Abstract
The rapid growth in population and industrialization have significantly increased global energy demand, placing immense pressure on finite and environmentally harmful conventional fossil fuel-based energy sources. In this context, the development of hybrid electrocatalysts presents a crucial solution for energy conversion and storage, addressing environmental challenges while meeting rising energy needs. In this study, the fabrication of a novel bifunctional catalyst, copper nickel aluminum spinel (Cu0.5Ni0.5Al2O4) supported on graphitic carbon nitride (GCN), using a solid-state synthesis process is reported. Because of its effective interface design and spinel cubic structure, the Cu0.5Ni0.5Al2O4/GCN nanocomposite, as synthesized, performs exceptionally well in electrochemical energy conversion, such as the oxygen evolution reaction (OER), the hydrogen evolution reaction (HER), and energy storage. In particular, compared to noble metals, Pt/C- and IrO2-based water-splitting cells require higher voltages (1.70 V), while for the Cu0.5Ni0.5Al2O4/GCN nanocomposite, a voltage of 1.49 V is sufficient to generate a current density of 10 mA cm−2 in an alkaline solution. When used as supercapacitor electrode materials, Cu0.5Ni0.5Al2O4/GCN nanocomposites show a specific capacitance of 1290 F g−1 at a current density of 1 A g−1 and maintain a specific capacitance of 609 F g−1 even at a higher current density of 5 A g−1, suggesting exceptional rate performance and charge storage capacity. The electrode’s exceptional capacitive properties were further confirmed through the determination of the roughness factor (Rf), which represents surface heterogeneity and active area enhancement, with a value of 345.5. These distinctive characteristics render the Cu0.5Ni0.5Al2O4/GCN composite a compelling alternative to fossil fuels in the ongoing quest for a viable replacement. Undoubtedly, the creation of the Cu0.5Ni0.5Al2O4/GCN composite represents a significant breakthrough in addressing the energy crisis and environmental concerns. Owing to its unique composition and electrocatalytic characteristics, it is considered a feasible choice in the pursuit of ecologically sustainable alternatives to fossil fuels.
1. Introduction
The worldwide problem regarding global energy demand is considerably exacerbated by the widespread usage of non-renewable energy sources like fossil fuels, particularly CO2, which significantly contributes to global warming and air pollution and is produced by carbon-based fuel reduction [1,2,3]. The increasing depletion of fossil fuels necessitates the exploration of alternative fuel sources that are environmentally friendly and sustainable. Current fossil fuel-based energy systems, which are not only limited but are also significant contributors to greenhouse gas emissions and climate change, are under tremendous strain due to the rising global energy demand brought on by fast growth in industrialization and the population. The International Energy Agency (IEA) predicts that by 2040, the world’s energy consumption will have increased by around 25%, with developing nations accounting for a sizable amount of this demand. The need to switch to ecologically friendly and sustainable energy sources has become more urgent as a result [4].
Among these types of energy sources, natural resources like solar energy, wind power, and water electrolysis present compelling opportunities for producing green fuels [5]. Supercapacitors have demonstrated superiority over other renewable energy storage technologies due to their quick charge/discharge, strong cycling stability, minimal environmental impact, and excellent fire safety, all of which have significant potential to promote water electrolysis. While integrated configurations involving water-splitting systems powered by renewable energy-powered energy storage systems (such as zinc–air, lithium-ion and sodium-ion batteries) have been reported, fewer integrated configurations involving a two-electrode electrolyzer powered by supercapacitors and charged by a solar cell have been documented. Conversely, as the electrocatalyst’s activity in the cathodic hydrogen evolution process (HER) and the anodic oxygen evolution reaction (OER) is a major determinant of overall water-splitting efficiency, it is desirable to rationally design specific electrocatalysts. Pt for the HER and IrO2/RuO2 for the OER are examples of noble metal-based electrocatalysts that have been studied to date and have demonstrated excellent reaction kinetics [6,7]. However, these electrocatalysts’ low durability and high costs limit their practical use. Thus, there is interest in creating bifunctional electrocatalysts for the HER and OER that are highly effective, long-lasting, and reasonably priced. Bifunctional electrocatalysts can further save production costs by avoiding complex electrode manufacturing and simplifying water-splitting equipment. Designing a multipurpose electrode material that can electrocatalyze the HER and OER at the same time and offer supercapacitors a large charge storage capacity is therefore essential.
Through effective water splitting, water electrocatalysis is essential to the production of clean fuels like hydrogen and oxygen. Fujishima and Honda carried out groundbreaking work on TiO2-based water-splitting devices and demonstrated the potential for converting electricity into usable energy [6,7]. However, in the OER a critical step in this process is inherently slow due to the complexity of breaking O-H bonds and forming O-O bonds, which requires a high overpotential and limits the efficiency of TiO2-based systems [8].
The challenge of developing stable, cost-effective, and efficient catalysts for water splitting, despite extensive research, remains significant. Nanodevice performance is often hindered by inefficient ion and electron transfer processes [9,10]. While RuO2, Pt, and IrO2 nanocatalysts exhibit promising properties, including low Tafel slopes and high current densities, their scarcity and high cost limit their widespread application in the OER and HER [11,12,13,14,15]. Consequently, the persistent need exists for the development of active, non-precious metal-based catalysts. Researchers are exploring inexpensive, abundant earth metals capable of achieving high energy yields at lower potentials to address this bottleneck. Additionally, building green energy storage devices, like capacitors, lithium-ion batteries, and metal-ion batteries, is crucial to meet growing global energy demand [16,17].
Furthermore, energy storage system supercapacitors (SCs) have garnered devotion due to their exceptional characteristics, such as low internal resistance, extended life cycles (>105 cycles), rapid charge and discharge rates, and high-power density (10 kW kg−1). These features make them favorable over traditional batteries and dielectric capacitors, leading to their application in mobile phones, laptops, electric vehicles, memory cards, and flashlights [18,19]. SCs are categorized into electrochemical double-layer capacitors (EDLCs storing charge at the electrode–electrolyte interface) and pseudocapacitors using reversible faradic processes [20,21,22]. However, in order to integrate SCs into everyday power supply systems, they need to be further developed to give high power and energy density [23].
The electrochemical performance of SCs is primarily influenced by the electrode material [24,25,26]. Nanostructured materials’ enormous surface area improves electrode performance by increasing the number of catalytic sites accessible for electrochemical processes [27,28]. However, challenges such as the limited stability of transition metal sulfides, poor electrical conductivity of metal oxides, and suboptimal performance of carbon-based materials hinder their effectiveness in fuel cells, batteries, and SCs [29,30,31,32]. Single transition metals often exhibit slow kinetics and limited electroactive sites, further restricting their electrochemical properties. Recent studies suggest that defect engineering and hybrid structures can address these limitations by enhancing material performance [33,34,35]. For example, Zhang and colleagues demonstrated that cobalt-deficient Co3-xO4 crystals improve water-splitting activities by facilitating water adsorption and activation at the catalyst surface [36]. Similarly, Tahir et al. developed a superior electrocatalyst using NiO/Co3O4 nanoparticles supported on nitrogen-doped carbon, enhancing OER and HER performance [37].
SCs and EWS electrode materials include metallic nitrides (Ni3N, MoN, FeN), phosphides (NiP, CoP), and non-noble metal alloys (NiCo, FeNi, NiMn) [38,39,40,41]. Binary metal hybrid nanostructures, in particular, offer improved performance due to the synergistic interactions of distinct electronic structures, which enhance bond rearrangement and catalytic activity. For instance, chlorine-doped carbonated cobalt hydroxide nanowires demonstrate enhanced pseudocapacitance and energy density through deep faradic redox reactions involving the entire nanowire structure [42]. Copper electrodes are also highly regarded for SCs due to their abundant availability and excellent charge storage capacity [43].
Our research introduces a novel bifunctional catalyst Cu0.5Ni0.5Al2O4 supported on graphitic carbon nitride (GCN) for energy conversion and storage. This hybrid material has outstanding bifunctional performance, with a Csp of 1290 F g−1 at 1 A g−1 and catalytic activity for the OER (10 mA cm−2 at 254 mV) and HER (101 mV). These results underscore the potential of Cu0.5Ni0.5Al2O4 as a dual-purpose nanocatalyst for commercial applications in EWS and SCs. Our findings highlight the importance of hybrid composite engineering and theoretical approaches to further optimize catalytic performance, bridging the gap between experimental challenges and practical applications.
2. Experimental Section
2.1. Reagents
All the purchased chemicals were used without further purity. Potassium hydroxide (KOH) 98%, (nickel (II) acetate) 99%, acetate di-hydrate, copper (I) chloride 99%, and aluminum oxide (Al2O3) 98% were acquired from Sigma-Aldrich Chemie GmbH, Steinheim, Germany Solvent 95% ethanol (C2H5OH) was bought from Merck KGaA, Darmstadt, Germany.
2.2. Fabrication of Spinel Cu0.5Ni0.5Al2O4
A solid-state approach for fabricating Cu0.5Ni0.5Al2O4 requires combining stoichiometric quantities of CuCl2, (nickel (II) acetate), and Al2O3 powders, followed by contiunous grinding to achieve uniformity. The powders are subsequently subjected to calcination at temperatures going from 800 °C to 900 °C in order to facilitate a reaction and eliminate any volatile compounds. Following cooling and regrinding, the calcined material is subjected to high-temperature sintering at temperatures varying from 1200 to 1400 °C for a duration of 12–24 h in order to fully develop the spinel structure. The product is cooled to ambient temperature and analyzed using X-ray diffraction (XRD) and other methods to verify the growth and quality of the Cu0.5Ni0.5Al2O4 compound.
2.3. Fabrication of GCN
Graphitic carbon nitride (GCN) is commonly synthesized via a thermal polymerization method using thiourea. Thiourea, serving as both a nitrogen and carbon source, undergoes high-temperature heating to produce GCN. Specifically, thiourea is heated in a muffle furnace at 500–600 °C for 2–4 h under ambient air conditions. During heating, thiourea decomposes and polymerizes to form GCN. The resulting yellow powder is then allowed to cool spontaneously to ambient temperature. This technique produces GCN with a stratified crystal structure, making it suitable for various applications such as overall water splitting and energy storage.
