Spray Drying of Jackfruit (Artocarpus heterophyllus Lam.) Seeds Protein Concentrate: Physicochemical, Structural, and Thermal Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetal Material and Chemical Substances
2.2. Jackfruit Seeds Protein Concentrate Obtention
2.3. Spray Drying of the Jackfruit Seeds Protein Concentrate (JSPC) Optimization
2.4. Physicochemical Analysis and Functional Properties of Jackfruit Seeds Protein Concentrate (JSPC) Powder
2.4.1. Moisture Content
2.4.2. Water Activity
2.4.3. Bulk Density
2.4.4. Color
2.4.5. Hydrosolubility
2.4.6. Foaming Properties
2.4.7. Emulsion Properties
2.4.8. Particle Size Distribution of Jackfruit Seeds Protein Concentrate (JSPC) Powder
2.5. Amino Acids of Jackfruit Seeds Protein Concentrate (JSPC) Powder
2.6. Differential Scanning Calorimetry (DSC) of Jackfruit Seeds Protein Concentrate (JSPC) Powder
2.7. Fourier Transform Infrared Spectroscopy (FTIR) of Jackfruit Seeds Protein Concentrate (JSPC) Powder
2.8. Statistical Analysis
3. Results and Discussion
3.1. Spray Drying of the Jackfruit Seed Protein Concentrate (JSPC) Optimization
3.2. Physicochemical Analysis and Functional Properties of Jackfruit Seeds Protein Concentrate (JSPC) Powder
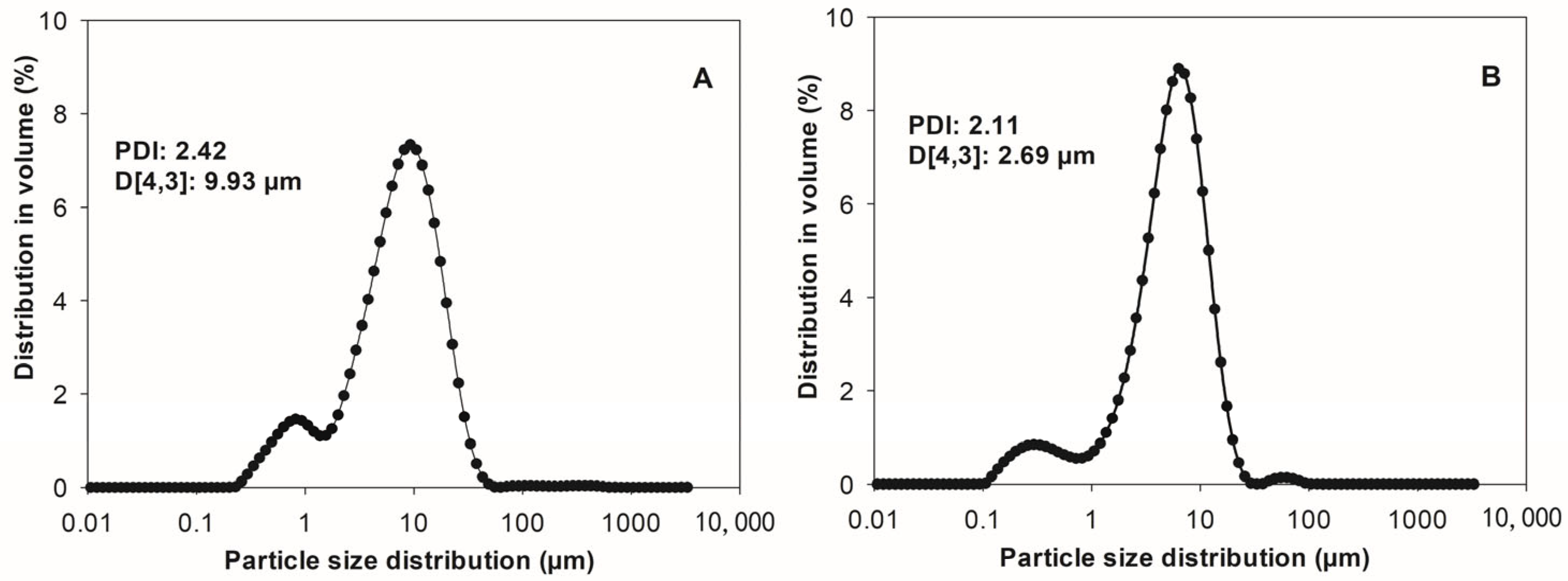
3.3. Particle Size Distribution of Jackfruit Seeds Protein Concentrate (JSPC) Solution and Powder
3.4. Amino Acids of Jackfruit Seeds Protein Concentrate (JSPC) Powder
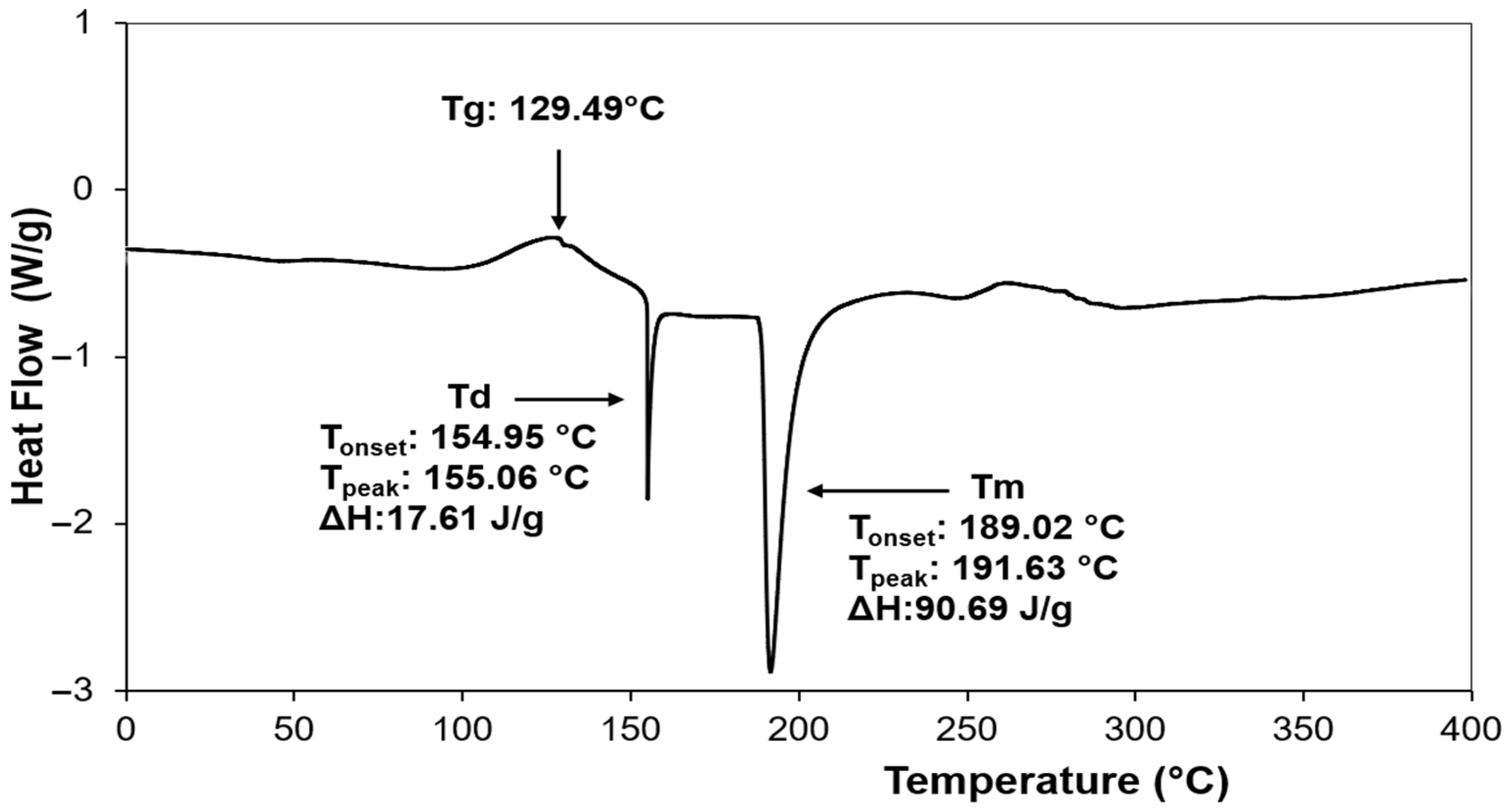
3.5. Differential Scanning Calorimetry of Jackfruit Seeds Protein Concentrate (JSPC) Powder
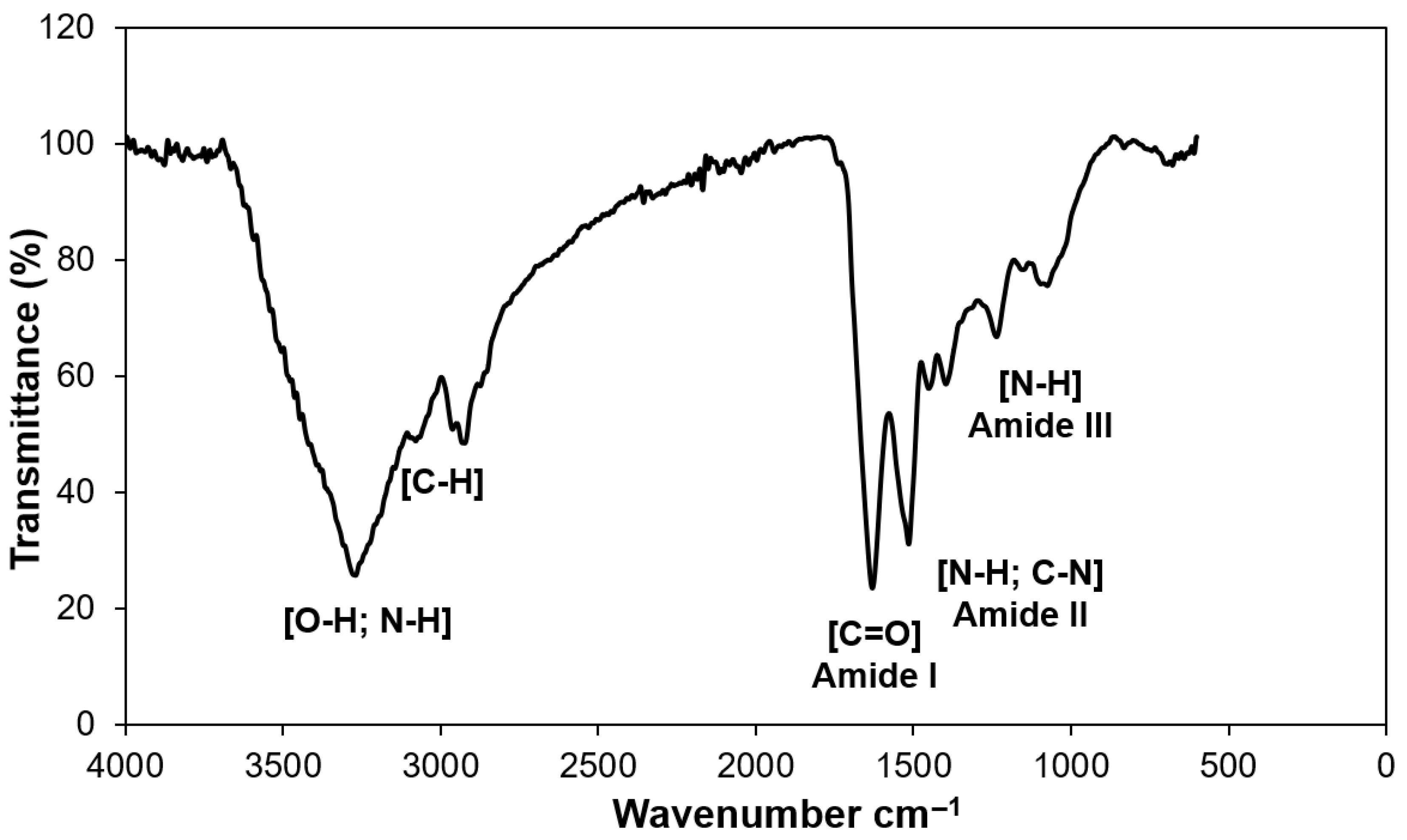
3.6. Fourier Transform Infrared Spectroscopy of Jackfruit Seeds Protein Concentrate (JSPC) Powder
