Molecular Dynamics Unveiled: Temperature–Pressure–Coal Rank Triaxial Coupling Mechanisms Governing Wettability in Gas–Water–Coal Systems
Abstract
1. Introduction
2. Molecular Models and Research Methods
2.1. Molecular Models
2.2. Research Methods
3. Simulation Results
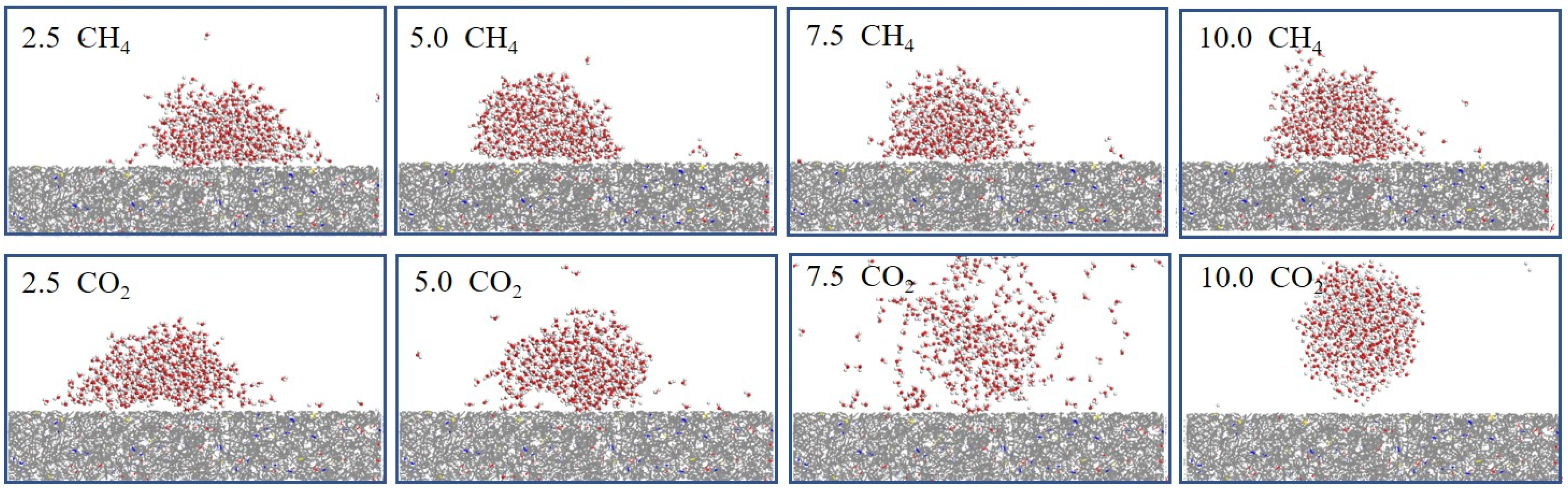
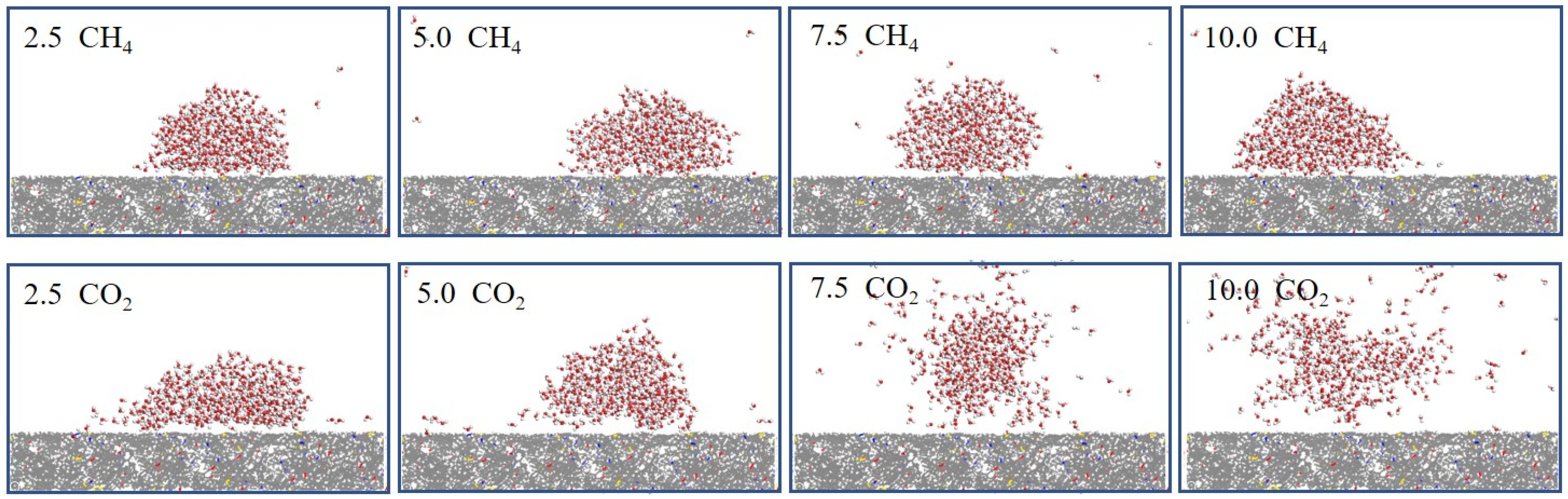
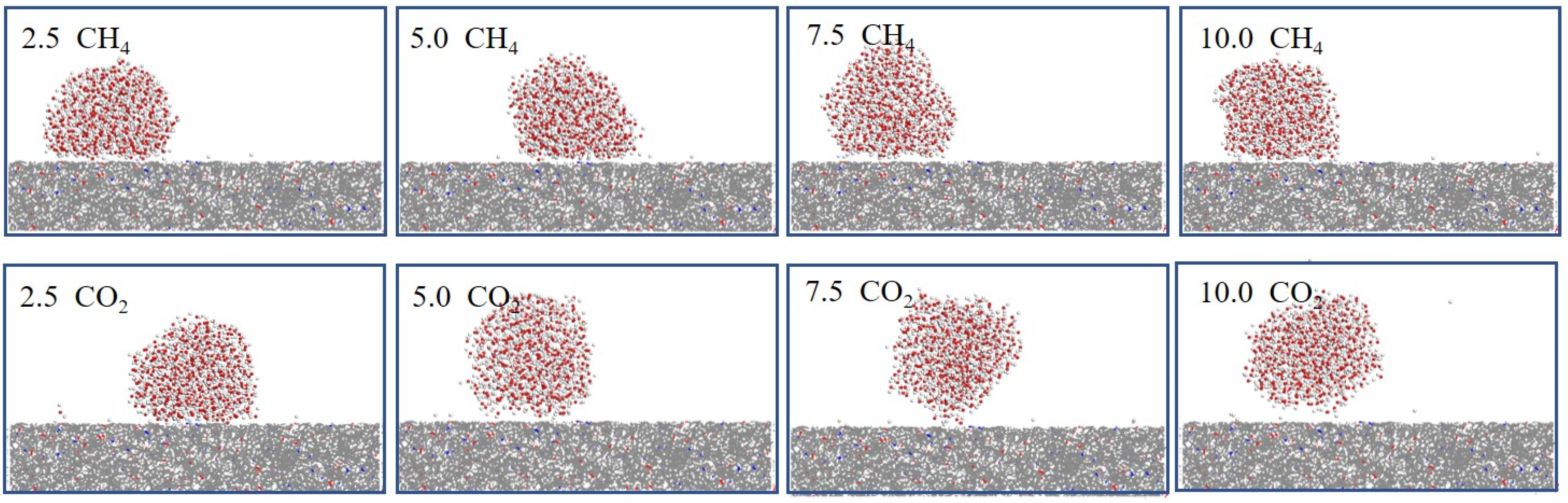
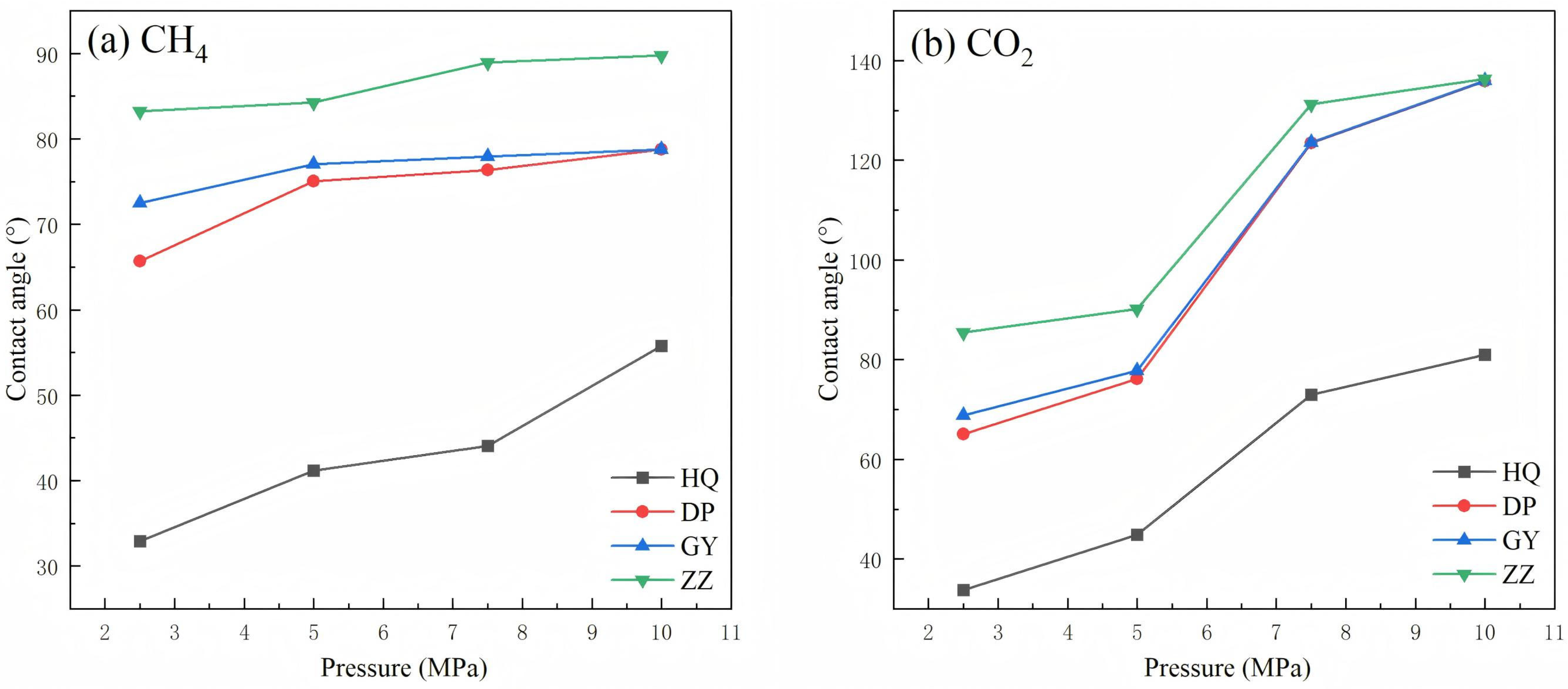
3.1. Wettability of Coal Under Different Pressures
3.2. Wettability of Coal Under Different Temperatures
4. Discussion
