Process Safety Assessment of the Entire Nitration Process of Benzotriazole Ketone
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Principles
- (1)
- Nitration step: The reactor jacket temperature was set to 33 °C. Benzotriazole ketone (186.0 g, 0.63 mol) and fuming sulfuric acid (640.0 g) were charged in a single portion and agitated with an anchor-type impeller at 110 rpm. After the mixture reached thermal equilibrium at 33 °C, concentrated nitric acid (42.0 g, 0.65 mol, 98 wt%) was added dropwise over 30 min while maintaining the bulk temperature at 33 °C. The reaction was then allowed to proceed isothermally until the exotherm ceased (≈6 h). At 33 °C, the post-reaction mixture was sampled to determine the specific heat capacity (Cp) and other thermophysical parameters.
- (2)
- Quenching step: With the reactor at ambient temperature, water (133.0 g) and toluene (140.0 g) were introduced, and the contents were cooled to 5 °C. The stirrer speed was adjusted to 150 rpm before 270.0 g of the nitration mixture was added over 4 h. During the addition process, the temperature rose from 5 °C to 60 °C. After completion, the system was held for 30 min to reach steady state, and Cp, along with other basic parameters of the quenched mixture, were measured.
- (3)
- Extraction step: The quenched liquor (360.0 g) was heated to 63 °C under stirring at 150 rpm. Toluene (140.0 g) was then added in a single charge, followed by a 30 min hold at 63 °C. Once thermal equilibrium was achieved, the mixture was analyzed for Cp and related properties.