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Munialo, C.D.; Stewart, D.; Campbell, L.; Euston, S.R. Extraction, Characterisation and Functional Applications of Sustainable Alternative Protein Sources for Future Foods: A Review. Future Foods 2022, 6, 100152. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, X.; Li, Y. Drying Methods Affect Physicochemical and Functional Properties of Quinoa Protein Isolate. Food Chem. 2021, 339, 127823. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, M.; Fan, H.; Wu, J. Emerging Proteins as Precursors of Bioactive Peptides/Hydrolysates with Health Benefits. Curr. Opin. Food Sci. 2022, 48, 100914. [Google Scholar] [CrossRef]
- Calderón-Chiu, C.; Calderón-Santoyo, M.; Herman-Lara, E.; Ragazzo-Sánchez, J.A. Potential Use of Leaf Protein Hydrolysate from the Green Biomass of Jackfruit Cultivation and Maltodextrin for the Microencapsulation of a Coccoloba uvífera L. Leaf Extract. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Fabil, M.; Dubey, P.K.; Roy, S.; Sharma, M. Jackfruit Seed Valorization: A Comprehensive Review of Nutritional Potential, Value Addition, and Industrial Applications. Food Humanit. 2024, 3, 100406. [Google Scholar] [CrossRef]
- Calderón-Chiu, C.; Calderón-Santoyo, M.; Herman-Lara, E.; Ragazzo-Sánchez, J.A. Jackfruit (Artocarpus heterophyllus Lam) Leaf as a New Source to Obtain Protein Hydrolysates: Physicochemical Characterization, Techno-Functional Properties and Antioxidant Capacity. Food Hydrocoll. 2021, 112, 106319. [Google Scholar] [CrossRef]
- Kalse, S.B.; Swami, S.B. Recent Application of Jackfruit Waste in Food and Material Engineering: A Review. Food Biosci. 2022, 48, 101740. [Google Scholar] [CrossRef]
- Goldstein, N.; Reifen, R. The Potential of Legume-Derived Proteins in the Food Industry. Grain Oil Sci. Technol. 2022, 5, 167–178. [Google Scholar] [CrossRef]