4.1. Effect of Pressure on Coal Wettability
4.2. Analysis of Wettability Differences Caused by Different Gases
4.3. Comparative Analysis of CH4 and CO2 Effects on Coal Wettability
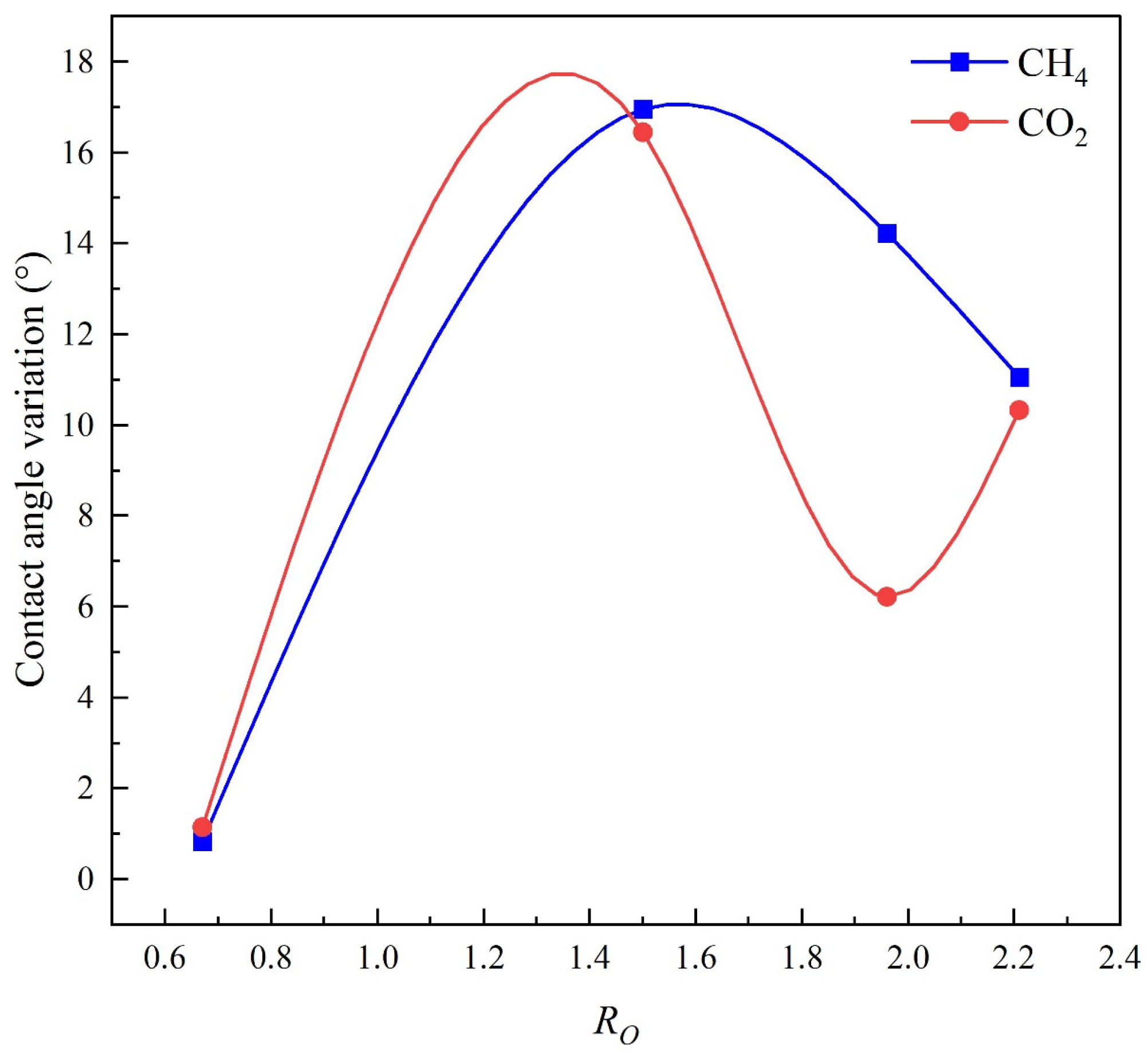
4.4. Correlation Analysis of Wettability Across Different Coal Ranks
5. Conclusions
- (1)
- Higher gas pressure enhanced CH4 and CO2 adsorption on the coal surface, reducing hydrogen bonding with water and increasing contact angles. In high-pressure CO2 environments (>5 MPa), most high-rank coals exhibited wettability reversal from water-wet to gas-wet. CH4 had a milder effect due to weaker adsorption.
- (2)
- Elevated temperature increased water molecule mobility and gas desorption, freeing up adsorption sites for water. This promoted hydrogen bond formation and reduced surface/interfacial tension, thereby improving wettability—especially in high-rank coals.
- (3)
- CO2, due to its higher polarity and stronger surface affinity, adsorbed more readily than CH4, occupying critical sites and disrupting water adsorption and hydrogen bonding. This led to more diffuse water density distributions and poorer wettability compared to CH4.
