Engineered Cu0.5Ni0.5Al2O4/GCN Spinel Nanostructures for Dual-Functional Energy Storage and Electrocatalytic Water Splitting
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
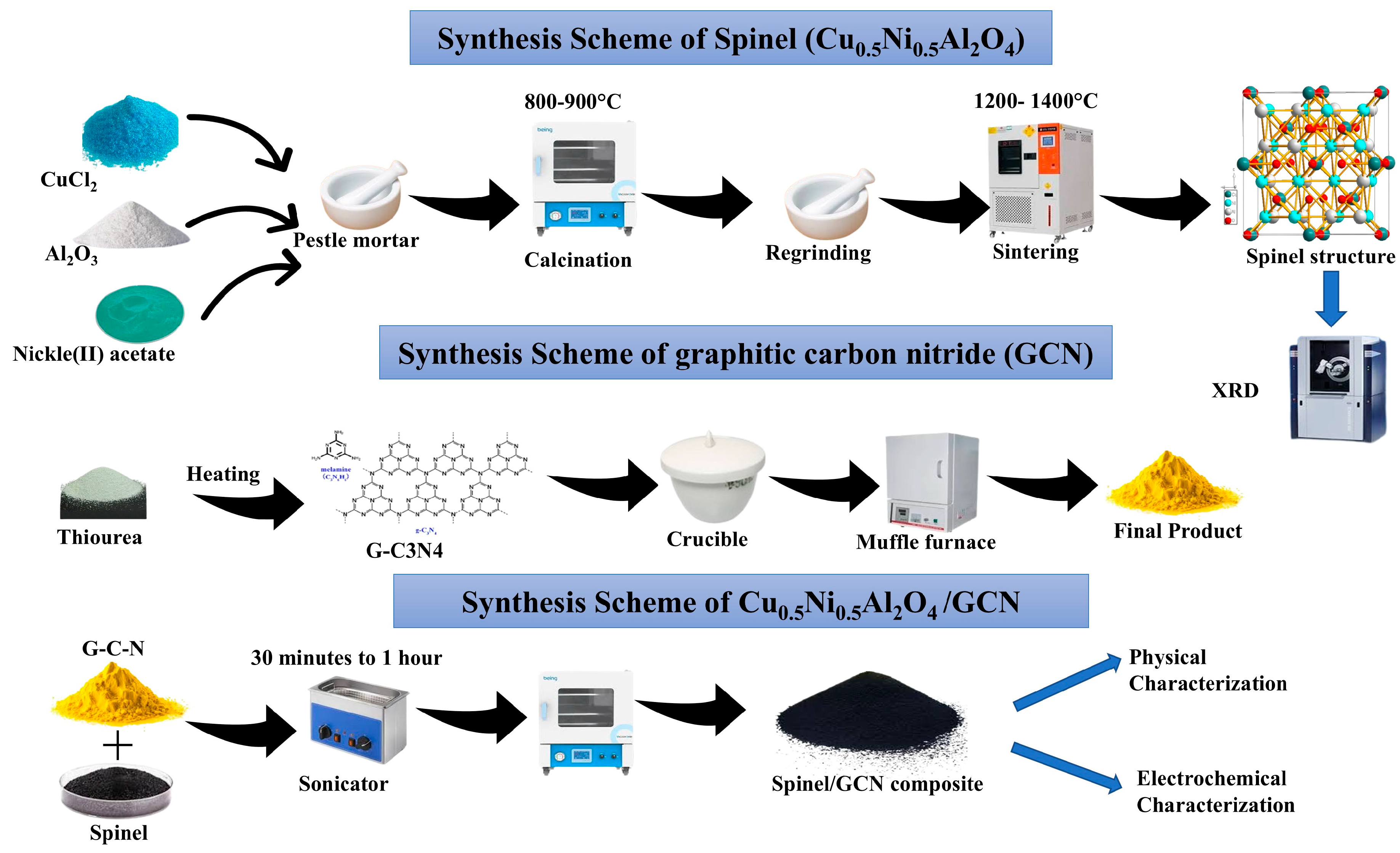
2.2. Fabrication of Spinel Cu0.5Ni0.5Al2O4
2.3. Fabrication of GCN
2.4. Synthesis of Spinel Cu0.5Ni0.5Al2O4/GCN Composite
2.5. Electrode Preparation
2.6. Examinations of Electrochemistry
2.7. Characterization (Physical)
3. Results and Discussions
3.1. X-Ray Diffraction Analysis
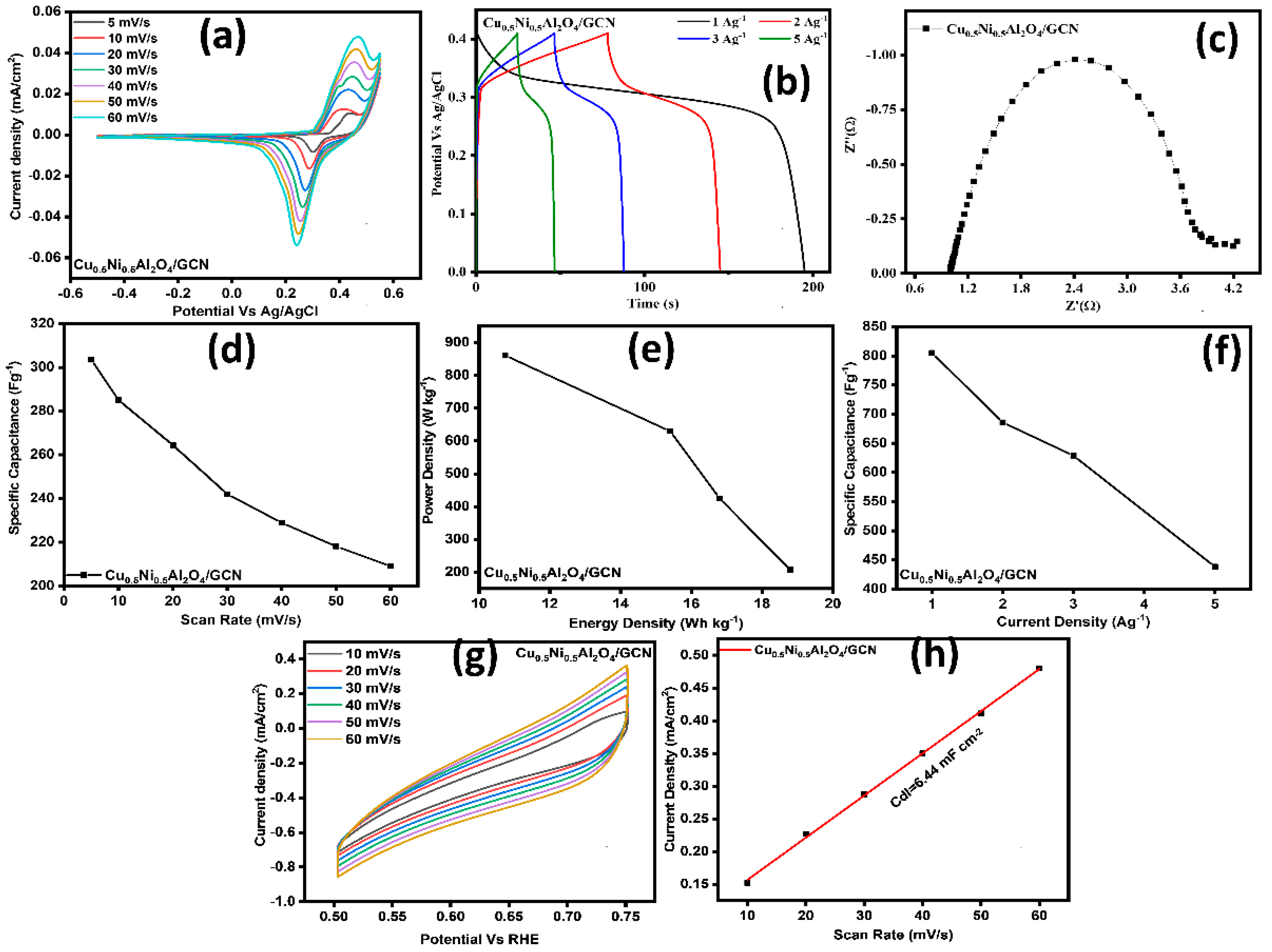
3.2. Electrocatalyst for SCs
3.3. SC Performance in Two-Electrode System
3.4. OER Analysis
3.5. HER Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haohong, D.; Dongguo, L.; Yan, T.; Yang, H.; Shufang, J.; Rongyue, W.; Haifeng, L.; Haoyi, L.; Chongmin, W.; Jun, L.; et al. High-Performance Rh2P Electrocatalyst for Efficient Water Splitting. J. Am. Chem. Soc. 2017, 139, 5494–5502. [Google Scholar]
- Zhou, F.; Xin, Q.; Fu, Y.; Hua, Z.; Dong, Y.; Ran, M.; Song, H.; Liu, S.; Qu, R.; Yang, Y.; et al. Efficient catalytic oxidation of chlorinated volatile organic compounds over RuO2-WOx/Sn0.2Ti0.8O2 catalysts: Insight into the Cl poisoning mechanism of acid sites. Chem. Eng. J. 2023, 464, 142471. [Google Scholar] [CrossRef]
- Li, B.; Jiang, S.D.; Fu, Q.; Wang, R.; Xu, W.Z.; Chen, J.X.; Liu, C.; Xu, P.; Wang, X.J.; Li, J.H.; et al. Tailoring Nanocrystalline/Amorphous Interfaces to Enhance Oxygen Evolution Reaction Performance for FeNi-Based Alloy Fibers. Adv. Funct. Mater. 2025, 35, 2413088. [Google Scholar] [CrossRef]
- Li, J.; Ju, X.; Feng, X.; Zhang, Y.; Huang, G.; Ma, D.; Zhao, X.; Xu, X.; Shi, J.-W. Constructing Z-scheme between graphite nitride carbon and supramolecular zinc porphyrin to promote photocatalytic H2 evolution. J. Colloid Interface Sci. 2025, 690, 137284. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wu, N.; Ji, B.; Cai, J.; Yao, W.; Wang, Z.; Zhang, Y.; Zhang, X.; Guo, S.; Zhou, X. Lattice Water Deprotonation Enables Potassium-Ion Chemistries. Angew. Chem. Int. Ed. 2025, 64, e202503904. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, J.; Jiang, S.; Xiong, H.; Fu, X.; Shang, S.; Xu, J.; He, G.; Chen, P.-C. Plasma-activated 2D CuMnO2 nanosheet catalysts with rich oxygen vacancies for efficient CO2 electroreduction. Appl. Catal. B Environ. Energy 2025, 371, 125255. [Google Scholar] [CrossRef]
- Li, L.; Gao, W.; Wan, Z.; Wan, X.; Ye, J.; Gao, J.; Wen, D. Confining N-doped carbon dots into PtNi aerogels skeleton for robust electrocatalytic methanol oxidation and oxygen reduction. Small 2024, 20, 2400158. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sato, E. Electrocatalytic properties of transition metal oxides for oxygen evolution reaction. Mater. Chem. Phys. 1986, 14, 397–426. [Google Scholar] [CrossRef]
- Cao, K.; Ge, X.; Li, S.; Tian, Z.; Cui, S.; Guo, G.; Yang, L.; Li, X.; Wang, Y.; Bai, S. Facile preparation of a 3D rGO/gC3N4 nanocomposite loaded with Ag NPs for photocatalytic degradation. RSC Adv. 2025, 15, 17089–17101. [Google Scholar] [CrossRef]
- Rosestolato, D.; Neodo, S.; Ferro, S.; Battaglin, G.; Rigato, V.; De Battisti, A. A comparison between structural and electrochemical properties of iridium oxide-based electrocatalysts prepared by sol-gel and reactive sputtering deposition. J. Electrochem. Soc. 2014, 161, E151. [Google Scholar] [CrossRef]
- De, A.; Kim, M.S.; Adhikari, A.; Patel, R.; Kundu, S. Sol-gel derived nanostructure electrocatalysts for oxygen evolution reaction: A review. J. Mater. Chem. A 2024, 12, 19720–19756. [Google Scholar] [CrossRef]