2.4. Synthesis of Spinel Cu0.5Ni0.5Al2O4/GCN Composite
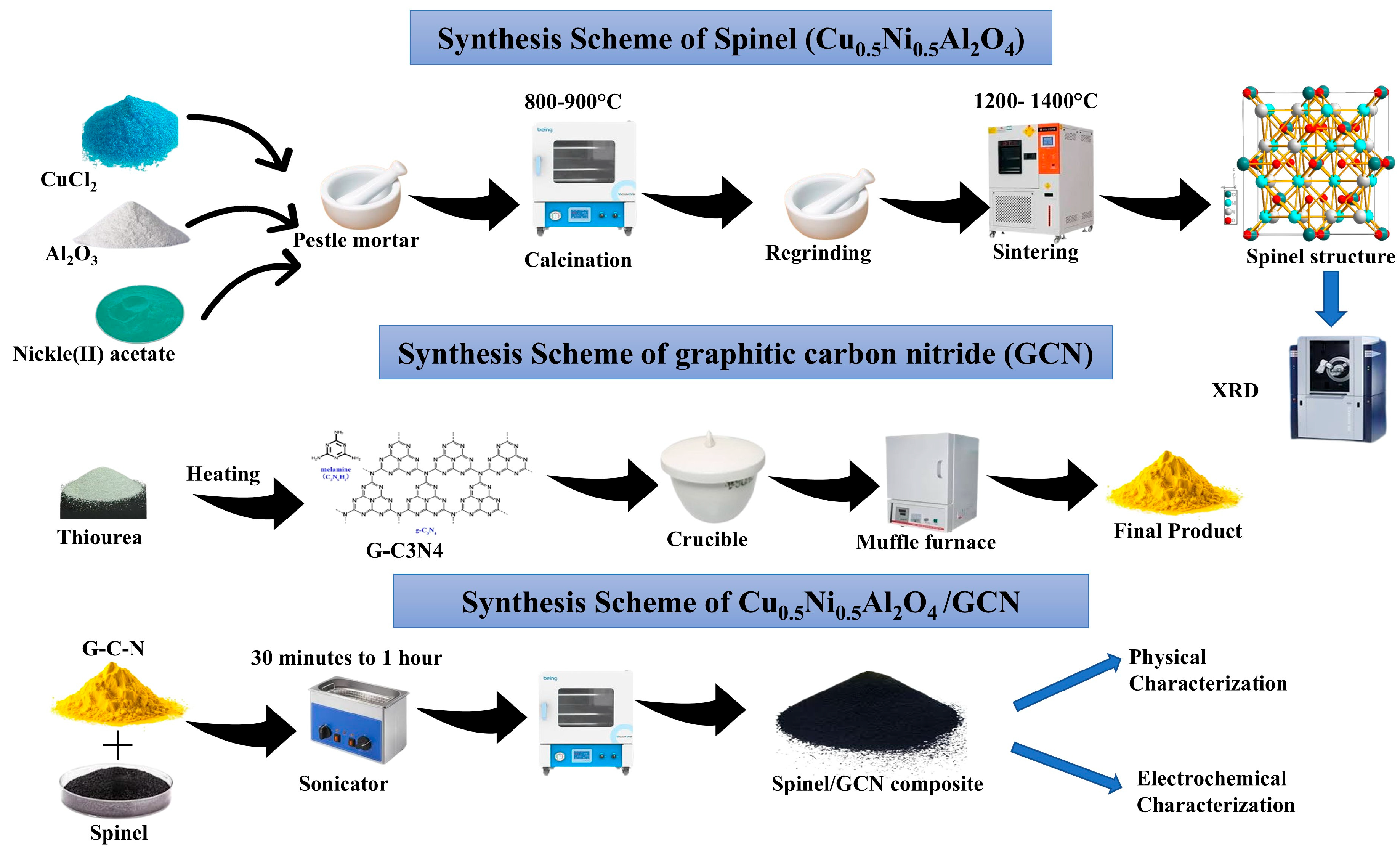
To synthesize a composite via sonication, first disperse the required amounts (1:1) of spinel and GCN powders in water to establish a homogenous suspension. This combination is then sonicated for about 1 h in an ultrasonic bath to break up agglomerates and improve material interaction. The suspension is dried by evaporating the solvent under reduced pressure or heating at a low temperature to a solid composite powder. The synthesis scheme of the spinel Cu0.5Ni0.5Al2O4/GCN composite is shown in Figure 1.
Figure 1.
Synthesis scheme for Cu0.5Ni0.5Al2O4/GCN composite.
2.5. Electrode Preparation
In order to create a working electrode, this work employed drop casting to deposit the newly synthesized electrocatalyst onto nickel foam (NF). To do this, NF was cut into 1 cm by 1 cm pieces, which were then washed for 5 min each in acetone, deionized (DI) water, and ethanol. The NF pieces were dried for 4 h at 70 °C after washing. To prepare a homogeneous catalyst ink without binding agents for electrochemical experiments, 200 μL of deionized water was added dropwise to 5 mg of the synthesized electrocatalyst powder. After coating the freshly cleaned NF surface with the catalyst ink, it was allowed to air-dry before being used for general water splitting and supercapacitor (SCs) applications.
2.6. Examinations of Electrochemistry
The electrochemical workstation Corrtest Bi-Potentiostat C-2350 from Wuhan Corrtest Instruments Corp., Ltd., Wuhan, China, was used for all electrochemical investigations, including chronoamperometry (CA), cyclic voltammetry (CV), electrical impedance spectroscopy (EIS), and galvanostatic charge/discharge (GCD), and were performed with an electrolyte of a 2 M KOH aqueous solution. Electrochemical performances were tested using a standard three-electrode setup, consisting of Pyrex glass covered in Teflon, Pt wire (counter electrode), Ag/AgCl (reference electrode), and NF plated with synthetic material. Linear sweep voltammetry (LSV) and CV electrochemical data were developed on a scan rate of 5 mV s−1. To ensure accuracy and reproducibility, these tests were repeated after each performance at room temperature. In this instance, the working electrode 0.5 cm2 geometrical surface area was taken into calculation of all current densities (j).
The potential ranges for Cu0.5Ni0.5Al2O4/GCN, Cu0.5Ni0.5Al2O4, and GCN were discovered to be between −0.6 and 0.6 V; Equation (1) was used to determine the specific capacitance (Csp) of the spinel, GCN, and spinel/GCN composite.
In this case, dV denotes the potential change derived from the redox peaks generated by CV, and I indicates the current. In contrast, CV polarization measurements use m, S, and ∆V to represent the loaded mass, scan rate, and applied potential window (−0.6–0.6 V).
Equations (2)–(4) were utilized to determine the values of the Csp, energy density (Ed), and power density (Pd) for each produced electrode based on GCD analysis [44].
The potential window variation is represented by delta V, and the mass of the loaded material is indicated by m, the constant discharging current by (I), and the discharging duration by delta t.
The charge transfer resistance (Rct) and solution resistance (Rs) between the electrode and electrolyte phases were evaluated at frequencies ranging from 0.1 to 1 MHz using EIS. The Nyquist plot display was performed in an open circuit with the data and was carried out at an amplitude of 10 mV and potential of 0.55 V. ZView software (version 4.1b) was used for curve fitting in the analysis of Nyquist plots utilizing equivalent circuit modeling.
Next, using Equation (5), the reference electrode potential of Ag/AgCl was converted to the RHE.
Here, the thermodynamic potential (E) in a 1 M KOH solution with a silver wire dip is represented by EAg/AgCl.
The electrocatalysts’ kinetic efficiency and catalytic efficiency were examined using CV Tafel graphs. Equation (6) was used to graph a steady-state polarization curve in the linear zone to create these plots [45].
where α is the charge transfer coefficient, n is the number of electrons transported during a redox reaction, j is the current density in milliampere-seconds (mA cm−2), F represents Faraday constant = 96,485, η is the overpotential, and 2.303/αnF is the slope value.
This allowed for determination of the value of Cdl. The evaluation of the ECSA was conducted by dividing the obtained capacitance of the flat electrodes (Cdl) by the Csp, which was derived from the previously published data (0.04 μF cm−2) [46], as shown in Equations (7) and (8).
One crucial factor in assessing the OER activity of a catalyst is its calculable turnover frequency (TOF), which is calculated using Equation (9). Under defined reaction circumstances, the TOF is applied to quantify the specific activity of a catalytic center for HER and OER reactions.
In this example, NA is the Avogadro number, n is the quantity of active catalysts in moles, and F is the Faraday constant. The single conventional techniques for verifying the long-term stability and wear resistance of the Cu0.5Ni0.5Al2O4/GCN composite comprise chronopotentiometry. With the stability of Cu0.5Ni0.5Al2O4/GCN using the chronoamperometry approach, the electrode material was evaluated at a fixed voltage of 0.75 V.
2.7. Characterization (Physical)
The crystallographic microstructure of the nanocomposite Cu0.5Ni0.5Al2O4/GCN, spinel Cu0.5Ni0.5Al2O4, and GCN generated by reduction was investigated employing an advanced D8 Bruker diffractometer with a Cu-kα monochromatic radiation source for powder X-ray diffraction from Bruker AXS GmbH, Germany. Ten milliamperes of current and 30 kV of voltage were employed for the trials. Using a scanning electron microscope (SEM), FEI Nova NanoSEM-450 from FEI, USA, was utilized to study the microstructure and shape of the final products. The composition of the generated electrode materials was studied utilizing energy-dispersive X-ray spectroscopy (EDX) utilizing a JSM-5910 energy-dispersive spectrometer from JEOL Ltd., Japan.
3. Results and Discussions
3.1. X-Ray Diffraction Analysis
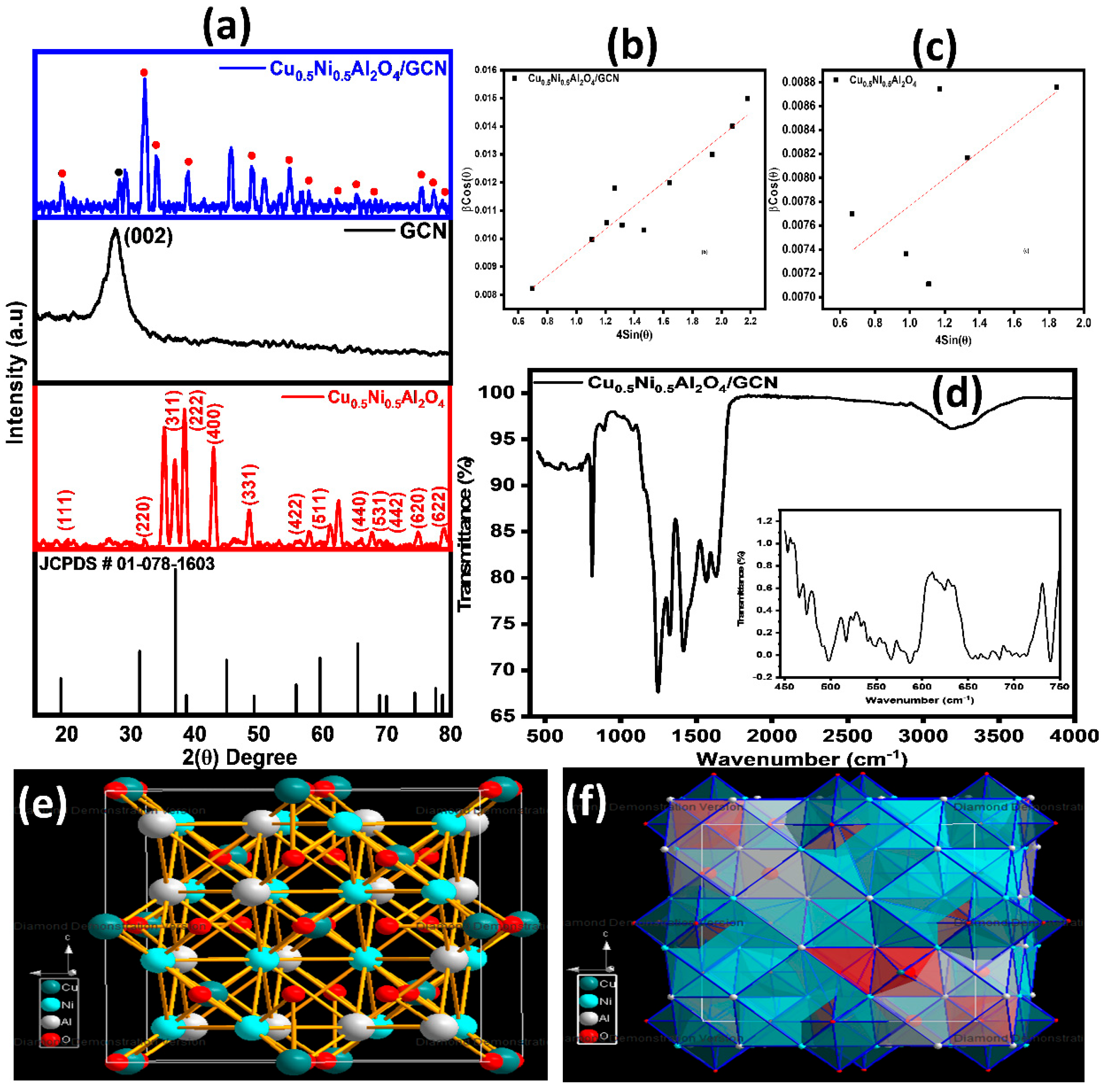
XRD examination was applied to determine the crystal structure of spinel Cu0.5Ni0.5Al2O4, GCN, and Cu0.5Ni0.5Al2O4/GCN composites. Figure 2a illustrates a diffractogram spanning a range of 2θ from 10° to 80°. The observed XRD peaks at 2θ (degree) 20.06°, 32.13°, 35.21°, 36.85°, 38.39°, 42.91°, 48.49°, 57.89°, 62.43°, 65.95°, 67.85°, 69.28°, 74.38°, and 78.69° correspond to crystal planes (111), (220), (311), (222), (400), (331), (422), (511), and (440), (531), (442), (620), and (622) of spinel Cu0.5Ni0.5Al2O4. These crystal planes align with the cubic structure of Cu0.5Ni0.5Al2O4, with standard lattice parameters of a = b = c = 8.0590 Å. This diffracted pattern is in accordance with JCPDS ref. # 01-078-1603. For GCN, the peak observed at an angle of 26.28° attributed to the diffraction plane labeled as (002) indicates an interlayer spacing along the c-axis of 3.372 Å. The determination of interlayer spacing was performed using Bragg law, applied to the (002) peak. The X-ray diffraction pattern of Cu0.5Ni0.5Al2O4/GCN composites exhibits identical distinct peaks as those observed in Cu0.5Ni0.5Al2O4, with slight displacement in the peak positions, validating the successful manufacturing of the nanocomposite. Scherrer expression (Equation (10) was employed to determine the crystallite sizes of Cu0.5Ni0.5Al2O4/GCN composites, GCN, and spinel Cu0.5Ni0.5Al2O4 samples, resulting in values of 17.58 nm, 17.2 nm, and 18.21 nm, respectively.