- (4)
- Alkaline-washing step: The combined toluene phase (430.0 g) was heated to 63 °C while maintaining agitation at 150 rpm. A single charge of aqueous caustic soda (200.0 g) was introduced, and the biphasic system was held for 30 min. After reaching thermal stability, Cp and other key parameters of the washed organic phase were determined.
2.2. Reagents
2.3. Experimental Devices
2.3.1. Dynamic Scanning Calorimetry
2.3.2. Reaction Calorimeter
2.3.3. Accelerating Rate Calorimetry
3. Results and Discussion
3.1. Stability of Material
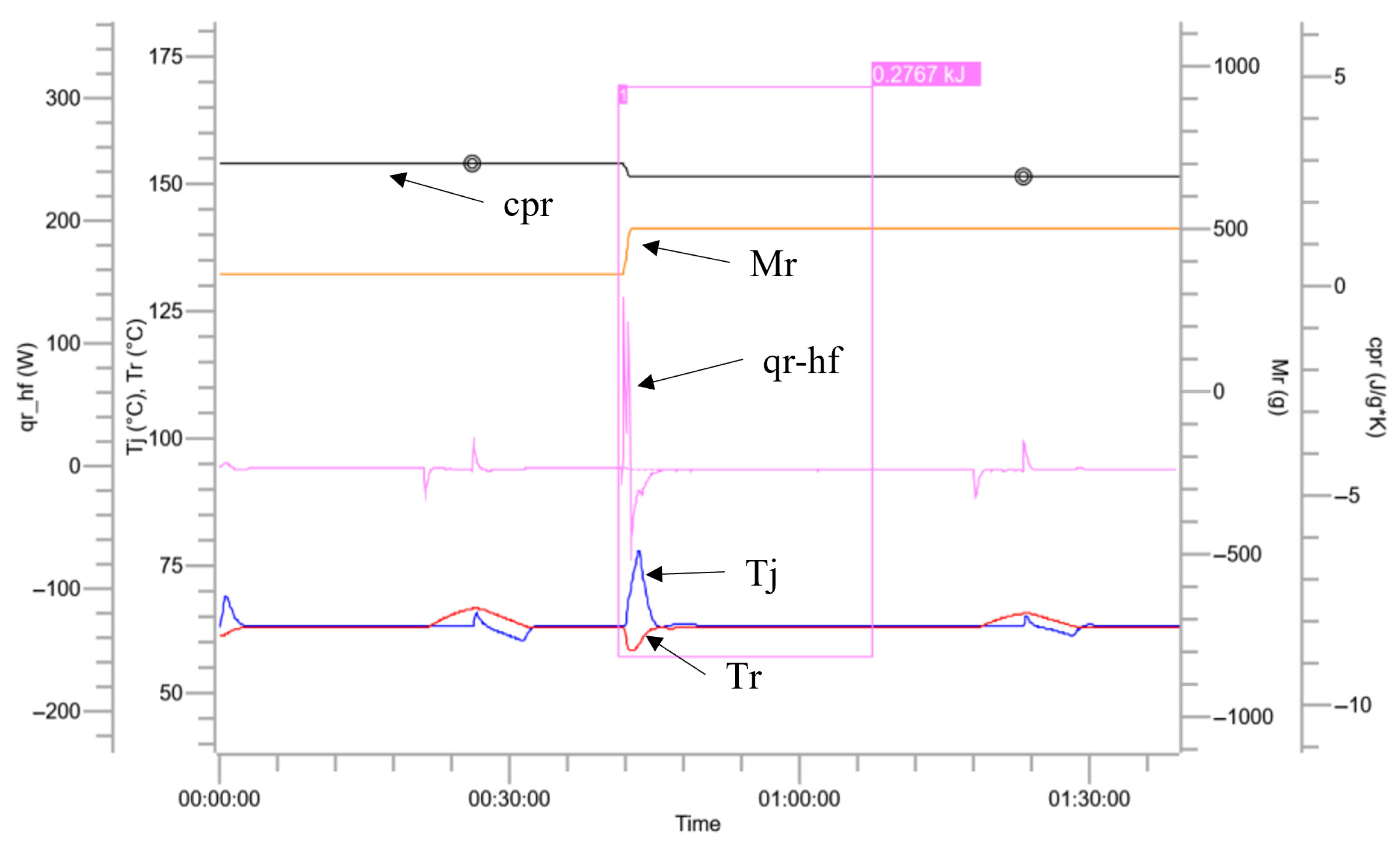
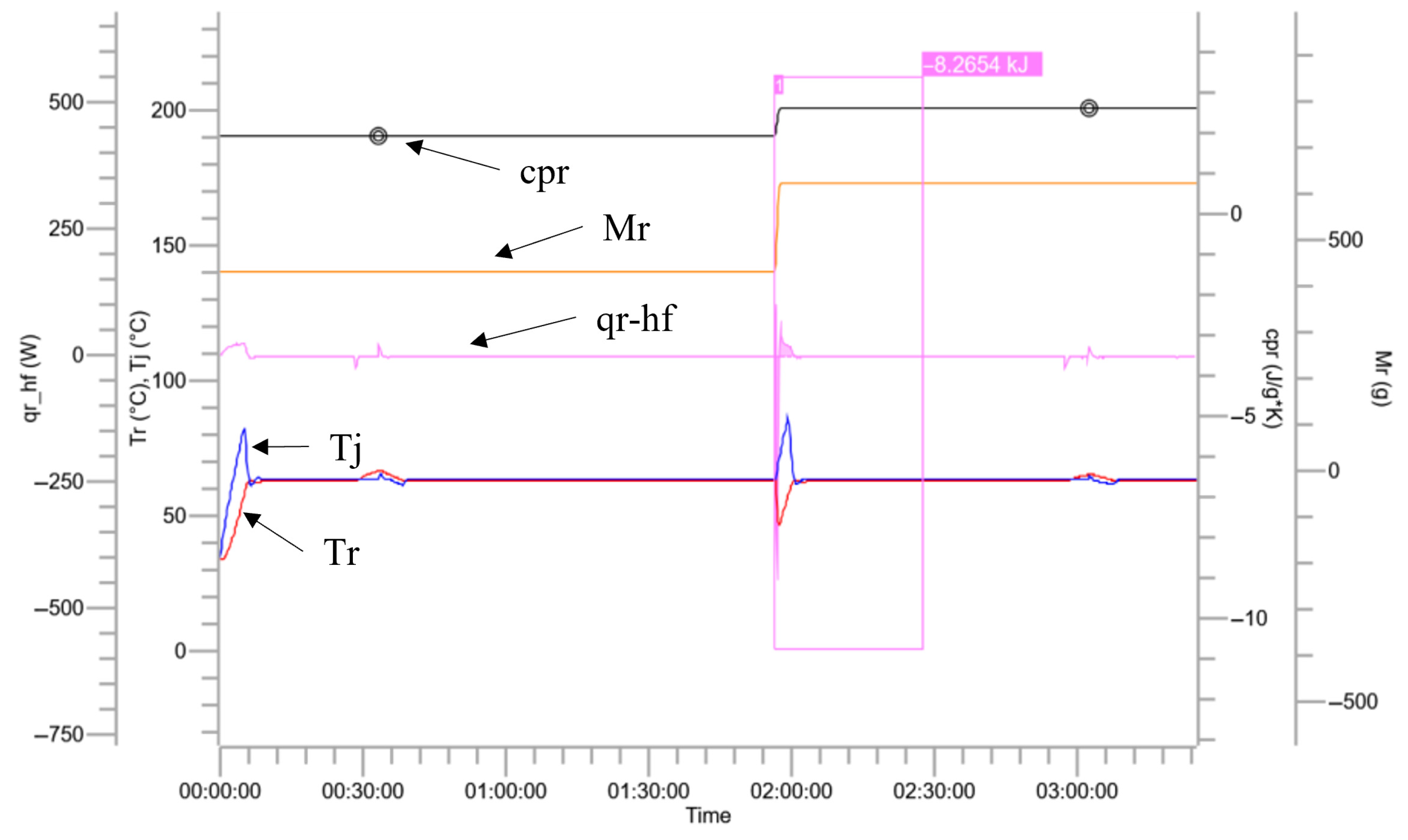
3.2. Characteristics of Reaction Heat
- (1)
- Process parameters: volumetric flow rate (υ0) = 0.00016 m3/s, concentration of chlorobenzotriazole ketone at the feed inlet (t = 0) (c0) = 1298.8 mol/m3, feed inlet temperature (T) = 25 °C, conversion rate of material at the overflow (X) = 97%, and density of the mixed solution (ρ) = 1800 kg/m3;
- (2)
- Reactor parameters: volume (V) = 3.99 m3, heat exchange area (A) = 8.77 m2, maximum temperature difference with cooling system (ΔT) = 58 K, and comprehensive heat transfer coefficient (U) = 0.0465 kW/(m2·K).
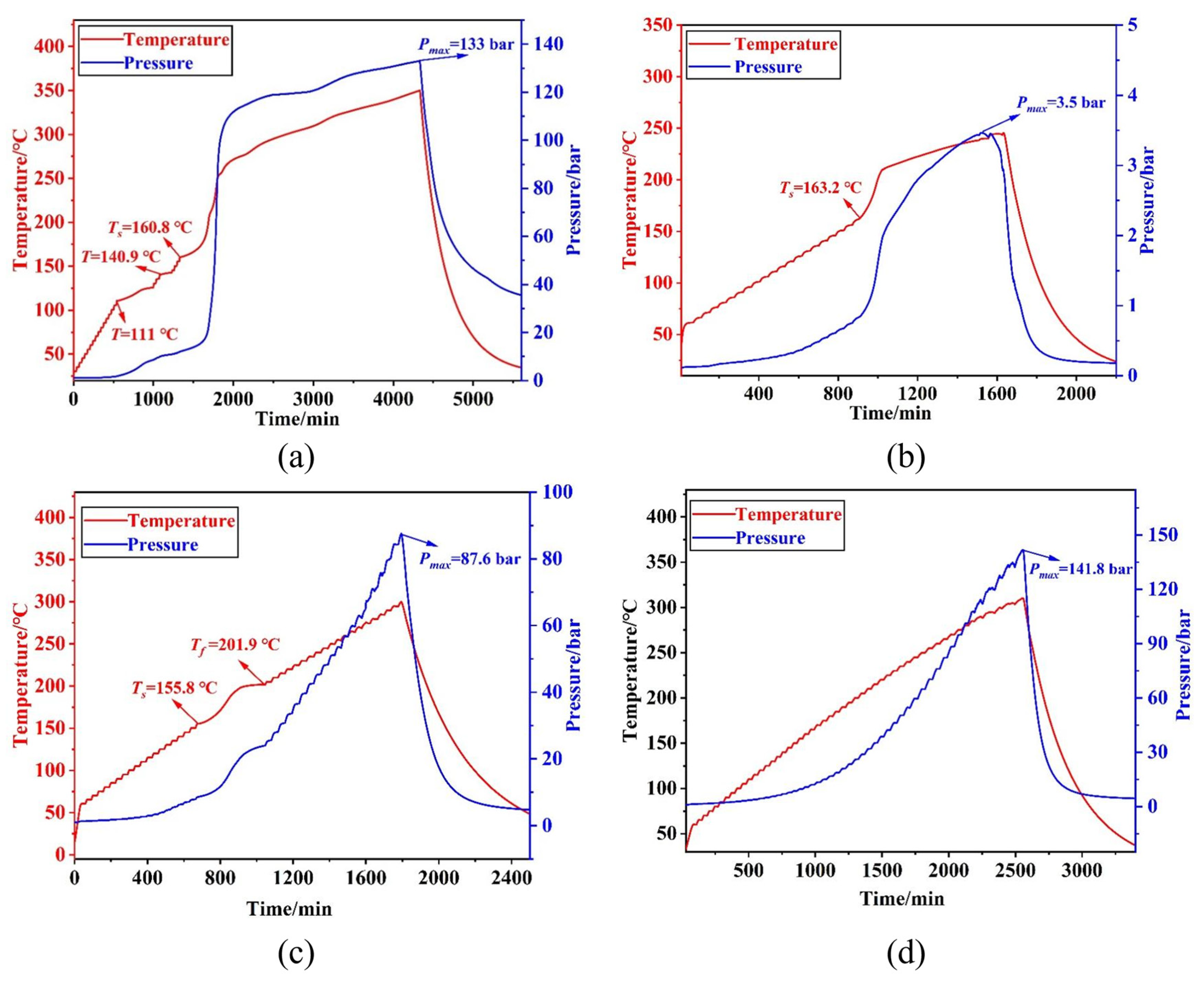
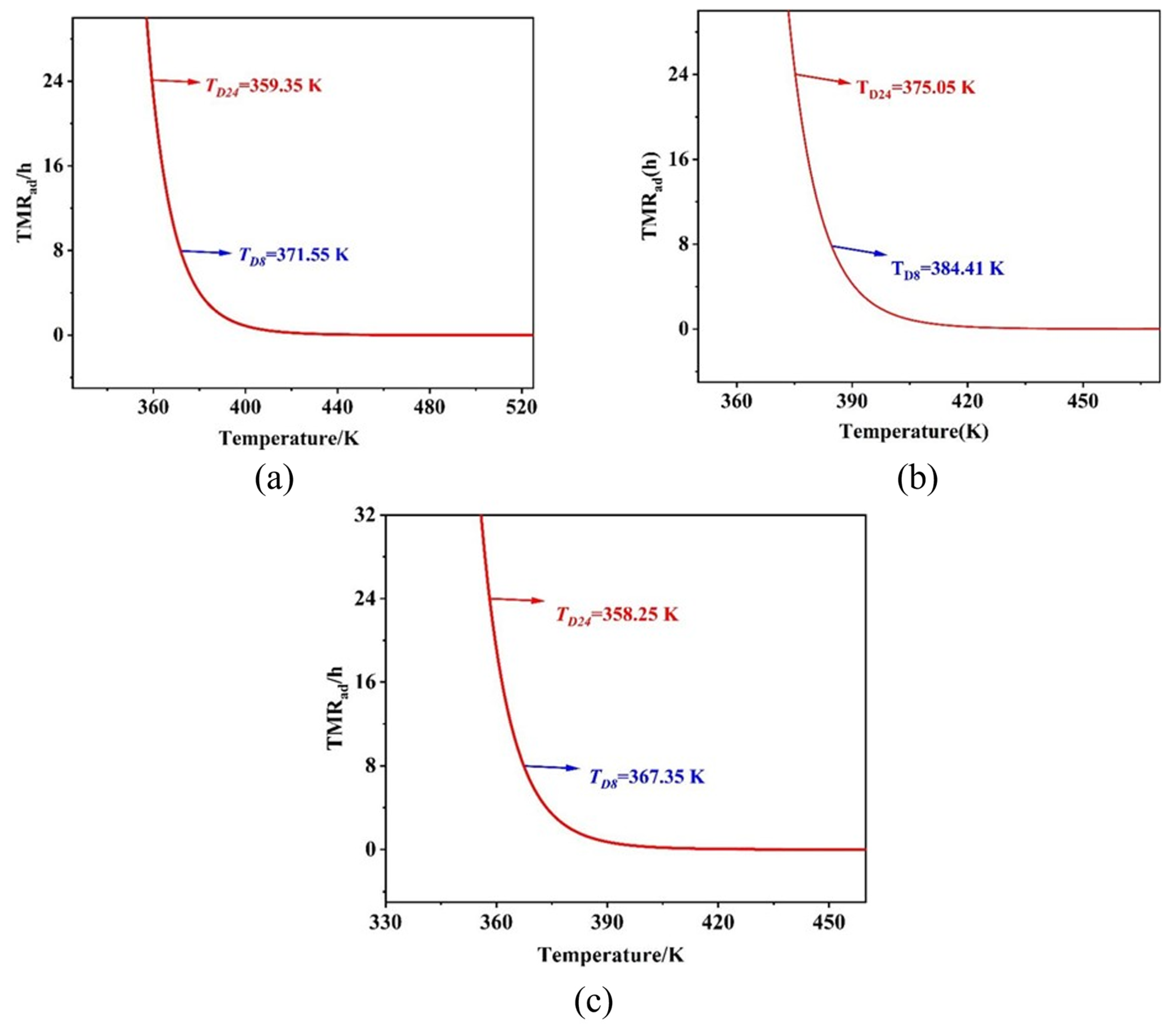
3.3. The Secondary Decomposition Behavior of Matter
3.4. Process Hazard Assessment
4. Conclusions
- (1)
- The decomposition heat release rates of the benzotriazole ketone solution and nitration solution were 306.86 kJ/kg and 455.77 kJ/kg, respectively.
- (2)