- Wu, L.-K.; Wu, W.-Y.; Xia, J.; Cao, H.-Z.; Hou, G.-Y.; Tang, Y.-P.; Zheng, G.-Q. A nanostructured nickel–cobalt alloy with an oxide layer for an efficient oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 10669–10677. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Q.; Sun, N.; Su, H.; Chen, P.; Wu, J.; Fu, Y.; Ma, J. Giant improvement in piezo-photocatalytic capability of colloidal g-C3N4 quantum dots. Prog. Nat. Sci. Mater. Int. 2025; in press. [Google Scholar] [CrossRef]
- Seitz, L.C.; Dickens, C.F.; Nishio, K.; Hikita, Y.; Montoya, J.; Doyle, A.; Kirk, C.; Vojvodic, A.; Hwang, H.Y.; Norskov, J.K.; et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 2016, 353, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.-B.; Xia, Y.-J.; Zhu, X.-X.; Pei, Y.-R.; Lang, X.-Y.; Jiang, Q. Self-supported Ni5P4/Co3O4 electrode with optimized electron structure as an efficient electrocatalyst for alkaline hydrogen evolution reaction. J. Alloys Compd. 2025, 1022, 179916. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H.J. Transition metal carbides and nitrides in energy storage and conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef]
- Christinelli, W.; da Trindade, L.; Trench, A.; Quintans, C.; Paranhos, C.; Pereira, E.J.E. High-performance energy storage of poly (o-methoxyaniline) film using an ionic liquid as electrolyte. Energy 2017, 141, 1829–1835. [Google Scholar] [CrossRef]
- Lee, J.A.; Shin, M.K.; Kim, S.H.; Cho, H.U.; Spinks, G.M.; Wallace, G.G.; Lima, M.D.; Lepró, X.; Kozlov, M.E.; Baughman, R.H.; et al. Ultrafast charge and discharge biscrolled yarn supercapacitors for textiles and microdevices. Nat. Commun. 2013, 4, 1970. [Google Scholar] [CrossRef]
- Yu, J.; Yang, X.; Jia, Y.; Wang, Z.-Q.; Li, W.; Jiang, Y.; Dai, S.; Zhan, W. Regulating socketed geometry of nanoparticles on perovskite oxide supports for enhanced stability in oxidation reactions. Nat. Commun. 2024, 15, 10229. [Google Scholar] [CrossRef]