- Ma, J.-J.; Mao, X.-Y.; Wang, Q.; Yang, S.; Zhang, D.; Chen, S.-W.; Li, Y.-H. Effect of Spray Drying and Freeze Drying on the Immunomodulatory Activity, Bitter Taste and Hygroscopicity of Hydrolysate Derived from Whey Protein Concentrate. LWT Food Sci. Technol. 2014, 56, 296–302. [Google Scholar] [CrossRef]
- Ziaee, A.; O’Connor, E.M.; Murphy, E.; O’Reilly, E. Spray Drying of Food. In Drying Technology in Food Processing; Elsevier: Amsterdam, The Netherlands, 2023; pp. 123–155. [Google Scholar] [CrossRef]
- Dantas, A.; Piella-Rifà, M.; Costa, D.P.; Felipe, X.; Gou, P. Innovations in Spray Drying Technology for Liquid Food Processing: Design, Mechanisms, and Potential for Application. Appl. Food Res. 2024, 4, 100382. [Google Scholar] [CrossRef]
- Yousefi, N.; Abbasi, S. Food Proteins: Solubility & Thermal Stability Improvement Techniques. Food Chem. Adv. 2022, 1, 100090. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of Process Conditions on the Physicochemical Properties of Açai (Euterpe oleraceae Mart.) Powder Produced by Spray Drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Özdemir, E.E.; Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Physicochemical, Functional and Emulsifying Properties of Plant Protein Powder from Industrial Sesame Processing Waste as Affected by Spray and Freeze Drying. LWT 2022, 154, 112646. [Google Scholar] [CrossRef]
- Dantas, A.; Gou, P.; Piella-Rifà, M.; Felipe, X. Powdered Oat Drink Production by Pulse Spray Drying. Future Foods 2025, 11, 100521. [Google Scholar] [CrossRef]
- Pui, L.P.; Lejaniya, A.K.S. Effects of Spray-Drying Parameters on Physicochemical Properties of Powdered Fruits. Foods Raw Mater. 2022, 10, 235–251. [Google Scholar] [CrossRef]