- The nitration reaction was a strongly exothermic reaction and underwent a semi-batch kettle reaction. The ∆Tad of benzotriazole ketone nitration was 86.70 °C. The series kettle continuous reaction was safer than the semi-batch kettle reaction.
- (3)
- The secondary decomposition reaction order of the digestion product of benzotriazole ketone under adiabatic conditions in the entire nitration process of benzotriazole ketone was 1.0, Ea was 111.66 kJ/mol, A was 1.1097 × 1010, and TD24 was 86.20 °C. Under adiabatic conditions, during quenching, TD24 was 101.90 °C, whereas during the extraction process, TD24 was 120.45 °C, and during alkaline washing, TD24 was greater than 250 °C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shao, R.; Pan, H.; Huang, J. Safety risk assessment of chemical production process based on local and global objectives. J. Loss Prev. Process Ind. 2022, 79, 104827. [Google Scholar] [CrossRef]
- Xia, Q.; Ren, Y.; Wang, Z.; Yang, D.; Yan, P.; Wu, Z.; Sun, B.; Feng, Q.; Qian, C. Safety risk assessment method for thermal abuse of lithium-ion battery pack based on multiphysics simulation and improved bisection method. Energy 2023, 264, 126228. [Google Scholar] [CrossRef]
- Chen, F.; Wei, D.; Ni, L.; Jiang, J.; Fu, G. A modified inherent thermal runaway hazard index (m-ITHI) for risk assessment of chemical processes based on cloud model. Process Saf. Environ. Prot. 2023, 169, 766–775. [Google Scholar] [CrossRef]