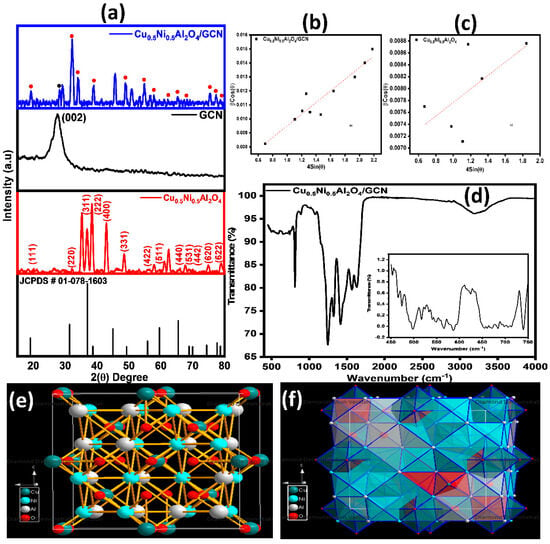
Figure 2.
(a) XRD pattern of Cu0.5Ni0.5Al2O4/GCN, GCN, and Cu0.5Ni0.5Al2O4, respectively; (b) W-H plot for Cu0.5Ni0.5Al2O4/GCN; (c) W-H plot for Cu0.5Ni0.5Al2O4; (d) FTIR spectrum for Cu0.5Ni0.5Al2O4/GCN; and (e,f) 3-D structure of Cu0.5Ni0.5Al2O4 and polyhedral view.
All the crystallographic structural details determined are provided in Table 1. Cu0.5Ni0.5Al2O4/GCN composites exhibit the smallest crystallite size of 17.58 nm, the highest dislocation density of 32.34 × 10–15, a moderate micro-strain of 2.13 × 10−3, and a relatively high lattice strain of 1.84 × 10−3. These characteristics indicate the highly defective structure of Cu0.5Ni0.5Al2O4/GCN composites, resulting in increased active sites, facilitating enhanced electrochemical reactions. The dislocation density (δ) and micro-strain (ε) were inferred from XRD data. Equation (11) was used to calculate the dislocation density (δ) [47].
where D is the size of the crystallite, and the line dislocation can be theoretically defined as

Table 1.
Structural parameters calculated via XRD pattern.
In order to calculate the lattice strain (LS) and micro-strain (ε), Equations (13) and (14) were used [47] as follows:
Using Williamson–Hall (W-H) techniques, the micro-strain of the produced materials was also determined using the following expression (Equation (15)).
where D is the crystallite size, λ is the X-ray wavelength, θ is the Bragg angle, k is the forming factor, while ɑ is the micro-strain. Plotting the W-H graph for Cu0.5Ni0.5Al2O4/GCN and Cu0.5Ni0.5Al2O4 over βcosθ vs. 4sinθ for the substantial peaks and values of the positive micro-strain comes out to be 4.16 × 10−3 and 1.13 × 10−3, respectively, and it is displayed in Figure 2b,c.
The 3-D simulated illustrations of spinel Cu0.5Ni0.5Al2O4 are created by means of the structural characteristics obtained from XRD. Three-dimensional representations produced by the diamond software (version 4.6) are shown in Figure 2e. According to the diagram, the crystal structure space group is Fd-3m (227). Site preferences for Cu, Ni, Al, and O are evident in the observed event. The 3-D picture of Cu0.5Ni0.5Al2O4 with polyhedral view is demonstrated in Figure 2f.
The FTIR spectra of Cu0.5Ni0.5Al2O4/GCN are shown in Figure 2d. A prominent peak may be seen at 810 cm−1. However, an alternative theory linked this peak to the collective out-of-plane wagging mode of N and C atoms in heptazine. The in-plane stretching modes of rings, which include common aromatic ring vibrations at 1423 cm−1 (s-triazine ring), 1643 cm−1 (C=N stretch), and 1348 cm−1 (C=N stretch), are responsible for strong bands at 1248 cm−1. Peaks at 3193 cm−1, representing uncondensed NH and NH2 groups, may overlap with the (H-bonded) OH stretch when adsorbed water is present [48]. The v3 vibration of was identified as the cause of the broad band about 1399 cm−1. Peaks smaller than 1000 cm−1, as depicted in Figure 2d (embedded graph), within the 400–750 cm−1 range are attributable to vibrations of M–O (M = Ni, Al, and Cu) [49].
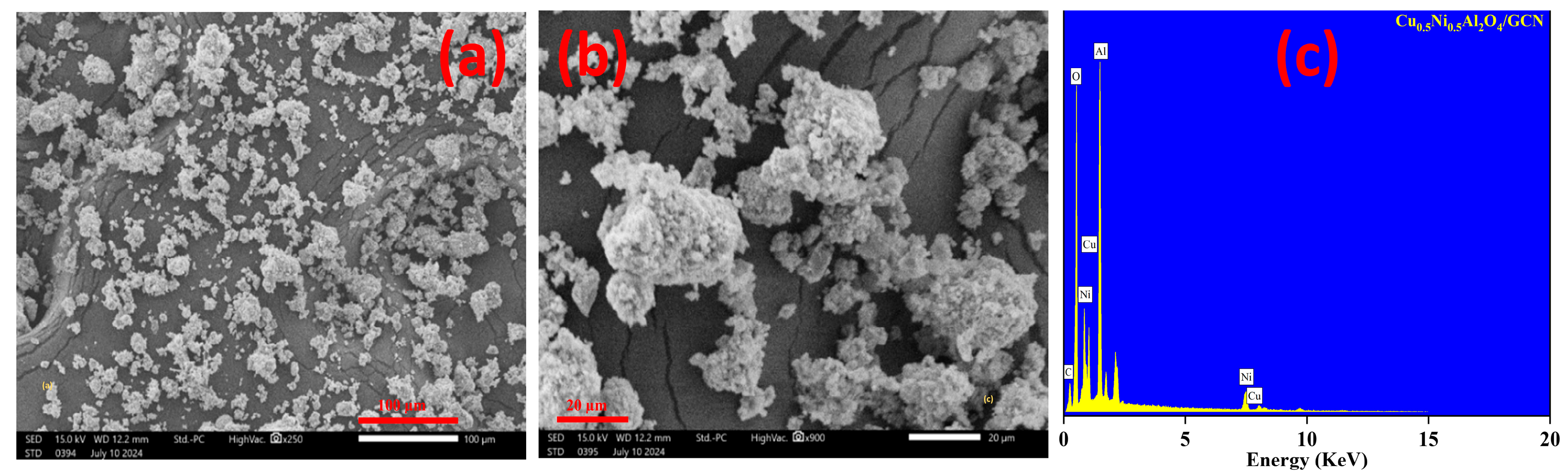
Using an SEM technique, morphological characterization of the manufactured materials was completed. The Cu0.5Ni0.5Al2O4/GCN micrograph, as seen in Figure 3a, depicts irregular agglomerated nanoparticles spread on regular agglomerated Cu0.5Ni0.5Al2O4. On the other hand, Cu0.5Ni0.5Al2O4/GCN in Figure 3b displays a zoom view of Figure 3a at 20 µm focuses. The morphology of the nanocomposite is represented by the irregularly shaped Cu0.5Ni0.5Al2O4/GCN particles scattered throughout the Cu0.5Ni0.5Al2O4/GCN surface. The rough surface serves as a source of additional active sites for intermediate diffusion. They expose the electrolyte to catalytically active binding sites, significantly enhance water splitting by facilitating more efficient electron and ion transport across the electrode–electrolyte interface, and act as a mechanism for energy storage. EDS was applied to evaluate the elemental composition of the nanocomposite, as exhibited in Figure 3c. Cu, Ni, Al, N, and O are the only elements visible in the composite peaks on the EDX spectrum. The absence of any additional elements indicates the material’s exceptional purity. This demonstrates that the nanocomposite was successfully synthesized without any impurity.

Figure 3.
(a,b) SEM images for Cu0.5Ni0.5Al2O4/GCN at 100 and 20 µm focuses and (c) EDX pattern for Cu0.5Ni0.5Al2O4/GCN composite.