- (4)
- Wettability was strongly positively correlated with coal rank and negatively correlated with hydrogen bond count and polar functional group content. As coal rank increased, the reduction in surface polarity weakened water adsorption, especially under CO2, promoting wettability reversal.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, Y.; Liu, D.; Xie, S. Quantitative Characterization of Methane Adsorption on Coal Using a Low-Field NMR Relaxation Method. Int. J. Coal Geol. 2014, 131, 32–40. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D. Rock Physics and Fluid Characterization of Coal Reservoirs Based on NMR Relaxation Spectra. Coal Sci. Technol. 2016, 44, 14–22. [Google Scholar] [CrossRef]
- Sun, X.; Yao, Y.; Liu, D.; Zhou, Y. Investigations of CO2-Water Wettability of Coal: NMR Relaxation Method. Int. J. Coal Geol. 2018, 188, 38–50. [Google Scholar] [CrossRef]
- Zheng, S.; Yao, Y.; Elsworth, D.; Liu, D.; Cai, Y. Dynamic Fluid Interactions During CO2-ECBM and CO2 Sequestration in Coal Seams. Part 2: CO2-H2O Wettability. Fuel 2020, 308, 279. [Google Scholar] [CrossRef]
- Sun, X.; Yao, Y.; Chen, J.; Xie, S.; Li, C. Analysis of Coal Wettability Based on Low-Field Nuclear Magnetic Resonance. Mod. Geol. 2015, 29, 190–197. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, X.; Wan, L. Research on the Micro-Mechanism of CO2 Geological Sequestration in Coal Seams. Coal Geol. Explor. 2023, 51, 146–157. [Google Scholar] [CrossRef]
- Li, J. Analysis of Factors Affecting Coal Surface Wettability. Master’s Thesis, Henan Polytechnic University, Jiaozuo, China, 2016. [Google Scholar]
- Li, J.; Li, K. Factors Affecting Coal Surface Wettability. J. China Coal Soc. 2016, 41 (Suppl. S2), 448–453. [Google Scholar] [CrossRef]
- Li, S.; Guo, D.; Bai, Y.; Yan, M.; Lin, H.; Shi, Y. Molecular Simulation of the Effect of Different Mass Fractions of SDBS on Coal Wettability. China Saf. Sci. J. 2020, 30, 21–27. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Sun, Z. Study on the Influencing Factors of Wettability of Coal Dust of Different Metamorphic Degrees. Coal Mine Mach. 2022, 43, 65–68. [Google Scholar] [CrossRef]
- Gao, M.; Wang, M.; Xie, J.; Gao, Y.; Deng, G.; Yang, B.; Wang, F.; Hao, H.; Xie, H. Research on In-Situ Disturbance Mechanical Behavior of Deep Coal Rock. J. China Coal Soc. 2020, 45, 2691–2703. [Google Scholar] [CrossRef]