- Mora, L.; Gallego, M.; Aristoy, M.C.; Reig, M.; Toldrá, F. Bioactive Peptides. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 333–345. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Q.; Lu, H.; Wang, J.; Zhao, J.; Li, P. Electrodialysis with Porous Membrane for Bioproduct Separation: Technology, Features, and Progress. Food Res. Int. 2020, 137, 109343. [Google Scholar] [CrossRef] [PubMed]
- Miss-Zacarías, D.M.D.J.; Calderón-Santoyo, M.; Ragazzo-Sánchez, J.A. Proteins and Protein Hydrolysate from Jackfruit Seeds (Artocarpus heterophyllus Lam.): Techno-Functional Properties and Amino Acid Profile. Food Bioprocess Technol. 2024, 18, 4345–4357. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- da Silva, E.S.; Xiong, J.; de Medeiros, F.G.M.; Grace, M.; Moncada, M.; Lila, M.A.; Hoskin, R.T. Spray Dried Insect Protein-Polyphenol Particles Deliver Health-Relevant Value-Added Food Ingredients. Future Foods 2024, 9, 100315. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Femenia, A.; Comas-Serra, F.; Rosselló, C.; Rodríguez-González, V.M.; González-Laredo, R.F.; Gallegos-Infante, J.A.; Medina-Torres, L. Effect of Different Drying Procedures on Physicochemical Properties and Flow Behavior of Aloe Vera (Aloe barbadensis Miller) Gel. LWT 2016, 74, 378–386. [Google Scholar] [CrossRef]
- Muhoza, B.; Harimana, Y.; Kayitesi, E.; Uriho, A.; Liu, Q. Insight into the Effect of Extraction and Spray Drying Conditions on the Nutritional and Techno-Functional Properties of Legume Protein Powder: A Review. Food Bioprocess Technol. 2024, 18, 1141–1159. [Google Scholar] [CrossRef]
- Looi, Y.F.; Ong, S.P.; Julkifle, A.; Alias, M.S. Effects of Pretreatment and Spray Drying on the Physicochemical Properties and Probiotics Viability of Moringa (Moringa oleifera Lam) Leaf Juice Powder. J. Food Process. Preserv. 2019, 43, e13915. [Google Scholar] [CrossRef]