3.2. Electrocatalyst for SCs
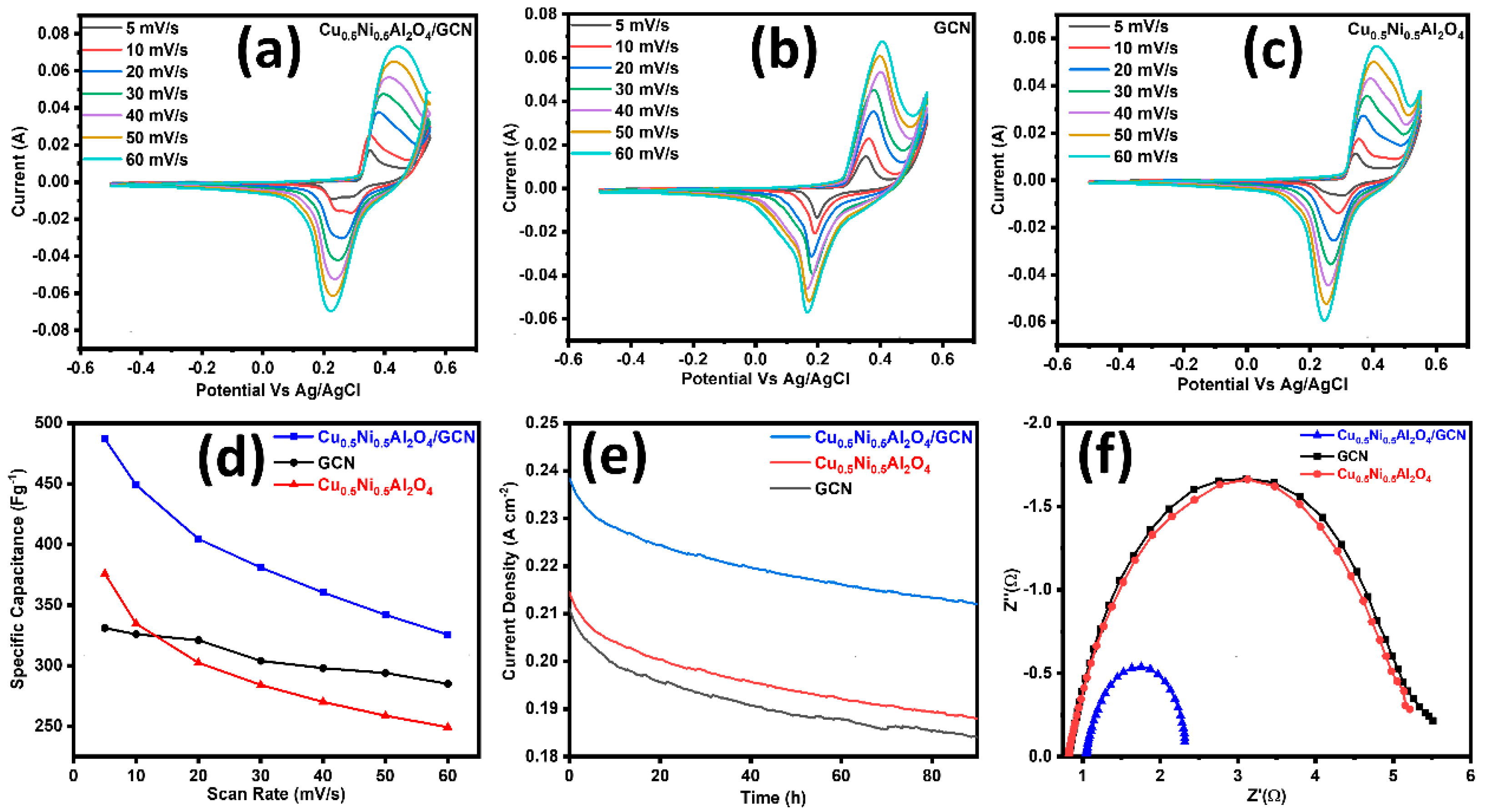
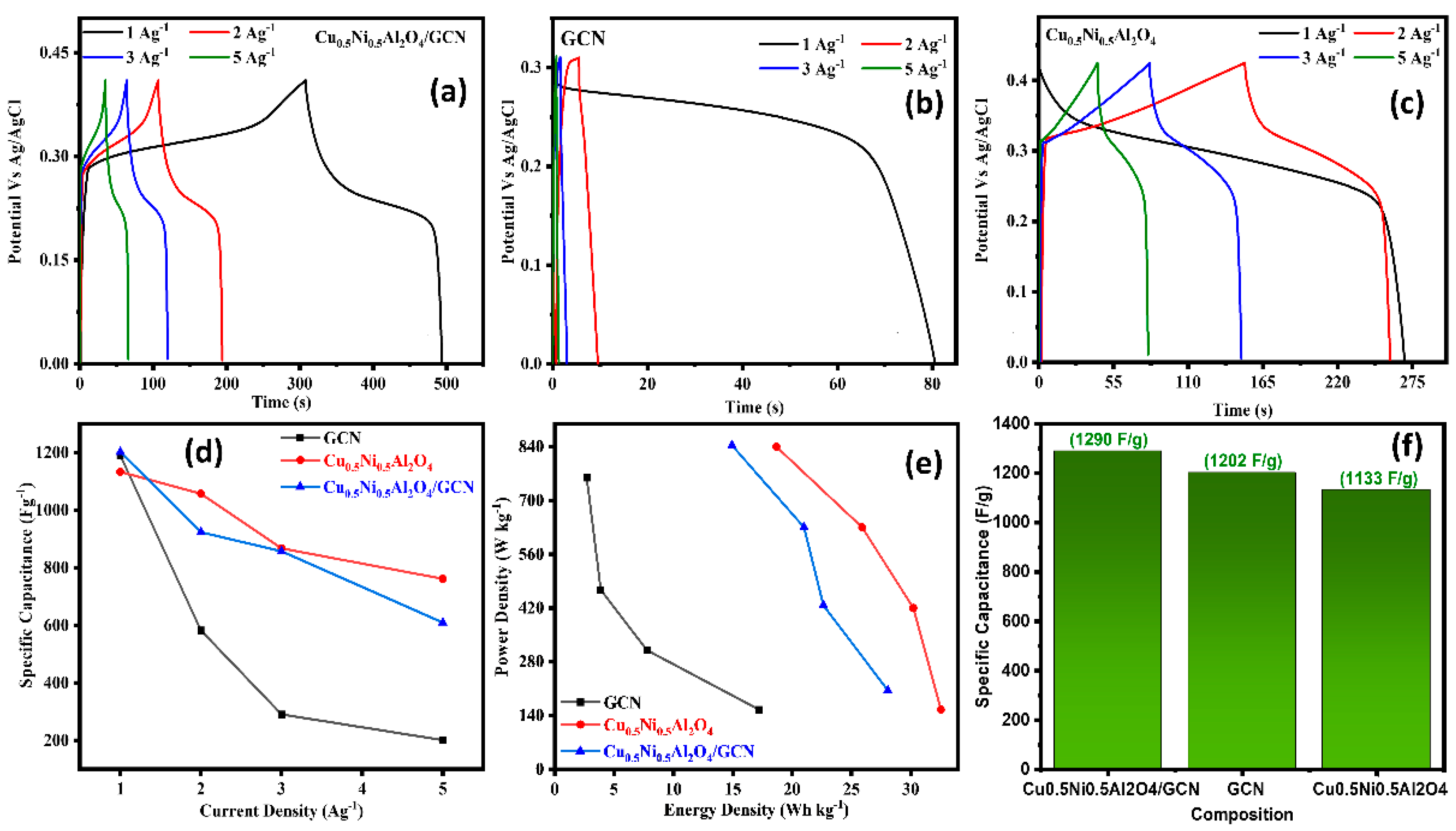
All electrochemical measurements were conducted under identical conditions to facilitate a comparison with Cu0.5Ni0.5Al2O4/GCN, GCN, and Cu0.5Ni0.5Al2O4, as presented in Table 2. Figure 4a–c display polarization curves from CV methods at a scan rate of 5–60 mV s−1 used to analyze the catalyst intrinsic characteristics on a potential window (−0.6–0.6 V) vs. Ag/AgCl as the reference electrode. The redox potential of Cu0.5Ni0.5Al2O4/GCN may be ascribed to the cooperative and synergistic impact of Cu, Ni, and Al ions. In Cu0.5Ni0.5Al2O4/GCN, a roughly linear relationship is shown between the scan speed and discharge current density, suggesting a quick charge–discharge process. The Csp value of pure Cu0.5Ni0.5Al2O4/GCN was 487 F g−1 at 5 mV s−1, but it gradually fell to 325 F g−1 at 60 mV s−1, as can be observed in the inset of Figure 4d. On other hand, when the scan rate was raised from 5 to 60 mV s−1, the Csp values of the spinel Cu0.5Ni0.5Al2O4/GCN nanocomposite electrode material and GCN fell. The Csp value of GCN decreased from 326 to 285 F g−1, while the Csp value of Cu0.5Ni0.5Al2O4 decreased from 375 to 249 F g−1; the Cu0.5Ni0.5Al2O4/GCN composite electrode exhibited a maximum Csp value that is equal or higher than that of the conventional spinel metal-oxide composite. Increased scanning rates decrease the critical crystal point because of the polarization effects and ohmic resistance, which hinder the movement of electrolyte ions and redox kinetics. In addition to that, the comparative CV of the Cu0.5Ni0.5Al2O4/GCN composite, GCN, and Cu0.5Ni0.5Al2O4 electrode material at 10 mV/s is also displayed in Figure S1.

Table 2.
Summary of the electrochemically extracted values for all the investigated electrocatalysts.
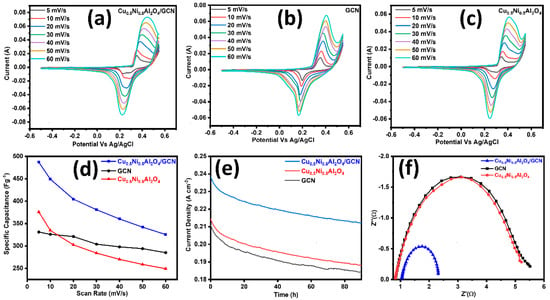
Figure 4.
(a) CV polarization curves for Cu0.5Ni0.5Al2O4/GCN, GC; (b) CV for Cu0.5Ni0.5Al2O4; (c) CV for Cu0.5Ni0.5Al2O4; (d) scan rate vs. Csp graph for Cu0.5Ni0.5Al2O4/GCN, GCN, and Cu0.5Ni0.5Al2O4/GCN; and (e) chronoamperometry and (f) EIS analysis test for Cu0.5Ni0.5Al2O4/GCN, GCN, and Cu0.5Ni0.5Al2O4.
In a chronoamperometric stability test, the synthesized materials Cu0.5Ni0.5Al2O4/GCN, GCN, and Cu0.5Ni0.5Al2O4 were exposed to a constant applied potential, and the current density was measured for ninety hours. The stability of the Cu0.5Ni0.5Al2O4/GCN electrode material was evaluated by means of the chronoamperometry method at a constant voltage of 0.75 V. Furthermore, we evaluated the fabricated supercapacitor’s stability using GCD cycles at a current density of 1 Ag−1; as can be seen in Figure S3, the Cu0.5Ni0.5Al2O4/GCN electrode material has good electrochemical stability because, prior to 5000 cycles, the capacitance decreased somewhat but still reached 80%.
The findings are shown in Figure 4e, whereby the Cu0.5Ni0.5Al2O4/GCN composite maintains a constant current density of 0.21 A cm−2, GCN shows Cd of 0.19 mA cm−2, spinel Cu0.5Ni0.5Al2O4 shows Cd of 0.23 mA cm−2, and all the synthesized material shows stability for 90 h at these Cds. The decrease in Cd at the electrode contact caused by electrolyte ion coagulation indicates that the composite material is very stable electrochemically.
In Figure 4f, the Nyquist plots for each composite material are shown. This study demonstrates that the Cu0.5Ni0.5Al2O4/GCN composite exhibits the smallest semicircular diameter when compared to spinel Cu0.5Ni0.5Al2O4 and GCN. The radius of the semicircle, which is frequently closely proportional to the Rs, indicates a greater electron transfer rate across the contact. The Rct metrics of the electrocatalysts is demonstrated, namely the Cu0.5Ni0.5Al2O4/GCN composite. Owing to its increased conductivity, with a solution resistance (Rs) of 1.88 Ω and a charge transfer resistance (Rct) of 1.27 Ω, the Cu0.5Ni0.5Al2O4/GCN electrode shows much lower impedance values, suggesting enhanced ionic conductivity and quicker charge transfer kinetics. In contrast, the virgin Cu0.5Ni0.5Al2O4 exhibits an Rs of 2.45 Ω and Rct of 4.65 Ω, whereas GCN alone shows higher Rs and Rct values of 2.40 Ω and 4.49 Ω, respectively. These results demonstrate that the electrochemical performance of the electrode is much improved by the integration of GCN with the spinel, which makes it a viable option for energy conversion and storage applications.
Figure 5a–c shows the recorded GCD curves at CDs of 1–5 A g−1 between the potential ranges of 0–0.5 V. The Cu0.5Ni0.5Al2O4/GCN composite possesses a particularly symmetrical and triangular structure in comparison to both spinel Cu0.5Ni0.5Al2O4 and GCN. These improvements not only significantly increase the conductivity but also demonstrate the hybrid functions as a reversible ideal capacitor. The prolonged period of discharge exhibited by Cu0.5Ni0.5Al2O4/GCN validates its exceptional specific capacitance. The enhanced surface electrode structure facilitates the migration of electrolyte ions, and the presence of Cu0.5Ni0.5Al2O4 spinel distributed over GCN may enhance electrical conductivity. The Csp of the Cu0.5Ni0.5Al2O4/GCN composite is 1290 F g−1 at a Cd of 1 A g−1, as depicted in Figure 4d. Spinel Cu0.5Ni0.5Al2O4 has a specific capacitance (Csp) value of 1133 F g−1, whereas GCN has 1202 F g−1. One proposed approach to enhance the efficiency of the diffusion process is the generation of active sites. A hypothesis posits that the incorporation of spinel Cu0.5Ni0.5Al2O4 into a GCN would facilitate diffusion and decrease the energy required for defect formation due to the present active sites. The experimental findings indicated that the energy density of the Cu0.5Ni0.5Al2O4/GCN composite was measured to be 28.07 Wh/kg. Additionally, the power density of Cu0.5Ni0.5Al2O4/GCN was found to be 205.19 W/kg, while the Pd and Ed of GCN were 154 W/kg and 17.22 Wh/kg, respectively, and for Cu0.5Ni0.5Al2O4 the Pd and Ed were 155.24 W/kg and 11.58 Wh/kg as demonstrated in Figure 5e. These energy and power density values were evaluated using Equations (3) and (4). The findings of this study indicate that the incorporation of a quantity of spinel Cu0.5Ni0.5Al2O4 into the GCN electrode significantly enhanced its porosity.