- Ray, A.; Nkwonta, C.; Forrestal, P.; Danaher, M.; Richards, K.; O’Callaghan, T.; Hogan, S.; Cummins, E. Current knowledge on urease and nitration inhibitors technology and their safety. Rev. Environ. Health 2021, 36, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Fu, G.; Ni, Y.; Ni, L.; Jiang, J.; Zhang, H.; Cheng, Z.; Chen, Z. Process optimization, thermal hazard evaluation and reaction mechanism of m-xylene nitration using HNO3-Ac2O as nitrating reagent. Process Saf. Environ. Prot. 2024, 182, 1008–1023. [Google Scholar] [CrossRef]
- Yao, H.; Ni, L.; Liu, Y.; Fu, G.; Jiang, J.; Cheng, Z.; Ni, Y.; Chen, Z. Process hazard and thermal risk evaluation of m-xylene nitration with mixed acid. Process Saf. Environ. Prot. 2023, 175, 238–250. [Google Scholar] [CrossRef]
- Liu, Z. Review and prospect of thermal analysis technology applied to study thermal properties of energetic materials. FirePhysChem 2021, 1, 129–138. [Google Scholar] [CrossRef]
- Lu, Y.M.; Liu, S.H.; Shu, C.M. Evaluation of thermal hazards based on thermokinetic parameters of 2-(1-cyano-1-methylethyl)azocarboxamide by ARC and DSC. J. Therm. Anal. Calorim. 2019, 138, 2873–2881. [Google Scholar] [CrossRef]
- Ghanbari, E.; Picken, S.J.; van Esch, J.H. Analysis of differential scanning calorimetry (DSC): Determining the transition temperatures, and enthalpy and heat capacity changes in multicomponent systems by analytical model fitting. J. Therm. Anal. Calorim. 2023, 148, 12393–12409. [Google Scholar] [CrossRef]
- Parker, T.F.; Quan, Y.; Wilhite, B.A.; Wang, Q. Process scale-up hazard analysis and product yield investigation of cyclohexanol oxidation to cyclohexanone using RC1 calorimetry. Ind. Eng. Chem. Res. 2022, 61, 17218–17225. [Google Scholar] [CrossRef]
