asp. 2023, 672, 131710. [Google Scholar] [CrossRef]
- Quast, K. Flotation of hematite using C6–C18 saturated fatty acids. Miner. Eng. 2006, 19, 582–597. [Google Scholar] [CrossRef]
- Bulatovic, S.M. 2-Collectors. In Handbook of Flotation Reagents; Bulatovic, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 5–41. [Google Scholar]
- Cook, B.K.; Gibson, C.E. A review of fatty acid collectors: Implications for spodumene flotation. Minerals 2023, 13, 212. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Deng, W.; Jiang, H.; Gao, Z.; Hu, Y. Adsorption mechanism of new mixed anionic/cationic collectors in a spodumene-feldspar flotation system. Chem. Eng. Sci. 2017, 164, 99–107. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Zhang, L.; Zhu, G. Role of oleic acid ionic−molecular complexes in the flotation of spodumene. Miner. Eng. 2015, 71, 7–12. [Google Scholar] [CrossRef]
- Achaye, I.; Wiese, J.; Mcfadzean, B. Effect of mineral particle size on froth stability. Miner. Process. Extr. Metall. 2021, 130, 253–261. [Google Scholar] [CrossRef]
- Ucurum, M.; Bayat, O. Effects of operating variables on modified flotation parameters in the mineral separation. Sep. Purif. Technol. 2007, 55, 173–181. [Google Scholar] [CrossRef]
- Zhang, J.G. Factors Affecting the Kinetics of Froth Flotation. Ph.D. Thesis, Department of Mining and Mineral Engineering, University of Leeds, Leeds, UK, 1989. [Google Scholar]
- Xia, W.C.; Yang, J.; Liang, C.; Zhu, B. The effects of conditioning time on the flotation of oxidized coal. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 36, 31–37. [Google Scholar] [CrossRef]



| Content | wt% | Content | wt% |
|---|---|---|---|
| Na2O | 5.88 | CaO + MgO | 5.50 |
| K2O | 5.63 | Fe2O3 + TiO2 | 6.99 |
| SiO2 | 54.27 | MnO | 0.11 |
| Al2O3 | 17.65 | LOI | 2.57 |
| Process Variables | Values and Units |
|---|---|
| Pulp density (Solid % by Wt.) | Conditioning: 35% |
| Flotation: 15% | |
| Impeller speed | Conditioning: 1750 rpm |
| Flotation: 1500 rpm | |
| pH | 9–10.50 |
| Conditioning time | 5 min |
| Flotation time | 1 min |
| Collector concentration | 1000–2000 g/t |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozun, S.; Ulutas, S.; Yurdakul, S. Recovery of High-Alkali-Grade Feldspar Substitute from Phonolite Tailings. Processes 2025, 13, 2334. https://doi.org/10.3390/pr13082334
Ozun S, Ulutas S, Yurdakul S. Recovery of High-Alkali-Grade Feldspar Substitute from Phonolite Tailings. Processes. 2025; 13(8):2334. https://doi.org/10.3390/pr13082334
Chicago/Turabian StyleOzun, Savas, Semsettin Ulutas, and Sema Yurdakul. 2025. "Recovery of High-Alkali-Grade Feldspar Substitute from Phonolite Tailings" Processes 13, no. 8: 2334. https://doi.org/10.3390/pr13082334
APA StyleOzun, S., Ulutas, S., & Yurdakul, S. (2025). Recovery of High-Alkali-Grade Feldspar Substitute from Phonolite Tailings. Processes, 13(8), 2334. https://doi.org/10.3390/pr13082334