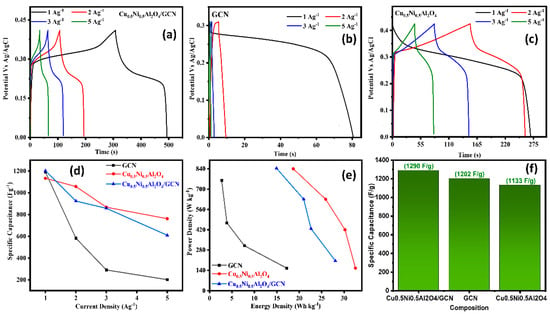
Figure 5.
(a–c) GCD test for Cu0.5Ni0.5Al2O4/GCN, GCN, and Cu0.5Ni0.5Al2O4, respectively; (d) current density (Ag−1) vs. Csp (Fg−1) for Cu0.5Ni0.5Al2O4/GCN, GCN, and Cu0.5Ni0.5Al2O4; (e) energy density vs. power density graph for Cu0.5Ni0.5Al2O4/GCN, GCN, and Cu0.5Ni0.5Al2O4; (f) composition vs. Csp (Fg−1) graph.
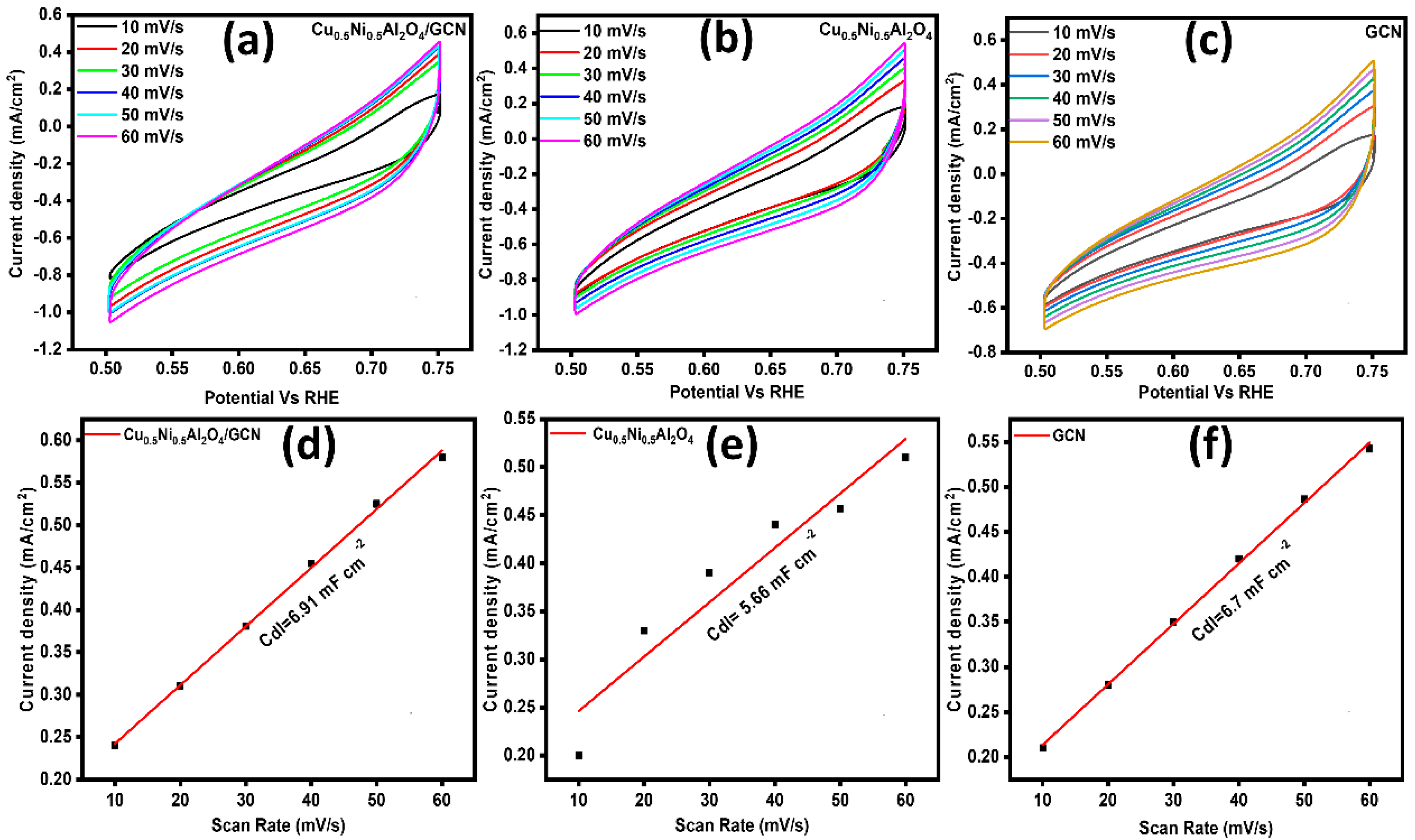
An essential parameter for the computation of the electrochemical surface area (ECSA) is established in Figure 6a–c. Therefore, the non-faradic region was chosen from the CV curve, namely within a potential window range of 0.50–0.75 V compared to the reference hydrogen electrode (RHE). Electrochemical CV was used to determine the restricted potential range at 10, 20, 30, 40, 50, and 60 millivolts per second. The cathodic and anodic currents were calculated by studying these graphs in order to identify the variation in CD (Δj). Plotting values of Δj against scan rate speeds using the linear fit approach yields a straight line. The resulting slope is divided in half to yield the Cdl. The ECSA CV curves were formed by dividing the Cdl value, which was 0.040 mF cm−2 for the flat electrode [50,51], by the Csp value, as shown in Figure 6d–f. Table 3 displays the ECSA and Rf values that were calculated.
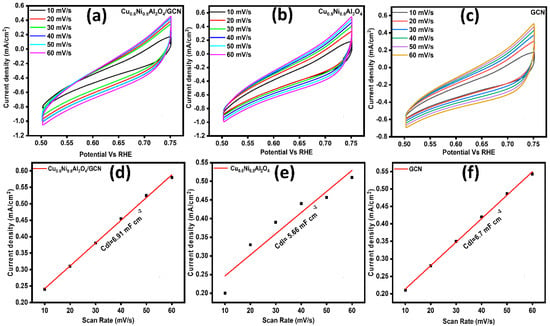
Figure 6.
(a–c) ECSA of Cu0.5Ni0.5Al2O4/GCN, Cu0.5Ni0.5Al2O4, and GCN; (d–f) double-layer capacitance (Cdl) of Cu0.5Ni0.5Al2O4/GCN, Cu0.5Ni0.5Al2O4, and GCN.

Table 3.
A list of the values provided for the Cdl, Rf, and ECSA for the nanocomposites that were synthesized using Cu0.5Ni0.5Al2O4/GCN, GCN, and Cu0.5Ni0.5Al2O4.
The following formulas (Equations (16) and (17)) are used to calculate the ECSA and roughness factor Rf.
3.3. SC Performance in Two-Electrode System
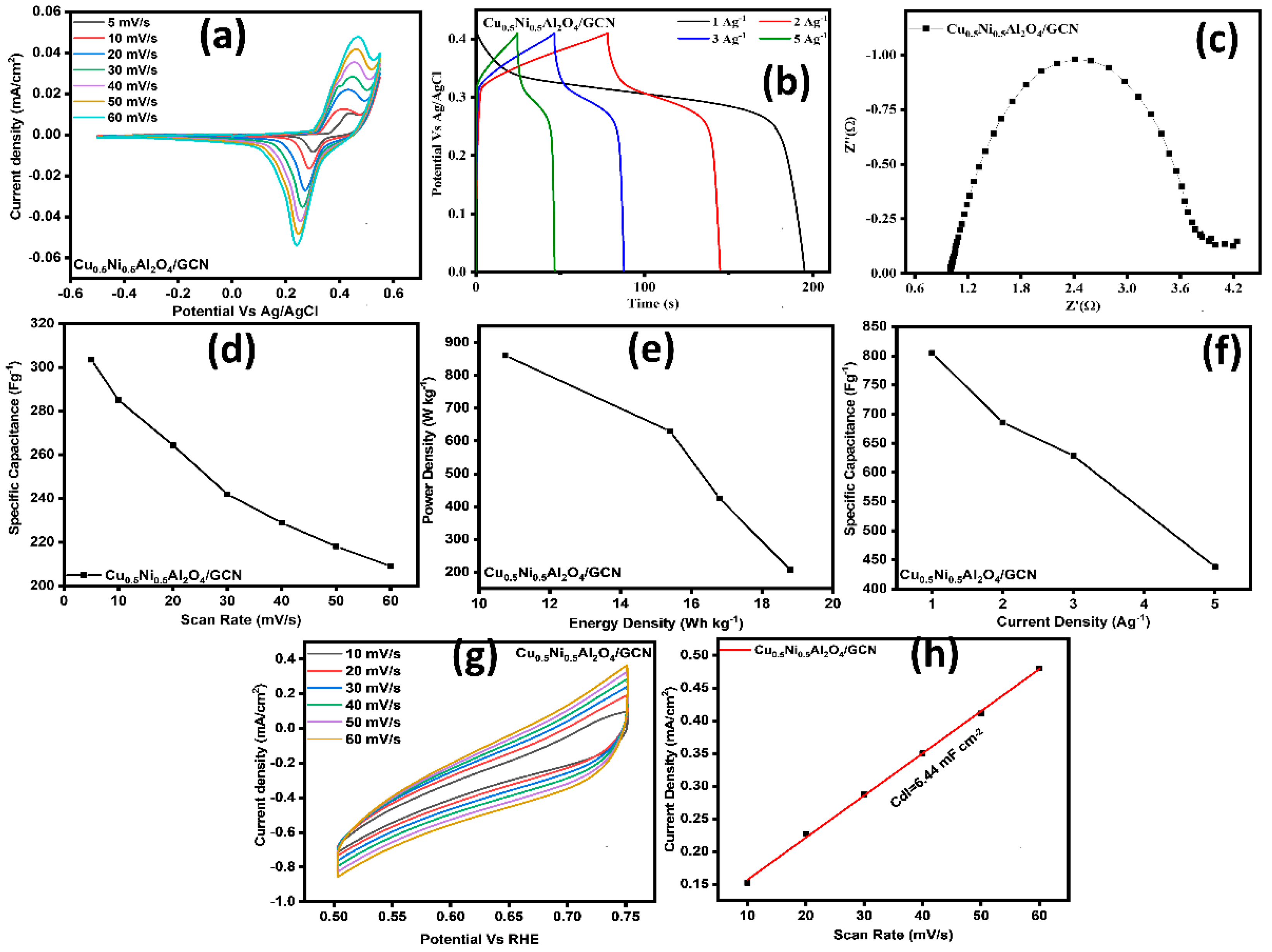
The Cu0.5Ni0.5Al2O4/GCN nanocomposite was tested using a two-electrode setup, with a carbon rod serving as the counter electrode. Figure 7a displays the CV curves for the Cu0.5Ni0.5Al2O4/GCN composite at scan rates from 5 to 60 mV s−1. The curves, corresponding to specific capacitance (Csp) values of 303, 285, 264, 241, 228, 218, and 209 F g−1, provide significant information. At a current density of 1 A g−1, the Csp was determined from the GCD curves shown in Figure 7b. For the Cu0.5Ni0.5Al2O4/GCN composite, the Rct was 4.53 Ω, as indicated by the EIS results in Figure 7c. The deliberate enhancement of the charge transfer conductivity achieved by merging GCN with the spinel Cu0.5Ni0.5Al2O4 lattice leads to a decrease in Rct. The complex features of the material, including its behavior during the redox process, become more pronounced as the scan rate increases. The calculated Csp value reached 804 F g−1, as described in Figure 7d. Furthermore, the device exhibits an energy density of 18.79 W kg−1 and a power density of 207.27 Wh kg−1, as shown in Figure 7e. The ECSA plot is presented in Figure 7g, which corresponds to the non-faradaic region, i.e., the potential window spanning from 0.50 to 0.75 V, based on the CV curve. Figure 7h illustrates a double-layer capacitance (Cdl) of 6.44 mF g−1 for the Cu0.5Ni0.5Al2O44/GCN composite electrode, corresponding to an ECSA of 161 cm2, consistent with the results obtained from a three-electrode system.