- Parker, T.; Mao, Y.; Wang, Q. Application of response surface methodology for hazard analysis of 2-butanol oxidation to 2-butanone using RC1 calorimetry. J. Loss Prev. Process Ind. 2022, 75, 104703. [Google Scholar] [CrossRef]
- Tramšek, M.; Goršek, A. Analysis of growth models for batch kefir grain biomass production in RC1 reaction system. J. Food Process Eng. 2008, 31, 754–767. [Google Scholar] [CrossRef]
- Wu, S.-H.; Shyu, M.-L.; Yet-Pole, I.; Chi, J.-H.; Shu, C.-M. Evaluation of runaway reaction for dicumyl peroxide in a batch reactor by DSC and VSP2. J. Loss Prev. Process Ind. 2009, 22, 721–727. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.; Pan, J.; Chen, S.; Zhang, L. Thermo-oxidative characteristics and kinetics of light, heavy, and extra-heavy crude oils using accelerating rate calorimetry. Fuel 2022, 312, 123001. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Wu, W.Q.; Li, H.; Guo, Z.-C.; Chen, W.-H.; Chen, L. Thermal hazards of benzaldehyde oxime: Based on decomposition products and kinetics analysis by adiabatic calorimeter. Process Saf. Environ. Prot. 2021, 153, 527–536. [Google Scholar] [CrossRef]
- GB/T 42300-2022; Standardization Administration of China. Specification for Safety Risk Assessment of Fine Chemical Reactions. Standards Press of China: Beijing, China, 2022. (In Chinese)

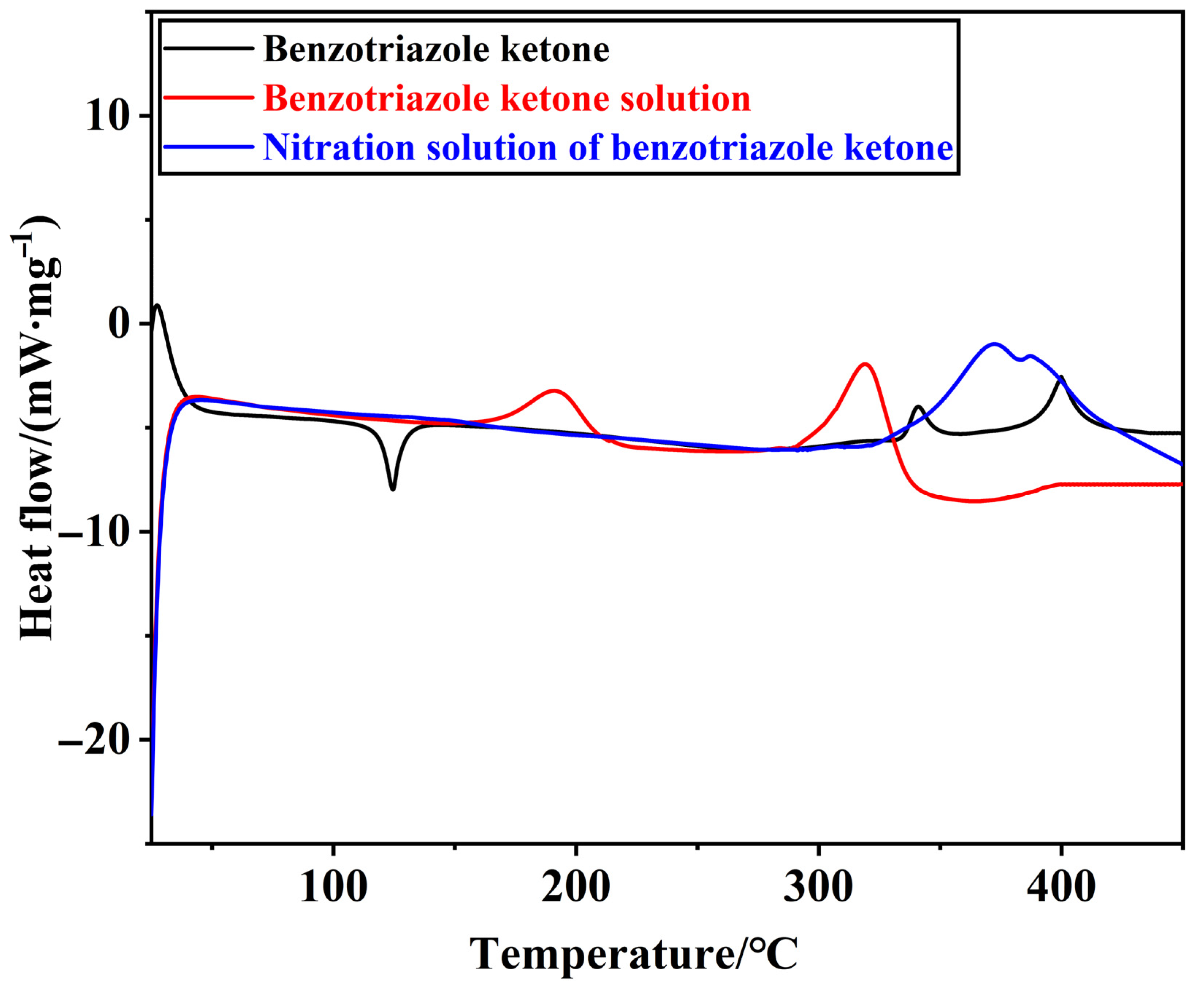