- Zhao, L.; Bi, S.; Li, J.; Wen, Y.; Zhang, H.; Zhang, D.; Lu, S.; Yin, P.; Shi, F.; Yan, J.; et al. Prussian blue analogues for advanced non-aqueous sodium ion batteries: Redox mechanisms, key challenges and modification strategies. Energy Storage Mater. 2025, 78, 104256. [Google Scholar] [CrossRef]
- Lang, X.; Hirata, A.; Fujita, T.; Chen, M. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat. Nanotechnol. 2011, 6, 232–236. [Google Scholar] [CrossRef]
- Bian, Y.; Xie, L.; Ma, L.; Cui, C. A novel two-stage energy sharing model for data center cluster considering integrated demand response of multiple loads. Appl. Energy 2025, 384, 125454. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, J.G.; Xiao, J.Q. Nanostructured electrodes for high-performance pseudocapacitors. Angew. Chem. Int. Ed. Engl. 2013, 52, 1882–1889. [Google Scholar] [CrossRef]
- Mahmood, N.; Tahir, M.; Mahmood, A.; Yang, W.; Gu, X.; Cao, C.; Zhang, Y.; Hou, Y. Role of anions on structure and pseudocapacitive performance of metal double hydroxides decorated with nitrogen-doped graphene. Sci. China Mater. 2015, 58, 114–125. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Chmiola, J.; Largeot, C.; Taberna, P.-L.; Simon, P.; Gogotsi, Y. Monolithic carbide-derived carbon films for micro-supercapacitors. Science 2010, 328, 480–483. [Google Scholar] [CrossRef]
- Yu, G.; Hu, L.; Liu, N.; Wang, H.; Vosgueritchian, M.; Yang, Y.; Cui, Y.; Bao, Z. Enhancing the supercapacitor performance of graphene/MnO2 nanostructured electrodes by conductive wrapping. Nano Lett. 2011, 11, 4438–4442. [Google Scholar] [CrossRef] [PubMed]
- Balogun, M.-S.; Qiu, W.; Yang, H.; Fan, W.; Huang, Y.; Fang, P.; Li, G.; Ji, H.; Tong, Y. A monolithic metal-free electrocatalyst for oxygen evolution reaction and overall water splitting. Energy Environ. Sci. 2016, 9, 3411–3416. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, X.; Hu, J.; Guo, Y.; Wan, L.J. α-Fe2O3 nanotubes in gas sensor and lithium-ion batteries applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar]