- Li, M.; Xu, H.; Shu, X. Discussion on the Application of Chemical Dust Suppressants in Coal Dust Suppression. China Coal 2007, 46–47+50. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, Z.; Deng, Y.; Xing, J. Study on Dust Pollution in Fully Mechanized Mining Face. J. Saf. Sci. Technol. 2008, 4, 67–70. [Google Scholar] [CrossRef]
- Yuan, L. Scientific Conception of Coal Mine Dust Control and Occupational Safety and Health. J. China Coal Soc. 2020, 45, 1–7. [Google Scholar] [CrossRef]
- Wang, J. Experimental Study on Physicochemical Characteristics and Dust Reduction of Coal Dust of Different Particle Sizes. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2019. [Google Scholar]
- Zhang, J.; Li, H.; Liu, Y.; Li, X.; Xie, J.; Dai, Z.; Ye, S.; Li, L.; Zhou, W.; Zhao, Y.; et al. Microcosmic Wettability Characteristics of Coal Dust and Preliminary Exploration of Dust Suppressant Development: A Case Study of Pingdingshan Mining Area. J. China Coal Soc. 2021, 46, 812–825. [Google Scholar] [CrossRef]
- Yan, M.; Yue, M.; Lin, H.; Yan, D.; Wei, J.; Qin, X.; Zhang, J. Experimental Study on the Influence of Functional Groups on the Wettability of Medium and Low Rank Coal. Coal Sci. Technol. 2023, 51, 103–113. [Google Scholar] [CrossRef]
- Zhou, G.; Cheng, W.; Xu, C.; Nie, W. Analysis of 13C-NMR Characteristics of Wettability Differences of Coal Dust of Different Metamorphic Degrees. J. China Coal Soc. 2015, 40, 2849–2855. [Google Scholar] [CrossRef]
- Zhou, G.; Xue, J.; Cheng, W.; Nie, W.; Wang, H. Influence of Stacking Structure on Coal Dust Wettability Based on X-Ray Diffraction Experiment. Chin. J. Eng. 2015, 37, 1535–1541. [Google Scholar] [CrossRef]
- Siemons, N.; Bruining, H.; Castelijns, H.; Wolf, K.-H. Pressure Dependence of the Contact Angle in a CO2-H2O-Coal System. J. Colloid Interface Sci. 2006, 297, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, Y.; Sun, X.; Xie, S.; Guo, W. Study on the Influence of CO2 and Helium on the Wettability of Low-Rank Coal. Coal Sci. Technol. 2015, 43, 129–134. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhang, R.; Xing, Y.; Gui, X. Enhancement of the Surface Hydrophobicity of Low-Rank Coal by Adsorbing DTAB: An Experimental and Molecular Dy-namics Simulation Study. Fuel 2019, 239, 145–152. [Google Scholar] [CrossRef]