| Materials | Mass/mg | Starting Temperature of Heat Release/°C | Peak Temperature/°C |
|---|---|---|---|
| Benzotriazole ketone solution | 3.80 | I: 144.76 II: 263.96 | I: 191.36 II: 319.40 |
| Nitration solution of benzotriazole ketone | 3.25 | 295.99 | 372.81 |
| Materials | Ending temperature of heat release/°C | Initial decomposition temperature/°C | Heat release/ (kJ·kg−1) |
| Benzotriazole ketone solution | I: 222.46 II: 345.65 | I: 165.28 II: 294.41 | 306.86 |
| Nitration solution of benzotriazole ketone | - | 336.26 | 455.77 |
| Trends | Colors | Units |
|---|---|---|
| Temperature inside the reaction vessel (Tr) |  | °C |
| Reactor jacket temperature (Tj) |  | °C |
| Reaction heat release power (qr_hf) |  | W |
| Total mass of materials inside the reaction vessel (Mr) |  | g |
| Thermal accumulation |  | % |
| Thermal conversion |  | % |
| Specific heat release rate (qr_hf-qb/Mr) |  | W/g |
| Specific heat capacity of the reaction mixture (cpr) |  | J/g*k |
| Reactions | Apparent Heat Release/kJ | Specific Heat Release/ (kJ·kg−1) | Specific Heat Capacity After Reaction/(kJ·kg−1·K−1) | ||
|---|---|---|---|---|---|
| The whole process of nitration of benzotriazole ketone | nitration process | 97.08 | 111.84 | 1.29 | |
| quenching reaction | 70.89 | 131.28 | 1.97 | ||
| extraction process | 0.28 | 0.56 | 2.59 | ||
| alkaline-washing process | 8.27 | 13.13 | 2.59 | ||
| Reactions | ΔTad/K | TP/°C | MTSR/°C | MTT/°C | |
| The whole process of nitration of benzotriazole ketone | nitration process | 86.70/11.95# | 33.00 | 119.7/44.95# | 320.00 |