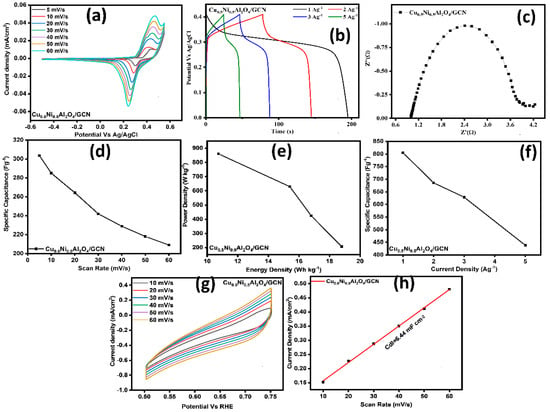
Figure 7.
(a) CV curves, (b) GCD profiles of samples, (c) EIS spectrum of electrodes, (d) scan rate vs. Csp, (e) power density vs. energy density graph, (f) current density (Ag−1) vs. Csp (Fg−1) graph, (g) (ECSA) analysis, and (h) double-layer capacitance (Cdl) graph for Cu0.5Ni0.5Al2O4/GCN nanocomposite in 2-electrode electrochemical analysis.
3.4. OER Analysis
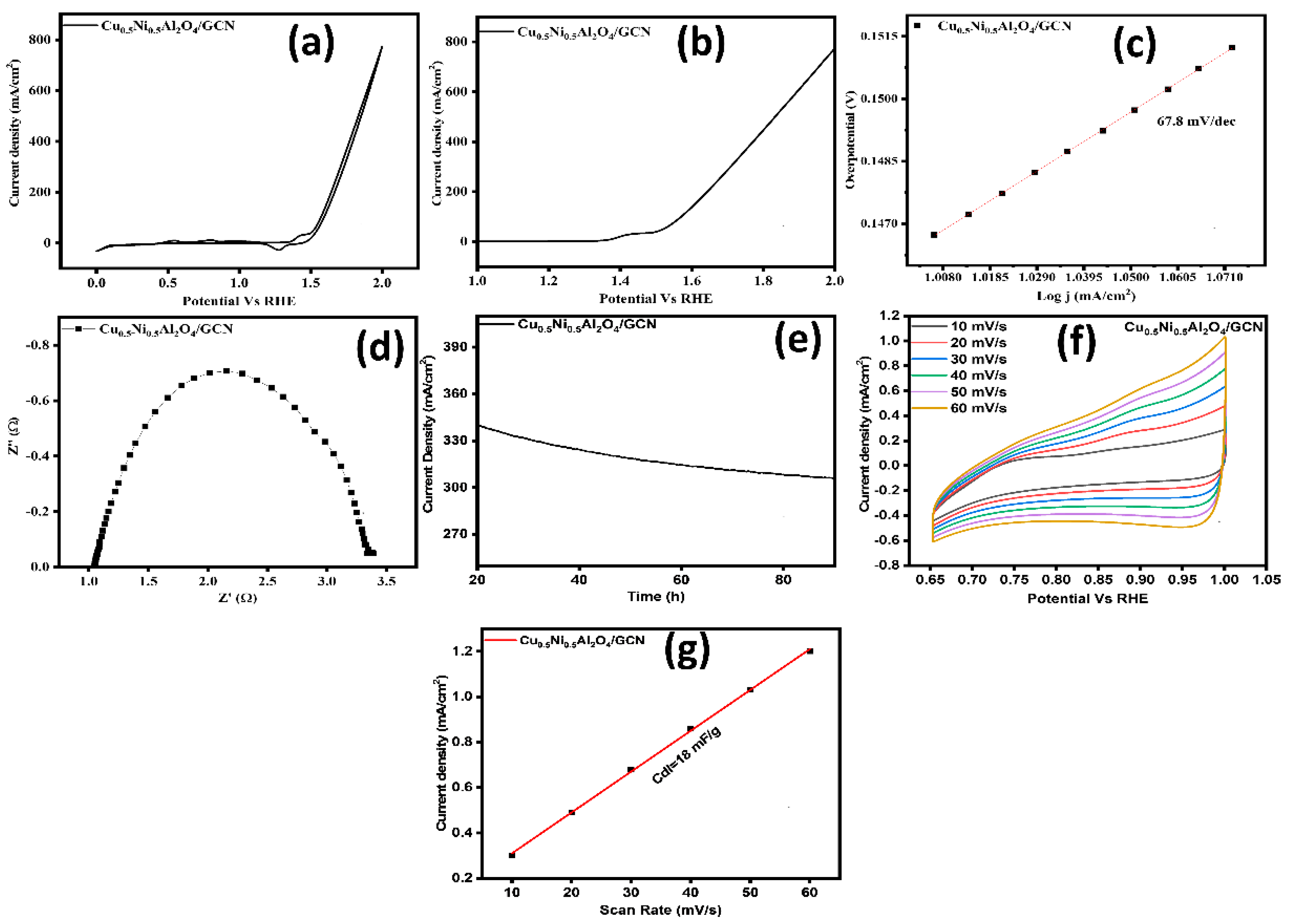
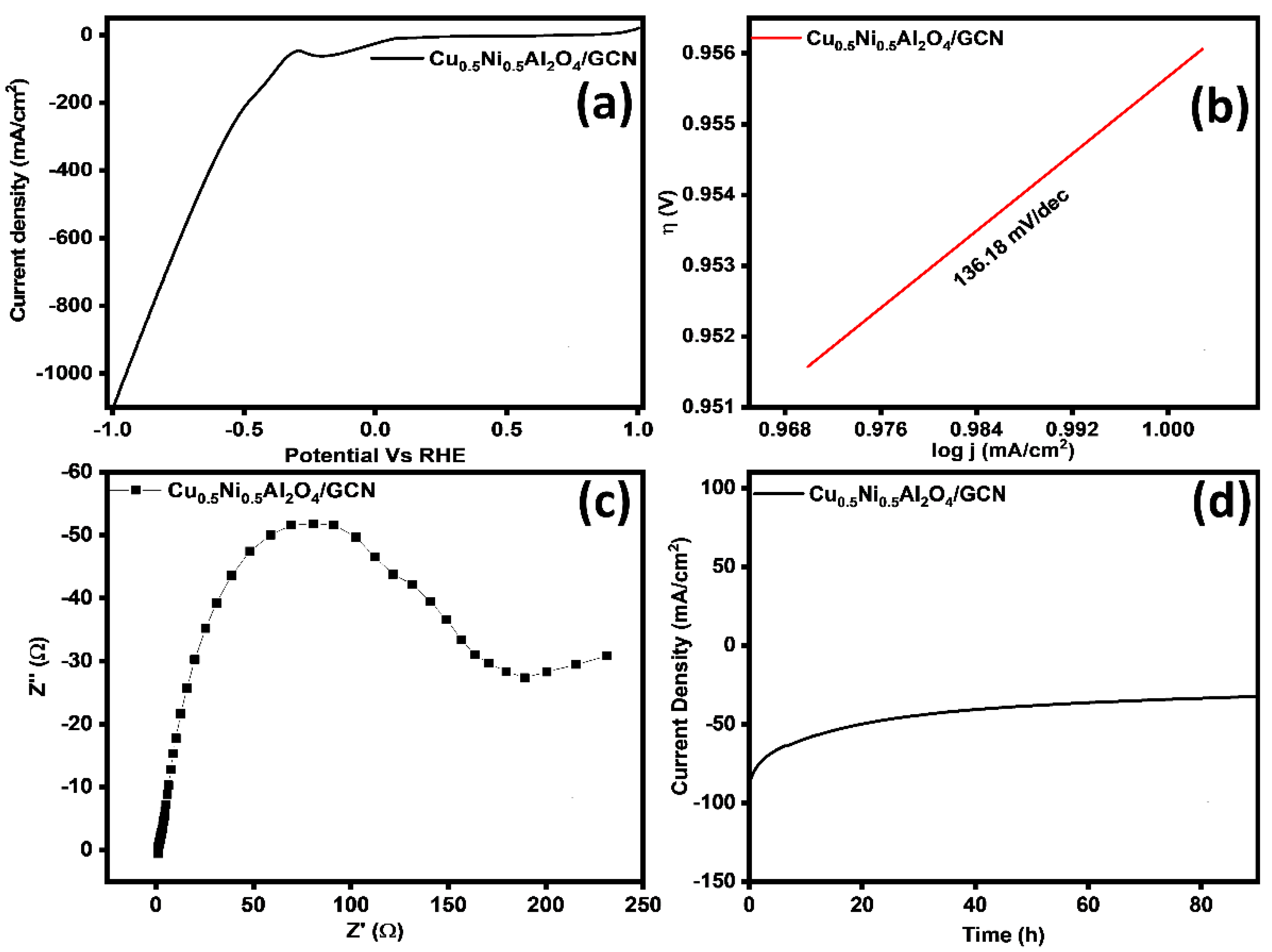
The kinetics, stability, and performance of electrocatalysts for the OER were studied using various electrochemical methods. Figure 8a,b illustrate the CV and LSV curves. Figure 8a shows the CV polarization; at a benchmark current density of 10 mA cm−2, the Cu0.5Ni0.5Al2O4/GCN nanocomposite exhibited significantly improved OER performance, with an overpotential of 254 mV and a lower onset potential of 1.49 V. These results indicate an enhanced intrinsic electrocatalytic activity of the Cu0.5Ni0.5Al2O4/GCN composite. Moreover, as shown in Figure 8c, the Cu0.5Ni0.5Al2O4/GCN electrode displayed the lowest Tafel slope (67.8 mV dec−1), suggesting faster kinetics in the four-electron transfer process. Furthermore, a CV comparison between bare nickel foam and the Cu0.5Ni0.5Al2O4/GCN composite is presented in Figure S2.
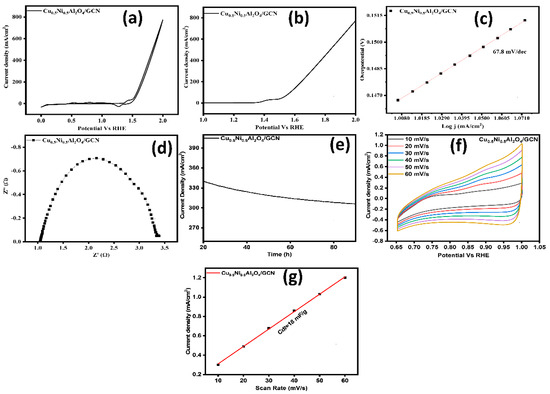
Figure 8.
(a) CV curves of the prepared materials, (b) LSV curves, (c) Tafel slope, (d) EIS analysis of the prepared materials, (e) chronoamperometry test, (f) ECSA analysis, and (g) double-layer capacitance (Cdl) graph for Cu0.5Ni0.5Al2O4/GCN for OER.
The introduction of Cu0.5Ni0.5Al2O4 into the GCN matrix significantly enhanced the electrochemical performance. The modification of the nanocomposite’s surface area, through its interaction with GCN, improves permeability and increases the number of electrocatalytic binding sites. An examination of the turnover frequency (TOF) was undertaken to evaluate the inherent catalytic capability of the material. The TOF of Cu0.5Ni0.5Al2O4GCN was determined to be 1.81 s−1, indicating that the incorporation of Cu0.5Ni0.5Al2O4 effectively enhances the intrinsic catalytic activity of GCN.
The electrochemical route for the Cu0.5Ni0.5Al2O4/GCN nanocomposite is suggested by the following equations (Equations (18)–(21)).
The interaction between the spinel Cu0.5Ni0.5Al2O4 nanoparticles and hydroxide ions (OH–) triggers the process of surface oxidation. This mechanism involves the accumulation of OH– ions on the readily available binding sites of reactive copper (Cu), nickel (Ni), and aluminum (Al) sites within the Cu0.5Ni0.5Al2O4 structure, leading to the formation of metal hydroxide species (M− OH–).
Following the production of M−OH–, the second phase involves interaction with additional hydroxide (OH–) ions. This interaction results in the release of protons (H+) and electrons (e−), thereby facilitating the formation of metal oxide species (M−O). The phenomenon of electron discharge is a key component of surface redox reactions.