- Van Niekerk, D.; Mathews, J.P. Molecular Dynamic Simulation of Coal-Solvent Interactions in Permian-Aged South African Coals. FPT 2011, 92, 729–734. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Lei, J.; Xie, J.; Li, L.; Gan, Y.; Deng, J.; Qi, R.; Liu, Y. Study on the Surface Wetting Mechanism of Bituminous Coal Based on the Microscopic Molecular Structure. RSC Adv. 2023, 13, 5933–5945. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Lu, Y.; Cheng, F.; Wang, X.; Miller, J.D. Effect of Oxidation on the Wetting of Coal Surfaces by Water: Experimental and Molecular Dynamics Simulation Studies. Physicochem. Probl. Miner. 2018, 54, 1039–1051. [Google Scholar]
- Meng, J.; Wang, L.; Zhang, S.; Lyu, Y.; Xia, J. Effect of Anionic/Nonionic Surfactants on the Wettability of Coal Surface. Chem. Phys. Lett. 2021, 785, 139130. [Google Scholar] [CrossRef]
- Xia, Y.; Rong, G.; Xing, Y.; Gui, X. Synergistic Adsorption of Polar and Nonpolar Reagents on Oxygen-Containing Graphite Surfaces: Implications for Low-Rank Coal Flotation. J. Colloid Interface Sci. 2019, 557, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Experimental Study on Competitive Wetting Characteristics of CO2 and Water in Coal of Different Metamorphic Degrees Under High Temperature and High Pressure. Master’s Thesis, China University of Mining and Technology, Beijing, China, 2022. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Tang, S.; Zhang, S.; Li, Z.; Fan, Z. Construction and Comparison of Coal Macromolecular Models of Different Ranks. Mod. Geol. 2025, 39, 839–855. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Tang, S.; Zhang, S.; Li, J. Study on the Wetting Mechanisms of Different Coal Ranks Based on Molecular Dynamics. Processes 2024, 12, 455. [Google Scholar] [CrossRef]
- Sakamaki, R.; Sum, A.K.; Narumi, T.; Yasuoka, K. Molecular dynamics simulations of vapor/liquid coexistence using the nonpolarizable water models. J. Chem. Phys. 2011, 134, 124708. [Google Scholar] [CrossRef]
- Si, L.; Gu, Y.; Wei, J.; Ding, N.; Jiang, W.; Yao, B.; Wen, Z. Variation Law and Microscopic Interaction Mechanism of Coal-Water Wettability Dynamic Spreading under Different Gas Environments. J. China Coal Soc. 2025, 1–16. [Google Scholar] [CrossRef]
- Yue, J.; Wang, C.; Shi, B.; Wang, Z.; Xu, J.; Han, Q.; Sun, Y.; Liang, Y. Wettability Characteristics and Influencing Mechanisms of Coal-Water Interface Under Methane Atmosphere. J. China Coal Soc. 2024, 49, 4325–4335. [Google Scholar] [CrossRef]
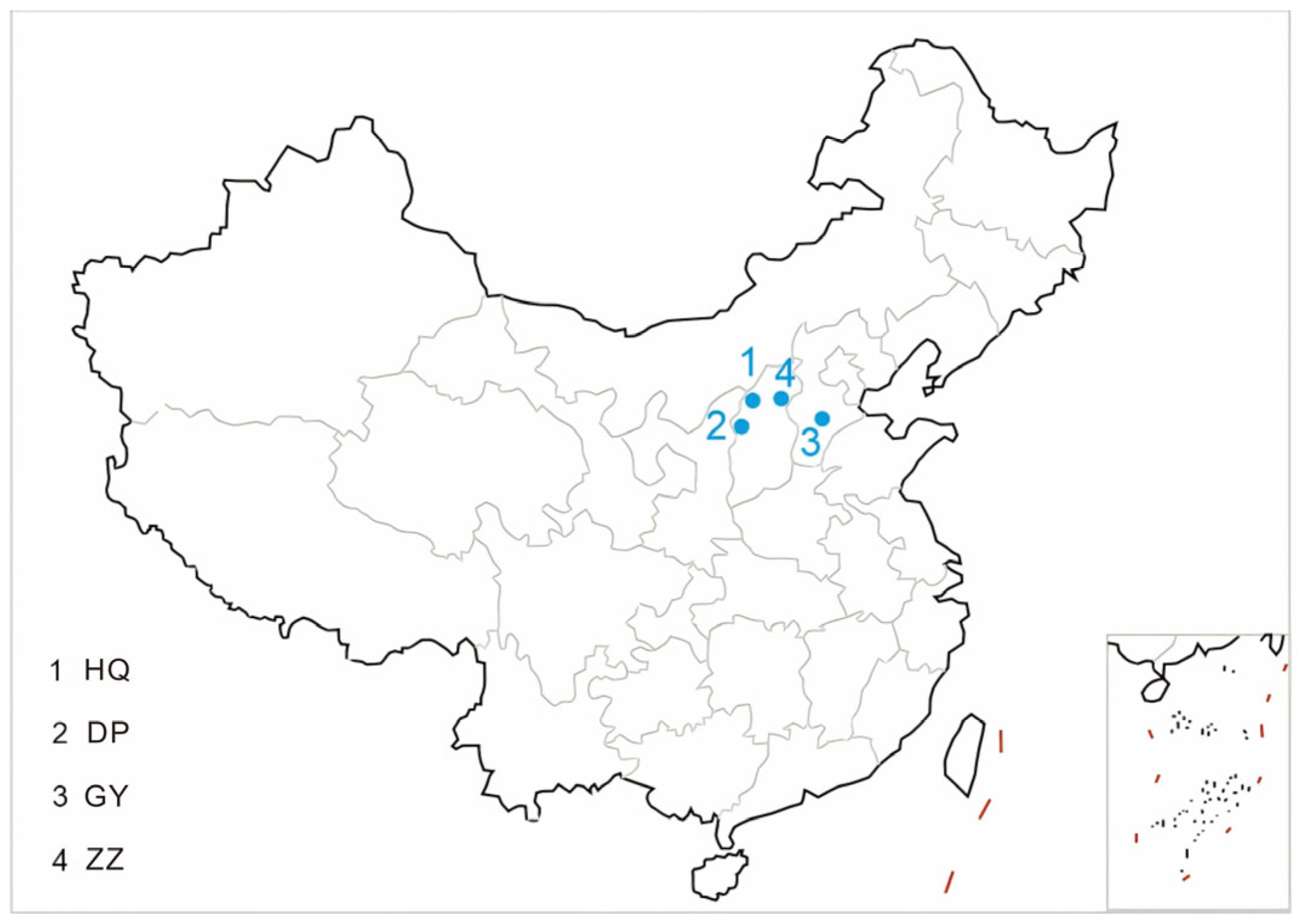
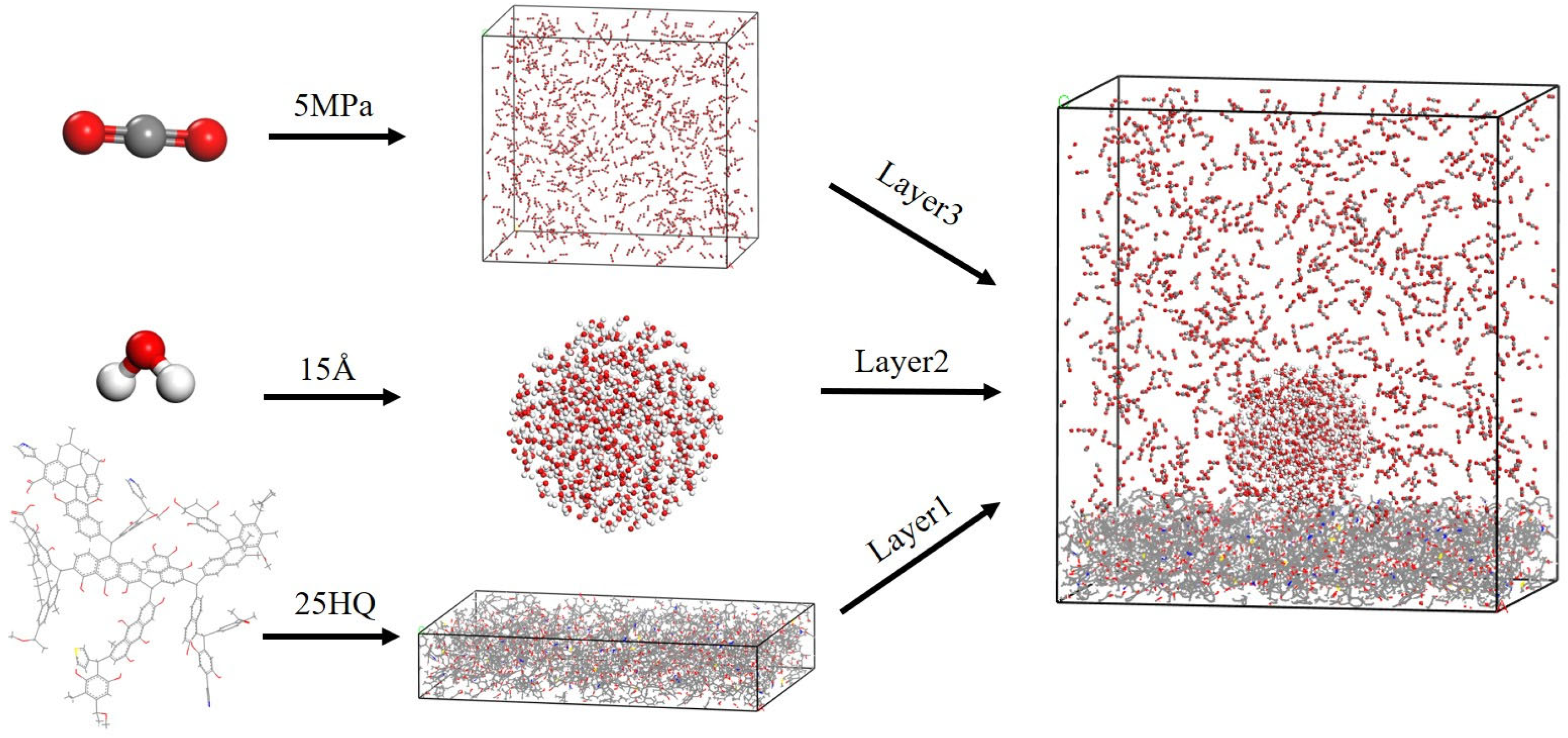











| No. | Sample | Sampling Site | Stratigraphy | Sedimentary Environment |
|---|---|---|---|---|