- Korkmaz, F. Safflower Protein as a Potential Plant Protein Powder: Optimization of Extraction and Spray-drying Process Parameters and Determination of Physicochemical and Functional Properties. J. Sci. Food Agric. 2024, 104, 7408–7416. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, C.; Korkmaz, F. Freeze, Spray, and Vacuum Dried Camelina Sativa Protein Powders and Their Physicochemical and Functional Properties. Food Bioprod. Process. 2024, 148, 559–567. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse Proteins: Secondary Structure, Functionality and Applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.G.; Sethi, S.; Jha, S.K.; Kumar, R. Physico-Chemical and Functional Properties of Cowpea Protein Isolate as Affected by the Dehydration Technique. Legum. Res. Int. J. 2016, 39, 370–378. [Google Scholar] [CrossRef]
- Wu, S.; Miao, S. Physical Properties and Stickiness of Spray-Dried Food Powders. In Spray Drying for the Food Industry; Elsevier: Amsterdam, The Netherlands, 2024; pp. 551–571. [Google Scholar] [CrossRef]
- de Paiva Gouvêa, L.; Caldeira, R.; de Lima Azevedo, T.; Galdeano, M.C.; Felberg, I.; Lima, J.R.; Grassi Mellinger, C. Physical and Techno-Functional Properties of a Common Bean Protein Concentrate Compared to Commercial Legume Ingredients for the Plant-Based Market. Food Hydrocoll. 2023, 137, 108351. [Google Scholar] [CrossRef]
- WHO/FAO World Health Organization/Food and Agricultural Organization. Report of a Joint WHO/FAO/UNU Expert Consultation 2007; WHO/FAO: Geneva, Switzerland, 2007. [Google Scholar]
- Hashemirad, F.-S.; Behfar, M.; Kavoosi, G. Proximate Composition, Physico-Chemical, Techno-Functional, Amino Acid Profile, Fatty Acid Profile, Nutritional Quality, Antioxidant, Anti-Amylase and Anti-Lipase Properties of Bee Bread, Royal Jelly, and Bee Propolis. LWT 2024, 200, 116190. [Google Scholar] [CrossRef]