- Chen, P.-C.; Shen, G.; Shi, Y.; Chen, H.; Zhou, C. Preparation and characterization of flexible asymmetric supercapacitors based on transition-metal-oxide nanowire/single-walled carbon nanotube hybrid thin-film electrodes. ACS Nano 2010, 4, 4403–4411. [Google Scholar] [CrossRef]
- Joo, S.H.; Choi, S.J.; Oh, I.; Kwak, J.; Liu, Z.; Terasaki, O.; Ryoo, R. Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 2001, 412, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, D.; Zhang, J.; Balogun, M.S.; Wang, P.; Tong, Y.; Huang, Y. Charge relays via dual carbon-actions on nanostructured BiVO4 for high performance photoelectrochemical water splitting. Adv. Funct. Mater. 2022, 32, 2112738. [Google Scholar] [CrossRef]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. TrAC Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Balogun, M.-S.; Tong, Y.; Huang, Y. Oxygen vacancy–based metal oxides photoanodes in photoelectrochemical water splitting. Mater. Today Sustain. 2022, 18, 100118. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.-C.; Pan, L.; Shen, G.-Q.; Mahmood, N.; Ma, Y.-H.; Shi, Y.; Jia, W.; Wang, L.; Zhang, X.; et al. Engineering cobalt defects in cobalt oxide for highly efficient electrocatalytic oxygen evolution. ACS Catal. 2018, 8, 3803–3811. [Google Scholar] [CrossRef]
- Tahir, M.; Pan, L.; Zhang, R.; Wang, Y.-C.; Shen, G.; Aslam, I.; Qadeer, M.; Mahmood, N.; Xu, W.; Wang, L.; et al. High-valence-state NiO/Co3O4 nanoparticles on nitrogen-doped carbon for oxygen evolution at low overpotential. ACS Energy Lett. 2017, 2, 2177–2182. [Google Scholar] [CrossRef]
- Anantharaj, S.; Reddy, P.N.; Kundu, S. Core-oxidized amorphous cobalt phosphide nanostructures: An advanced and highly efficient oxygen evolution catalyst. Inorg. Chem. 2017, 56, 1742–1756. [Google Scholar] [CrossRef]