The nucleophilic attack of M−O may be amplified by their conjunction with another electron. The formation of M−OOH, a critical stage in the catalytic cycle, happens after this reaction. This process leads to the accumulation of additional active sites within the Cu0.5Ni0.5Al2O4/GCN composite matrix.
The interaction of M−OOH with OH– is the final phase. Protons (H+), molecular oxygen (O2), and one additional electron are emitted following this contact. Significantly, the completion of the OER is proven by the development of O2. This extensive mechanistic work stresses the complicated but well-organized sequence of chemical processes that the Cu0.5Ni0.5Al2O4/GCN nanocomposite coordinates, demonstrating its catalytic potential to increase the efficient OER. An important scientific instrument for properly assessing the rate at which charges travel between an electrode and an electrolyte is EIS. It was observed that the Cu0.5Ni0.5Al2O4/GCN composite exhibits a charge transfer resistance (Rct) of roughly 2.91 Ω. At the electrode–electrolyte interface, the Cu0.5Ni0.5Al2O4/GCN composite exhibited effective and rapid proton and electron transport during the (OER) process. The electrical efficiency of Cu0.5Ni0.5Al2O4/GCN, as indicated by a Tafel slope of 67.8 mV dec−1, was further improved by EIS analysis, therefore validating its effectiveness as a catalyst for advancement of the OER. Through the use of chronoamperometry analysis, the electrode stability was evaluated. To evaluate the generated material long-term endurance against Ag/AgCl, a chronoamperometry test was performed at voltages up to 0.75 V. Figure 8e shows the results of the chronoamperometric study for Cu0.5Ni0.5Al2O4/GCN, which demonstrate the electrode material prolonged endurance over a 90 h period. Overall, the performance of the fabricated material and longevity were amazing. Due to its exceptional stability, it grew good electrode materials, particularly for the OER.
The exact quantity of the conveniently available electrocatalyst necessary for electrochemical activity is established by the ECSA approach. When the electrocatalyst includes more active and functional sites, it performs optimally and has greater ECSA values, which were determined by looking at CV graphs generated in the non-faradaic spectrum area of Figure 8f at different scan speeds (10–60 mVs−1). Figure 8g depicts the experimental data, which demonstrate that the Cu0.5Ni0.5Al2O4/GCN composite exhibited a Cdl of 18 mF cm−2.
3.5. HER Analysis
The current work studied the electrocatalytic activity of the Cu0.5Ni0.5Al2O4/GCN composite for the HER. Relevant LSV diagrams are provided in Figure 9a. The Cu0.5Ni0.5Al2O4/GCN composite demonstrated considerable HER activity, requiring an overpotential of 101 mV to obtain a current density of 10 mA cm−2. The Tafel slope in Figure 9b demonstrates the activity of the Cu0.5Ni0.5Al2O4/GCN composite, with a measured value of 136.18 mV dec−1. These results indicate a relatively fast electrochemical reaction. In alkaline solutions the HER begins with hydrogen dissociation from water, resulting in an adsorbed proton on the electrode surface, known as the Volmer step. This phase requires a Tafel slope value greater than 90 mV dec−1. On the electrode surface, the adsorbed hydrogen reacts with water and electrons to produce a H2 molecule during the Heyrovsky step. The device displays the Tafel slope in millivolts per decimal point. Recombination of the adsorbed protons on the electrode surface during the Tafel step typically requires an average Tafel slope of 30 mV dec−1. This work highlights the importance of the Volmer step in determining the overall electrocatalytic efficiency of the material.
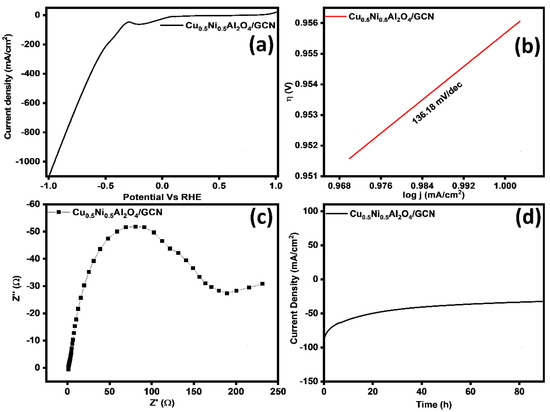
Figure 9.
(a) LSV curves, (b) Tafel slope, (c) EIS analysis, and (d) chronoamperometry test for Cu0.5Ni0.5Al2O4/GCN nanocomposite for HER.
The intrinsic catalytic capability of a material is measured using a turnover frequency (TOF) study. The TOF value of Cu0.5Ni0.5Al2O4/GCN was found to be 2.99 s−1. The foregoing conclusions were further verified by empirical data on electronic conductivities. This shows that the synthesized composite is a potent electrocatalyst for the HER. The conductivity and electrochemical properties of samples were evaluated using EIS, as shown in Figure 9c. The Rct values of Cu0.5Ni0.5Al2O4/GCN was 178.36 Ω. This indicates that the combination of Cu0.5Ni0.5Al2O4 with GCN increases conductivity and promotes interfacial electron transport in the Cu0.5Ni0.5Al2O4/GCN composite. Consequently, the catalyst displays enhanced conductivity and rapid charge transfer capabilities, thereby improving reaction kinetics.
Consideration of catalyst durability is crucial for practical applications. Therefore, the results of prolonged stability tests carried out under a continuous applied voltage were analyzed (Figure 9d). Having demonstrated long-term stability for 90 h, Cu0.5Ni0.5Al2O4/GCN is confirmed to be an outstanding electrocatalyst suited for practical applications.
4. Conclusions
In conclusion, a spinel/GCN-based composite material was synthesized using the solid-state method, offering a feasible alternative to fossil fuels. Cu0.5Ni0.5Al2O4 was added to GCN to improve its surface area and significantly enhance its electrocatalytic activity.
Electrochemical tests demonstrate that the Cu0.5Ni0.5Al2O4/GCN nanocomposite achieved an overpotential of η10 = 101 mV for the HER and η10 = 254 mV for the OER. The hybrid nanocomposite exhibited a large ECSA of 172.75 cm2 and favorable Tafel slopes of 67.8 mV dec−1 (OER) and 136.18 mV dec−1 (HER), highlighting its efficient catalytic properties. In an energy storage application, Cu0.5Ni0.5Al2O4/GCN nanocomposite showed a specific capacitance (Csp) of 1290 F g−1 with an excellent energy and power density of 28.07 Wh/kg and 205.19 W/kg, respectively, obtained through GCD at a current density of 1 Ag−1 in a three-electrode configuration. Moreover, EIS analysis showed exceptionally lower charge transfer resistance (Rct) of 1.27 Ω and smaller solution resistance of (Rs) of 1.88 Ω. In addition to that, a two-electrode system with a carbon rod as the counter electrode was used to assess the Cu0.5Ni0.5Al2O4/GCN nanocomposite’s electrochemical performance. The results showed higher specific capacitance (Csp) values of 303 F g−1 obtained by CV in 2.0 M KOH at scan speeds of 5 mV s−1. A high Csp of 804 F g−1 with a power density of 207.27 Wh kg−1 and an energy density of 18.79 W kg−1 was found using GCD experiments at 1 A g−1. The device showed promise for high-performance energy storage applications. Further demonstrating the electrode material’s exceptional capacitive behavior was the ECSA, which was determined from the non-faradaic part of the CV curve and was 161 cm2 with a corresponding double-layer capacitance (Cdl) of 6.44 mF g−1. The synergistic interaction between GCN and the spinel Cu0.5Ni0.5Al2O4 resulted in a low charge transfer resistance (Rct) of 4.53 Ω, according to the EIS measurement.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13072200/s1, Figure S1: Comparative CV of Cu0.5Ni0.5Al2O4/GCN composite, GCN and Cu0.5Ni0.5Al2O4 electrode material at 10 mV/s; Figure S2: CV comparison of bare nickel foam and Cu0.5Ni0.5Al2O4/GCN composite; Figure S3: Electrochemical stability of Cu0.5Ni0.5Al2O4/GCN electrode material using GCD cycles at current density of 1 Ag−1.
Author Contributions
Conceptualization: A.S., S.A., S.F.; methodology: A.S., S.A., S.F.; investigation: A.S., S.A., S.F.; data curation: A.S., S.A., S.F.; software: A.S., S.A., S.F.; writing—original draft: A.S., S.A., S.F.; resources: A.-D.S., S.Z., L.F.; investigations: A.-D.S., S.Z., L.F.; data curation: A.-D.S., S.Z., L.F.; writing—review and editing: S.K.A.-D., S.F., R.H.H., G.H.; project administration, H.N.H., R.H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Research and Graduate Studies at King Khalid University (grant no. RGP2/333/45). This work was also supported by the Guizhou University of Engineering Science High-level Talent Research Startup Fund (No. YuanKe [2025] No. 01).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through the Large Research Project under grant number RGP2/333/45. They also acknowledge Guizhou University for its support through Guizhou Province Science and Technology Plan Project No. ZK [2024] Key Project 078A and the Joint Fund of Bijie City and Guizhou University of Engineering Science (Bijie Science and Technology Union Contract [2023] No.16). Thanks also go to the University of Nizwa. We are also grateful to the University of Punjab, Lahore, Pakistan, for providing the facilities and support to accomplish this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Haohong, D.; Dongguo, L.; Yan, T.; Yang, H.; Shufang, J.; Rongyue, W.; Haifeng, L.; Haoyi, L.; Chongmin, W.; Jun, L.; et al. High-Performance Rh2P Electrocatalyst for Efficient Water Splitting. J. Am. Chem. Soc. 2017, 139, 5494–5502. [Google Scholar]
- Zhou, F.; Xin, Q.; Fu, Y.; Hua, Z.; Dong, Y.; Ran, M.; Song, H.; Liu, S.; Qu, R.; Yang, Y.; et al. Efficient catalytic oxidation of chlorinated volatile organic compounds over RuO2-WOx/Sn0.2Ti0.8O2 catalysts: Insight into the Cl poisoning mechanism of acid sites. Chem. Eng. J. 2023, 464, 142471. [Google Scholar] [CrossRef]
- Li, B.; Jiang, S.D.; Fu, Q.; Wang, R.; Xu, W.Z.; Chen, J.X.; Liu, C.; Xu, P.; Wang, X.J.; Li, J.H.; et al. Tailoring Nanocrystalline/Amorphous Interfaces to Enhance Oxygen Evolution Reaction Performance for FeNi-Based Alloy Fibers. Adv. Funct. Mater. 2025, 35, 2413088. [Google Scholar] [CrossRef]
- Li, J.; Ju, X.; Feng, X.; Zhang, Y.; Huang, G.; Ma, D.; Zhao, X.; Xu, X.; Shi, J.-W. Constructing Z-scheme between graphite nitride carbon and supramolecular zinc porphyrin to promote photocatalytic H2 evolution. J. Colloid Interface Sci. 2025, 690, 137284. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wu, N.; Ji, B.; Cai, J.; Yao, W.; Wang, Z.; Zhang, Y.; Zhang, X.; Guo, S.; Zhou, X. Lattice Water Deprotonation Enables Potassium-Ion Chemistries. Angew. Chem. Int. Ed. 2025, 64, e202503904. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, J.; Jiang, S.; Xiong, H.; Fu, X.; Shang, S.; Xu, J.; He, G.; Chen, P.-C. Plasma-activated 2D CuMnO2 nanosheet catalysts with rich oxygen vacancies for efficient CO2 electroreduction. Appl. Catal. B Environ. Energy 2025, 371, 125255. [Google Scholar] [CrossRef]