- Yu, N.; Jiang, C.; Ning, F.; Hu, Z.; Shao, S.; Zou, X.; Meng, X.; Xiong, H. Protein Isolate from Stauntonia brachyanthera Seed: Chemical Characterization, Functional Properties, and Emulsifying Performance after Heat Treatment. Food Chem. 2021, 345, 128542. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and Functional Properties of Food Protein-Derived Antioxidant Peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- Qoms, M.S.; Arulrajah, B.; Shamsudin, R.; Ibadullah, W.Z.W.; Saari, N. Valorization of Green Biomass Azolla pinnata Fern: Multi-parameter Evaluation of Processing Conditions on Protein Extractability and Their Influence on the Physicochemical, Structural, Techno-functional Properties and Protein Quality. J. Sci. Food Agric. 2022, 102, 6974–6983. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Barbin, D.F.; Hubinger, M.D. Implications of Non-Equilibrium States and Glass Transitions in Frozen and Dried Fish and Meat Products. In Non-Equilibrium States and Glass Transitions in Foods; Elsevier: Amsterdam, The Netherlands, 2017; pp. 325–348. [Google Scholar] [CrossRef]
- Calderón-Chiu, C.; Calderón-Santoyo, M.; Barros-Castillo, J.C.; Díaz, J.A.; Ragazzo-Sánchez, J.A. Structural Modification of Jackfruit Leaf Protein Concentrate by Enzymatic Hydrolysis and Their Effect on the Emulsifier Properties. Colloids Interfaces 2022, 6, 52. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Li, S. Physicochemical and Functional Properties of Chinese Quince Seed Protein Isolate. Food Chem. 2019, 283, 539–548. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Mahdi Jafari, S.; Sarabandi, K.; Mohammadi, M.; Khakbaz Heshmati, M.; Pezeshki, A. Influence of Spray Drying Encapsulation on the Retention of Antioxidant Properties and Microstructure of Flaxseed Protein Hydrolysates. Colloids Surf. B Biointerfaces 2019, 178, 421–429. [Google Scholar] [CrossRef]