- Dutta, A.; Mutyala, S.; Samantara, A.K.; Bera, S.; Jena, B.K.; Pradhan, N. Synergistic effect of inactive iron oxide core on active nickel phosphide shell for significant enhancement in oxygen evolution reaction activity. ACS Energy Lett. 2017, 3, 141–148. [Google Scholar] [CrossRef]
- Jing, M.; Hou, H.; Banks, C.E.; Yang, Y.; Zhang, Y.; Ji, X. Alternating voltage introduced NiCo double hydroxide layered nanoflakes for an asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 22741–22744. [Google Scholar] [CrossRef]
- Liang, F.; Huang, L.; Tian, L.; Li, J.; Zhang, H.; Zhang, S. Microwave-assisted hydrothermal synthesis of cobalt phosphide nanostructures for advanced supercapacitor electrodes. CrystEngComm 2018, 20, 2413–2420. [Google Scholar] [CrossRef]
- Mahmood, N.; Tahir, M.; Mahmood, A.; Zhu, J.; Cao, C.; Hou, Y. Chlorine-doped carbonated cobalt hydroxide for supercapacitors with enormously high pseudocapacitive performance and energy density. Nano Energy 2015, 11, 267–276. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, T.; Zhu, Z.; Xiao, R.; Yao, X.; Huang, Y.; Balogun, M.S. Deciphering the lithium storage chemistry in flexible carbon fiber-based self-supportive electrodes. Carbon Energy 2022, 4, 820–832. [Google Scholar] [CrossRef]
- Sami, A.; Khan, M.M.; Haidar, Z.; Bahajjaj, A.A.A.; Riaz, S.; Naseem, S.; Osman, S.M.; Khan, M.S. Enhanced supercapacitor performance of rGO-Supported BaVO3 nanostructures as advanced electrode materials. Ceram. Int. 2025, 51, 8480–8491. [Google Scholar] [CrossRef]
- Bano, N.; Manzoor, S.; Sami, A.; Shah, S.I.A.; Junaid, A.; Rehman, M.Y.U.; Alshgari, R.A.; Ehsan, M.F.; Ashiq, M.N. Fabrication of vanadium telluride anchored on carbon nanotubes nanocomposite for overall water splitting. J. Am. Ceram. Soc. 2024, 107, 4027–4041. [Google Scholar] [CrossRef]