| quenching reaction | 66.64 | 63.00 | 66.06 | 100.00 | |
| extraction process | 0.22 | 63.00 | 63.22 | 100.00 | |
| alkali washing process | 5.07 | 63.00 | 68.07 | 100.00 | |
| Level | Temperature | Consequences and Explanation |
|---|---|---|
| 1 | Tp ≤ MTSR < MTT < TD24 | Low risk. When MTSR is less than MTT and TD24, there will be no secondary decomposition of reaction products or material flushing. However, the reactants should avoid prolonged heating to prevent them from reaching MTT. |
| 2 | Tp ≤ MTSR < TD24 < MTT | There is a potential for decomposition of the reactants. MTT is higher than TD24. Once the reaction is out of control for a long time, it may cause secondary decomposition of the reaction products. Continuing to release heat will reach MTT and cause material flushing, etc. |
| 3 | Tp ≤ MTT ≤ MTSR < TD24 | Possible material flushing may occur. MTSR is greater than MTT, which can easily cause material flushing and even sudden pressure increase. At this time, MTSR is less than TD24, and the possibility of secondary decomposition of reaction products is not high. The exothermic rate during the MTT reaction affects safety. It is recommended to increase emergency pressure relief and emergency cooling to avoid material flushing. |
| 4 | Tp ≤ MTT < TD24 < MTSR | There is a high possibility of secondary decomposition of materials and reaction products, which may lead to explosions. The temperature may exceed MTT, causing material flushing and secondary decomposition of reaction products, which seriously affects process safety in terms of heat release rate. Emergency pressure relief and emergency cooling have a certain protective effect, but cannot avoid secondary decomposition. |