| 1 | HQ | Hequ coal mine | Permian Taiyuan formation | Fluvial-deltaic plain deposition |
| 2 | DP | Dianping coal mine | Permian lower Shihezi formation | Meandering delta plain deposition |
| 3 | GY | Gaoyang Coal mine | Carboniferous-Permian Taiyuan formation | Barrier-lagoon deposition |
| 4 | ZZ | Zhaozhuang Coal mine | Carboniferous-permian Taiyuan formation | Lagoon deposition |
| Sample | RO (%) | Carbonyl | Carboxyl | Hydroxyl | Ether Oxygen | Aldehyde |
|---|---|---|---|---|---|---|
| HQ | 0.67 | 2 | 2 | 28 | 2 | 0 |
| DP | 1.50 | 0 | 0 | 2 | 0 | 1 |
| GY | 1.96 | 1 | 0 | 1 | 1 | 0 |
| ZZ | 2.21 | 1 | 0 | 1 | 3 | 0 |
| Sample | RO (%) | Total H-Bonds | Intra-Coal H-Bonds | Intra-Water H-Bonds | Interfacial H-Bonds |
|---|---|---|---|---|---|
| HQ | 0.67 | 909 | 199 | 625 | 85 |
| DP | 1.50 | 702 | 4 | 694 | 4 |
| GY | 1.96 | 698 | 0 | 692 | 6 |
| ZZ | 2.21 | 698 | 2 | 685 | 11 |
| Sample | CH4 Environment | CO2 Environment | ||||||
|---|---|---|---|---|---|---|---|---|
| 2.5 Mpa | 5.0 Mpa | 7.5 Mpa | 10.0 Mpa | 2.5 Mpa | 5.0 Mpa | 7.5 Mpa | 10.0 Mpa | |
| HQ | 32.92° 2.1° | 41.17° 2.3° | 44.07° 2.5° | 55.74° 2.8° | 33.81° 2.0° | 44.90° 2.4° | 72.91° 2.7° | 81.02° 2.9° |
| DP | 65.71° 2.5° | 75.02° 2.6° | 76.34° 2.7° | 78.75° 2.8° | 65.10° 2.4° | 76.09° 2.5° | 123.48° 2.7° | 135.86° 2.8° |
| GY | 72.51° 2.6° | 77.03° 2.7° | 77.94° 2.7° | 78.78° 2.8° | 68.82° 2.5° | 77.73° 2.6° | 123.54° 2.7° | 135.95° 2.8° |
| ZZ | 83.24° 2.8° | 84.24° 2.8° | 88.96° 2.9° | 89.78° 2.9° | 85.47° 2.7° | 90.15° 2.8° | 131.28° 2.9° | 136.37° 3.0° |
| Sample | RO (%) | Contact Angle (CH4, 5 MPa) (°) | Contact Angle (CO2, 5 MPa) (°) | ||||
|---|---|---|---|---|---|---|---|
| 298 K | 318 K | 338 K | 298 K | 318 K | 338 K | ||
| HQ | 0.67 | 41.17 | 40.75 | 40.34 | 44.90 | 44.34 | 43.75 |
| DP | 1.50 | 75.02 | 63.73 | 58.08 | 76.09 | 63.34 | 59.64 |
| GY | 1.96 | 77.03 | 66.74 | 62.08 | 77.73 | 74.90 | 71.51 |
| ZZ | 2.21 | 84.24 | 75.38 | 73.18 | 90.15 | 83.09 | 79.82 |
| Sample | RO (%) | CH4 Environment (Number of H-Bonds) | CO2 Environment (Number of H-Bonds) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2.5 MPa | 5.0 MPa | 7.5 MPa | 10.0 MPa | 2.5 MPa | 5.0 MPa | 7.5 MPa | 10.0 MPa | ||
| HQ | 0.67 | 147 | 84 | 92 | 56 | 83 | 74 | 31 | 21 |
| DP | 1.50 | 8 | 6 | 6 | 5 | 6 | 2 | 2 | 0 |
| GY | 1.96 | 15 | 10 | 12 | 6 | 14 | 9 | 5 | 0 |
| ZZ | 2.21 | 11 | 8 | 6 | 4 | 9 | 6 | 2 | 0 |
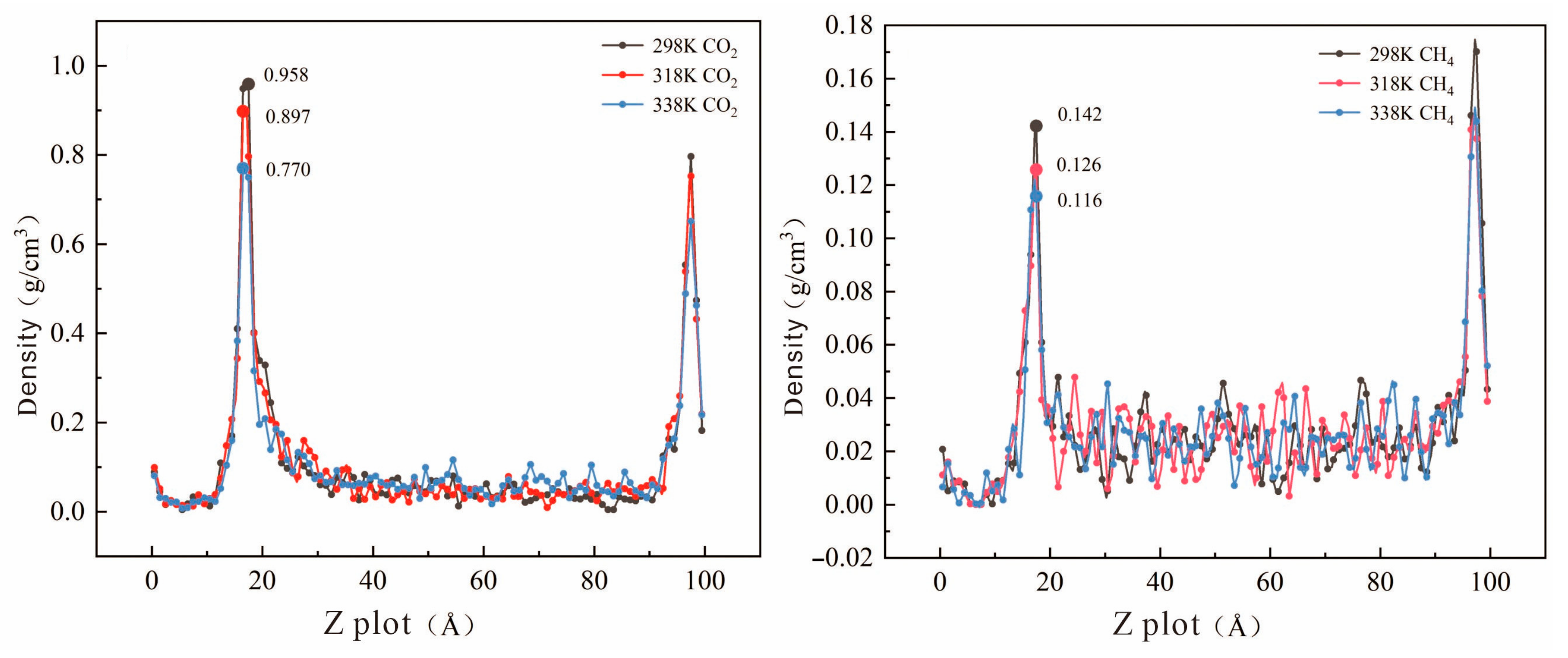