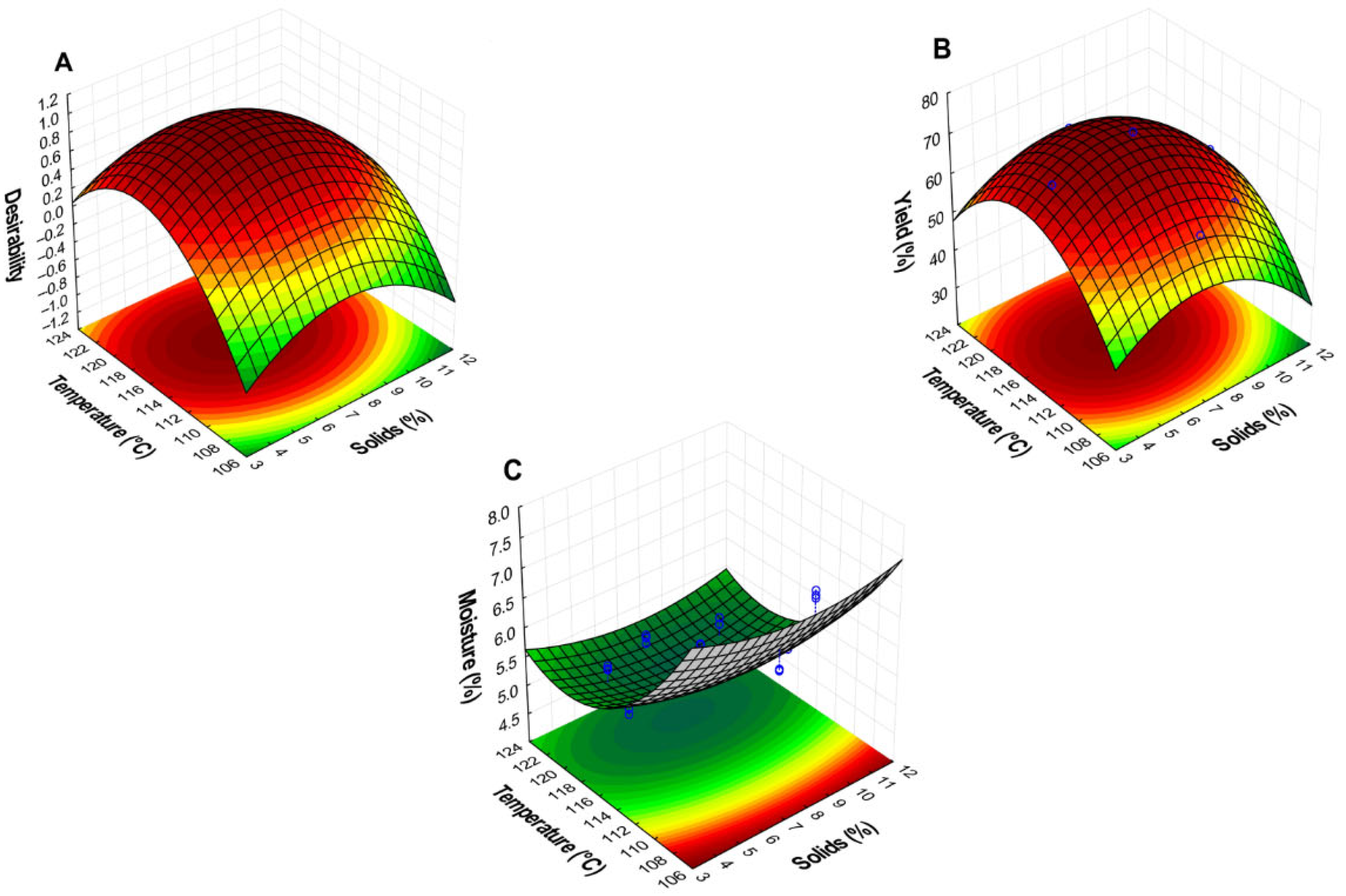

| Run No. | Independents Variables | Responses Variables | ||
|---|---|---|---|---|
| Solids (%, X1) | Ti (°C, X2) | Yield (%, Y1) | Moisture (%, Y2) | |
| 1 | 5.0 | 110 | 58.18 ± 0.01 b | 6.89 ± 0.0 e |
| 2 | 5.0 | 120 | 65.45 ± 0.01 e | 5.38 ± 0.03 bc |
| 3 | 10.0 | 110 | 56.36 ± 0.01 a | 6.81 ± 0.06 c |
| 4 | 10.0 | 120 | 58.18 ± 0.01 b | 5.31 ± 0.06 b |
| 5 * | 7.5 | 115 | 70.30 ± 0.01 g | 5.37 ± 0.02 bc |
| 6 * | 7.5 | 115 | 70.30 ± 0.01 g | 5.39 ± 0.06 bc |
| 7 * | 7.5 | 115 | 71.51 ± 0.01 h | 5.44 ± 0.03 c |
| 8 | 3.9 | 115 | 68.79 ± 0.00 f | 5.38 ± 0.05 bc |
| 9 | 11.0 | 115 | 59.31 ± 0.01 c | 5.21 ± 0.06 a |
| 10 | 7.5 | 107.9 | 58.18 ± 0.01 b | 6.23 ± 0.01 d |
| 11 | 7.5 | 122.0 | 63.03 ± 0.02 d | 5.20 ± 0.07 a |
| 12 * | 7.5 | 115 | 71.51± 0.00 h | 5.40 ± 0.05 c |
| 13 * | 7.5 | 115 | 70.30 ± 0.00 g | 5.38 ± 0.07 c |
| 14 * | 7.5 | 115 | 72.72 ± 0.00 i | 5.4 ± 0.04 c |
| Optimal | Predicted | |||
| 7.5 | 115 | 72.08 | 5.22 | |
| Experimental | ||||
| 71.51 ± 1.21 | 5.33 ± 0.11 | |||
| Property | JSPC Powder |
|---|---|
| Moisture (%) | 5.33 ± 0.11 |
| Water activity (aw) | 0.15 ± 0.02 |
| Bulk density (g/mL) | 0.40 ± 0.01 |
| L* (Lightness) | 70.56 ± 0.38 |
| a* (redness) | 7.80 ± 0.11 |
| b* (yellowness) | 15.18 ± 0.15 |
| Hydrosolubility (%) | 82.46 ± 1.68 |
| Foaming capacity (%) | 48.33 ± 1.66 |
| Foaming stability (%) | 46.11 ± 0.96 |
| Emulsifying activity index (m2/g) | 105.74 ± 10.20 |
| Emulsifying stability index (min) | 85.12 ± 8.06 |
| Amino Acids | JSPC Powder (g/100 g) | WHO/FAO * (g/100 g) | |
|---|---|---|---|
| Essential amino acids | Valine (Val) | 9.58 | 3.9 |
| Leucine (Leu) | 10.42 | 5.9 | |