| 5 | Tp < TD24 < MTSR < MTT Tp < TD24 < MTT < MTSR | There is a high possibility of explosion. MTSR greater than TD24 is prone to secondary decomposition of reaction products, which can continue to release heat and exceed MTT temperature, increasing the risk. Relying solely on emergency cooling, emergency pressure relief, etc., cannot meet safety requirements. Therefore, the process should be optimized, area isolation should be increased, and the entire process should be automated. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Y.; Xiao, Q.; Hu, H.; Zhang, T.; Guan, G. Process Safety Assessment of the Entire Nitration Process of Benzotriazole Ketone. Processes 2025, 13, 2201. https://doi.org/10.3390/pr13072201
Sheng Y, Xiao Q, Hu H, Zhang T, Guan G. Process Safety Assessment of the Entire Nitration Process of Benzotriazole Ketone. Processes. 2025; 13(7):2201. https://doi.org/10.3390/pr13072201
Chicago/Turabian StyleSheng, Yingxia, Qianjin Xiao, Hui Hu, Tianya Zhang, and Guofeng Guan. 2025. "Process Safety Assessment of the Entire Nitration Process of Benzotriazole Ketone" Processes 13, no. 7: 2201. https://doi.org/10.3390/pr13072201
APA StyleSheng, Y., Xiao, Q., Hu, H., Zhang, T., & Guan, G. (2025). Process Safety Assessment of the Entire Nitration Process of Benzotriazole Ketone. Processes, 13(7), 2201. https://doi.org/10.3390/pr13072201