- Li, L.; Gao, W.; Wan, Z.; Wan, X.; Ye, J.; Gao, J.; Wen, D. Confining N-doped carbon dots into PtNi aerogels skeleton for robust electrocatalytic methanol oxidation and oxygen reduction. Small 2024, 20, 2400158. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sato, E. Electrocatalytic properties of transition metal oxides for oxygen evolution reaction. Mater. Chem. Phys. 1986, 14, 397–426. [Google Scholar] [CrossRef]
- Cao, K.; Ge, X.; Li, S.; Tian, Z.; Cui, S.; Guo, G.; Yang, L.; Li, X.; Wang, Y.; Bai, S. Facile preparation of a 3D rGO/gC3N4 nanocomposite loaded with Ag NPs for photocatalytic degradation. RSC Adv. 2025, 15, 17089–17101. [Google Scholar] [CrossRef]
- Rosestolato, D.; Neodo, S.; Ferro, S.; Battaglin, G.; Rigato, V.; De Battisti, A. A comparison between structural and electrochemical properties of iridium oxide-based electrocatalysts prepared by sol-gel and reactive sputtering deposition. J. Electrochem. Soc. 2014, 161, E151. [Google Scholar] [CrossRef]
- De, A.; Kim, M.S.; Adhikari, A.; Patel, R.; Kundu, S. Sol-gel derived nanostructure electrocatalysts for oxygen evolution reaction: A review. J. Mater. Chem. A 2024, 12, 19720–19756. [Google Scholar] [CrossRef]
- Wu, L.-K.; Wu, W.-Y.; Xia, J.; Cao, H.-Z.; Hou, G.-Y.; Tang, Y.-P.; Zheng, G.-Q. A nanostructured nickel–cobalt alloy with an oxide layer for an efficient oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 10669–10677. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Q.; Sun, N.; Su, H.; Chen, P.; Wu, J.; Fu, Y.; Ma, J. Giant improvement in piezo-photocatalytic capability of colloidal g-C3N4 quantum dots. Prog. Nat. Sci. Mater. Int. 2025; in press. [Google Scholar] [CrossRef]
- Seitz, L.C.; Dickens, C.F.; Nishio, K.; Hikita, Y.; Montoya, J.; Doyle, A.; Kirk, C.; Vojvodic, A.; Hwang, H.Y.; Norskov, J.K.; et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 2016, 353, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.-B.; Xia, Y.-J.; Zhu, X.-X.; Pei, Y.-R.; Lang, X.-Y.; Jiang, Q. Self-supported Ni5P4/Co3O4 electrode with optimized electron structure as an efficient electrocatalyst for alkaline hydrogen evolution reaction. J. Alloys Compd. 2025, 1022, 179916. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H.J. Transition metal carbides and nitrides in energy storage and conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef]
- Christinelli, W.; da Trindade, L.; Trench, A.; Quintans, C.; Paranhos, C.; Pereira, E.J.E. High-performance energy storage of poly (o-methoxyaniline) film using an ionic liquid as electrolyte. Energy 2017, 141, 1829–1835. [Google Scholar] [CrossRef]
- Lee, J.A.; Shin, M.K.; Kim, S.H.; Cho, H.U.; Spinks, G.M.; Wallace, G.G.; Lima, M.D.; Lepró, X.; Kozlov, M.E.; Baughman, R.H.; et al. Ultrafast charge and discharge biscrolled yarn supercapacitors for textiles and microdevices. Nat. Commun. 2013, 4, 1970. [Google Scholar] [CrossRef]
- Yu, J.; Yang, X.; Jia, Y.; Wang, Z.-Q.; Li, W.; Jiang, Y.; Dai, S.; Zhan, W. Regulating socketed geometry of nanoparticles on perovskite oxide supports for enhanced stability in oxidation reactions. Nat. Commun. 2024, 15, 10229. [Google Scholar] [CrossRef]
- Zhao, L.; Bi, S.; Li, J.; Wen, Y.; Zhang, H.; Zhang, D.; Lu, S.; Yin, P.; Shi, F.; Yan, J.; et al. Prussian blue analogues for advanced non-aqueous sodium ion batteries: Redox mechanisms, key challenges and modification strategies. Energy Storage Mater. 2025, 78, 104256. [Google Scholar] [CrossRef]
- Lang, X.; Hirata, A.; Fujita, T.; Chen, M. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat. Nanotechnol. 2011, 6, 232–236. [Google Scholar] [CrossRef]
- Bian, Y.; Xie, L.; Ma, L.; Cui, C. A novel two-stage energy sharing model for data center cluster considering integrated demand response of multiple loads. Appl. Energy 2025, 384, 125454. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, J.G.; Xiao, J.Q. Nanostructured electrodes for high-performance pseudocapacitors. Angew. Chem. Int. Ed. Engl. 2013, 52, 1882–1889. [Google Scholar] [CrossRef]
- Mahmood, N.; Tahir, M.; Mahmood, A.; Yang, W.; Gu, X.; Cao, C.; Zhang, Y.; Hou, Y. Role of anions on structure and pseudocapacitive performance of metal double hydroxides decorated with nitrogen-doped graphene. Sci. China Mater. 2015, 58, 114–125. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Chmiola, J.; Largeot, C.; Taberna, P.-L.; Simon, P.; Gogotsi, Y. Monolithic carbide-derived carbon films for micro-supercapacitors. Science 2010, 328, 480–483. [Google Scholar] [CrossRef]
- Yu, G.; Hu, L.; Liu, N.; Wang, H.; Vosgueritchian, M.; Yang, Y.; Cui, Y.; Bao, Z. Enhancing the supercapacitor performance of graphene/MnO2 nanostructured electrodes by conductive wrapping. Nano Lett. 2011, 11, 4438–4442. [Google Scholar] [CrossRef] [PubMed]
- Balogun, M.-S.; Qiu, W.; Yang, H.; Fan, W.; Huang, Y.; Fang, P.; Li, G.; Ji, H.; Tong, Y. A monolithic metal-free electrocatalyst for oxygen evolution reaction and overall water splitting. Energy Environ. Sci. 2016, 9, 3411–3416. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, X.; Hu, J.; Guo, Y.; Wan, L.J. α-Fe2O3 nanotubes in gas sensor and lithium-ion batteries applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar]
- Chen, P.-C.; Shen, G.; Shi, Y.; Chen, H.; Zhou, C. Preparation and characterization of flexible asymmetric supercapacitors based on transition-metal-oxide nanowire/single-walled carbon nanotube hybrid thin-film electrodes. ACS Nano 2010, 4, 4403–4411. [Google Scholar] [CrossRef]
- Joo, S.H.; Choi, S.J.; Oh, I.; Kwak, J.; Liu, Z.; Terasaki, O.; Ryoo, R. Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 2001, 412, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, D.; Zhang, J.; Balogun, M.S.; Wang, P.; Tong, Y.; Huang, Y. Charge relays via dual carbon-actions on nanostructured BiVO4 for high performance photoelectrochemical water splitting. Adv. Funct. Mater. 2022, 32, 2112738. [Google Scholar] [CrossRef]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. TrAC Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Balogun, M.-S.; Tong, Y.; Huang, Y. Oxygen vacancy–based metal oxides photoanodes in photoelectrochemical water splitting. Mater. Today Sustain. 2022, 18, 100118. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.-C.; Pan, L.; Shen, G.-Q.; Mahmood, N.; Ma, Y.-H.; Shi, Y.; Jia, W.; Wang, L.; Zhang, X.; et al. Engineering cobalt defects in cobalt oxide for highly efficient electrocatalytic oxygen evolution. ACS Catal. 2018, 8, 3803–3811. [Google Scholar] [CrossRef]
- Tahir, M.; Pan, L.; Zhang, R.; Wang, Y.-C.; Shen, G.; Aslam, I.; Qadeer, M.; Mahmood, N.; Xu, W.; Wang, L.; et al. High-valence-state NiO/Co3O4 nanoparticles on nitrogen-doped carbon for oxygen evolution at low overpotential. ACS Energy Lett. 2017, 2, 2177–2182. [Google Scholar] [CrossRef]
- Anantharaj, S.; Reddy, P.N.; Kundu, S. Core-oxidized amorphous cobalt phosphide nanostructures: An advanced and highly efficient oxygen evolution catalyst. Inorg. Chem. 2017, 56, 1742–1756. [Google Scholar] [CrossRef]
- Dutta, A.; Mutyala, S.; Samantara, A.K.; Bera, S.; Jena, B.K.; Pradhan, N. Synergistic effect of inactive iron oxide core on active nickel phosphide shell for significant enhancement in oxygen evolution reaction activity. ACS Energy Lett. 2017, 3, 141–148. [Google Scholar] [CrossRef]
- Jing, M.; Hou, H.; Banks, C.E.; Yang, Y.; Zhang, Y.; Ji, X. Alternating voltage introduced NiCo double hydroxide layered nanoflakes for an asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 22741–22744. [Google Scholar] [CrossRef]
- Liang, F.; Huang, L.; Tian, L.; Li, J.; Zhang, H.; Zhang, S. Microwave-assisted hydrothermal synthesis of cobalt phosphide nanostructures for advanced supercapacitor electrodes. CrystEngComm 2018, 20, 2413–2420. [Google Scholar] [CrossRef]
- Mahmood, N.; Tahir, M.; Mahmood, A.; Zhu, J.; Cao, C.; Hou, Y. Chlorine-doped carbonated cobalt hydroxide for supercapacitors with enormously high pseudocapacitive performance and energy density. Nano Energy 2015, 11, 267–276. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, T.; Zhu, Z.; Xiao, R.; Yao, X.; Huang, Y.; Balogun, M.S. Deciphering the lithium storage chemistry in flexible carbon fiber-based self-supportive electrodes. Carbon Energy 2022, 4, 820–832. [Google Scholar] [CrossRef]
- Sami, A.; Khan, M.M.; Haidar, Z.; Bahajjaj, A.A.A.; Riaz, S.; Naseem, S.; Osman, S.M.; Khan, M.S. Enhanced supercapacitor performance of rGO-Supported BaVO3 nanostructures as advanced electrode materials. Ceram. Int. 2025, 51, 8480–8491. [Google Scholar] [CrossRef]
- Bano, N.; Manzoor, S.; Sami, A.; Shah, S.I.A.; Junaid, A.; Rehman, M.Y.U.; Alshgari, R.A.; Ehsan, M.F.; Ashiq, M.N. Fabrication of vanadium telluride anchored on carbon nanotubes nanocomposite for overall water splitting. J. Am. Ceram. Soc. 2024, 107, 4027–4041. [Google Scholar] [CrossRef]
- Aftab, F.; Duran, H.; Kirchhoff, K.; Zaheer, M.; Iqbal, B.; Saleem, M.; Arshad, S.N. A facile synthesis of FeCo nanoparticles encapsulated in hierarchical N-doped carbon nanotube/nanofiber hybrids for overall water splitting. ChemCatChem 2020, 12, 932–943. [Google Scholar] [CrossRef]
- Ilayas, T.; Anjum, S.; Raja, M.Y.A.; Khurram, R.; Sattar, M.; Mansoor, A. Rietveld refinement, 3D view and electrochemical properties of rare earth lanthanum doped nickel ferrite to fabricate high performance electrodes for supercapacitor applications. Ceram. Int. 2023, 49, 28864–28877. [Google Scholar] [CrossRef]
- Ben-Refael, A.; Benisti, I.; Paz, Y.J. Transient photoinduced phenomena in graphitic carbon nitride as measured at nanoseconds resolution by step-scan FTIR. Catal. Today 2020, 340, 97–105. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Zhao, J. Correlation of structural and chemical characteristics with catalytic performance of hydrotalcite-based CuNiAl mixed oxides for SO2 abatement. Chem. Eng. J. 2013, 223, 164–171. [Google Scholar] [CrossRef]
- Shah, S.I.A.; Ansari, M.N.; Nisa, M.U.; Bano, N.; Shabbir, B.; Alrashidi, K.A.; Mohammad, S.; Khan, M.S.; Ashiq, M.F.; Allakhverdiev, S.I. Harnessing dysprosium telluride/GPE as an effective electrode for energy storage and conversion. J. Energy Storage 2024, 101, 113868. [Google Scholar] [CrossRef]
- Fatima, R.; Sami, A.; Haidar, Z.; Ahmed, F.; Junaid, A.; Qasim, B.; Alfagham, A.T.; Khan, M.S.; Elgorban, A.M.; Shah, S.I.A. Facile synthesis of rGO/DyMnO3 nanocomposite directly grown on nickel foam for supercapacitor applications. J. Am. Ceram. Soc. 2025, 108, e20357. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).