- Aftab, F.; Duran, H.; Kirchhoff, K.; Zaheer, M.; Iqbal, B.; Saleem, M.; Arshad, S.N. A facile synthesis of FeCo nanoparticles encapsulated in hierarchical N-doped carbon nanotube/nanofiber hybrids for overall water splitting. ChemCatChem 2020, 12, 932–943. [Google Scholar] [CrossRef]
- Ilayas, T.; Anjum, S.; Raja, M.Y.A.; Khurram, R.; Sattar, M.; Mansoor, A. Rietveld refinement, 3D view and electrochemical properties of rare earth lanthanum doped nickel ferrite to fabricate high performance electrodes for supercapacitor applications. Ceram. Int. 2023, 49, 28864–28877. [Google Scholar] [CrossRef]
- Ben-Refael, A.; Benisti, I.; Paz, Y.J. Transient photoinduced phenomena in graphitic carbon nitride as measured at nanoseconds resolution by step-scan FTIR. Catal. Today 2020, 340, 97–105. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Zhao, J. Correlation of structural and chemical characteristics with catalytic performance of hydrotalcite-based CuNiAl mixed oxides for SO2 abatement. Chem. Eng. J. 2013, 223, 164–171. [Google Scholar] [CrossRef]
- Shah, S.I.A.; Ansari, M.N.; Nisa, M.U.; Bano, N.; Shabbir, B.; Alrashidi, K.A.; Mohammad, S.; Khan, M.S.; Ashiq, M.F.; Allakhverdiev, S.I. Harnessing dysprosium telluride/GPE as an effective electrode for energy storage and conversion. J. Energy Storage 2024, 101, 113868. [Google Scholar] [CrossRef]
- Fatima, R.; Sami, A.; Haidar, Z.; Ahmed, F.; Junaid, A.; Qasim, B.; Alfagham, A.T.; Khan, M.S.; Elgorban, A.M.; Shah, S.I.A. Facile synthesis of rGO/DyMnO3 nanocomposite directly grown on nickel foam for supercapacitor applications. J. Am. Ceram. Soc. 2025, 108, e20357. [Google Scholar] [CrossRef]
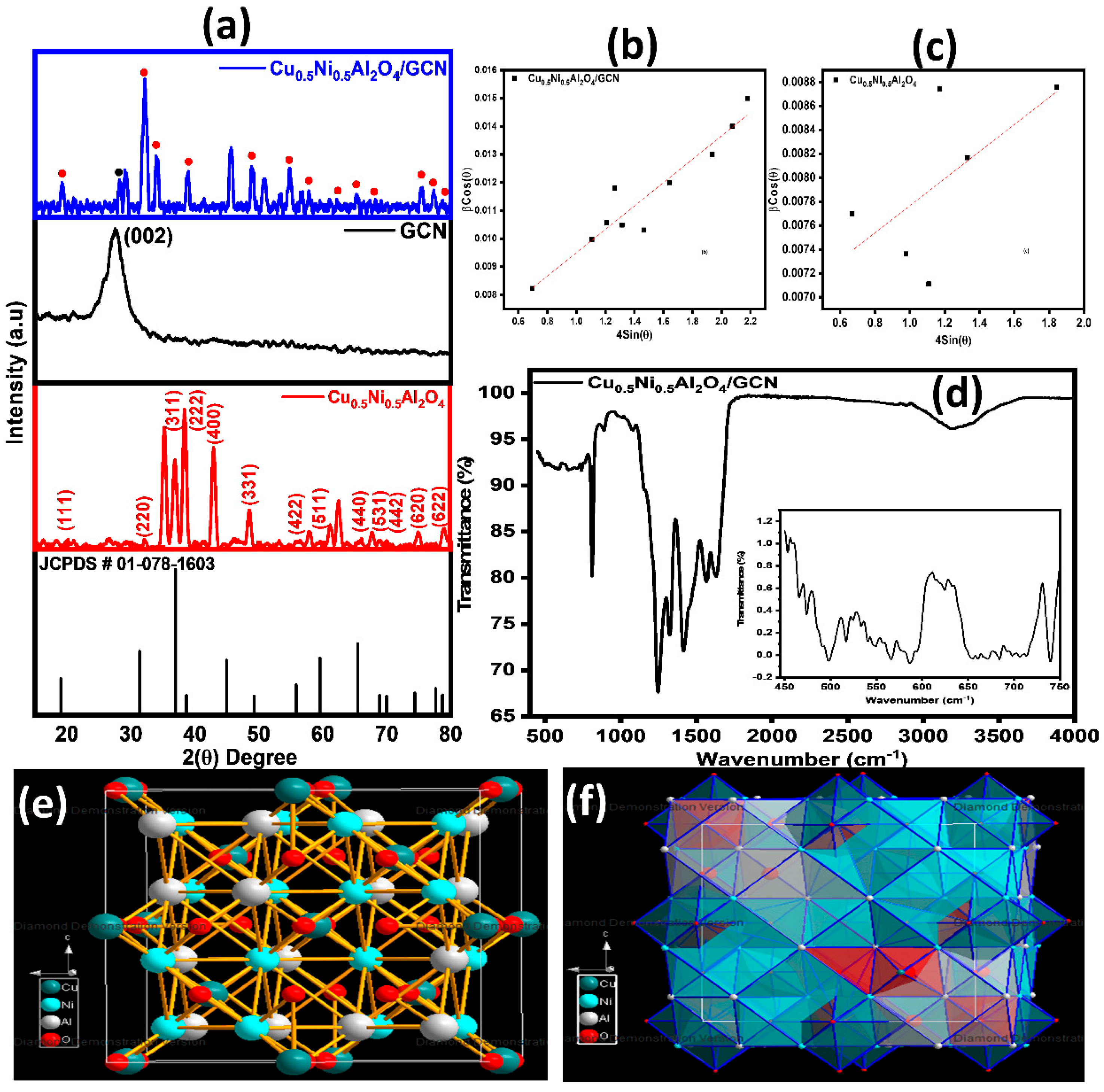
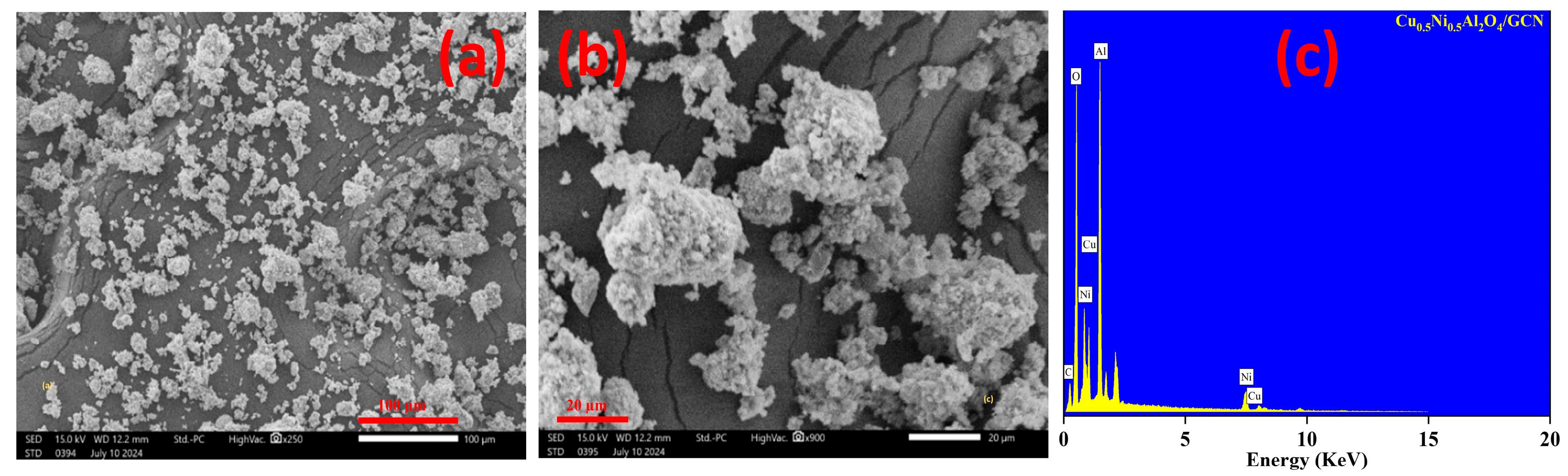





| Samples | (D) nm | (1/D2) (10−15) | () 10−3 | (L.S) 10−3 |
|---|---|---|---|---|
| Cu0.5Ni0.5Al2O4/GCN | 17.58 | 32.34 | 2.13 | 1.84 |