| Sample | RO (%) | CO2 Density Peak (g/cm3) | CH4 Density Peak (g/cm3) | ||||
|---|---|---|---|---|---|---|---|
| 298 K | 318 K | 338 K | 298 K | 318 K | 338 K | ||
| HQ | 0.67 | 0.798 | 0.767 | 0.748 | 0.102 | 0.081 | 0.056 |
| DP | 1.50 | 1.000 | 0.842 | 0.840 | 0.141 | 0.119 | 0.101 |
| GY | 1.96 | 0.958 | 0.897 | 0.770 | 0.142 | 0.126 | 0.116 |
| ZZ | 2.21 | 0.999 | 0.821 | 0.795 | 0.150 | 0.147 | 0.124 |
| CH4 Environment | CO2 Environment | |||
|---|---|---|---|---|
| Pressure (MPa) | CH4 Density (g/cm3) | H2O Density (g/cm3) | CO2 Density (g/cm3) | H2O Density (g/cm3) |
| 2.5 | 0.061 | 0.436 | 0.411 | 0.370 |
| 5 | 0.102 | 0.390 | 0.798 | 0.308 |
| 7.5 | 0.158 | 0.337 | 1.15 | 0.156 |
| 10 | 0.207 | 0.302 | 1.17 | 0.146 |
| Sample | HQ | DP | GY | ZZ |
|---|---|---|---|---|
| RO | 0.67 | 1.50 | 1.96 | 2.21 |
| Contact angle in CH4 (°) | 41.17 | 75.02 | 77.03 | 84.24 |
| Contact angle in CO2 (°) | 44.9 | 76.09 | 77.73 | 90.15 |
| Hydrogen bonds in CH4 | 84 | 6 | 10 | 8 |
| Hydrogen bonds in CO2 | 74 | 6 | 9 | 6 |
| Carbonyl groups (C=O) | 2 | 0 | 1 | 1 |
| Carboxyl groups (–COOH) | 2 | 0 | 0 | 0 |
| Hydroxyl groups (–OH) | 28 | 2 | 1 | 1 |
| Ether oxygen groups (–O–) | 2 | 0 | 1 | 3 |
| Aldehyde groups (–CHO) | 0 | 1 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, S.; Tang, S.; Xi, Z.; Li, J.; Zhang, Q.; Zhang, K.; Tian, W. Molecular Dynamics Unveiled: Temperature–Pressure–Coal Rank Triaxial Coupling Mechanisms Governing Wettability in Gas–Water–Coal Systems. Processes 2025, 13, 2209. https://doi.org/10.3390/pr13072209
Zhang L, Zhang S, Tang S, Xi Z, Li J, Zhang Q, Zhang K, Tian W. Molecular Dynamics Unveiled: Temperature–Pressure–Coal Rank Triaxial Coupling Mechanisms Governing Wettability in Gas–Water–Coal Systems. Processes. 2025; 13(7):2209. https://doi.org/10.3390/pr13072209
Chicago/Turabian StyleZhang, Lixin, Songhang Zhang, Shuheng Tang, Zhaodong Xi, Jianxin Li, Qian Zhang, Ke Zhang, and Wenguang Tian. 2025. "Molecular Dynamics Unveiled: Temperature–Pressure–Coal Rank Triaxial Coupling Mechanisms Governing Wettability in Gas–Water–Coal Systems" Processes 13, no. 7: 2209. https://doi.org/10.3390/pr13072209
APA StyleZhang, L., Zhang, S., Tang, S., Xi, Z., Li, J., Zhang, Q., Zhang, K., & Tian, W. (2025). Molecular Dynamics Unveiled: Temperature–Pressure–Coal Rank Triaxial Coupling Mechanisms Governing Wettability in Gas–Water–Coal Systems. Processes, 13(7), 2209. https://doi.org/10.3390/pr13072209