| Isoleucine (Ile) | 9.44 | 3.0 | |
| Methionine (Met) | 1.71 | 1.6 | |
| Threonine (Thr) | 9.67 | 2.3 | |
| Phenylalanine (Phe) | 13.12 | 3.8 ** | |
| Lysine (Lys) | 15.50 | 4.5 | |
| Non-essential amino acids | Alanine (Ala) | 3.48 | |
| Glycine (Gly) | 5.63 | ||
| Proline (Pro) | 6.57 | ||
| Serine (Ser) | 9.61 | ||
| Aspartic acid (Asp) | 14.21 | ||
| Glutamic acid (Glu) | 13.46 | ||
| Tyrosine (Tyr) | 19.25 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miss-Zacarías, D.M.d.J.; Calderón-Santoyo, M.; Zamora-Gasga, V.M.; Ascanio, G.; Ragazzo-Sánchez, J.A. Spray Drying of Jackfruit (Artocarpus heterophyllus Lam.) Seeds Protein Concentrate: Physicochemical, Structural, and Thermal Characterization. Processes 2025, 13, 2319. https://doi.org/10.3390/pr13072319
Miss-Zacarías DMdJ, Calderón-Santoyo M, Zamora-Gasga VM, Ascanio G, Ragazzo-Sánchez JA. Spray Drying of Jackfruit (Artocarpus heterophyllus Lam.) Seeds Protein Concentrate: Physicochemical, Structural, and Thermal Characterization. Processes. 2025; 13(7):2319. https://doi.org/10.3390/pr13072319
Chicago/Turabian StyleMiss-Zacarías, Dulce María de Jesús, Montserrat Calderón-Santoyo, Victor Manuel Zamora-Gasga, Gabriel Ascanio, and Juan Arturo Ragazzo-Sánchez. 2025. "Spray Drying of Jackfruit (Artocarpus heterophyllus Lam.) Seeds Protein Concentrate: Physicochemical, Structural, and Thermal Characterization" Processes 13, no. 7: 2319. https://doi.org/10.3390/pr13072319
APA StyleMiss-Zacarías, D. M. d. J., Calderón-Santoyo, M., Zamora-Gasga, V. M., Ascanio, G., & Ragazzo-Sánchez, J. A. (2025). Spray Drying of Jackfruit (Artocarpus heterophyllus Lam.) Seeds Protein Concentrate: Physicochemical, Structural, and Thermal Characterization. Processes, 13(7), 2319. https://doi.org/10.3390/pr13072319