| GCN | 17.2 | 33.47 | 0.20 | 5.07 |
| Cu0.5Ni0.5Al2O4 | 18.21 | 30.16 | 2.22 | 0.38 |
| Samples | Scan Rate (mV/s) | Csp (F/g) | Rct (Ω) | Rs (Ω) |
|---|---|---|---|---|
| Cu0.5Ni0.5Al2O4/GCN | 5 | 487 | 1.27 | 1.88 |
| GCN | 5 | 326 | 4.49 | 2.40 |
| Cu0.5Ni0.5Al2O4 | 5 | 375 | 4.65 | 2.45 |
| Synthesized Material | ECSA (cm2) | (Rf) | Cdl (mF) |
|---|---|---|---|
| Cu0.5Ni0.5Al2O4/GCN | 172.75 | 345.5 | 6.91 |
| GCN | 150 | 300 | 6.7 |
| Cu0.5Ni0.5Al2O4 | 141.5 | 283 | 5.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sami, A.; Ahmad, S.; Shan, A.-D.; Zhang, S.; Fu, L.; Farooq, S.; Al-Dawery, S.K.; Harharah, H.N.; Harharah, R.H.; Hayder, G. Engineered Cu0.5Ni0.5Al2O4/GCN Spinel Nanostructures for Dual-Functional Energy Storage and Electrocatalytic Water Splitting. Processes 2025, 13, 2200. https://doi.org/10.3390/pr13072200
Sami A, Ahmad S, Shan A-D, Zhang S, Fu L, Farooq S, Al-Dawery SK, Harharah HN, Harharah RH, Hayder G. Engineered Cu0.5Ni0.5Al2O4/GCN Spinel Nanostructures for Dual-Functional Energy Storage and Electrocatalytic Water Splitting. Processes. 2025; 13(7):2200. https://doi.org/10.3390/pr13072200
Chicago/Turabian StyleSami, Abdus, Sohail Ahmad, Ai-Dang Shan, Sijie Zhang, Liming Fu, Saima Farooq, Salam K. Al-Dawery, Hamed N. Harharah, Ramzi H. Harharah, and Gasim Hayder. 2025. "Engineered Cu0.5Ni0.5Al2O4/GCN Spinel Nanostructures for Dual-Functional Energy Storage and Electrocatalytic Water Splitting" Processes 13, no. 7: 2200. https://doi.org/10.3390/pr13072200
APA StyleSami, A., Ahmad, S., Shan, A.-D., Zhang, S., Fu, L., Farooq, S., Al-Dawery, S. K., Harharah, H. N., Harharah, R. H., & Hayder, G. (2025). Engineered Cu0.5Ni0.5Al2O4/GCN Spinel Nanostructures for Dual-Functional Energy Storage and Electrocatalytic Water Splitting. Processes, 13(7), 2200. https://doi.org/10.3390/pr13072200











